Abstract
An injury to the human nervous system, which plays a major role in our daily lives by being involved in our thought and action processes, has been one of the greatest issues in the medical field. Social costs are considerably high because of these injuries and the many ongoing studies searching for cures to nervous system injuries. As a result of these efforts, electrospinning technology has been found to a suitable alternative to fabricating scaffolds for nerve regeneration. The electrospun nanofibrous scaffold can provide the regenerating nervous system with cell-friendly environments that have sufficient porosity, mechanical strength, guidance cues, etc. First, the anatomies of the central and peripheral nervous systems and their regeneration mechanisms are introduced and compared to each other. Second, the mechanisms, requirements, and favored properties are discussed. Finally, various fabrication methods and the current evolving concept of electrospun nerve conduits with functionalization strategies such as cell loading, neurotrophic biomolecule or nanoparticle immobilization, and conductive polymer use are discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The human nervous system is responsible for the thought processes and action control that occurs in our bodies. The nervous system consists of two main parts: the central nervous system (CNS) and peripheral nervous system (PNS), which are based on location and functions. The PNS collects and integrates somatic and autonomic information from different sensory nerves and organs throughout the trunk and extremities. The CNS determines the responses based on the collected information and sends commands throughout our body via the PNS. These processes are conducted literally millions of times each minute of our daily lives [1].
Despite being connected systems and having similarities in functions and gross anatomy, the CNS and PNS show distinct differences. For instance, the healing capacities of PNS injuries are considerably greater than those of CNS lesions owing to the difference in the intrinsic properties of neurons and extrinsic cellular environments.
With the nervous system playing such a major role, an injury to the system can cause not only a socio-economic problem but a decline in an individual’s quality of life. For example, spinal cord injuries (SCI) can cause various clinical manifestations including loss of motor control and sensation, pain, numbness in related areas, and rarely death. The one-year survival rate of SCIs in the U.S. (2015, NSCISC annual statistical report) was reported to be higher than 95.5%, due in part to the fact that damage to the spinal cord below the cervical level rarely leads to death. However, many American Spinal Injury Association (ASIA) Classification “complete” injury cases result in permanent disabilities because neuronal cells generally fail to regenerate in a CNS injury [2,3,4]. The lifetime healthcare costs for a 25-year-old patient with cervical level tetraplegia are expected to be more than $4 million. Even though there are various biological, pharmacological, mechanical, and surgical treatments to overcome these problems, there is no established treatment strategy yet [5,6,7].
Even if these therapeutic strategies do not guarantee full recovery from spinal injury, by facilitating functional recovery in the early stages of post-trauma, overall lifetime expenses are reduced and quality of life is increased. On the other hand, peripheral nerve injuries can be healed and can recover to some degree from neurotmesis, although complete functionality is not recovered and some complications may arise even after recovery [8].
Physicians decide how to go about treatment by taking into account many different factors, but the deciding factor is the size of the defective gap between the two stumps. Surgical approaches, tensioned direct repair, and grafting techniques have been the conventional treatments for nerve injuries in the past. The autograft transplantation has become the most popular surgical technique since Berger and Millesi demonstrated its superiority over direct tension repairs if the injured nerve gap is narrow enough to avoid tension [9]. The autograft is an instantly available source in most cases and also offers the following advantages: a peripheral nerve-friendly environment, guidance cues, perineurium scaffolding, and the support of Schwann cells. Although autograft transplantation has been the treatment of choice for the past few decades, many complications and limitations still remain [10].
The reported complications from autograft nerve repair include tender neuroma formation, dysesthesia, paresthesia, paralysis, contracture, etc. excluding iatrogenic complications. They are more frequently found in wounds with inadequate tension and vascularization. Also, the donor site from where the autograft tissue is harvested suffers from a permanent nerve injury and potential postoperative complications from the required additional incision. More recently, alternative sutureless nerve repair techniques by coaptation using fibrin glue and laser have come into the limelight offering competitive benefits [11]. Sutureless techniques are arguably more efficient than conventional techniques, eliminate the tension caused by suturing, and improve the alignment of fascicles.
Another emerging technology, the bioartificial nerve conduit, is a supporting tube-like scaffold proposed as a promising new alternative or complementary therapeutic technology to autografts. With extensive researches and advantages, nerve conduit techniques are considered to be the current gold standard for nerve repair [12]. This scaffolding technique focuses on entubulation, the guiding of axonal regrowth through an enclosed tubal structure. One of the significant advantages of the entubulating nerve conduit is that it is able to be used to upgrade other conventional suture techniques and sutureless techniques or even as a stand-alone procedure.
Various artificial nerve conduit researches are being carried out to enhance functionality and improve properties such as biomimetics, nanotopography, material selection, and enhancement by the addition of biomolecules [13]. The biodegradable aligned electrospun nanofibrous nerve conduit is currently the option with the best nanotopographic properties.
The aligned electrospun nanofibrous nerve conduit is fabricated by electrospinning, which is one of the most suitable techniques for producing nerve conduits. Electrospinning is also one of the simplest methods that can produce aligned topography with tunable porosity from a large variety of materials and additive substances. The tailored surface topography and porosity of aligned electrospun nanofibrous nerve conduits foster nerve regeneration by providing guidance cues, permissiveness, and a cell-friendly environment [14, 15]. Also, the polymer composition for nerve conduit and doping substances also influences nerve regeneration with controllable drug loading capacity of electrospun nanofibers. Although nerve conduit technology is commonly used to facilitate nerve regeneration in the PNS, it is also expected to take part in CNS injury treatment combined with other therapeutic strategies such as cell transplantation, neurotrophic factors, and nanoparticles [16, 17].
We will discuss in brief nerve regeneration physiology, electrospinning methods for obtaining aligned nanofibers, and the role of aligned nanofibers in cell guidance in vitro and in vivo, by comparing aligned fibers with randomly oriented nanofibrous fibers.
1.1 Anatomy of a Nerve and Neuroregeneration
The human nervous system has two main components: the central nervous system and the peripheral nervous system. The CNS is composed of the brain and spinal cord while the PNS can be categorized into spinal nerves and cranial nerves according to the initiation lesion/level or three groups according to the direction of signal conduction: afferent nerves, efferent nerves, and mixed nerves. A nerve is a cord-like axonal bundle in the PNS that delivers electric and chemical signals from the CNS to the innervated organ and vice versa. Axons are covered by the endoneurium, an outer layer of connective tissue, and bundle up into fascicles, which are wrapped in the perineurium. These fascicles are bundled together to finally form the hierarchical structure of the nerve covered by the epineurium, the outermost layer of connective tissue as seen in Fig. 1 [1].
The schematic of layered-structure of a nerve (a), histologic picture of a nerve (b) (tissue source: simian). LM × 40. Image by OpenStax College, licensed under CC BY 3.0 [18]
As seen in Fig. 2, the two nervous systems show several big differences in their functions, structures and, more importantly, in their regeneration processes. The difference in regenerative capacities between the two systems is explained mainly by their intrinsic regenerative potentials and different glial cells, also known as signal transduction assistants, and their different reactions to a damaged neuron [19, 20].
Slender peripheral nerves are usually located at mechanically vulnerable positions surrounded by tissues. They can be easily injured by various traumatic events including cuts and compressions. Once a nerve is damaged, the remnant distal nerve tissues undergo Wallerian degeneration, the fragmentation and disintegration of the axon, to start the regenerative process in which Schwann cells, the basal lamina, and the neurilemma around the damaged sites begin to construct a primitive regeneration tube [22,23,24]. Upregulation of regeneration-associated genes (RAGs) with nerve growth factors (NGFs) also highly contributes to this process. Once the regeneration tube is completed, the nerves grow guided by the tube to reach its destination [25]. However, this entire regenerative process takes several months and incomplete functional restoration often occurs when the regenerative processes are not able to complete their tasks. Regeneration in the CNS used to be regarded as an unachievable goal due to the limited intrinsic capacity for axonal growth and inhibitory milieu. The inhibitory environmental cues in the CNS were experimentally demonstrated by David and Aguayo, transplanting peripheral nerve graft to the CNS and vice versa. The transplanted PNS nerve graft vastly promoted CNS neuron regeneration but the CNS grafts dampened the regeneration capabilities of the PNS neuron [26]. The incapability of the CNS neuron to recover postpones prompt initiation of axonal regeneration. Moreover, the injury site is further beset by secondary reactive events.
