Abstract
Canine babesiosis caused by different Babesia species is a protozoal tick-borne disease with worldwide distribution and global significance. Historically, Babesia infection in dogs was identified based on the morphologic appearance of the parasite in the erythrocyte. All large forms of Babesia were designated B. canis, whereas all small forms of Babesia were considered to be B. gibsoni. However, the development of molecular methods has demonstrated that additional Babesia species infect dogs and cause distinct diseases. The geographical distribution of canine Babesia species and thus the occurrence of babesiosis are largely dependent on the habitat of relevant tick vector species, with the exception of B. gibsoni where evidence for dog-to-dog transmission indicates that infection can be transmitted among fighting dog breeds independently of the limitations of vector tick infestation. Knowledge of the prevalence and clinicopathological aspects of Babesia species infecting dogs around the world is of epidemiological and medical interest. Babesia infection causes a disease with clinical manifestations that may vary considerably with the different species and strains involved and with factors that determine the host response to infection such as age, individual immune status, and the presence of concurrent infections or other diseases. Hemolytic anemia with systemic inflammatory responses may lead to tissue hypoxia and organ dysfunction, which account for the clinical signs observed in severe canine babesiosis. Babesiosis caused by large Babesia species is treated with imidocarb dipropionate or diminazene aceturate, while small Babesia species are more resistant to anti-babesial therapy and often require treatment with combinations of other drugs such as atovaquone, azithromycin, and clindamycin. Accurate detection and species recognition are important for the selection of the correct therapy and predicting the course of disease.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Canine babesiosis
- Babesia canis
- Babesia vogeli
- Babesia rossi
- Babesia gibsoni
- Babesia conradae
- Babesia vulpes
- Rangelia vitalii
10.1 Morphology, Life Cycle, and Host-Pathogen Interactions
10.1.1 Morphology
Babesia are tick-borne protozoan parasites that belong to the phylum Apicomplexa, class Piroplasmea, and order Piroplasmida and infect erythrocytes of domestic and wild animals and humans. Babesia belong to the Aconoidasida as they lack a conoid structure in their apical complex in all of their life stages except for the ookinete stage—in contrast to apicomplexans of the Conoidasida which have a conoid in all life stages (Mehlhorn et al. 1980). The babesial species that infect dogs are divided into those that present with relatively large merozoite forms in erythrocytes (5 × 2 μm) termed large canine Babesia species and those species which have distinctly smaller merozoite stages (0.3 × 3 μm) termed small canine Babesia species (Table 10.1). Historically, Babesia infection in dogs was identified based on the morphologic appearance of the parasite in the erythrocyte, and all large forms of Babesia were designated B. canis, whereas all small forms of Babesia were considered to be B. gibsoni. However, the development of molecular methods has demonstrated that other genetically distinct Babesia species exist. This division into large and small species of canine Babesia is not supported by phylogenetic studies. The large Babesia species Babesia canis, Babesia vogeli, Babesia rossi, and Babesia sp. (Coco) as well as the small Babesia species B. gibsoni have been placed into a single monophyletic group, which is sometimes for convenience referred to as true Babesia or Babesia sensu stricto and corresponds to Clade V as defined in Schnittger et al. (2012). In contrast, other small Babesia species, Babesia vulpes and Babesia conradae, are sometimes for convenience referred to as Babesia sensu lato as they place into two different unrelated monophyletic groups—Babesia vulpes into Clade I as defined by Schnittger et al. (2012) and Baneth et al. (2015) and Babesia conradae into Clade II (Schnittger et al. 2012). Lack et al. (2012) and Schreeg et al. (2016) have confirmed this phylogenetic classification. Thus, altogether seven canine Babesia species are currently known to infect canines of which one—Babesia sp. (Coco)—has not yet been named.
The morphology of the different life stages of canine babesial parasites has been described in studies using light microscopy and electron microscopy. Most of these studies focused on Babesia canis—i.e., before this taxon has been later divided into the large species Babesia vogeli, Babesia rossi, and Babesia canis as known today—and on Babesia gibsoni, which represents a small Babesia species. The morphology of the red blood cell stages has been reported in more detail than the morphology of the tick stages of some canine Babesia species (Kjemtrup et al. 2006).
The life cycle of Babesia species in canines generally includes a trophozoite stage, which develops immediately after infection of the erythrocyte, and a merozoite stage, which is the result of the asexual division of the trophozoite and the formation of two pyriform bodies that may further divide within the erythrocyte. Parasites taken up during the blood meal of the feeding vector tick develop in the tick intestine into the gamete stage—termed ray body because of its thin projections. Zygotes formed by fusion of two ray body gametes develop into sporokinetes which are motile and infect the salivary glands of the tick to form the infective sporozoite stage or—in Babesia sensu stricto with transovarial transmission—also infect the ovaries of the tick and are transmitted via the eggs transovarially (Mehlhorn et al. 1994).
Intraerythrocytic B. canis merozoites have an outer pellicle layer consisting of three membranes, have no conoid, but possess apical and posterior polar rings, mitochondria, rhoptries, micronemes, subpellicular microtubules, and a membrane-bound nucleus (Mehlhorn and Schein 1984). Babesia canis merozoites maintain their structure and size also when grown in culture (Walter et al. 2002). In contrast, merozoites of small Babesia species such as B. gibsoni and B. conradae may take on variable forms that consist of four to six shapes (Walter et al. 2002; Radi et al. 2004; Kjemtrup et al. 2006). B. gibsoni may develop in culture differently than in the host, and its morphology as seen in the blood of infected dogs changes into larger merozoites that almost fill the entire erythrocyte (Walter et al. 2002).
The ray bodies of B. canis initially develop within erythrocytes in the tick gut and are spherical, polymorphic, or pyramidal with diameters of about 4–7 μm. They are characterized by short thornlike projections, which measure about 1.0–1.2 μm in length (Mehlhorn and Schein 1984). More developed ray bodies leave the decaying erythrocytes and possess several raylike projections before they fuse with another ray body to develop the zygote and sporokinete.
The sporozoites of B. canis measure about 2.5 μm in length. They have a broad apical pole and a pointed posterior pole and are bound by a pellicle composed of an outer cell membrane and an inner layer consisting of two membranes. They contain microtubules and several rhoptries that attach to the apical complex, which discharges proteolytic enzymes that enable the invasion of the parasite into host erythrocytes upon their transmission by the tick saliva (Mehlhorn and Schein 1984).
