Abstract
Sarcocystis (derived from the Greek words sarx, which means flesh, and kystis, which means bladder) are apicomplexan protozoans that cause sarcocystosis or sarcocystiosis. Infections are characterized by the formation of numerous sarcocysts, which are essentially parasite-full sacs ranging in size from micrometers to several centimeters, in the muscles or nervous tissue of a great variety of animals. The genus is composed of more than 100 species that differ in pathogenicity, host specificity, and sarcocyst structure and location. Sarcocystis are obligatory intracellular, with a typical coccidian life cycle, consisting of merogony, gametogony, and sporogony. The life cycle involves an intermediate and a definitive host, usually an herbivore and a carnivore, respectively. At first, a series of asexual reproduction steps culminate with sarcocyst formation. Ingestion of cyst-infected tissues by the definitive host leads to sexual reproduction of the parasite in the digestive tract, followed by excretion of infective forms in the feces. The cycle is closed when an intermediate host becomes infected by the fecal-oral route. Most Sarcocystis are species-specific for intermediate and family-specific for definitive hosts. Infection of farm animals is sometimes associated with the reduction in quality and quantity of meat, wool, and fiber, resulting in important economic losses. Additionally, some Sarcocystis species are zoonotic. Thus, the study of sarcocystosis constitutes an active field of research.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Morphology, Life Cycle, and Host-Pathogen Interactions
Sarcocystis was first reported in Switzerland in 1843 by Miescher, who found white threads in the skeletal muscle of a house mouse—Mus musculus—which came to be known as tubules of Miescher (Levine 1986). Until the 1970s, the taxonomic position of the group was not clear, and the major criteria for naming new species were cyst structure and host species. However, studies based on intermediate host specificity indicated that some Sarcocystis parasites with structurally similar cysts are actually different species, for example, S. tenella and S. capracanis. Additionally, some species of Sarcocystis, such as S. falcatula and S. neurona, can infect numerous host species (Dubey 2015). This information, as well as sequencing data, is currently helping to change the status of some species and rename others.
Some of the most important species of Sarcocystis that affect farm animals and pets are shown in Table 4.1. Even though sarcocystosis is mainly a veterinary problem, some species are pathogenic to man, such as S. hominis and S. suihominis. Humans are the definitive hosts of these parasites and become infected by the ingestion of raw or undercooked meat of cattle and pig, respectively, which act as intermediate hosts. Humans can also serve as accidental intermediate or aberrant hosts for several species of Sarcocystis, through the ingestion of oocysts (Fayer 2004).
4.1.1 Morphology
Sarcocystis parasites undergo numerous morphological changes that allow distinct functions necessary to carry out host cell invasion, asexual multiplication, or sexual reproduction along their life cycle. Stages that undergo asexual multiplication are schizonts and metrocytes; host cell invasion is carried out by merozoites, bradyzoites, and sporozoites, while sexual reproduction involves micro- and macrogametes produced by micro- and macrogamonts and leads to the formation of oocysts. Importantly, sporozoites and bradyzoites are produced in wall-enclosed structures containing multiple parasites—the sporocyst and the sarcocyst—which guarantee an efficient transmission to the intermediate and definitive host, respectively.
The main features of Sarcocystis stages and structures are described below. As a rule, the phylum-characteristic apical complex is clearly observed in infective stages that interact with and internalize in host cells, while it is not apparent in stages engaged in asexual or sexual multiplication.
The schizont is the first parasite stage found in the intermediary host. It develops after a sporozoite has invaded an intermediary host cell, which is generally, but not exclusively, an endothelial cell of a mesenteric lymph node. Early schizonts are ovoid and contain a large nucleus and a single nucleolus. In a process known as endopolygeny, the nucleus gets lobulated and shows several nucleoli. A spindle apparatus with microtubules and two centromeres is associated to each lobe. It guides the genetic material to the lobe end, where a merozoite is formed, and eventually buds, giving the schizont the appearance of a rosette of merozoites. Schizonts develop free in the host cell cytoplasm and are not contained in a parasitophorous vacuole (PV).
Merozoites disseminate the infection in the intermediary host. They are motile, crescent-shaped organisms, with a rhoptry-less apical complex. After budding from a schizont, they can be found free in the blood or located within mononuclear cells. In the latter case, they can divide to form two merozoites by endodyogeny. Upon invasion of a suitable host cell, they start a new schizogony cycle (Fayer 2004).
Sarcocysts are the most characteristic structures produced by Sarcocystis parasites. They constitute the last stage of the asexual phase in the intermediary host and are generated after a merozoite has invaded a myocyte or a nervous cell. The membrane of the PV that encloses the parasite and the material underlying it form a wall, providing a safe microenvironment for multiplication. According to the species, sarcocysts can be found in skeletal, cardiac, or smooth myocytes or in neural cells. They can present different shapes—globular, filamentous, and fusiform—and sizes, from a few microns to several centimeters. These and other physical features, such as the presence or absence of internal partitions and the ultrastructure of their walls, aid in species identification (Fayer 2004; Dubey 2015). A certain degree of size variation according to the age of the cyst and the type of parasitized host cell is sometimes observed. For example, within the same species, cysts in cardiac muscles are always smaller than those in skeletal muscles. The wall can invaginate forming villar protrusions, or cytophaneres, of different shapes and sizes. There are over 80 distinct types of cyst wall structures (Dubey 2015). Immediately underneath, there is a granular layer from which septa generally arise, separating the sarcocyst into compartments (Fig. 4.1a). Some sarcocysts, however, have no septa. Table 4.2 and Figs. 4.2 and 4.3 show examples of sarcocysts and their special features. Parasites are located in the fluid contained between partitions (Fig. 4.1b) or, when no partitions are present, free within the cyst. The number of parasites contained in a sarcocyst varies with the species and the stage of maturation: young cysts as small as 5 μm in diameter might contain only two parasites, while a mature cyst can contain over 107 parasites, as is the case of S. aucheniae macrocysts (Carletti et al. 2013).
