Abstract
Fungal infections of the central nervous system (CNS) represent a diagnostic and therapeutic challenge and are associated with high morbidity and mortality. The incidence of invasive fungal infections has increased over time with the rise of at-risk populations including organ transplants, chemotherapy, and human immunodeficiency virus infections among other conditions. Respiratory tract or sinuses are usually the most common port of entry, and then fungi spread hematogenously or by contiguity to the CNS. Infection may also occur after neurosurgical procedures and catheters, trauma, skin burns and penetrating wounds, and near drowning. CNS fungal infections may present as an acute or chronic meningitis, brain abscess, occupying space lesions, hydrocephalus, stroke, vasculitis, and arachnoiditis. In this chapter, the most common etiologies of CNS infections will be reviewed, including Cryptococcus, Candida, Aspergillus, Mucor, endemic dimorphic fungi (Histoplasma, Coccidioides, Blastomyces), and other more rare pathogens including Exserohilum rostratum and Scedosporium spp. Management include the use of amphotericin B, flucytosine, triazoles (voriconazole, posaconazole, fluconazole, itraconazole), and surgery for large brain mass. Long-term azole therapy may be needed for chronic infections to avoid recurrence.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Amphotericin B
- Aspergillosis
- Brain abscess
- Candida
- Cryptococcal meningitis
- Fungal infections
- Histoplasma
- Molds
- Mucormycosis
- Phaeohyphomycosis
Introduction
Fungi are ubiquitous saprophyte organisms that can be found in soil and water. They have an important role in the decomposition of organic material and decaying vegetation. Of the 100,000 fungi species, around 300 are pathogen to humans. Fungal infections of the central nervous system (CNS) represent a diagnostic and therapeutic challenge and are associated with high morbidity and mortality. CNS fungal pathogens can be classified as yeasts (small unicellular organisms), filamentous or hyphae fungi (molds) that grow in colonies and dimorphic fungi (Table 7.1).
Some endemic fungi have specific geographic distribution in template and subtropical regions. However, other fungi may have a worldwide distribution and be ubiquitous. In the last decades, the epidemiology of fungal infections has rapidly changed. Involvement of the CNS is now a more frequent complication of disseminated mycosis in susceptible hosts. The incidence of invasive fungal infections has increased over time with the rise of at-risk populations including organ transplants, chemotherapy, and human immunodeficiency virus (HIV) infections [1].
There are specific risk factors for CNS fungal infections, as these affect mostly immunocompromised individuals. Acquired immune deficiency syndrome (AIDS), solid organ transplantation, chronic diseases (diabetes, renal failure, and cirrhosis), hematological malignancies, chronic corticosteroid or immunosuppressive therapy, some congenital immune deficiency conditions, and the use of intravenous drugs are well-known risk factors. However, some pathogen fungi may also affect to healthy immunocompetent people, and predisposing factors include trauma, near drowning, skin burns and penetrating wounds, and the use of catheter and neurosurgical devices [2].
Cryptococcus is the most frequent cause of fungal meningitis. Cryptococcal meningitis is now an AIDS-defining illness, and the incidence rose dramatically with the pandemic of AIDS. During the pre-highly active antiretroviral therapy (HAART) era, thousands of people yearly died from cryptococcal meningitis in sub-Saharan Africa [3]. The incidence of HIV-associated cryptococcosis has diminished in developed countries since the introduction of antiretroviral therapy [4].
Geographical differences have been observed in the frequency of CNS fungal infections, too. In a 2-year study performed in a reference hospital in northern India, the most common cause in 50 CNS fungal infections was mucormycosis (50%), followed by Cryptococcus (34%), and aspergillosis (16%) [5]. However, incidence of invasive aspergillosis continues to rise in Middle East countries among immunocompromised patients [6].
Meningitis and meningoencephalitis are the most frequent forms of presentation of CNS fungal infections. Cryptococcus is the most common cause of chronic lymphocytic fungal meningitis. Candida albicans is the most frequent etiology of acute or neutrophilic fungal meningitis. CNS fungal infections may also present as space-occupying focal lesions, brain abscesses, or granulomas. Some angioinvasive fungi such as Aspergillus may cause ischemic stroke secondary to vasculitis. In rare occasions, myelopathy and epidural abscess, and osteomyelitis may happen.
There are new clinical phenotypes of fungal infections in special immunosuppressed hosts. New groups at risk include patients with acquired immunodeficiency due to immunosuppressive therapies such as anti-tumor necrosis factor-α (TNF-α) treatment. Patients who have severe burns and damage of skin barrier protection are also susceptible to disseminated Candida and filamentous fungal infections (Aspergillus spp., Mucorales). Some recently discovered primary immunodeficiency conditions can also predispose selectively to invasive CNS fungal diseases [1].
Respiratory tract is the most common port of entry, and fungi spread through hematogenous dissemination to the CNS. In some cases, direct fungal inoculation in the CNS may occur as consequence of trauma, infected wounds, neurosurgery, and use of intravenous drugs or contaminated corticosteroid injections. Local spread from sinuses, orbit, and vessels may also occur, and blood vessel invasion is common in immunosuppressed patients [7].
Microscopy, culture, and histopathological identification of fungal organisms are the standard strategies for the diagnosis of fungal pathogen infection. Recently, new diagnostic techniques have emerged such as lateral flow immunoassays, matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) , and multiplex polymerase chain reaction techniques (PCR) [8]. Neuroimaging (CT scan and MRI of brain) have been very useful to detect and evaluate progression of cerebritis, abscess formation, large mass lesions, vasculitis and ischemic infarctions, mycotic aneurysms, and the presence of leptomeningeal enhancement [9].
Timely antifungal therapy may avoid long-term neurological sequel. In patients with focal mass lesions, neurosurgical therapy may also be needed. Reversal of immunosuppression and early detection and treatment of immune reconstitution inflammatory syndrome (IRIS) are other basic management points. Antifungal agents include amphotericin B formulations, flucytosine, and triazoles [10].
In this chapter, the most common fungal pathogens affecting the CNS will be reviewed.
Yeasts
Cryptococcosis
Epidemiology and Risk Factors
Cryptococcus is a worldwide spread yeast that is usually found in soils contaminated with bird feces which are an environmental reservoir for the fungi. Cryptococcosis is a fungal infection caused mainly by two pathogen species which belong to the group of basidiomycete, Cryptococcus var. neoformans and Cryptococcus var. gattii. Cryptococcus is a leading cause of meningitis in those areas of the world in which HIV infection is endemic. One decade ago, it was estimated that Cryptococcus caused annually around 1 million cases of meningitis and half million deaths per year in sub-Saharan HIV-endemic countries [11]. In the last decade, the introduction of HAART caused a significant reduction in the number of cases of HIV-associated cryptococcal meningitis in developed countries. Prevalence of cryptococcal meningitis remains still high in HIV-endemic African countries, and patients at high risk are those with CD4 T cell count <100 cells/ul and not taking HAART.
Cryptococcus gattii has also caused outbreaks in the region of the United States (US) Pacific Northwest, British Columbia, and Vancouver, affecting mainly healthy immunocompetent population and causing involvement of lungs and the CNS [12]. C. gattii infection is usually found in tropical and subtropical areas among healthy people exposed to plant propagules.
Risk factors for cryptococcal meningitis in HIV non-infected patients are receiving solid organ transplantation [13], having cell-mediated immunity disorders, chronic liver, kidney or lung disease, and malignancy [14]. In addition, Cryptococcus is associated with sarcoidosis [15] and some autoimmune diseases (lupus, dermatomyositis) [16]. However, a confounding factor in these cases may be the chronic use of steroids.
Cryptococcal meningitis has also been reported in health immunocompetent people. Nevertheless, it is thought that many “normal” or “healthy” hosts may harbor unknown primary immune defects [11]. Idiopathic CD4+ lymphopenia, pulmonary alveolar proteinosis with antibodies to granulocyte-macrophage colony-stimulating factor, and the presence of autoantibodies against interpheron-gamma have been associated with cryptococcosis [17].
Recently, cryptococcal meningitis/meningoencephalitis and disseminated cryptococcosis have been reported in several patients with relapsing remitting multiple sclerosis treated with the immunosuppressive drug fingolimod [18,19,20].
Pathogenesis
Cryptococcus genome displays plasticity and high capacity for microevolution, which can happen in the stressful environment of the human subarachnoid space [21]. C. neoformans is found in purine-rich bird guano and suffers a dramatic change in nutrient availability during host infection when disseminating to colonize the purine poor CNS [22]. The virulence of this human fungal pathogen is complex, and multiple factors have been identified including the ability to growth in higher temperatures, the capsule and melanin production, and the phospholipase/urease activity.
Cryptococcus can grow at 37 °C and survive in relatively hypoxic environments such as the brain and produces a polysaccharide capsule that protects from host. Cryptococcus has a high tropism for the brain and uses the virulence factor laccase which has a predilection for the dopaminergic tracts, particularly in the basal ganglia , to produce melanin. The oxidation of catecholamines within dopaminergic tracts produces melanin which acts as an immunosuppressive compound. The high concentration of inositol in the CNS seems also to be necessary for the survival of the fungus in the brain. Recently, it has been shown that guanosine monophosphate (GMP) synthase, the second enzyme in the guanylate branch of de novo purine biosynthesis, is required for virulence factor production and infection by Cryptococcus [22].
