Abstract
The development of science and technology has not only improved the comfort of humans but also added a wide variety of hazardous chemicals and life threatening pathogens into the living environment. Surveillance of bacterial pathogens is a daunting task for healthcare industries, food industries and environmental quality control sectors. During the past few decades, pathogens with high virulence have emerged, leading to steady increase in the mortality and morbidity rates, posing burden on the nation’s economy. Therefore it becomes necessary to develop devices that can quickly sense pathogens in quantities much lower than pico- and femto-moles. A ideal sensor has short sensing time, low measurable quantities and reliable results.
In this chapter we discuss various types of biosensors for pathogen detection. Optical biosensors have been explored extensively and used as labeled (fluorophores, quantum dots, carbon dots), label free (surface plasmon resonance) and hybrid biosensors for a highly sensitive pathogen detection. Piezoelectric-cantilever biosensors are simple, rapid and as effective as conventional pathogen detection techniques and are notable for detection of food pathogens like Listeria monocytogenes. Successful electrochemical biosensors have also been developed with unmodified electrodes and later electrodes were modified with bio-recognition elements such as specific DNA, antibodies or nanoparticles, for detection of pathogens like methicillin resistant Staphylococcus aureus and Salmonella. Almost all biosensors, including immunosensors, are being improved, by sample enrichment or signal amplification, in order to obtain a simple and rapid pathogen detection tool with lower limits of detection.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Sensors are becoming a vital part in human lives in this fast moving world due to its rapid mode of detection and ease of use. They are used to detect the measurable responses of an analyte in a sample with respect to either physical conditions or chemical interactions. A good sensor is the one that possesses optimum characteristics like reproducibility, sensitivity, selectivity, stability and linearity (Bhalla et al. 2016). Biosensors are analytical devices that work by detecting the presence and/or concentration of a biological analyte, such as biomolecules, microorganism, or any biological structure. It is composed of three major components namely: bio-recognition element that recognizes the analyte and produces a specific signal, transducer that receives and transmits the signal in a readable format which is read by another component, a reader device. With developments in science and technology, there is an increase in exposure to different kinds of hazardous chemicals and life threatening pathogens (Dasgupta et al. 2015, 2017; Shukla et al. 2017; Jain et al. 2016; Ranjan et al. 2014). This urges researchers for the development of a vast range of sensors. Detection of pathogen gains extreme importance in various sectors like health care for clinical diagnosis (Yanase et al. 2014), food industry to ensure food quality (Scognamiglio et al. 2014), and water and environmental quality control (Bereza-Malcolm et al. 2014; Teo and Wong 2014). Most of the available diagnostic methods are time consuming, requires skilled labor and large sample volumes. All these pave way for the researchers to develop sensors that can offer fast response, ease to operate and high sensitivity to detect analytes.
Detection of pathogens in food, water and air has been vital for the researchers due to its crucial effects on the health of people. Though the standard techniques in microbiology using cell culture and plating could confirm the identity of microbial strains (Gracias and McKillip 2004; Monis and Giglio 2006), it usually takes several days to finish the processes. Also, almost all the conventional methods involve complex instrumentation which defies on-site detection process. Conventional methods of pathogen detection are still in use in spite of their longer response times, which is solely due to their high selectivity and sensitivity. Thus, it becomes essential to develop biosensors that could be used for pathogen detection in a rapid and precise manner. Efficient pathogen sensors must satisfy several requisites. Firstly they need to exhibit high sensitivity and lower limits of detection. As the microorganisms like bacteria multiplies at a faster rate, even meager numbers of cells could lead to risk of health in patients (Ward et al. 2014; Thakur et al. 2015). It is also necessary to meet zero tolerance of certain bacterial strains such as E. coli O157:H7, Salmonella, Listeria monocytogenes etc., in food products to meet the standards of food safety (Batt 2007; Nugen and Baeumner 2008). Second important requirement is faster mode of analysis. This is essential in order to take immediate actions for treating patients and preventing the transmission of pathogens. Next requisite would be high specificity in identifying different bacterial strains. This could be satisfied by means of micro or nano arrays or chips in sensors with a number of target specific probes. Lastly, portability, ease of use and also automation are significant for real time as well as long term monitoring.
A pathogenic biosensor works by transmitting/transducing receptor recognition signals to a specific target pathogen, into a detectable signal. Presently available techniques to detect pathogens include immunosensing or nucleic acid (Deoxyribonucleic acid/aptamers) detection. There are various modes of transduction that includes optical mode involving fluorescence (Li et al. 2015), surface plasmon resonance (Rifat et al. 2015; Liu et al. 2016a), colorimetric (Taneja and Tyagi 2007), mechanical mode involving cantilever (Fritz 2008)/ piezoelectric crystal/quartz crystalline microbalance (Fawcett et al. 1988; Farka et al. 2013) and electrochemical mode consisting of amperometric, voltammetric and impedimetric (Wan et al. 2011; Chen and Shah 2013) and immunosensors (Menti et al. 2016; Sign and Sumana 2016). Immunosensors are based on antigen-antibody interactions, where antibodies specific to antigens are immobilized on sensor surfaces. Antibody development and selection are vital for constructing an immunosensor for pathogen detection. Moreover, as the cells contain lesser concentrations of nucleic acids or other analytes, the biosensors need an enrichment step for amplification of targeted analytes like nucleic acids using polymerase chain reaction (PCR), reverse transcriptase-PCR and various other techniques. Amplification of the transducing signals is also done for confirming the hybridization or affinity binding between the probe and the analyte. The specific target DNA/ribonucleic acid (RNA) can also be identified using different physical bio-sensing methods. Overall, the efficiency of a pathogen sensor is based on the specificity of biochemical reactions, high concentration of analytes under investigation and sensitive/selective detection or transduction methods. The sensor probe ought to be developed with smaller dimensions providing high sensitivity and low detection limit. Here we discuss about the different types of biosensors involved in pathogen detection, brief account on their principles of transduction along with some remarkable research, critically analyzing its construction, specificity, efficiency like limit of detection and ability to detect pathogens.
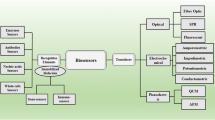
2.2 Classification of Biosensors
Biosensors can be broadly classified, as physical and chemical biosensors, based on the mode of signal transduction used in detection of biological analytes. The biological components can be enzymes, whole cells, organelles, lipids, peptides, tissues, antibodies or nucleic acids. Biosensor functions by following a biological event (e.g. antibody-antigen interaction) whose signals can be sensed by any physical or physico-chemical transducer as illustrated in Fig. 2.1.
Schematic representation of a Biosensor. Following a biological event, the alterations caused in any of the intrinsic parameters like fluorescence intensity, refractive index, surface tension, viscosity, etc. of the sensor probes can be sensed by a physical or physico-chemical transducer/detector which converts into a readable signal
2.2.1 Physical Biosensors
Biosensors that can sense a biological event by following the changes in physical phenomena like mass, resonance frequency, refractive index, fluorescence, etc. of the targeted analyte are classified as physical biosensors. These are further classified into optical and mechanical biosensors.
2.2.1.1 Optical Biosensors
Biosensors that quantify the analyte in a sample by its interaction with photons are categorized as optical biosensors. There are several advanced optical sensing methods available today to overcome the limitations of its preceding version. Few of them are discussed below.
2.2.1.1.1 Labeled Optical Biosensors
2.2.1.1.1.1 Fluorescent Labels
Fluorescence and phosphorescence emissions are about 1000 fold more sensitive with low limits of detection than other available spectrophotometric methods (Guilbault 1990; Yu et al. 2013). A typical fluorescence label method (Li et al. 2015) involves excitation of fluorophore at a particular wavelength followed by emission of light at a different wavelength. When the analytes are present in trace amounts, reporter molecules labeled with fluorescent dyes, are used for sensitive detection. Fluorescence detection has number of advantages (Waggoner 2006) that includes higher level of detection, sensitivity, rapid response times, a selective fluorescence signal, multiple assays using different colored dyes and direct labeling process, which yields specific functional moieties on the analyte.
The reagents used for fluorescent labeling have their key roles for sensitive and reliable mode of detection. Fluorescent labeling agents include organic dyes, nanoparticles like quantum dots, carbon nanotubes and rare-earth elements. Fluorescent biosensor works with optical signals for quantitative or semi-quantitative detection of pathogens. Recently, a number of nanomaterials which can act as efficient fluorescence quenchers have been studied to build biosensing platforms (Jans and Huo 2012; Wang et al. 2015; Wang and Alocilja 2015). Organic dyes were the mostly used labeling molecules in fluorescence biosensors (Panchuk-Voloshina et al. 1999) which included fluorescein, rhodamine dyes, sulfo rhodamine, Alexa dyes (Alexa350, Alexa488, Alexa532 and Alexa594) and cyanine dyes (carbo cyanines Cy3, Cy5 and Cy7). Cyanine dye labels like Cy3, Cy5 and Cy7 were used along with immunological reagents like antibodies and also DNA as bio-recognition elements (Kallioniemi et al. 1992). Sulfonate groups with negative charge, enhances solubility in water, prevents aggregation and minimizes the fluorescence dye-dye interactions bound to the antibodies (Mujumdar et al. 1993). These groups were combined with cyanine dyes in its ring structure, enhancing the intensity of the dyes in aqueous media. Cy3 and Cy5 have been extensively used for DNA and RNA labeling for gene expression studies (Schena et al. 1995). Recently, Cy3 dye bound capillary tube, along with a mesoporous chip was explored in the detection of a carcinoembryonic antigen, a protein available in the blood of some people with certain kinds of cancer (Yu et al. 2013; Yu et al. 2014). Drawbacks faced in using these highly sensitive fluorescent labels for detection includes lower fluorescence intensity, pH-sensitivity, photo-bleaching and non-fluorescent product formation. These limitations restricted their use in a bio-sensing system and paves way for alternatives.
Fluorescence can be directly used as bio-recognition tool/reagent that takes part in the bio assays for the detection of pathogens. Calcein Blue is a fluorophore whose fluorescence is quenched in the presence of iron (III) has been proved to have the potential to directly detect bacteria (Sankaranarayanan et al. 2015). In the presence of an iron chelating molecule (siderophore) released by bacteria, iron is removed from the fluorophore and fluorescence is restored in Calcein Blue. This serves as an easier, sensitive and cheaper method, which could be done in a 96 well plate, to identify and quantify iron chelators, and hence the presence of pathogenic bacteria within 7–8 h of incubation. Formation of siderophores by the Gram positive bacteria such as Bacillus species, Staphylococcus aureus (Dale et al. 2004; Zawadzka et al. 2009) and Gram negative bacteria like Escherichia coli, Legionella species, Proteus species and Mycobacterium tuberculosis (Himpsl et al. 2010; Wells et al. 2013; Adler et al. 2014) have been well studied. Here fluorescence acts as a sensitive indicator of iron sequestration by the virulence marker, siderophores and hence in the detection of the presence of bacterial pathogens in the sample. Fluorescence based antibiogram method was found to be exceptional compared to disc diffusion method or the standard liquid turbidity method. Percentages of individual pathogens susceptible/resistant to different antibiotics are profiled as antibiogram. This technique helps in reporting antibiotic susceptibility, intermediateness and resistance within 6–8 h, allowing rapid selection of suitable antibody against infectious bacteria. Fluorescein diacetate is used as viability indicator along with the antibiotic agent and the bacterial samples that has grown for 7 h (Alagumaruthanayagam and Sankaran 2012). Fluorescence released was measured after half an hour to check for viability of the infectious bacteria in the sample.
Specific antibodies conjugated with fluorescein enriched hollow silica nanospheres (diameter 350 nm) were used along with magnetic probes for selectively detecting Escherichia coli O157:H7 (Hu et al. 2016). Here, acidity of the solution is important for the fluorescein release, as the hollow silica nanospheres dissolves only in alkaline solution of sodium hydroxide leading to release of the fluorescent labels from the immune complexes. It involves capture of Escherichia coli O157:H7 cells followed by magnetic separation and fluorescein release in a detection solution (pH 10). Quantification of pathogens is based on the fluorescence intensity of the fluorescein released from the fluorescein-enriched hollow silica nanospheres. Here the detection time is only 75 min with limit of detection as low as 3 cfu/ml. The same strategy could be employed in detection for a different pathogen.