First, the glial cellular function of oligodendrocytes as a scavenger is generally unsuccessful. As a result, they fail to rejuvenate and to make matters worse, the astrocyte-supported oligodendrocytes secrete inhibitory substances instead of helpful trophic factors. Secretory inhibitors including myelin-associated inhibitors (MAIs) and chondroitin sulfate proteoglycans (CSPGs) coincide the upregulation of RAGs such as c-Jun, activating transcription factor-3 (ATF-3), SRY-box containing gene 11 (Sox11), small proline-repeat protein 1A (SPRR1A), growth-associated protein-43 (GAP-43) and CAP-23 [27,28,29,30,31]. Finally, they initiate fibrosis and a dense scar formation, or sometimes a cyst formation, which acts as a natural obstacle that prevents the nerve stumps from reuniting by blocking neural cell migration. Figure 3 is a graphical representation of the regeneration process of the axon.
1.2 Nerve Guide Conduits
1.2.1 Requirements of Nerve Conduit
The history of manufactured nerve conduits is traced back to the tube-shaped decalcified bone made by Gluck and his colleagues for the connection of transected nerve ends in the 1880s before the microsurgery [32]. This trial for the treatment of neurotmesis injuries by an encapsulation strategy continued until Dahlin and Lundborg’s silicon-based nerve conduit. Dahlin and Lundborg characterized the mechanism of nerve regeneration in a tube-structured nerve conduit. The nerve conduit encases both nerve stumps within the lumen of the tube to provide a gross alignment and fluid accumulation between the stumps for nerve regeneration. The fluid accumulated inside the tube’s inner chamber initiates a rudimentary fibrin matrix formation that connects the two nerve stumps. When the accumulation reaches a sufficient amount, this primitive fibrin matrix can serve as a bridge for cell migration [23]. Once the cells have migrated, connecting structures called Büngner’s linear band forms within the disordered rudimentary fibrin matrix. The juvenile neurites grow along these linear bands surrounded by newly-created pseudo nerve sheaths.
This process depends heavily on the sufficiency of fluid leakage volume from both nerve ends. If the volume of fluid is insufficient to fill up the inner lumen, the newly formed fibrin matrix and neo nerve can often be too thin and weak due to the mechanical contraction. The thinner nerve regeneration alters the functional regain because the axonal regeneration is proportional to the thinnest cross sectional diameter of the cable [33]. Also, there has been another issue that can cause neuroma formation by tightly holding neurites to prevent them from potential escape. Even with these limitations, the nerve encapsulation strategy demonstrates apparent benefits. The axoplasm and milieu-containing nerve conduits provide treatment to the injured nerve tissue by keeping it away from the inflammation of the wound bed.
A series of mixed nerve repairs in the forearm were researched by the Lundborg group and a 5-year follow-up study reported that the peripheral nervous tubal scaffolding strategy is highly comparable to direct sutures. They showed a greater sensory recovery in less than 5 mm gaps even with non-permissive, permanent silicon tubes [23].
After a series of researches with efforts to improve the nerve conduit, some requirements have been explored and are being optimized. As a tissue scaffold, a nerve conduit must be composed of biocompatible materials to avoid rejection and further wound inflammation. The porosity and permeability of a scaffold are also important for invasion and migration of nerve cells, including Schwann cells and neural stem cells, and also neurological biomolecules (such as laminin-1, NGF, & BDNF). The porosity of a scaffold should be kept in a proper range to avoid complications. If the pores are too big, the fluids and cells needed to form a fibrin matrix in the early stages escape before the cells can attach, leading to failed neuroregeneration in the later stages. On the other hand, if pores are too narrow, the supportive cells cannot penetrate through to their destinations [14]. Also, the biodegradability of the nerve conduit also supports the dynamic neuroregeneration condition. When the active neuroregeneration process ends, a permanent nerve conduit that remains surrounding the neo-tissue may generate unwanted compression as an unnecessary structure.
Recently, the microscopic alignment of the scaffold surface and the electrical conductivity of the scaffold have emerged as new supporting strategies [34, 35]. Nanotopography is considered to provide nerve cells and neurites with guidance cues since the nerve cells need to directionally regenerate. Biodegradable aligned electrospun nanofibers have been attracting attention for their ability to fulfill the described requirements as nerve conduit materials.
2 Electrospun Nerve Conduits
2.1 Nanotopography of Nerve Conduit and Alignments
The nervous system comprises central and peripheral branches and functions to deliver information to all parts of the body. An extensive network of neurons and glia support the communication process. Nerve injury could result in painful neuropathies because of reduction in sensory perception and motor function depending on the location of the injury. There are many limitations in microsurgery techniques for the treatment of serious peripheral nerve injuries. NGCs are limited to treating nerve gaps of less than 4 cm in length, and sometimes the postoperative nerve is not well connected after implanting [36]. For this reason, recent developments in biomaterials and tissue engineering approaches seek to overcome the limitations associated with these methods of treatment. The incorporation of topographical guidance features and intraluminal structures have been studied to induce Schwann cell migration and regrowth of the axon toward their distal target. Several similar studies have been performed using various combinations of intraluminal guide structures and external conduit materials. One approach to this intraluminal guidance structure, including gels, sponges, films, filaments, and fibers, taken alone or with multiple support factors, is to add nanoscale guidance cues to micrometric in situ guiding structures [35, 37, 38].
Nanofiber-based scaffolds are most commonly used to add nanoscale guidance functionality to micrometric in situ guidance structures. Nanofibers are generally fabricated using three methods: self-assembly, phase separation, and electrospinning. Of these three, electrospinning is more widely used because of the excellent tunability of the nanotopography and diameter of the nanofibers [39]. Many studies have demonstrated the superiority of nanofibrous scaffolds in terms of cell activity. Also, nanofibrous scaffolds with specific patterning exhibit excellent mechanical strength as well as significant advantages in terms of cell proliferation processes associated with cell proliferation such as cell attachment, migration, and orientation. For example, highly aligned nanofibers, when compared to randomly distributed nanofibers, play an important role in neurite outgrowth in the case of neurons [40]. The reason is that the extracellular matrix of neural cells and neurites tend to grow parallel to the nanofibers aligned along the nanofiber array [14].
The environment at the single-cell level is considered to be key in elucidating the fundamental mechanisms of tissue regeneration [41]. While the extracellular matrix (ECM) has been a main target to reproduce or mimic, it has been revealed that the various properties of ECM including not only the soluble chemical factors, but also the physical characteristics, regulate cellular processes such as attachment, migration, proliferation, and differentiation. The architecture (structural and morphological properties) of the ECM is also a major determinant of the fate of both stem cells and differentiated cells in its vicinity, aiding them to inherit the characteristics of the original tissue [42].
The cell’s behaviors and functions heavily depend on the cell polarity and shape [43]. A single abnormal polarity among many different cell types can cause an organ malformation during fetal development and is also closely related to the pathophysiology of various human diseases, including cancer metastasis. There are reported cellular regulators of polarity such as Par (partitioning defective) complex and associated Rho GTPase signaling involved in various cellular activities. Although the physiological mechanism of the architecture of native ECM in situ is yet unclear, the influence of topography on cell polarity in various cell types has been researched and proven by employing an experimental process known as contact guidance [44].
The polarizations of the affected cells were investigated with nano-patterned surfaces that had various modifications such as gradients and isotropic and anisotropic nanotopographies. The many different cells grown on the nano-patterned substrate were noted to prefer elongation and parallel alignment to the patterned nanogrooves. For instance, the PNS neurons are also polarized along the fabricated nanogrooves as they regenerate in neurite bundles. The cell proliferation rate is greatly dependent on and sensitive to the size of the nanostructures and cell type. For example, neural stem cells cultured on electrospun nanofibrous meshes with larger nanostructures proliferated much less than on flat surfaces while proliferation increased with decreased fiber size [45]. In contrast, the cell proliferation rate of mouse osteoblasts was enhanced on hollow 100 nm diameter nanotubes.