Clinical Features
Cryptococcus is usually acquired by inhalation, and the yeast reaches the alveolar spaces. Following a latent period in which Cryptococcus is contained within the lymph nodes of the lung, the organism may spread and involve the lungs, CNS, skin, urinary tract, and bones. During the primary infection, dissemination from lungs to the blood and CNS may cause a life-threatening meningoencephalitis in immunosuppressed patients. Dissemination may also happen after reactivation of a granuloma upon immunosuppression [23].
Acute cryptococcal meningitis may present with headache, fever, nausea and vomiting, and mental changes. Blurred vision, seizures, and decreased level of consciousness may occur as a consequence of raised intracranial pressure. In rare cases, visual loss may occur through direct optic nerve, chiasm, or tract invasion by the fungi [24]. Focal brain granulomas are called cryptococcomas and may present as focal neurological deficit [25].
In some patients, a significant delay in diagnosis may happen; chronic infection is associated with cognitive dysfunction, gait disturbances, and even dementia in these cases. Visual and hearing problems, cranial nerve involvement, and either obstructive or non-obstructive hydrocephalus may also occur. HIV-negative patients may have a more prolonged and chronic course of the disease [7]. In a case-control study, the main predictors of cryptococcal meningitis were positive blood culture, detection of cryptococcal antigen in serum, current malignancy, and headache [26].
Diagnosis
Cerebrospinal flow (CSF) analysis may demonstrate raised protein, low glucose levels, and lymphocyte pleocytosis. However, the CSF white cell count can be low in HIV patients. Confirmatory diagnosis is based on detection of cryptococcal antigen (the capsular polysaccharide called glucuronoxylomannan) and/or the direct visualization of Cryptococcus in the CSF using India ink stain, which has 70–90% sensitivity. In those patients having negative CSF culture, antigen titters from serum and CSF can be detected by means of enzyme immunoassay, latex agglutination tests, or lateral flow assay. In non-HIV patients, CSF culture and latex agglutination tests can be negative, and repeated CSF samples may be indicated, mainly for C. gattii [11].
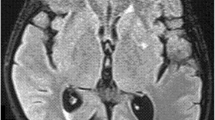
The CT and/or MRI of the brain may reveal leptomeningeal enhancement, hydrocephalus, and cryptococcomas . In negative CSF culture patients and in those who present with cryptococcomas, diagnosis can be done by pathological examination of meningeal biopsy sample. Encapsulated cryptococcomas, although rare, may happen in immunocompetent hosts. These are large cystic lesions, usually related to C. gattii and may mimic a glioblastoma [27]. Vasculitis causing small vessel infarctions in the basal ganglia, thalamus, and posterior circulation has also been described in cryptococcal meningoencephalitis [28,29,30] (Fig. 7.1).
IRIS, resulting in worsening of cerebral edema and neurological deterioration have been reported during early stages of treatment in both HIV and non-HIV patients [31]. IRIS may happen in HIV patients with cryptococcosis after initiation of the HAART. Paradoxical cryptococcal IRIS associated with the development of intracranial cryptococcomas have been described in HIV patients following treatment of cryptococcal meningitis [32]. Cryptococcal-IRIS findings include focal meningeal and parenchymal enhancement, linear perivascular enhancement in the brain sulci, and enhancement of the distended Virchow-Robin space pseudocysts [33].
Treatment
Crptococcosis of the CNS is associated with significant mortality and morbidity among HIV and non-HIV infected patients, despite antifungal therapy. Early diagnosis and treatment are needed to avoid long-term neurological sequelae and death. Raised intracranial pressure should be treated soon as it is significantly associated with poor neurological outcomes [34, 35].
The three-steep therapeutic approach (induction, consolidation, and maintenance) has been used in the last two decades. Amphotericin B (Amb) with or without flucytosine is the treatment of choice. Amphothericin B plus flucytosine showed better outcomes, with increased survival ratio and quicker clearance of infection, compared to single therapy with amphotericin B. Flucytosine dose should be adjusted if renal impairment secondary to amphotericin occurs [11].
Amphotericin B deoxycholate (D-Amb) may cause side effects including hypokalemia, hypomagnesemia, renal dysfunction, and anemia. The intravenous (IV) lipid amphotericin B formulations (L-Amb), either liposomal AmB (dose: 3–4 mg/kg/day IV) or lipid complex AmB (5 mg/kg/day IV) have a better tolerability profile and can be used in those patients having kidney dysfunction [11].
Initial intravenous fungicidal therapy (D-Amb dose: 0.7–1 mg/kg/day) plus flucytosine (100 mg/kg/day orally in four divided doses) is recommended for at least 2 weeks. The initial induction phase may be longer (4–6 weeks) in non-HIV patients and is followed by a consolidation therapy with fluconazole (400 mg/day) for at least 8 weeks. Fungistatic oral drugs such as fluconazole (200 mg/day) or itraconazole are also recommended as subsequent oral treatment to complete a 12–18 months course. Cryptococcus gattii may become fluconazole-resistant [11].
In some patients, serial high-volume lumbar punctures may be indicated to control CSF pressure during the first weeks of treatment. Cerebral edema, outflow obstruction secondary to arachnoiditis, and obstruction within the Monro, Luschka, and Magendie foramen may happen. In severe hydrocephalus cases, a shunt may be required.
CNS Candidiasis
Epidemiology and Risk Factors
Candida spp., a yeast that forms part of the normal commensal human microbial flora, can be found frequently in the skin, oral cavity, and the digestive and genitourinary tracts. There are more than 80 species and the most common are Candida albicans, Candida tropicalis, Candida glabatra, Candida krusei, and Candida parapsilosis [36].
Candida spp. can cause invasive disease in immunocompromised patients, and the main risk factors are neutropenia and AIDS [37]. In immunocompetent patients, invasive candidiasis is associated with critical illness, the use of central venous catheters, prosthetic material and other indwelling catheters, prolonged use of broad-spectrum antibiotics, and burns [38].
Data from the Prospective Antifungal Therapy Alliance registry, a multicenter observational study of HIV patients, showed that Candida (33%) was the second most common infection after Cryptococcus (50%), and followed by Histoplasma (9%), and Aspergillus (4.4%) [39]. Disseminated Candida infections in HIV patients occurred mainly as candidemia [39].
Candida spp. meningitis occurs mostly in neonates, and also in patients that underwent neurosurgical p rocedure such as ventriculostomy, had an infected wound or a shunt placed, and also in immunocompromised subjects [2, 40].
Invasive fungal infection rates are highest among neonates, especially those of low birth weight. The Neonatal Infection Surveillance Network in England study (2004–2010) reported that the overall incidence was 2.4/1000 neonatal unit admissions and was highest among babies <1000 g. The majority of infections were caused by C. albicans (n = 59; 69%) and C. parapsilosis (n = 17; 20%). Identified risk factors were the use of central venous catheters, parenteral nutrition, and previous antibiotic use [41]. Mortality and neurodevelopmental outcome of extremely low birth weight infants who suffer CNS candidiasis is very poor, and severe neurodevelopmental impairment or death has been reported [42]. In the England study, the case fatality rate was 21% [41].
Caspase recruitment domain-containing protein 9 (CARD9) deficiency is an autosomal recessive primary immunodeficiency disorder which confer human susceptibility to invasive fungal disease [43]. Cases of congenital CARD9 deficiency have been associated with chronic mucocutaneous candidiasis including spontaneous CNS candidiasis [44]. In some cases, CNS candidiasis occurred in adulthood and was confounded radiologically with brain malignancies [44]. Inherited CARD9 deficiency in otherwise healthy children and adults may present with Candida spp. meningoencephalitis, colitis, or both [45]. Chronic C. albicans meningitis has also been reported in a 4-year-old girl harboring a homozygous mutation in the CARD9 gene (Q295X) [46].
Clinical Features
C. albicans initially infects the oral cavity and esophagus; submucosal blood vessels become involved when infection progresses. Hematogenous dissemination may cause meningitis and also multiple embolic micro-abscesses in the brain parenchyma in neutropenic patients. CNS involvement may occur in at least half of cases of disseminated candidiasis, and mortality is very high [7]. Candida endophthalmitis may happen following bloodstream infections. After Staphylococcus aureus, neurocandidiasis is the second most common etiology of scattered cerebral micro-abscesses. Candida micro-abscesses may present with non-specific symptoms including headache, fever, and encephalopathy. Cerebral abscesses may also happen in preterm infants [47].
There are other less common forms of presentation such as large solitary brain abscesses, basilar artery thrombosis, ischemic stroke secondary to vasculitis, and subarachnoid hemorrhage following the rupture of a mycotic aneurysm [30]. Shunt dysfunction can be the first manifestation once the device is infected.
Diagnosis
CSF analysis may reveal low glucose levels, increased CSF adenosine and protein levels, and monocytic or neutrophilic pleocytosis, although in immunocompromised patients the inflammatory response can be absent. Diagnosis is based on the identification of Candida spp. in the CSF culture. Negative culture cases are seen commonly and for this reason large volume CSF samples, around 30 ml, may be needed [7]. The detection of 1,3-β-D-glucan, a fungal cell wall component, is not specific and can also be detected in Aspergillus infections [48, 49].
MRI is a helpful technique to detect multiple parenchymal micro-abscesses, hydrocephalus, or diffuse meningeal gadolinium enhancement. CNS candidiasis may also present radiographically as bilateral punctate areas of restricted diffusion in the basal ganglia on the brain MRI [50].
Treatment
The treatment of choice of CNS candidiasis is liposomal Amb (3–5 mg/kg/day) with or without flucytosine (25 mg/kg four times a day) for several weeks, followed by a maintenance therapy with fluconazole (6–12 mg/kg; 400–800 mg daily) [2, 51].
