Abstract
Ischemic heart disease (IHD) continues to be a major health threat to women worldwide. Sex-specific differences in IHD presentation, pathophysiology, treatment, and outcomes have increasingly been identified. While IHD care has focused around detection and treatment of obstructive coronary artery disease (CAD), it is clear that symptomatic patients with evidence of ischemia do not always have obstructive CAD. This problem appears to disproportionately impact women; compared to men, women who present with acute coronary syndrome/unstable angina as well as stable angina are more likely to have non-obstructive CAD on coronary angiography, and yet have a high IHD morbidity and mortality. Data indicates that coronary microvascular dysfunction (CMD), due to endothelial and non-endothelial dependent mechanisms, may be an explanation in at least half of these symptomatic women who have evidence of myocardial ischemia. CMD is associated with adverse cardiovascular outcomes, including myocardial infarction, stroke, and heart failure. This chapter focuses on CMD diagnosis and treatment (pharmacological and non-pharmacological approaches) in women with no obstructive CAD.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
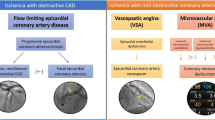
Cardiovascular disease (CVD) continues to be the leading cause of death in women, regardless of race or ethnicity [1]. Among CVD, ischemic heart disease (IHD) is a major contributor to death, and while IHD death rate has declined in older women over the past several years, the death rates appear to be increasing in young women [1]. IHD mortality in women has not fully been explained by ischemia from significant obstructive coronary artery disease (CAD) [2]. In fact, data indicate that women are more likely than men to have the finding of no obstructive CAD on coronary angiography in the settings of unstable angina/acute coronary syndromes as well as in stable ischemic heart disease (SIHD) [3]. Women with IHD experience a greater symptom burden, greater disability, have more IHD risk factors, and more psychosocial risk factors such as depression, anxiety, and post-traumatic stress disorder compared to men [4,5,6]. Previously it was considered a benign finding when a women is found to have no obstructive CAD despite signs and symptoms of ischemia, although mounting evidence from the past two decades indicates that coronary microvascular dysfunction (CMD) may be an explanation in at least half of these cases, and CMD is associated with an elevated cardiovascular risk, which includes myocardial infarction, stroke, and congestive heart failure [7,8,9,10,11]. CMD occurs due to endothelial dependent and endothelial-independent mechanisms. In addition to CMD, etiologies such as prolonged coronary vasospasm, spontaneous coronary artery dissection (SCAD), stress cardiomyopathy (Takotsubo cardiomyopathy), plaque erosion and thrombi, and myocarditis should also be considered in the differential when no obstructive CAD is found in the setting of acute coronary syndrome (Table 8.1). This chapter focuses on CMD diagnosis and treatment in symptomatic women with ischemia and no obstructive CAD.
Definition and Classification
Previously, women with chest pain in the absence of obstructive CAD were labeled with an ill-defined term, “cardiac syndrome X (CSX) ”, which is now considered an outdated term; improved diagnostic methods have shown us that at least half of these CSX patients may have CMD [3, 12]. The coronary microvasculature contributes greater than 70% of resistance to coronary blood flow; microvascular dysfunction can occur due to structural, functional, and extravascular alterations, and results in impaired coronary blood flow reserve (CFR) [13]. Plaque erosions, distal luminal obstruction from micro-embolization, arterial remodeling with smooth muscle cell hypertrophy, and capillary rarefaction contribute to CMD [13, 14]. Myocardial structural abnormalities caused by etiologies including aortic stenosis, hypertrophic or infiltrative cardiomyopathy can also lead to a reduction in myocardial blood flow reserve [13]. Given that CMD is a heterogeneous disorder with many underlying pathophysiologic mechanisms, four main classifications of CMD are proposed that take into consideration primary CMD vs. secondary causes: (1) CMD in the absence of obstructive CAD and myocardial diseases; (2) CMD in the presence of structural myocardial diseases (such as hypertrophic or dilated cardiomyopathy); (3) CMD in the presence of obstructive CAD; (4) iatrogenic CMD such as in the setting of post-percutaneous coronary intervention [7, 13].
The microvasculature cannot be visually assessed on coronary angiography, but the response of the microvasculature to vasoactive substances can be detected by invasive Doppler flow and resistance measurements; advanced non-invasive cardiac imaging can also quantify myocardial flow reserve and diagnose CMD. While coronary endothelial dysfunction and CMD are important contributors in the pathophysiology of IHD in women, recent reports demonstrate that CMD is highly prevalent in both men and women [15]. Contemporary registries are also documenting a high prevalence of non-obstructive CAD during angiographic evaluation in men. Given the emerging epidemic of non-obstructive CAD, systematic sex-specific studies of CMD prevalence, risk, and evidence based treatment are needed [2, 16, 17]. An important issue in the diagnosis and management of CMD is the confusion in the literature to describe this group of patients, and lack of consistent terminology and standardized diagnostic criteria. To address this problem and to improve CMD research and patient care, the Coronary Vasomotion Disorders International Study Group (COVADIS) investigators have proposed international standards for the diagnostic criteria of coronary vasomotor disorders [18, 19].
Pathophysiology
The vascular endothelium is a monolayer of cells that line the inside surface of arteries, veins, and capillaries. It functions as a barrier and a dynamic organ responsive to stimuli and responsible for the production of a number of regulatory factors [20]. Through stimulation via shear stress, temperature changes, and factors such as bradykinin and acetylcholine, the endothelium mediates vasomotor tone, effectively altering the diameter of the lumen, changing vascular resistance and, consequently, modifying blood flow (Fig. 8.1) [21, 22]. The endothelium also mediates hematologic effects, including the inhibition of clotting factors, platelet aggregation, and inflammatory cell adhesion [23]. Furthermore, the endothelium engages in vascular regeneration through endothelial progenitor cells as mediators capable of vascular repair [22].
Diagram of endothelial function . Endothelium-derived dilating and constricting factors. ACE angiotensin converting enzyme, cAMP cyclic adenosine monophosphate, cGMP cyclic guanosine monophosphate, EDHF endothelium-derived hyperpolarizing factor, EDRF endothelium-derived relaxing factor, GTP guanosine triphosphate, NO nitric oxide, NOS nitric oxide synthase, PGI 2 prostacyclin, TxA 2 thromboxane A2. (Reprinted with permission from Elsevier) [21]. (Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998; 105(1A): 32s-39s)
Endothelial dysfunction (ED) occurs when homeostatic mechanisms are altered, resulting in the loss of several key regulatory functions [20, 21]. ED plays a fundamental role in early atherosclerosis and plaque formation; peripheral as well as coronary endothelial dysfunction are both associated with adverse cardiovascular outcomes [24, 25]. The hallmark of endothelial dysfunction pertains to oxidative stress, causing upregulation of renin-angiotensin system, release of pro-inflammatory cytokines, and consequently, a reduction of NO [20, 21]. Lower NO bioavailability results in a pro-thrombotic, pro-inflammatory environment, paving the way to the development of atherosclerotic plaque lesions [21, 23]. Gender may also play a significant role in the development of ED as certain single nucleotide polymorphisms (SNPs) associated with a higher risk of coronary ED have been found to be sex specific [26].
ED is associated with cardiovascular risk factors, including aging, a sedentary lifestyle, obesity, hypertension, hypercholesterolemia, diabetes mellitus, and tobacco use, although CMD is not effectively reflected by traditional CVD risk factors [25, 27,28,29,30,31,32]. Impaired microvascular vasodilation and/or abnormal vasoconstriction which result in failure to auto-regulate blood flow implicates autonomic nervous system dysfunction as an important mechanistic pathway in CMD. Women with CMD also tend to have angina at low cardiac workloads and mental stress-related angina, which likely involves autonomic regulation [33,34,35,36,37,38]. Myocardial ischemia due to mental stress is independent of CAD severity [39], and the normal increase in coronary diameter to mental stress is blunted in those with endothelial dysfunction [40,41,42]. Psychosocial risk factors such as depression and anxiety are highly prevalent in women, are associated with adverse outcom es and may mediate the link between mental stress and CMD [43,44,45,46]. Normally, epicardial coronary arteries contribute less than 10% of vascular resistance, whereas the microcirculation accounts for the majority of resistance and thus regulates blood flow according to the myocardial oxygen demand [13, 47]. In addition to metabolic and local autoregulatory mechanisms that control coronary blood flow, the ANS, via the sympathetic and the parasympathetic (vagal) systems, plays a critical role in vasomotor regulation [33,34,35,36, 48]. Previous studies conducted in CSX have observed impaired parasympathetic tone as well as sympathetic predominance [49,50,51]. In CSX patients, abnormal cardiac adrenergic nerve function detected by using the sympathetic nuclear imaging isotope, 123I-meta-iodobenzylguanidine (mIBG), has been reported previously [52]; no associations have been found between a low measured coronary flow reserve and abnormal cardiac sympathetic function [53].
Pathophysiologic links between CMD and progression to heart failure with preserved ejection fraction (HFpEF) have been proposed and are being investigated [29,30,31]. It has been hypothesized that in patients with CMD, repetitive bouts of microvascular ischemia may lead to microinfarctions, fibrosis, and diastolic dysfunction, and progressive heart failure. In the Women’s Ischemia Syndrome Evaluation (WISE) study, those suspected of ischemia who had no obstructive CAD, one third were found to have an elevated left ventricular end diastolic pressures. In WISE , those women with signs and symptoms of ischemia who had no obstructive CAD and were followed for 6-years, heart failure hospitalization was predominately due to preserved ejection fraction [54]. In another WISE cohort, an elevated interleukin-6 level predicted heart failure hospitalization and all-cause mortality, suggesting that inflammation may be a mediator in CMD-associated HFpEF [55]. Recently, acetylcholine-induced coronary microvascular spasm was shown to be associated with diastolic dysfunction in patients with no obstructive CAD [56].