Even though the underlying molecular mechanisms of cellular response remain elusive, some clues can be found from investigating the integrin family of adhesion molecules which play a key role in adhesion and mechanical signal transduction and its related intrinsic pathways. Since the nerve acts as an electric wire, neural cell orientation and polarization in the correct direction are crucial for nerve regeneration [46]. Aligned patterned nano-sized scaffolds can support nerve regeneration not only at the macroscopic level but also at the nano level by providing neuronal cells with anchors in the form of nanogrooves.
2.2 Aligned Electrospun Nanofibers
Aligned nanofibers provide topographic cues for nerve regeneration. Randomly oriented nanofibers are generally fabricated without preferential direction by a typical electrospinning set-up because the polymer jet that travels from nozzle tip to collector is disordered [14, 47]. It is challenging to produce a conduit from a neat aligned nanofibrous mat. In addition, aligned nanofibers have not been used for surgical applications as a nerve guide conduit due to their insufficient mechanical strength. For these reasons, a conduit with a highly aligned electrospun mat is produced with double coating using randomly oriented nanofibers by a modified electrospinning method. This double coated conduit favorable features like selective permeability and good hydrophilicity were made as nerve guide conduits [14, 48]. The inner part of the nerve guide conduit is covered with ordered nanofibers for enhancement of the proliferation of neural cells and the outer part of the conduit is double-coated with random nanofibers over ordered nanofibers for strengthening the mechanical properties of the inner part of the aligned nanofibrous conduit as shown in Fig. 4.
2.3 Fabrication Methods
A nerve conduit made of aligned nanofibers is favorable for nerve regeneration because of their superior nerve cell proliferation and attachment. However, it is challenging to fabricate a neat mat form with aligned nanofibers for biological applications as a nerve guide conduit because of their insufficient tensile strength. For this reason, extensive efforts have focused on producing aligned nanofibers and controlling the orientation of the fibers to meet requirements for medical applications. The most common fabrication technique for aligned nanofibers is the introduction and the modification of the collectors like a rotating drum, cone and disk as shown in Fig. 5.
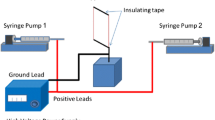
Many researchers have used high-speed rotating drums to collect ordered nanofibers which are parallel to each other along a common axis. Increasing the speed of the rotating collector results in highly aligned nanofibers. Edwards et al. reported that the structures of aligned polycaprolactone (PCL) nanofibers are influenced by the speed of the rotating collector [40, 49]. Theron et al. investigated an electrospinning set-up with a thin rotating disk that has a tapered edge to fabricate continuous aligned nanofibers. Afifi et al. proposed a modified rotating collector covered with insulating materials and fixed with conductive fins to produce aligned nanofibers. By setting up a gap between grounded conductive materials, one can obtain ordered nanofibers between the conductive materials. Li et al. reported the density of collected aligned nanofibers can differ according to the width of the gap between conductive materials. Sun et al. investigated that a rotating grooved collector could achieve the results of fabricating ordered nanofibrous mat in the electrospinning [50]. The charged nanofibers are stretched and spun across a gap between grooved collectors and form axially aligned nanofibers. Kim et al. demonstrated a modification of the electrospinning process in order to collect both aligned and random nanofibers on a mat via a single step electrospinning process. The copper wires were attached to a rotating collector and a semi-conductive mat was attached to the fixed copper wires in the horizontal and vertical axes for fabrication of aligned nanofibers. In this process of fabricating aligned nanofibers, the collector base was rotated at a rate of about 1000 rpm to get a neat mat with aligned nanofibers as shown in Fig. 6.
Morphological analysis of PU and PLGA nanofibrous mat with diameter graph (a) SEM image of randomly oriented PU nanofibers (d) SEM images of aligned PU nanofibers at a speed of 1000 rpm. (g) SEM image of randomly oriented PLGA nanofibers (j) SEM images of aligned PLGA nanofibers at a speed of 1000 rpm (b, e, h, k) FFT output images (c, f, i, l) Pixel intensity plots against the angle of acquisition for the aligned and random nanofibers. From Ref. [14] by Kim et al., licensed under CC BY 4.0 [14]
Aligned nanofibers can achieve a better cell viability and migration compared to randomly oriented nanofibers and act as a guide for neurite growth (Fig. 7). These processing methods for ordered nanofibers can be promising candidates for manufacturing scaffolds for neural tissue engineering.
(a, b) Confocal microscopy images of PC12 cells attached after 1 day of culture on a randomly oriented and aligned nanofibrous PLGA scaffold (c, d) Confocal microscopy images of PC12 cells attached after 5 day of culture on a random and aligned nanofibrous PLGA scaffold (e, f) Confocal microscopy images of S42 cells attached after 1 day of culture on a random and aligned nanofibrous PLGA scaffold (g, h) Confocal microscopy images of S42 cells attached after 5 day of culture on a random and aligned nanofibrous PLGA scaffold. Actin Green 488 (green) was applied for actin filament and DAPI (blue) for staining nuclei (i) Z stack from a PLGA scaffold with aligned nanofibers in which S42 cells. Images were collected at 0.37 μm intervals using the 488 laser. From Ref. [14] by Kim et al., licensed under CC BY 4.0 [14]
2.4 Improvement of Electrospun Nerve Conduit
2.4.1 Cell-Seeded Constructs
Therapeutic cell transplantation technology has taken its place in SCI treatment in the last few decades as the importance of the supportive cellular environment in neuroregeneration is highlighted. Several approaches to understanding more efficient cell delivery routes also have been under investigation [21, 51]. Reports have observed that the treatment outcome of cell transplantation is highly influenced by the volumetric cell density, type, and delivery route [52, 53].
While the cell density and type are adjustable, the optimal delivery routes and methods for cell transplantation vary from direct injection to scaffolds—seeding according to the target site. For SCI cell transplantation, injection through lumbar puncture is less invasive, cost-effective, and readily available [52, 54]. The preliminary cell transplantation therapeutic outcomes of the epicenter, rostral, and caudal injection sites at 1–2 weeks after SCI appeared to have similar levels of functional restoration. However, the result may imply the injection requires a higher population of therapeutic cells to reach a favorable cell density for filling up the spinal cavity.
As a combinatorial treatment, a cell-seeded nerve conduit or a nerve conduit-mediated cell transplantation along with additional mechanical supports and guidance cues can be a promising alternative in providing the injured tissue with the appropriate cell density needed in order to efficiently utilize the limited supply of cells.
Among the many types of cells available Schwann cells, neural stem cells (NSCs), neural progenitor cells (NPCs), ectomesenchymal stem cells (EMSCs), mesenchymal cells (MSCs) and olfactory ensheathing cells (OECs) are considered to be the most promising in bringing out the best outcome from cell-seeded nerve conduit treatments. Particularly, Schwann cells are most studied neurotrophic cell type and have demonstrated the most superior effect in treatments for both spinal cord and peripheral nerve injuries. For example, Schwann cells are reported to improve propriospinal axons around the injury site and also enhance the axonal regrowth of dorsal root ganglia (DRG). However, Schwann cells alone are incapable of helping axonal sprouts penetrate into the proximal destination so Schwann cell therapy should be aided by a secondary scaffold such as aligned nerve conduits with a directional topology for better therapeutic results [53].
To determine the best option and understand the behaviors and environmental cues of the potential cells, the types and characteristics of a few neurotrophic cells will be briefly discussed.
2.4.1.1 Schwann Cells
Schwann cells, named after physiologist Theodor Schwann, or neurolemmocytes, are the principal glial cells of the PNS. They can be categorized into myelinating and nonmyelinating Schwann cells depending on the myelin sheath wrapping around the axons of neurons in the PNS. All glial cells including Schwann cells functionally support neurons by supporting the conduction of electric signals and synaptic activity. However, different glial cells play roles in the development and regeneration of nerves, extracellular matrix synthesis, and immunologic monocytic antigen presentation.