10.1.2 Life Cycle
10.1.2.1 Transmission by the Vector Tick
Dogs are infected following a tick bite when Babesia sporozoites are injected with saliva into their skin during the blood meal. In the canine host, parasites attach to erythrocyte membranes and invade the cell where they form ring-shaped or ameboid trophozoites. Merogony with asexual replication takes place when the parasite replicates by binary fission within the erythrocyte and forms merozoites observed as pairs of attached pear-shaped parasites—also termed pyriform bodies—in some Babesia species (Fig. 10.1). Merozoites may further divide forming eight or more parasites in the same host erythrocyte, and eventually the cell disintegrates, and merozoites are released in the blood to invade new cells. Some merozoites develop to spheroid forms in erythrocytes considered as gamonts (Mehlhorn and Schein 1984).
Natural transmission of Babesia species usually occurs during an infected tick bite; however, congenital transplacental infection with passage of the parasite through the placenta has been documented in some species but is considered rarer than transmission by tick bite (Fukumoto et al. 2005; Konishi et al. 2008).
The sexual part of the babesial life cycle takes place in the tick. Ticks feeding on infected blood take up intraerythrocytic parasites, and while most merozoites are destroyed in the tick, spheroid forms differentiate further in the tick gut into gametes with discrete projections—ray bodies—which associate in couples and fuse to form zygotes. Zygotes develop into motile sporokinetes, which cross the tick gut into the hemocoele and migrate to the salivary glands where the infective sporozoites are produced by sporogony (Chauvin et al. 2009). Some species of Babesia including B. canis have been shown to be infective to animals only up to 2–3 days after tick attachment (Schein et al. 1979; Mehlhorn and Schein 1984). It is presumed that the change in temperature or the presence of a blood meal in the tick gut acts as an activation stimulus for the maturation of the infective sporozoites.
The life cycle of Babesia sp. in the mammalian host takes place exclusively in erythrocytes, whereas Theileria sp. have a preerythrocytic life stage in leukocytes—the schizont (Chauvin et al. 2009; Uilenberg 2006). Some Babesia species—Babesia sensu lato—are transmitted transstadially from one developmental tick stage to another after feeding on the vertebrate host, whereas other Babesia species, Babesia sensu stricto, are transmitted transovarial through the tick eggs and may therefore be passed through to the next generation without having to feed on an infected host (Uilenberg 2006; Chauvin et al. 2009; Schnittger et al. 2012). Furthermore, all Theileria sensu stricto are transmitted exclusively transstadially.
10.1.2.2 Transmission by Blood Transfusion or Dog-to-Dog Bite
Canine Babesia infection can also be transmitted via transfusion of blood products from an infected blood donor to a recipient dog. This has been shown for several canine babesial species including B. gibsoni and B. canis (Freeman et al. 1994; Stegeman et al. 2003). Babesia species are among the most important pathogens listed for testing in the blood of canine blood donors by the American college of Veterinary Internal Medicine and the Association of Veterinary Hematology and Transfusion Medicine (Wardrop et al. 2005, 2016). In addition to transmission by blood transfusion, studies have provided evidence that B. gibsoni is likely transmitted directly from dog to dog by bites. This has been especially observed for fighting dog breeds such as the Pit Bull Terrier and the Tosa (Birkenheuer et al. 2005; Jefferies et al. 2007; Lee et al. 2009; Yeagley et al. 2009).
10.1.3 Host-Pathogen Interactions
Babesia infection causes a disease with clinical manifestations that may vary considerably between the different species and strains involved as they may have different degrees of virulence. Important for the course of infection are also factors that determine the host immune response to the infection such as age, individual immune status, and the presence of concurrent infections or other diseases (Irwin 2009). Hemolytic anemia with massive erythrocyte destruction and a systemic inflammatory response, which may lead to multiple-organ dysfunction syndrome, account for most of the clinical signs observed in canine babesiosis. The disease onset is often acute with affected dogs suffering from fever and lethargy.
Acute canine babesiosis is characterized by the induction and increased activity of a multitude of inflammatory mediators including cytokines, chemokines, and acute phase proteins. The interaction between these inflammatory mediators, the infective organism, and the canine host have been studied in both experimental and natural infection settings with different species of Babesia including B. canis, B. gibsoni, and B. rossi (Zygner et al. 2014; Brown et al. 2015; Goddard et al. 2016). In some situations, these interactions may be beneficial to the host, but in others, they may prove detrimental. A study on dogs naturally infected with B. canis from Poland found increases of serum tumor necrosis factor alpha (TNF-α) concentration during canine babesiosis, which were associated with renal failure. The authors concluded that the increased TNF-α concentration influenced the development of hypotension and renal failure probably via TNF-α-mediated production of nitric oxide and induction of vasodilation and hypotension, leading to renal ischemia and hypoxia (Zygner et al. 2014). Another study that evaluated an experimental B. gibsoni infection of beagles found that it was associated with marked increases in the concentration of several cytokines such as TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, IL-6, IL7, and IL-18, which showed a delayed onset that followed the infection and occurred subsequent to the detection of peripheral parasitemia. Increases in the levels of the acute phase protein c-reactive protein (CRP) occurred a few days prior to the detection of parasitemia (Brown et al. 2015). A study on B. rossi from South Africa investigated cytokines in naturally infected dogs and evaluated whether their levels were associated with disease outcome. Ninety-seven dogs naturally infected with B. rossi were studied, and 15 healthy dogs were included as controls. IL-10 and monocyte chemotactic protein 1 (MCP-1) concentrations were significantly elevated for the Babesia-infected dogs compared to the healthy controls. In contrast, the IL-8 concentration was significantly decreased in the Babesia-infected dogs. Concentrations of IL-6 and MCP-1 were increased in the non-survivor dogs compared to the survivors. Concentrations of IL-2, IL-6, IL-18, and GM-CSF were significantly higher in those cases that presented during the more acute stage of the disease. The result demonstrates a mixed cytokine response in B. rossi infection, and the authors suggested that an excessive pro-inflammatory response might result in a poor outcome (Goddard et al. 2016).