Other Etiologies of Angina, Ischemia, and No Obstructive CAD
In addition to CMD, when a woman presents with signs and symptoms of ischemia in the setting of no obstructive CAD, etiologies such as coronary artery spasm, spontaneous coronary artery dissection, and stress cardiomyopathy should be in the differential and are briefly discussed here.
Coronary Artery Spasm
Coronary artery spasm can occur in the presence of significant atheroma or in the absence of angiographic lesions, and prolonged vasospasm can progress to myocardial ischemia and infarction. While vasospastic angina can occur in both the epicardial vessels and the microvasculature, the underlying cause of coronary artery vasospasm remains unclear [57]. Several mechanisms, including autonomic nervous system activation by stimuli, endothelial dysfunction, smooth muscle hypercontractility, inflammation, and oxidative stress have been implicated [58]. Medications such as sympathomimetics, non-selective beta-blockers, and ergot alkaloids can also precipitate vasospasm [58]. In a porcine model, both adventitial inflammation and endothelial dysfunction have been directly implicated in the pathogenesis of coronary artery spasm [59]. While driven by a number of stimuli, the primary mechanism driving spasm is from vascular smooth muscle hyperreactivity [19]. In women with chest pain and normal coronary arteries angiographically, there is increased prevalence of both epicardial and microvascular coronary constriction [57]. A classic entity attributed to coronary artery spasm, Prinzmetal’s angina is a variant angina with preserved exercise cap acity and chest pain associated with transient ST elevations [57]. The Coronary Vasomotion Disorders International Study Group has proposed diagnostic criteria to define vasospastic angina which includes the presence of nitrate-responsive angin a, transient ischemic EKG changes (ST elevation, ST depression, nega tive U waves) and coronary artery spasm in response to a provocative stimulus [18].
Spontaneous Coronary Artery Dissection (SCAD)
Predominantly occurring in young women, spontaneous dissections in the coronary arteries occur when a separation of the arterial wall results in hemorrhage (Fig. 8.2), subsequently causing myocardial infarction. The mechanisms that precipitate SCAD are not well understood, and SCAD is not associated with CAD risk factors. Given the predominance in young women and in peripartum females, hormonal changes may potentially contribute to the development of SCAD [60]. SCAD can be either due to an intimal tear or dissecting medial hematoma, possibly from rupture from the vaso vasorum, and can occur in vessels with and without atherosclerosis. In fact, optical coherence tomography has distinguished two distinct subtypes of SCAD: (1) a false lumen between the adventitia and media associated with an intimal tear and (2) separation of the media and adventitia with an intramural hematoma with or without an intimal tear [61]. Adventitial vaso vasorum proliferation has also been linked with SCAD, but no causal relationship has been established [62]. In the abse nce of atherosclerosis, fibromuscular dysplasia (FMD), connective tissue disorders, the peripartum state, extreme exertion, inflammatory dis orders, and coronary artery vasospasm have all been implicated as potential underlying etiologies [60, 63]. Furthermore, coronary tortuosity and extra coronary vascular abnormalities such as aneurysms and aortic tortuosity in the neck, abd omen, and pelvis often coincide with SCAD [60].
Intracoronary Imaging of SCAD (a) False lumen with intramural hematoma (plus sign) and intimal rupture (arrow). (b) False lumen with intramural hematoma (plus sign). (c) False lumen with intramural hematoma (plus sign) (Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 2016; 68(3):297-312)
Stress Cardiomyopathy
Takotsubo cardiomyopathy is typically comprised of a transient, apical left ventricular dysfunction typically induced by strong emotional or physical stimuli (Fig. 8.3) [64]. Patients present with signs of myocardial infarction with symptoms, EKG changes, and cardiac biomarker elevations but are found to have no obstructive CAD on angiography; mortality in the acute phase is comparable to acute myocardial infarction due to obstructive CAD [65]. In addition to the apical-sparing variant, there are other ventricular patterns that have been described including biventricular pattern [64]. Most cases of Takotsubo cardiomyopathy involve post-menopausal women, have normal coronary arteries, and is not associated with acute plaque rupture from underlying atherosclerosis [64]. The underlying mechanisms are unclear, but prolonged coronary spasm and CMD have been implicated in the setting of a catecholamine surge [64]. Given the rare occurrence of coronary spasm, CMD is more likely; however, the determination of CMD as the cause or a conseq uence has been difficult [64]. It remains unclear why a majority of cases present in women [66, 67]. A possible mechanism for post-menopausal female prevalence may include the age-specific decrease in vagal tone and baroreflex sensitivity from reduced estrogen levels, thereby augmenting the catecholamine-mediated stress response. Recently, increased SNS activity and impaire d BRS has been shown in women with takotsubo syndro me compared to women with chronic heart failure [68].
Clinical Presentation
Compared to acute myocardial infarction in men, stable angina is the most common initial presentation of IHD in women [69]. The non-obstructive pattern of CAD, as opposed to the detectable flow-limiting atherosclerotic disease, oftentimes delay recognition of IHD in women, since routine diagnostic testing currently focuses on obstructive CAD. Although both women and men have typical and atypical symptoms of angina, women are more likely to present atypically, including dyspnea, upper back pain, nausea/vomiting, indigestion, palpitations, or unusual fatigue [70]. Women also verbalize symptoms more than men and have greater somatic awareness [71]. In a recently published study of 155 women with no obstructive CAD who had CMD, 30% were found to have typical angina (defined as substernal chest pain precipitated by physical or emotional stress and relieved with rest or nitroglycerin); and those with t ypical angina had worse endothelial dysfunction and quality of life [72].
Risk Factors
A comprehensive discussion on traditional and novel IHD risk factors in women [5] is not the focus of this chapter, but we highlight some unique risk factors below to point out that current IHD risk assessment tools (i.e. the Framingham Risk Score, the Reynold’s Risk Score [73], and the atherosclerotic cardiovascular disease (ASCVD) risk score ) do not take into account unique IHD risk factors that may contribute to CMD [74, 75]. IHD in women is associated with traditional risk factors, such as age, hypertension, diabetes, cigarette smoking, dyslipidemia, obesity, and physical inactivity. Patients with CMD are more likely to have these risk factors compared to the general population; however, in the WISE study, cardiac risk factors were modestly related to CMD (diagnosed by invasive coronary reactivity testing or perfusion index by cardiac magnetic resonance imaging). In women suspected of IHD with no obstructive CAD and with IVUS measured atherosclerosis, waist circumference and systolic bloo d pressure were independently associated with plaque severity (even after adjustment of factors such as age, diabetes, hyperlipidemia, hormone replacement, and tobacco smoking); thus, authors concluded that metabolic syndrome by itself is not an independent IHD predictor in women [76].
There are unique ASCVD risk factors for women that should also be considered, such as gestational hypertension, pre-eclampsia, gestational-diabetes, and pre-term labor. Pre-eclampsia affects 3–7% of all pregnancies and has been shown to result from vascular dysfunction—increased vascular resistance and vasoconstriction occurs in vasculature of the mother [77]. A large meta-analysis showed that after pre-eclampsia, women had an increased risk for ischemic heart disease after 11.7 years (RR: 2.16, 95% CI: 1.86–2.52), stroke after 10.4 years (RR: 1.81, 95% CI: 1.45–2.27), and overall mortality after 14.5 years (RR: 1.49, 95% CI: 1.05–2.14) [78]. The shared pathway of vascular dysfunction may explain the association of pre-eclampsia and development of ASCVD and adverse cardiovascular events that occur years later [77].
Autoimmune disorders , such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) , and depression are conditions that disproportionately affect women and are also recognized risk factors for IHD, and were added to the Women’s Heart Disease Prevention guidelines in 2011 [79, 80]. SLE patients who present with chest pain are often thought to have pericarditis even though there are often no objective EKG findings and the physical exam does not point to pericarditis. It may be possible that some of these patients are having angina due to CMD [81,82,83,84]. Ishimori et al. found that 44% of women with SLE with typical and atypical chest pain without obstructive CAD had visual perfusion defects on stress cardiac magnetic resonance, compared to 0% in 10 asymptomatic reference control subjects (p = 0.014). The presence of SLE was a significant predictor of an abnormal myocardial perfusion reserve index (MPRI) [82]. Carotid plaque determined by carotid ultrasonography was found to be more prevalent in patients with SLE than in the matched controls (37.1% vs. 15.2% respectively, p < 0.001) [85]. The diagnosis of RA was also associated with a 50% increased risk of ASCVD death in a meta-analysis of 111,758 patients (meta-standardized mortality ratio 1.59, 95% CI: 1.39–1.61) [86].
The American Heart Association (AHA) recognizes depression as a risk factor for adverse outcomes in patients with acute coronary syndrome [87]. Women generally have higher contributions of psychosocial risk factors (45.2% versus 28.8% in men) to MI [88]. Depressive symptoms also predicted the presence of CAD in women 55 years-old or younger (OR: 1.07, 95% CI: 1.02–1.13) and increased the risk of death in this group of women (adjusted HR 1.07; 95% CI: 1.02–1.14) [89]. In 514 women suspected of IHD, the WISE study reported that anxiety predicted cardiac symptom severity and healthcare utilization in women [45]. Anxiety in this study was measured by the Spielberger Trait Anxiety Inventory (STAI), anxiolytic use, and anxiety disorder treatment history. The use of anxiolytics predicted hospitalizations for chest pain and catheterizations (HR: 2.0, 95% CI: 1.1–4.7), as well as symptoms of nighttime angina and nitroglycerine use. STAI scores and anxiety treatment history correlated with nighttime angina, angina frequency and shortness of breath. Furthermore, anxiolytic use and a higher STAI value (using a median of 18) predicted greater costs, including medications and hospitalizations, regardless of severity of CAD [45].