One of the many roles of glial cells and Schwann cells, in general, include rejuvenating the distal portion of the damaged area and making it permissive for further neuronal regrowth and re-innervation. Following this cellular debridement, the Schwann cells layer upon on each other to form what are known as bands of Büngner, tunnels that guide axon regeneration toward the destination. Axons begin to regenerate at a rate of approximately 1 mm per day under favorable cellular milieu [55]. Schwann cells contribute to axonal regeneration by providing direction and the synthesis of ECM and neurotrophic biomolecules such as NGF, BDNF, and cell adhesion molecules. Once nerve fibers in the PNS get injured, the genes related to Schwann cell formation are quickly upregulated. The most well-known genes that contribute to Schwann cell formation and maintenance are SOX10 and Neuregulin 1. SOX10 is known as a determining transcription factor for glial cell generation from trunk crest cells and Neuregulin 1 (NRG1) promotes the formation of Schwann cells and support the survival of immature Schwann cells [56, 57].
Owing to their neurotrophic properties, Schwann cells are popularly used in attempts to treat neuronal injuries in a variety of ways. Novikova et al. seeded Schwann cells on poly-β-hydroxybutyrate (PHB) nerve conduit and implanted the nerve scaffold to cervical SCI rat models. They reported the nerve conduit enhanced axonal regeneration by supporting cell attachment and proliferation in the vicinity of the injury [58]. Xu et al. evaluated the influence of Schwann cell-seeded PAN/PVC mini-channel on a SCI rat model. Significant axonal regrowth in both directions, myelination, and improved vasculature were observed only in the cell-seeded group [59]. Blits et al. also loaded Schwann cells to their PAN/polyvinyl chloride (PVC)-based nerve conduit with other substances such as fibrinogen, gentamycin, and aprotinin to promote neuroregeneration. Two adenovirus-associated vectors were additionally administrated to two groups separately to evaluate the roles of BDNF and NT-3 and significantly improved functional gains of the hind-limb in both groups were observed [60].
Meanwhile, through various assessments such as histologic findings, electrophysiologic analyses and walking track analyses, the neuroregenerative effect of Schwann cells seeded on nerve conduits were confirmed by Keeley et al. [61]. The supportive cellular mechanisms of Schwann cells are also gradually being revealed. Williams et al. reported for the first time that the supportive roles of Schwann cells in nerve regeneration are performed mainly during the early stages [54]. The guidance cue provided by Schwann cells was emphasized by Brayan et al. through their experiment on Schwann cell-seeded poly-L-lysine precoated polyethylene (PE) nerve conduit implanted in a 20 mm neurotmesis rat model [53, 62].
To recapitulate briefly, once PNS nerve injury occurs, Schwann cells convert to a cell phenotype that is specialized to promote nerve repair and rapidly divide, migrate, express an appropriate set of genes, and facilitate axonal regrowth in the early phase of nerve regeneration. Because of their many supportive properties, Schwann cells have been extensively studied to be exploited in various neural injury treatments [3]. Schwann cells are the most studied and potentially most favorable candidate for cell loading nervous scaffold use, however, their application is restricted by low availability owing to insufficient nerve donors and the time-consuming culture processes for cell expansion.
2.4.1.2 Neural Stem Cells (NSCs) & Neural Progenitor Cells (NPCs)
Neural stem cells (NSCs) refer to multipotent cells closely involved in the embryonic development of the nervous system. More specifically, the neurons and glia of the animal nervous system are generated in embryonic development by NSCs and some NSCs will remain in the adult brain and continue to produce neurons [63, 64]. Neural stem cells can be divided into two daughter cells through asymmetric or symmetric cell division. When NSCs undergo asymmetric cell division, one of the daughter cells is differentiated into another cell type, primarily astrocytes, neurons, or oligodendrocytes, while the other daughter cell remains multipotent [65]. NSCs can also improve axonal regeneration by releasing metalloprotease-2 and multiple neurotrophic factors [66].
Olson et al. reported the supportive influence of NSCs and Schwann cells on neuroregeneration. In the study, Schwann cells and NSCs were seeded separately on a multi-channel PLGA scaffold which was implanted in an SCI-injury rat model. Increased axonal regeneration was observed in both the Schwann cell and NSC seeded-scaffolds, although the number of newly developed axons appeared to be slightly higher in the Schwann cell scaffold [67]. Another group, Lee et al., confirmed the positive effects of NSCs in a canine SCI model instead of the conventional rodent model.
Stem cell transplantation always carries with it concerns of toxicity and graft-versus-host disease (GVHD) along with post-transplantation fever. Therefore, regimens for stem cell transplantation using preventive strategies such as alloimmunization and upregulation of Regulatory T cells, have been extensively studied [68]. Neural progenitor cells (NPCs) are also multipotent as differentiated into hierarchically lower cell types similarly to NSCs. The differences of NPCs from NSCs can be found in the more specific differentiating potentials and the finite replication capabilities of the cells [69]. However, these two types of cells are sometimes considered to be equal and the concept of NPCs is still evolving.
2.4.1.3 Other Cells
Ectomesenchymal Stem Cells (EMSCs) are capable of differentiating into Schwann cells and also supporting neuroregeneration. Nie et al. reported the effect of EMSCs-loaded PLGA nerve conduit model in a rat sciatic neurotmesis model. The EMSCs-loaded nerve conduit showed statistically similar results as the autograft transplant group in a 3-month follow-up study using the sciatic functional index (SFI) while the result of the cell-free PLGA group was substantially inferior to the other groups [70].
Mesenchymal Stem Cells (MSCs), usually referred to as bone marrow stromal cells, are also available for cell-loaded nerve conduit technology. Although MSCs do not differentiate into nerve cells, many studies have reported improved neurogenesis with MSCs transplantation. Through a lumbar puncture, human BMSCs transplanted into a SCI rat model by Pal et al. appeared to guide axonal growth and also significantly improved nerve functions. The transplanted hBMSCs survived in the spinal cavity for at least 1 month and the functional regain was found to be dependent on the volume of the transplanted cells [71]. Pereira Lopes et al. reported the supportive effect of BMSCs as seeded on biodegradable collagen tube implanted in a sciatic neurotmesis rat model with a 3 mm nerve gap. Compared to the control group, a significantly greater number of regenerating clusters including both myelinated and non-myelinated fibers were observed in the BMSC-loaded nerve conduit after 6 weeks. They also confirmed the secretion of two neural growth factors, BDNF and NGF β [72].
Olfactory ensheathing cells (OECs) also known as olfactory ensheathing glia (OEG), are a type of glial cells distributed throughout the olfactory epithelium, olfactory nerve, and also olfactory bulb. By phagocytosing pathogens, they contribute to the immunoprotection of the olfactory nerve which lies under a mucosal layer of the upper nasal cavity. The human olfactory system keeps regenerating its neurons even in adulthood because the exposed nerve ends must degenerate to maintain the function [73]. During the regeneration processes, OECs play an important role in functionalization of new olfactory receptor neurons by cleaning up debris and providing damaged neurons with a favorable environment for neuroregeneration. Since the 1990s, the therapeutic possibility of transplantation of OECs to SCI to promote axonal regeneration and neurogenesis began to be reported possibly owing to the similar function of OECs in the olfactory system. In 2014, a Polish patient suffering from SCI-induced paraplegia regained mobility via therapeutic OEG transplantation. It was the first report of paraplegia recovery by SCI treatment [74]. The supportive mechanisms of OECs are still under investigation, but widely thought to be due to the upregulation of NGF receptors rather than the release of neurotrophic factors.
2.4.1.4 Neurotrophic Factors
Neurotrophic factors (NTFs) are various biomolecules related to neuronal growth, survival, and differentiation. This family of biomolecules is widely studied to understand the mechanisms of neuronal regeneration in both the CNS and PNS. NTFs are categorized into three main groups: the neurotrophin family, the CNTF family, and the GDNF family, based on the neurotrophic mechanisms at the cellular level [75]. Neurotrophin-3 (NT-3) of the neurotrophin family, is known for its significant role in neuronal survival in the PNS, and improves neuroregeneration in the CNS, specifically in the corticospinal tracts. Fan et al. reported that the addition of NT-3 onto PLGA nerve conduits promotes neural regrowth and motor function [76].
Another neurotrophin family member, brain-derived neurotrophic factor (BDNF) is one of the most studied biomolecules in various different aspects among neurotrophic factors. BDNF shares structural similarities to other family members such as NGF, NT-3 and NT-4/5 but BDNF is more closely related to the survival of neurons, particularly of dorsal root ganglion neurons, compared to other factors although the mechanism is still unclear [77]. Liang et al. suggested that collagen-bound BDNF enables sufficient BDNF delivery to the injured tissue [78].