Echinocandins such as caspofungin, micafungin, or anidulafungin, although commonly used in disseminated candidiasis, have very low detectable levels in the brain and can be even undetectable in the CSF. Echinocandins are not active against Cryptococcus. For these reasons, echinocandins are not usually used to treat CNS fungal infections.
Removal of prosthetic material such as contaminated shunts, indwelling catheters, and other neurosurgical devices and the drainage of collections is also necessary. Restitution of immune function is also important. Clinical remission of CNS candidiasis was obtained in a CARD9 deficiency patient with adjunctive granulocyte-macrophage colony-stimulating factor therapy [44]. Interferon-gamma immunotherapy has been used in patients with refractory disseminated candidiasis [52]. Ruptured mycotic aneurysms may be treated with coil e mbolization [53].
Molds
CNS Aspergillosis
Epidemiology and Risk Factors
Aspergillus spp. is a genus of filamentous and septated molds that can be found throughout the world. Aspergillus is a ubiquitous airborne saprophytic fungus. A. fumigates is the most common species, and other pathogens from the same genus are A. flavus, A. niger, and A. terreus. Immunocompetent hosts usually eliminate the inhaled Aspergillus conidia by means of the innate immune mechanism.
Aspergillosis is a relatively infrequent opportunistic infection of the CNS that may account for around 10% of all fungal infections of the CNS and it is associated with high mortality and morbidity [54]. Risk factors for invasive CNS aspergillosis [2] include neutropenia, hematological malignancies, AIDS, chronic treatment with corticosteroids, transplant recipients [55], and severe debilitating conditions such as chronic granulomatous diseases [56], tuberculosis, cancer, diabetes, or alcoholism. CNS aspergillosis has also been described in patient with Crohn’s disease after treatment with infliximab and corticosteroids [57].
Rarely granulomatous cerebral aspergillosis may happen in immunocompetent adult patients [54]. Sinocranial aspergillosis has also been described in inmunocompetent patients in temperate countries [2].
Clinical Features
The involvement of the CNS may occur either by direct propagation from sinuses, nose or ear canal, or by hematogenous spread from a primary pulmonary focus. It is estimated that around 40% of patients with pulmonary aspergillosis may develop extrapulmonary involvement, mostly brain abscesses and sinusitis [58]. Mortality rate can be higher than 50% [59,60,61].
Intracranial mass lesions and skull base involvement may happen in CNS aspergillosis [2]. Intracranial fungal granulomas are rare space-occupying lesions, and among these, Aspergillus granuloma is the most frequent. Aspergillomas may result in focal neurological deficit (hemiparesis) or symptomatic seizures, and headache, fever, and altered mental status are common. The involvement of the base of skull may cause multiple cranial nerve palsies [7]. Sinucranial aspergillosis may present with nasal stuffiness, periorbital pain, and can be followed by diverse degrees of ophthalmoplegia, proptosis and visual loss, and orbital apex or cavernous sinus syndrome. Optic nerve aspergillosis has been reported, and enlargement of optic nerve at the level of the cavernous sinus and extending into the optic chiasm has been described [62]. Rare cases of primary Aspergillus sellar abscess simulating pituitary tumor in immunocompetent patient have also been described [63].
Aspergillosis is the most common invasive fungal infection affecting cerebral blood vessels. Hematogenous dissemination can cause ischemic infarctions and brain hemorrhages [30]. Aspergillus is an angioinvasive pathogen, and the enzyme elastase is able to digest the internal elastic lamina of cerebral arteries leading to focal micro-hemorrhages. As a consequence, mycotic aneurysm may occur in the weakened walls of the cerebral arteries and their rupture may cause subarachnoid hemorrhage. Cerebral aneurysms associated with Aspergillus infection are highly vulnerable to rupture. Recurrent cerebral aneurysm formation and rupture due to invasive aspergillosis of the nasal sinus have also been reported [64].
Occlusive thrombosis and hemorrhagic infarctions can happen once Aspergillus invade and fill brain vessels [30]. Aspergillosis usually blocks the cerebral blood flow at the origin of the small perforating arteries and frequently affects the basal ganglia, thalamus, and the corpus callosum [65]. The anterior and middle cerebral arteries are also frequently involved.
Aspergillus vertebral osteomyelitis, spinal epidural abscess, and intramedullary infections have described less frequently [66].
Diagnosis
The galactomannan antigen test and the PCR technique in CSF are helpful to establish the diagnosis. Another potentially helpful biomarker is (1 → 3)-β-D-glucan, a cell wall component found mainly in Candida and Aspergillus spp. The detection of (1 → 3)-β-D-glucan in the CSF may also contribute to the diagnosis, although validation studies are needed [49]. In some patients with invasive aspergillosis [67], CSF and serum 1,3-β-D-glucan test may be positive, in contrast to galactomannan antigen.
In many occasions, a definitive diagnosis can be only done by means of pathological exam [68] or a positive culture obtained from the brain tissue. CNS biopsy followed by histopathological examination and/or culture can contribute to an early diagnosis and timely treatment and improve survival rate [69].
The brain MRI may show multiple brain abscess, ring-enhancing lesions, and dural or blood vessel infiltration from near surrounding areas from orbit region or paranasal sinus, and/or ischemic lesions [70]. The CT scan is helpful to identify any subarachnoid hemorrhage.
Treatment
Voriconazole is the recommended treatment for invasive aspergillosis and has a good penetration in the CNS [71]. Invasive Aspergillus sinusitis and otitis with meningeal extension have been successfully treated with voriconazole [67]. Initial dose (6 mg/kg IV every 12 h the first day) should be followed by 4 mg/kg IV every 12 h and then oral dosage 200 mg twice a day. Nevertheless, this drug has a non-linear pharmacokinetics and extensive inter and intra-patient variation in serum levels is common. As some of the voriconazole adverse effects may be related to high serum concentrations, therapeutic drug monitoring is advisable. Periostitis and alopecia have been associated with prolonged use [71]. Liposomal Amb (3–5 mg/kg/day IV), posaconazole [72, 73], itraconazol, or micafungin [74] are alternatives for refractory cases, and adjunctive surgery should be considered when needed as this could reduce mortality [75, 76].
Azole-resistant Aspergillus fumigatus is a concern and experts recommend switching in these cases from voriconazole to liposomal amphotericin B . L-Amb is recommended as first-line therapy for azol-resistant CNS aspergillosis in regions with environmental resistance rates of ≥10%, and the addition of a second agent such as flucytosine should also be considered [77].
Mucorales (Mucormycosis)
Epidemiology and Risk Factors
Traditionally, it has been considered two orders of Zygomycetes, the Mucorales and the Entomophthorales. Recently, a change in nomenclature has been proposed. Now it is accepted as more appropriate the term mucormycosis, as the old concept “zygomycosis ” has been discarded following molecular reclassification using sequence- based DNA phylogeny [78]. These fungi are ubiquitous in soil and can be found frequently in decaying vegetation and in decomposing organic matter such as fruit and bread [2]. Mucorales are aseptate molds and have broad, ribbonlike nonseptate hyphae.
The most pathogenic are Mucor, Rhizopus, Absidia, Cunninghamella spp., and Lichtheimia corymbifera. In a review of 96 mucormycosis cases from literature, the most frequent pathogens were Rhizopus spp. (31%), followed by Mucor spp. (15%) [79].
Risk factors for Mucor infection are hematological malignancies, immunosuppression states, prolonged neutropenia, chronic corticosteroid therapy, hematopoietic stem cell and kidney transplantation, diabetes, renal failure, injectable drug users, trauma, malnutrition, iron overload, and the use of deferoxamine chelation therapy. Uncommonly, mucormycosis can affect immunocompetent subjects, mainly when the skin barrier is affected in wounds and burns.
Clinical Features
The port of entry of sporangiospores is through inhalation. There are several forms of the disease including pulmonary, cutaneous and soft tissues, renal, gastrointestinal, rhino-orbital-cerebral, and disseminated disease [80]. CNS involvement can occur as sino-orbital infections or through hematogenous dissemination from pulmonary mucormycosis. In a review of published cases, rhino-orbital was the predominant site of infection (38.5%, of which 43% also had CNS involvement), followed by disseminated disease (22%) [79]. Rhinocerebral mucormycosis is the most common form of the disease in diabetic ketoacidosis, followed by the cerebral form. Hematogenous spread and cerebral involvement are more common in IV drug abusers [81].)
Rhinocerebral mucormycosis constitutes a medical emergency. A sequential involvement from nasal cavity and ethmoidal sinuses to the orbital region, eye, bone, and brain usually occurs [82]. Ethmoidal mucormycosis can extend into cavernous sinuses because the venous drainage of ethmoidal sinuses extends to the cavernous sinuses. Cavernous sinus thrombosis is characterized by the involvement of ocular motor nerves and the first and second branches of trigeminal nerve. Diplopia secondary to ophthalmoparesis, loss of vision or blurred vision, and paranasal sinus symptoms are initial symptoms, and may be followed by contralateral hemiparesis if internal carotid artery thrombosis occurs [7]. Rhino-orbito-cerebral mucormycosis may sometimes present as orbital apex syndrome initially [83].
Isolated cerebral mucormycosis and disseminated mucormycosis with cerebral involvement are other forms of presentation of the disease. Temporal bone mucormycosis may affect elderly diabetic patients and present as facial palsy [84]. Cerebral mucormycosis may present with fever, headache, impaired vision, lethargy, seizures, and focal symptoms such as hemiparesis, aphasia, or Gerstmann syndrome [85]. This condition can be complicated with multiple cerebral infarctions and abscesses [86]. Mucormycosis with cerebral involvement without sinus disease may result in ischemic stroke [87].