Hemolytic anemia in canine babesiosis can be intravascular—within blood vessels—or extravascular, in parenchymal organs of which the spleen plays a major role. It often presents with a combination of erythrocyte destruction in both locations. Hemolytic anemia in canine babesiosis is multifactorial and associated with several mechanisms. It can occur due to direct red blood cell lyses produced by replicating intracellular parasites or due to the binding of antibodies to the erythrocyte cell membrane displaying parasite antigens leading to complement activation (Adachi et al. 1994, 1995; Carli et al. 2009). Furthermore, hemolytic anemia can be caused by the production of serum hemolytic factors, the oxidative damage of erythrocytes, an increased red blood cell phagocytosis, the creation of spherocytes, and a decrease in the osmotic fragility of red blood cells (Onishi et al. 1990; Makinde and Bobade 1994; Murase et al. 1996; Otsuka et al. 2001, 2002). Antibodies against red blood cells have been documented in dogs infected with B. gibsoni and B. vogeli (Adachi et al. 1994; Carli et al. 2009). Intense hemolysis results in hemoglobinemia, hemoglobinuria, bilirubinemia, and bilirubinuria.
Thrombocytopenia is frequently found in canine babesiosis and may be caused by immune-mediated mechanisms such as antibodies coating platelets, splenic sequestration, or coagulatory consumption of platelets from hemolytic or vascular injury. Thrombocytopenia has been demonstrated in experimental canine babesiosis caused by B. gibsoni as well as in natural infections with other Babesia species (Wilkerson et al. 2001; Sikorski et al. 2010; Brown et al. 2015; Eichenberger et al. 2016).
Particular virulence factors encoded by B. rossi have been identified and associated with the high pathogenic phenotype of this species. A polymorphic phosphoprotein localized on the cytoplasmic surface of B. rossi-infected red blood cells that has been named Babesia rossi erythrocyte membrane antigen 1 (BrEMA1) is suspected to be a virulence factor in B. rossi canine babesiosis (Matjila et al. 2009). The gene that encodes this protein is not found in other species of Babesia infecting dogs such as B. canis and B. vogeli. A preliminary study suggests that there are also clinically important differences in the virulence between various B. rossi genotypes/strains (Matjila et al. 2009). Different proportions of the prevalence of genotypes of the BC28 gene—encoding the major merozoite surface antigens of B. canis—have been found in different regions of Europe. These differences may be related to variable biologic properties of parasite strains within the same Babesia species (Carcy et al. 2015).
Tissue hypoxia is a severe consequence of canine babesiosis associated with multiple-organ damage. It has been studied in depth in B. rossi and B. canis infections (Leisewitz et al. 2001; Jacobson 2006; Mathe et al. 2007). The causes of hypoxia include anemia, hypotensive shock, vascular stasis by sludging of erythrocytes, excessive endogenous production of carbon monoxide, parasitic damage to hemoglobin, and decreased ability of hemoglobin to offload oxygen in Babesia-infected dogs (Ayoob et al. 2010). The central nervous system, kidney, and muscle are the organs most affected by the resultant tissue hypoxia (Jacobson 2006). The histological changes observed in the kidneys in naturally acquired B. canis infections included vacuolar-hydropic degeneration, necrosis, and detachment of renal tubular epithelial cells in the proximal convoluted tubules, while no significant histological changes were seen in the glomeruli (Mathe et al. 2007). Tubular hemoglobin casts and hemoglobin droplets in the renal tubular epithelial cells were more rarely observed (Mathe et al. 2007).
Tissue hypoxia, hypotensive shock, multiple-organ dysfunction, and high mortality rates have been documented mostly in association with B. rossi infection (Jacobson 2006; Reyers et al. 1998). Infection with this species may present acutely or even as a peracute and fatal syndrome with massive hemolysis, renal failure, and acid-base abnormalities (Leisewitz et al. 2001; Jacobson 2006). Free oxygen radical release and mechanisms associated with harmful cytokine effects have been associated with endothelial damage and increased vascular permeability in canine babesiosis. These may result in non-cardiogenic pulmonary edema (Jacobson 2006). B. rossi infection of dogs can lead to cerebral babesiosis and mortality due to a more severe consumptive coagulopathy compared to that found in dogs surviving clinical disease (Jacobson 2006; Goddard et al. 2013).
The spleen has an important function in controlling babesiosis (Homer et al. 2000). Splenectomized and immune-compromised dogs are more susceptible to infection with Babesia spp. Splenectomized dogs that are experimentally infected with Babesia spp. rapidly develop parasitemia and clinical disease and may reach high parasitemia levels (Vercammen et al. 1995). Accordingly, splenectomy is an important risk factor for the development of natural and potentially fatal babesiosis in humans and has been documented to be associated with clinical natural canine babesiosis (Rosner et al. 1984; Camacho et al. 2002; Sikorski et al. 2010).
10.2 Diagnosis and Epidemiology
10.2.1 Diagnosis
The diagnosis of canine babesiosis and detection of Babesia infection are carried out by several diagnostic techniques ranging from simple microscopy of blood that can be carried out in field conditions to advanced and very sensitive molecular techniques (Lempereur et al. 2017). Although observation of stained blood smear can usually distinguish between infection with large or small canine Babesia species, the morphology of parasites in the blood as observed by light microscopy is not sufficient in separating between the different large canine babesial species and between the varieties of small species. Another consideration when selecting an appropriate diagnostic method is that subclinical infection will be often associated with extremely low parasitemia, and therefore molecular techniques for the detection of parasite DNA should be preferred over the relatively less sensitive technique of blood smear microscopy. Canine blood donors or blood transfusion products should be tested by sensitive PCR detection protocols as parasitemia, if present, is expected to be low in apparently healthy donors (Solano-Gallego and Baneth 2011).
10.2.1.1 Detection by Light Microscopy
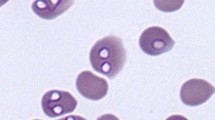
Observation of large or small species of Babesia in stained blood smears has been the standard for diagnosis for many years. This method is deemed reliable when a moderate to high parasitemia is present; however, there is not always a correlation between the level of parasitemia and the severity of clinical signs. Furthermore, the diagnosis of chronically infected and carrier dogs remains a diagnostic challenge due to low and often intermittent parasitemia that is frequently difficult to observe by microscopic evaluation. In those cases, the use of molecular diagnostic assays is strongly recommended. Smears made from ear tip or toe nail capillary blood may be beneficial in exhibiting large form Babesia parasites versus blood from a central vein (Bohm et al. 2006). A fresh smear is recommended for the accurate diagnosis of infection. Parasites may be detected in phagocytosed erythrocytes within macrophages (Fig. 10.2). Distinguishing between small and between large canine Babesia species based solely on morphology is not possible, and molecular analysis is required for speciation.