Assessment
Non-invasive Assessment
Exercise Treadmill Testing
One of the most widely available and relatively inexpensive forms of stress testing, exercise treadmill testing (ETT ), is recommended as the first step for evaluation of women with suspected IHD, per the 2014 AHA consensus statement on stress testing in women [90]. This test is appropriate for women who are able to physically achieve adequate levels of exercise, defined as 4–5 metabolic equivalents (METs) and for those with a normal resting electrocardiogram (ECG). Significant ST-segment depression with exercise is considered diagnostic for ischemia, either from obstructive CAD or from non-obstructive CAD; ETT is unable to accurately distinguish between ischemia due to obstructive CAD vs. non-obstructive CAD [3, 91]. Repro duction of symptoms, heart rate and blood pressure responses, as well as heart rate recovery should also be considered when assessing a women for IHD [92, 93]. ETT has been considered to be less accurate in women because of a higher false positive rate; it should be noted that the gold standard in these cases was evaluation with angiography for detection of obstructive CAD, which is an anatomic assessment (and not a physiologic assessment of impaired myocardial blo od flow). Given that women with IHD often present with no obstructive CAD, the ST segment depressions may represent ischemia due to CMD. Furthermore, mental stress induced angina is more prevalent in women [40], and ETT may not be as accurate in detection of ST segment changes due to mental stress [39, 40, 94, 95]. Despite limitations of ETT, it is an excellent first-line test for detection of IHD, and functional capacity measured by METs is an important prognostic indicator in both men and women [92].
Stress Echocardiography
Although not commonly used in the clinical setting, Doppler echocardiography is a noninvasive means to measure CFR , which is t he ratio of hyperemic to resting coronary blood flow. CFR of the left anterior descending artery (LAD) in 1660 patients (906 women and 754 men) with chest pain of unknown origin was measured by Doppler in response to dipyridamole. A CFR ≤2 was independently associated with an adverse prognosis in women (hazard ratio [HR] 16.48, 95% confidence interval [CI] 7.17–37.85, p < 0.0001) [96]. Patients with diabetes, obesity, hypertension, were found to have a low CFR of the LAD on dobutamine stress echocardiography [97]. Furthermore, the degree of impairment of CFR was exaggerated as the number of risk factors increased. Contrast echocardiography is another technique where microbubbles can be used to generate time-acoustic intensity curves to calculate blood flow velocity; this can detect myocardial perfusion abnormalities and quantify coronary blood flow, however, is not routinely used in most clinical centers [98,99,100].
Cardiac Positron Emission Tomography (PET) Imaging
Rest/stress myocardial positron emission tomography (PET) is a well-validated and reliable method for quantification of myocardial flow reserve and is used to diagnose CMD [101,102,103,104,105,106,107,108]. Stress agents include dipyridamole, dobutamine, adenosine, or regadenoson, while commonly used nuclear tracers include N-13 ammonia or rubidium-82. PET is also the preferred imaging choice for patients with a body mass index (BMI) >40, with large breasts or breast implants, or chest wall deformities [109]. It can provide reproducible measurements of regional myocardial blood flow in milliliters per minute per gram of tissue, which is a functional parameter used to assess coronary microcirculation [110]. Normal CFR ranges from 2 to 4, i.e. myocardial blood flow increases 2–4 times during peak hyperemia induced by vasodilators such as adenosine [111, 112]. It should be noted that CFR is dependent on rate pressure product, and thus blood pressure changes can impact CFR. A reduction in CFR can be due to obstructive epicardial stenosis or due to microvascular dysfunction in a setting of non-obstructive CAD. A low CFR is associated with a worse prognosis, and a preserved CFR has a very high negative predictive value for excluding ischemia [15, 96, 113,114,115,116,117].
Given its high diagnostic accuracy, reproducibility, short tracer half-life, and fast acquisition protocols that minimize radiation exposure, PET is an essential nuclear testing modality to non-invasively diagnose CMD. O ne of the limitations of cardiac PET is that it is not available at many centers and is often not covered by medical insurance, which makes it challenging to diagnose a low coronary flow reserve non-invasively at some centers. At centers where cardiac PET, CMR or invasive coronary reactivity testing is not available, we recommend empiric symptom management with anti-anginal medications based on symptoms, risk factor profile, and ETT results. Women with persistent symptoms can also be referred to centers of excellence that have the ability to diagnose CMD for a definitive diagnosis and a therapeutic plan.
Cardiac Magnetic Resonance (CMR) Imaging
CMR imaging is an emerging technique that has numerous advantages, including high spatial resolution and evaluation of ventricular function, perfusion, viability and scar assessment as well as m yocardial tissue characterization in a single exam. A large, prospective trial compared CMR to single-photon emission computed tomography (SPECT) in 752 patients with suspected CAD undergoing coronary angiography. CMR consisted of rest and adenosine stress perfusion, cine imaging, and late gadolinium enhancement. Its sensitivity and negative predictive value was significantly superior, compared to SPECT (p < 0.0001 for both) [118]. CMR can detect segmental as well as global ischemia and is being used as a technique to detect CMD in some centers [119, 120]. In the WISE study, semi-quantitative myocardial perfusion reserve index (MPRI) was determined from CMR in symptomatic women with no obstructive CAD who were diagnosed by CMD by invasive coronary reactivity testing (CRT). Women with symptoms had lower pharmacologic stress M PRI compared to controls, and lower MPRI predicted one or more abnormal CRT variables. The sensitivity and specificity of an MPRI threshold of 1.84 predicting CRT abnormality were 73% and 74%, respectively [121]. Although a promising field of imaging, further studies assessing the diagnos tic and progn ostic abilities of CMR are needed.
Biomarkers
Oxidative stress and inflammation are implicated in the pathogenesis of endothelial dysfunction and IHD [122,123,124,125]. Glutathione maintains thiol groups of biomolecules in their reduced states and prevents peroxidation of membrane lipids [126]. It also transports nitric oxide (NO), the major endogenous vasodilator, from larger epicardial coronaries to the distal smaller vessels and microcirculation [127]. Studies have shown an association between glutathione levels and CFR, indicating that lower glutathione levels reflect higher amounts of oxidative stress and endothelial dysfunction [128, 129]. Markers of oxidative stress, such as aminothiols and asymmetric dimethylarginine (ADMA) have been studied in endothelial injury and dysfunction. Asymmetric ADMA is a byproduct of the metabolism of l-arginine, the substrate for NO production, that has been found to be elevated in patients with hypertension, dyslipidemia and atherosclerosis [130,131,132,133]. ADMA acts as a competitive inhibitor to endothelial nitric oxide synthase, leading to decreased NO production and bioavailability [130]. Plasma ADMA levels have correlated with endothelial dysfunction [131] and subclinical atherosclerosis [134].
Markers of inflammation have also been studied as possible diagnostic or prognostic tools. Highly sensitive-C reactive protein (hs-CRP) is a nonspecific marker of inflammation that has been studied in the prediction of cardiovascular risk in women [135]. Most notably, the Reynolds Risk Score used hs-CRP in addition to traditional risk factors, to estimate the 10-year risk of major adverse cardiac events from studying 24,558 initially healthy women for a median of 10.2 years [73]. However, hs-CRP can fluctuate with infections, inflammatory conditions, and even throughout the day and reflects metabolic changes of many pathways [136]. While hs-CRP has been linked to atherosclerosis , a study of women undergoing angiography for suspected ischemia found that hs-CRP was not associated with angiography CAD, but was predictive of adverse CV outcomes [137]. In another WISE study of women with ischemia and no obstructive CAD with preserved ejection fracti on, interleukin-6 levels predicted heart failure hospitalization and all-cause mortality [55].
Progenitor cells (PC) are essential to endothelial cell regeneration and can be measured peripherally. In 123 women with non-obstructive CAD enrolled in WISE study, lower CFR was associated with higher levels of circulating PCs [138], implying that PCs are mobilized in response to the CMD and resultant chronic myocardial ischemia.
Invasive Assessment
Coronary Reactivity Testing
Coronary reactivity testing (CRT) may be used in patients with angina and evidence of myocardial ischemia to definitively diagnose coronary endothelial and microvascular dysfunction. CRT can help clarify the etiology of symptoms and guide management in those with persistent symptoms and objective evidence of ischemia. CRT involves intra-arterial infusions of non-endothelium-dependent vasodilators, such as adenosine, or nitroglycerin, or endothelium-dependent vasodilators, such as acetylcholine, bradykinin, or substance P (Fig. 8.4), although the latter two are not commonly used. A Doppler flow wire, placed in the epicardial vessel, measures the coronary flow velocity in response to vasoactive agents. Quantitative coronary angiographic response of epicardial diameter changes is also measured during CRT. Coronary blood flow can then be calculated by the product of average peak velocity and vessel diameter.
Coronary Angiogram and coronary reactivity testing. (a) The figure shows Doppler flow wire in the left anterior descending artery (red arrow). (b) In response to acetylcholine infusion, there is abnormal coronary artery vasoconstriction (black arrows), indicating endothelial dysfunction. (c) Resolution by intracoronary nitroglycerin (Wei, Mehta, Johnson, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease. Results from the NHLBI-sponsored WISE study. JACC: Cardiovascular Interventions. 2012. 5(6): 646-652)
To test endothelial-dependent r esponse of coronaries, acetylcholine is injected into the coronary artery in increasing concentrations, and the diameter and velocity changes are measured. Acetylcholine activates the endothelial muscarinic receptors that metabolize l-arginine, stimulate NO synthase and thus generate NO [139], which then activates cyclic GMP and mediates vascular smooth muscle cell relaxation. In normal or preserved endothelial function, coronary epicardial vessels dilate in response to acetylcholine. However, with an impaired endothelial function, acetylcholine’s direct smooth muscle constrictor effects on epicardial vessels overcome the dilator effects of endothelium-dependent NO release [140]. The ratio between coronary blood flow during maximal dilation with adenosine compared to baseline is defined as the coronary flow reserve (CFR) [141]. CFR represents the coronary circulation’s capacity to respond to an increase in oxygen demand with an appropriate increase in blood flow, where an appropriate CFR is over 3 in healthy adults and CFR <2 is considered impaired. It should be noted that CFR varies by factors such as age, sex, and rate-pressure product [142]. There are multiple studies have reported on the prognostic value of CFR, in those with and without obstructive CAD [143]. A CFR of less than 2.3 in a WISE study in women with no obstructive CAD wa s prognostic of adverse outcomes, including death, nonfatal MI, nonfatal stroke, hospitalization for congestive heart failure, angina and other vascular events (event rate 26.7% vs. event rate 12.2% for CFR ≥2.3; p = 0.01) [115].