Metrocytes and bradyzoites are the parasite stages found in sarcocysts. Metrocytes—mother cells—are rapidly multiplying forms dominant in immature cysts. They are round to oval and have a variable size according to the stage of division. During the transformation of a merozoite into a metrocyte, many of the organelles of the apical complex, such as micronemes, conoid, and polar and apical rings, disappear, while ribosomes, endoplasmic reticulum, and mitochondria become more abundant, and the nucleus becomes larger. Bradyzoites, slow cells or also known as cystozoites, are the dominant forms in mature cysts. They are approximately 17 by 4 μm in size and display gliding motility and a characteristic apical complex (Figs. 4.4 and 4.5). In mature cysts, metrocytes localize in the cortex and stain lightly with hematoxylin and eosin, while bradyzoites are found in the medulla and get heavily stained (Dubey 2015).
Micro- and macrogamonts are formed upon bradyzoite infection of goblet cells of the small intestine of the definitive host. Macrogamonts are round or ovoid, measure up to 20 μm in diameter, and contain a single large nucleus. Initially intracellular, they are usually freed into the lamina propria after lysis of the host cell. Microgamonts are elongated, slightly smaller than macrogamonts, and contain several nuclei that move to the periphery. Slender microgametes with two flagella, measuring up to 10 μm in diameter, are formed around each nucleus (Dubey 2015).
Oocysts result from the fusion of micro- and macrogametes. They have an ellipsoid shape, measure around 20 μm long, and are surrounded by a thin wall with a dense external layer and an internal layer of one to four membranes. When eliminated in the feces, they are sporulated and contain two sporocysts. Sporocysts measure 10 by 15 μm and are indistinguishable between species. Each sporocyst has four sporozoites arranged lengthwise (Fig. 4.6) (Dubey 2015; Fayer et al. 2015).
Sporozoites are banana-shaped cells measuring 11–19 μm long by 7–10 μm wide, with all the structural features of bradyzoites. In addition, they possess one or more virus-like crystalloid bodies that consist of electron-dense and electron-lucent granules. These structures likely represent a source of energy or amino acids, as was postulated for Eimeria sp. (Dubremetz and Torpier 1978).
4.1.2 Life Cycle
The biological cycle of Sarcocystis parasites remained unknown until 1972 when it was recognized that the predator-prey relationship corresponded to a definitive and an intermediate host, respectively. The life cycle consists largely of schizogony—also known as merogony—gametogony, and sporogony. The latter two comprise the sexual phase of the cycle and take place in the intestine of the definitive host—predator. Schizogony corresponds to the asexual phase and occurs in various tissues of the intermediate host—prey—until the formation of bradyzoite-containing cysts that are mainly located in muscle fibers, as mentioned before (Dubey et al. 1989) (Fig. 4.6).
A compatible herbivore becomes infected by ingesting pastures or water contaminated with sporulated Sarcocystis sporocysts. Exposure to trypsin and bile in the small intestine causes the liberation of four motile sporozoites from each sporocyst that invades endothelial cells of mesenteric lymph node arteries. Here, first-generation schizogony takes place, giving rise to numerous motile merozoites that bud from a schizont and are released to the bloodstream. Peripheral blood smears show the presence of merozoites between 24 and 46 days postinfection. Merozoites invade endothelial cells of downstream arterioles, capillaries, and veins, distributing throughout the body and producing additional generations by schizogony. Schizonts of some Sarcocystis species can also be found in connective tissue cells, macrophages, neural cells, and cells of many different organs. The last schizogony cycle takes place when a merozoite invades a muscle cell—skeletal, smooth, or cardiac—or, exceptionally, a nervous cell and forms a sarcocyst within its surrounding PV. Intracellularly, the invading merozoite first differentiates into a metrocyte, which reproduces by endodyogeny inside the PV. In this type of asexual reproduction, two daughter cells arise from an existing one, which is consumed during the process. Concomitantly, a wall develops, isolating the nascent sarcocyst from the surrounding tissues. Eventually, metrocytes stop division and differentiate into infective bradyzoites, which display an apical complex that will aid in the invasion of definitive host cells. A sarcocyst full of bradyzoites is considered mature. The time elapsed between infection and formation of a mature sarcocyst varies between species but lasts in general around 2 months. Cysts can then persist in the tissues for months or even years. Bradyzoites are infectious for the definitive host, while schizonts and immature sarcocysts are not. The number and distribution of cysts in a particular host depend on different factors, including the amount of sporozoites ingested, the Sarcocystis and host species involved, the stage of infection, and the immune status of the animal. Gametogony is possible when a compatible carnivore feeds on cyst-containing tissues of an intermediate host (Fig. 4.7). Upon digestion of the cyst wall in the stomach and intestine, bradyzoites are liberated from the sarcocyst and penetrate host cells, generally goblet cells, or enterocytes of the small intestine. Each bradyzoite differentiates intracellularly into either a macro- or a macrogamont. Each macrogamont yields a single macrogamete, while microgamonts become multinucleated and yield several microgametes. The latter are motile and migrate to the surface of a macrogamete. After membrane fusion, the microgamete nucleus enters and fertilizes the macrogamete, yielding a zygote. A thin wall (<1 μm) then develops around the zygote giving place to the formation of the oocyst. The whole process of gametogony and fertilization can be completed in 1 day. The infected cells move to the lamina propria, where sporulation happens, giving rise to two elongated sporocysts containing four sporozoites each. The timing of sporocyst excretion by the definitive host after ingestion of sarcocysts is highly variable within the same species but in general starts after 7 and 14 days (Dubey et al. 1989; Dubey 2015; Fayer et al. 2015).