Mucormycosis has a very poor prognosis. Mortality rate for rhinocerebral infection is more than 60%, whereas mortality associated with disseminated infection in the CNS is 98%. Differential diagnosis includes other mold infections as those caused by Aspergillus .
Diagnosis
Pathological assessment and culture of tissue samples may show lack of septa in the hyphae, and this fact allows the distinction with aspergillosis. The Grocott-Gomori methenamine-silver stain and the calcofluor white fluorescent stain are the stains of choice to confirm the lack of septae. Cultures may become positive in only 40–70% of biopsy-proven cases [2].
The CT scan of the sinuses and/or craniofacial MRI may detect sinus opacification, fluid-filled sinuses, bone erosions, and obliteration of deep fascia planes [82] (Fig. 7.2). Bony destruction may happen as the disease progresses. MRI and MR venogram are helpful to identify thrombosis of cavernous sinus and perineural intradural spread. Neuroimaging techniques are also helpful to visualize the best area for the biopsy.
Treatment
The principles of treatment of rhinocerebral mucormycosis include antifungal therapy, the control and reversal of host conditions (control of diabetes and neutropenia), and appropriate surgical debridement of necrotic tissue. High-dose amphotericin B (1.2–1.5 mg/kg/day) or AmB lipid complex (3–10 mg/kg/day) is the antifungal treatment of choice, and total dose of AmB may range between 2 and 4 g [88]. Posaconazole has in vitro activity against Mucorales and may also be an effective therapy, although CNS penetration is poor; recommended oral daily dose is 800 mg divided in 2 or 4 doses.
A case of disseminated mucormycosis with cerebral involvement owing to Rhizopus microsporus has been successfully treated in a kidney recipient with combined liposomal amphotericin B and posaconazole therapy [89]. Case reports have also highlighted isavuconazole as effective treatment for disseminated mucormycosis [90]. Mucor usually has a poor response to voriconazole which is the treatment of choice for cerebral aspergillosis.
Dimorphic Fungi
Histoplasmosis
Epidemiology and Risk Factors
Histoplasmosis is probably the most common endemic mycosis in South and Central America, Mexico, and the USA. In the USA, the disease is endemic in Ohio and Mississippi river valleys. Endemic areas have also been described in Southeast Asia, India, and along the Yangtze River in China [91]. Factors that account for specific endemicity in these geographical areas are moderate temperature and the presence of bird or bat guano containing soil. Activities at risk of Histoplasma infection include farming, working as geologist in caves, demolition of old buildings, exposure to chicken coops or caves, or travel to endemic areas [91].
Infection occurs by inhaling microconidia and is usually asymptomatic in healthy subjects. However, inhalation of large inoculums may cause acute pulmonary histoplasmosis in immunocompetent individuals. Most of subjects develop a self-limited or subclinical disease. Immunocompromised patients who have impaired cellular immunity such as AIDS, lupus, and solid organ transplantation recipients [92] are at risk of developing the disease either pulmonary or disseminated [93]. Histoplasmosis can also affect infants, given their immune immaturity, and children [94] and main risk factors are malnutrition (37%) and environmental exposure (33%) [95].
Clinical Features
Fever, fatigue, weight loss, and respiratory symptoms can be observed in disseminated histoplasmosis. The lungs, kidney, liver, skin, and CNS can be affected. Chest X-rays may detect pulmonary nodules, hilar and mediastinal adenopathy, or pulmonary reticulonodular, interstitial, or military infiltrates [91]. Progressive histoplasmosis can present with hemophagocytic lymphohistiocytosis and epithelioid cell granulomatosis [96].
CNS histoplasmosis can occur in 10% of cases of disseminated histoplasmosis, although in children the proportion is higher (50%) [95]. CNS histoplasmosis can present as acute or subacute meningitis, brain and/or spine parenchymal lesions, and chronic meningitis with or without vasculitis and ischemic infarctions [97, 98]. Headache, focal deficits [99], seizures, stroke, and even cognitive dysfunction [100] are common forms of presentation. Hemichorea has also been reported as clinical presentation of HIV-associated CNS histoplasmosis [101]. Cases of histoplasmosis infecting ventriculoperitoneal shunt have been described [102].
Diagnosis
H. capsulatum can be visualized as ovoid yeast cells in tissues and body fluid specimens, including the brain parenchyma. Galactomannan antigen for Histoplasma can be detected in serum, urine, and the CSF, although cross-reactions with Blastomyces have been seen. CSF analysis may show low glucose levels, raised proteins, and myeloid pleocytosis. In occasions, the diagnosis is obtained by positive culture and/or histopathological analysis of brain tissue obtained by means of stereotactic biopsy. Cultures can be positive in 50–85% of cases of disseminated or chronic pulmonary histoplasmosis, and the highest culture yield is from bone marrow or blood with a positivity of 75% [91]. The brain MRI may reveal meningeal enhancement, hydrocephalus, or multiple lesions with a ring-enhancing pattern [99, 103] (Fig. 7.3).
Treatment
Recommended therapy for CNS histoplasmosis is liposomal amphotericin B (dose, 5 mg/kg/day; total dose, 175 mg/kg given over 4–6 weeks) followed by itraconazole (200 mg two or three times per day) for at least 12 months. Histoplasma antigen levels should be cleared, and CSF analysis should come back normal before deciding stopping itraconazole. Azole serum levels should be monitored during treatment. Fluconazole, voriconazole, and posaconazole are alternative treatments for salvage therapy. Relapses may occur, and in some patients, lifelong azole therapy is necessary, mainly for those patients for whom effective immune reconstitution is not observed.
In a multicenter retrospective study of outcomes and factors associated with relapse in 96 cases of AIDS-associated histoplasmosis, 67% of CNS histoplasmosis patients relapsed compared to 15% without CNS involvement [104]. Patients with antigenuria above 2.0 ng/mL at 1-year follow-up were also almost 13 times more likely to relapse. The following factors have been associated with safe discontinuation of antifungal therapy: (1) adherent patients who completed at least 1 year of antifungal treatment; (2) to have CD4 count >150 cells/mL, (3) HIV RNA < 400 c/mL, and (4) Histoplasma antigenuria <2 ng/mL; and (5) absence of CNS hist oplas mosis [104].
Coccidioidomycosis
Epidemiology and Risk Factors
Coccidioides spp. are dimorphic saprophytic fungi that are common in dry and warm regions in Mexico, South America, and south-western USA. Coccidioides immitis is the most common cause of chronic meningitis in endemic regions for coccidioidomycosis. Risk factors are immunosuppression states, AIDS, diabetes, and chronic steroid therapy.
Prolonged soil exposure in dry hot areas is a risk factor in healthy people and population at risk include farm and construction workers and other professionals such as archaeologists. Pulmonary infection may happen after inhalation of Coccidioides arthroconidia, and disseminated state affecting the CNS occurs mostly in immunosuppressed patients [2].
Clinical Features
CNS involvement can present as chronic basilar meningitis with involvement of multiple cranial nerves. Initial clinical symptoms include low-grade fever, chronic daily headache, and memory and attention problems. Without treatment, the disease may progress and complicate with hydrocephalus, vasculitis, and ischemic infarctions in approximately 40% of cases.
Spinal coccidioidomycosis is less frequent and usually manifests as bone involvement leading to osteomyelitis and epidural abscess. A rare case of disseminated coccidioidomycosis who presented with rapidly progressive quadriparesis due to cervical intramedullary spinal cord involvement has been recently reported [105].
Diagnosis
Diagnosing coccidioidal meningitis can be difficult owing to delay in the positivity of a CSF culture or CSF antibody, particularly if the primary coccidioidal infection is unrecognized [106]. In many cases, there is a delay in the diagnosis, as patients may present with subacute meningitis in the absence of pulmonary symptoms, and CSF culture may be negative.
Coccidioidomycosis should be considered in the differential diagnosis of chronic meningitis in endemic areas. Diagnosis relies on the detection of positive antibodies or a positive culture in CSF. CSF Coccidioides antigen testing may be helpful in both the diagnosis and management of CNS coccidioidomycosis [107]. In occasions, the CSF may show eosinophilic pleocytosis and hypoglucorrachia. CSF (1,3)-β-D-glucan testing may also be useful in diagnosis of coccidioidal meningitis, and this test has a 96% sensitivity and 82% specificity [106]. Neuroimaging techniques are helpful to rule out hydrocephalus or ischemic complications. Post-contrast MRI sequences may reveal leptomeningeal enhancement around the basilar cisterns, and in some cases spinal nerve root enhancement [103].
Treatment
Induction treatment of choice can be either fluconazole (400–600 mg/day) or liposomal amphotericin B (3–5 mg/kg/day IV for 6–10 weeks; total dose, 100–150 mg/kg). Consolidation treatment requires the use of oral fluconazole (400–900 mg/day) for 1 year, and should be followed up by lifelong suppressive therapy (fluconazole 200–400 mg/day) to prevent recurrences. Recurrence of coccidioidal meningitis after discontinuation of fluconazole has been reported [108]. Hydrocephalus requires the placement of ventriculoperitoneal shunt. Despite of this therapy, mortality still remains high, and in the azole era has been estimated in 40%.
Paracoccidioidomycosis
Paracoccidioidomycosis is an endemic mycosis in South America caused by Paracoccidioides brasiliensis and is characterized by a chronic course with involvement of multiple organs mainly in immunocompromised patients.