Patients in the lowest tertile of CFR had the largest number of adverse events, when compared to those in the highest tertile [144]. Suwaidi et al. categorized patients with insignificant CAD into normal endothelial function, mild and severe endothelial dysfunction according to their response in coronary blood flow to acetylcholine. Fourteen percent with severe endothelial dysfunction had a total of ten cardiac events, whereas those with normal function and mild endothelial dysfunction had none (p < 0.05) [145]. The Women’s Ischemia Syndrome Evaluation (WISE) study also found that an abnormal response to acetylcholine predicted cardiac events in a median follow-up of approximately 4 years [146]. Approximately half of patients with acute coronary syndrome with no obstructiv e CAD were found to have coronary vasospasm on intracoronary acetylcholine provocation testing in the Coronary Artery Spasm as a Frequent Cause for Acute Coronary Syndrome (CASPAR) study [147].
Currently there are no standardized protocols assessing coronary microvascular function and each institution performs these tests according to their own protocols. The doses of the vasoactive agents used in the WISE study were 18 mcg and 64 mcg for intracoronary adenosine, graded infusions of 0.364 and 36.4 mcg over 3 min for acetylcholine and 200 mcg of nitroglycerin in the left coronary artery [ 148]. No reactivity-testing related deaths were found in the 293 women in the WISE study, and two serious adverse events (0.7%) occurred (one dissection and one MI resulting from spasm) [148].
Management
Lifestyle Modifications
Women with IHD should all be counseled on a Mediterranean diet, physical activity, and tobacco cessation. The AHA and American College of Sports Medicine recommend at least 30 min of moderate-intensity physical activity for at least 5 days of the week, or 20 min of vigorous aerobic exercise 3 days a week, or a combination of the two [149], and for those with metabolic syndrome, regular exercise is crucial [150]. Exercise has been shown to improve endothelial function, independently of its reduction in cardiovascular risk factors [151]. Twelve weeks of aerobic interval training and a low-calorie diet of 800–1000 kcal per day increased the CFR of 70 obese, non-diabetic patients with CAD and a baseline CFR below 2.5: CFR increased by 0.26 (95% CI: 0.04–0.48) in the aerobic interval training group and by 0.39 (95% CI: 0.13–0.65) in the low-calorie diet group. Another study found a 29% increase in CFR in patients with coronary endothelial dysfunction, diagnosed on CRT, after only 4 weeks of exercise, compared to those in the control group [152]. Smoking is also a well-known and most important modifiable risk factor for ASCVD, and the proposed mechanisms are thought to be secondary to smoking increasing adherence of platelets and macrophages to the vessel wall, developing a procoagulant and inflammatory environment [153]. Smoking cessation, for only 2 weeks, has been found to reduce platelet aggregations, and thus, decrease oxidative stress [154], a major culprit in endothelial dysfunction.
Pharmacotherapy
While some medications have been shown to improve endothelial function and cardiovascular outcomes in patients without obstruc tive CAD (Fig. 8.5), others have been shown to improve symptoms. Here we discuss a variety of pharmacotherapies that may be used in women with angina and IHD, although large outcomes based clinical trials in those with CMD are needed. Our current strategy is to use anti-ischemic, anti-anginal, and anti-atherosclerosis medications in those with signs and symptoms of ischemia who have been diagnosed with CMD.
Summary on the effects of each drug on endothelial function and cardiovascular outcomes (Lerman et al. Prog Cardiovasc Dis . 2015;57(5):431-42) *Torcetrapib and dalcetrapib
Statins
There are no large randomized controlled outcome trials of statins in subjects with no obstructive CAD who have CMD. However, there are intermediate trials demonstrating benefit of statins on endothelial function, and thus the current ACC/AHA recommendations for statin therapy [155] can be followed for patients with CMD, especially given the high prevalence of subclinical coronary atherosclerosis. Patients with CSX with dyslipidemia on simvastatin were found to have significant improvement in endothelial function, measured by brachial artery flow-mediated dilatation, as well as a decrease in LDL levels [156]. Studies have also shown that atorvastatin improved CFR as early as 2 months [157]. Interestingly, independent of its effects on LDL cholesterol, statins have been shown to improve endothelial dysfunction, by increasing the bioavailability of NO [158, 159] and by reducing circulating levels of adhesion molecules P-selectin and ICAM-1 [160]. Statins have also been shown to improve exercis e tolerance and reduce angina [161].
Antiplatelet Agents
Although they may not have significant obstructive plaque burden, patients with CMD have been shown to have coronary atherosclerosis by intravascular ultrasound in the WISE study [76, 162]. In symptomatic patients with demonstrable ischemia, aspirin can be used as recommended in ACC/AHA stable angina guidelines [163], even if without obstructive CAD. There are no clinical trials of dual anti-platelet therapy in women with no obstructive CAD, and thus a decision of adding clopidogrel or another anti-platelet agent in addition to aspirin in women with no obstructive CAD should be made on an individual basis and clinical history.
Symptom Management
Beta-blockers
Beta-blockers reduce the frequency and severity of angina symptoms [163], and specifically carvedilol and nebivolol are preferred in patients with non-obstructive coronary disease with their anti-oxidant properties by stimulating the release of endothelial NO [164]. In one study, four months of carvedilol improved flow-mediated, endothelial-dependent dilatation in patients with CAD, while placebo and 2 h after carvedilol had no significant effect on endothelial dysfunction [165]. Intracoronary administration of nebivolol also increased CFR, in a dose-dependent response, in patients with CAD in another study [166].
Interestingly, in 24 men and women with angina and non-obstructive CAD, nebivolol did not improve endothelial function. However, it did lead to plaque progression and constrictive remodeling assessed by intravascular ultrasound in 1 year, likely from the higher number of low shear stress segments in those who received nebivolol [167].
Angiotensin Converting Enzyme Inhibitors (ACE-I)
ACE-Is have also been shown to improve CFR and exercise capacity in patients with CSX [168]. CFR improved in women with CMD (defined as CFR <3 after intracoronary adenosine) and lower baseline CFR values who were assigned to quinapril, as opposed to placebo. Those who received the ACE-I also reported improved angina measured by the Seattle Angina Questionnaire [169]. The combination of atorvastatin and ramipril was used in a randomized trial of patients with angina, normal coronary angiograms, and ischemia during stress testing. This combination therapy improved both Seattle Angina Questionnaire scores and exercise duration, compared to placebo. Increased brachial artery flow-mediated dilation and decreased extracellular superoxide dismutase were found in these patients on both a statin and an ACE-I [170]. After treatment with an angiotensin receptor blocker, significant improvements in MBF in response to adenosine were noted in patients with hypertension and CAD before a reduction in blood pressure was seen, suggesting improvement on microvascular function [171]. Sixteen weeks of treatment with pioglitazone , an insulin sensitizer thiazolidinedione, in 26 nondiabetic patients also improved MBF in response to adenosine, as well as myocardial glucose use—again suggesting a beneficial effect on coronary microvascular function [172].
Calcium Channel Blockers (CCBs)
CCBs improve angina and exercise tolerance in patients with CMD [173, 174]. However, several trials comparing beta-blockers, nitrates and CCBs have shown that beta-blockade may be more effective compared to CCBs. Atenolol and propranolol were superior in improving angina, compared to amlodipine and verapamil, respectively [175, 176]. However, in those who are suspected of coronary vasospastic angina, CCBs are first line therapy in addition to nitrates [177]. Several trials have shown that diltiazem , verapamil , and nifedipine reduce episodes of Prinzmetal’s angina [178,179,180].
l-Arginine
l-Arginine is the substrate for endothelial NOS to produce NO. Thus, increasing levels of the substrate and thus increasing NO production could possibly treat endothelial dysfunction. Indeed, l-arginine treatment for 6 months improved endothelial function in patients with non-obstructive CAD [181]. Randomized trials assessing l-arginine’s role in management of CMD are needed.
Nitrates
Nitrates upregulate cGMP which leads to relaxation of vascular smooth muscle cells and causes a vasodilatory effect. While there are no randomized trials, an observational study of 99 patients with CSX showed that in 40–50% of the patients nitrates were effective anti-anginals agent [182]. Physicians must advise patients to take a nitrate-free interval of at least 12 h daily, as nitrate tolerance may develop after continued use.
Ranolazine
Ranolazine reduces calcium overload in myocytes by altering the sodium current and is used as an anti-angina medication [183]. It has been specifically tested in subjects with CMD and non-obstructive CAD. A randomized, placebo-controlled pilot study in 20 women with CMD demonstrated improvement in angina by Seattle Angina Questionnaire measures [184]. A subsequent larger RWISE trial in 128 patients (96% women) with CMD found that ranolazine improved angina and CMR determined myocardial perfusion reserve index in those with more severe coronary microvascular dysfunction (CFR <2.5), but did not significantly improve the severity or frequency of angina or CFR in the overall cohort [185].