2.2.1.1.1.2 Graphene-Fluorophore
Graphene and graphene oxide are used for their functionalization, high volume to surface ratio, desirable physical and electrical properties. Combination of graphene and graphene oxide material with fluorescent labeled probes like aptamer/DNA enabled detection by their fluorescence quenching properties (Zhang et al. 2017). Quenching occurred upon adsorption of the fluorophore conjugated probes on the graphene surface. The analytes present in the sample interacts with the probe leading to its detachment from the graphene surface and generating more fluorescence and hence acting as indicators for detection of the target analytes.
Graphene and graphene oxide can be complemented with different types of sensing mechanisms like optical/fluorescence biosensors and electrochemical sensors (Shao et al. 2010; Liu et al. 2012; Kamali et al. 2015). Based on the sensing mechanism and principle graphene-based biosensors either use its unique properties like high electron mobility, high electron-transfer rates, or high surface to volume ratio for detection of biomolecules (Kuila et al. 2011; Zheng et al. 2014).
2.2.1.1.1.3 Quantum Dots
Nanoparticles in the form of quantum dots and carbon nanotubes have been used for immobilization in bio-recognition applications (Rosi and Mirkin 2005; Ansari et al. 2010; Merkoci 2010). They serve as good alternatives for their biocompatibility, higher distinct surface area, non-toxicity, good electro-catalytic activity, high chemical and thermal stability, and rapid communication by means of electrons. Moreover, nanoparticles associated fluorescence is an emerging research area that enhances sensitivity, ease of use and diversity of the fluorescence-based sensing methods. The development of quantum dots in labeling technology is due to their desirable optical and chemical properties that include broader absorption with narrow photoluminescence spectra, minimum photo-bleaching, high quantum yield and ability to withstand chemical degradation. These are semiconductor nanocrystals that are spherical with size ranging from 1 to 10 nm (Sutherland 2002). An interesting feature of quantum dots is that their fluorescence emission wavelengths can be tuned by varying their particle size and chemical composition. The ability of quantum dots to tune their size has been exploited for labeling and detection of multiple analytes at the same time (Rosenthal 2001). The uninterrupted long term tracking of biological processes is possible with quantum dots due to their low photo degradation rate (Chan and Nie 1998; Wu et al. 2003). Quantum dots are considered to be more advantageous than traditional organic dyes as fluorescent probes (Vinayaka and Thakur 2010), as they are 20 folds brighter and 100 folds more resistant to photo-bleaching (Chan and Nie 1998). But it also has three major limitations, synthesis from toxic elements, no inherent solubility and aggregation in water leading to lower quantum yields, which restricts its use for biological applications (Shen et al. 2012).
2.2.1.1.1.4 Carbon Dots
Carbon-based nanomaterials , fluorescent carbon dots with similar optical properties like quantum dots have emerged which possesses photoluminescence properties. By means of purification of single-walled carbon nanotubes through preparative electrophoresis carbon dots of size below 10 nm, were first formed in 2004 (Xu et al. 2004). Carbon nanotubes offer high surface to volume ratio, strong adsorption ability, desirable electronic and mechanical properties (Davis et al. 1998). It is commonly used as, single-wall carbon nanotubes and multi-wall carbon nanotubes. Due to its high surface to volume ratio and good adsorption, carbon nanotubes are employed for adsorption of bacteria and enrichment of various types of pathogens (Srivastava et al. 2004; Deng et al. 2008; Upadhyayula et al. 2009). It also serves as a material with high antimicrobial nature (Kang et al. 2007).
2.2.1.1.1.5 Combination of Quantum Dots with Bio-recognition Elements
One major development in fluorescence based detection technique includes immunoassays that uses bio-recognition elements along with fluorescent labels (Yang and Li 2006). Quantum dots are bound to biomolecules like antibodies by strategies like covalent binding, electrostatic binding, non-covalent biotin-avidin binding and nickel-based histidine tagging (Xing et al. 2007; Lee and Kang 2009; Zhang et al. 2010). Semiconductor quantum dots with different emission wavelengths (525 nm and 705 nm) were bound to anti-Escherichia coli O157 and anti-Salmonella antibodies by streptavidin and biotin coupling and used as fluorescent labels in immunoassay for detection of E. coli O157:H7 and Salmonella (Yang and Li 2006).
2.2.1.1.1.6 Fluorescence-Based Fiber Optic and Planar Waveguide Biosensors
Fluorescence based optical fiber and planar waveguide consists of an optical transmitter for the bio-recognition and for transporting the signal responses to a photodetector which converts the photons into an electrical signal. The optical biosensors offer advantages like low cost, compact size, ease of use and ability for real time monitoring or detection of specific species in test samples as well as quantification of the binding events (Taitt et al. 2016). These sensors find their use in detection of pathogen or contamination in food samples, environment and also in clinical diagnosis (Ligler and Taitt 2008; Benito-Pena et al. 2016). Fluorescence based transduction is most common in optical biosensors (White and Errington 2005; Borisov and Wolfbeis 2008). Parameters like fluorescence intensities measured at a particular excitation/emission wavelengths and decay time as a function of intensities could be used for bio-sensing (Demchenko 2008). It offers various ways to improve the performance, selectivity and sensitivity of the optical biosensors. Optical waveguides are made up of dielectric structures which transmit energy between ultra violet-visible and infra-red regions in the electromagnetic spectrum. Based on their configuration they are categorized as cylindrical and planar wave guides (Fig. 2.2). Optical fibers are composed of cylindrical central dielectric core covered by a lower refractive index material, whereas a planar waveguide consists of block of dielectric core placed in between two layers with reduced refractive indexes (Banica 2012).
A fiber-optic biosensor, shown schematically in Fig. 2.3, used to detect Staphylococcus aureus was established in 1996 (Hsin Chang et al. 1996). The fluorescein derivative fluorescein isothiocyanate was bound to the anti-Protein A immunoglobulin G, produced by Staphylococcus aureus that emits fluorescent signals of the antigen-antibody reaction with lower detection time (24 h). Similarly, the above method was used to detect Staphylococcal enterotoxin B with cyanine dye Cy5 as fluorescent label (Rowe-Taitt et al. 2000). A planar waveguide attracted the Staphylococcal enterotoxin B present in samples, a small diode laser excited the fluorophore Cy5, the pattern of fluorescent biochemical assay was recorded and extraction procedure lasted for less than 20 min. Another fiber optic biosensor (Ko and Grant 2006) based on fluorescence resonance energy transfer with donor fluorophore as Alexa Fluor 546 and Alexa Fluor 594 as acceptor fluorophore due to its high spectral overlap and energy transfer (Panchuk-Voloshina et al. 1999) for the detection of Salmonella typhimurium in the samples of ground pork was established. A glass fibre based lateral flow DNA biosensor was reported (Chua et al. 2011) for the detection of food borne Vibrio cholerae by conjugation of the capture reagents to carrier beads and fluorescein labeled detector reagent bound to gold nanoparticles.
Schematic representation of a fluorescence-based fiber optic biosensor. In this illustration, a laser source coupled with fiber optics is used in the detection of protein A from Staphylococcus aureus. This antigen-antibody reaction induces fluorescence signals from fluorescein isothiocyanate (FITC) conjugated anti-protein A immunoglobulin G (IgG), when complexed with protein A which then can be detected by a conventional optical set up
2.2.1.1.2 Label Free Optical Biosensors
2.2.1.1.2.1 Surface Plasmon Resonance Biosensors
While sensing biological analytes it becomes necessary to analyze them in their naturally occuring form. Early optical sensors required a fluorescent probe to be tagged to the analyte. Such tagging may interefere with the native interpretations. Therefore it becomes necessary to identify techniques that can go label-free, background-free and detect heterogeneity among interactions. In this context, surface plasmon resonance biosensors follow a label-free technique. They have been developed for sensitive and specific real time analyte detection. These surface plasmon resonance instruments work by optically monitoring the changes that occur on its surface, (usually metals like gold, copper or silver), with respect to the analyte flowing over the surface. A surface plasmon occurs at the metal-dielectric interface, as a charge density wave also called as surface plasmon wave (Liedberg et al. 1995). Surface plasmon resonance biosensors employ an optical phenomenon where the surface plasmon wave undergoes changes corresponding to the changes in the refractive index of the dielectric, and due to the binding of the analyte to the surface (Homola 2003). The most commonly used surface plasmon resonance sensing method involves the prism-coupling system (Fig. 2.4) where an incident light at the metal/glass interface at the bottom is coupled to the surface plasmon waves on the metal or liquid interface at the top. The light rays with an incident angle bends due to high refractive index of the prism which then interacts with the surface plasmon dispersion curve at the interface (Pi et al. 2016). Here the change in signal will be solely due to the variation in the surface refractive index at the top interface.
Prism coupled-surface plasmon resonance biosensor with immobilized antibodies to detect specific antigens in the sample. The antigen-antibody reaction, causes a change in the refractive index at the interface (thin noble metal), which is being monitored as variations in output optical signals by the detector
Some examples and improved methodologies employed for pathogen detection along with their limitations are listed below. Surface plasmon resonance has proven to have the potential to identify a particular target bacterium in a sample containing closely-related species. Differentiation of spores of Bacillus globigii, a simulant of the bio-warfare agent Bacillus anthracis, from a sample mixture of various other Bacillus species, was made possible with a portable surface plasmon resonance biosensor. Detection was performed by direct capture using anti-Bacillus. However this technique was impractical to use due to its higher limit of detection (107 spores/ml) compared to other detection methods (Byrne et al. 2009) and the lethal dose of Bacillus anthracis (100 spores) (Housewright and Glassman 1966). This also signifies the need for a biosensor to have lower limits of detection for pathogens in order to detect them sensitively.
2.2.1.1.3 Hybrid Optical Biosensors
Integration of surface plasmon resonance biosensors with other detection elements/techniques like, fluorophore, immunolabels, magnetic nano particles, PCR, etc., was found to enhance sensitivity and reduce limit of detection.
An improved version of surface plasmon resonance sensor was developed by integrating with immunolabels, for Salmonella detection in food samples with lower limit of detection of 103 cfu.ml−1(Farka et al. 2016). The surface plasmon resonance chip was immobilized with capture antibody, to which the samples containing Salmonella will be added. Salmonella if present in the sample will bind to the immobilized capture antibody followed by binding to secondary/detection antibody conjugated to Horse Radish Peroxidase. Here surface plasmon resonance detection is enhanced by Horse Radish Peroxidase catalysed reaction where 4-chloro-1-naphthol gets converted into insoluble benzo-4-chlorocyclohexadienone as shown in Fig. 2.5. This has been found to increase the detection signal and 40 times enhancement of sensitivity in detecting Salmonella compared to label-free detection. Enzymatic reaction oriented bio-recognition along with antibodies have proved to enhance the ability of surface plasmon resonance biosensor in pathogen detection compared to direct detection with antibodies or amplification with secondary antibodies. Use of antibody functionalized with Fe3O4 (iron oxide) magnetic nanoparticles (immuno magnetic nanoparticles) in surface plasmon resonance sensors based on sandwich immunoassay for Salmonella detection (Fig. 2.6) was found to be more sensitive with limit of detection as low as 14 cfu/ml (Liu et al. 2016a). These immune magnetic nanoparticles work as vehicles to selectively detect, isolate and deliver Salmonella from a sample mixture onto the sensor surface. Moreover, it also serves as labels that amplify sensor signal by the enhancement of refractive index variations for particular analyte pathogen. Sensitivity of the surface plasmon resonance sensors employing these immuno magnetic nanoparticles were proved to have four times more sensitivity compared to direct detection surface plasmon resonance sensors. Surface plasmon resonance biosensors with labeled antibodies, (be it enzyme/magnetic nanoparticles), that were based on sandwich immunoassay were found to have high sensitivity compared to other direct detection surface plasmon resonance sensors. With appropriate antibodies immobilized on gold surface of surface plasmon resonance sensors, pathogens in food samples could be detected in less than 5 h (Sharma and Mutharasan 2013). All these prove the ability of surface plasmon resonance sensors to be a sensitive, selective, quicker and label-free detection tool. Moreover, these sensor surfaces can be regenerated and reused by washing with NaOH (5 mM). A surface plasmon resonance based protein chip with Protein G immobilized on its surface was developed to selectively detect different pathogens (Fig. 2.7) like Escherichia coli O157:H7, Salmonella typhimurium, Legionella pneumophila, and Yersinia enterocolitica (Oh et al. 2005) that exist in the contaminated environment. The immobilised Protein G preserves the orientation of the monoclonal capture antibodies on sensor surface. It enhances the sensitivity by associating specifically with the fragment crystallizable portion of antibody immunoglobulin G (Boyle and Reis 1987) and improving the efficiency of antigen-antibody binding.
