Abstract
Vitiligo is a common pigmentary disorder characterised by the loss of functioning melanocytes from the basal layer of epidermis, leaving behind depigmented patches on the skin. It has a complex aetiopathology. Even though there are various theories describing the pathomechanisms of melanocyte loss, the initial trigger for melanocyte directed attack and the final steps causing melanocyte destruction is still speculative. The poor understanding of a common pathway causing melanocyte loss reflects in the lack of a targeted therapy in the medical management of vitiligo in this era of biologicals. The unravelling of interferon (IFN)-γ/CXCL10 axis in the causation of melanocyte directed attack and the observation of clinical usefulness of tofacitinib, which blocks the same pathway, give new hope in the direction of targeted therapy in vitiligo. In vitiligo, unlike psoriasis, the physician needs to address not only the issue of halting the inflammatory cascade causing the overt manifestation of the disease but also that of reviving the lost melanocytes, to regain normal skin colour. This chapter discusses the recent advances in the understanding of vitiligo pathogenesis and includes an update on the conventional and newer modalities in the medical management of vitiligo. A brief overview of the approach to the medical management of vitiligo is given at the end of the chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Vitiligo
- Classification
- Recent advances
- Pathogenesis
- Medical management
- Autoimmunity
- Oxidative stress
- Newer medical agents
- Phototherapy
- Treatment approach
Introduction
Vitiligo is an acquired disorder of melanogenesis caused by melanocyte destruction, involving a complex interaction between environmental and genetic factors, ultimately resulting in the characteristic depigmented patches [1]. The disease runs an unpredictable course but is often progressive with stable phases in between [2]. It usually begins during childhood or early adulthood. Vitiligo on the exposed body parts often leads to social embarrassment, psychological turmoil and aesthetic disfigurement [3]. An aggressive management is warranted in vitiligo because of the stigma, social isolation and psychological stress associated with the disease.
Recently, there has been an increase in the awareness activities on vitiligo by various patient support organisations. Vitiligo research has also gained much attention nowadays in pigment cell research community. This chapter deals with the updates on the classification and recent advances in pathogenesis and medical management of vitiligo.
Epidemiology
Worldwide prevalence of vitiligo is 0.5–2%, but up to 8.8% has been reported in India, with no sex predilection [4, 5]. In approximately 50% of cases, vitiligo appears before the age of 20 years, and 70–80% of patients develop the disease by the age of 30 years [6]. Non-segmental vitiligo can occur at any age, whereas a younger age of occurrence is seen in segmental vitiligo [7].
Classification
The Bordeaux Vitiligo Global Issues Consensus Conference (VGICC) [8] classified vitiligo in to two major forms, namely, segmental vitiligo (SV) and non-segmental vitiligo (NSV). Focal vitiligo was assigned a separate category – ‘undetermined’ vitiligo – until more definitive classification can be made on clinical grounds [8].
Segmental (unilateral) vitiligo
It has an early onset, rapid progression, early stabilisation and unilateral or segmental distribution with no specific triggering factors [9]. Depigmentation process in SV occurs rapidly within the segment over a period of 6–24 months and then usually halts without any further progression [8]. Prevalence of SV ranges from 5% to 16% of all patients with vitiligo [9]. Autoimmune disorders are reported less frequently in patients with SV and their family members than in those with NSV [10]. However, there are recent reports of associated autoimmune diseases in SV as well [11]. Demonstration of inflammation in early stages of SV [12] and the concept of mixed vitiligo has challenged the so far considered non-immune background of SV. Segmental vitiligo most commonly affects the face (Fig. 8.1), followed by the trunk, neck, extremities and scalp in descending order. VGICC has subdivided SV into uni-, bi- or plurisegmental based on the distribution. Based on the pattern of involvement, SV over face is classified by Hann [13] and over trunk by van Geel [14]. These patterns do not strictly follow a dermatomal or blaschkolinear distribution [11]. Several hypotheses for the pathogenesis of SV have been proposed, including neuronal mechanisms, somatic mosaicism and microvascular skin homing, through or without immune destruction of melanocytes [15]. Since hair follicular melanocyte reservoir is affected early in the disease process, SV responds poorly to medical modalities. High rate of repigmentation with surgical techniques is frequently achieved, owing to its less immunologic background.
Non-segmental (bilateral) vitiligo
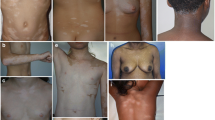
Although not fully satisfactory, the term ‘NSV’ is currently used as an umbrella term for different clinical subtypes of vitiligo that are all clearly distinct from SV including acrofacial, generalised, mucosal (more than one mucosal site), universal, mixed and rare variants [8]. Non-segmental vitiligo typically starts over acral areas and gradually involves other parts of the body. Contrary to SV, in NSV, hair follicle melanocytes are initially spared from immune-mediated attack. However, hair depigmentation may occur with disease progression when hair follicle melanocyte stem cells are exhausted following repeated melanocyte contribution to the depigmented epidermis. Acrofacial vitiligo (Fig. 8.2) involves distal digits and periorificial facial areas. Lip-tip vitiligo (Fig. 8.3) is a variety in which tips of fingers, toes, nipples, penis, and lips become depigmented. Common vitiligo (formerly referred to as vitiligo vulgaris ) (Fig. 8.4) is the most common form of NSV and is composed of several scattered depigmented macules distributed symmetrically all over the body. Mucosal vitiligo affects mucosae of both mouth and genitalia. If it is found in isolation, i.e. affecting only one mucosa (Fig. 8.5), it is considered as focal vitiligo under ‘unclassified vitiligo’ as per VGICC. Universal vitiligo (Fig. 8.6) is the most severe form of NSV, where complete or nearly complete depigmentation can be noted. Mixed vitiligo has been defined as the coexistence of NSV and SV in the same patient and is classified as a subgroup of NSV [16]. This association may be viewed as an example of a superimposed segmental manifestation of a generalised polygenic disorder, in which segmental involvement precedes disease generalisation and is more resistant to therapy [8]. Vitiligo minor and follicular vitiligo are rare variants. Vitiligo minor [17], reported mainly in dark-skinned individuals, refers to incomplete pigment defects leaving pale skin, apart from coexistent depigmented patches of vitiligo. Follicular vitiligo [18] is a rare subtype of NSV where hair follicular melanocytes are preferentially destroyed compared to epidermal melanocytes, with marked generalised hair whitening in contrast to limited skin involvement.
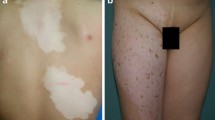
Genital (mucosal) vitiligo. Isolated involvement of the genital mucosa for years classifies this case as focal vitiligo under ‘unclassified vitiligo’ as per VGICC (Vitiligo Global Issues Consensus Conference 2012). The patient had no difficulty in retracting the foreskin and hence lichen sclerosus was ruled out clinically
Universal vitiligo. Nearly complete depigmentation of the body in this lady of Indian origin (Fitzpatrick skin type IV). She had got breakthrough repigmentation recently over her malar area (scapular areas in this picture), after a brief period of sun exposure for which she attended our clinic for depigmentation therapy
Focal vitiligo refers to a small isolated patch (Fig. 8.5) that does not follow a typical segmental distribution and which has not evolved into SV/NSV after a period of 1–2 years [8]. This form of vitiligo may evolve into either SV or NSV. Accurate prediction of fate of focal vitiligo is still not possible [19].
In vitiligo punctata, lesions present as sharply demarcated depigmented punctate macules involving any area of the body [8]. Other forms of vitiligo that have not found mention in VGICC are inflammatory vitiligo, multichrome vitiligo and blue vitiligo [20].
Recent Insights into the Pathogenic Mechanisms in Vitiligo
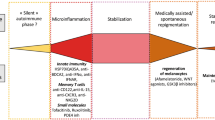
Pathogenic mechanisms causing vitiligo are still elusive. Various theories have been proposed for the melanocyte defects in vitiligo, but there is a lack of consensus among scientists on the pathophysiology of vitiligo. Even though the convergence or integrated theory [21], which encompasses autoimmune hypothesis [22], self-destruct hypothesis [23], biochemical theory and genetics, fairly depicts various causative steps in melanocyte loss, the overall contribution of each step as well as triggering event in the pathogenic cascade is still debatable.