Estrogen
The risk of IHD increases after menopause, along with the development of ASCVD risk factors, such as hypertension, diabetes, and dyslipidemia. Thus, estrogen has been implicated in the pathophysiology of CMD, especially as the mean age of diagnosis of CSX patient was 48.5 years and 62% of the women were postmenopausal [182]. Estrogen has been hypothesized to have a beneficial effect on vascular reactivity, as well as lipid profiles [186]. Transdermal estrogen improved angina as well as endothelium-dependent coronary vasomotion in 15 postmenopausal women with normal coronary angiograms: in 24 h, there was no vasoconstriction with acetylcholine with a mean diameter change significantly different from the pre-estrogen diameter reduction that was observed (p = 0.003) [186].
However, the results of Women’s Health Initiative (WHI) and the Heart and Estrogen/Progestin Replacement Study (HERS) led to the U.S. Preventive Services Task Force (USPSTF) recommendations against the use of combined estrogen and progestin or estrogen alone for prevention of ASCVD in menopausal women. There was no difference in incidence of CHD events between the estrogen-progestin and placebo group in the HERS trial, despite a decrease in low-density lipoprotein (LDL) and an increase in high-density lipoprotein (HDL) in the experimental group [187]. The combined estrogen-progestin therapy group of the WHI was at increased risk for total ASCVD, including CHD (HR: 1.29; 95% CI 1.02–1.63), stroke (HR: 1.41; 95% CI 1.07–1.85), and total cardiovascular disease (HR: 1.22; 95% CI 1.09–1.36) over an average follow up of 5.2 years [188]. The use of unopposed estrogen increased the risk of stroke (HR: 1.39; 95% CI 1.10–1.77) and total cardiovascular disease (HR: 1.12; 95% CI 1.01–1.24) [189]. Women in the more recent Kronos Early Estrogen Prevention Study (KEEPS) experienced improvement in menopausal vasomotor symptoms with hormonal therapy, but again there was no improvement in subclinical markers of atherosclerosis, measured by carotid intima-media thickness or coronary artery calcifications [190].
Ivabradine
Ivabradine reduces heart rate by selectively inhibiting the funny channels (If) in the sino-atrial node [191,192,193] and was recently approved for treatment of chronic stable angina in patients with normal sinus rhythm in the U.S. It was non-inferior to atenolol at all doses in a randomized double-blind trial in 939 patients with stable angina [194], and found to improve CFR in patients with stable CAD [195]. However, another study showed no effect on CMD, but an improvement of symptoms [196].
Other Medical Therapies
Low-dose tricyclic antidepressants (TCA) have also been studied, as impaired cardiac nociception is thought to play a role in CMD. Imipramine has been studied and reported to reduce the frequency of pain [197, 198]. TCAs may modulate the effects of norepinephrine uptake and anticholinergic effect that lead to analgesia.
Nicorandil , an adenosine triphosphate sensitive nitrate-potassium channel agonist used in Europe, was found to improve peak exercise capacity in patients with cardiac syndrome X and angina, but failed to significantly improve exercise-induced ST changes [199, 200]. Fasudil , a rho kinase inhibitor currently available in Japan, inhibits smooth muscle vasoconstriction and has been shown to increase ischemic threshold and exercise duration in patients with stable angina [201,202,203,204]. Trimetazidine inhibits cardiomyocyte free fatty acid beta-oxidation and promotes glucose oxidation, which leads to decreased acidosis and preservation of energy by the ischemic cell [205]. While its role in patients with CMD is not yet clear [206,207,208], it has shown benefit in chronic stable angina as anti-ischemic and anti-anginal therapy [209, 210]. Compared to placebo, trimetazidine reduced the number of weekly angina attacks, weekly nitroglycerin tablet consumption and improved exercise time to 1 mm segment depression [210].
Non-pharmacologic Treatments
Enhanced External Counterpulsation (EECP)
EECP is a non-invasive, FDA approved treatment for management of refractory angina. It has been shown to improve functional capacity, anginal class, and time to ST-segment depression during exercise stress testing in patients with CAD [211,212,213], with its benefit lasting as long as 3 years [214]. Proposed mechanisms include improved collateral blood flow and endothelial function from the diastolic augmentation of myocardial perfusion via inflation of pneumatic cuffs on the lower extremities during EECP [215,216,217].
Stem Cell Therapy
Bone-marrow stem-cell transplantation has improved exercise capacity, myocardial perfusion, and cardiac function in patients with MI [218]. However, stem cell therapy remains experimental and has not been studied in patients with angina and no obstructive CAD. Microvascular rarefaction , a reduced number of arterioles and capillaries [219] has been speculated to play a role in coronary microvascular angina [220], and restoring impaired microvascular function has been a focus of pre-clinical stem cell therapy studies [221].
Cognitive Behavioral Therapy and Group Support
An 8-week program of the cognitive behavioral therapy improved angina frequency and severity in women with ischemia and non-obstructive CAD [222]. In a study of 49 women with CSX, 12 monthly group support meetings helped reduce health-care demands and maintained social support for these individuals [223]. A multi-disciplinary team approach of including psychiatrist/psychologist, a chronic pain specialist, along with cardiologist is needed to provide comprehensive care to women who have persistent symptoms of chest pain.
Conclusion
Women have a lower prevalence of obstructive CAD compared to men, yet have high rates of myocardial ischemia and subsequent mortality. CMD is highly prevalent in women with signs and symptoms of ischemia, and CMD should be considered in the differential when no obstructive CAD is found. CMD can be definitively diagnosed by invasive coronary reactivity testing, which assess endothelial and non-endothelial dependent mechanisms. Non-invasive imaging with cardiac PET can provide CFR and diagnose CMD, which is a diagnosis associated with adverse cardiovascular prognosis. In addition to IHD lifestyle modifications, CMD treatment revolves around anti-anginal, anti-ischemic, and anti-atherosclerotic medications, as well as non-pharmacologic strategies to improve symptoms and quality of life. Larger clinical trials are needed in this population to determine therapeutic algorithms and to improve CMD outcomes in women.
References
Bairey Merz CN. Sex, death, and the diagnosis gap. Circulation. 2014;130(9):740–2.
Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135(11):1075–92.
Pepine CJ, Ferdinand KC, Shaw LJ, et al. Emergence of nonobstructive coronary artery disease: a woman’s problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66(17):1918–33.
Bucholz EM, Butala NM, Rathore SS, Dreyer RP, Lansky AJ, Krumholz HM. Sex differences in long-term mortality after myocardial infarction: a systematic review. Circulation. 2014;130(9):757–67.
Mehta PK, Wei J, Wenger NK. Ischemic heart disease in women: a focus on risk factors. Trends Cardiovasc Med. 2015;25(2):140–51.
Mehta LS, Beckie TM, DeVon HA, et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133(9):916–47.
Phan A, Shufelt C, Merz CN. Persistent chest pain and no obstructive coronary artery disease. JAMA. 2009;301(14):1468–74.
Humphries KH, Pu A, Gao M, Carere RG, Pilote L. Angina with “normal” coronary arteries: sex differences in outcomes. Am Heart J. 2008;155(2):375–81.
Reis SE, Holubkov R, Conrad Smith AJ, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141(5):735–41.
Hasdai D, Holmes DR Jr, Higano ST, Burnett JC Jr, Lerman A. Prevalence of coronary blood flow reserve abnormalities among patients with nonobstructive coronary artery disease and chest pain. Mayo Clin Proc. 1998;73(12):1133–40.
Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol. 2012;59(7):655–62.
Kemp HG Jr. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol. 1973;32(3):375–6.
Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356(8):830–40.
Jones E, Eteiba W, Merz NB. Cardiac syndrome X and microvascular coronary dysfunction. Trends Cardiovasc Med. 2012;22(6):161–8.
Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129(24):2518–27.
Patel MR, Dai D, Hernandez AF, et al. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am Heart J. 2014;167(6):846–852.e842.
Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886–95.
Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2015;38(33):2565–8.
Beltrame JF, Crea F, Kaski JC, et al. The who, what, why, when, how and where of vasospastic angina. Circ J. 2016;80(2):289–98.
Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60(16):1455–69.
Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105(1A):32S–9S.
Ozkor MA, Murrow JR, Rahman AM, et al. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation. 2011;123(20):2244–53.
Paneni F, Diaz Canestro C, Libby P, Luscher TF, Camici GG. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol. 2017;69(15):1952–67.
Matsuzawa Y, Guddeti RR, Kwon TG, Lerman LO, Lerman A. Treating coronary disease and the impact of endothelial dysfunction. Prog Cardiovasc Dis. 2015;57(5):431–42.
Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753–67.
Yoshino S, Cilluffo R, Prasad M, et al. Sex-specific genetic variants are associated with coronary endothelial dysfunction. J Am Heart Assoc. 2016;5(4):e002544.
Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8(11):1445–53.
Bairey Merz CN, Shaw LJ, Reis SE, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47(3 Suppl):S21–9.
Crea F, Bairey Merz CN, Beltrame JF, et al. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J. 2017;38(7):473–7.
Nelson MD, Szczepaniak LS, Wei J, et al. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study. Circ Cardiovasc Imaging. 2014;7(3):510–6.
Taqueti VR, Di Carli MF. Clinical significance of noninvasive coronary flow reserve assessment in patients with ischemic heart disease. Curr Opin Cardiol. 2016;31(6):662–9.
Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111(3):363–8.
McCraty R, Atkinson M, Tiller WA, Rein G, Watkins AD. The effects of emotions on short-term power spectrum analysis of heart rate variability. Am J Cardiol. 1995;76(14):1089–93.
Pagani M, Furlan R, Pizzinelli P, Crivellaro W, Cerutti S, Malliani A. Spectral analysis of R-R and arterial pressure variabilities to assess sympatho-vagal interaction during mental stress in humans. J Hypertens Suppl. 1989;7(6):S14–5.