Schematic representation of surface plasmon resonance chip that can detect pathogenic antigens in the sample, by surface plasmon coupled sandwich immunoassay, enhanced by Horse radish peroxidase labeled antibodies catalyzing the formation of insoluble benzo-4-chlorocyclohexadienone; SPR- Surface plasmon resonance, HRP- Horse radish peroxidase
(i) Formation of antibody functionalized iron oxide magnetic nanoparticles by EDC/NHS coupling; (ii) Binding of specific antibodies conjugated iron oxide magnetic nanoparticles with pathogenic antigens; (iii) Enrichment of pathogens complexed with antibody-iron oxide nanoparticles from sample mixture by magnetic separation; (iv) Pathogen detection by surface plasmon resonance based gold chip coated with anti-pathogen polyclonal antibody. SPR - Surface plasmon resonance, EDC/NHS - 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide/N-Hydroxysuccinimide, Fe3O4 MNPs - iron oxide magnetic nanoparticles
As production of specific antibodies is expensive, time consuming, complicated and are prone to lose its activity due to external conditions (Arya et al. 2011) DNA based surface plasmon resonance biosensors were developed. Here the carboxylated dextran fixed onto the gold surface of sensor chip was coated with streptavidin covalently linked to biotinylated single stranded oligonucleotide probe as shown in Fig. 2.8 (Zhang et al. 2012). The probe was designed in such a way that it is specific to a highly conserved gene, a potential target for pathogenicity in pathogens like Salmonella which undergoes hybridization that lasts only for 15 min.. Detection of Salmonella cells upto 102 cfu/ml could be performed in 4.5 h using this surface plasmon resonance DNA biosensor. Regeneration of the sensor surface makes it possible to reuse the sensor for atleast 300 assay cycles, thereby reducing the cost of pathogen detection. By standardizing the sample preparation procedures to extract DNA followed by its amplification, this sensor could be used as an efficient tool to detect and monitor pathogens in real samples.
Schematic representation of a surface plasmon resonance - DNA biosensor chip, coated with gold, carboxylated dextran and streptavidin on top that binds biotin conjugated ss-DNA probe, followed by hybridization with complementary pathogenic target gene from the sample. SPR - surface plasmon resonance, ss-DNA - single stranded-deoxyribonucleic acid
Surface plasmon resonance biosensors based on grating coupled long range surface plasmons were employed along with magnetic nanoparticles to detect bacterial pathogens like Escherichia coli O157:H7 at concentrations as low as 50 cfu/ml (Wang et al. 2012a). Here the limit of detection is four times better than that obtained by the regular surface plasmon resonance with direct detection. Long range surface plasmons provide reduction in losses compared to the normal surface plasmons and exhibits narrow resonance, thereby rendering accurate measurements of changes in refractive index. Long range surface plasmons based biosensors could probe higher distances from the surface (Dostalek et al. 2007) and possess several fold enhanced refractive index resolution compared to regular grating-coupled surface plasmons suitable for analyzing large analytes (Huang et al. 2011; Vala et al. 2009).
Further PCR microchip hybridized with optical fiber surface plasmon resonance sensor with bimetallic coating (Ag/Al) (Nguyen et al. 2017) serves as a label-free, reusable, point of care diagnostic instrument by genetic analysis. In this, DNA of the pathogen to be detected is amplified within 30 min on the PCR microchip followed by detection with the surface plasmon resonance optical fibre sensor without the aid of any kind labels. Here the pathogen detection is free from sample volume restriction and labeling, until the DNA sample is in close contact to the surface of the sensor. It has paved way for further miniaturization and point of care diagnosis by genetic analysis towards effective pathogen detection without much human intervention.
2.2.1.2 Mechanical Biosensors
Sensors that sense the analytes by monitoring the change in mass associated with it during the interaction process. They are more advantageous (Arlett et al. 2011) than any other biosensors as they do not require any sample preparation. The commonly available mechanical sensors follow either quartz crystal microbalance or cantilever technology for the detection of analytes. Moreover, these sensors work as label-free unlike other optical techniques.
2.2.1.2.1 Piezoelectric Quartz Crystal Biosensors
In piezoelectric quartz crystal biosensors, the detection of an analyte is usually based on adsorbate identification. Here corresponding to the selective binding, mass change occurs and is detected by change in electrical/acoustic parameters of a piezoelectric quartz crystal. Piezoelectric effect is well observed in crystals on applying pressure to it, thereby distorting the crystal lattice and inducing dipole moment in the crystal molecules (Alder and McCallum 1983). Quartz crystal is the most commonly used (Deakin and Buttry 1989), due to its desirable electrical, mechanical and chemical properties. The piezoelectric quartz crystal is placed in between two metal (gold/silver) electrodes and when a potential difference is applied, the crystal lattice gets distorted and undergoes oscillation with a characteristic resonance frequency. This oscillating piezoelectric quartz crystal has a characteristic frequency dependent on its own physical properties and other phases adjacent to it. This particular proportional relationship between the resonance frequency and the mass of the crystal is exploited from this technique for pathogen detection.
Piezoelectric biosensors are being used for rapid detection (Farka et al. 2013) of pathogens either by active mode or passive mode. In active mode, the piezoelectric crystal oscillates and the corresponding resonance frequency is measured by means of a frequency counter (Arnau 2008). The passive mode is an expensive one where the resonators are monitored for their impedance (Zhang et al. 2002). Passive mode piezoelectric biosensor employs one equipment that can recognize the changes in mass and viscosity (Itoh and Ichihashi 2008). These sensors detect the change in resonance frequency that is caused by the binding of an analyte or increase in the mass on its surface. A more stable and sensitive series piezoelectric quartz crystal sensors (Shen et al. 1993; Jang et al. 2009) were designed by interconnecting several piezoelectric quartz crystal with conducting electrodes successively, which responded with resonance frequencies to every change in the electrical parameters (He et al. 2003; Lakshmanan et al. 2014). With the aid of series piezoelectric quartz crystal having multiple channels several samples could be analyzed at the same time (Tong et al. 2014) and hence can be used for high-throughput analyses.
To further increase the sensitivity, series piezoelectric quartz crystal with interdigital electrodes where a series of cathodes and anodes are connected with micro gap in between were designed. This enables detection of even minute variations on its surface (He et al. 2007; Ren et al. 2010). Graphene coated interdigital gold electrodes are connected in series with piezoelectric quartz crystal to develop a series piezoelectric quartz crystal sensor upon which the probes like pathogen specific aptamers or antibodies, are impregnated as assemblies for the corresponding pathogen detection. This was demonstrated initially by binding Staphylococcus aureus aptamers to grapheme by π-π stacking of DNA bases (Lian et al. 2015). Upon introducing analyte/target DNA (Staphylococcus aureus), the aptamers bind strongly with the target DNA. The force of interaction of aptamer with graphene becomes relatively weaker and the aptamer-target DNA complex gets itself freed from the electrode surface, thereby causing changes in electrical parameters of electrode surface with corresponding shift in oscillation frequencies. These sensors have limit of detection of pathogens upto 41 cfu/ml. Similarly interdigital electrodes impregnated with pleurocidin, an antimicrobial peptide with broad spectrum of antimicrobial activity, help in rapid detection of microbes in clinical blood samples in a time span of 15 min has also been established (Shi et al. 2017). This sensor has found its importance in clinical diagnosis (Jordana-Lluch et al. 2013) and food safety applications (Farahi et al. 2012). In this the transducing element is an integrated single walled carbon nanotubes that crosslinks pleurocidin with interdigital electrodes connected to a multichannel series piezoelectric quartz crystal. This sensor works by specific binding of pleurocidin with microbe which causes detachment of pleurocidin from the single walled carbon nanotubes associated with the electrodes. The response in the form of frequency changes in multichannel series piezoelectric quartz crystal are detected by the transducer which is proportional to the microbes present in the sample. It fulfills the need to detect the microbes which is the first step in the microbial blood stream infection tests (Gonsalves and Sakr 2010), followed by identification and drug susceptibility of microbes.
As a further step towards increasing the sensitivity, a sensor holding a similar transducer as above is used along with gold nanoparticles (He et al. 2016), aptamers specific for the complex of 10 kDa culture filtrate protein and 6 kDa early secreted antigen target (Renshaw et al. 2002; Philip et al. 2005), an early phase antigen, secreted by the pathogenic Mycobacterium tuberculosis, is used as probe for detection of Mycobacterium tuberculosis. These aptamers along with complementary DNA bound gold nanoparticles over the interdigital electrodes showed more sensitivity (Zheng et al. 2007; Li et al. 2009) when the target protein in the test sample interacts with the aptamer. The DNA bound gold nanoparticles detached from the electrode surface followed by electrical changes on the electrode due to the substitution of conductive DNA-gold nanoparticles by non-conductive target proteins. This had led to a more sensitive detection of frequency changes by the multichannel series piezoelectric quartz crystal with a detection time of 96.3 h. This sensor serves as a rapid and early detection tool for detection of pathogenic M. tuberculosis which is not possible with other sensors based on antigen-antibody interactions (Ren et al. 2008; Kirsch et al. 2013).
2.2.1.2.2 Piezoelectric – Cantilever Biosensors
Cantilevers are rigid structural elements held at one end to a rigid support. Micro-cantilever sensors have bio-receptors bound to it and have characteristic resonance frequencies of oscillation upon interaction with bio-analytes (Ahmed et al. 2014). The cantilever undergoes mechanical stress due to increase in mass on the sensor surface and induces variation in the resonance frequencies. This property has been used in the detection of various bacteria like Escherichia coli O157:H7 (Zhang and Hai-Feng 2004; Campbell and Mutharasan 2005a), Salmonella typhimurium (Zhu et al. 2007), Vibrio cholerae (Sungkanak et al. 2010), and the biowarfare agent Francisella tularensis (Ji et al. 2004). Antibody functionalized cantilevers of the size of a millimeter excited by piezoelectric effect has the ability to detect various bacteria like Escherichia coli cells (Campbell and Mutharasan 2007), Listeria monocytogenes cells in milk (Sharma and Mutharasan 2013). By using an impedance analyzer the changes in the resonance frequencies when the analyte binds specifically to the antibody at the cantilever tip could be measured. In a 1 ml sample cell, Escherichia coli O157:H7 upto 700 cells/ml has been detected with a cantilever functionalized with monoclonal antibody specific to Escherichia coli O157:H7 (Campbell and Mutharasan 2005b). Amplitude ratio and phase angle variations with respect to mass changes at the cantilever were measured using an impedance analyzer. Further, flow cells wherein analyte solution flows at a particular rate have been used along with the functionalized piezoelectric-cantilever to detect pathogens more sensitively (Campbell and Mutharasan 2006).
Piezoelectric cantilever sensors employing specific gene based detection of pathogenic bacteria have also been developed. A food pathogen, Listeria monocytogenes could be detected with one such sensor employing a probe specific for the target virulence hemolysin gene, hlyA, within 90 min (Sankaranarayanan et al. 2015). The hybridization response of the specific virulence gene from the sample genome extract of the pathogens, with the probe on the cantilever was detected by different means. It includes, a fluorescent indicator to detect the hybridized double stranded DNA, a secondary single stranded DNA to detect the unhybridized portion of the target DNA and also gold nanoparticles tagged secondary single stranded DNA to amplify the target hybridization. It is necessary to extract the genomic DNA of the test sample prior to detection with the cantilever. It proved to be a rapid technique compared to other conventional detection techniques with almost same detection limit.