Genetic susceptibility in vitiligo is established by various studies. However, only 23% concordance has been seen in monozygotic twins, lower than that of psoriasis and atopic dermatitis [24, 25]. In an epidemiological analysis of Caucasian probands and their families, the risk of vitiligo in a patient’s siblings was about 6.1%, 18 times the population frequency, suggesting a strong genetic component in disease pathogenesis [25]. It refers mostly to increased susceptibility of melanocytes to immune-mediated attacks. However, polymorphisms in non-immune genes like TYR (the gene encoding tyrosinase), MC1R (the gene encoding melanocortin 1 receptor), and MTHFR (the gene encoding methylene tetrahydrofolate reductase) have also been described [24, 26]. Of the 23 new risk loci of vitiligo susceptibility genes in the recent genome-wide analysis among European-ancestry subjects [27], 15 loci are associated with immune system and 6 loci regulate apoptosis. Gene expression profiling of generalised vitiligo was different from segmental vitiligo [28]. The inverse relationship of genetic susceptibility to vitiligo with melanoma, as shown by a recent genome-wide analysis study [27], is consistent with the threefold reduction in melanoma incidence among patients with vitiligo [29] and the prolonged survival of patients with melanoma who develop vitiligo during immunotherapy [30].
Autoimmune hypothesis is currently the leading hypothesis to describe vitiligo pathogenesis. The role of autoimmunity in vitiligo is supported by the presence of antibodies against melanocytes, association with polymorphisms at immune loci, presence of prominent T-cell perilesional infiltrates and cytokine expression and by the association with other autoimmune diseases [25]. Interferon (IFN)-γ/CXCL10 axis and IL-17-mediated responses are considered as the two key components of the autoimmune response that perpetuate disease activity in vitiligo [31]. In a recent genome-wide analysis, nearly all vitiligo susceptibility genes have been found to encode components of the immune system , suggesting a deregulated immune response in vitiligo [32].
Innate immunity
Deregulated innate immune system plays an important role in the initiation and maintenance of melanocyte directed attack in vitiligo. In vitiligo, the disease process is likely to begin with release of stress signals by melanocytes or possibly by keratinocytes. These exosomes or damage-associated molecular patterns (DAMPs) attract natural killer cells to the stressed melanocytes and also activate nearby dendritic cells into antigen-presenting cells. S100B, a DAMP protein expressed in melanocytes, was found to be a possible biomarker for vitiligo activity and a potential target for treatment [33]. An important role of inducible heat-shock protein 70 (HSP70i) , released by stressed melanocytes, in vitiligo induction and disease progression has been suggested [34]. A recent study highlighted the role of HSP70 in promoting interferon alpha production by plasmacytic dendritic cells and subsequent induction of keratinocytes to produce CXCL9 and CXCL10 [35]. Moreover, melanocytes upon stress by phenols and other chemical agents, respond by accumulation of unfolded proteins in endoplasmic reticulum (unfolded protein response, UPR). Even though this homeostatic response is intended for cell survival, prolonged stress leads to production of IL-6 and IL-8, providing a direct link between cellular stress and immune activation [36].
Adaptive immunity
Patients with vitiligo have higher numbers of cytotoxic CD8+ T cells in blood, compared with healthy controls, which correlate with disease activity; and isolated CD8+ T cells from vitiligo patients can identify and kill normal human melanocytes in vitro [37]. Functional studies in mice have confirmed the crucial role of the IFN-γ–CXCL10– CXCR3 axis in both the progression and maintenance of depigmentation [38]. Elevated levels of CXCL10 were noted in NSV patients, especially in the presence of autoimmune thyroid disorders, suggesting a common TH1-mediated immune pathogenesis in both the diseases [39]. Melanocyte derived CXCL12 and CXCL5 were found to be associated with onset and progression of vitiligo through activation of melanocyte-specific immunity [40]. The role of CD4+ T cells in the pathogenesis of vitiligo is still unclear, although a possible role of deregulated regulatory T cells has been suggested [41, 42].
Oxidative stress
Melanocytes in vitiligo patients are inherently susceptible to oxidative stresses owing to the imbalance of the pro-oxidant and antioxidant systems. Oxidative stress compromises the function of cellular proteins and membrane lipids. Impairment in autophagy, a lysosome-dependent degradation pathway that protects cells from oxidative insults, is a speculated mechanism for melanocyte damage [43]. Oxidative stress-driven modification of the TRP1–calnexin complex can lead to reduced TRP1 stability with subsequent production of toxic melanin intermediates [44]. Modification and inactivation of acetylcholinesterase further promotes and maintains skin oxidative damage [45]. Oxidative stress impairs WNT-β catenin pathway which helps in melanoblast differentiation. Using a WNT agonist, Regazzetti et al. have demonstrated melanocyte differentiation from stem cells in vitiligo skin explants [46]. Oxidation end products were found to be increased in NSV compared to healthy controls and were directly correlated with extent, duration and activity of vitiligo [47]. A recent study that suggested similar immune pathogenesis in halo nevus and vitiligo has demonstrated H2O2-associated autoimmune phenotype in both the conditions. Moreover, elevated H2O2 concentration correlated well with CXCL10 levels in skin lesions [48].
Cellular metabolic alterations
Unaffected epidermis in vitiligo is characterised by deregulation of the biopterin metabolism [49] that could lead to inhibition of antioxidant enzyme activities and of melanin synthesis [50, 51].Aberrant signalling pathways have been noted even in non-lesional skin of vitiligo patients, which result in increased expression of p53, hence a pre-senescent cellular profile, which could be melanocyte specific [5]. Increased p53 expression was found to be associated with defects in stem cell reservoir in a mice study [52]. Interestingly, the p53 deregulation correlates with a protection from non-melanoma skin cancer [53]. Several lines of evidence suggest that mitochondria are key in mediating melanocyte dysfunction [5]. Keratinocytes also exhibit oxidative stress , phosphorylation of p38, overexpression of p53 and a senescent phenotype in perilesional vitiligo skin [54]. It is important to note that various melanocyte growth factors are secreted by keratinocytes. UV light triggers differentiation of melanocyte stem cells through WNT proteins derived from irradiated keratinocytes [55]. A recent immunohistochemical study in vitiligo patients has demonstrated aberrant Notch-1 signalling (required for development and maintenance of melanocyte lineage) in acral vitiligo and attributed it to be the cause of their treatment resistance [56]. Senescence of dermal fibroblasts was noted in NSV, and it can lead to decreased secretion of growth factors and cytokines for melanocyte survival resulting in vitiligo progression [57]. In summary, metabolic alterations within melanocytes and its neighbouring cells or a disruption in the ‘cross talk’ between them may lead to melanocyte loss in vitiligo.
Melanocytorrhagy
Defective attachment within the epidermis leads to detachment of melanocytes from the basal layer, to be eliminated through superficial epidermis, where they are more prone to apoptosis [58]. Demonstration of altered expression and/ or distribution of E-cadherin or co-localised aquaporin-3 in vitiligo skin has attracted further interest in this hypothesis recently [59, 60].
Medical Management
Literature on the conventional medical modalities and light-based therapies are discussed briefly, focusing mainly on topic introduction and recent updates as an exhaustive discussion is beyond the scope of this chapter. We will discuss novel medical modalities in vitiligo and summarise the treatment protocol for vitiligo as per current evidence.
Update on Conventional Medical Treatments and Light-Based Therapy in Vitiligo
Conventional medical treatments include topical medications like corticosteroids, tacrolimus, vitamin D analogues and systemic agents like corticosteroids. Light-based therapy includes NBUVB, photochemotherapy and lasers. Recent studies have found that a combination of light-based therapy with topical medication yields greater efficacy compared to either treatment as monotherapy [31, 61,62,63,64,65]. Light-based therapies are increasingly being used as adjuvants to surgical modalities.