Pagani M, Mazzuero G, Ferrari A, et al. Sympathovagal interaction during mental stress. A study using spectral analysis of heart rate variability in healthy control subjects and patients with a prior myocardial infarction. Circulation. 1991;83(4 Suppl):II43–51.
Tuininga YS, Crijns HJ, Brouwer J, et al. Evaluation of importance of central effects of atenolol and metoprolol measured by heart rate variability during mental performance tasks, physical exercise, and daily life in stable postinfarct patients. Circulation. 1995;92(12):3415–23.
Krantz DS, Kop WJ, Santiago HT, Gottdiener JS. Mental stress as a trigger of myocardial ischemia and infarction. Cardiol Clin. 1996;14(2):271–87.
Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318(16):1005–12.
Ramadan R, Sheps D, Esteves F, et al. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. J Am Heart Assoc. 2013;2(5):e000321.
Al Mheid I, Quyyumi AA. Sex differences in mental stress-induced myocardial ischemia: are women from venus? J Am Coll Cardiol. 2014;64(16):1679–80.
Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO 3rd, Panza JA. Role of nitric oxide in the vasodilator response to mental stress in normal subjects. Am J Cardiol. 1997;80(8):1070–4.
Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO 3rd. Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol. 1995;76(3):125–30.
Rutledge T, Vaccarino V, Johnson BD, et al. Depression and cardiovascular health care costs among women with suspected myocardial ischemia: prospective results from the WISE (Women’s Ischemia Syndrome Evaluation) Study. J Am Coll Cardiol. 2009;53(2):176–83.
Vaccarino V, Johnson BD, Sheps DS, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50(21):2044–50.
Rutledge T, Kenkre TS, Bittner V, et al. Anxiety associations with cardiac symptoms, angiographic disease severity, and healthcare utilization: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation. Int J Cardiol. 2013;168(3):2335–40.
Rutledge T, Linke SE, Krantz DS, et al. Comorbid depression and anxiety symptoms as predictors of cardiovascular events: results from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Psychosom Med. 2009;71(9):958–64.
Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33(1):87–94.
Di Carli MF, Tobes MC, Mangner T, et al. Effects of cardiac sympathetic innervation on coronary blood flow. N Engl J Med. 1997;336(17):1208–15.
Gulli G, Cemin R, Pancera P, Menegatti G, Vassanelli C, Cevese A. Evidence of parasympathetic impairment in some patients with cardiac syndrome X. Cardiovasc Res. 2001;52(2):208–16.
Camici PG, Marraccini P, Gistri R, Salvadori PA, Sorace O, L’Abbate A. Adrenergically mediated coronary vasoconstriction in patients with syndrome X. Cardiovasc Drugs Ther. 1994;8(2):221–6.
Cemin R, Erlicher A, Fattor B, Pitscheider W, Cevese A. Reduced coronary flow reserve and parasympathetic dysfunction in patients with cardiovascular syndrome X. Coron Artery Dis. 2008;19(1):1–7.
Lanza GA, Giordano A, Pristipino C, et al. Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I]metaiodobenzylguanidine myocardial scintigraphy. Circulation. 1997;96(3):821–6.
Di Monaco A, Bruno I, Sestito A, et al. Cardiac adrenergic nerve function and microvascular dysfunction in patients with cardiac syndrome X. Heart. 2009;95(7):550–4.
Bakir M, Nelson MD, Jones E, et al. Heart failure hospitalization in women with signs and symptoms of ischemia: a report from the women’s ischemia syndrome evaluation study. Int J Cardiol. 2016;223:936–9.
AlBadri A, Lai K, Wei J, et al. Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: a report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). PLoS One. 2017;12(5):e0177684.
Arrebola-Moreno AL, Arrebola JP, Moral-Ruiz A, Ramirez-Hernandez JA, Melgares-Moreno R, Kaski JC. Coronary microvascular spasm triggers transient ischemic left ventricular diastolic abnormalities in patients with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2014;236(1):207–14.
Ong P, Athanasiadis A, Mahrholdt H, Borgulya G, Sechtem U, Kaski JC. Increased coronary vasoconstrictor response to acetylcholine in women with chest pain and normal coronary arteriograms (cardiac syndrome X). Clin Res Cardiol. 2012;101(8):673–81.
Hung MJ, Hu P, Hung MY. Coronary artery spasm: review and update. Int J Med Sci. 2014;11(11):1161–71.
Shimokawa H. 2014 Williams Harvey Lecture: importance of coronary vasomotion abnormalities-from bench to bedside. Eur Heart J. 2014;35(45):3180–93.
Tweet MS, Gulati R, Hayes SN. What clinicians should know alphabout spontaneous coronary artery dissection. Mayo Clin Proc. 2015;90(8):1125–30.
Kanwar SS, Hayes SN, Olson TM, Gulati RA. breakthrough in spontaneous coronary artery dissection pathogenesis: is it an inherited condition? Expert Rev Cardiovasc Ther. 2017;15(1):1–2.
Kwon TG, Gulati R, Matsuzawa Y, et al. Proliferation of coronary adventitial vasa vasorum in patients with spontaneous coronary artery dissection. JACC Cardiovasc Imaging. 2016;9(7):891–2.
Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv. 2013;6(1):44–52.
Kurisu S, Kihara Y. Tako-tsubo cardiomyopathy: clinical presentation and underlying mechanism. J Cardiol. 2012;60(6):429–37.
Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of takotsubo syndrome. Circulation. 2017;135(24):2426–41.
Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary microcirculation in patients with takotsubo-like left ventricular dysfunction. Circ J. 2005;69(8):934–9.
Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94(3):343–6.
Vaccaro A, Despas F, Delmas C, et al. Direct evidences for sympathetic hyperactivity and baroreflex impairment in Tako Tsubo cardiopathy. PLoS One. 2014;9(3):e93278.
Fihn SD, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:2354–471.
Johnson BDK, Kelsey SF, Bairey Merz CN. Clinical risk assessment in women: chest discomfort: report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. In: Shaw LJ, Redberg RF, editors. Coronary disease in women: evidence-based diagnosis and treatment. Totowa, NJ: Humana Press; 2003. p. 129–42.
Milner KA, Funk M, Richards S, Wilmes RM, Vaccarino V, Krumholz HM. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84(4):396–9.
AlBadri A, Leong D, Bairey Merz CN, et al. Typical angina is associated with greater coronary endothelial dysfunction but not abnormal vasodilatory reserve. Clin Cardiol. 2017. https://doi.org/10.1002/clc.22740.
Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–9.
Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2013;129(25 Suppl 2):S49–73.
Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):3024–5.
Khaliq A, Johnson BD, Anderson RD, et al. Relationships between components of metabolic syndrome and coronary intravascular ultrasound atherosclerosis measures in women without obstructive coronary artery disease: the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation Study. Cardiovasc Endocrinol. 2015;4(2):45–52.
Enkhmaa D, Wall D, Mehta PK, et al. Preeclampsia and vascular function: a window to future cardiovascular disease risk. J Womens Health (Larchmt). 2016;25(3):284–91.
Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974.
Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update. Circulation. 2011;123(11):1243–62.
Garcia M, Mulvagh SL, Merz CNB, Buring JE, Manson JE. Cardiovascular disease in women. Circ Res. 2016;118(8):1273–93.
Kobayashi H, Giles JT, Arinuma Y, Yokoe I, Hirano M, Kobayashi Y. Cardiac magnetic resonance imaging abnormalities in patients with systemic lupus erythematosus: a preliminary report. Mod Rheumatol. 2010;20(3):319–23.
Ishimori ML, Martin R, Berman DS, et al. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging. 2011;4(1):27–33.
Ishimori ML, Anderson L, Weisman MH, Mehta PK, Bairey Merz CN, Wallace DJ. Microvascular angina: an underappreciated cause of SLE chest pain. J Rheumatol. 2013;40(5):746–7.
Goykhman P, Mehta PK, Minissian M, Thomson LEJ, Berman DS, Ishimori ML, Wallace DJ, Weisman MH, Shufelt CL, Bairey Merz CN. Subendocardial ischemia and myocarditis in systemic lupus erythematosus detected by cardiac magnetic resonance imaging. J Rheumatol. 2012;39(2):448–50.
Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, Crow MK, Schwartz JE, Paget SA, Devereux RB, Salmon JE. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–406.
Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690–7.
Lichtman JH, Froelicher ES, Blumenthal JA, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129(12):1350–69.
Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52.
Shah AJ, Ghasemzadeh N, Zaragoza-Macias E, et al. Sex and age differences in the association of depression with obstructive coronary artery disease and adverse cardiovascular events. J Am Heart Assoc. 2014;3(3):e000741.
Mieres JH, Gulati M, Bairey Merz N, et al. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation. 2014;130(4):350–79.
Shaw LJ, Bairey Merz CN, Pepine CJ, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47(3 Suppl):S4–S20.
Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353(5):468–75.
Kohli P, Gulati M. Exercise stress testing in women: going back to the basics. Circulation. 2010;122(24):2570–80.
Vaccarino V, Shah AJ, Rooks C, et al. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med. 2014;76(3):171–80.
Vaccarino V, Wilmot K, Al Mheid I, et al. Sex differences in mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Heart Assoc. 2016;5(9):pii: e003630.
Cortigiani L, Rigo F, Gherardi S, et al. Prognostic effect of coronary flow reserve in women versus men with chest pain syndrome and normal dipyridamole stress echocardiography. Am J Cardiol. 2010;106(12):1703–8.
Ahmari SA, Bunch TJ, Modesto K, et al. Impact of individual and cumulative coronary risk factors on coronary flow reserve assessed by dobutamine stress echocardiography. Am J Cardiol. 2008;101(12):1694–9.
Kaul S. Myocardial Contrast Echocardiography. Circulation. 2008;118(3):291–308.