2.2.2 Chemical Biosensors
Chemical biosensors monitor the changes in the chemical phenomenon of the analytes upon interaction with the sensor probes. Further discussion on this will be carried out under two categories as electrochemical and biochemical sensors.
2.2.2.1 Electrochemical Biosensors
Here the variations in electrical properties like current, potential or impedance at the electrode surface upon binding to the bio-analytes in the test sample will be examined. It can be further classified as label dependent and label free electrochemical biosensors (Xu et al. 2017) based on the technique used for detection. Labels like enzymes, metal particles, etc. are specifically tagged to the target analyte in case of label dependent sensors. Label free technique involves the binding of bacterial cells on the electrode surface that elicits changes in electrical parameters which could then be measured (Sang et al. 2016). With respect to the methods used for measurement of electrical parameters, electrochemical biosensors can be sub divided as amperometric, potentiometric, voltametric, conductometric and impedimetric.
2.2.2.1.1 Amperometric and Potentiometric Detection
Pathogen detection by amperometric or potentiometric methods (Monzo et al. 2015) depends on the variations in current or potentials in which the electrode is kept at a constant potential or current, relative to the reference electrode (Bard and Faulkner 2001). This type of sensor has biological receptors like aptamers/antibodies that can specifically adsorb microbes coupled to an enzyme transducer. Upon binding of microbes, the enzymatic reactions are initiated, which either produces or takes up a species, followed by its detection, by a selective electrode (Barlett 2008). It proves to be a sensitive method as it is dependent on logarithmic concentration. Recently, the use of a semiconductor device, field effect transistors, for microbial detection enhanced the signal of the sensors ensuring increased sensitivity (Grieshaber et al. 2008; Lin et al. 2008). It is also a rapid and cost effective method (Barlett 2008; Wei et al. 2009), but these amperometric sensors tend to have poor selectivity. This is because all the constituents in the solution with a standard potential less than operating potential will add to the faradaic current, generated by the reduction or oxidation of those constituents at the electrode surface (Monzo et al. 2015).
2.2.2.1.2 Impedimetric Detection
Here the impedance changes caused by the voltage signal when the analyte binds, relative to the frequency of an electrode with dielectric properties, are measured (Bard and Faulkner 2001; Barlett 2008). Two approaches are employed for the identification of pathogens using impedimetric techniques. One involves the use of unmodified electrodes, or electrodes bound to biological receptors, for the measurement of the variations in impedance due to unspecific or specific attachment of bacteria to the electrode, respectively (Wang et al. 2012b). Another approach is the direct detection of the metabolites or toxins that are released by the pathogens during their growth and is considered to be an important tool in detecting infected samples (Felice et al. 1999; Gomez et al. 2002). The association of the microbes or the metabolites with the electrode can be measured by differences in the capacitance magnitude at the junction of electrode or variations in impedance. Upon adsorption of either metabolites or microbes, reduction in total electrode surface area occurs which further shifts the impedance to a higher level. Sensitivity and selectivity are found to be high in impedance spectroscopy towards detecting biological elements (Felice et al. 1999). Carbon electrode modified with reduced graphene oxide has been reported in the detection of methicillin resistant Staphylococcus aureus by means of impedance spectroscopy (Wang et al. 2011). Single stranded probe DNA bound to the functionalized electrode undergoes hybridization with the complementary target DNA present in the test sample, followed by measuring the change in impedance values. This proves to be a more sensitive and selective method in the detection of pathogen with the help of DNA as bio-receptor. A rapid (45 min) and label free approach was demonstrated for the detection of Salmonella by using a combination of polypyrrole based polymer, poly [pyrrole-co-3-carboxyl-pyrrole] copolymer and a specific aptamer (Sheikhzadeh et al. 2016). Impedimetric measurements upon interaction of the aptamer with the target, cause changes in electrical parameters of the polypyrrole based copolymer. It is considered to be a cost effective and rapid detection technique that could be used for analyzing the contaminants in food samples and also in the environment. As an alternative to the above, gene/immune based approach with engineered synthetic peptides specific for pathogens have been used while monitoring the changes in impedance (Liu et al. 2016b).
2.2.2.1.3 Voltammetric Detection
Voltammetric method for pathogen detection depends on the variations in the potential at the junction of the electrode surface and the analyte solution with respect to time, along with the measure of current (Bard and Faulkner 2001). Cyclic sweep voltammetry is a commonly used technique for acquiring information like oxidation or reduction potentials, reaction mechanisms and kinetics (Bard and Faulkner 2001; Compton and Banks 2011). Here the voltage scan is performed from a minimum to a maximum level of potential at a specific scan rate. The response in the form of current is recorded with respect to the voltage instead of time. Voltammetric sensing is done either with the help of biological receptors or by direct detection of the metabolite species undergoing oxidation or reduction on a modified or unmodified electrode. Several advanced voltammteric detection methods employing pulse strategies are available. These include differential pulse voltammetry, normal pulse voltammetry and square wave voltammetry. For better time resolution and high frequency operation, differential pulse voltammetry and square wave voltammetry are more preferred for electroanalysis (Chen and Shah 2013). Genomic DNA of the targeted pathogens like Salmonella or Escherichia coli cultures without any PCR amplification could be detected by means of sandwich like assay consisting of magnetic nanoparticles, the genomic target DNA and gold nanoparticles imprinted on carbon electrodes (Blow 2015).
An antibody based sensor with an immunoelectrode containing Graphene oxide-silver nanoparticles immobilized with antibodies of the pathogen Salmonella typhimurium utilizes cyclic voltammetry for the detection the same pathogen (Sign and Sumana 2016). This could be extended to detect various other pathogens with corresponding specific antibodies.
2.2.2.2 Biochemical Sensors
2.2.2.2.1 Immunosensors
Immunosensors are solid-state devices that combine immunochemical reactions and transducers. Here the sensitivity and selectivity depends on specific ligand-receptor interactions. A typical immunosensor is composed of two elements: a bio-recognition element and a transducer. Here the bio-recognition element is formed by impregnation of specific antigens/antibodies, and their interaction is converted into a measurable signal by the transducer.
The immunoassay strategies involve direct or indirect methods of detection. In the direct method, the immunochemical reaction is directly quantified with respect to the physical changes that occur due to the antigen-antibody interaction. Indirect method involves the use of a label in association with the antibody or antigen.
Immunosensors are combined with magnetic particles or gold nanoparticles for enrichment or signal amplification in the detection of pathogens (Wang and Alocilja 2015). An in-situ immuno-gold nanoparticle integrated network-based enzyme-linked immunosorbent assay biosensor together with initial sample enrichment using immuno-magnetic separation have been developed for the detection of pathogens with high sensitivity (Cho and Irudayaraj 2013). It involves the formation of immuno-gold nanoparticle network onto the antigenic site at the bacterial outer membrane surface, followed by analytical validation using microtiter immunoassay. Here magnetic and gold nanoparticles are coupled with antibodies specific to the target bacterium. Initially separation and enrichment of bacteria will be done by immuno-magnetic separation followed by secondary antibodies functionalized with gold nanoparticles (30 nm in size) and are able to bind to complementary targets on the cell surface to form a network structure that can grow with time amplifying the signal in the network structure (Lee and Irudayaraj 2009; Lee et al. 2011). It proved to be highly sensitive to detect pathogens at extremely lower concentration like 3 cells/ml of Escherichia coli O157:H7 and Salmonella typhimurium in buffer, and 3 cfu/ml of Escherichia coli O157: H7 and 15 cfu/ml of Salmonella typhimurium in real sample conditions within 2 h of inoculation. The ability to detect and monitor target bacteria with enhanced analytical sensitivity compared to other current techniques makes it suitable as a tool for routine monitoring and improved food safety. Based on the measurement of the amount of specific virulence factors formed by the pathogenic organism, the pathogen can be detected. Immunochemical approaches have been developed to detect Pseudomonas aeruginosa infections by quantification of a specific virulence factor pyocyanin secreted exclusively by these organisms (Pastells et al. 2016). Antibodies specific to 1-hydroxyphenazine, the major metabolite of pyocyanin virulence factor enables us to quantify both 1-hydroxyphenazine and pyocyanin in clinical test samples in 20 min. This assay completes in 2 h and offers simultaneous detection of several samples. It proves to be a remarkable development in diagnosis of infections in test samples.
2.2.2.2.2 Immunoassays/ Immunochemical Methods to Detect Pathogenic Infections
Antibodies suitable for immunoassays are chosen based on factors like, the assay format where these monoclonal/polyclonal antibodies will be employed according to antigen specificity. These assays are integrated with sensors to form immunosensors . After establishing the ability of antibodies to recognize particular antigens, it becomes easier to classify, detect and ultimately prevent pathogenic infections. Several immunoassays have emerged through the years, to detect pathogens in the clinical stool samples or industrial food products. Immunoassays have been developed for food industry to detect and quantify various food components like protein, enzymes, vitamins and contaminants like microorganism, toxins, pesiticides, hormones and others (Fukal and Kas 1989). Immunoassay format can be designed for identification by means of understanding the immunochemical reaction between antigen-antibody complex that is formed during an immunological reaction between the analyte (antigen) and the reagent antibody (Kas et al. 1986). Immunoassays serve as a tool for analyzing the presence or concentration of an antigen. Initially the agglutination of the antigen-antibody complex was visualized followed by visible clumping of cells and antibodies (Burnet 1934; Pauling 1940), which would be discussed in the latter part of this chapter. This was fine-tuned with the establishment of radial immune diffusion assays commonly referred to as the double disk diffusion assays (Ouchterlony 1949). This immune diffusion is based on the principle that molecules with similar structure will migrate with similar rates through the agarose gel, without any restriction of movement of the molecules (Lam and Mutharia 1994). Double disk diffusion assay is used in the determination of minimum concentration of antigen required for precipitation of the antibody-antigen complex. Here the center part of the agarose gel consists of a well or disk with an antibody surrounding which antigens are placed at equal distances around the center forming a streak or band like precipitate or the precipitin line would be formed, ensuring the binding of antigen with the specific antibody. This type of double disk diffusion assay was used to identify various components of Escherichia coli like enzymes (Lee and Englesberg 1962), surface proteins (Guinee et al. 1976; Isaacson 1977), enterotoxins (Honda et al. 1981; Tsuji et al. 1985) and Shiga toxins (Oku et al. 1989).
Immunoassays work by the hydrophobic interaction of an antibody and antigen, where either component could be bound to hydrophobic surfaces to quantify the relationship between antigen and antibody. Immunoassays were developed further with multiple screening of samples in a microtiter plate (96-well plates). Upon binding of the antibodies to solid phase surfaces that are mostly hydrophobic, quantification of these antibodies could be done by attachment of labels to antibody which can be detected with a transducer. The important approaches include radio immuno assay, enzyme-linked immunosorbant assay and latex agglutination.
2.2.2.2.2.1 Radio Immunoassay
Radio immunoassay pioneered in incorporating labels (Lequin 2005) like the radioisotopes 125I and 3H were commonly used (Soergel et al. 1982; De Boever et al. 1983). Here the radio labelled antibodies are allowed to interact with the analytes and the screening was carried out with the help of a liquid scintillation counter, which quantifies the particles emitted due to radioactive decay (Chase 1980). It was then followed by the emergence and usage of non-radioactive labels like enzyme-conjugated chromophore systems that indirectly forms a detectable label in an immune assay reaction (Lequin 2005).
2.2.2.2.2.2 Enzyme-Linked Immunosorbent Assay
Several enzyme associated formats of immunoassays emerged with the successful implementation of enzyme labels for detection of antigens (Lequin 2005). Enzyme linked immunosorbent assay development could be seen as a way of improving the detection methods in clinical and public health setting, where cell culture or radio immune assay was commonly used (Downes et al. 1989). Enzyme linked immunosorbent assay has evolved into different forms for antigen detection and quantification as illustrated in the Fig. 2.9.