10.2.1.2 PCR-Based Molecular Detection
PCR is a sensitive and specific diagnostic technique frequently used for the detection of canine babesiosis and particularly useful for low parasitemia levels and for the determination of parasite species. A large number of PCR assays and protocols using a variety of gene targets have been described. Several PCR assays and additional procedures have been developed and used for the detection of canine babesiosis. Real-time PCR techniques have been developed to detect and quantify Babesia infection in canine blood (Qurollo et al. 2017). PCR-restriction fragment length polymorphism is also used to separate between canine Babesia species (Carret et al. 1999). A reverse line blotting (RLB) technique in which PCR products are hybridized to a membrane containing specific probes for the known babesial species and possibly for other pathogens has been developed for simultaneous detection of piroplasm species and coinfections. The RLB confirmed the presence of B. vogeli in addition to B. rossi in dogs from South Africa (Matjila et al. 2004). In addition, a high-resolution melting curve quantitative fluorescence resonance energy transfer-PCR has been developed to discriminate between species based on melting curve analysis (Wang et al. 2010).
10.2.1.3 Detection by Serology
Measurement of antibodies reactive with Babesia antigen indicates a past or present infection. The indirect fluorescent antibody test (IFAT) is the most commonly used test for canine babesiosis (Vercammen et al. 1995); however, cross-reactivity between different Babesia species and with other protozoan parasites occurs (Vercammen et al. 1995; Yamane et al. 1993). Enzyme-linked immunosorbent assays (ELISA) have been used in research and epidemiologic surveys (Schetters et al. 1996). The use of recombinant proteins such as the thrombospondin-related adhesive protein (TRAP) of B. gibsoni has been employed as an alternative for whole-parasite antigen with good sensitivities and specificities (Goo et al. 2008). False-negative results are possible in peracute or acute infection. In these cases, the use of convalescent antibody titers is strongly recommended to confirm acute infection.
10.2.2 Epidemiology
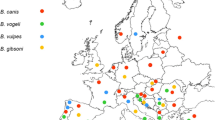
The geographical distribution of the causative agents and thus the occurrence of babesiosis are largely dependent on the habitat of their tick vector species, with the exception of B. gibsoni, where evidence for dog-to-dog transmission between fighting dog breeds independent of the vector tick has been presented (Birkenheuer et al. 2005; Jefferies et al. 2007; Yeagley et al. 2009). Babesia vogeli and B. gibsoni have a worldwide distribution, whereas B. rossi and B. canis have been mostly restricted to Africa and Europe, respectively. The unnamed large Babesia species most closely related to B. bigemina and B. conradae have been reported only from North America, whereas B. vulpes has been reported in Europe, Asia, and North America (Solano-Gallego and Baneth 2011).
10.2.2.1 Babesia rossi
Babesia rossi—( Babesia canis rossi) is a large form Babesia species, which has been described from South Africa as well as from other sub-Saharan African countries including Sudan and Nigeria (Adamu et al. 2014; Oyamada et al. 2005). Its tick vectors are Haemaphysalis elliptica and Haemaphysalis leachi, which were thought to be the same species in the past (Apanaskevich et al. 2007). It is considered the most virulent large Babesia species that affects dogs.
10.2.2.2 Babesia canis
Babesia canis—(B. canis canis) is a large Babesia mostly prevalent in central and northern Europe and transmitted by the tick Dermacentor reticulatus. It causes a moderate to severe disease that often has an acute onset (Zygner et al. 2014; Eichenberger et al. 2016).
10.2.2.3 Babesia vogeli
Babesia vogeli—( B. canis vogeli) is a large Babesia with a very wide distribution. It is transmitted by Rhipicephalus sanguineus sensu lato ticks and found mostly in tropical and subtropical regions including the Mediterranean basin, the Middle East, large areas of Asia, Australia, and South, Central, and North America (Solano-Gallego and Baneth 2011). It can cause subclinical infection or mild to moderate disease. It may cause a severe illness in young puppies and in greyhounds. Babesia vogeli-infected dogs often present with coinfections, frequently with Ehrlichia canis or Hepatozoon canis which are also transmitted by the same tick vector (Singla et al. 2016).
10.2.2.4 Babesia gibsoni
Babesia gibsoni is a small form Babesia that is endemic in Southeast Asia and appears to have spread from there to other continents including North and South America, Australia, and Europe. It is a common and often subclinical cause of infection in pit bull terriers but can also inflict a severe disease in this as well as in other dog breeds. It is transmitted by Haemaphysalis longicornis and possibly by H. bispinosa and R. sanguineus s.l. There is also evidence for its direct transmission by dog bites (Birkenheuer et al. 2005; Jefferies et al. 2007; Yeagley et al. 2009).
10.2.2.5 Babesia conradae
Babesia conradae is a small Babesia, which has mostly been reported in dogs from California and appears not to be prevalent outside North America. It was initially thought to be a strain of B. gibsoni but later found to be a genetically distinct species causing a severe and potentially fatal disease in dogs. The tick vector of B. conradae has not been described to date (Kjemtrup et al. 2006).
10.2.2.6 Babesia vulpes
Babesia vulpes described initially as Theileria annae and also termed Babesia microti-like and Babesia cf. microti is a third small species of Babesia that infects dogs (Zahler et al. 2000; Baneth et al. 2015). It was initially described from a dog that originated from Northern Spain and has subsequently been found in other European countries and in North America (Yeagley et al. 2009; Miró et al. 2015). Babesia vulpes is a common cause of infection of wild red foxes—i.e., Vulpes vulpes. Although it has been speculated that it is transmitted by the Hedgehog tick Ixodes hexagonus and/or by Dermacentor reticulatus, there is currently no definitive evidence of its transmission by any of these or by other tick species (Camacho et al. 2003; Hodžić et al. 2017).
10.2.2.7 Babesia sp. (Coco)
A currently unnamed large form Babesia species was detected for the first time in North Carolina in a dog under chemotherapy for lymphoma (Birkenheuer et al. 2004). Seven dogs infected with this pathogen were subsequently reported in eastern United States. All the dogs presented with immunocompromised conditions such as splenectomy or chemotherapy due to neoplasia (Sikorski et al. 2010). Analyses of the 18S rRNA gene of the unnamed large Babesia have revealed a unique sequence that shared a 93.9% identity with B. bigemina (Birkenheuer et al. 2004).