Thomas JD. Myocardial contrast echocardiography perfusion imaging. J Am Coll Cardiol. 2013;62(15):1362–4.
Vogel R, Indermuhle A, Reinhardt J, et al. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: algorithm and validation. J Am Coll Cardiol. 2005;45(5):754–62.
Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62(18):1639–53.
Rimoldi OE, Camici PG. Positron emission tomography for quantitation of myocardial perfusion. J Nucl Cardiol. 2004;11(4):482–90.
Camici PG. Positron emission tomography and myocardial imaging. Heart. 2000;83(4):475–80.
Camici P, Ferrannini E, Opie LH. Myocardial metabolism in ischemic heart disease: basic principles and application to imaging by positron emission tomography. Prog Cardiovasc Dis. 1989;32(3):217–38.
Cho SG, Park KS, Kim J, et al. Coronary flow reserve and relative flow reserve measured by N-13 ammonia PET for characterization of coronary artery disease. Ann Nucl Med. 2017;31(2):144–52.
Garcia EV. Are absolute myocardial blood flow PET measurements ready for clinical use? J Nucl Cardiol. 2014;21(5):857–8.
Branscomb E, Heller G, Bateman T, et al. Advances in cardiac imaging: taking a closer look at PET perfusion imaging : American Society of Nuclear Cardiology, Philadelphia, PA, 24 September 2010. J Nucl Cardiol. 2012;19(Suppl 1):S46–7.
Nakazato R, Heo R, Leipsic J, Min JK. CFR and FFR assessment with PET and CTA: strengths and limitations. Curr Cardiol Rep. 2014;16(5):484.
Chow BJ, Dorbala S, Di Carli MF, et al. Prognostic value of PET myocardial perfusion imaging in obese patients. JACC Cardiovasc Imaging. 2014;7(3):278–87.
Camici PG, Rimoldi OE. The clinical value of myocardial blood flow measurement. J Nucl Med. 2009;50(7):1076–87.
Geltman EM, Henes CG, Senneff MJ, Sobel BE, Bergmann SR. Increased myocardial perfusion at rest and diminished perfusion reserve in patients with angina and angiographically normal coronary arteries. J Am Coll Cardiol. 1990;16(3):586–95.
Czernin J, Muller P, Chan S, et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation. 1993;88(1):62–9.
Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124(20):2215–24.
Naya M, Murthy VL, Taqueti VR, et al. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med. 2014;55(2):248–55.
Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55(25):2825–32.
Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol. 2009;103(5):626–31.
Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131(1):19–27.
Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet (London, England). 2012;379(9814):453–60.
Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–53.
Pilz G, Klos M, Ali E, Hoefling B, Scheck R, Bernhardt P. Angiographic correlations of patients with small vessel disease diagnosed by adenosine-stress cardiac magnetic resonance imaging. J Cardiovasc Magn Reson. 2008;10:8.
Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8(4):pii: e002481.
Tanriverdi H, Evrengul H, Kuru O, et al. Cigarette smoking induced oxidative stress may impair endothelial function and coronary blood flow in angiographically normal coronary arteries. Circ J. 2006;70(5):593–9.
Vichova T, Motovska Z. Oxidative stress: predictive marker for coronary artery disease. Exp Clin Cardiol. 2013;18(2):e88–91.
Reho JJ, Rahmouni K. Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin Sci (Lond). 2017;131(14):1689–700.
Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411–8.
Molyneux CA, Glyn MC, Ward BJ. Oxidative stress and cardiac microvascular structure in ischemia and reperfusion: the protective effect of antioxidant vitamins. Microvasc Res. 2002;64(2):265–77.
Bohlen HG, Zhou X, Unthank JL, Miller SJ, Bills R. Transfer of nitric oxide by blood from upstream to downstream resistance vessels causes microvascular dilation. Am J Physiol Heart Circ Physiol. 2009;297(4):H1337–46.
Mkhwanazi BN, Serumula MR, Myburg RB, Van Heerden FR, Musabayane CT. Antioxidant effects of maslinic acid in livers, hearts and kidneys of streptozotocin-induced diabetic rats: effects on kidney function. Oxidative Med Cell Longev. 2014;36(3):419–31.
Dhawan SS, Eshtehardi P, McDaniel MC, et al. The role of plasma aminothiols in the prediction of coronary microvascular dysfunction and plaque vulnerability. Atherosclerosis. 2011;219(1):266–72.
Ignarro LJ, Napoli C. Novel features on nitric oxide, endothelial nitric oxide synthase and atherosclerosis. Curr Atheroscler Rep. 2004;6:278–87.
Lekakis J, Abraham P, Balbarini A, et al. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil. 2011;18(6):775–89.
Wang H, Liu J. Plasma asymmetric dimethylarginine and L-arginine levels in Chinese patients with essential hypertension without coronary artery disease. J Cardiovasc Dis Res. 2011;2(3):177–80.
Sitia S, Tomasoni L, Atzeni F, et al. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. 2010;9(12):830–4.
Sen N, Poyraz F, Tavil Y, et al. Carotid intima-media thickness in patients with cardiac syndrome X and its association with high circulating levels of asymmetric dimethylarginine. Atherosclerosis. 2009;204(2):e82–5.
Ridker P, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109(16):1955–9.
Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. 2006;47(Suppl. 8):C19–31.
Johnson BD, Kip KE, Marroquin OC, et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109(6):726–32.
Mekonnen G, Hayek SS, Mehta PK, et al. Circulating progenitor cells and coronary microvascular dysfunction: results from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation—Coronary Vascular Dysfunction Study (WISE-CVD). Atherosclerosis. 2016;253:111–7.
Flavahan NA. Atherosclerosis or lipoprotein-induced endothelial dysfunction: potential mechanism underlying reduction in ADRF/nitric oxide activity. Circulation. 1992;85:1927–38.
Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic arteries. N Engl J Med. 1986;315:1046–51.
Kern MJ, Lerman A, Bech JW, et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114(12):1321–41.
Chareonthaitawee P, Kaufmann PA, Rimoldi O, Camici PG. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res. 2001;50(1):151–61.
Lee JM, Jung JH, Hwang D, et al. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. J Am Coll Cardiol. 2016;67(10):1158–69.
Murakami T, Mizuno S, Kaku B. Clinical morbidities in subjects with Doppler-evaluated endothelial dysfunction of coronary artery. J Am Coll Cardiol. 1998;31(s1):419A.
Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54.
von Mering GO, Arant CB, SP MG, Miller DM, CNB M, Kelsey SF, Reichek N, Reis SE, Rogers WJ, Sharaf BL, Sopko G, Kerensky RA. Abnormal coronary vasomotion in response to acetylcholine predicts increased cardiac events in women: data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 2001;37:243A.
Ong P, Athanasiadis A, Borgulya G, Voehringer M, Sechtem U. 3-year follow-up of patients with coronary artery spasm as cause of acute coronary syndrome: the CASPAR (coronary artery spasm in patients with acute coronary syndrome) study follow-up. J Am Coll Cardiol. 2011;57:147–52.
Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5(6):646–53.
Haskell WL, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Assoication. Med Sci Sports Exerc. 2007;39(8):1423–34.
Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52.
Green DJ, Walsh JH, Maiorana A, Best MJ, Taylor RR, O’Driscoll JG. Exercise-induced improvement in endothelial dysfunction is not mediated by changes in CV risk factors: pooled analysis of diverse patient populations. Am J Physiol Heart Circ Physiol. 2003;285:H2679–87.
Hambrecht R, Wolf A, Gielen S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–60.
Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509–15.
Morita H, Ikeda H, Haramaki N, Eguchi H, Imaizumi T. Only two-week smoking cessation improves platelet aggregability and intraplatelet redox imbalance of long-term smokers. J Am Coll Cardiol. 2005;45(4):589–94.
Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45.
Fabian E, Varga A, Picano E, Vajo Z, Ronaszeki A, Csanady M. Effect of simvastatin on endothelial function in cardiac syndrome X patients. Am J Cardiol. 2004;94(5):652–5.
Caliskan M, Erdogan D, Gullu H, et al. Effects of atorvastatin on coronary flow reserve in patients with slow coronary flow. Clin Cardiol. 2007;30(9):475–9.
John S, Schlaich M, Langenfeld M, et al. Increased bioavailability of nitric oxide after lipid-lowering therapy in hypercholesterolemic patients: a randomized, placebo-controlled, double-blind study. Circulation. 1998;98:211–6.
Masumoto A, Hirooka Y, Hironaga K, et al. Effect of pravastatin on endothelial function in patients with coronary artery disease (cholesterol-independent effect of pravastatin). Am J Cardiol. 2001;88(11):1291–4.
Romano M, Mezzetti A, Marulli C, et al. Fluvastatin reduces soluble P-selectin and ICAM-1 levels in hypercholesterolemic patients: role of nitric oxide. J Invest Med. 2000;48(3):183–9.
Kayikcioglu M, Payzin S, Yavuzgil O, Kultursay H, Can LH, Soydan I. Benefits of statin treatment in cardiac syndrome-X. Eur Heart J. 2003;24(22):1999–2005.
Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. JAMA. 2005;293(4):477–84.
Fraker TD Jr, Fihn SD, Chronic Stable Angina Writing C, et al. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 2007;50(23):2264–74.
Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, et al. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation. 2003;107(21):2747–52.
Matsuda Y, Akita H, Terashima M, Shiga N, Kanazawa K, Yokoyama M. Carvedilol improves endothelium-dependent dilatation in patients with coronary artery disease. Am Heart J. 2000;140(5):753–9.
Togni M, Vigorito F, Windecker S, et al. Does the beta-blocker nebivolol increase coronary flow reserve? Cardiovasc Drugs Ther. 2007;21(2):99–108.