4.1.3 Host-Pathogen Interactions
Even though Sarcocystis is a very broad genus that includes some of the most prevalent parasites of vertebrate animals, they are understudied compared to other members of the Apicomplexa. For this reason, information on host-pathogen interactions is rather scarce. Since Sarcocystis sp. are obligate intracellular parasites, surface molecules that participate in recognition and invasion of host cells are likely essential for their survival. A prominent group of coccidian surface proteins are the SAGs, a family of glycosylphosphatidylinositol (GPI)-anchored surface antigens, initially characterized in Toxoplasma gondii and Neospora spp. (Howe et al. 2005). T. gondii SAGs have been implicated in receptor-ligand interactions with the host cell surface and in the stimulation of immune responses during infection, suggesting they are attractive targets for anti-coccidian drugs or immunotherapy approaches (Jacquet et al. 2001; Rachimel et al. 2004). Homology searches in an expressed sequence tag (EST) database of S. neurona allowed the identification of four SAG family members in this parasite, and other two members were later discovered (Howe et al. 2005; Crowdus et al. 2008). These proteins were demonstrated to be expressed on the surface of merozoites and to be highly immunogenic. The presence of SAG family proteins in different coccidian genera suggests a conserved essential function (Howe et al. 2005). Interestingly, studies performed with S. neurona merozoites, bradyzoites, and sporozoites showed that expression of individual SAGs is stage-specific. This is consistent with findings in T. gondii and suggests that surface antigen switching could be essential for the completion of the parasite life cycle (Gautam et al. 2011). Another GPI-anchored protein, surface protein 1 or SnSPR1, has been also identified in the S. neurona EST database. Contrary to SAGs, SnSPR1 shows no orthologs in other coccidian genera. It is expressed at the surface of merozoites in all stages of schizont development, is immunogenic, and might also participate in host-pathogen interactions (Zhang and Howe 2008).
Microneme proteins are also key elements in the invasion process of apicomplexan parasites, likely involved in the attachment and entry into the host cell (Dubremetz et al. 1998). The ortholog of a T. gondii microneme protein was identified in the S. neurona EST database and named SnMIC10. This protein has been shown to be differentially expressed in the apical end of merozoites during endopolygeny, supporting the view that micronemes are only needed during cell invasion (Hoane et al. 2003). A large array of conserved and species-specific proteins of Sarcocystis parasites await characterization and could serve as targets for control strategies.
Effector cells of the host immune system are mobilized during a Sarcocystis infection. The predominant cells infiltrating visceral and muscular tissues are lymphocytes and macrophages (Dubey et al. 1982). The cell infiltration of mononuclear cells starts during the third week of infection and can last for several months, even after the parasite is no longer detectable in visceral tissues (Gasbarre et al. 1984). Whether these cellular events participate in the recovery of the host from sarcocystosis has not been established, and passive transfer of resistance via cells or antibodies has not been reported. The intense cellular response seen in immune animals that survive lethal challenges indicates a cell-mediated immunity against the parasite. Protective immunity has been shown to be induced only by homologous Sarcocystis species. As an example, in S. hirsuta experimentally infected cattle, no protection against challenge with S. cruzi was shown (Dubey 2015; Fayer and Dubey 1984; Ford 1985).
S. neurona-experimentally infected horses develop clinical disease and generate antibodies in serum and cerebrospinal fluid. Alterations in the immune cell subset expression that changed during disease progression were observed. Infected horses showed decreased antigen-specific proliferation responses compared to nonexperimentally infected horses, suggesting that the process between antigen-presenting cells—monocyte/dendritic cells—and/or T-cell antigen recognition may be damaged in S. neurona-infected horses (Lewis et al. 2014).
4.2 Diagnosis and Epidemiology
4.2.1 Diagnosis
Diagnosis of acute sarcocystosis is difficult since symptoms are not very specific and, therefore, easily confused with other pathological processes. Regularly, a diagnosis of sarcocystosis is based on the elimination of other causative agents, a good epidemiologic evaluation of the intermediate host, and its relationship to definitive hosts, as well as clinical findings (Cordero del Campillo et al. 1999). However, there are some techniques that have been employed or developed over the years and are described below.
4.2.1.1 Definitive Host
The diagnosis of sarcocystosis in the definitive host is based mostly on the identification of Sarcocystis sporocysts in the feces. This can be achieved by using a coproparasitological assay, consisting of flotation in zinc sulfate or other high-density solutions, followed by microscopic observation (Dubey et al. 2015). However, species cannot be discriminated by this method because of morphological similarities of sporocysts, thus molecular tests need to be employed.
4.2.1.2 Intermediate Host
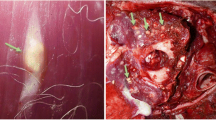
Diagnosis can sometimes be inferred from epidemiological data of the region of interest, as well as information obtained by coproparasitological analysis of definitive hosts. Usually, confirmatory diagnosis is achieved by postmortem examination of the skeletal muscle. Cysts of some species are visible to the naked eye, as in the case of S. aucheniae and S. gigantea, that infect South American camelids and sheep, respectively (Fig. 4.2). In a great number of species, however, cysts are microscopic, so other examination methods are applied.
Microscopy and electron microscopy allow to diagnose Sarcocystis sp. and to differentiate morphological features between species. They are specific but time-consuming, limiting their application on large numbers of samples (Moré et al. 2010).
Artificial digestion is a sensitive method that allows analyzing large amounts of tissue. It detects bradyzoites released from cysts, but it does not allow to differentiate between species of Sarcocystis (Savini et al. 1996). In the digestion procedure, tissues are incubated with proteases, such as trypsin or pepsin. Released bradyzoites can be used as antigen, and the species can be identified by molecular methods.
Histopathology and examination of fresh tissue allows the differentiation between thick- and thin-walled cysts, but not between species. The sensitivity of histopathological examinations is lower due to the smaller volume of sample that can be processed (Dubey et al. 1989).
Polymerase chain reaction (PCR) is an important tool for epidemiological studies. It allows to detect DNA of the parasite in small volumes of sample and also to differentiate between Sarcocystis and related organisms, such as Toxoplasma and Neospora, or discriminate between Sarcocystis species (Ortega-Mora et al. 2007). Different PCR protocols have been developed over the years. For example, recent studies showed that it is possible to detect DNA of S. aucheniae in the blood of South American camelids employing a semi-nested PCR (Martín et al. 2016). Other studies allowed the differentiation of Sarcocystis species affecting cattle using multiplex real-time PCR (Moré et al. 2013) or applied PCR followed by RFLP—restriction fragment length polymorphism—to determine the Sarcocystis species affecting sheep (Hamidinejat et al. 2014). In addition to PCR, another highly sensitive DNA amplification technique that takes place at constant temperature, and thus does not require the use of a thermocycler, is being increasingly applied to the diagnosis of different pathogens (Notomi et al. 2015). This technique—known as loop-mediated isothermal amplification or LAMP—has been successfully applied to the molecular detection of S. fayeri in horsemeat (Furukawa et al. 2016).