10.2.2.8 Rangelia vitalii
Rangelia vitalii is a piroplasm described in dogs in the southeast of Brazil, Uruguay, and Northern Argentina. This infection causes a disease referred to as Nambiuvú—i.e., bloody ears. The life cycle of R. vitalii is different from Babesia species because it has a tissue stage in the cytoplasm of endothelial cells as well as a developmental phase in blood cells. Piroplasm-like intracellular organisms of R. vitalii have been described in erythrocytes, monocytes, and neutrophils by observation of stained blood smears, and it has been reported to be transmitted by the tick Amblyomma aureolatum. Clinical manifestations are associated with fever, anemia, jaundice, splenomegaly, lymphadenomegaly, hemorrhage in the gastrointestinal tract, and persistent bleeding from the nose, oral cavity, and the ear pinna (Franca et al. 2010; Da Silva et al. 2011; Eiras et al. 2014). It also has been described as a cause of disease in wild canids in South America (Fredo et al. 2015).
10.3 Clinical Effects, Prevention, and Treatment
10.3.1 Clinical Effects
The clinical manifestations of babesiosis are mainly dependent on the infecting species and host-related factors. The main effects of babesiosis are related to anemia, tissue anoxia, and effect of toxins and inflammatory mediators produced during infection. Babesiosis due to B. rossi can present with clinical signs similar to those described for other babesial species such as fever, dehydration, lethargy, pale mucous membranes, and anorexia or with complicated severe clinical disorders including acute renal failure with anuria, icterus, hypotension, acute respiratory distress syndrome (ARDS), vomiting, diarrhea, pancreatitis, myalgia, rhabdomyolysis, ascites, pulmonary edema, encephalomyelitis, and peracute shock. Dogs with B. rossi infection may present with mild to severe anemia, thrombocytopenia, leucocytosis, bilirubinemia, pigmenturia, bilirubinuria, hypoglycaemia, acid-base imbalances, azotemia, and hyperlactemia.
The main clinical and clinicopathological findings reported in dogs suffering from B. canis infection include dehydration, lethargy, anorexia, fever, lethargy and dehydration with mild to severe thrombocytopenia, hyperfibrinogenemia, mild to severe anemia, hemolysis, and neutropenia (Adaszek et al. 2009; Eichenberger et al. 2016). Hemoglobinuria has also been described in urinalysis of naturally infected dogs. Babesia vogeli causes a mild to moderate clinical disease, which often accompanies other concomitant diseases or immunosuppressive conditions or affects splenectomized dogs. Severe fatal hemolytic anemia has been reported in puppies. The most common laboratory findings are regenerative anemia and thrombocytopenia (Solano-Gallego et al. 2008). The clinicopathological findings in B. gibsoni infection include fever, regenerative anemia, thrombocytopenia, splenomegaly, lymphadenomegaly, hepatomegaly, and lethargy. Babesia conradae infection has been described as more virulent than B. gibsoni infection resulting in higher parasitemia, more pronounced anemia, and higher rate of mortality. The clinicopathological findings resemble those reported in B. gibsoni infections. The most common clinical findings reported in dogs infected with B. vulpes from the north west of Spain include fever, lethargy, weakness, azotemia and pigmenturia. Infected dogs had moderate to severe regenerative anemia and thrombocytopenia (Guitián et al. 2003; Miró et al. 2015).
10.3.2 Prevention
Prevention of babesiosis is based on acaricidal treatments administered topically, systematically, orally as chewable tablets, or spread in the environment. Acaricidal treatments are aimed at reducing the exposure to vector ticks, their bites, and the transmission of the pathogen to dogs. A variety of products based on commercially available acaricidal chemicals have been tested for efficacy in the prevention of babesial infections under experimental or field conditions, and have been licensed for use in dogs (Solano-Gallego et al. 2016). As Babesia species are transmitted by blood product transfusions, it is recommended to screen canine blood donors for Babesia infection on a regular basis (Wardrop et al. 2016). Non-vectorial dog-to-dog transmission of babesia by fighting can be responsible for the spread of babesiosis into previously non-endemic areas. Vaccines against canine Babesia species are commercially available in some countries in Europe.
10.3.3 Treatment
The differences between Babesia species that infect dogs are also reflected in their susceptibility to drugs. Accurate detection and species recognition are important for the selection of the correct therapy and predicting the course of disease. While large form Babesia species of dogs are usually susceptible to certain drugs, small form Babesia are often resistant to these drugs, and treatment of their infections requires the use of other drugs and combinations of drugs. Large Babesia species infections of dogs are commonly treated with one dose of imidocarb dipropionate at 5–6 mg per kilogram dog weight intramuscular (IM) or subcutaneously (SC) with good clinical response and a repeated dose 14 days later. Large Babesia species and Rangelia vitalii have been reported to respond to diminazene aceturate treatment at 3.5 mg/kg IM once.
Babesia gibsoni and B. conradae infections are often resistant to imidocarb dipropionate and diminazene aceturate. The treatment of choice for these small Babesia species is the combination of the antimalarial atovaquone and the macrolide azithromycin. The most commonly used dose of atovaquone is 13.5 mg/kg, administered orally (PO) every 8 h with fatty food to maximize drug absorption, in combination with azithromycin at 10 mg/kg PO for 10 days. Buparvaquone at 5 mg/kg IM, 2 days apart in combination with azithromycin at 10 mg/kg PO for 10 days, has also been studied for the treatment of dogs infected with B. vulpes and found less effective than the combination of atovaquone and azithromycin (Checa et al. 2017). Resistance to atovaquone associated with irresponsiveness to treatment has been described in B. gibsoni in dogs from Japan and Taiwan and results from mutations in the parasite cytochrome b gene (Sakuma et al. 2009; Iguchi et al. 2012; Liu et al. 2016).
Parasitological cure with complete elimination of the parasite is commonly not achieved by treatment of small Babesia spp. infections in dogs, and clinical relapses frequently occur. Medical management of infection may require supportive treatments including blood transfusions, intravenous fluids, and the use of anti-inflammatory drugs. The prognosis of dogs infected with large forms of Babesia species and treated with effective drugs is generally good in uncomplicated disease. Canine disease with small Babesia spp. may be more resistant to treatment and carry a poorer prognosis. Currently, there are no zoonotic canine Babesia species known.
References
Adachi K, Tateishi M, Horii Y, Nagatomo H, Shimizu T, Makimura S. Elevated erythrocyte-bound IgG value in dogs with clinical Babesia gibsoni infection. J Vet Med Sci. 1994;56:757–9.
Adachi K, Tateishi M, Horii Y, Nagatomo H, Shimizu T, Makimura S. Immunologic characteristics of anti-erythrocyte membrane antibody produced in dogs during Babesia gibsoni infection. J Vet Med Sci. 1995;57:121–3.