Hung OY, Molony D, Corban MT, et al. Comprehensive assessment of coronary plaque progression with advanced intravascular imaging, physiological measures, and wall shear stress: a pilot double-blinded randomized controlled clinical trial of nebivolol versus atenolol in nonobstructive coronary artery disease. J Am Heart Assoc. 2016;5(1):pii: e002764.
Chen JW, Hsu NW, Wu TC, Lin SJ, Chang MS. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. 2002;90(9):974–82.
Pauly DF, Johnson BD, Anderson RD, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011;162(4):678–84.
Pizzi C, Manfrini O, Fontana F, Bugiardini R. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac Syndrome X: role of superoxide dismutase activity. Circulation. 2004;109(1):53–8.
Higuchi T, Abletshauser C, Nekolla SG, Schwaiger M, Bengel FM. Effect of the angiotensin receptor blocker Valsartan on coronary microvascular flow reserve in moderately hypertensive patients with stable coronary artery disease. Microcirculation. 2007;14(8):805–12.
Naoumova RP, Kindler H, Leccisotti L, et al. Pioglitazone improves myocardial blood flow and glucose utilization in nondiabetic patients with combined hyperlipidemia: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2007;50(21):2051–8.
Cannon RO 3rd, Watson RM, Rosing DR, Epstein SE. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol. 1985;56(4):242–6.
Ozcelik F, Altun A, Ozbay G. Antianginal and anti-ischemic effects of nisoldipine and ramipril in patients with syndrome X. Clin Cardiol. 1999;22(5):361–5.
Bugiardini R, Borghi A, Biagetti L, Puddu P. Comparison of verapamil versus propranolol therapy in syndrome X. Am J Cardiol. 1989;63(5):286–90.
Lanza GA, Colonna G, Pasceri V, Maseri A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84(7):854–6. A858
Stone PH. Calcium antagonists for Prinzmetal’s variant angina, unstable angina and silent myocardial ischemia: therapeutic tool and probe for identification of pathophysiologic mechanisms. Am J Cardiol. 1987;59(3):101B–15B.
Parodi O, Simonetti I, Michelassi C, et al. Comparison of verapamil and propranolol therapy for angina pectoris at rest: a randomized, multiple-crossover, controlled trial in the coronary care unit. Am J Cardiol. 1986;57(11):899–906.
Pepine CJ, Feldman RL, Whittle J, Curry RC, Conti CR. Effect of diltiazem in patients with variant angina: a randomized double-blind trial. Am Heart J. 1981;101(6):719–25.
Yasue H, Omote S, Takizawa A, Nagao M, Miwa K, Tanaka S. Exertional angina pectoris caused by coronary arterial spasm: effects of various drugs. Am J Cardiol. 1979;43(3):647–52.
Lerman A, Burnett JC Jr, Higano ST, McKinley LJ, Holmes DR Jr. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97(21):2123–8.
Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25(4):807–14.
Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113(20):2462–72.
Mehta PK, Goykhman P, Thomson LE, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. J Am Coll Cardiol Img. 2011;4(5):514–22.
Bairey Merz CN, Handberg EM, Shufelt CL, et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. 2016;37(19):1504–13.
Roqué M, Heras M, Roig E, et al. Short-term effects of transdermal estrogen replacement therapy on coronary vascular reactivity in postmenopausal women with angina pectoris and normal results on coronary angiograms. J Am Coll Cardiol. 1998;31(1):139–43.
Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–12.
Investigators WHI. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–33.
Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12.
Wolff EF, He Y, Black DM, et al. Self-reported menopausal symptoms, coronary artery calcification, and carotid intima-media thickness in recently menopausal women screened for the Kronos early estrogen prevention study (KEEPS). Fertil Steril. 2013;99(5):1385–91.
Sulfi S, Timmis AD. Ivabradine—the first selective sinus node I(f) channel inhibitor in the treatment of stable angina. Int J Clin Pract. 2006;60(2):222–8.
Fox K, Ford I, Steg PG, Tendera M, Ferrari R, Investigators B. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2008;372(9641):807–16.
Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet (London, England). 2010;376(9744):875–85.
Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K, Investigators I. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26(23):2529–36.
Skalidis EI, Hamilos MI, Chlouverakis G, Zacharis EA, Vardas PE. Ivabradine improves coronary flow reserve in patients with stable coronary artery disease. Atherosclerosis. 2011;215(1):160–5.
Villano A, Di Franco A, Nerla R, et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013;112(1):8–13.
Cannon RO 3rd, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330(20):1411–7.
Cox ID, Hann CM, Kaski JC. Low dose imipramine improves chest pain but not quality of life in patients with angina and normal coronary angiograms. Eur Heart J. 1998;19(2):250–4.
Hongo M, Takenaka H, Uchikawa S, Nakatsuka T, Watanabe N, Sekiguchi M. Coronary microvascular response to intracoronary administration of nicorandil. Am J Cardiol. 1995;75(4):246–50.
Chen JW, Lee WL, Hsu NW, et al. Effects of short-term treatment of nicorandil on exercise-induced myocardial ischemia and abnormal cardiac autonomic activity in microvascular angina. Am J Cardiol. 1997;80(1):32–8.
Vicari RM, Chaitman B, Keefe D, et al. Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol. 2005;46(10):1803–11.
Fukumoto Y, Mohri M, Inokuchi K, et al. Anti-ischemic effects of fasudil, a specific Rho-kinase inhibitor, in patients with stable effort angina. J Cardiovasc Pharmacol. 2007;49(3):117–21.
Shimokawa H, Hiramori K, Iinuma H, et al. Anti-anginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: a multicenter study. J Cardiovasc Pharmacol. 2002;40(5):751–61.
Shimokawa H, Satoh K. 2015 ATVB Plenary Lecture: translational research on rho-kinase in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2015;35(8):1756–69.
Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86(5):580–8.
Rogacka D, Guzik P, Wykretowicz A, Rzezniczak J, Dziarmaga M, Wysocki H. Effects of trimetazidine on clinical symptoms and tolerance of exercise of patients with syndrome X: a preliminary study. Coron Artery Dis. 2000;11(2):171–7.
Nalbantgil S, Altinti&gbreve A, Yilmaz H, Nalbantgil II, Onder R. The effect of trimetazidine in the treatment of microvascular angina. Int J Angiol. 1999;8(1):40–3.
Leonardo F, Fragasso G, Rossetti E, et al. Comparison of trimetazidine with atenolol in patients with syndrome X: effects on diastolic function and exercise tolerance. Cardiologia. 1999;44(12):1065–9.
Peng S, Zhao M, Wan J, Fang Q, Fang D, Li K. The efficacy of trimetazidine on stable angina pectoris: a meta-analysis of randomized clinical trials. Int J Cardiol. 2014;177(3):780–5.
Ciapponi A, Pizarro R, Harrison J. Trimetazidine for stable angina. Cochrane Database Syst Rev. 2005;(4):Cd003614.
Stys TP, Lawson WE, Hui JC, et al. Effects of enhanced external counterpulsation on stress radionuclide coronary perfusion and exercise capacity in chronic stable angina pectoris. Am J Cardiol. 2002;89(7):822–4.
Arora RR, Chou TM, Jain D, et al. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33(7):1833–40.
Urano H, Ikeda H, Ueno T, Matsumoto T, Murohara T, Imaizumi T. Enhanced external counterpulsation improves exercise tolerance, reduces exercise-induced myocardial ischemia and improves left ventricular diastolic filling in patients with coronary artery disease. J Am Coll Cardiol. 2001;37(1):93–9.
Lawson WE, Hui JC, Zheng ZS, et al. Three-year sustained benefit from enhanced external counterpulsation in chronic angina pectoris. Am J Cardiol. 1995;75(12):840–1.
Beck DT, Martin JS, Casey DP, Avery JC, Sardina PD, Braith RW. Enhanced external counterpulsation improves endothelial function and exercise capacity in patients with ischaemic left ventricular dysfunction. Clin Exp Pharmacol Physiol. 2014;41(9):628–36.
Loh PH, Cleland JG, Louis AA, et al. Enhanced external counterpulsation in the treatment of chronic refractory angina: a long-term follow-up outcome from the International Enhanced External Counterpulsation Patient Registry. Clin Cardiol. 2008;31(4):159–64.
Lawson WE, Barsness G, Michaels AD, et al. Effectiveness of repeat enhanced external counterpulsation for refractory angina in patients failing to complete an initial course of therapy. Cardiology. 2007;108(3):170–5.
Kang H-J, Kim H-S, Zhang S-Y, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363(9411):751–6.
Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Structural skin capillary rarefaction in essential hypertension. Hypertension. 1999;33(4):998–1001.
Pries AR, Badimon L, Bugiardini R, et al. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J. 2015;36(45):3134–46.
Kanazawa H, Tseliou E, Malliaras K, et al. Cellular postconditioning: allogeneic cardiosphere-derived cells reduce infarct size and attenuate microvascular obstruction when administered after reperfusion in pigs with acute myocardial infarction. Circ Heart Fail. 2015;8(2):322–32.
Asbury EA, Kanji N, Ernst E, Barbir M, Collins P. Autogenic training to manage symptomology in women with chest pain and normal coronary arteries. Menopause. 2009;16(1):60–5.
Asbury EA, Webb CM, Collins P. Group support to improve psychosocial well-being and primary-care demands among women with cardiac syndrome X. Climacteric. 2011;14(1):100–4.
Acknowledgment
This work was supported by NIH grant K23HL105787.
Conflicts: Lee: none; Khambhati: none; Mehta: none.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Lee, S.K., Khambhati, J., Mehta, P.K. (2018). Angina and Ischemia in Women with No Obstructive Coronary Artery Disease. In: Mehta, J., McSweeney, J. (eds) Gender Differences in the Pathogenesis and Management of Heart Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-71135-5_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-71135-5_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-71134-8
Online ISBN: 978-3-319-71135-5
eBook Packages: MedicineMedicine (R0)