In immunohistochemistry, anatomical, immunological, and biochemical techniques are combined to identify discrete tissue components. The method is based on the interaction of target antigens with specific antibodies tagged with a label that allows visualizing the distribution and localization of specific components within cells and in the proper tissue context. A number of immunohistochemical methods have been developed for the improvement of the sensitivity and specificity of the histological detection of life cycle stages of Sarcocystis species. However, these methods are complicated due to the high cross-reactivity among Sarcocystis species when polyclonal antisera are used. In addition, cross-reactivity of anti-Sarcocystis sp. antibodies is sometimes extended to other cyst-forming coccidia such as T. gondii (Uggla and Buxton 1990). Monoclonal antibodies have been produced for S. cruzi, S. tenella, and S. arieticanis; however also in this case, most are cross-reactive with antigens of heterologous Sarcocystis species (Ortega-Mora et al. 2007).
Enzyme-linked immunosorbent assay (ELISA) detects and measures antibodies and is the most commonly used serological test for the diagnosis of Sarcocystis. However, cross-reactivity with heterologous Sarcocystis species is also a problem here (Tenter 1995). Different types of antigens have been employed in ELISA tests. As an example, an indirect ELISA (iELISA) based on S. cruzi bradyzoite antigens was developed in Sri Lanka for the detection of anti-Sarcocystis spp. antibodies in cattle (Kalubowila et al. 2004). In addition, in Argentina, an iELISA based on an immunogenic protein fraction extracted from S. aucheniae bradyzoites was applied to the detection of anti-Sarcocystis antibodies in South American camelids (Romero et al. 2014). More recently, in the USA, recombinant forms of S. neurona SnSAG surface antigens were used in an iELISA format to measure antibodies in serum and cerebrospinal fluid. The latter revealed active infection in the central nervous system (Yeargan et al. 2015).
In a Western blot, a mixture of proteins is separated by gel electrophoresis and then transferred to a membrane. The membrane is incubated with specific antibodies against the protein or proteins of interest. Detection is achieved by reaction with an enzyme-conjugated anti-species antibody, followed by incubation with a colorimetric or chemiluminescent substrate (Mahmood and Yang 2012). Detection of antibodies against two S. neurona-specific antigens, of 29 and 17 kDa, is currently the standard serological diagnostic method for infections with this parasite in horses (Rossano et al. 2000; Hamir and Dubey 2001).
4.2.2 Epidemiology
Sarcocystis infections of farm animals are worldwide distributed and often present high prevalences, both in developing and industrialized countries (Dubey et al. 1989). The percentages of infected animals depend on various aspects, including host, viability of the sporocysts in the environment, number of sporocysts released by the definitive host, immune status of the intermediate host, hygiene, and proximity between definitive host and intermediate hosts, among others (McKenna and Charleston 1994; Savini et al. 1996). According to the species, infections can cause mortality, morbidity, abortions, lower meat yield, and economic losses due to confiscation of meat when macroscopic cysts are found (Poulsen and Stensvold 2014). In cattle, Sarcocystis infections are regularly asymptomatic, with prevalences over 90%, being S. cruzi the most commonly found species (Moré et al. 2010). In Argentina, recent studies showed a direct connection between the type of breeding of llamas and prevalence of anti-Sarcocystis sp. antibodies (Romero et al. 2017). In Bolivia, a study made in abattoirs showed that 23–50% of llama carcasses contained macroscopic cysts, with infection rates higher in females and in older animals (Rooney et al. 2013).
Seroprevalences reported for S. neurona in US horses vary from 15 to 89%, depending on the geographic region. They are also lower during the winter season compared to the rest of the year (Pusterla et al. 2014; Reed et al. 2016). Seroprevalences of around 35% were reported in Brazil and Argentina, indicating that the parasite is also present in South America (Dubey et al. 1999a, b). Most infections have been observed in young animals of 1–5 years or older than 13 years of age. Interestingly, the likelihood of infection was significantly reduced in farms where wildlife had no access to feed and when a creek or river was present as a water source. On the other hand, stress related to heavy exercise, transport, injury, surgery, or parturition was found to increase the risk of disease caused by S. neurona. In addition, racehorses and show horses had higher infection risks than breeding and pleasure horses (Reed et al. 2016).
Sarcocystosis is usually acquired horizontally through ingestion of contaminated food or water. In addition, anti-Sarcocystis sp. antibodies were detected in horse fetuses and newborn foals, indicating the occurrence of transplacental transmission. However, this event appears to be rare (Duarte et al. 2004).
A number of molecular typing techniques, including PCR-RFLP, microsatellite, and whole-genome fingerprinting, have been developed to differentiate between S. neurona isolates. Application of these techniques can aid in understanding parasite transmission and epidemiology and reveal its population structure (Elsheikha and Mansfield 2007).
4.3 Clinical Effects, Prevention, and Treatment
4.3.1 Clinical Effects
Sarcocystis natural infections of intermediate hosts are in most cases asymptomatic. However, S. neurona is a special case, due to the parasite tropism for horse nervous tissues, infecting both gray and white matter, which provokes focal or multifocal signs of neurological disease. This syndrome was initially known as segmental myelitis and, later, focal encephalitis-myelitis, until the presence of protozoa in characteristic lesions led to the current name of equine protozoal myeloencephalitis (EPM). The disease can actually also be caused by Neospora hughesi, although the majority of cases are due to S. neurona. Clinical EPM signs include dysphagia, upper airway dysfunction, muscle weakness and atrophy, ataxia, weakness of limbs, and even seizures. Severely affected horses show difficulty in standing, walking, or swallowing and present head tilt and facial nerve paralysis. In some cases, signs stabilize but relapse in a few days or weeks (Dubey et al. 2015; Reed et al. 2016).