Other neurotrophic factors, acidic fibroblast growth factor (a-FGF) and basic fibroblast growth factor also demonstrate neuroprotective effects and increase proliferation of NSCs and NPCs [79]. There are also other potential neurotrophic growth factors reported and used to support neuronal regeneration.
Vascular endothelial growth factor (VEGF) produced by cells is another supporting biomolecule that promotes neuronal reconstruction. Excluding traumatic neural injury in the CNS, these angiogenetic factors have demonstrated clinical significances in prognosis and treatment strategies of many other different diseases such as breast cancer, rheumatoid arthritis, diabetic retinopathy, age-related macular degeneration, and angiosarcoma. The members of the VEGF family are activated through tyrosine kinase receptors, similar to the cellular activation mechanism of most NTFs [80]. After the spinal cord is injured, VEGF represses apoptosis of nerve cells in order to spare neural tissues. Improved behavior, increased vascularization, increased spare tissue and decreased apoptosis levels were observed in rat SCI models with VEGF injection [81]. VEGF administration to SCI rat model also resulted in attenuated cavity formation and the production of a more permissive tissue environment for axonal ingrowth as reported by Sundberg et al. [82]. However, the clinical VEGF therapy for acute SCI is controversial because other studies exhibit few adverse effects including chronic pain after VEGF injection and acute exacerbation to injured neural tissue possibly due to VEGF-induced microvascular permeability.
Many ongoing researches on immobilization techniques and a number of potential substances including glial cell line-derived neurotrophic factor (GDNF), nerve growth factor (NGF), ciliary neurotrophic factor (CNTF), and alpha-1 glycoprotein (α1-GP) are expected to achieve great improvements in the treatment of neural injury.
2.4.2 Nanoparticles
Nerve guide conduits have benefited from the many recent advances in nanotechnology, in particular aligned nanofiber membranes and nanoparticles (NPs). Various materials from gold, silica, poly(lactide-co-glycolide) (PLGA), and titanium dioxide have been used to make nanoparticles for the delivery of drugs and growth factors to the injured site [83,84,85]. Other materials such as carbon nanotubes were utilized to promote and direct the growth of neuronal cells [86].
Nanoparticles have the great advantage of being able to penetrate the blood-brain barrier for more efficient and effective treatment of the CNS. To be used as drug carriers to the brain, NPs must be smaller than 100 nm, stable in blood, avoid platelet aggregation as well as meet many other conditions [87]. Besides being exceptional drug carriers, NPs have also been reported to promote the growth of neuronal cells and elongate neurites uniaxially for enhanced recovery rates. A property of metallic nanoparticles that is particularly attractive is their distinctive optical properties. When metallic NPs are illuminated by external light, they generate an oscillation that is called localized surface plasmon resonance. Depending on the wavelength of the absorbed light the localized surface plasmon resonance can match the therapeutic window of biological tissues to help with regeneration. For example, silica-coated Au nanorods irradiated with near infrared light can stimulate electrical activity in auditory neurons by inducing temperature increases between 0.5 and 6 °C [88]. Metallic NPs have also been used to deliver small-interfering RNA (siRNA) into neural stem cells for controlling their differentiation as depicted in Fig. 8.
Magnetic core-shell nanoparticles (MCNPs) for the delivery of small-interfering RNA (siRNA) into rat neural stem cells (rNSCs). (a) MCNPs functionalized with siRNA. (b) Transmission electron microscopy image of MCNPs. Scale bar: 10 nm. (c) MCNPs dispersed in water attracted to a magnet. (d) Schematic of magnetically facilitated delivery of siRNA to induce neural differentiation of rNSCs using MCNPs. Fluorescence images of neuronal (top) and oligodendrocyte (bottom) differentiation after siSOX9 and siCAV delivery, respectively. Adapted with permission from Ref. [85]. Copyright 2016 American Chemical Society [85]
Polymer NPs have the advantage of being biocompatible and biodegradable, making them a prime choice in neuroprotective therapeutic strategy and drug release. Rittchen et al. reported the delivery of pro-remyelinating factors to the CNS using PLGA nanoparticles and targeting antibodies to induce oligodendrocyte precursor cell maturation and improve remyelination. PLGA, like other FDA-approved polymers, degrades to biocompatible agents in the body, eliminating the need for a surgery to recover the particles. Silica NPs have large surface areas for protein binding, but they also can be easily functionalized for targeted delivery of cargos to neuronal cells. One study utilized transferrin-modified mesoporous silica nanoparticles (TF-MSNs) conjugated with HI-6 to prevent brain damage caused by soman poisoning [89]. These NPs were chosen because of their mesoporous structure for storage and rapid release, important for fighting toxic nerve agents.
Carbon nanotubes (CNTs) are cylindrical nanostructures comprised of graphene sheets that are wrapped onto themselves. The most frequently used CNTs are single (SWCNT) and multi-walled (MWCNT) carbon nanotubes, which are made of one layer of graphene and several concentric graphene cylinders, respectively. CNTs have many important applications in neuroscience currently and of these is acting as a platform to promote neuronal growth and performance. They have had unexpected and exciting impacts on neuronal signaling and behavior.
2.4.3 Conductive Polymers
There are many different types of polymers that have unique properties and characteristics such as biocompatibility and conductivity. Polymers that are conductive can conduct charge because electrons can jump within and between the chains of the polymer with ease. The polymers contain a conjugated backbone, meaning that single and double bonds alternate along the polymer chain. Both single and double bonds contain chemically strong localized σ-bonds that hold the atoms together, while double bonds also have a weaker π-bond that allow electrons to be more easily delocalized and move freely. Moreover, doping conducting polymers can increase their conductivity even further by introducing oxidizing or reducing agents and is dependent on the type and molecular size of the dopant.
Conductive polymers typically allow excellent control of the electrical stimulus, possess very good electrical and optical properties, and can be made biocompatible and biodegradable [34]. Many conductive polymers such as polypyrrole (PPy) are not inherently biodegradable, but there are ways to make them be so. One method is to prepare a composite containing both the conducting polymer with a biodegradable polymer. However, a downside to this method is that the conductive polymers are not degraded in the body. A second route is to modify the polymer structure itself. Studies have reported that the addition of ionizable or hydrolysable side groups to the backbone of PPy have successfully made the PPy degradable [90].
Considering the electroactive nature of our nervous system, conductive polymers such as PPy and PEDOT are attractive for use in neural engineering applications. Recent studies have used these conductive polymers as neural electrodes and scaffolds for nerve regeneration in neural-tissue engineering [91, 92]. Conductive polymers allow for the electrical stimulation of cells cultured on the polymers, as shown by Schmidt et al. [93]. PC12 cells were cultured on PPy and subjected to electrical stimulation, resulting in a significant increase in neurite lengths when compared to the passive control group. Another group explored the possibilities of combining electrical stimulation and nanotopography as shown in Fig. 9 [94]. Several studies have also combined neural growth factors with conductive polymers for enhanced neurite outgrowth and obtained positive results.
Left column. (a) Uncoated PLGA mesh (left) and PPy-PLGA mesh (right). (b) SEM of single strands of PPy-PLGA fibers. (c) SEM image of section of the PPy-PLGA meshes. Right column. (a) PC12 cells cultured on PPy-random fibers and (b) PPy-aligned fibers for 2 days. Black arrows indicated neurites. Reprinted from Ref. [94] with permission from Elsevier [94]
3 Conclusion
Since the introduction of electrospinning technology, a number of studies on collecting various types of polymers into nanofibrous architectures have been reported. The nanofiber producing technique is also useful for tissue engineering applications because it creates scaffolds with a fibrous structure that is similar in structure to that of natural extracellular matrix. The biomimetic structure of electrospun nanofibers is favorable for cell viability, attachment, growth, migration, and division. In particular, uniaxially aligned nanofibers have been reported to aid in neuronal lining growth. Aligned nanofibrous nerve conduits meet the requirements of porosity, biocompatibility, biodegradability, and mechanical strength that scaffolds need while providing guidance cues. However, in vivo transplantation of the nerve conduit itself requires further studies such as a development of optimal surgical access to the spinal cord, long-term adverse effects, and graft-versus-host disease. Various methods have been studied to develop nanofibrous nerve conduits, and the incorporation of Schwann cells, neurotrophic factors, CNTs, and conductive polymers have been reported to showing remarkable results.