In a retrospective study of 1219 paracoccidioidomycosis cases, the most commonly affected sites were the lungs (64%) and oral mucosa (50%). Generalized lymphadenopathy and skin lesions were observed in almost one-third of cases. Involvement of the larynx (16%), gut (7.5%), spleen (4.7%), CNS (3.4%), bones and joints (2.2%), and adrenal (2.1%) was also described [109]. Pulmonary and adrenal insufficiencies are common sequelae.
CNS paracoccidioidomycosis is potentially fatal and can occur in 12% of cases [110]. CNS paracoccidioidomycosis has also been described in AIDS patients [111]. Concomitant pulmonary and CNS paracoccidioidomycosis with cerebellar abscess [112] and also rare cases of encephalomyelopathy have been reported [113].
The CSF may reveal positive P. brasiliensis smear and culture. The brain MRI may show enhancing supra or infratentorial nodular lesions and meningeal enhancement [111, 114]. Itraconazole and amphotericin B have been advocated as treatments of choice [115].
Blastomycosis
Blastomycosis is a mycosis caused by Blastomyces dermatitidis , a dimorphic fungus endemic in Africa, Canada, and midwestern and southeastern USA, mainly in the Mississippi and Ohio River basins.
Blastomycosis may happen in both immunocompetent and immunosuppressed (AIDS, diabetes, transplant recipient) populations. Healthy humans become infected through the inhalation of airborne conidia. Pulmonary symptoms are common; however, dissemination to the skin, genitourinary system, bone [116], and CNS may occur.
CNS blastomycosis is a potentially fatal complication and usually present as subacute or chronic meningitis. Brain abscess, osteomyelitis, and mass lesions may also occur. Headache and focal neurologic deficits are the most common presenting symptoms [117]. Large cerebellar mass lesions [118] and enhancing cerebellopontine mass have been reported as rare intracranial manifestation of blastomycosis [119].
Diagnostic tests include CSF analysis and biopsy for tissue culture and pathology [117]. Enzyme immunoassay may detect Blastomyces dermatitidis antigen in the CSF [119]. There is a high cross-reactivity with Histoplasma antigen enzyme immunoassay. Brain biopsy may be needed in those cases of mass lesions.
Treatment is based on amphotericin B lipid formulation (5 mg/kg/day for 4–6 weeks) and extended course of azole therapy such as voriconazole (200–400 mg twice a day), itracozanole (400–600 mg/day), or fluconazole (800 mg/day). In occasions, surgical drainage of brain mass lesions or abscess may be necessary.
Rare and Emerging Fungi Affecting the CNS
Dematiaceous Molds
Dematiaceous molds are usually identified by the presence of melanin-like pigment within the cell wall. These fungi are well-known pathogen organisms to livestock or plants, causing chromoblastomycosis and black-grain mycetoma. These black molds cause invasive infections and may affect the CNS in both immunocompetent and immunosuppressed subjects. The term phaeohyphomycosis has been used to describe the infection caused by these dark pigmented fungi. CNS phaeohyphomycosis agents include Cladophialophora bantiana , Exserohilum rostratum, Rhinocladiella mackenziei, and Ochroconis gallopava [120, 121]. Prognosis of CNS phaeohyphomycosis is poor, with mortality rates of 50–70%, despite combination therapy of surgery and antifungal treatment [122].
The largest phaeohyphomycosis outbreak was caused by Exserohilum rostratum in the USA in 2012. This outbreak was associated with the contamination of three lots of preservative-free methylprednisolone for musculoskeletal injection and resulted in 750 infections by E. rostratum. There were 151 cases with meningitis and paraspinal infections, 325 cases with paraspinal infections without meningitis, and 64 deaths following spinal methylprednisolone lumbar injections [120]. Spinal infections presented as epidural abscess, vertebral osteomyelitis, diskitis, arachnoiditis, and cauda equina syndrome. Around 5% of patients presented with posterior circulation stroke, affecting the vertebrobasilar system, and most of deaths (85%) were associated with the occurrence of stroke [120, 123]. Voriconazole, liposomal amphotericin B or both can be used as initial treatment.
C. bantiana has a marked neurotropism for the CNS, causing almost exclusively brain abscesses. Approximately 120 cases of cerebral C. bantiana have been reported in the literature [124], and 60% occurred in healthy subjects in the absence of pulmonary lesions. Cerebral cases have been described in hypogammaglobulinemia [125] and prediabetes [126]. Slowly expanding brain abscess (97.5%), coinfection of brain parenchyma and meninges (14%), and meningitis alone (2.5%) were the most common clinical manifestations. Melanised fungal cells can be seen in a brain biopsy and abscess materials [127]. Mortality rate has been estimated in 65%.
Ochroconis gallopava is a neurotropic dematiaceous mold responsible for life-threatening pulmonary and CNS infections in domestic poultry and in immunologically compromised humans [128]. O. gallopava is an emerging cause of mycosis in solid organ transplant recipients, and disseminated forms affecting the brain have been reported [129].
Other Molds
Scedosporium apiospermum (Pseudallescheria boydii is the sexual or perfect stage) is a soil fungus that cause severe cerebral infections in the form of brain abscess in trauma and near- drowning patients with aspiration of polluted water and also in immunosuppressed hosts such as lung transplant recipients. Incubation period is around 1–3 weeks in near drowning, whereas in transplant hosts may be up to 1 year [7]. Disseminated infection in near-drowning patients has also been reported [130].
The fungi may invade the CNS through the cribriform plate or from pneumonic foci [8]. Clinical picture resembles aspergillosis of the CNS, and the hyphal appearance may resemble septated Aspergillus. Multiple brain abscesses may also occur [131]. Case reports of colitis, gastrointestinal manifestations, and CNS infection have also been described [132, 133]. Voriconazole has been advocated as initial therapy.
In Japan, a case of Scedosporium aurantiacum brain abscess after near drowning in a survivor of a tsunami has been published [134]. Scedosporium prolificans is a frequently fatal pathogen in immunocompromised subjects. Cases of CNS involvement by Scedosporium prolificans have been reported [135]. This fungus infects the CNS through hematogenous dissemination or through traumatic inoculation (Fig. 7.4).
Fusarium spp. is also a ubiquitous fungus that can cause fatal infection in immunosuppressed patients with persistent neutropenia. Sinuses and respiratory tract, catheters, and periungual lesions (paronychia) are common portals of entry. Disseminated fusariosis frequently causes fungemia and skin lesions. Fever, erythematous nodular cutaneous lesions, sinus and pulmonary infections, and septic arthritis are common manifestations. Fusarium solani brain abscess has been reported in acute lymphocytic leukemia and autologous peripheral stem cell transplant patients [136]. Fusarium is highly angioinvasive and can cause hemorrhagic infarctions. Other complications are bilateral endophthalmitis and chorioretinitis [8].
Other Rare Fungi
Case reports about other rare fungi that may affect the CNS have been published. Invasive infections have also described in Saprochaete and Geotrichum species [137]. Phellinus spp. is an emerging cause of refractory fungal infection in patients with X-linked chronic granulomatous disease [138]. Bipolaris spp. is dematiaceous fungus that usually affects the skin and nasal sinuses and rarely the CNS. Disseminated phaeohyphomycosis with brain abscess and biliary tree invasion due to Bipolaris spp. in an immunocompetent patient has been recently published [139].
Sporothrix schenckii and Sporothrix brasiliensis can also affect the CNS [140]. Sporotrichosis usually manifest as a lymphocutaneous form. Cases of disseminated infection have been reported in AIDS patients, and mortality has been linked with CNS involvement [141].
Conclusions
An increased incidence of CNS fungal infections is expected as the number of patients at risk due to AIDS and other immunosuppression states have risen. Early diagnosis and identification of fungal meningitis and specific pathogens are very important, and prognosis depends on opportune administration of antifungal treatment. The many different types of clinical presentations (ranging from acute and chronic meningitis to brain abscess and vasculitis), the diversity of microorganisms, and the resistance to treatment are additional challenges that need to be tackled.
References
Pilmis B, Puel A, Lortholary O, Lanternier F. New clinical phenotypes of fungal infections in special hosts. Clin Microbiol Infect. 2016;22:681–7.
Murthy JMK, Sundaram C. Fungal infections of the central nervous system. Handb Clin Neurol. 2014;121:1383–401.
Bicanic T, Harrison TS. Cryptococcal meningitis. Br Med Bull. 2004;72:99–118.
La Hoz RM, Pappas PG. Cryptococcal infections: changing epidemiology and implications for therapy. Drugs. 2013;73:495–504.
Sethi PK, Khanna L, Batra A, Anand I, Sethi NK, Torgovnick J, et al. Central nervous system fungal infections: observations from a large tertiary hospital in northern India. Clin Neurol Neurosurg. 2012;114:1232–7.
Al-Abdely HM, Alothman AF, Salman JA, Al-Musawi T, Almaslamani M, Butt AA, et al. Clinical practice guidelines for the treatment of invasive Aspergillus infections in adults in the Middle East region: Expert panel recommendations. J Infect Public Health. 2014;7:20–31.
Panackal AA, Williamson PR. Fungal infections of the central nervous system. Continuum (Minneap Minn). 2015;21:1662–78.
McCarthy MW, Walsh TJ. Molecular diagnosis of invasive mycoses of the central nervous system. Expert Rev Mol Diagn. 2017;17:129–39.
Gavito-Higuera J, Mullins CB, Ramos-Duran L, Olivas Chacon CI, Hakim N, Palacios E. Fungal infections of the central nervous system: a pictorial review. J Clin Imaging Sci. 2016;6:24.
Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O. on behalf of the ESCMID EFISG study group and ECM. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20(Suppl. 3):76–9.
Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol. 2016;13:13–24. https://doi.org/10.1038/nrneuol.2016.167.
Harris JR, Lockhart SR, Debess E, Marsden-Haug N, Goldoft M, Wohrle R, et al. Cryptococcus gattii in the United States: clinical aspects of infection with an emerging pathogen. Clin Infect Dis. 2011;53:1188–95.
Henao-Martínez AF, Beckham JD. Cryptococcosis in solid organ transplant recipients. Curr Opin Infect Dis. 2015;28:300–7.
Schmalzle SA, Buchwald UK, Gilliam BL, Riedel DJ. Cryptococcus neoformans infection in malignancy. Mycoses. 2016;59:542–52.
Hadid H, Nona P, Usman M, Paje D. Cryptococcal eosinophilic meningitis in a patient with sarcoidosis. BMJ Case Rep. 2015;2015. pii:bcr2015212765. https://doi.org/10.1136/bcr-2015-212765.
Jiang S, Lei TC, Xu SZ. Successful treatment of cryptococcal meningitis with amphotericin B in a patient with systemic lupus erythematosus. West Indian Med J. 2015;65:222–5. https://doi.org/10.7727/wimj.2014.352.
Saijo T, Chen J, Chen SC, Rosen LB, Yi J, Sorrell TC, et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. MBio. 2014;5:e00912–4.
Ward MD, Jones DE, Goldman MD. Cryptococcal meningitis after fingolimod discontinuation in a patient with multiple sclerosis. Mult Scler Relat Disord. 2016;9:47–9.
Achtnichts L, Obreja O, Conen A, Fux CA, Nedeltchev K. Cryptococcal meningoencephalitis in a patient with multiple sclerosis treated with fingolimod. JAMA Neurol. 2015;72:1203–5.
Huang D. Disseminated cryptococcosis in a patient with multiple sclerosis treated with fingolimod. Neurology. 2015;85:1001–3.
Perfect JR, Bicanic T. Cryptococcosis diagnosis and treatment: what do we know. Fungal Genet Biol. 2015;78:49–54.
Chitty JL, Tatzenko TL, Williams SJ, Koh YQ, Corfield EC, Butler MS, et al. GMP synthase is required for virulence factor production and infection by Cryptococcus neoformans. J Biol Chem. 2017;292:3049–59.
Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin N Am. 2016;30:179–206.
Merkler AE, Gaines N, Baradaran H, Schuetz AN, Lavi E, Simpson SA, et al. Direct invasion of the optic nerves, chiasm, and tracts by Cryptococcus neoformans in an immunocompetent host. Neurohospitalist. 2015;5:217–22.
Lomes NR, Melhem MS, Szeszs MW, Martins Mdos A, Buccheri R. Cryptococcosis in non-HIV/non-transplant patients: A Brazilian case series. Med Mycol. 2016;54:669–76.
Henao-Martínez AF, Gross L, Mcnair B, McCollister B, DeSanto K, Montoya JG, et al. Risk factors for cryptococcal meningitis: a single United States center experience. Mycopathologia. 2016;181:807–14.
Ulett KB, Cockburn JW, Jeffree R, Woods ML. Cerebral cryptococcoma mimicking glioblastoma. BMJ Case Rep. 2017;2017. pii:bcr2016218824.
Cachia D, Singh C, Tetzlaff MT, Penas-Prado M. Middle cerebral artery territory infarct due to Cryptococcus infections: an uncommon indication for cerebrospinal fluid analysis in stroke patients. Diagn Cytopathol. 2015;43:632–4.
Rosario M, Song SX, McCullough LD. An unusual case of stroke. Neurologist. 2012;18:229–32.
Carod Artal FJ. Clinical management of infectious cerebral vasculitides. Expert Rev Neurother. 2016;16:205–21.
Meya DB, Manabe YC, Boulware DR, Janoff EN. The immunopathogenesis of cryptococcal immune reconstitution inflammatory syndrome: understanding a conundrum. Curr Opin Infect Dis. 2016;29:10–22.
Pettersen KD, Pappas PG, Chin-Hong P, Baxi SM. A paradoxical decline: intracranial lesions in two HIV-positive patients recovering from cryptococcal meningitis. BMJ Case Rep. 2015;2015. pii:bcr2015212108.
Post MJ, Thurnher MM, Clifford DB, Nath A, Gonzalez RG, Gupta RK, et al. CNS-immune reconstitution inflammatory syndrome in the setting of HIV infection, part 1: overview and discussion of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome and cryptococcal-immune reconstitution inflammatory syndrome. AJNR Am J Neuroradiol. 2013;34:1297–307.
Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis. 2014;58:736–45.
Aye C, Henderson A, Yu H, Norton R. Cryptococcosis-the impact of delay to diagnosis. Clin Microbiol Infect. 2016;22:632–5.
Spampinato C, Leonardi D. Candida infections, causes, targets, and resistance mechanisms: traditional and alternative antifungal agents. Biomed Res Int. 2013;2013:204237.
Corti M, Solari R, De Carolis L, Cangelosi D, Arechavala A, Negroni R. Candida parapsilosis meningitis in a patient with AIDS. Report of a case and review of the literature. Rev Iberoam Micol. 2013;30:122–4.
Colomba C, Trizzino M, Imburgia C, Madonia S, Siracusa L, Giammanco GM. Candida glabrata meningitis and endocarditis: a late severe complication of candidemia. Int J Infect Dis. 2014;29:174–5.
Marukutira T, Huprikar S, Azie N, Quan SP, Meier-Kriesche HU, Horn DL. Clinical characteristics and outcomes in 303 HIV-infected patients with invasive fungal infections: data from the Prospective Antifungal Therapy Alliance registry, a multicenter, observational study. HIV AIDS (Auckl). 2014;6:39–47.
Zhang SC. Cerebral candidiasis in a 4-year-old boy after intestinal surgery. J Child Neurol. 2015;30(3):391.
Oeser C, Vergnano S, Naidoo R, Anthony M, Chang J, Chow P, et al. Neonatal invasive fungal infection in England 2004-2010. Clin Microbiol Infect. 2014;20:936–41.
Adams-Chapman I, Bann CM, Das A, Goldberg RN, Stoll BJ, Walsh MC, et al. Neurodevelopmental outcome of extremely low birth weight infants with Candida infection. J Pediatr. 2013;163:961–7. e3
Gavino C, Hamel N, Zeng JB, Legault C, Guiot MC, Chankowsky J, et al. Impaired RASGRF1/ERK-mediated GM-CSF response characterizes CARD9 deficiency in French-Canadians. J Allergy Clin Immunol. 2016;137:1178–88. e1–7
Gavino C, Cotter A, Lichtenstein D, Lejtenyi D, Fortin C, Legault C, et al. CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy. Clin Infect Dis. 2014;59:81–4.
Lanternier F, Mahdaviani SA, Barbati E, Chaussade H, Koumar Y, Levy R, et al. Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both. J Allergy Clin Immunol. 2015;135:1558–68. e2
Herbst M, Gazendam R, Reimnitz D, Sawalle-Belohradsky J, Groll A, Schlegel PG, et al. Chronic Candida albicans meningitis in a 4-year-old girl with a homozygous mutation in the CARD9 gene (Q295X). Pediatr Infect Dis J. 2015;34:999–1002.
Wang GH, Dai CL, Liu YF, Li YM. Cerebral and renal abscess and retino-choroiditis secondary to Candida albicans in preterm infants: eight case retrospective study. Clin Exp Obstet Gynecol. 2013;40:519–23.
Lyons JL, Erkkinen MG, Vodopivec I. Cerebrospinal fluid (1,3)-β-D-glucan in isolated candida meningitis. Clin Infect Dis. 2015;60:161–2.
Salvatore CM, Chen TK, Toussi SS, DeLaMora P, Petraitiene R, Finkelman MA, et al. (1→3)-β-D-glucan in cerebrospinal fluid as a biomarker for Candida and Aspergillus infections of the central nervous system in pediatric patients. J Pediatric Infect Dis Soc. 2016;5:277–86.
Lin DJ, Sacks A, Shen J, Lee TC. Neurocandidiasis: a case report and consideration of the causes of restricted diffusion. J Radiol Case Rep. 2013;7:1–5.
Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50:291–322.
Buddingh EP, Leentjens J, van der Lugt J, Dik WA, Gresnigt MS, Netea MG, et al. interferon-gamma immunotherapy in a patient with refractory disseminated candidiasis. Pediatr Infect Dis J. 2015;34:1391–4.
Hayashi S, Maehara T, Mukawa M, Aoyagi M, Yoshino Y, Nemoto S, et al. Successful coil embolization of a ruptured basilar artery aneurysm in a child with leukemia: a case report. Neurol Med Chir (Tokyo). 2014;54:150–4.
Ellenbogen JR, Waqar M, Cooke RP, Javadpour M. Management of granulomatous cerebral aspergillosis in immunocompetent adult patients: a review. Br J Neurosurg. 2016;30:280–5.
Mazzaferri F, Adami I, Tocco P, Cazzadori A, Merighi M, Forni A, et al. Thalamo-mesencephalic aspergillus abscess in a heart transplant subject: a case report and literature review. Infez Med. 2015;23:51–5.
Segundo JB, da Silva MA, Filho WE, Nascimento AC, Vidal FC, Bezerra GF, et al. Cerebral aspergillosis in a patient with leprosy and diabetes: a case report. BMC Res Notes. 2014;7:689.