Experimental infections of different farm animals and pets, on the other hand, have shown a number of severe signs (Table 4.3). As a rule in Sarcocystis infections, necrosis of cells and tissues produced by multiplication of schizonts is very common, but it does not appear to be extensive enough to cause severe illness or death in large animals—cattle, sheep, goats, and pigs. However, an intense inflammatory reaction is usually associated to second-generation schizont maturation. Eosinophilic myositis (EM) is a specific inflammatory condition of striated muscles. It happens during the penetration of myocytes by merozoites and might be related to products liberated from merozoites or myocytes. It has been found mostly in cattle, occasionally in sheep, and rarely in pigs and horses. With the progression of EM, eosinophils and myocytes degenerate, resulting in granulomas with a central area of necrosis. Later, the tissue becomes surrounded by zones of giant cells, epithelial cells, lymphocytes, and fibrocytes (Dubey 2015).
Abortion can result when animals become infected with pathogenic species of Sarcocystis during pregnancy, and there are many unanswered questions and observations that appear contradictory about the effect of sarcocystosis on fetal health. When infection is induced experimentally, most animals can develop clinical sarcocystosis and abortion, showing parasites and lesions in maternal placentomes but rarely infecting the fetus or fetal membranes. Unlike experimentally infected animals, those with natural infections show parasites, lesions, or both in the fetuses (Jerrett et al. 1984).
Definitive hosts usually do not present clinical signs. Dogs, cats, coyotes, foxes, and raccoons fed with tissues infected with different Sarcocystis species-excreted sporocysts but were otherwise asymptomatic. However, a few dogs and coyotes vomited or were anorexic for 1–2 days following ingestion of meat. In addition, in trials made in human volunteers who ingested beef and pork infected with S. hominis or S. suihominis, respectively, clinical symptoms were observed, including vomiting, diarrhea, and respiratory distress (Dubey 2015). Indeed, soluble extracts prepared from the tissue cysts of various Sarcocystis species have been shown to contain powerful toxins—sarcotoxins—which have even proven lethal when administered to laboratory animals (Hiepe et al. 1981; Harada et al. 2013, Kamata et al. 2014). Accordingly, experimental inoculation of rabbits with an extract derived from S. fusiformis or S. cruzi cysts developed a shock-like state and/or death, likely caused by the sudden exposure to high doses of sarcotoxins (Saleque et al. 1991; Nakamura et al. 1999).
4.3.2 Prevention
Currently, there is no vaccine to protect animals against sarcocystosis, but experimental studies indicate that cattle, sheep, goats, and pigs develop a humoral response when inoculated with small numbers of live sporocysts. For this reason, there is hope of developing a vaccine for sarcocystosis in the future (Dubey 2015). In the USA, a killed whole S. neurona merozoite vaccine was marketed by Fort Dodge, but the product has been retired from the market, because no differences between vaccinated and control horses were found during the trials (Dubey et al. 2015).
For the time being, interrupting the cycle of the parasite is the only practical method of control. This can be achieved by preventing definitive hosts to consume raw or insufficiently cooked meat infected with Sarcocystis or to cohabitate with intermediate hosts.
It is important to take into account that during veterinary inspections in slaughterhouses, meat containing macroscopic cysts can be confiscated, but microscopic cysts pass unnoticed. For this reason, it can be assumed that most meat that is consumed is infected with Sarcocystis (Godoy et al. 2007). It has been demonstrated that freezing meat is effective to prevent the occurrence of food poisoning when consuming raw meat containing sarcocysts. Indeed, freezing S. fayeri sarcocyst-infected horsemeat for 48 h at −20 °C resulted in the disappearance of bradyzoites, as well as of a 15 kDa sarcotoxin found to be responsible for the clinical signs associated with food poisoning (Kamata et al. 2014).
Confiscated carcasses should be buried or incinerated to prevent definitive hosts from eating infected meat, and the prophylactic use of anticoccidials in definitive and intermediate hosts could help to control sarcocystosis in farm animals and pets (Dubey 2015).
4.3.3 Treatment
Currently there is no specific prophylactic or therapeutic treatment for sarcocystosis. Infections usually go undetected, but in case of the appearance of clinical signs, some drugs have proved effective to partially reduce illness and parasitic load (Table 4.4). In horses, treatment with an anticoccidial drug has been shown to increase ten times the likelihood of recovery from clinical EPM signs and survival. Treatment, however, was more effective when milder, rather than severe, clinical signs were present (Saville et al. 2000; Pusterla et al. 2014; Reed et al. 2016).
References
Bucca M, Brianti E, Giuffrida A, Ziino G, Cicciari S, Panebianco A. Prevalence and distribution of Sarcocystis spp. cysts in several muscles of cattle slaughtered in Sicily, Southern Italy. Food Control. 2011;22:105–8. https://doi.org/10.1016/j.foodcont.2010.05.015.
Carletti T, Martin M, Romero S, Morrison DA, Marcoppido G, Florin-Christensen M, Schnittger L. Molecular identification of Sarcocystis aucheniae as the macrocyst-forming parasite of llamas. Vet Parasitol. 2013;198:396–400. https://doi.org/10.1016/j.vetpar.2013.09.007.
Cordero del Campillo M, Rojas F, Fernández M, Sánchez M, Rodríguez S, López I. Parasitología veterinaria. Madrid: McGraw-Hill; 1999. p. 968.
Crowdus CA, Marsh AE, Saville WJ, Lindsay DS, Dubey JP, Granstrom DE, Howe DK. SnSAG5 is an alternative surface antigen of Sarcocystis neurona strains that is mutually exclusive to SnSAG1. Vet Parasitol. 2008;158(1–2):36–43. https://doi.org/10.1016/j.vetpar.2008.08.012.