Conventional Topical Agents
-
1.
Topical corticosteroids: Topical steroids are the first-line treatment for body lesions, barring intertriginous and genital areas, and have the best response over sun-exposed sites and in newer lesions [66, 67]. Preceding laser dermabrasion and combination with NBUVB are found to increase the treatment efficacy in refractory vitiligo [68]. A recent cross-sectional study did not show an increased risk of glaucoma or cataract in vitiligo patients with periorbital topical steroid use [69].
-
2.
Topical calcineurin inhibitors: Apart from inhibition of T-cell activation, the role of tacrolimus in inducing melanocyte migration and differentiation has been described recently [70]. Vitiligo European Task Force considers it as the first-line treatment for facial vitiligo, unlike its use as second-line agent in atopic dermatitis [66, 67]. Twice-daily application ensures optimal results [71]. Occlusion, preceding microdermabrasion, sunlight and adjuvant NB-UVB or 308-nm laser have all been reported to augment therapeutic response to topical calcineurin inhibitors [66]. Black-box warning by FDA of potential risk of carcinoma and lymphoma warrants avoidance of tacrolimus use in children less than the age of 2 years. However, two recent reviews on long-term usage of topical calcineurin inhibitors in atopic dermatitis have not reported an increased risk of malignancy [72, 73]. Twice-weekly application of 0.1% tacrolimus ointment was found to be successful in retaining the attained pigmentation in vitiligo that may address the local relapse (40% within the first year) [74].
-
3.
Topical vitamin D analogues: Calcipotriene , calcipotriol and tacalcitol have all been used topically in vitiligo, and act by modulation of the local immune response and direct influence on melanogenesis. Recently, protective role of tacalcitol against oxidative damage in human epidermal melanocytes has also been demonstrated [75]. It is mutually beneficial when combined with topical corticosteroids; irritation of vitamin D analogues and atrophogenic effects of topical steroids are reduced [76]. Combination with light-based therapy has shown variable response [67, 77, 78]. Lack of superior results on combination with phototherapy may be due to photoprotective effect of vitamin D analogues decreasing the efficacy of phototherapy and its degradation on light exposure. Hence, they should be applied after the phototherapy session.
Conventional Systemic Agents
-
1.
Systemic steroids: Though widely used in active vitiligo , literature on its use in vitiligo is sparse. Steroids are commonly used as pulse therapy – suprapharmacological doses at regular intervals – to augment the response and reduce side effects. Oral mini-pulse therapy (OMP) involves oral administration of betamethasone or dexamethasone at a single dose of 5 mg on 2 consecutive days per week. A low-dose OMP schedule with oral dexamethasone 2.5 mg per day on 2 consecutive days in a week is also found to be effective in halting progression of vitiligo with minimal side effects [79]. Oral methylprednisolone mini-pulse therapy (0.5 mg/kg on 2 consecutive days per week) combined with NBUVB has also shown similar results [80]. A recent RCT has found that NBUVB plus OMP and NBUVB alone are clinically superior over OMP alone in treating stable vitiligo patients [81].
Light-Based Therapies
-
1.
Photochemotherapy: Topical or systemic photochemotherapy combines use of long-wave UVA (320–340 nm) with psoralen (PUVA) or khellin (KUVA) to stimulate melanogenesis. Prolonged therapy lasting for months with 100–200 treatment sessions given two to three times per week may be needed for optimal outcome. If no response is observed even after approximately 50 sessions or 6 months, PUVA should be discontinued [67, 82]. KUVA is less phototoxic and less mutagenic than PUVA, but hepatotoxicity limits its widespread use. Oral PUVA was found to be more efficacious than PUVA sol (UVA obtained through sunlight) in a recent prospective comparative study [83].
-
2.
Nontargeted phototherapy: Narrowband UVB (NBUVB) is currently the treatment of choice in active, widespread vitiligo involving more than 10–20% body surface area. Combined use of various medical modalities and, recently, fractional CO2 laser and platelet-rich plasma were found to enhance the efficacy of NBUVB [84, 85]. A recent systematic review of RCTs reveals equivalent efficacy of NBUVB to UVA, PUVA or 308-nm excimer laser in the treatment of vitiligo with acceptable side effect profile [86]. Carcinogenic risk of NBUVB therapy is poorly defined. However, recent studies have shown no increased risk of malignancies with long-term use of NBUVB [87]. Total body irradiation in NBUVB chambers is preferred in generalised vitiligo. Even though the vitiligo lesions are devoid of melanin to protect from UV rays, MED (minimal erythema dose) of vitiligo patients varies depending on the phototypes of the individuals. Hence a higher starting dose of NBUVB (50% of MED) is increasingly used nowadays. Treatment is normally given twice or three times weekly and is continued up to 1–2 years as long as there is ongoing repigmentation. Maintenance phototherapy is not recommended [67].
Broadband (BB)-UVA was also found to be useful in vitiligo. A recent RCT evaluating efficacy of different doses of BB-UVA in vitiligo showed BB-UVA at a dose of 15 J/cm2 /session had comparable efficacy to PUVA [88].
-
3.
Targeted phototherapy [89]: In this modality, super-erythemogenic doses of radiation are delivered selectively to the lesions in a short duration thereby enhancing efficacy, achieving faster response and attaining longer duration of remission. It is helpful to treat difficult areas such as scalp, nose, genitals, oral mucosa and ears. It also helps to tailor the dose according to the refractoriness of the lesions. Targeted phototherapy includes lasers (excimer laser and low-energy helium–neon (He–Ne) lasers) and non-laser devices (monochromatic Excimer Lamp or Light 308 nm, mercury arc lamps, plasma lamps and microphototherapy). Combination with topical modalities is found to enhance efficacy. Digital phototherapy device skintrek®, a novel targeted UV therapy modality, reduces carcinogenic risk, premature skin ageing and avoids tanning of healthy surrounding skin by delivering light energy only to the lesional skin. Positive results in psoriasis and mycosis fungoides make this method a promising therapeutic option for vitiligo [90, 91]. A literature review on targeted phototherapy in vitiligo showed it to have good response in localized involvement, resistant lesions and in children [92]. A recent study has demonstrated similar efficacy with once a week targeted NBUVB exposure compared to twice a week schedule, thus obviating the need of frequent hospital visits [93].
In home-based phototherapy , motivated patients are trained to use phototherapy units, either standard chambers or hand-held devices, at their homes. It is a convenient and cost-effective approach which increases treatment compliance and hence efficacy [94]. Further well-conducted studies are needed to ascertain the safety and efficacy of this approach.
Other Interventions
-
1.
Camouflage: Methods to conceal depigmented patches may be permanent like cosmetic tattoos; tanning agents like dihydroxyacetone; general cosmetics like tinted cover creams, compact, liquid and stick foundations, fixing powders, and dyes for leukotrichia; and various topical camouflage agents like the newest product, Microskin™. Clinical studies on camouflaging in vitiligo are sparse. A recent literature review favours temporary methods for camouflaging [95].
-
2.
Depigmenting agents: Methods of permanent depigmentation should be explored only in patients with extensive refractory vitiligo. Further depigmenting cycles may be needed sometimes for possible spontaneous repigmentation over vitiligo lesions. Patients with darker skin phototypes V and VI are ideal candidates, although those with lighter phototypes I and II may also obtain better cosmetic improvement. Motivated patients, after proper psychological evaluation and counselling, can apply 20% monobenzone ethyl ester (MBEH) cream twice or thrice a day. Sun exposure may decrease the efficacy and hence sunscreens are advised. If no satisfactory depigmentation occurs even after 4 months, treatment should be discontinued. Retinoic acid may also augment the depigmenting action of MBEH by accelerating keratinocyte turnover and, hence, melanocyte loss, or by impairing glutathione-dependent defence. But 0.025% retinoic acid plus 10% MBEH may worsen the eczematous side effects of MBEH. The Q-switched ruby laser (QSR) alone or in combination with methoxyphenol and cryotherapy has also been used for depigmentation [96, 97].