Abbreviations
- a-FGF:
-
Acidic fibroblast growth factor
- ASIA:
-
American Spinal Injury Association
- ATF-3:
-
Activating transcription factor-3
- BDNF:
-
Brain-derived neurotrophic factor
- CNS:
-
Central nervous system
- CNTF:
-
Ciliary neurotrophic factor
- DRG:
-
Dorsal root ganglia
- ECM:
-
Extracellular matrix
- EMSCs:
-
Ectomesenchymal stem cells
- GAP-43:
-
Growth-associated protein-43
- GDNF:
-
Glial cell line-derived neurotrophic factor
- GVHD:
-
Graft-versus-host disease
- MAIs:
-
Myelin-associated inhibitors
- MSCs:
-
Mesenchymal stem cells
- MWCNT:
-
Multi-walled carbon nanotubes
- NGFs:
-
Nerve growth factors
- NPCs:
-
Neural progenitor cells
- NPs:
-
Nanoparticles
- NRG1:
-
Neuregulin 1
- NSCs:
-
Neural stem cells
- NT-3:
-
Neurotrophin-3
- NTFs:
-
Neurotrophic factors
- OECs:
-
Olfactory ensheathing cells
- OEG:
-
Olfactory ensheathing glia
- PAN:
-
Polyacrylonitrile
- PCL:
-
Polycaprolactone
- PE:
-
Polyethylene
- PHB:
-
Poly-β-hydroxybutyrate
- PLGA:
-
Poly(lactide-co-glycolide)
- PNS:
-
Peripheral nervous system
- PPy:
-
Polypyrrole
- PVC:
-
Polyvinyl chloride
- RAGs:
-
Regeneration-associated genes
- rNSCs:
-
Rat neural stem cells
- SCI:
-
Spinal cord injuries
- siRNA:
-
Small-interfering RNA
- Sox11:
-
SRY-box containing gene 11
- SPRR1A:
-
Small proline-repeat protein 1A
- SWCNT:
-
Single-walled carbon nanotube
- TF-MSNs:
-
Transferrin-modified mesoporous silica nanoparticles
- VEGF:
-
Vascular endothelial growth factor
- α1-GP:
-
Alpha-1 glycoprotein
References
Hall JE, Guyton AC (2011) Guyton and Hall textbook of medical physiology, 12th edn. Saunders/Elsevier, Philadelphia, PA
Kwon BK, Okon E, Hillyer J, Mann C, Baptiste D, Weaver LC, Fehlings MG, Tetzlaff W (2011) A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J Neurotrauma 28(8):1545–1588. https://doi.org/10.1089/neu.2009.1149
Han D, Cheung KC (2011) Biodegradable cell-seeded nanofiber scaffolds for neural repair. Polymers-Basel 3(4):1684–1733. https://doi.org/10.3390/polym3041684
National Spinal Cord Injury Statistical Center (NSCISC). Available online: https://www.nscisc.uab.edu/. Accessed 10 Jan 2017 (2015)
Kwon BK, Okon EB, Plunet W, Baptiste D, Fouad K, Hillyer J, Weaver LC, Fehlings MG, Tetzlaff W (2011) A systematic review of directly applied biologic therapies for acute spinal cord injury. J Neurotrauma 28(8):1589–1610. https://doi.org/10.1089/neu.2009.1150
Sykova E, Homola A, Mazanec R, Lachmann H, Konradova SL, Kobylka P, Padr R, Neuwirth J, Komrska V, Vavra V, Stulik J, Bojar M (2006) Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant 15(8–9):675–687
Geron C (2009) World’s first clinical trial of human embryonic stem cell therapy cleared. Regen Med 4(2):161
Ruijs AC, Jaquet JB, Kalmijn S, Giele H, Hovius SE (2005) Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg 116(2):484–494; discussion 495-486
Berger A, Millesi H (1978) Nerve grafting. Clin Orthop Relat Res 133:49–55
Mikami Y, Nagano A, Ochiai N, Yamamoto S (1997) Results of nerve grafting for injuries of the axillary and suprascapular nerves. J Bone Joint Surg Br 79(4):527–531
Barton MJ, Morley JW, Stoodley MA, Lauto A, Mahns DA (2014) Nerve repair: toward a sutureless approach. Neurosurg Rev 37(4):585–595. https://doi.org/10.1007/s10143-014-0559-1
Phillips JB, Bunting SC, Hall SM, Brown RA (2005) Neural tissue engineering: a self-organizing collagen guidance conduit. Tissue Eng 11(9–10):1611–1617. https://doi.org/10.1089/ten.2005.11.1611
de Ruiter GC, Malessy MJ, Yaszemski MJ, Windebank AJ, Spinner RJ (2009) Designing ideal conduits for peripheral nerve repair. Neurosurg Focus 26(2):E5. https://doi.org/10.3171/FOC.2009.26.2.E5
Kim JI, Hwang TI, Aguilar LE, Park CH, Kim CS (2016) A controlled design of aligned and random nanofibers for 3D bi-functionalized nerve conduits fabricated via a novel electrospinning set-up. Sci Rep 6:23761. https://doi.org/10.1038/srep23761
Yang F, Murugan R, Wang S, Ramakrishna S (2005) Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 26(15):2603–2610. https://doi.org/10.1016/j.biomaterials.2004.06.051
Koh HS, Yong T, Chan CK, Ramakrishna S (2008) Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials 29(26):3574–3582. https://doi.org/10.1016/j.biomaterials.2008.05.014
Ahmed I, Liu HY, Mamiya PC, Ponery AS, Babu AN, Weik T, Schindler M, Meiners S (2006) Three-dimensional nanofibrillar surfaces covalently modified with tenascin-C-derived peptides enhance neuronal growth in vitro. J Biomed Mater Res A 76a(4):851–860. https://doi.org/10.1002/jbm.a.30587
College O (2013) Illustration from anatomy & physiology. https://commons.wikimedia.org/wiki/File:1319_Nerve_StructureN.jpg
Kandel ER, Schwartz JH (1981) Principles of neural science. Elsevier, North Holland, New York
Brosius Lutz A, Barres BA (2014) Contrasting the glial response to axon injury in the central and peripheral nervous systems. Dev Cell 28(1):7–17. https://doi.org/10.1016/j.devcel.2013.12.002
Lutz AB, Barres BA (2014) Contrasting the glial response to axon injury in the central and peripheral nervous systems. Dev Cell 28(1):7–17. https://doi.org/10.1016/j.devcel.2013.12.002
Huebner EA, Strittmatter SM (2009) Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ 48:339–351. https://doi.org/10.1007/400_2009_19
Dahlin LB, Lundborg G (2001) Use of tubes in peripheral nerve repair. Neurosurg Clin N Am 12(2):341
Moore AM, Kasukurthi R, Magill CK, Farhadi HF, Borschel GH, Mackinnon SE (2009) Limitations of conduits in peripheral nerve repairs. Hand (N Y) 4(2):180–186. https://doi.org/10.1007/s11552-008-9158-3
Ma TC, Willis DE (2015) What makes a RAG regeneration associated? Front Mol Neurosci 8:43. https://doi.org/10.3389/fnmol.2015.00043
David S, Aguayo AJ (1981) Axonal elongation into peripheral nervous-system bridges after central nervous-system injury in adult-rats. Science 214(4523):931–933. https://doi.org/10.1126/science.6171034
Jankowski MP, McIlwrath SL, Jing X, Cornuet PK, Salerno KM, Koerber HR, Albers KM (2009) Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res 1256:43–54. https://doi.org/10.1016/j.brainres.2008.12.032
Seijffers R, Allchorne AJ, Woolf CJ (2006) The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci 32(1-2):143–154. https://doi.org/10.1016/j.mcn.2006.03.005
GrandPre T, Nakamura F, Vartanian T, Strittmatter SM (2000) Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403(6768):439–444
Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Skene JHP (2001) Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci 4(1):38–43
Bonilla IE, Tanabe K, Strittmatter SM (2002) Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci 22(4):1303–1315
Safa B, Buncke G (2016) Autograft substitutes: conduits and processed nerve allografts. Hand Clin 32(2):127–140. https://doi.org/10.1016/j.hcl.2015.12.012