Bourne EL, Dimou J. Invasive central nervous system aspergillosis in a patient with Crohn’s disease after treatment with infliximab and corticosteroids. J Clin Neurosci. 2016;30:163–4.
Saghrouni F, Ben Youssef Y, Gheith S, Bouabid Z, Ben Abdeljelil J, Khammari I, et al. Twenty-nine cases of invasive aspergillosis in neutropenic patients. Med Mal Infect. 2011;41:657–62.
Kourkoumpetis TK, Desalermos A, Muhammed M, Mylonakis E. Central nervous system aspergillosis: a series of 14 cases from a general hospital and review of 123 cases from the literature. Medicine (Baltimore). 2012;91:328–36.
Shamim MS, Enam SA, Ali R, Anwar S. Craniocerebral aspergillosis: a review of advances in diagnosis and management. J Pak Med Assoc. 2010;60:573–9.
Naik V, Ahmed FU, Gupta A, Garg A, Sarkar C, Sharma B, et al. Intracranial fungal granulomas: a single institutional clinicopathologic study of 66 patients and review of the literature. World Neurosurg. 2015;83:1166–72.
Yuan L, Prayson RA. Optic nerve aspergillosis. J Clin Neurosci. 2015;22:1191–3.
Ouyang T, Zhang N, Wang L, Jiao J, Zhao Y, Li Z, et al. Primary Aspergillus sellar abscess simulating pituitary tumor in immunocompetent patient. J Craniofac Surg. 2015;26:e86–8.
Shinya Y, Miyawaki S, Nakatomi H, Okano A, Imai H, Shin M, et al. Recurrent cerebral aneurysm formation and rupture within a short period due to invasive aspergillosis of the nasal sinus; pathological analysis of the catastrophic clinical course. Int J Clin Exp Pathol. 2015;8:13510–22.
Okazaki T, Shiraishi S, Iwasa N, Kitamura E, Mizutani T, Hanada Y, et al. Extended voriconazole therapy and long term survival of a patient with invasive central aspergillosis causing stroke. Rinsho Shinkeigaku. 2015;55:472–7.
McCaslin AF, Lall RR, Wong AP, Lall RR, Sugrue PA, Koski TR. Thoracic spinal cord intramedullary aspergillus invasion and abscess. J Clin Neurosci. 2015;22:404–6.
Morgand M, Rammaert B, Poirée S, Bougnoux ME, Tran H, Kania R, et al. Chronic invasive aspergillus sinusitis and otitis with meningeal extension successfully treated with voriconazole. Antimicrob Agents Chemother. 2015;59:7857–61.
Forest F, Cinotti E, Habougit C, Ginguéné C, Perrot JL, Labeille B, et al. Rapid characterization of human brain aspergillosis by confocal microscopy on a thick squash preparation. Cytopathology. 2016;27:221–2.
Wang RX, Zhang JT, Chen Y, Huang XS, Jia WQ, Yu SY. Cerebral aspergillosis: a retrospective analysis of eight cases. Int J Neurosci. 2017;127:339–43.
Gabelmann A, Klein S, Kern W, Krüger S, Brambs HJ, Rieber-Brambs A, et al. Relevant imaging findings of cerebral aspergillosis on MRI: a retrospective case-based study in immunocompromised patients. Eur J Neurol. 2007;14:548–55.
Malani AN, Kerr LE, Kauffman CA. Voriconazole: how to use this antifungal agent and what to expect. Semin Respir Crit Care Med. 2015;36:786–95.
Conant MM, Sha BE, Proia LA. Use of posaconazole delayed-release tablets for treatment of invasive aspergillosis. Mycoses. 2015;58:313–4.
Ellenbogen JR, Waqar M, Denning DW, Cooke RP, Skinner DW, Lesser T, et al. Posaconazole responsive cerebral aspergillosis in an immunocompetent adult. J Clin Neurosci. 2014;21:1825–7.
Ogawa T, Matsumoto K, Tsujimoto K, Hishiya N, Yamada Y, Uno K, et al. Chronic invasive sinus and intracerebral aspergillosis controlled by combination therapy with micafungin and a daily dose of 400 mg itraconazole oral solution. J Infect Chemother. 2015;21:134–7.
Panackal AA, Bennett JE, Williamson PR. Treatment options in invasive aspergillosis. Curr Treat Options Infect Dis. 2014;6:309–25.
Schwartz S, Reisman A, Troke PF. The efficacy of voriconazole in the treatment of 192 fungal central nervous system infections: a retrospective analysis. Infection. 2011;39:201–10.
Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Brüggemann RJ, Chowdhary A, et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat. 2015;21-22:30–40.
Kwon-Chung KJ. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin Infect Dis. 2012;54(suppl 1):S8–S15.
Vehreschild JJ, Birtel A, Vehreschild MJ, Liss B, Farowski F, Kochanek M, et al. Mucormycosis treated with posaconazole: review of 96 case reports. Crit Rev Microbiol. 2013;39:310–24.
Higo T, Kobayashi T, Yamazaki S, Ando S, Gonoi W, Ishida M, et al. Cerebral embolism through hematogenous dissemination of pulmonary mucormycosis complicating relapsed leukemia. Int J Clin Exp Pathol. 2015;8:13639–42.
Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23–34.
McCarthy M, Rosengart A, Schuetz AN, Kontoyiannis DP, Walsh TJ. Mold infections of the central nervous system. N Engl J Med. 2014;371:150–60.
Jiang N, Zhao G, Yang S, Lin J, Hu L, Che C, et al. A retrospective analysis of eleven cases of invasive rhino-orbito-cerebral mucormycosis presented with orbital apex syndrome initially. BMC Ophthalmol. 2016;16:10.
Katsantonis NG, Hunter JB, O’Connell BP, He J, Lewis JS Jr, Wanna GB. Temporal bone mucormycosis. Ann Otol Rhinol Laryngol. 2016;125:850–3.
Stretz C, Mook A, Modak JM, Rodriguez JM, Nouh AM. Gerstmann Syndrome in a patient with aggressive mucormycosis. Neurohospitalist. 2017;7:102–3.
Mulki R, Masab M, Eiger G, Perloff S. Lethargy and vision loss: successful management of rhinocerebral mucormycosis. BMJ Case Rep. 2016;2016. pii:bcr2016215855.
Ermak D, Kanekar S, Specht CS, Wojnar M, Lowden M. Looks like a stroke, acts like a stroke, but it’s more than a stroke: a case of cerebral mucormycosis. J Stroke Cerebrovasc Dis. 2014;23:e403–4.
Benachinmardi KK, Rajalakshmi P, Veenakumari HB, Bharath RD, Vikas V, Mahadevan A, et al. Successful treatment of primary cerebral mucormycosis: Role of microbiologist. Indian J Med Microbiol. 2016;34:550–3.
Ville S, Talarmin JP, Gaultier-Lintia A, Bouquié R, Sagan C, Le Pape P, et al. Disseminated mucormycosis with cerebral involvement owing to Rhizopus microsporus in a kidney recipient treated with combined liposomal amphotericin B and posaconazole therapy. Exp Clin Transplant. 2016;14:96–9.
Peixoto D, Gagne LS, Hammond SP, Gilmore ET, Joyce AC, Soiffer RJ, et al. Isavuconazole treatment of a patient with disseminated mucormycosis. J Clin Microbiol. 2014;52:1016–9.
Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin N Am. 2016;30:207–27.
Schuster JE, Wushensky CA, Di Pentima MC. Chronic primary central nervous system histoplasmosis in a healthy child with intermittent neurological manifestations. Pediatr Infect Dis J. 2013;32:794–6.
Nyalakonda H, Albuerne M, Suazo Hernandez LP, Sarria JC. Central nervous system histoplasmosis in acquired immunodeficiency syndrome. Am J Med Sci. 2016;351:177–86.
Osorio N, López Y, Jaramillo JC. Histoplasmosis of the central nervous system in an immunocompetent patient. Biomedica. 2014;34:506–13.
López LF, Valencia Y, Tobón ÁM, Velásquez O, Santa CD, Cáceres DH, et al. Childhood histoplasmosis in Colombia: Clinical and laboratory observations of 45 patients. Med Mycol. 2016;54:677–83.
Schulze AB, Heptner B, Kessler T, Baumgarten B, Stoica V, Mohr M, et al. Progressive histoplasmosis with hemophagocytic lymphohistiocytosis and epithelioid cell granulomatosis: a case report and review of the literature. Eur J Haematol. 2017;99:91–100. https://doi.org/10.1111/ejh.12886. [Epub ahead of print]
Carod-Artal F, Venturini M, Gomes E, de Mello M. Chronic central nervous system histoplasmosis in an immunocompetent patient. Neurologia. 2008;23:263–8.
Nguyen FN, Kar JK, Zakaria A, Schiess MC. Isolated central nervous system histoplasmosis presenting with ischemic pontine stroke and meningitis in an immune-competent patient. JAMA Neurol. 2013;70:638–41.
Andrade AI, Donato M, Previgliano C, Hardjasudarma M. Histoplasmosis brain abscesses in an immunocompetent adult. A case report and literature review. Neuroradiol J. 2014;27:334–8.
Loughan AR, Perna R, Hertza J. Cognitive impairment and memory loss associated with histoplasmosis: a case study. Clin Neuropsychol. 2014;28:514–24.
Estrada-Bellmann I, Camara-Lemarroy CR, Flores-Cantu H, Calderon-Hernandez HJ, Villareal-Velazquez HJ. Hemichorea in a patient with HIV-associated central nervous system histoplasmosis. Int J STD AIDS. 2016;27:75–7.