Duarte PC, Conrad PA, Barr BC, Wilson WD, Ferraro GL, Packham AE, Carpenter TE, Gardner IA. Risk of transplacental transmission of Sarcocystis neurona and Neospora hughesi in California horses. J Parasitol. 2004;90(6):1345–51. https://doi.org/10.1645/GE-3372.
Dubey JP. Sarcocystosis of animals and humans. 2nd ed. Boca Raton: CRC Press; 2015.
Dubey JP, Speer CA, Epling GP. Sarcocystosis in newborn calves fed Sarcocystis cruzi sporocysts from coyotes. Am J Vet Res. 1982;43:2147–64. PMID: 6819793.
Dubey JP, Speer CA, Fayer R. Structure and life cycle. Sarcocystosis of animals and man. Boca Raton: CRC; 1989.
Dubey JP, Kerber CE, Granstrom DE. Serologic prevalence of Sarcocystis neurona, Toxoplasma gondii, and Neospora caninum in horses in Brazil. J Am Vet Med Assoc. 1999b;215:970–2. PMID: 10511862.
Dubey JP, Venturini MC, Venturini L, McKinney J, Pecoraro M. Prevalence of antibodies to Sarcocystis neurona, Toxoplasma gondii and Neospora caninum in horses from Argentina. Vet Parasitol. 1999a;86:59–62. https://doi.org/10.1016/S0304-4017(99)00127-2.
Dubey JP, Howe DK, Furrc M, Savilled WJ, Marshd AE, Reede SM, Griggf ME. An update on Sarcocystis neurona infections in animals and Equine Protozoal Myeloencephalitis (EPM). Vet Parasitol. 2015;209(0):1–42. https://doi.org/10.1016/j.vetpar.2015.01.026.
Dubremetz JF, Torpier G. Freeze fracture study of the pellicle of an eimerian sporozoite (protozoa, coccidia). J Ultrastruct Res. 1978;62:94–109. PMID: 418187.
Dubremetz JF, Garcia-Reguet N, Conseil V, Fourmaux MN. Apical organelles and host-cell invasion by Apicomplexa. Int J Parasitol. 1998;28:1007–13. PMID: 9724870.
Elsheikha HM, Mansfield LS. Molecular typing of Sarcocystis neurona: current status and future trends. Vet Parasitol. 2007;149:43–55. https://doi.org/10.1016/j.vetpar.2007.06.039.
Fayer R. Sarcocystis spp. in human infections. Clin Microbiol Rev. 2004;17(4):894–902. https://doi.org/10.1128/CMR.17.4.894-902.2004.
Fayer R, Douglas H, Dubey JP. Human infections with Sarcocystis species. Clin Microbiol Rev. 2015;28(2):295–311. https://doi.org/10.1128/CMR.00113-14.
Fayer R, Dubey JP. Protective immunity against clinical sarcocystosis in cattle. Vet Parasitol. 1984;15:187–201. PMID: 6437053.
Ford GE. Immunity of sheep to homologous challenge with dog borne Sarcocystis species following varying levels of prior exposure. Int J Parasitol. 1985;15:629–34. PMID: 3937817.
Furukawa M, Minegishi Y, Izumiyama S, Yagita K, Mori H, Uemura K, Etoh Y, Maeda E, Sasaki M, Ichinose K, Harada S, Kamata Y, Otagiri M, Sugita-Konishi Y, Ohnishi T. The development of a novel, validated, rapid and simple method for the detection of Sarcocystis fayeri in horse meat in the sanitary control setting. Biocontrol Sci. 2016;21(2):131–4. https://doi.org/10.4265/bio.21.131.
Gautam A, Dubey JP, Saville WJ, Howe DK. The SnSAG merozoite surface antigens of Sarcocystis neurona are expressed differentially during the bradyzoite and sporozoite life cycle stages. Vet Parasitol. 2011;183(1–2):37–42. https://doi.org/10.1016/j.vetpar.2011.06.024.
Gasbarre LC, Suter P, Fayer R. Humoral and cellular immune responses in cattle and sheep inoculated with Sarcocystis. Am J Vet Res. 1984;45:1592–6. PMID: 6433756.
Gjerde B. Molecular characterization of Sarcocystis bovifelis, Sarcocystis bovini n. sp, Sarcocystis hirsuta and Sarcocystis cruzi from cattle (Bos taurus) and Sarcocystis sinensis from water buffaloes (Bubalus bubalis). Parasitol Res. 2016;115:1473. https://doi.org/10.1007/s00436-015-4881-5.
Godoy R, Vilca M, Gonzáles A, Leyva V, Sam R. Saneamiento y detoxificación de carne de llama (Lama glama) infectada con Sarcocystis aucheniae mediante cocción, horneado, fritura y congelado. Rev Investig Vet del Perú. 2007;18(1):51–6. 10.15381/rivep.v18i1.1275.
Golubkov VI, Rybaltovskii DV, Kislyakova ZI. The source of infection for swine Sarcocystis. Veterinarya. 1974;11:85–7. (In Russian).
Hamidinejat H, Moetamedi H, Alborzi A, Hatami A. Molecular detection of Sarcocystis species in slaughtered sheep by PCR–RFLP from south-western of Iran. J Parasitol Dis. 2014;38(2):233–7. https://doi.org/10.1007/s12639-012-0231-z.
Hamir AN, Dubey JP. Myocarditis and encephalitis associated with Sarcocystis neurona infection in raccoons (Procyonlotor). Vet Parasitol. 2001;95:335–40. PMID: 11223214.
Harada S, Furukawa M, Tokuoka E, Matsumoto K, Yahiro S, Miyasaka J, Saito M, Kamata Y, Watanabe M, Irikura D, Matsumoto H, Sugita-Konishi Y. Control of toxicity of Sarcocystis fayeri in horsemeat by freezing treatment and prevention of food poisoning caused by raw consumption of horsemeat. Shokuhin Eiseigaku Zasshi. 2013;54(3):198–203. https://doi.org/10.3358/shokueishi.54.198.