-
3.
Psychological interventions: Prevalence of psychiatric morbidity in vitiligo patients ranges from 25% to 75%, depending on the extension and site of the disease, phototype, ethnicity and cultural background [67]. Being a disease that is associated with psychological morbidity, various psychological interventions may have a role in controlling the disease activity. However, there is a paucity of literature on psychological intervention in vitiligo. A recent Cochrane review couldn’t retrieve any new literature on psychological interventions since the 2010 review which had commented on cognitive behavioural therapy [98]. Recently, group climatotherapy [99] and cognitive behavioural self-help [100]/therapy [101] have been found to improve quality of life and social anxiety in vitiligo patients.
Newer Medical Modalities
-
1.
Tofacitinib: A case report has highlighted the usefulness of this systemic Janus kinase 1/3 inhibitor , proposed to act by blockade of interferon-gamma signalling and downstream CXCL10 expression [102]. It is considered to be the first therapy in vitiligo acting on the pathogenic pathway. Later, a case series has suggested that tofacitinib might require concomitant light exposure for the repigmentation outcome [103]. Ruxolitinib, another JAK inhibitor, was studied in a patient with alopecia areata and vitiligo and showed fairly good, yet not long-standing, repigmentation over the face [104]. An open-label study of twice-daily topical ruxolitinib 1.5% cream showed significant repigmentation in facial vitiligo with poor response at other body parts [105]. As the data on long-term safety of these drugs is not available at present, it is too early to draw a solid conclusion about the position of these drugs in the treatment of vitiligo.
-
2.
Simvastatin: Simvastatin , by blocking activation of STAT1, inhibits interferon-gamma signalling and has shown to halt vitiligo and attain repigmentation in a mouse model [106]. A case report highlighted repigmentation of vitiligo lesions, while the patient was on treatment with high-dose simvastatin [107]. But a recent phase 2 clinical trial using 80 mg per day dose of simvastatin did not support its use in vitiligo, owing mainly to dosage limitations in humans compared to mice [108]. Addition of simvastatin did not have any impact on the repigmentation outcome of topical betamethasone [109].
-
3.
Afamelanotide: It is an analogue of α-melanocyte-stimulating hormone that has shown to induce melanogenesis. It was licensed in Europe to reduce the photosensitivity pain in erythropoietic protoporphyria [110]. A recent randomised multicentre trial has found that the combination of afamelanotide (16 mg) implant and narrowband UVB phototherapy resulted in statistically superior and faster repigmentation, compared to narrowband UVB monotherapy [111]. Melanocortin 1 receptor (MC1R) is not present in melanocyte stem cells; thus, concomitant induction of these stem cells with phototherapy is required for the optimal repigmentation outcome of afamelanotide [112]. Adverse effects of afamelanotide include fatigue, gastrointestinal intolerance and hyperpigmentation.
-
4.
Minocycline: It has been shown that minocycline can rescue melanocytes from oxidative stress in vitro [113]. The mechanism of action includes inhibition of free radical and cytokine production, interference with protein synthesis, anti-apoptotic properties and modulation of matrix metalloproteinases activity. A randomised clinical trial comparing oral minocycline (100 mg/day) to oral mini-pulse corticosteroids found that the two treatments were comparable in arresting the progression of actively spreading vitiligo with minimal side effects in each group [114]. But a prospective comparative trial showed that NB-UVB was statistically more useful than oral minocycline in unstable vitiligo in terms of efficacy and the resulting stability [115].
-
5.
Prostaglandin analogues: Latanoprost , a prostaglandin F2-alpha analogue, is well known for causing, among the possible side effects, increased pigmentation of the iris and periocular hyperpigmentation [116]. Latanoprost was found to be better than placebo and comparable with NB-UVB in inducing skin repigmentation, with enhanced efficacy when combined with NB-UVB [117]. Efficacy of latanoprost and tacrolimus was found to be comparable, when used in combination with phototherapy and microneedling [118]. Any doubts regarding malignant melanoma induction due to latanoprost administration have been excluded [119]. A recent study has shown that bimatoprost as a monotherapy or in combination with mometasone is more effective than mometasone alone in the treatment of non-facial vitiligo [120]. However, more robust clinical studies comparing prostaglandin analogues with other alternatives are needed.
-
6.
Oral vitamins, antioxidants and other supplements: Owing to their antioxidant properties , silymarin [121]; pseudocatalase/superoxide dismutase (PSD) [122]; piperine [123]; curcumin [124]; L-phenylalanine; khellin; Polypodium leucotomos; ginkgo biloba; vitamin B12; folic acid; vitamins C, D [125] and E; alpha lipoic acid; and zinc have shown varying efficacy in the treatment of vitiligo, either as monotherapy or in combination with other treatments [31]. Topical basic fibroblast growth factor (bFGF)-related decapeptide is increasingly being used in augmenting repigmentation, though evidence regarding its efficacy is lacking. Low-dose oral administration of cytokines has been suggested as a newer therapeutic approach to normalise the melanocyte homeostasis in vitiligo [126]. However, larger randomised controlled trials are warranted before recommending them as primary agents in the treatment of vitiligo, and hence, to date, these agents are best regarded as adjuvants to primary agents during the active phase of the disease or as monotherapy during remission.
-
7.
Conventional steroid sparing agents: Methotrexate can be used in patients with active vitiligo wherever corticosteroids are contraindicated as evidenced by a randomised study comparing oral mini-pulse corticosteroids and low-dose oral methotrexate (10 mg/week) [127]. Azathioprine has also found to be beneficial in enhancing repigmentation when used along with phototherapy [128]. Evidence for the beneficial effects of cyclosporine and cyclophosphamide in vitiligo is based on individual case reports [129, 130].
-
8.
Biologicals: A phase 1 clinical trial is underway to ascertain the efficacy of abatacept , a soluble fusion protein consisting of human cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), which prevents T-cell activation in active vitiligo [131]. Various anti-TNF alpha agents [132] as well as alefacept [133] have been found to be ineffective in treating vitiligo, though strength of evidence is less. Rituximab was found to be beneficial in active disseminated vitiligo [134]. Emergence or progression of vitiligo in patients treated with biological agents , especially TNF alpha inhibitors [135], understates the primary role of these targeted molecules in the pathogenesis of vitiligo and hence their usefulness in therapeutic targeting.
-
9.
Future trends: Topical photocil , selectively filters solar radiation to deliver narrowband UVB to vitiligo lesions, obviating the need of phototherapy machines [136]. Transdermal protein transduction of melanocyte-lineage-specific genes is a possible modality for treatment of vitiligo [137]. Induction of melanogenesis even from glabrous vitiligo skin as demonstrated in an in vitro study [138] gives a glimmer of hope in the management of refractory acral vitiligo. Multilineage-differentiating stress-enduring (Muse) cells , a distinct stem cell type among human dermal fibroblasts, can be readily reprogrammed into functional melanocytes and can be a potential source of melanocytes for cell-based therapy in vitiligo [139].