Chen ZL, Yu WM, Strickland S (2007) Peripheral regeneration. Annu Rev Neurosci 30:209–233. https://doi.org/10.1146/annurev.neuro.30.051606.094337
Balint R, Cassidy NJ, Cartmell SH (2014) Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater 10(6):2341–2353. https://doi.org/10.1016/j.actbio.2014.02.015
Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S (2009) Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng A 15(11):3605–3619. https://doi.org/10.1089/ten.tea.2008.0689
Oh SH, Kim JH, Song KS, Jeon BH, Yoon JH, Seo TB, Namgung U, Lee IW, Lee JH (2008) Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials 29(11):1601–1609. https://doi.org/10.1016/j.biomaterials.2007.11.036
Assmann U, Szentivanyi A, Stark Y, Scheper T, Berski S, Drager G, Schuster RH (2010) Fiber scaffolds of polysialic acid via electrospinning for peripheral nerve regeneration. J Mater Sci Mater Med 21(7):2115–2124. https://doi.org/10.1007/s10856-010-4072-y
Dinis TM, Elia R, Vidal G, Dermigny Q, Denoeud C, Kaplan DL, Egles C, Marin F (2015) 3D multi-channel bi-functionalized silk electrospun conduits for peripheral nerve regeneration. J Mech Behav Biomed Mater 41:43–55. https://doi.org/10.1016/j.jmbbm.2014.09.029
Goh YF, Shakir I, Hussain R (2013) Electrospun fibers for tissue engineering, drug delivery, and wound dressing. J Mater Sci 48(8):3027–3054. https://doi.org/10.1007/s10853-013-7145-8
Jenkins PM, Laughter MR, Lee DJ, Lee YM, Freed CR, Park D (2015) A nerve guidance conduit with topographical and biochemical cues: potential application using human neural stem cells. Nanoscale Res Lett 10(1):972. https://doi.org/10.1186/s11671-015-0972-6
Gattazzo F, Urciuolo A, Bonaldo P (2014) Extracellular matrix: a dynamic microenvironment for stem cell niche. BBA-Gen Subjects 1840(8):2506–2519. https://doi.org/10.1016/j.bbagen.2014.01.010
Lutolf MP, Blau HM (2009) Artificial stem cell niches. Adv Mater 21(32–33):3255–3268. https://doi.org/10.1002/adma.200802582
Kim DH, Provenzano PP, Smith CL, Levchenko A (2012) Matrix nanotopography as a regulator of cell function. J Cell Biol 197(3):351–360. https://doi.org/10.1083/jcb.201108062
Kim MH, Sawada Y, Taya M, Kino-oka M (2014) Influence of surface topography on the human epithelial cell response to micropatterned substrates with convex and concave architectures. J Biol Eng 8:13. https://doi.org/10.1186/1754-1611-8-13
Beachley V, Wen XJ (2010) Polymer nanofibrous structures: fabrication, biofunctionalization, and cell interactions. Prog Polym Sci 35(7):868–892. https://doi.org/10.1016/j.progpolymsci.2010.03.003
Hoffman-Kim D, Mitchel JA, Bellamkonda RV (2010) Topography, cell response, and nerve regeneration. Annu Rev Biomed Eng 12(12):203–231. https://doi.org/10.1146/annurev-bioeng-070909-105351
Jha BS, Colello RJ, Bowman JR, Sell SA, Lee KD, Bigbee JW, Bowlin GL, Chow WN, Mathern BE, Simpson DG (2011) Two pole air gap electrospinning: fabrication of highly aligned, three-dimensional scaffolds for nerve reconstruction. Acta Biomater 7(1):203–215. https://doi.org/10.1016/j.actbio.2010.08.004
Gupta D, Venugopal J, Prabhakaran MP, Dev VR, Low S, Choon AT, Ramakrishna S (2009) Aligned and random nanofibrous substrate for the in vitro culture of Schwann cells for neural tissue engineering. Acta Biomater 5(7):2560–2569. https://doi.org/10.1016/j.actbio.2009.01.039
Bhutto MA, Wu T, Sun B, Ei-Hamshary H, Al-Deyab SS, Mo X (2016) Fabrication and characterization of vitamin B5 loaded poly (l-lactide-co-caprolactone)/silk fiber aligned electrospun nanofibers for schwann cell proliferation. Colloids Surf B: Biointerfaces 144:108–117. https://doi.org/10.1016/j.colsurfb.2016.04.013
Sun B, Jiang XJ, Zhang SC, Zhang JC, Li YF, You QZ, Long YZ (2015) Electrospun anisotropic architectures and porous structures for tissue engineering. J Mater Chem B 3(27):5389–5410. https://doi.org/10.1039/c5tb00472a
Rowan A (2006) Nerve regeneration - a strain on regeneration. Nat Rev Neurosci 7(8):596–597. https://doi.org/10.1038/nrn1972
Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK (2011) A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotraum 28(8):1611–1682. https://doi.org/10.1089/neu.2009.1177
Bryan DJ, Wang KK, ChakalisHaley DP (1996) Effect of Schwann cells in the enhancement of peripheral-nerve regeneration. J Reconstr Microsurg 12(7):439–446. https://doi.org/10.1055/s-2007-1006616
Williams LR, Longo FM, Powell HC, Lundborg G, Varon S (1983) Spatial-temporal progress of peripheral-nerve regeneration within a silicone chamber - parameters for a bioassay. J Comp Neurol 218(4):460–470. https://doi.org/10.1002/cne.902180409
Bunge RP (1994) The role of the Schwann-cell in trophic support and regeneration. J Neurol 242(1):S19–S21. https://doi.org/10.1007/Bf00939235
Jankowski MP, Cornuet PK, McILwrath S, Koerber HR, Albers KM (2006) SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience 143(2):501–514. https://doi.org/10.1016/j.neuroscience.2006.09.010
Barres BA, Raff MC (1999) Axonal control of oligodendrocyte development. J Cell Biol 147(6):1123–1128. https://doi.org/10.1083/jcb.147.6.1123
Novikova LN, Pettersson J, Brohlin M, Wiberg M, Novikov LN (2008) Biodegradable poly-beta-hydroxybutyrate scaffold seeded with Schwann cells to promote spinal cord repair. Biomaterials 29(9):1198–1206. https://doi.org/10.1016/j.biomaterials.2007.11.033
Xu XM, Zhang SX, Li HY, Aebischer P, Bunge MB (1999) Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur J Neurosci 11(5):1723–1740. https://doi.org/10.1046/j.1460-9568.1999.00591.x
Blitts B, Oudega M, Boer GJ, Bunge MB, Verhaagen J (2003) Adeno-associated viral vector-mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hind-limb function. Neuroscience 118(1):271–281. https://doi.org/10.1016/S0306-4522(02)00970-3
Keeley R, Atagi T, Sabelman E, Padilla J, Kadlcik P, Agras J, Eng L, Wiedman TW, Nguyen K, Sudekum A, Rosen J (1994) Synthetic nerve graft containing collagen and synthetic Schwann-cells improves functional, electrophysiological, and histological parameters of peripheral-nerve regeneration (Vol 5, Pg 353, 1993). Restor Neurol Neurosci 6(2):161
Lohmeyer JA, Shen ZL, Walter GF, Berger A (2007) Bridging extended nerve defects with an artificial nerve graft containing Schwann cells pre-seeded on polyglactin filaments. Int J Artif Organs 30(1):64–74
Nie X, Zhang YJ, Tian WD, Jiang M, Dong R, Chen JW, Jin Y (2007) Improvement of peripheral nerve regeneration by a tissue-engineered nerve filled with ectomesenchymal stem cells. Int J Oral Maxillofac Surg 36(1):32–38. https://doi.org/10.1016/j.ijom.2006.06.005
Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J (2000) Generalized potential of adult neural stem cells. Science 288(5471):1660–1663
Temple S (1989) Division and differentiation of isolated CNS blast cells in microculture. Nature 340(6233):471–473. https://doi.org/10.1038/340471a0