Veeravagu A, Ludwig C, Camara-Quintana JQ, Jiang B, Lad N, Shuer L. Fungal infection of a ventriculoperitoneal shunt: histoplasmosis diagnosis and treatment. World Neurosurg. 2013;80:222.e5–13.
Starkey J, Moritani T, Kirby P. MRI of CNS fungal infections: review of aspergillosis to histoplasmosis and everything in between. Clin Neuroradiol. 2014;24:217–30.
Myint T, Anderson AM, Sanchez A, Farabi A, Hage C, Baddley JW, et al. Histoplasmosis in patients with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS): multicenter study of outcomes and factors associated with relapse. Medicine (Baltimore). 2014;93:11–8.
Tan LA, Kasliwal MK, Nag S, O’Toole JE, Traynelis VC. Rapidly progressive quadriparesis heralding disseminated coccidioidomycosis in an immunocompetent patient. J Clin Neurosci. 2014;21:1049–51.
Stevens DA, Zhang Y, Finkelman MA, Pappagianis D, Clemons KV, Martinez M. Cerebrospinal Fluid (1,3)-beta-d-glucan testing is useful in diagnosis of coccidioidal meningitis. J Clin Microbiol. 2016;54:2707–10.
Bamberger DM, Pepito BS, Proia LA, Ostrosky-Zeichner L, Ashraf M, Marty F, et al. Cerebrospinal fluid Coccidioides antigen testing in the diagnosis and management of central nervous system coccidioidomycosis. Mycoses. 2015;58:598–602.
Nelson S, Vytopil M. Recurrence of coccidioidal meningitis after discontinuation of fluconazole. JAMA Neurol. 2013;70:1586.
Bellissimo-Rodrigues F, Bollela VR, Da Fonseca BA, Martinez R. Endemic paracoccidioidomycosis: relationship between clinical presentation and patients’ demographic features. Med Mycol. 2013;51:313–8.
Pedroso VS, Lyon AC, Araújo SA, Veloso JM, Pedroso ER, Teixeira AL. Paracoccidioidomycosis case series with and without central nervous system involvement. Rev Soc Bras Med Trop. 2012;45:586–90.
Silva-Vergara ML, Rocha IH, Vasconcelos RR, Maltos AL, Neves Fde F, Teixeira Lde A, et al. Central nervous system paracoccidioidomycosis in an AIDS patient: case report. Mycopathologia. 2014;177:137–41.
Abud LG, Queiroz RM, Abud TG. Concomitant pulmonary and central nervous system paracoccidioidomycosis with cerebellar abscess. Rev Soc Bras Med Trop. 2015;48:789.
Souza PV, Pinto WB, Matas SL. Paracoccidioidomycosis: a rare cause of infectious encephalomyelopathy. Arq Neuropsiquiatr. 2014;72:904–5.
Reis F, Collier PP, Souza TF, Lopes GP, Bronzatto E, Silva Junior NA, et al. Neuroparacoccidioidomycosis (NPCM): magnetic resonance imaging (MRI) findings. Mycopathologia. 2013;175:181–6.
Shikanai-Yasuda MA. Paracoccidioidomycosis treatment. Rev Inst Med Trop Sao Paulo. 2015;57(Suppl 19):31–7.
Codifava M, Guerra A, Rossi G, Paolucci P, Iughetti L. Unusual osseous presentation of blastomycosis in an immigrant child: a challenge for European pediatricians. Ital J Pediatr. 2012;38:69.
Bush JW, Wuerz T, Embil JM, Del Bigio MR, McDonald PJ, Krawitz S. Outcomes of persons with blastomycosis involving the central nervous system. Diagn Microbiol Infect Dis. 2013;76:175–81.
Munich SA, Johnson AK, Ahuja SK, Venizelos A, Byrne RW. Large cerebellar mass lesion: A rare intracranial manifestation of blastomycosis. Surg Neurol Int. 2013;4:141.
Akture E, Salamat S, Baskaya MK. Intracranial blastomycosis presenting as an enhancing cerebellopontine mass. Turk Neurosurg. 2013;23:252–5.
Chiller TM, Roy M, Nguyen D, Guh A, Malani AN, Latham R, et al. Clinical findings for fungal infections caused by methylprednisolone injections. N Engl J Med. 2013;369:1610–9.
Didehdar M, Gokanian A, Sofian M, Mohammadi S, Mohammadi R, Aslani N, et al. First fatal cerebral phaeohyphomycosis due to Rhinocladiella mackenziei in Iran, based on ITS rDNA. J Mycol Med. 2015;25:81–6.
Deng S, Pan W, Liao W, de Hoog GS, Gerrits van den Ende AH, Vitale RG, et al. Combination of amphotericin b and flucytosine against neurotropic species of melanized fungi causing primary cerebral phaeohyphomycosis. Antimicrob Agents Chemother. 2016;60:2346–51.
Pappas PG. Lessons learned in the multistate fungal infection outbreak in the United States. Curr Opin Infect Dis. 2013;26:545–50.
Kantarcioglu AS, Guarro J, De Hoog S, Apaydin H, Kiraz N. An updated comprehensive systematic review of Cladophialophora bantiana and analysis of epidemiology, clinical characteristics, and outcome of cerebral cases. Med Mycol. 2016;55:579–604.
Shimogawa T, Sayama T, Haga S, Akiyama T, Makihara K, Morioka T. Brain abscess due to infection with dematiaceous fungi Cladophialophora bantiana associated with hypogammaglobulinemia following gastrectomy: A Case Report. No Shinkei Geka. 2016;44:59–66.
Mansour A, Jordan K. Disseminated Cladophialophora bantiana disease in a patient with prediabetes. BMJ Case Rep. 2014;2014. pii:bcr2014206426.
Kantarcioglu AS, Guarro J, de Hoog GS, Apaydin H, Kiraz N, Balkan II, et al. A case of central nervous system infection due to Cladophialophora bantiana. Rev Iberoam Micol. 2016;33:237–41.
Brokalaki EI, Sommerwerck U, von Heinegg EH, Hillen U. Ochroconis gallopavum infection in a lung transplant recipient: report of a case. Transplant Proc. 2012;44:2778–80.
Cardeau-Desangles I, Fabre A, Cointault O, Guitard J, Esposito L, Iriart X, et al. Disseminated Ochroconis gallopava infection in a heart transplant patient. Transpl Infect Dis. 2013;15:E115–8.
Chen TC, Ho MW, Chien WC, Lin HH. Disseminated Scedosporium apiospermum infection in a near-drowning patient. J Formos Med Assoc. 2016;115:213–4.
Slone HW, Kontzialis M, Kiani B, Triola C, Oettel DJ, Bourekas EC. MRI with magnetic resonance spectroscopy of multiple brain abscesses secondary to Scedosporium apiospermum in two immunocompromised patients. Clin Imaging. 2013;37:361–6.
Husain N, Chen TC, Hou JK. An unusual cause of diarrhea in an immunocompromised patient. Scedosporium apiospermum colitis and brain abscess. Gastroenterology. 2013;145(519):697–8.
Lin D, Kamili Q, Lai S, Musher DM, Hamill R. Cerebral Scedosporium apiospermum infection presenting with intestinal manifestations. Infection. 2013;41:723–6.
Nakamura Y, Suzuki N, Nakajima Y, Utsumi Y, Murata O, Nagashima H, et al. Scedosporium aurantiacum brain abscess after near-drowning in a survivor of a tsunami in Japan. Respir Investig. 2013;51:207–11.
Carod-Artal FJ, Ferreira-Coral L, Mauro-Couto J, Gomes E. de Agassiz-Vasques M. Chronic spinal epidural abscess caused by Scedosporium prolificans in an immunocompetent patient. Spine (Phila Pa 1976). 2009;34:E330–2.
Garcia RR, Min Z, Narasimhan S, Bhanot N. Fusarium brain abscess: case report and literature review. Mycoses. 2015;58:22–6.
Durán Graeff L, Seidel D, Vehreschild MJ, Hamprecht A, Kindo A, Racil Z, et al. Invasive infections due to Saprochaete and Geotrichum species: Report of 23 cases from the FungiScope Registry. Mycoses. 2017;60:273–9.
Haidar G, Zerbe CS, Cheng M, Zelazny AM, Holland SM, Sheridan KR. Phellinus species: An emerging cause of refractory fungal infections in patients with X-linked chronic granulomatous disease. Mycoses. 2017;60:155–60.
Frank T, Esquenazi Y, Nigo M, Wanger A, Portnoy B, Shepard S. Disseminated phaeohyphomycosis with brain abscess and biliary invasion due to Bipolaris spp. in an immunocompetent patient. Ann Clin Lab Sci. 2016;46:439–42.
Freitas DF, Lima MA. de Almeida-Paes R, Lamas CC, do Valle AC, Oliveira MM, et al. Sporotrichosis in the central nervous system caused by Sporothrix brasiliensis. Clin Infect Dis. 2015;61:663–4.
Moreira JA, Freitas DF, Lamas CC. The impact of sporotrichosis in HIV-infected patients: a systematic review. Infection. 2015;43:267–76.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Carod-Artal, F.J. (2018). Fungal Infections of the Central Nervous System. In: García-Moncó, J. (eds) CNS Infections. Springer, Cham. https://doi.org/10.1007/978-3-319-70296-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-70296-4_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-70295-7
Online ISBN: 978-3-319-70296-4
eBook Packages: MedicineMedicine (R0)