Practical Approach to the Management of Vitiligo
The most recent Cochrane review on interventions in vitiligo concludes that cure for vitiligo and an effective method of limiting the spread of the disease are still an enigma [98]. Research for newer agents in the management raises new hopes in the vitiligo community. However, lack of evidence showing efficacy of these agents urges clinicians to use conventional modalities as the first lines of treatment. In 2013, the Vitiligo Guideline Subcommittee of the European Dermatology Forum developed a new treatment guideline for vitiligo, detailing first line to fourth line of therapy [67]. Here we would like to summarise briefly the approach to manage vitiligo based on the above guideline in the light of newer studies on vitiligo pathogenesis and medical modalities. In a patient with recent onset SV or NSV with fewer patches (<10–20% body surface area) topical modalities (corticosteroids or calcineurin inhibitors) or targeted phototherapies are preferred. Though there is no concrete evidence on clinical usefulness of antioxidants , clinicians are increasingly adopting various oral supplements with antioxidant property for stabilisation of disease at this stage, owing to recent emphasis on oxidant stress in vitiligo pathogenesis. A common approach is giving topical steroids in the morning and topical calcineurin inhibitors in the evening (Fig. 8.7). Topical calcineurin inhibitors are preferred for facial vitiligo (especially in children) and topical corticosteroids for other areas. Continuous administration of topical steroids should be restricted to 3 months, with alternate day or twice weekly regimen for another 3 months if needed, based on response. Potent steroids like mometasone furoate should be preferred over super potent steroids like clobetasol propionate owing to similar efficacy, lesser local side effects and minimal systemic absorption. Topical vitamin D analogues can be added if local side effects of steroids manifest or no further improvement is noted with topical steroids. Prostaglandin analogues and basic fibroblast-related decapeptide are other steroid sparing agents that can be used as first-line agents for facial vitiligo along with topical calcineurin inhibitors. Topical PUVA can be used for refractory acral vitiligo.
Pre- and posttreatment photographs of a patient. A 14-year-old girl with depigmented patches of common vitiligo on both legs (a). Repigmentation of around 75% was attained after 4 months of morning application of topical mometasone furoate 0.1% cream and night application of topical tacrolimus 0.1% ointment along with daily oral folic acid and vitamin B12 supplements (b)
In patients with active widespread NSV (>10–20% body surface area), NBUVB is the treatment of choice. Stabilisation of disease with OMP before starting or during NBUVB therapy is a logical approach to prevent koebnerisation over photoexposed areas, when disease is rapidly spreading. Therapy can be stopped if no repigmentation occurs after 3 months or <25% repigmentation occurs after 6 months. If there is response, NBUVB should be maintained at least for a year. Topical modalities can be added to NBUVB to augment repigmentation. Oral PUVA can be tried as a second-line therapy. As with NBUVB , continuous treatment for 1–2 years is needed for maximal repigmentation. OMP, methotrexate or PUVA sol can be considered, if facility for phototherapy is not available and in the rapid spreading phase of SV. In stable vitiligo, immunosuppressants are not indicated as they are not beneficial in repigmentation. Use of newer agents like afamelanotide or tofacitinib should be restricted to refractory cases of active vitiligo as clinical trials only, since the long-term safety profile or sound evidence on the efficacy are not available at present. Depigmenting agents can be offered after proper counselling, if >50% body surface area is involved.
Surgical therapy can be offered to any individual lesion with more than 1 year clinical stability, and is an important treatment approach in SV. For all individual cases, psychological interventions and camouflaging options should be offered.
References
Spritz RA. The genetics of generalized vitiligo and associated autoimmune diseases. Pigment Cell Res. 2007;20:271–8.
Sehgal VN, Srivastava G. Vitiligo: compendium of clinico-epidemiological features. Indian J Dermatol Venereol Leprol. 2007;73:149–56.
Parsad D, Pandhi R, Dogra S, Kanwar AJ, Kumar B. Dermatology life quality index score in vitiligo and its impact on the treatment outcome. Br J Dermatol. 2003;148:373–4.
Behl PN, Bhatia RK. 400 cases of vitiligo. A clinico-therapeutic analysis. Indian J Dermatol. 1972;17:51–6.
Picardo M, Dell’Anna ML, Ezzedine K, Hamzavi I, Harris JE, Parsad D, et al. Vitiligo. Nat Rev Dis Primers. 2015;1:15011.
Herane MI. Vitiligo and leukoderma in children. Clin Dermatol. 2003;21:283–95.
Nicolaidou E, Antoniou C, Miniati A, Lagogianni E, Matekovits A, Stratigos A, et al. Childhood- and later-onset vitiligo have diverse epidemiologic and clinical characteristics. J Am Acad Dermatol. 2012;66:954–8.
Ezzedine K, Lim HW, Suzuki T, Katayama I, Hamzavi I, Lan CC, et al. Revised classification/nomenclature of vitiligo and related issues: the vitiligo global issues consensus conference. Pigment Cell Melanoma Res. 2012;25:E1–13.
Hann SK, Lee HJ. Segmental vitiligo: clinical findings in 208 patients. J Am Acad Dermatol. 1996;35:671–4.
Koga M, Tango T. Clinical features and course of type a and type B vitiligo. Br J Dermatol. 1988;118:223–8.
van Geel N, De Lille S, Vandenhaute S, Gauthier Y, Mollet I, Brochez L, et al. Different phenotypes of segmental vitiligo based on a clinical observational study. J Eur Acad Dermatol Venereol. 2011;25:673–8.
van Geel NA, Mollet IG, De Schepper S, Tjin EP, Vermaelen K, Clark RA, et al. First histopathological and immunophenotypic analysis of early dynamic events in a patient with segmental vitiligo associated with halo nevi. Pigment Cell Melanoma Res. 2010;23:375–84.
Hann SK, Chang JH, Lee HS, Kim SM. The classification of segmental vitiligo on the face. Yonsei Med J. 2000;41:209–12.
van Geel N, Bosma S, Boone B, Speeckaert R. Classification of segmental vitiligo on the trunk. Br J Dermatol. 2014;170:322–7.
van Geel N, Mollet I, Brochez L, Dutre M, De Schepper S, Verhaeghe E, et al. New insights in segmental vitiligo: case report and review of theories. Br J Dermatol. 2012;166:240–6.
Ezzedine K, Gauthier Y, Leaute-Labreze C, Marquez S, Bouchtnei S, Jouary T, et al. Segmental vitiligo associated with generalized vitiligo (mixed vitiligo): a retrospective case series of 19 patients. J Am Acad Dermatol. 2011;65:965–71.
Ezzedine K, Mahe A, van Geel N, Cardot-Leccia N, Gauthier Y, Descamps V, et al. Hypochromic vitiligo: delineation of a new entity. Br J Dermatol. 2015;172:716–21.
Ezzedine K, Amazan E, Seneschal J, Cario-Andre M, Leaute-Labreze C, Vergier B, et al. Follicular vitiligo: a new form of vitiligo. Pigment Cell Melanoma Res. 2012;25:527–9.
Lommerts JE, Schilder Y, de Rie MA, Wolkerstorfer A, Bekkenk MW. Focal vitiligo: long-term follow-up of 52 cases. J Eur Acad Dermatol Venereol. 2016;30:1550–4.
Passeron T, Ortonne J-P. Generalized vitiligo. In: Picardo M, Taïeb A, editors. Vitiligo. Heidelberg: Springer; 2010. p. 35–9.
Le Poole IC, Das PK, van den Wijngaard RM, Bos JD, Westerhof W. Review of the etiopathomechanism of vitiligo: a convergence theory. Exp Dermatol. 1993;2:145–53.
Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90–100.
Lerner AB. On the etiology of vitiligo and gray hair. Am J Med. 1971;51:141–7.
Spritz RA. Modern vitiligo genetics sheds new light on an ancient disease. J Dermatol. 2013;40:310–8.
Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208–14.
Chen JX, Shi Q, Wang XW, Guo S, Dai W, Li K, et al. Genetic polymorphisms in the methylenetetrahydrofolate reductase gene (MTHFR) and risk of vitiligo in Han Chinese populations: a genotype-phenotype correlation study. Br J Dermatol. 2014;170:1092–9.
Jin Y, Andersen G, Yorgov D, Ferrara TM, Ben S, Brownson KM, et al. Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat Genet. 2016;48:1418–24.
Wang P, Li Y, Nie H, Zhang X, Shao Q, Hou X, et al. The changes of gene expression profiling between segmental vitiligo, generalized vitiligo and healthy individual. J Dermatol Sci. 2016;84:40–9.
Paradisi A, Tabolli S, Didona B, Sobrino L, Russo N, Abeni D. Markedly reduced incidence of melanoma and nonmelanoma skin cancer in a nonconcurrent cohort of 10,040 patients with vitiligo. J Am Acad Dermatol. 2014;71:1110–6.
Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773–81.
Manga P, Elbuluk N, Orlow SJ. Recent advances in understanding vitiligo. F1000Res. 2016;5 https://www.ncbi.nlm.nih.gov/pubmed/?term=Recent+advances+in+understanding+vitiligo.+F1000
Jin Y, Birlea SA, Fain PR, Gowan K, Riccardi SL, Holland PJ, et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med. 2010;362:1686–97.
Speeckaert R, Voet S, Hoste E, van Geel N. S100B is a potential disease activity marker in nonsegmental vitiligo. J Invest Dermatol. 2017;137:1445–53.
Richmond JM, Frisoli ML, Harris JE. Innate immune mechanisms in vitiligo: danger from within. Curr Opin Immunol. 2013;25:676–82.
Jacquemin C, Rambert J, Guillet S, Thiolat D, Boukhedouni N, Doutre MS, et al. HSP70 potentiates interferon-alpha production by plasmacytoid dendritic cells: relevance for cutaneous lupus and vitiligo pathogenesis. Br J Dermatol. 2017;177(5):1367–1375.
Toosi S, Orlow SJ, Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol. 2012;132:2601–9.
Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188:1203–8.
Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869–76.
Ferrari SM, Fallahi P, Santaguida G, Virili C, Ruffilli I, Ragusa F, et al. Circulating CXCL10 is increased in non-segmental vitiligo, in presence or absence of autoimmune thyroiditis. Autoimmun Rev. 2017;16:946–50.
Rezk AF, Kemp DM, El-Domyati M, El-Din WH, Lee JB, Uitto J, et al. Misbalanced CXCL12 and CCL5 chemotactic signals in vitiligo onset and progression. J Invest Dermatol. 2017;137:1126–34.
Lili Y, Yi W, Ji Y, Yue S, Weimin S, Ming L. Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS One. 2012;7:e37513.
Zhou L, Li K, Shi YL, Hamzavi I, Gao TW, Henderson M, et al. Systemic analyses of immunophenotypes of peripheral T cells in non-segmental vitiligo: implication of defective natural killer T cells. Pigment Cell Melanoma Res. 2012;25:602–11.
Qiao Z, Wang X, Xiang L, Zhang C. Dysfunction of autophagy: a possible mechanism involved in the pathogenesis of vitiligo by breaking the redox balance of melanocytes. Oxidative Med Cell Longev. 2016;2016:7.
Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208–14.
Schallreuter KU, Elwary SM, Gibbons NC, Rokos H, Wood JM. Activation/deactivation of acetylcholinesterase by H2O2: more evidence for oxidative stress in vitiligo. Biochem Biophys Res Commun. 2004;315:502–8.
Regazzetti C, Joly F, Marty C, Rivier M, Mehul B, Reiniche P, et al. Transcriptional analysis of vitiligo skin reveals the alteration of WNT pathway: a promising target for repigmenting vitiligo patients. J Invest Dermatol. 2015;135:3105–14.
Vaccaro M, Bagnato G, Cristani M, Borgia F, Spatari G, Tigano V, et al. Oxidation products are increased in patients affected by non-segmental generalized vitiligo. Arch Dermatol Res. 2017;309:485–90.
Yang Y, Li S, Zhu G, Zhang Q, Wang G, Gao T, et al. A similar local immune and oxidative stress phenotype in vitiligo and halo nevus. J Dermatol Sci. 2017;87:50–9.
Hasse S, Gibbons NC, Rokos H, Marles LK, Schallreuter KU. Perturbed 6-tetrahydrobiopterin recycling via decreased dihydropteridine reductase in vitiligo: more evidence for H2O2 stress. J Invest Dermatol. 2004;122:307–13.
Rokos H, Beazley WD, Schallreuter KU. Oxidative stress in vitiligo: photo-oxidation of pterins produces H(2)O(2) and pterin-6-carboxylic acid. Biochem Biophys Res Commun. 2002;292:805–11.
Dell’anna ML, Picardo M. A review and a new hypothesis for non-immunological pathogenetic mechanisms in vitiligo. Pigment Cell Res. 2006;19:406–11.
Kim J, Nakasaki M, Todorova D, Lake B, Yuan CY, Jamora C, et al. p53 Induces skin aging by depleting Blimp1+ sebaceous gland cells. Cell Death Dis. 2014;5:e1141.
Salem MM, Shalbaf M, Gibbons NC, Chavan B, Thornton JM, Schallreuter KU, Enhanced DNA. Binding capacity on up-regulated epidermal wild-type p53 in vitiligo by H2O2-mediated oxidation: a possible repair mechanism for DNA damage. FASEB J. 2009;23:3790–807.
Bondanza S, Maurelli R, Paterna P, Migliore E, Giacomo FD, Primavera G, et al. Keratinocyte cultures from involved skin in vitiligo patients show an impaired in vitro behaviour. Pigment Cell Res. 2007;20:288–300.
Fukunaga-Kalabis M, Hristova DM, Wang JX, Li L, Heppt MV, Wei Z, et al. UV-induced Wnt7a in the human skin microenvironment specifies the fate of neural crest-like cells via suppression of notch. J Invest Dermatol. 2015;135:1521–32.
Seleit I, Bakry OA, Abdou AG, Dawoud NM. Immunohistochemical expression of aberrant Notch-1 signaling in vitiligo: an implication for pathogenesis. Ann Diagn Pathol. 2014;18:117–24.
Rani S, Bhardwaj S, Srivastava N, Sharma VL, Parsad D, Kumar R. Senescence in the lesional fibroblasts of non-segmental vitiligo patients. Arch Dermatol Res. 2017;309:123–32.
Gauthier Y, Cario-Andre M, Lepreux S, Pain C, Taieb A. Melanocyte detachment after skin friction in non lesional skin of patients with generalized vitiligo. Br J Dermatol. 2003;148:95–101.
Wagner RY, Luciani F, Cario-Andre M, Rubod A, Petit V, Benzekri L, et al. Altered E-cadherin levels and distribution in melanocytes precede clinical manifestations of vitiligo. J Invest Dermatol. 2015;135:1810–9.
Kim NH, Lee AY. Reduced aquaporin3 expression and survival of keratinocytes in the depigmented epidermis of vitiligo. J Invest Dermatol. 2010;130:2231–9.
Li L, Wu Y, Li L, Sun Y, Qiu L, Gao XH, et al. Triple combination treatment with fractional CO2 laser plus topical betamethasone solution and narrowband ultraviolet B for refractory vitiligo: a prospective, randomized half-body, comparative study. Dermatol Ther. 2015;28:131–4.
Bae JM, Yoo HJ, Kim H, Lee JH, Kim GM. Combination therapy with 308-nm excimer laser, topical tacrolimus, and short-term systemic corticosteroids for segmental vitiligo: a retrospective study of 159 patients. J Am Acad Dermatol. 2015;73:76–82.
Hossani-Madani AR, Halder RM. Topical treatment and combination approaches for vitiligo: new insights, new developments. G Ital Dermatol Venereol. 2010;145:57–78.
Abdel Latif AA, Ibrahim SM. Monochromatic excimer light versus combination of topical steroid with vitamin D3 analogue in the treatment of nonsegmental vitiligo: a randomized blinded comparative study. Dermatol Ther. 2015;28:383–9.
Yazdani Abyaneh M, Griffith RD, Falto-Aizpurua L, Nouri K. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: mechanisms and efficacies. J Eur Acad Dermatol Venereol. 2014;28:1610–22.
Van Driessche F, Silverberg N. Current management of pediatric vitiligo. Paediatr Drugs. 2015;17:303–13.
Taieb A, Alomar A, Bohm M, Dell’anna ML, De Pase A, Eleftheriadou V, et al. Guidelines for the management of vitiligo: the European dermatology forum consensus. Br J Dermatol. 2013;168:5–19.