Illustration of Enzyme labeled immunosorbent assay (ELISA), (i) Direct ELISA - antigen adsorbed on plastic/microtiter plate binds to the enzyme-antibody complex added (ii) Indirect ELISA - the enzyme-antibody complex uses an antibody against the isotype of antibody that is used to detect the antigen (iii) Sandwich ELISA - antigens in sample bind to capture antibody followed by binding with monoclonal antibody and secondary antibody conjugated with enzyme, (iv) Competitive ELISA - more the antigen present in the sample, the less antibody will be present to bind to the antigen coated in the microtiter well, followed by the enzyme-antibody conjugate that binds the isotype of antibody that is used to detect the antigen; Addition of enzyme’s substrate produces colored product
A sandwich assay (Skinner et al. 2013) was developed based on enzyme-linked immunosorbent assay for detection of Escherichia coli producing Shiga toxin Stx2 subtype known as Stx2f. Although the Stx2f toxin was not involved in any critical human disease (Melton-Celsa 2014), the assay proved to be useful in detecting the presence of phages and plasmids in Escherichia coli isolates (Skinner et al. 2013). Prior to the development of immunoassay for the Stx2f toxin, monoclonal antibodies were generated by murine immunization with a Stx2f A subunit and fusion of the mice spleenocytes with the myeloma cells. It was followed by screening of antibodies for immune reactions with the antigen Stx2f toxin, where four unique antibodies (Stx2f-1, Stx2f-2, Stx2f-3 and Stx2f-4) were found to be specific for Stx2f toxin.
2.2.2.2.2.3 Latex Agglutination
This method uses latex beads immobilized with specific antibodies and is one of the widely used technique to sense the pathogens by their specificity towards antibodies (Hegde et al. 2012). It is the common technique employed in clinical laboratories for O antigen identification (Atkinson et al. 2012). Latex-bound antibodies generates complexes with any antigen present in the sample and forms visually detectable precipitates (Boer and Heuvelink 2000).
In addition, an optical immunoassay has been developed for visually identifying microorganisms. The BioStar optical immunoassay SHIGATOX kit (Inverness Medical Professional Diagnostics, Inc.) serves as a visual identification tool to detect the presence of shiga toxins Stx1 and Stx2, but the drawback is that it does not differentiate the two toxins (Teel et al. 2007). Here a mixture of anti-Stx antibodies (either Stx1 or Stx2 specific) is bound to a silicon wafer. The principle is based on the reflection of light from the silicon wafer, which appears gold in color when bare and purple as the thickness increases due to the binding of shiga toxins from the pathogen (Teel et al. 2007).
2.2.2.2.3 Antibiogram
Antibiogram was employed as a surveillance tool for the detection of early microbial growth. It was initially developed as a 96 well plate format which was later transferred to a portable, low cost point of care resazurin based polymethylmethacrylate microfluidic chip (Elavarasan et al. 2013) for live cells. Resazurin is a blue colored water soluble dye that undergoes two stages of reduction in the presence of viable cells (Fig. 2.10), first stage resulting in an irreversible pink colored resorufin, formed by loss of one oxygen atom and a reversible second stage resulting in colorless hydroresorufin (Sarker et al. 2007). It serves as an indicator of cell viability, growth and toxicity (Palomino et al. 2002). Here, in this immunoassay, blue color indicates the blank/antibiotic susceptibility of the microbial sample where there is no cell growth; pink/colorless indicates antibiotic resistance of the sample and violet coloration shows intermediate to poor growth of the sample due to partial/complete reduction of resazurin by cell growth. This method is used for testing contamination of milk with bacteria and detection of multi drug resistant microbes. This microfluidic chip can be used as a one-time disposable device and thereby no cross contamination.
Immunoassay based on Resazurin reduction reaction (Left), blue colored resazurin loses one oxygen atom in the presence of oxidoreductases in viable cells to form pink colored resorufin followed by further reduction to a colourless hydroresorufin. Resazurin dye reduction antibiogram in 96 well plate format (Right), blue color- blank/antibiotic susceptibility, pink/colorless- antibiotic resistance, violet- intermediate to poor growth of microbes in the sample
2.3 Conclusion
Researchers working on pathogenic biosensors focus mainly on lowering the time taken for detection, the limit of detection, the need for skilled labor and sample volume required for pathogen detection. Pathogen surveillance tools including microchips and other diagnostic kits, based on surface plasmon resonance or immunochemical reactions are remarkable. New trends involve the integrated use of micro and nano fabrication techniques along with sample enrichment using techniques like magnetic separation, followed by signal amplification and detection using various transducers in the area of biosensors for pathogen detection (Zuo et al. 2013; Kim et al. 2014; Hsieh et al. 2015; Altobelli et al. 2016). Progress has been witnessed in label free technologies like surface plasmon resonance/piezoelectric quartz crystal based optical, mechanical and biochemical biosensors, in spite of the improvements in label-based detection techniques which includes fluorophores, quantum/carbon dots based techniques. As a next step, these significant pathogen detection tools ought to be made available in the market for timely detection of pathogen s in no minute at a cheaper cost, thereby preventing epidemic conditions.
References
Adler C, Corbalan NS, Peralta DR, Pomares MF, de Cristóbal RE, Vincent PA (2014) The alternative role of enterobactin as an oxidative stress protector allows Escherichia coli colony development. PLoS One 9:e84734. https://doi.org/10.1371/journal.pone.0084734
Ahmed A, Rushworth JV, Hirst NA, Millner PA (2014) Biosensors for whole-cell bacterial detection. Clin Microbiol Rev 27:631–646. https://doi.org/10.1128/CMR.00120-13
Alagumaruthanayagam A, Sankaran K (2012) High-throughput fluorescence-based early antibiogram determination using clinical Escherichia coli isolates as case study. Microb Drug Resist 18:586–596. https://doi.org/10.1089/mdr.2011.0238
Alder JF, McCallum JJ (1983) Piezoelectric crystals for mass and chemical measurements. A review. Analyst 108:1169–1189. https://doi.org/10.1039/AN9830801169
Altobelli ER, Mohan KE, Mach ML, Sin Y, Anikst V, Buscarini M, Wong PK, Gau V, Banaei N, Liao JC (2016) Integrated biosensor assay for rapid uropathogen identification and phenotypic antimicrobial susceptibility testing. Eur Urol Focus. https://doi.org/10.1016/j.euf.2015.12.010
Ansari AA, Alhoshan M, Alsalhi MS, Aldwayyan AS (2010) Prospects of nanotechnology in clinical immunodiagnostics. Sensors 10:6535–6581. https://doi.org/10.3390/s100706535
Arlett J, Myers E, Roukes M (2011) Comparative advantages of mechanical biosensors. Nat Nanotechnol 6:203–215. https://doi.org/10.1038/nnano.2011.44
Arnau A (2008) A review of interface electronic systems for AT-cut quartz crystal microbalance applications in liquids. Sensors 8:370–411. https://doi.org/10.3390/s8010370
Arya SK, Singh A, Naidoo R, Wu P, McDermott MT, Evoy S (2011) Chemically immobilized T4-bacteriophage for specific Escherichia coli detection using surface plasmon resonance. Analyst 136:486–492. https://doi.org/10.1039/c0an00697a
Atkinson R, Besser J, Bopp C, Carlson C, Crandall C, George K, Gerner-Smidt P, Gladbach S, Gould LH and Hartley C (2012) Guidance for public health laboratories on the isolation and characterization of Shiga toxin-producing Escherichia coli (STEC) from clinical specimens. Silver Spring (MD): APHL:1–102
Banica FG (2012) Chemical sensors and biosensors: fundamentals and applications. Wiley. https://doi.org/10.1002/9781118354162
Bard AJ, Faulkner LR (2001) Fundamentals and applications. Electrochem Methods 2. 978-0-471-04372-0
Barlett P (2008) Bioelectrochemistry: fundamentals. Wiley, Experimental techniques and applications. 978-0-470-84364-2
Batt CA (2007) Food pathogen detection. Science 316:1579–1580. https://doi.org/10.1126/science.1140729
Benito-Peña E, Valdés MG, Glahn-Martínez B, Moreno-Bondi MC (2016) Fluorescence based fiber optic and planar waveguide biosensors. A review. Anal Chim Acta 943:17–40. https://doi.org/10.1016/j.aca.2016.08.049
Bereza-Malcolm LT, Mann GI, Franks AE (2014) Environmental sensing of heavy metals through whole cell microbial biosensors: a synthetic biology approach. ACS Synth Biol 4:535–546. https://doi.org/10.1021/sb500286r
Bhalla N, Jolly P, Formisano N, Estrela P (2016) Introduction to biosensors. Essays Biochem 60:1–8. https://doi.org/10.1042/EBC20150001
Blow M (2015) Streamlining a DNA-based biosensor detection system for the detection of non-PCR amplified genomic pathogenic DNA targets in food. 2015 annual meeting-international association for food protection, Portland, Oregon
Boer E, Heuvelink A (2000) Methods for the detection and isolation of Shiga toxin-producing Escherichia coli. J Appl Microbiol 88. doi:https://doi.org/10.1111/j.1365-2672.2000.tb05341.x
Borisov SM, Wolfbeis OS (2008) Optical biosensors. Chem Rev 108:423–461. https://doi.org/10.1021/cr068105t
Boyle M, Reis K (1987) Bacterial Fc receptors. Nature Biotechnol 5:697–703. https://doi.org/10.1038/nbt0787-697
Burnet F (1934) Antigenic differences between related bacterial strains: a criticism of the mosaic hypothesis. Br J Exp Pathol 15:354
Byrne B, Stack E, Gilmartin N, O’Kennedy R (2009) Antibody-based sensors: principles, problems and potential for detection of pathogens and associated toxins. Sensors 9:4407–4445. https://doi.org/10.3390/s90604407
Campbell GA, Mutharasan R (2005a) Detection of pathogen Escherichia coli O157: H7 using self-excited PZT-glass microcantilevers. Biosens Bioelectron 21:462–473. https://doi.org/10.1016/j.bios.2004.11.009
Campbell GA, Mutharasan R (2005b) Escherichia coli O157: H7 detection limit of millimeter-sized PZT cantilever sensors is 700 cells/mL. Anal Sci 21:355–357. https://doi.org/10.2116/analsci.21.355
Campbell GA, Mutharasan R (2006) Detection of Bacillus anthracis spores and a model protein using PEMC sensors in a flow cell at 1mL/min. BiosensBioelectron 22:78–85. https://doi.org/10.1016/j.bios.2005.12.002
Campbell GA, Mutharasan R (2007) A method of measuring Escherichia coli O157: H7 at 1 cell/mL in 1 liter sample using antibody functionalized piezoelectric-excited millimeter-sized cantilever sensor. Environ Sci Technol 41:1668–1674. https://doi.org/10.1021/es061947p
Chan WC, Nie S (1998) Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281:2016–2018. https://doi.org/10.1126/science.281.5385.2016
Chase GD (1980) Applications of liquid scintillation counting to radioimmunoassay. In: Peng C-T, Horrocks DL, Alpen EL (eds) Liquid scintillation counting, recent applications and development: sample preparation and applications, pp 489–502. ISBN:9780323146128
Chen A, Shah B (2013) Electrochemical sensing and biosensing based on square wave voltammetry. Anal Methods 5:2158–2173. https://doi.org/10.1039/C3AY40155C
Cho IH, Irudayaraj J (2013) In-situ immuno-gold nanoparticle network ELISA biosensors for pathogen detection. Int J Food Microbiol 164:70–75. https://doi.org/10.1016/j.ijfoodmicro.2013.02.025
Chua A, Yean CY, Ravichandran M, Lim B, Lalitha P (2011) A rapid DNA biosensor for the molecular diagnosis of infectious disease. Biosens Bioelectron 26:3825–3831. https://doi.org/10.1016/j.bios.2011.02.040
Compton RG and Banks CE (2011) Understanding voltammetry, World.Scientific. ISBN:978-1-84816-585-4.