Adamu M, Troskie M, Oshadu DO, Malatji DP, Penzhorn BL, Matjila PT. Occurrence of tick-transmitted pathogens in dogs in Jos, Plateau State, Nigeria. Parasit Vectors. 2014;7:119.
Adaszek Ł, Winiarczyk S, Skrzypczak M. The clinical course of babesiosis in 76 dogs infected with protozoan parasites Babesia canis canis. Pol J Vet Sci. 2009;12:81–7.
Apanaskevich DA, Horak IG, Camicas JL. Redescription of Haemaphysalis (Rhipistoma) elliptica (Koch, 1844), an old taxon of the Haemaphysalis (Rhipistoma) leachi group from east and southern Africa, and of Haemaphysalis (Rhipistoma) leachi (Audouin, 1826) (Ixodida, Ixodidae). Onderstepoort J Vet Res. 2007;74:181–208.
Ayoob AL, Hackner SG, Prittie J. Clinical management of canine babesiosis. J Vet Emerg Crit Care (San Antonio). 2010;20:77–89.
Baneth G, Florin-Christensen M, Cardoso L, Schnittger L. Reclassification of Theileria annae as Babesia vulpes sp. nov. Parasit Vectors. 2015;8:207.
Birkenheuer AJ, Neel J, Ruslander D, Levy MG, Breitschwerdt EB. Detection and molecular characterization of a novel large Babesia species in a dog. Vet Parasitol. 2004;124:151–60.
Birkenheuer AJ, Correa MT, Levy MG, Breitschwerdt EB. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000–2003). J Am Vet Med Assoc. 2005;227:942–7.
Bohm M, Leisewitz AL, Thompson PN, Schoeman JP. Capillary and venous Babesia canis rossi parasitaemias and their association with outcome of infection and circulatory compromise. Vet Parasitol. 2006;141:18–29.
Brown AL, Shiel RE, Irwin PJ. Clinical, haematological, cytokine and acute phase protein changes during experimental Babesia gibsoni infection of beagle puppies. Exp Parasitol. 2015;157:185–96.
Camacho AT, Pallas E, Gestal JJ, Guitian FJ, Olmeda AS. Babesia canis infection in a splenectomized dog. Bull Soc Pathol Exot. 2002;95:17–9.
Camacho AT, Pallas E, Gestal JJ, Guitián FJ, Olmeda AS, Telford SR, Spielman A. Ixodes hexagonus is the main candidate as vector of Theileria annae in northwest Spain. Vet Parasitol. 2003;112:157–63.
Carcy B, Randazzo S, Depoix D, Adaszek L, Cardoso L, Baneth G, Gorenflot A, Schetters TP. Classification of Babesia canis strains in Europe based on polymorphism of the Bc28.1-gene from the Babesia canis Bc28 multigene family. Vet Parasitol. 2015; 211(3–4):111–23.
Carli E, Tasca S, Trotta M, Furlanello T, Caldin M, Solano-Gallego L. Detection of erythrocyte binding IgM and IgG by flow cytometry in sick dogs with Babesia canis canis or Babesia canis vogeli infection. Vet Parasitol. 2009;162:51–7.
Carret C, Walas F, Carcy B, Grande N, Precigout E, Moubri K, Schetters TP, Gorenflot A. Babesia canis canis, Babesia canis vogeli, Babesia canis rossi: differentiation of the three subspecies by a restriction fragment length polymorphism analysis on amplified small subunit ribosomal RNA genes. J Eukaryot Microbiol. 1999;46:298–303.
Chauvin A, Moreau E, Bonnet S, Plantard O, Malandrin L. Babesia and its hosts: adaptation to long-lasting interactions as a way to achieve efficient transmission. Vet Res. 2009;40:37.
Checa R, Montoya A, Ortega N, González-Fraga JL, Bartolomé A, Gálvez R, Marino V, Miró G. Efficacy, safety and tolerance of imidocarb dipropionate versus atovaquone or buparvaquone plus azithromycin used to treat sick dogs naturally infected with the Babesia microti-like piroplasm. Parasit Vectors. 2017;10:14.
Da Silva AS, Franca RT, Costa MM, Paim CB, Paim FC, Dornelles GL, Soares JF, Labruna MB, Mazzanti CM, Monteiro SG, Lopes ST. Experimental infection with Rangelia vitalii in dogs: acute phase, parasitemia, biological cycle, clinical-pathological aspects and treatment. Exp Parasitol. 2011;128:347–52.
Eichenberger RM, Riond B, Willi B, Hofmann-Lehmann R, Deplazes P. Prognostic markers in acute Babesia canis infections. J Vet Intern Med. 2016;30:174–82.
Eiras DF, Craviotto MB, Baneth G, Moré G. First report of Rangelia vitalii infection (canine rangeliosis) in Argentina. Parasitol Int. 2014;63:729–34.
Franca RT, Silva AS, Paim FC, Costa MM, Soares JF, Mazzanti CM, Lopes STA. Rangelia vitalii in dogs in southern Brazil. Comp Clin Path. 2010;19:383–7.
Fredo G, Bianchi MV, De Andrade CP, De Souza SO, Leite-Filho RV, Bandinelli MB, Amorim DB, Driemeier D, Sonne L. Natural infection of wild canids (Cerdocyon thous and Lycalopex gymnocercus) with the Intraendothelial Piroplasm Rangelia vitalii in southern Brazil. J Wildl Dis. 2015;51:880–4.
Freeman MJ, Kirby BM, Panciera DL, Henik RA, Rosin E, Sullivan LJ. Hypotensive shock syndrome associated with acute Babesia canis infection in a dog. J Am Vet Med Assoc. 1994;204:94–6.
Fukumoto S, Suzuki H, Igarashi I, Xuan X. Fatal experimental transplacental Babesia gibsoni infections in dogs. Int J Parasitol. 2005;35:1031–5.
Goddard A, Wiinberg B, Schoeman JP, Kristensen AT, Kjelgaard-Hansen M. Mortality in virulent canine babesiosis is associated with a consumptive coagulopathy. Vet J. 2013;196:213–7.
Goddard A, Leisewitz AL, Kjelgaard-Hansen M, Kristensen AT, Schoeman JP. Excessive pro-inflammatory serum cytokine concentrations in virulent canine babesiosis. PLoS One. 2016;11:e0150113.