Heydorn AO, Gestrich R, Mehlhorn H, Rommel M. Proposal for a new nomenclature of the Sarcosporidia. Z Parasitenkd. 1975;48:73–82. https://doi.org/10.1007/BF00389639.
Hiepe F, Litzke LF, Scheibner G, Jungmann R, Hiepe T, Montag T. Ultersuchungen zur toxischen Wirkung von Extrakten aus Sarcocystis ovifelis Makrozysten auf Kaninchen. Mh Vet Med. 1981;36:908–10.
Hoane JS, Carruthers VB, Striepen B, Morrison DP, Entzeroth R, Howe DK. Analysis of the Sarcocystis neurona microneme protein SnMIC10: protein characteristics and expression during intracellular development. Int J Parasitol. 2003;33:671–9. PMID: 12814647.
Howe DK, Gaji RY, Mroz-Barrett M, Gubbels M, Striepen B, Stamper S. Sarcocystis neurona merozoites express a family of immunogenic surface antigens that are orthologues of the Toxoplasma gondii surface antigens (SAGs) and SAG-related sequences. Infect Immun. 2005;73(2):1023–33. https://doi.org/10.1128/IAI.73.2.1023–1033.2005.
Jacquet A, Coulon L, De Neve J, Daminet V, Haumont M, Garcia L, Bollen A, Jurado M, Biemans R. The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol Biochem Parasitol. 2001;116:35–44. PMID: 11463464.
Jerrett IV, McOrist S, Waddington J, Browning JM, Malecki JC, McCausland IP. Diagnostic studies of the fetus, placenta and maternal blood from 265 bovine abortions. Cornell Vet. 1984;74:8–20.
Kalubowila DGW, Udagama-Randeniya PV, Perera NAN, Rajapakse RPV. Seroprevalence of Sarcosystis spp. in cattle and buffaloes from the wet and dry zones of Sri Lanka: a preliminary study. J Vet Med. 2004;51:89–93. https://doi.org/10.1111/j.1439-0450.2004.00726.x.
Kamata Y, Saito M, Irikura D, Yahata Y, Ohnishi T, Bessho T, Watanabe M, Sugita-Konishi Y. A toxin isolated from Sarcocystis fayeri in raw horsemeat may be responsible for food poisoning. J Food Prot. 2014;77(5):814–9. https://doi.org/10.4315/0362-028X.JFP-13-351.
Lewis SR, Ellison SP, Dascanio JJ, Lindsay DS, Gogal RM Jr, Werre SR, Surendran N, Breen ME, Heid BM, Andrews FM, Buechner-Maxwell VA, Witonsky SG. Effects of experimental Sarcocystis neurona-induced infection on immunity in an equine model. J Vet Med. 2014;2014:239495. https://doi.org/10.1155/2014/239495.
Levine N. The taxonomy of Sarcocystis (Protozoa: Apicomplexa) species. Parasitol Today. 1986;7:54–6. PMID: 3091802.
Lindsay DS, Dubey JP. Determination of the activity of pyrimethamine, trimethoprim, and sulfonamides and combinations of pyrimethamine and sulfonamides against Sarcocystis neurona in cell cultures. Vet Parasitol. 1999;82:205–21. PMID: 10348099.
Mahmood T, Yang PC. Western blot: technique, theory, and trouble shooting. N Am J Med Sci. 2012;4(9):429–34. https://doi.org/10.4103/1947-2714.100998.
Mansfield LS, Schott HC, Murphy AJ, Rossano MG, Tanhauser SM, Patterson JS, Nelson K, Ewart SL, Marteniuk JV, Bowman DD, Kaneene JB. Comparison of Sarcocystis neurona isolates derived from horse neural tissue. Vet Parasitol. 2001;95(2–4):167–78. https://doi.org/10.1016/S0304-4017(00)00388-5.
Martín M, Decker C, Romero S, Carletti T, Schnittger L, Florin-Christensen M. Molecular detection of Sarcocystis aucheniae in the blood of llamas from Argentina. Rev Argent Microbiol. 2016;48(3):200–5. https://doi.org/10.1016/j.ram.2016.03.009.
McKenna PB, Charleston WAG. The outdoor survival of Sarcocystis gigantea sporocysts. Vet Parasitol. 1994;55:21–7. PMID: 7886918.
Meshkov S. The jackal (Canis aureus) as a new host of Sarcocystis infecting swine. Vet Sbirka. 1980;78:20–1. (In Russian).
Moré G, Abrahamovich P, Jurado S, Bacigalupe D, Marin JC, Rambeaud M, Venturini L, Venturini MC. Prevalence of Sarcocystis spp. in Argentinean cattle. Vet Parasitol. 2010;177(1–2):162–5. https://doi.org/10.1016/j.vetpar.2010.11.036.
Moré G, Regensburger C, Gos ML, Pardini L, Verma SK, Ctibor J, Serrano-Martínez ME, Dubey JP, Venturini MC. Sarcocystis masoni, n. sp. (Apicomplexa: Sarcocystidae), and redescription of Sarcocystis aucheniae from llama (Lama glama), guanaco (Lama guanicoe) and alpaca (Vicugna pacos). Parasitology. 2016;143(5):617–26. https://doi.org/10.1017/S003118201600007X.
Moré G, Schares S, Maksimov A, Conraths FJ, Venturini MC, Schares G. Development of a multiplex real time PCR to differentiate Sarcocystis spp. affecting cattle. Vet Parasitol. 2013;197(1–2):85–94. https://doi.org/10.1016/j.vetpar.2013.04.024.
Munday BL, Obendorf DL. Development and growth of Sarcocystis gigantea in experimentally infected sheep. Vet Parasitol. 1984;15:203–11. PMID: 6437054.
Nakamura Y, Saito M, Shibata Y, Itagaki H. Induction of tumor necrosis factor α and nitric oxide in rabbits inoculated with a cyst extract of Sarcocystis cruzi. Vet Parasitol. 1999;85:235–43. https://doi.org/10.1016/S0304-4017(99)00128-4.
Notomi T, Mori Y, Tomita N, Kanda H. Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol. 2015;53:1–5. https://doi.org/10.1007/s12275-015-4656-9.