Heine W, Conant K, Griffin JW, Hoke A (2004) Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp Neurol 189(2):231–240. https://doi.org/10.1016/j.expneurol.2004.06.014
Olson HE, Rooney GE, Gross L, Nesbitt JJ, Galvin KE, Knight A, Chen B, Yaszemski MJ, Windebank AJ (2009) Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng A 15(7):1797–1805. https://doi.org/10.1089/ten.tea.2008.0364
Lee SH, Chung YN, Kim YH, Kim YJ, Park JP, Kwon DK, Kwon OS, Heo JH, Kim YH, Ryu S, Kang HJ, Paek SH, Wang KC, Kim SU, Yoon BW (2009) Effects of human neural stem cell transplantation in canine spinal cord hemisection. Neurol Res 31(9):996–1002. https://doi.org/10.1179/174313209x385626
Zahir T, Nomura H, Guo XD, Kim H, Tator C, Morshead C, Shoichet M (2008) Bioengineering neural stem/progenitor cell-coated tubes for spinal cord injury repair. Cell Transplant 17(3):245–254
Nie X, Zhang YJ, Tian WD, Jiang M, Dong R, Chen JW, Jin Y (2007) Improvement of peripheral nerve regeneration by a tissue-engineered nerve filled with ectomesenchymal stem cells. Int J Oral Max Surg 36(1):32–38. https://doi.org/10.1016/j.ijom.2006.06.005
Pal R, Gopinath C, Rao NM, Banerjee P, Krishnamoorthy V, Venkataramana NK, Totey S (2010) Functional recovery after transplantation of bone marrow-derived human mesenchymal stromal cells in a rat model of spinal cord injury. Cytotherapy 12(6):792–806. https://doi.org/10.3109/14653249.2010.487899
Lopes FRP, Campos LCD, Correa JD, Balduino A, Lora S, Langone F, Borojevic R, Martinez AMB (2006) Bone marrow stromal cells and resorbable collagen guidance tubes enhance sciatic nerve regeneration in mice. Exp Neurol 198(2):457–468. https://doi.org/10.1016/j.expneurol.2005.12.019
Su ZD, He C (2010) Olfactory ensheathing cells: biology in neural development and regeneration. Prog Neurobiol 92(4):517–532. https://doi.org/10.1016/j.pneurobio.2010.08.008
Hempstead BL (2006) Dissecting the diverse actions of pro- and mature neurotrophins. Curr Alzheimer Res 3(1):19–24
Fan J, Zhang H, He J, Xiao Z, Chen B, Xiaodan J, Dai J, Xu R (2011) Neural regrowth induced by PLGA nerve conduits and neurotrophin-3 in rats with complete spinal cord transection. J Biomed Mater Res B Appl Biomater 97(2):271–277. https://doi.org/10.1002/jbm.b.31810
Joosten EA, Houweling DA (2004) Local acute application of BDNF in the lesioned spinal cord anti-inflammatory and anti-oxidant effects. Neuroreport 15(7):1163–1166
Liang W, Han Q, Jin W, Xiao Z, Huang J, Ni H, Chen B, Kong J, Wu J, Dai J (2010) The promotion of neurological recovery in the rat spinal cord crushed injury model by collagen-binding BDNF. Biomaterials 31(33):8634–8641. https://doi.org/10.1016/j.biomaterials.2010.07.084
Cheng H, Cao Y, Olson L (1996) Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science 273(5274):510–513
De Laporte L, des Rieux A, Tuinstra HM, Zelivyanskaya ML, De Clerck NM, Postnov AA, Preat V, Shea LD (2011) Vascular endothelial growth factor and fibroblast growth factor 2 delivery from spinal cord bridges to enhance angiogenesis following injury. J Biomed Mater Res A 98(3):372–382. https://doi.org/10.1002/jbm.a.33112
Widenfalk J, Lipson A, Jubran M, Hofstetter C, Ebendal T, Cao Y, Olson L (2003) Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neuroscience 120(4):951–960
Sundberg LM, Herrera JJ, Narayana PA (2011) Effect of vascular endothelial growth factor treatment in experimental traumatic spinal cord injury: in vivo longitudinal assessment. J Neurotrauma 28(4):565–578. https://doi.org/10.1089/neu.2010.1533
Kim MS, El-Fiqi A, Kim JW, Ahn HS, Kim H, Son YJ, Kim HW, Hyun JK (2016) Nanotherapeutics of PTEN inhibitor with mesoporous silica nanocarrier effective for axonal outgrowth of adult neurons. ACS Appl Mater Inter 8(29):18741–18753. https://doi.org/10.1021/acsami.6b06889
Sun BB, Taing A, Liu HY, Nie GC, Wang JY, Fang YL, Liu L, Xue Y, Shi J, Liao YP, Ku J, Xia T, Liu Y (2016) Nerve growth factor-conjugated mesoporous silica nanoparticles promote neuron-like PC12 cell proliferation and neurite growth. J Nanosci Nanotechnol 16(3):2390–2393. https://doi.org/10.1166/jnn.2016.10958
Shah S, Solanki A, Lee KB (2016) Nanotechnology-based approaches for guiding neural regeneration. Acc Chem Res 49(1):17–26. https://doi.org/10.1021/acs.accounts.5b00345
Fabbro A, Prato M, Ballerini L (2013) Carbon nanotubes in neuroregeneration and repair. Adv Drug Deliver Rev 65(15):2034–2044. https://doi.org/10.1016/j.addr.2013.07.002
Gilmore JL, Yi X, Quan L, Kabanov AV (2008) Novel nanomaterials for clinical neuroscience. J Neuroimmune Pharm 3(2):83–94. https://doi.org/10.1007/s11481-007-9099-6
Yong J, Needham K, Brown WGA, Nayagam BA, McArthur SL, Yu AM, Stoddart PR (2014) Gold-nanorod-assisted near-infrared stimulation of primary auditory neurons. Adv Healthc Mater 3(11):1862–1868. https://doi.org/10.1002/adhm.201400027
Yang J, Fan LX, Wang FJ, Luo Y, Sui X, Li WH, Zhang XH, Wang YG (2016) Rapid-releasing of HI-6 via brain-targeted mesoporous silica nanoparticles for nerve agent detoxification. Nanoscale 8(18):9537–9547. https://doi.org/10.1039/c5nr06658a
Zelikin AN, Lynn DM, Farhadi J, Martin I, Shastri V, Langer R (2002) Erodible conducting polymers for potential biomedical applications. Angew Chem Int Ed 41(1):141–144. https://doi.org/10.1002/1521-3773(20020104)41:1<141::Aid-Anie141>3.0.Co;2-V
Sun BB, Wu T, Wang J, Li DW, Wang J, Gao Q, Bhutto MA, El-Hamshary H, Al-Deyab SS, Mo XM (2016) Polypyrrole-coated poly(L-lactic acid-co-epsilon-caprolactone)/silk fibroin nanofibrous membranes promoting neural cell proliferation and differentiation with electrical stimulation. J Mater Chem B 4(41):6670–6679. https://doi.org/10.1039/c6tb01710j
Yan L, Zhao BX, Liu XH, Li X, Zeng C, Shi HY, Xu XX, Lin T, Dai LM, Liu Y (2016) Aligned nanofibers from polypyrrole/graphene as electrodes for regeneration of optic nerve via electrical stimulation. ACS Appl Mater Inter 8(11):6834–6840. https://doi.org/10.1021/acsami.5b12843
Schmidt CE, Shastri VR, Vacanti JP, Langer R (1997) Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci U S A 94(17):8948–8953. https://doi.org/10.1073/pnas.94.17.8948
Lee JY, Bashur CA, Goldstein AS, Schmidt CE (2009) Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 30(26):4325–4335. https://doi.org/10.1016/j.biomaterials.2009.04.042
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Kim, J.I., Hwang, T.I., Lee, J., Park, C.H., Kim, C.S. (2017). Electrospun Nanofibrous Nerve Conduits. In: Almodovar, J. (eds) Electrospun Biomaterials and Related Technologies. Springer, Cham. https://doi.org/10.1007/978-3-319-70049-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-70049-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-70048-9
Online ISBN: 978-3-319-70049-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)