Goo YK, Jia H, Aboge GO, Terkawi MA, Kuriki K, Nakamura C, Kumagai A, Zhou J, Lee EG, Nishikawa Y, Igarashi I, Fujisaki K, Xuan X. Babesia gibsoni: serodiagnosis of infection in dogs by an enzyme-linked immunosorbent assay with recombinant BgTRAP. Exp Parasitol. 2008;118:555–60.
Guitián FJ, Camacho AT, Telford SR III. Case-control study of canine infection by a newly recognised Babesia microti-like piroplasm. Prev Vet Med. 2003;61:137–45.
Hodžić A, Zörer J, Duscher GG. Dermacentor reticulatus, a putative vector of Babesia cf. microti (syn. Theileria annae) piroplasm. Parasitol Res. 2017;116:1075–7.
Homer MJ, Aguilar-Delfin I, Telford SR III, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13:451–69.
Iguchi A, Matsuu A, Ikadai H, Talukder MH, Hikasa Y. Development of in vitro atovaquone-resistant Babesia gibsoni with a single-nucleotide polymorphism in cytb. Vet Parasitol. 2012;185:145–50.
Irwin PJ. Canine babesiosis: from molecular taxonomy to control. Parasit Vectors. 2009;26(2 Suppl 1):S4.
Jacobson LS. The south African form of severe and complicated Canine babesiosis: clinical advances 1994-2004. Vet Parasitol. 2006;138:126–39.
Jefferies R, Ryan UM, Jardine J, Broughton DK, Robertson ID, Irwin PJ. Blood, Bull Terriers and babesiosis: further evidence for direct transmission of Babesia gibsoni in dogs. Aust Vet J. 2007;85:459–63.
Kjemtrup AM, Wainwright K, Miller M, Penzhorn BL, Carreno RA. Babesia conradae, sp. nov., a small canine Babesia identified in California. Vet Parasitol. 2006;138:103–11.
Konishi K, Sakata Y, Miyazaki N, Jia H, Goo YK, Xuan X, Inokuma H. Epidemiological survey of Babesia gibsoni infection in dogs in Japan by enzyme-linked immunosorbent assay using B. gibsoni thrombospondin-related adhesive protein antigen. Vet Parasitol. 2008;155:204–8.
Lack JB, Reichard MV, van den Bussche RA. Phylogeny and evolution of the piroplasmida as inferred from 18S rRNA sequences. Int J Parasitol. 2012;42:353–63.
Lee MJ, DH Y, Yoon JS, Li YH, Lee JH, Chae JS, Park J. Epidemiologic and clinical surveys in dogs infected with Babesia gibsoni in South Korea. Vector Borne Zoonotic Dis. 2009;9:681–6.
Leisewitz AL, Jacobson LS, de Morais HS, Reyers F. The mixed acid-base disturbances of severe canine babesiosis. J Vet Intern Med. 2001;15:445–52.
Lempereur L, Beck R, Fonseca I, Marques C, Duarte A, Santos M, Zúquete S, Gomes J, Walder G, Domingos A, Antunes S, Baneth G, Silaghi C, Holman P, Zintl A. Guidelines for the detection of Babesia and Theileria parasites. Vector Borne Zoonotic Dis. 2017;17:51–65.
Liu PC, Lin YL, Lin CN, BL S. A SimpleProbe(®) real-time PCR assay for differentiating the cytochrome b M121I mutation in clinical specimens from dogs infected with Babesia gibsoni. Ticks Tick Borne Dis. 2016;7:639–43.
Makinde MO, Bobade PA. Osmotic fragility of erythrocytes in clinically normal dogs and dogs infected with parasites. Res Vet Sci. 1994;57:343–8.
Mathe A, Dobos-Kovacs M, Voros K. Histological and ultrastructural studies of renal lesions in Babesia canis infected dogs treated with imidocarb. Acta Vet Hung. 2007;55:511–23.
Matjila PT, Penzhorn BL, Bekker CP, Nijhof AM, Jongejan F. Confirmation of occurrence of Babesia canis vogeli in domestic dogs in South Africa. Vet Parasitol. 2004;122:119–25.
Matjila PT, Carcy B, Leisewitz AL, Schetters T, Jongejan F, Gorenflot A, Penzhorn BL. Preliminary evaluation of the BrEMA1 gene as a tool for associating Babesia rossi genotypes and clinical manifestation of canine babesiosis. J Clin Microbiol. 2009;47:3586–92.
Mehlhorn H, Schein E. The piroplasms: life cycle and sexual stages. Adv Parasitol. 1984;23:37–103.
Mehlhorn H, Peters W, Haberkorn A. The formation of kinetes and oocysts in Plasmodium gallinaceum and considerations on phylogenetic relationships between Haemosporidia, Piroplasmida, and other Coccidia. Protistologica. 1980;16:135–54.
Mehlhorn H, Schein E, Ahmed JS. Theileria. In: Kreier JP, editor. Parasitic protozoa, vol. 7. San Diego: Academic; 1994. p. 217–304.
Miró G, Checa R, Paparini A, Ortega N, González-Fraga JL, Gofton A, Bartolomé A, Montoya A, Gálvez R, Mayo PP, Irwin P. Theileria annae (syn. Babesia microti-like) infection in dogs in NW Spain detected using direct and indirect diagnostic techniques: clinical report of 75 cases. Parasit Vectors. 2015;8:217.
Murase T, Ueda T, Yamato O, Tajima M, Maede Y. Oxidative damage and enhanced erythrophagocytosis in canine erythrocytes infected with Babesia gibsoni. J Vet Med Sci. 1996;58:259–61.
Onishi T, Ueda K, Horie M, Kajikawa T, Ohishi I. Serum hemolytic activity in dogs infected with Babesia gibsoni. J Parasitol. 1990;76:564–7.
Otsuka Y, Yamasaki M, Yamato O, Maede Y. Increased generation of superoxide in erythrocytes infected with Babesia gibsoni. J Vet Med Sci. 2001;63:1077–81.
Otsuka Y, Yamasaki M, Yamato O, Maed Y. The effect of macrophages on the erythrocyte oxidative damage and the pathogenesis of anemia in Babesia gibsoni-infected dogs with low parasitemia. J Vet Med Sci. 2002;64:221–6.
Oyamada M, Davoust B, Boni M, Dereure J, Bucheton B, Hammad A, Itamoto K, Okuda M, Inokuma H. Detection of Babesia canis rossi, B. canis vogeli, and Hepatozoon canis in dogs in a village of eastern Sudan by using a screening PCR and sequencing methodologies. Clin Diagn Lab Immunol. 2005;12:1343–6.