Dale SE, Doherty-Kirby A, Lajoie G, Heinrichs DE (2004) Role of siderophore biosynthesis in virulence of Staphylococcus aureus: identification and characterization of genes involved in production of a siderophore. Infect Immun 72:29–37. https://doi.org/10.1128/IAI.72.1.29-37.2004
Dasgupta N, Ranjan S, Mundra S, Ramalingam C, Kumar A (2015) Fabrication of food grade vitamin E nanoemulsion by low energy approach, characterization and its application. Int J Food Prop 19(3):700–708
Dasgupta N, Ranjan S, Ramalingam C (2017) Applications of nanotechnology in agriculture and water quality management. Environ Chem Lett 15(4):591–605
Davis JJ, Green ML, Hill HAO, Leung YC, Sadler PJ, Sloan J, Xavier AV, Tsang SC (1998) The immobilisation of proteins in carbon nanotubes. Inorg Chim Acta 272:261–266. https://doi.org/10.1016/S0020-1693(97)05926-4
De Boever J, Kohen F, Vandekerckhove D (1983) Solid-phase chemiluminescence immunoassay for plasma estradiol-17 beta during gonadotropin therapy compared with two radioimmunoassays. Clin Chem 29:2068–2072
Deakin MR, Buttry DA (1989) Electrochemical applications of the quartz crystal microbalance. Anal Chem 61:1147A–1154A. https://doi.org/10.1021/ac00195a001
Demchenko AP (2008) Introduction to fluorescence sensing. Springer Science & Business Media. ISBN:978-1-4020-9002-8.
Deng S, Upadhyayula VK, Smith GB, Mitchell MC (2008) Adsorption equilibrium and kinetics of microorganisms on single-wall carbon nanotubes. IEEE Sensors J 8:954–962. https://doi.org/10.1109/JSEN.2008.923929
Dostálek J, Kasry A, Knoll W (2007) Long range surface plasmons for observation of biomolecular binding events at metallic surfaces. Plasmonics 2:97–106. https://doi.org/10.1007/s11468-007-9037-8
Downes FP, Green J, Greene K, Strockbine N, Wells J, Wachsmuth I (1989) Development and evaluation of enzyme-linked immunosorbent assays for detection of shiga-like toxin I and shiga-like toxin II. J Clin Microbiol 27:1292–1297
Elavarasan T, Chhina SK, Parameswaran M, Sankaran K (2013) Resazurin reduction based colorimetric antibiogram in microfluidic plastic chip. Sensor Actuat B: Chem 176:174–180. https://doi.org/10.1016/j.snb.2012.10.011
Farahi R, Passian A, Tetard L, Thundat T (2012) Critical issues in sensor science to aid food and water safety. ACS Nano 6:4548–4556. https://doi.org/10.1021/nn204999j
Farka Z, Kovář D, Přibyl J, Skládal P (2013) Piezoelectric and surface plasmon resonance biosensors for Bacillus atrophaeus spores. Int J Electrochem Sci 8:100–112
Farka ZK, Juřík TS, Pastucha MJ, andSkládal P (2016) Enzymatic precipitation enhanced surface Plasmon resonance Immunosensor for the detection of salmonella in powdered milk. Anal Chem 88:11830–11836. https://doi.org/10.1021/acs.analchem.6b03511
Fawcett NC, Evans JA, Chen LC, Drozda KA, Flowers AN (1988) A quartz crystal detector for DNA. Anal Lett 21:1099–1110
Felice CJ, Madrid RE, Olivera JM, Rotger VI, Valentinuzzi ME (1999) Impedance microbiology: quantification of bacterial content in milk by means of capacitance growth curves. J Microbiol Methods 35:37–42. https://doi.org/10.1016/S0167-7012(98)00098-0
Fritz J (2008) Cantilever biosensors. Analyst 133:855–863. https://doi.org/10.1039/B718174D
Fukal L, Kas J (1989) The advantages of immunoassay in food analysis. TrAC Trends Anal Chem 8:112–116. https://doi.org/10.1016/0165-9936(89)85010-1
Gómez R, Bashir R, Bhunia AK (2002) Microscale electronic detection of bacterial metabolism. Sensor Actuat B: Chem 86:198–208. https://doi.org/10.1016/S0925-4005(02)00175-2
Gonsalves MD, Sakr Y (2010) Early identification of sepsis. Curr Infect Dis Rep 12:329–335. https://doi.org/10.1007/s11908-010-0122-3
Gracias KS, McKillip JL (2004) A review of conventional detection and enumeration methods for pathogenic bacteria in food. Can J Microbiol 50:883–890. https://doi.org/10.1139/w04-080
Grieshaber D, MacKenzie R, Voeroes J, Reimhult E (2008) Electrochemical biosensors-sensor principles and architectures. Sensors 8:1400–1458. https://doi.org/10.3390/s80314000
Guilbault GG (1990) Practical fluorescence. Press, CRC. 9780824783501
Guinée P, Jansen W, Agterberg C (1976) Detection of the K99 antigen by means of agglutination and immunoelectrophoresis in Escherichia coli isolates from calves and its correlation with entertoxigenicity. Infect Immun 13:1369–1377
He F, Zhao J, Zhang L, Su X (2003) A rapid method for determining Mycobacterium tuberculosis based on a bulk acoustic wave impedance biosensor. Talanta 59:935–941. https://doi.org/10.1016/S0039-9140(02)00643-4
He F, Ren J, Liu Z (2007) The study and application of a new IDE–PQC sensor. Sensor Actuat B Chem 123:1057–1063. https://doi.org/10.1016/j.snb.2006.11.017
He F, Xiong Y, Liu J, Tong F, Yan D (2016) Construction of Au-IDE/CFP10-ESAT6 aptamer/DNA-AuNPs MSPQC for rapid detection of Mycobacterium tuberculosis. Biosens Bioelectron 77:799–804. https://doi.org/10.1016/j.bios.2015.10.054
Hegde NV, Cote R, Jayarao BM, Muldoon M, Lindpaintner K, Kapur V, DebRoy C (2012) Detection of the top six non-O157 Shiga toxin–producing Escherichia coli O Groups by ELISA. Foodborne Pathog Dis 9:1044–1048. https://doi.org/10.1089/fpd.2012.1231
Himpsl SD, Pearson MM, Arewang CJ, Nusca TD, Sherman DH, Mobley HL (2010) Proteobactin and a yersiniabactin-related siderophore mediate iron acquisition in Proteus mirabilis. Mol Microbiol 78:138–157. https://doi.org/10.1111/j.1365-2958.2010.07317.x
Homola J (2003) Present and future of surface plasmon resonance biosensors. Anal Bioanal Chem 377:528–539. https://doi.org/10.1007/s00216-003-2101-0
Honda T, Tsuji T, Takeda Y, Miwatani T (1981) Immunological nonidentity of heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect Immun 34:337–340
Housewright RD, Glassman HN (1966) Concluding remarks of the second international conference on aerobiology (airborne infection). Bacteriol Rev 30:696
Hsieh K, Ferguson BS, Eisenstein M, Plaxco KW, Soh HT (2015) Integrated electrochemical microsystems for genetic detection of pathogens at the point of care. Acc Chem Res 48:911–920. https://doi.org/10.1021/ar500456w
Hsin Chang Y, Chang TC, Kao EF, Chou C (1996) Detection of protein A produced by Staphylococcus aureus with a fiber-optic-based biosensor. Biosci Biotechnol Biochem 60:1571–1574. https://doi.org/10.1271/bbb.60.1571
Hu RR, Yin ZZ, Zeng YB, Zhang J, Liu HQ, Shao Y, Ren SB, Li L (2016) A novel biosensor for Escherichia coli O157: H7 based on fluorescein-releasable biolabels. Biosens Bioelectron 78:31–36. https://doi.org/10.1016/j.bios.2015.11.018
Huang CJ, Dostalek J, Sessitsch A, Knoll S (2011) Long-range surface plasmon-enhanced fluorescence spectroscopy biosensor for ultrasensitive detection of E. Coli O157: H7. Anal Chem 83:674–677. https://doi.org/10.1021/ac102773r
Isaacson R (1977) K99 surface antigen of Escherichia coli: purification and partial characterization. Infect Immun 15:272–279
Itoh A, Ichihashi M (2008) A frequency of the quartz crystal microbalance (QCM) that is not affected by the viscosity of a liquid. Meas Sci Technol 19:075205. https://doi.org/10.1088/0957-0233/19/7/075205
Jain A, Ranjan S, Dasgupta N, Ramalingam C (2016) Nanomaterials in food and agriculture: an overview on their safety concerns and regulatory issues. Crit Rev Food Sci Nutr 15:1–21
Jang HD, Chang KS, Lee YG, Lee SJ and Hsu CL (2009) Quantitative determination of Lactobacillus spp. in Milk using a series piezoelectric quartz crystal sensor, vol 229. European Food Research and Technology, pp 349–355. doi:https://doi.org/10.1007/s00217-009-1047-7
Jans H, Huo Q (2012) Gold nanoparticle-enabled biological and chemical detection and analysis. Chem Soc Rev 41:2849–2866. https://doi.org/10.1039/C1CS15280G.