Bayoumi W, Fontas E, Sillard L, Le Duff F, Ortonne JP, Bahadoran P, et al. Effect of a preceding laser dermabrasion on the outcome of combined therapy with narrowband ultraviolet B and potent topical steroids for treating nonsegmental vitiligo in resistant localizations. Br J Dermatol. 2012;166:208–11.
Khurrum H, AlGhamdi KM, Osman E. Screening of glaucoma or cataract prevalence in vitiligo patients and its relationship with periorbital steroid use. J Cutan Med Surg. 2016;20:146–9.
Lan CC, CS W, Chen GS, Yu HS. FK506 (tacrolimus) and endothelin combined treatment induces mobility of melanoblasts: new insights into follicular vitiligo repigmentation induced by topical tacrolimus on sun-exposed skin. Br J Dermatol. 2011;164:490–6.
Radakovic S, Breier-Maly J, Konschitzky R, Kittler H, Sator P, Hoenigsmann H, et al. Response of vitiligo to once- vs. twice-daily topical tacrolimus: a controlled prospective, randomized, observer-blinded trial. J Eur Acad Dermatol Venereol. 2009;23:951–3.
Margolis DJ, Abuabara K, Hoffstad OJ, Wan J, Raimondo D, Bilker WB. Association between malignancy and topical use of pimecrolimus. JAMA Dermatol. 2015;151:594–9.
Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163–78.
Cavalie M, Ezzedine K, Fontas E, Montaudie H, Castela E, Bahadoran P, et al. Maintenance therapy of adult vitiligo with 0.1% tacrolimus ointment: a randomized, double blind, placebo-controlled study. J Invest Dermatol. 2015;135:970–4.
Li QL, YH W, Niu M, XJ L, Huang YH, He DH. Protective effects of tacalcitol against oxidative damage in human epidermal melanocytes. Int J Dermatol. 2017;56:232–8.
Xing C, Xu A. The effect of combined calcipotriol and betamethasone dipropionate ointment in the treatment of vitiligo: an open, uncontrolled trial. J Drugs Dermatol. 2012;11:e52–4.
Sahu P, Jain VK, Aggarwal K, Kaur S, Dayal S. Tacalcitol: a useful adjunct to narrow-band ultraviolet-B phototherapy in vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:262–8.
Khullar G, Kanwar AJ, Singh S, Parsad D. Comparison of efficacy and safety profile of topical calcipotriol ointment in combination with NB-UVB vs. NB-UVB alone in the treatment of vitiligo: a 24-week prospective right-left comparative clinical trial. J Eur Acad Dermatol Venereol. 2015;29:925–32.
Kanwar AJ, Mahajan R, Parsad D. Low-dose oral mini-pulse dexamethasone therapy in progressive unstable vitiligo. J Cutan Med Surg. 2013;17:259–68.
Lee J, Chu H, Lee H, Kim M, Kim DS, Retrospective Study OSHA. Of methylprednisolone mini-pulse therapy combined with narrow-band UVB in non-segmental vitiligo. Dermatology. 2016;232:224–9.
El Mofty M, Essmat S, Youssef R, Sobeih S, Mahgoub D, Ossama S, et al. The role of systemic steroids and phototherapy in the treatment of stable vitiligo: a randomized controlled trial. Dermatol Ther. 2016;29:406–12.
Shenoi SD, Prabhu S. Photochemotherapy (PUVA) in psoriasis and vitiligo. Indian J Dermatol Venereol Leprol. 2014;80:497–504.
Singh S, Khandpur S, Sharma VK, Ramam M. Comparison of efficacy and side-effect profile of oral PUVA vs. oral PUVA sol in the treatment of vitiligo: a 36-week prospective study. J Eur Acad Dermatol Venereol. 2013;27:1344–51.
Ibrahim ZA, El-Ashmawy AA, El-Tatawy RA, Sallam FA. The effect of platelet-rich plasma on the outcome of short-term narrowband-ultraviolet B phototherapy in the treatment of vitiligo: a pilot study. J Cosmet Dermatol. 2016;15:108–16.
Abdelghani R, Ahmed NA, Darwish HM. Combined treatment with fractional carbon dioxide laser, autologous platelet-rich plasma, and narrow band ultraviolet B for vitiligo in different body sites: a prospective, randomized comparative trial. J Cosmet Dermatol. 2017. http://doi: 10.1111/jocd.12397.
Xiao BH, Wu Y, Sun Y, Chen HD, Gao XH. Treatment of vitiligo with NB-UVB: a systematic review. J Dermatolog Treat. 2015;26:340–6.
Jo SJ, Kwon HH, Choi MR, Youn JI. No evidence for increased skin cancer risk in Koreans with skin phototypes III-V treated with narrowband UVB phototherapy. Acta Derm Venereol. 2011;91:40–3.
El Mofty M, Bosseila M, Mashaly HM, Gawdat H, Makaly H. Broadband ultraviolet A vs. psoralen ultraviolet A in the treatment of vitiligo: a randomized controlled trial. Clin Exp Dermatol. 2013;38:830–5.
Leone G, Tanew A. UVB total body and targeted phototherapies. In: Picardo M, Taïeb A, editors. Vitiligo. Heidelberg: Springer; 2010. p. 359–65.
Werfel T, Holiangu F, Niemann KH, Schmerling O, Lullau F, Zedler A, et al. Digital ultraviolet therapy: a novel therapeutic approach for the targeted treatment of psoriasis vulgaris. Br J Dermatol. 2015;172:746–53.
Reidel U, Bechstein S, Lange-Asschenfeldt B, Beyer M, Vandersee S. Treatment of localized mycosis fungoides with digital UV photochemotherapy. Photodermatol Photoimmunol Photomed. 2015;31:333–40.
Mysore V, Shashikumar BM. Targeted phototherapy. Indian J Dermatol Venereol Leprol. 2016;82:1–6.
Majid I, Imran S. Targeted ultraviolet B phototherapy in vitiligo: a comparison between once-weekly and twice-weekly treatment regimens. Indian J Dermatol Venereol Leprol. 2015;81:600–5.
Dillon JP, Ford C, Hynan LS, Pandya AG. A cross-sectional, comparative study of home vs in-office NB-UVB phototherapy for vitiligo. Photodermatol Photoimmunol Photomed. 2015;33:282–3.
Hossain C, Porto DA, Hamzavi I, Lim HW. Camouflaging agents for vitiligo patients. J Drugs Dermatol. 2016;15:384–7.
Solano F, Briganti S, Picardo M, Ghanem G. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006;19:550–71.
AlGhamdi KM, Kumar A. Depigmentation therapies for normal skin in vitiligo universalis. J Eur Acad Dermatol Venereol. 2011;25:749–57.
Whitton ME, Pinart M, Batchelor J, Leonardi-Bee J, Gonzalez U, Jiyad Z, et al (2015) Interventions for vitiligo. Cochrane Database Syst Rev (2):CD003263.
Kruger C, Smythe JW, Spencer JD, Hasse S, Panske A, Chiuchiarelli G, et al. Significant immediate and long-term improvement in quality of life and disease coping in patients with vitiligo after group climatotherapy at the Dead Sea. Acta Derm Venereol. 2011;91:152–9.
Shah R, Hunt J, Webb TL, Thompson AR. Starting to develop self-help for social anxiety associated with vitiligo: using clinical significance to measure the potential effectiveness of enhanced psychological self-help. Br J Dermatol. 2014;171:332–7.
Jha A, Mehta M, Khaitan BK, Sharma VK, Ramam M. Cognitive behavior therapy for psychosocial stress in vitiligo. Indian J Dermatol Venereol Leprol. 2016;82:308–10.
Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 2015;151:1110–2.
Liu LY, Strassner JP, Refat MA, Harris JE, King BA. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77:675.
Harris JE, Rashighi M, Nguyen N, Jabbari A, Ulerio G, Clynes R, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370–1.
Rothstein B, Joshipura D, Saraiya A, Abdat R, Ashkar H, Turkowski Y, et al. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib. J Am Acad Dermatol. 2017;76:1054–60.e1.