Qurollo BA, Archer NR, Schreeg ME, Marr HS, Birkenheuer AJ, Haney KN, ThomasBS BEB. Improved molecular detection of Babesia infections in animals using a novel quantitative real-time PCR diagnostic assay targeting mitochondrial DNA. Parasit Vectors. 2017;10:128.
Radi ZA, Styer EL, Frazier KS. Electron microscopic study of canine Babesia gibsoni infection. J Vet Diagn Investig. 2004;16:229–33.
Reyers F, Leisewitz AL, Lobetti RG, Milner RJ, Jacobson LS, van Zyl M. Canine babesiosis in South Africa: more than one disease. Does this serve as a model for falciparum malaria? Ann Trop Med Parasitol. 1998;92:503–11.
Rosner F, Zarrabi MH, Benach JL, Habicht GS. Babesiosis in splenectomized adults. Review of 22 reported cases. Am J Med. 1984;76:696–701.
Sakuma M, Setoguchi A, Endo Y. Possible emergence of drug-resistant variants of Babesia gibsoni in clinical cases treated with atovaquone and azithromycin. J Vet Intern Med. 2009;23:493–8.
Schein E, Mehlhorn H, Voigt WP. Electron microscopical studies on the development of Babesia canis (Sporozoa) in the salivary glands of the vector tick Dermacentor reticulatus. Acta Trop. 1979;36:229–41.
Schetters TP, Scholte NC, Kleuskens JA, Bos HJ. Not peripheral parasitaemia but the level of soluble parasite antigen in plasma correlates with vaccine efficacy against Babesia canis. Parasite Immunol. 1996;18:1–6.
Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: a world emerging. Infect Genet Evol. 2012;12:1788–809.
Schreeg ME, Marr HS, Tarigo JL, Cohn LA, Bird DM, Scholl EH, Levy MG, Wiegmann BM, Birkenheuer AJ. Mitochondrial genome sequences and structures aid in the resolution of Piroplasmida phylogeny. PLoS One. 2016;11:e0165702.
Sikorski LE, Birkenheuer AJ, Holowaychuk MK, McCleary-Wheeler AL, Davis JM, Littman MP. Babesiosis caused by a large Babesia species in 7 immunocompromised dogs. J Vet Intern Med. 2010;24:127–31.
Singla LD, Sumbria D, Mandhotra A, Bal MS, Kaur P. Critical analysis of vector-borne infections in dogs: Babesia vogeli, Babesia gibsoni, Ehrlichia canis and Hepatozoon canis in Punjab, India. Acta Parasitol. 2016;61:697–706.
Solano-Gallego L, Baneth G. Babesiosis in dogs and cats—expanding parasitological and clinical spectra. Vet Parasitol. 2011;181:48–60.
Solano-Gallego L, Trotta M, Carli E, Carcy B, Caldin M, Furlanello T. Babesia canis canis and Babesia canis vogeli clinicopathological findings and DNA detection by means of PCR-RFLP in blood from Italian dogs suspected of tick-borne disease. Vet Parasitol. 2008;157:211–21.
Solano-Gallego L, Sainz Á, Roura X, Estrada-Peña A, Miró G. A review of canine babesiosis: the European perspective. Parasit Vectors. 2016;9:336.
Stegeman JR, Birkenheuer AJ, Kruger JM, Breitschwerdt EB. Transfusion-associated Babesia gibsoni infection in a dog. J Am Vet Med Assoc. 2003;222:959–63.
Uilenberg G. Babesia—a historical overview. Vet Parasitol. 2006;138:3–10.
Vercammen F, De Deken R, Maes L. Clinical and serological observations on experimental infections with Babesia canis and its diagnosis using the IFAT. Parasite. 1995;2:407–10.
Walter S, Mehlhorn H, Zweygarth E, Schein E. Electron microscopic investigations on stages of dog piroplasms cultured in vitro: Asian isolates of Babesia gibsoni and strains of B. canis from France and Hungary. Parasitol Res. 2002;88:32–7.
Wang C, Ahluwalia SK, Li Y, Gao D, Poudel A, Chowdhury E, Boudreaux MK, Kaltenboeck B. Frequency and therapy monitoring of canine Babesia spp. infection by high-resolution melting curve quantitative FRET-PCR. Vet Parasitol. 2010;168:11–8.
Wardrop KJ, Reine N, Birkenheuer A, Hale A, Hohenhaus A, Crawford C, Lappin MR. Canine and feline blood donor screening for infectious disease. J Vet Intern Med. 2005;19:135–42.
Wardrop KJ, Birkenheuer A, Blais MC, Callan MB, Kohn B, Lappin MR, Sykes J. Update on canine and feline blood donor screening for blood-borne pathogens. J Vet Intern Med. 2016;30:15–35.
Wilkerson MJ, Shuman W, Swist S, Harkin K, Meinkoth J, Kocan AA. Platelet size, platelet surface-associated IgG, and reticulated platelets in dogs with immune-mediated thrombocytopenia. Vet Clin Pathol. 2001;30:141–9.
Yamane I, Thomford JW, Gardner IA, Dubey JP, Levy M, Conrad PA. Evaluation of the indirect fluorescent antibody test for diagnosis of Babesia gibsoni infections in dogs. Am J Vet Res. 1993;54:1579–84.
Yeagley TJ, Reichard MV, Hempstead JE, Allen KE, Parsons LM, White MA, Little SE, Meinkoth JH. Detection of Babesia gibsoni and the canine small Babesia 'Spanish isolate' in blood samples obtained from dogs confiscated from dog fighting operations. J Am Vet Med Assoc. 2009;235:535–9.
Zahler M, Rinder H, Schein E, Gothe R. Detection of a new pathogenic Babesia microti-like species in dogs. Vet Parasitol. 2000;89:241–8.
Zygner W, Gójska-Zygner O, Bąska P, Długosz E. Increased concentration of serum TNF alpha and its correlations with arterial blood pressure and indices of renal damage in dogs infected with Babesia canis. Parasitol Res. 2014;113:1499–503.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Baneth, G. (2018). Babesia of Domestic Dogs. In: Florin-Christensen, M., Schnittger, L. (eds) Parasitic Protozoa of Farm Animals and Pets. Springer, Cham. https://doi.org/10.1007/978-3-319-70132-5_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-70132-5_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-70131-8
Online ISBN: 978-3-319-70132-5
eBook Packages: MedicineMedicine (R0)