Ji HF, Yang X, Zhang J, Thundat T (2004) Molecular recognition of biowarfare agents using micromechanical sensors. Expert Rev Mol Diagn 4:859–866. https://doi.org/10.1586/14737159.4.6.859
Jordana-Lluch E, Carolan HE, Giménez M, Sampath R, Ecker DJ, Quesada MD, Mòdol JM, Arméstar F, Blyn LB, Cummins LL (2013) Rapid diagnosis of bloodstream infections with PCR followed by mass spectrometry. PLoS One 8:e62108. https://doi.org/10.1371/journal.pone.0062108
Kallioniemi OP, Kallioniemi A, Kurisu W, Thor A, Chen LC, Smith HS, Waldman FM, Pinkel D, Gray JW (1992) ERBB2 amplification in breast cancer analyzed by fluorescence in situ hybridization. Proc Natl Acad Sci 89:5321–5325. https://doi.org/10.1073/pnas.89.12.5321
Kamali KZ, Pandikumar A, Sivaraman G, Lim HN, Wren SP, Sun T, Huang NM (2015) Silver@ graphene oxide nanocomposite-based optical sensor platform for biomolecules. RSC Adv 5:17809–17816. https://doi.org/10.1039/C4RA11356J
Kang S, Pinault M, Pfefferle LD, Elimelech M (2007) Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 23:8670–8673. https://doi.org/10.1021/la701067r
Kas J, Fukal L, Rauch P (1986) Immunochemical assay of enzymes. TrAC Trends Anal Chem 5:205–209. https://doi.org/10.1016/0165-9936(86)80014-0
Kim TH, Park J, Kim CJ, Cho YK (2014) Fully integrated lab-on-a-disc for nucleic acid analysis of food-borne pathogens. Anal Chem 86:3841–3848. https://doi.org/10.1021/ac403971h
Kirsch J, Siltanen C, Zhou Q, Revzin A, Simonian A (2013) Biosensor technology: recent advances in threat agent detection and medicine. Chem Soc Rev 42:8733–8768. https://doi.org/10.1039/c3cs60141b
Ko S, Grant SA (2006) A novel FRET-based optical fiber biosensor for rapid detection of Salmonella typhimurium. Biosens Bioelectron 21:1283–1290. https://doi.org/10.1016/j.bios.2005.05.017
Kuila T, Bose S, Khanra P, Mishra AK, Kim NH, Lee JH (2011) Recent advances in graphene-based biosensors. Biosens Bioelectron 26:4637–4648. https://doi.org/10.1016/j.bios.2011.05.039
Lakshmanan RS, Efremov V, Cullen SM, Killard AJ (2014) Measurement of the evolution of rigid and viscoelastic mass contributions from fibrin network formation during plasma coagulation using quartz crystal microbalance. Sensor Actuat B: Chemical 192:23–28. https://doi.org/10.1016/j.snb.2013.10.081
Lam JS, Mutharia LM (1994) Antigen-antibody reactions. Methods for general and molecular bacteriology. American Society for Microbiology, Washington, DC, pp 104–132. https://doi.org/10.1128/9781555817497.ch08
Lee N, Englesberg E (1962) Dual effects of structural genes in Escherichia coli. Proc Natl Acad Sci 48:335–348
Lee K, Irudayaraj J (2009) Periodic and dynamic 3-D gold nanoparticle-DNA network structures for surface-enhanced Raman spectroscopy-based quantification. J Phys Chem C 113:5980–5983. https://doi.org/10.1021/jp809949v
Lee KR, Kang IJ (2009) Effects of dopamine concentration on energy transfer between dendrimer–QD and dye-labeled antibody. Ultramicroscopy 109:894–898. https://doi.org/10.1016/j.ultramic.2009.03.012
Lee K, Drachev VP, Irudayaraj J (2011) DNA-gold nanoparticle reversible networks grown on cell surface marker sites: application in diagnostics. ACS Nano 5:2109–2117. https://doi.org/10.1021/nn1030862
Lequin RM (2005) Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin Chem 51:2415–2418. https://doi.org/10.1373/clinchem.2005.051532
Li G, Li X, Wan J, Zhang S (2009) Dendrimers-based DNA biosensors for highly sensitive electrochemical detection of DNA hybridization using reporter probe DNA modified with Au nanoparticles. Biosens Bioelectron 24:3281–3287. https://doi.org/10.1016/j.bios.2009.04.022
Li B, Yu Q, Duan Y (2015) Fluorescent labels in biosensors for pathogen detection. Crit Rev Biotechnol 35:82–93. https://doi.org/10.3109/07388551.2013.804487
Lian Y, He F, Wang H, Tong F (2015) A new aptamer/graphene interdigitated gold electrode piezoelectric sensor for rapid and specific detection of Staphylococcus aureus. Biosens Bioelectron 65:314–319. https://doi.org/10.1016/j.bios.2014.10.017
Liedberg B, Nylander C, Lundström I (1995) Biosensing with surface plasmon resonance—how it all started. Biosens Bioelectron 10:i–ix. doi:https://doi.org/10.1016/0956-5663(95)96965-2
Ligler FS, Taitt CR (2008) Fluorescence lifetime biosensing: entering the mainstream. Optical Biosensors: Today and Tomorrow 287. 978-0-444-53125-4
Lin YH, Chen SH, Chuang YC, YC L, Shen TY, Chang CA, Lin CS (2008) Disposable amperometric immunosensing strips fabricated by Au nanoparticles-modified screen-printed carbon electrodes for the detection of foodborne pathogen Escherichia coli O157: H7. Biosens Bioelectron 23:1832–1837. https://doi.org/10.1016/j.bios.2008.02.030
Liu Y, Dong X, Chen P (2012) Biological and chemical sensors based on graphene materials. Chem Soc Rev 41:2283–2307. https://doi.org/10.1039/C1CS15270J
Liu X, Hu Y, Zheng S, Liu Y, He Z, Luo F (2016a) Surface plasmon resonance immunosensor for fast, highly sensitive, and in situ detection of the magnetic nanoparticles-enriched Salmonella enteritidis. Sensor Actuat B Chem 230:191–198. https://doi.org/10.1016/j.snb.2016.02.043
Liu X, Marrakchi M, Xu D, Dong H, Andreescu S (2016b) Biosensors based on modularly designed synthetic peptides for recognition, detection and live/dead differentiation of pathogenic bacteria. Biosens Bioelectron 80:9–16. https://doi.org/10.1016/j.bios.2016.01.041
Melton-Celsa AR (2014) Shiga toxin (Stx) classification, structure, and function. Microbiol Spectr 2. https://doi.org/10.1128/microbiolspec.EHEC-0024-2013
Menti C, Henriques JA, Missell FP, Roesch-Ely M (2016) Antibody-based magneto-elastic biosensors: potential devices for detection of pathogens and associated toxins. Appl Microbiol Biotechnol 100:6149–6163. https://doi.org/10.1007/s00253-016-7624-3
Merkoçi A (2010) Nanoparticles-based strategies for DNA, protein and cell sensors. Biosens Bioelectron 26:1164–1177. https://doi.org/10.1016/j.bios.2010.07.028
Monis PTand Giglio S (2006) Nucleic acid amplification-based techniques for pathogen detection and identification. Infect Genet Evol 6:2–12. https://doi.org/10.1016/j.meegid.2005.08.004
Monzo J, Insua I, Fernandez-Trillo F, Rodriguez P (2015) Fundamentals, achievements and challenges in the electrochemical sensing of pathogens. Analyst 140:7116–7128. https://doi.org/10.1039/c5an01330e
Mujumdar RB, Ernst LA, Mujumdar SR, Lewis CJ, Waggoner AS (1993) Cyanine dye labeling reagents: sulfoindocyanine succinimidyl esters. Bioconjug Chem 4:105–111. https://doi.org/10.1021/bc00020a001
Nguyen TT, Trinh KTL, Yoon WJ, Lee NY, Ju H (2017) Integration of a microfluidic polymerase chain reaction device and surface plasmon resonance fiber sensor into an inline all-in-one platform for pathogenic bacteria detection. Sensor Actuat B Chem 242:1–8. https://doi.org/10.1016/j.snb.2016.10.137
Nugen SR, Baeumner A (2008) Trends and opportunities in food pathogen detection. Anal Bioanal Chem 391:451. https://doi.org/10.1007/s00216-008-1886-2
Oh BK, Lee W, Chun BS, Bae YM, Lee WH, Choi JW (2005) The fabrication of protein chip based on surface plasmon resonance for detection of pathogens. Biosens Bioelectron 20:1847–1850. https://doi.org/10.1016/j.bios.2004.05.010
Oku Y, Yutsudo T, Hirayama T, O'Brien AD, Takeda Y (1989) Purification and some properties of a Vero toxin from a human strain of Escherichia coli that is immunologically related to Shiga-like toxin II (VT2). Microb Pathog 6:113–122. https://doi.org/10.1016/0882-4010(89)90014-4
Ouchterlony O (1949) Antigen-antibody reactions in gels. Acta Pathologica Microbiologica Scandinavica 26:507–515. https://doi.org/10.1111/j.1699-0463.1949.tb00751.x
Palomino JC, Martin A, Camacho M, Guerra H, Swings J, Portaels F (2002) Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 46:2720–2722. https://doi.org/10.1016/j.ejcdt.2013.05.008
Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, Bhalgat MK, Millard PJ, Mao F, Leung WY, Haugland RP (1999) Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J Histochem Cytochem 47:1179–1188. https://doi.org/10.1177/002215549904700910
Pastells C, Pascual N, Sanchez-Baeza F, Marco MP (2016) Immunochemical determination of pyocyanin and 1-hydroxyphenazine as potential biomarkers of Pseudomonas aeruginosa infections. Anal Chem 88:1631–1638. https://doi.org/10.1021/acs.analchem.5b03490.
Pauling L (1940) A theory of the structure and process of formation of antibodies. J Am Chem Soc 62:2643–2657. https://doi.org/10.1021/ja01867a018.
Philip A, Odabasi Z, Matiuzzi G, Paetznick VL, Tan SW, Warmington J, Rex JH, Ostrosky-Zeichner L (2005) Syscan3, a kit for detection of anti-Candida antibodies for diagnosis of invasive candidiasis. J Clin Microbiol 43:4834–4835. https://doi.org/10.1128/JCM.43.9.4834-4835.2005
Pi S, Zeng X, Zhang N, Ji D, Chen B, Song H, Cheney A, Xu Y, Jiang S, Sun D (2016) Dielectric-grating-coupled surface plasmon resonance from the back side of the metal film for ultrasensitive sensing. IEEE Photon J 8:1–6. https://doi.org/10.1109/JPHOT.2015.2509870
Ranjan S, Dasgupta N, Chakraborty AR, Melvin Samuel S, Ramalingam C, Shanker R, Kumar A (2014) Nanoscience and nanotechnologies in food industries: opportunities and research trends. J Nanopart Res 16(6):1–23
Ren J, He F, Yi S, Cui X (2008) A new MSPQC for rapid growth and detection of Mycobacterium tuberculosis. Biosens Bioelectron 24:403–409. https://doi.org/10.1016/j.bios.2008.04.018
Ren J, He F, Zhang L (2010) The construction and application of a new PPY-MSPQC for l-asparaginase activity assay. Sensor Actuat B Chem 145:272–277. https://doi.org/10.1016/j.snb.2009.12.006
Renshaw PS, Panagiotidou P, Whelan A, Gordon SV, Hewinson RG, Williamson RA, Carr MD (2002) Conclusive evidence that the major T-cell antigens of the mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1: 1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6· CFP-10 complex implications for pathogenesis and virulence. J Biol Chem 277:21598–21603. https://doi.org/10.1074/jbc.M201625200
Rifat AA, Mahdiraji GA, Sua YM, Shee YG, Ahmed R, Chow DM, Adikan FRM (2015) Surface Plasmon resonance photonic crystal fiber biosensor: a practical sensing approach. IEEE Photon Technol Lett 27:1628–1631. https://doi.org/10.1109/LPT.2015.2432812
Rosenthal SJ (2001) Bar-coding biomolecules with fluorescent nanocrystals. Nature Biotechnol 19:621–623. https://doi.org/10.1038/90213
Rosi NL, Mirkin CA (2005) Nanostructures in biodiagnostics. Chem Rev 105:1547–1562. https://doi.org/10.1021/cr030067f
Rowe-Taitt CA, Golden JP, Feldstein MJ, Cras JJ, Hoffman KE, Ligler FS (2000) Array biosensor for detection of biohazards. Biosens Bioelectron 14:785–794. https://doi.org/10.1016/S0956-5663(99)00052-4
Sang S, Wang Y, Feng Q, Wei Y, Ji J, Zhang W (2016) Progress of new label-free techniques for biosensors: a review. Crit Rev Biotechnol 36:465–481. https://doi.org/10.3109/07388551.2014.991270
Sankaranarayanan R, Alagumaruthanayagam A, Sankaran K (2015) A new fluorimetric method for the detection and quantification of siderophores using Calcein Blue, with potential as a bacterial detection tool. Appl Microbiol Biotechnol 99:2339–2349. https://doi.org/10.1007/s00253-015-6411-x
Sarker SD, Nahar L, Kumarasamy Y (2007) Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42:321–324. https://doi.org/10.1016/j.ymeth.2007.01.006
Schena M, Shalon D, Davis RW, Brown PO (1995) Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270:467. https://doi.org/10.1126/science.270.5235.467
Scognamiglio V, Arduini F, Palleschi G, Rea G (2014) Biosensing technology for sustainable food safety. TrAC Trends Anal Chem 62:1–10. https://doi.org/10.1016/j.trac.2014.07.007