Nourani H, Matin S, Nouri A, Azizi H. Prevalence of thin-walled Sarcocystis cruzi and thick-walled Sarcocystis hirsuta or Sarcocystis hominis from cattle in Iran. Trop Anim Health Prod. 2010;42:1225–7. https://doi.org/10.1007/s11250-010-9552-z.
Nourollahi Fard SR, Asghari M, Nouri F. Survey of Sarcocystis infection in slaughtered cattle in Kerman, Iran. Trop Anim Health Prod. 2009;41:1633–6. https://doi.org/10.1007/s11250-009-9358-z.
Ortega-Mora LM, Gottstein B, Conraths FJ, Buxton D. Protozoal abortions in farm ruminants: guidelines for diagnosis and control. Wallingford: CAB International; 2007. https://doi.org/10.1079/9781845932114.0000.
Poulsen CS, Stensvold CR. Current status of epidemiology and diagnosis of human sarcocystosis. J Clin Microbiol. 2014;52(10):3524–30. https://doi.org/10.1128/JCM.00955-14.
Pusterla N, Mackie S, Packham A, Conrad PA. Serological investigation of transplacental infection with Neospora hughesi and Sarcocystis neurona in broodmares. Vet J. 2014;202(3):649–50. https://doi.org/10.1016/j.tvjl.2014.09.015.
Rachimel N, Buzoni-Gatel D, Dutta C, Mennechet FJ, Luangsay S, Minns LA, Grigg ME, Tomavo S, Boothroyd JC, Kasper LH. The induction of acute ileitis by a single microbial antigen of Toxoplasma gondii. J Immunol. 2004;173:2725–35. https://doi.org/10.4049/jimmunol.173.4.2725.
Reed SM, Furr MD, Howe K, Johnson AL, MacKay RJ, Morrow JK, Pusterla N, Witonsky S. Equine Protozoal Myeloencephalitis: an updated consensus statement with a focus on parasite biology, diagnosis, treatment, and prevention. J Vet Intern Med. 2016;30:491–502. https://doi.org/10.1111/jvim.13834.
Rodrigues JS, Meireles GS, Carvalho Filho PR, Ribeiro CT, Flausino W, Lopes CWG. Sarcocystis cruzi (Apicomplexa: Sarcocystidae) no cachorro do mato (Cerdocyon thous). Pesqui Vet Bras. 2008;28:561–4. https://doi.org/10.1590/S0100-736X2008001100004.
Romero SA, Carletti T, Decker Franco C, Moré G, Schnittger L, Florin-Christensen M. Seropositivity to Sarcocystis infection of llamas correlates with breeding practices. Vet Parasitol Reg Studies and Reports. 2017;10:68–70.
Rooney AL, Limon G, Vides H, Cortez A, Guitian J. Sarcocystis spp. in llamas (Lama glama) in Southern Bolivia: a cross sectional study of the prevalence, risk factors and loss in income caused by carcass downgrades. Prev Vet Med. 2013;116(3):296–304. https://doi.org/10.1016/j.prevetmed.2013.11.014.
Rossano MG, Mansfield LS, Kaneene JB, Murphy AJ, Brown CM, Schott HC, Fox JC. Improvement of western blot test specificity for detecting equine serum antibodies to Sarcocystis neurona. J Vet Diagn Investig. 2000;12:28–32. https://doi.org/10.1177/104063870001200105.
Saito M, Itagaki H. Experimental infection of raccoon dogs with Sarcocystis cruzi and S. miescheriana. J Vet Anim Sci. 1994;56:671–4. PMID: 7999889.
Saleque A, Bhatia BB, Juyal PD, Rahman H. Toxicity of cyst extract of Sarcocystis fusiformis from buffalo in rabbits and mice. Vet Parasitol. 1991;38:61–5. https://doi.org/10.1016/0304-4017(91)90009-K.
Saville WJ, Morley PS, Reed SM, Granstrom DE, Kohn CW, Hinchcliff KW, Wittum TE. Evaluation of risk factors associated with clinical improvement and survival of horses with equine protozoal myeloencephalitis. J Am Vet Med Assoc. 2000;217(8):1181–5. PMID: 11043689.
Savini G, Robertson ID, Dunsmore JD. Viability of the sporocysts of Sarcocystis cruzi after exposure to different temperatures and relative humidities. Vet Parasitol. 1996;67:153–60. https://doi.org/10.1016/S0304-4017(96)01046-1.
Soulsby EJ. Parasitología y enfermedades parasitarias en los animales domésticos. 7th ed. México: Interamericana; 1987. p. 823.
Tenter AM. Current research on Sarcocystis species of domestic animals. Int J Parasitol. 1995;25:1311–30. https://doi.org/10.1016/0020-7519(95)00068-D.
Uggla A, Buxton D. Immune responses against Toxoplasma and Sarcocystis infection in ruminants: diagnosis and prospect for vaccination. Rev Sci Tech. 1990;2:441–619. PMID: 2132690.
Yeargan M, Rocha I, Morrow J, Graves A, Reed SM, Howe DK. A new trivalent SnSAG surface antigen chimera for efficient detection of antibodies against Sarcocystis neurona and diagnosis of equine protozoal myeloencephalitis. J Vet Diagn Investig. 2015;27(3):377–81. https://doi.org/10.1177/1040638715584995.
Zhang D, Howe DK. Investigation of SnSPR1, a novel and abundant surface protein of Sarcocystis neurona merozoites. Vet Parasitol. 2008;152(3–4):210–9. https://doi.org/10.1016/j.vetpar.2007.12.036.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Decker Franco, C., Schnittger, L., Florin-Christensen, M. (2018). Sarcocystis. In: Florin-Christensen, M., Schnittger, L. (eds) Parasitic Protozoa of Farm Animals and Pets. Springer, Cham. https://doi.org/10.1007/978-3-319-70132-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-70132-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-70131-8
Online ISBN: 978-3-319-70132-5
eBook Packages: MedicineMedicine (R0)