Agarwal P, Rashighi M, Essien KI, Richmond JM, Randall L, Pazoki-Toroudi H, et al. Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J Invest Dermatol. 2015;135:1080–8.
Noel M, Gagne C, Bergeron J, Jobin J, Poirier P. Positive pleiotropic effects of HMG-CoA reductase inhibitor on vitiligo. Lipids Health Dis. 2004;3:7.
Vanderweil SG, Amano S, Ko WC, Richmond JM, Kelley M, Senna MM, et al. A double-blind, placebo-controlled, phase-II clinical trial to evaluate oral simvastatin as a treatment for vitiligo. J Am Acad Dermatol. 2017;76:150–1.e3.
Iraji F, Banihashemi SH, Faghihi G, Shahmoradi Z, Tajmirriahi N, Jazi SBA. Comparison of betamethasone Valerate 0.1% cream twice daily plus oral simvastatin versus betamethasone Valerate 0.1% cream alone in the treatment of vitiligo patients. Adv Biomed Res. 2017;6:34.
Lotti TM, Hercogova J, Schwartz RA, Tsampau D, Korobko I, Pietrzak A, et al. Treatments of vitiligo: what’s new at the horizon. Dermatol Ther. 2012;25(Suppl 1):S32–40.
Lim HW, Grimes PE, Agbai O, Hamzavi I, Henderson M, Haddican M, et al. Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: a randomized multicenter trial. JAMA Dermatol. 2015;151:42–50.
Passeron T. Indications and limitations of afamelanotide for treating vitiligo. JAMA Dermatol. 2015;151:349–50.
Song X, Xu A, Pan W, Wallin B, Kivlin R, Lu S, et al. Minocycline protects melanocytes against H2O2-induced cell death via JNK and p38 MAPK pathways. Int J Mol Med. 2008;22:9–16.
Singh A, Kanwar AJ, Parsad D, Mahajan R. Randomized controlled study to evaluate the effectiveness of dexamethasone oral minipulse therapy versus oral minocycline in patients with active vitiligo vulgaris. Indian J Dermatol Venereol Leprol. 2014;80:29–35.
Siadat AH, Zeinali N, Iraji F, Abtahi-Naeini B, Nilforoushzadeh MA, Jamshidi K, et al. Narrow-band ultraviolet B versus oral minocycline in treatment of unstable vitiligo: a prospective comparative trial. Dermatol Res Pract. 2014;2014:240856.
Chou SY, Chou CK, Kuang TM, Hsu WM. Incidence and severity of iris pigmentation on latanoprost-treated glaucoma eyes. Eye (Lond). 2005;19:784–7.
Anbar TS, El-Ammawi TS, Abdel-Rahman AT, Hanna MR. The effect of latanoprost on vitiligo: a preliminary comparative study. Int J Dermatol. 2015;54:587–93.
Korobko IV, Lomonosov KMA. Pilot comparative study of topical latanoprost and tacrolimus in combination with narrow-band ultraviolet B phototherapy and microneedling for the treatment of nonsegmental vitiligo. Dermatol Ther. 2016;29:437–41.
Tressler CS, Wiseman RL, Dombi TM, Jessen B, Huang K, Kwok KK, et al. Lack of evidence for a link between latanoprost use and malignant melanoma: an analysis of safety databases and a review of the literature. Br J Ophthalmol. 2011;95:1490–5.
Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703–10.
Sehgal VN. Role of tacrolimus (FK506) 0.1% ointment WW in vitiligo in children and imperatives of combine therapy with Trioxsalen and Silymarin suspension in progressive vitiligo. J Eur Acad Dermatol Venereol. 2009;23:1218–9.
Naini FF, Shooshtari AV, Ebrahimi B, Molaei R. The effect of pseudocatalase/superoxide dismutase in the treatment of vitiligo: a pilot study. J Res Pharm Pract. 2012;1:77–80.
Faas L, Venkatasamy R, Hider RC, Young AR, Soumyanath A. Vivo evaluation of piperine and synthetic analogues as potential treatments for vitiligo using a sparsely pigmented mouse model. Br J Dermatol. 2008;158:941–50.
Asawanonda P, Klahan SO. Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: a preliminary randomized controlled study. Photomed Laser Surg. 2010;28:679–84.
Karaguzel G, Sakarya NP, Bahadir S, Yaman S, Okten A. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28–31.
Lotti T, Hercogova J, Fabrizi G. Advances in the treatment options for vitiligo: activated low-dose cytokines-based therapy. Expert Opin Pharmacother. 2015;16:2485–96.
Singh H, Kumaran MS, Bains A, Parsad DA. Randomized comparative study of oral corticosteroid Minipulse and low-dose oral methotrexate in the treatment of unstable vitiligo. Dermatology. 2015;231:286–90.
Radmanesh M, Saedi K. The efficacy of combined PUVA and low-dose azathioprine for early and enhanced repigmentation in vitiligo patients. J Dermatolog Treat. 2006;17:151–3.
Gupta AK, Ellis CN, Nickoloff BJ, Goldfarb MT, Ho VC, Rocher LL, et al. Oral cyclosporine in the treatment of inflammatory and noninflammatory dermatoses. A clinical and immunopathologic analysis. Arch Dermatol. 1990;126:339–50.
Dogra S, Kumar B. Repigmentation in vitiligo universalis: role of melanocyte density, disease duration, and melanocytic reservoir. Dermatol Online J. 2005;11:30.
Open-label pilot study of abatacept for the treatment of vitiligo [Internet]. U.S. National Institutes of Health. 2016 [cited December 29, 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT02281058.
Alghamdi KM, Khurrum H, Taieb A, Ezzedine K. Treatment of generalized vitiligo with anti-TNF-alpha agents. J Drugs Dermatol. 2012;11:534–9.
Bin Dayel S, AlGhamdi K. Failure of alefacept in the treatment of vitiligo. J Drugs Dermatol. 2013;12:159–61.
Ruiz-Arguelles A, Garcia-Carrasco M, Jimenez-Brito G, Sanchez-Sosa S, Perez-Romano B, Garces-Eisele J, et al. Treatment of vitiligo with a chimeric monoclonal antibody to CD20: a pilot study. Clin Exp Immunol. 2013;174:229–36.
Mery-Bossard L, Bagny K, Chaby G, Khemis A, Maccari F, Marotte H, et al. New-onset vitiligo and progression of pre-existing vitiligo during treatment with biological agents in chronic inflammatory diseases. J Eur Acad Dermatol Venereol. 2017;31:181–6.
Wang X, McCoy J, Lotti T, Goren A. Topical cream delivers NB-UVB from sunlight for the treatment of vitiligo. Expert Opin Pharmacother. 2014;15:2623–7.
Mou Y, Jiang X, Du Y, Xue L. Intelligent bioengineering in vitiligo treatment: transdermal protein transduction of melanocyte-lineage-specific genes. Med Hypotheses. 2012;79:786–9.
Kumar R, Parsad D, Rani S, Bhardwaj S, Srivastav N. Glabrous lesional stem cells differentiated into functional melanocytes: new hope for repigmentation. J Eur Acad Dermatol Venereol. 2016;30:1555–60.
Tsuchiyama K, Wakao S, Kuroda Y, Ogura F, Nojima M, Sawaya N, et al. Functional melanocytes are readily reprogrammable from multilineage-differentiating stress-enduring (muse) cells, distinct stem cells in human fibroblasts. J Invest Dermatol. 2013;133:2425–35.
Acknowledgement
Authors are indebted to Dr. Raihan Ashraf, MBBS and Dr. Rajsmita Bhattacharjee, MD for the English language editing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Razmi T, M., Parsad, D. (2018). Recent Advances in Pathogenesis and Medical Management of Vitiligo. In: Kumarasinghe, P. (eds) Pigmentary Skin Disorders. Updates in Clinical Dermatology. Springer, Cham. https://doi.org/10.1007/978-3-319-70419-7_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-70419-7_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-70418-0
Online ISBN: 978-3-319-70419-7
eBook Packages: MedicineMedicine (R0)