Shao Y, Wang J, Wu H, Liu J, Aksay IA, Lin Y (2010) Graphene based electrochemical sensors and biosensors: a review. Electroanalysis 22:1027–1036. https://doi.org/10.1002/elan.200900571
Sharma H, Mutharasan R (2013) Rapid and sensitive immunodetection of Listeria monocytogenes in milk using a novel piezoelectric cantilever sensor. Biosens Bioelectron 45:158–162. https://doi.org/10.1016/j.bios.2013.01.068
Sheikhzadeh E, Chamsaz M, Turner A, Jager E, Beni V (2016) Label-free impedimetric biosensor for Salmonella typhimurium detection based on poly [pyrrole-co-3-carboxyl-pyrrole] copolymer supported aptamer. Biosens Bioelectron 80:194–200. https://doi.org/10.1016/j.bios.2016.01.057
Shen D, Zhu W, Nie L, Yao S (1993) Behaviour of a series piezoelectric sensor in electrolyte solution: part I. Theory. Anal Chim Acta 276:87–97. https://doi.org/10.1016/0003-2670(93)85042-I
Shen J, Zhu Y, Yang X, Li C (2012) Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem Commun 48:3686–3699. https://doi.org/10.1039/C2CC00110A
Shi X, Zhang X, Yao Q, He F (2017) A novel method for the rapid detection of microbes in blood using pleurocidin antimicrobial peptide functionalized piezoelectric sensor. J Microbiol Methods 133:69–75. https://doi.org/10.1016/j.mimet.2016.12.005
Shukla A, Dasgupta N, Ranjan S, Singh S, Chidambram R (2017) Nanotechnology towards prevention of anaemia and osteoporosis: from concept to market. Biotechnol Biotechnol Equip 31(5):863–879
Sign C, Sumana G (2016) Antibody conjugated graphene nanocomposites for pathogen detection. J Phys Conf Ser IOP Publishing. https://doi.org/10.1088/1742-6596/704/1/012014
Skinner C, Patfield S, Stanker L, He X (2013) Development of monoclonal antibodies and immunoassays for sensitive and specific detection of Shiga toxin Stx2f. PLoS One 8:e76563. https://doi.org/10.1371/journal.pone.0076563
Soergel ME, Schaffer FL, Blank HF (1982) Solid-phase radioimmunoassay for detection of plague antigen in animal tissue. J Clin Microbiol 16:953–956
Srivastava A, Srivastava O, Talapatra S, Vajtai R, Ajayan P (2004) Carbon nanotube filters. Nat Mater 3:610–614. https://doi.org/10.1038/nmat1192
Sungkanak U, Sappat A, Wisitsoraat A, Promptmas C, Tuantranont A (2010) Ultrasensitive detection of Vibrio cholerae O1 using microcantilever-based biosensor with dynamic force microscopy. Biosens Bioelectron 26:784–789. https://doi.org/10.1016/j.bios.2010.06.024
Sutherland AJ (2002) Quantum dots as luminescent probes in biological systems. Curr Opin Solid State Mater Sci 6:365–370. https://doi.org/10.1016/S1359-0286(02)00081-5
Taitt CR, Anderson GP, Ligler FS (2016) Evanescent wave fluorescence biosensors: advances of the last decade. Biosens Bioelectron 76:103–112. https://doi.org/10.1016/j.bios.2015.07.040
Taneja NK, Tyagi JS (2007) Resazurin reduction assays for screening of anti-tubercular compounds against dormant and actively growing Mycobacterium tuberculosis, Mycobacterium bovis BCG and Mycobacterium smegmatis. J Antimicrob Chemother 60:288–293. https://doi.org/10.1093/jac/dkm207
Teel LD, Daly JA, Jerris RC, Maul D, Svanas G, O’Brien AD, Park CH (2007) Rapid detection of Shiga toxin-producing Escherichia coli by optical immunoassay. J Clin Microbiol 45:3377–3380. https://doi.org/10.1128/JCM.00837-07
Teo SC, Wong LS (2014) Whole cell-based biosensors for environmental heavy metals detection. Annual Res Rev Biol 4:2663–2674. https://doi.org/10.1016/S0003-2670(98)00725-9
Thakur B, Amarnath CA, Mangoli S, Sawant SN (2015) Polyaniline nanoparticle based colorimetric sensor for monitoring bacterial growth. Sensor Actuat B Chem 207:262–268. https://doi.org/10.1016/j.snb.2014.10.045
Tong F, Lian Y, Zhou H, Shi X, He F (2014) Multichannel series piezoelectric quartz crystal cell sensor for real time and quantitative monitoring of the living cell and assessment of cytotoxicity. Anal Chem 86:10415–10421. https://doi.org/10.1021/ac502926k
Tsuji T, Honda T, Miwatani T, Wakabayashi S, Matsubara H (1985) Analysis of receptor-binding site in Escherichia coli enterotoxin. J Biol Chem 260:8552–8558
Upadhyayula VK, Deng S, Smith GB, Mitchell MC (2009) Adsorption of Bacillus subtilis on single-walled carbon nanotube aggregates, activated carbon and NanoCeram™. Water Res 43:148–156. https://doi.org/10.1016/j.watres.2008.09.023
Vala M, Etheridge S, Roach J, Homola J (2009) Long-range surface plasmons for sensitive detection of bacterial analytes. Sensor Actuat B Chem 139:59–63. https://doi.org/10.1016/j.snb.2008.08.029
Vinayaka A, Thakur M (2010) Focus on quantum dots as potential fluorescent probes for monitoring food toxicants and foodborne pathogens. Anal Bioanal Chem 397:1445–1455. https://doi.org/10.1007/s00216-010-3683-y
Waggoner A (2006) Fluorescent labels for proteomics and genomics. Curr Opin Chem Biol 10:62–66. https://doi.org/10.1016/j.cbpa.2006.01.005
Wan Y, Lin Z, Zhang D, Wang Y, Hou B (2011) Impedimetric immunosensor doped with reduced graphene sheets fabricated by controllable electrodeposition for the non-labelled detection of bacteria. Biosens Bioelectron 26:1959–1964. https://doi.org/10.1016/j.bios.2010.08.008
Wang Y, Alocilja EC (2015) Gold nanoparticle-labeled biosensor for rapid and sensitive detection of bacterial pathogens. J Biol Eng 9:16. https://doi.org/10.1186/s13036-015-0014-z
Wang Z, Zhang J, Chen P, Zhou X, Yang Y, Wu S, Niu L, Han Y, Wang L, Boey F (2011) Label-free, electrochemical detection of methicillin-resistant staphylococcus aureus DNA with reduced graphene oxide-modified electrodes. Biosens Bioelectron 26:3881–3886. https://doi.org/10.1016/j.bios.2011.03.002
Wang Y, Knoll W, Dostalek J (2012a) Bacterial pathogen surface plasmon resonance biosensor advanced by long range surface plasmons and magnetic nanoparticle assays. Anal Chem 84:8345–8350. https://doi.org/10.1021/ac301904x
Wang Y, Ye Z, Ying Y (2012b) New trends in impedimetric biosensors for the detection of foodborne pathogenic bacteria. Sensors 12:3449–3471. https://doi.org/10.3390/s120303449
Wang R, Xiang Y, Zhou X, Liu LH, Shi H (2015) A reusable aptamer-based evanescent wave all-fiber biosensor for highly sensitive detection of Ochratoxin A. Biosens Bioelectron 66:11–18. https://doi.org/10.1016/j.bios.2014.10.079
Ward AC, Connolly P, Tucker NP (2014) Pseudomonas aeruginosa can be detected in a polymicrobial competition model using impedance spectroscopy with a novel biosensor. PLoS One 9:e91732. https://doi.org/10.1371/journal.pone.0091732
Wei D, Bailey MJ, Andrew P, Ryhanen T (2009) Electrochemical biosensors at the nanoscale. Lab Chip 9:2123–2131. https://doi.org/10.1039/B903118A
Wells RM, Jones CM, Xi Z, Speer A, Danilchanka O, Doornbos KS, Sun P, Wu F, Tian C, Niederweis M (2013) Discovery of a siderophore export system essential for virulence of Mycobacterium tuberculosis. PLoS Pathog 9:e1003120. https://doi.org/10.1371/journal.ppat.1003120.
White NS, Errington RJ (2005) Fluorescence techniques for drug delivery research: theory and practice. Adv Drug Deliv Rev 57:17–42. https://doi.org/10.1016/j.addr.2004.08.003
Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, Ge N, Peale R, Bruchez MP (2003) Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nature Biotechnol 21:41–46. https://doi.org/10.1038/nbt764
Xing Y, Chaudry Q, Shen C, Kong KY, Zhau HE, Chung LW, Petros JA, O'regan RM, Yezhelyev MV, Simons JW (2007) Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nat Protoc 2:1152–1165. https://doi.org/10.1038/nprot.2007.107
Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, Scrivens WA (2004) Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126:12736–12737. https://doi.org/10.1021/ja040082h
Xu M, Wang R, Li Y (2017) Electrochemical biosensors for rapid detection of Escherichia coli O157:H7. Talanta 162:511–522. https://doi.org/10.1016/j.talanta.2016.10.050
Yanase Y, Hiragun T, Ishii K, Kawaguchi T, Yanase T, Kawai M, Sakamoto K, Hide M (2014) Surface plasmon resonance for cell-based clinical diagnosis. Sensors 14:4948–4959. https://doi.org/10.3390/s140304948
Yang L, Li Y (2006) Simultaneous detection of Escherichia coli O157∶ H7 and Salmonella typhimurium using quantum dots as fluorescence labels. Analyst 131:394–401. https://doi.org/10.1039/B510888H
Yu X, Xia HS, Sun ZD, Lin Y, Wang K, Yu J, Tang H, Pang DW, Zhang ZL (2013) On-chip dual detection of cancer biomarkers directly in serum based on self-assembled magnetic bead patterns and quantum dots. Biosens Bioelectron 41:129–136. https://doi.org/10.1016/j.bios.2012.08.007
Yu Q, Wang X, Duan Y (2014) Capillary-based three-dimensional immunosensor assembly for high-performance detection of carcinoembryonic antigen using laser-induced fluorescence spectrometry. Anal Chem 86:1518–1524. https://doi.org/10.1021/ac402973n
Zawadzka AM, Kim Y, Maltseva N, Nichiporuk R, Fan Y, Joachimiak A, Raymond KN (2009) Characterization of a Bacillus subtilis transporter for petrobactin, an anthrax stealth siderophore. Proc Natl Acad Sci 106:21854–21859. https://doi.org/10.1073/pnas.0904793106
Zhang J, Hai-Feng J (2004) An anti E. coli O157: H7 antibody-immobilized microcantilever for the detection of Escherichia coli (E. coli). Anal Sci 20:585–587. https://doi.org/10.2116/analsci.20.585
Zhang J, Su X, O'shea S (2002) Antibody/antigen affinity behavior in liquid environment with electrical impedance analysis of quartz crystal microbalances. Biophys Chem 99:31–41. https://doi.org/10.1016/S0301-4622(02)00109-6
Zhang Y, Zeng Q, Sun Y, Liu X, Tu L, Kong X, Buma WJ, Zhang H (2010) Multi-targeting single fiber-optic biosensor based on evanescent wave and quantum dots. Biosens Bioelectron 26:149–154. https://doi.org/10.1016/j.bios.2010.05.032.
Zhang D, Yan Y, Li Q, Yu T, Cheng W, Wang L, Ju H, Ding S (2012) Label-free and high-sensitive detection of salmonella using a surface plasmon resonance DNA-based biosensor. J Biotechnol 160:123–128. https://doi.org/10.1016/j.jbiotec.2012.03.024
Zhang H, Zhang H, Aldalbahi A, Zuo X, Fan C, Mi X (2017) Fluorescent biosensors enabled by graphene and graphene oxide. Biosens Bioelectron 89:96–106. https://doi.org/10.1016/j.bios.2016.07.030
Zheng J, Lin L, Cheng G, Wang A, Tan X, He P, Fang Y (2007) Study on an electrochemical biosensor for thrombin recognition based on aptamers and nano particles. Sci China Ser B Chem 50:351–357. https://doi.org/10.1007/s11426-007-0062-4
Zheng Q, Wu H, Wang N, Yan R, Ma Y, Guang W, Wang J, Ding K (2014) Graphene-based biosensors for biomolecules detection. Curr Nanosci 10:627–637. https://doi.org/10.2174/1573413710666140422231701
Zhu Q, Shih WY, Shih WH (2007) In situ, in-liquid, all-electrical detection of Salmonella typhimurium using lead titanate zirconate/gold-coated glass cantilevers at any dipping depth. Biosens Bioelectron 22:3132–3138. https://doi.org/10.1016/j.bios.2007.02.005
Zuo P, Li X, Dominguez DC, Ye BC (2013) A PDMS/paper/glass hybrid microfluidic biochip integrated with aptamer-functionalized graphene oxide nano-biosensors for one-step multiplexed pathogen detection. Lab Chip 13:3921–3928. https://doi.org/10.1039/c3lc50654a
Acknowledgement
The authors gratefully acknowledge VIT University, Vellore for the support through Seed Grant for Research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
J, B., Chanda, K., MM, B. (2018). Physical, Chemical and Biochemical Biosensors to Detect Pathogens. In: Gothandam, K., Ranjan, S., Dasgupta, N., Ramalingam, C., Lichtfouse, E. (eds) Nanotechnology, Food Security and Water Treatment. Environmental Chemistry for a Sustainable World. Springer, Cham. https://doi.org/10.1007/978-3-319-70166-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-70166-0_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-70165-3
Online ISBN: 978-3-319-70166-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)