Abstract
Nuclear medicine plays an important role in the evaluation of inflammatory processes for many years. The tools used, starting from the gamma camera, passing through the SPECT, up to the latest PET scan, have increased diagnostic accuracy in this difficult field. Even the more specific radiotracers that are being used are a goal in further improving the appropriateness of nuclear medicine in this area. Many studies have shown that positron emission tomography with 2-deoxy- 2-[fluorine-18]fluoro-D-glucose integrated with computed tomography (18F-FDG-PET/CT), commonly used in the oncology field, has also the features to study some diseases with inflammatory origin which are usually difficult to identify using traditional diagnostic techniques. Indeed the activated inflammatory cells accumulate FDG with high concentration depending upon the degree of stimulation that is a function of inflammatory activity. In particular some recent papers have suggested that FDG-PET is a very promising tool in the diagnosis of the infected kidney and liver cysts in autosomal dominant polycystic kidney disease (ADPKD). Basing on our large personal experience, we report images of some suggestive clinical cases and the major works written in the literature about this specific clinical setting. At last limits, advantages and potential of this diagnostic examination, together with some new future development, are reported in this chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Infections, abscesses, and other inflammatory process are a major concern to the clinician and to the patient. Inflammatory imaging requires a multidisciplinary approach to accurately diagnose, stage, and follow up infectious processes. Inflammation acts as the initial host defense against invasive pathogens and other inciting stimulus. It plays an important role in tissue repair and elimination of harmful pathogens. Although the inflammatory response is essential for host defense, it is very much a double-edged sword. Inappropriate inflammatory reaction or delay in the resolution of inflammation will damage adjacent normal cells in the tissue.
Timely diagnosis and localization of infection is a critical step in the appropriate clinical management of this group of diseases. Radiological imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography (US) are all being currently used for the evaluation of infection. These imaging techniques are, however, based on morphological changes and are therefore of limited value in the early stage of the infectious process, which may show only insignificant structural infection-related tissue modifications or none at all.
Nuclear medicine plays an important role in the evaluation of infection. Scintigraphic tests are excellent noninvasive modalities of whole-body scanning that allow diagnosis of infectious foci and assessment of the extent of disease in any part of the body. Nuclear medicine procedures have been the tool of choice for assessment of musculoskeletal infections such as osteomyelitis, infected prosthetic joint implants, and diabetic foot. These functional imaging modalities are also used for the evaluation of patients presenting with fever of unknown origin. Infection-seeking radiotracers are used for diagnosis and localization of suspected abdominal abscesses, vascular graft infection, and pulmonary infection [1].
Nuclear medicine provides unique information on pathophysiological and patho-biochemical processes—as opposed to other imaging procedures such as CT, MRI, and US, which supply high-resolution data on morphological changes that take place as a result of a specific disease. Because they provide a different type of information, scintigraphic and anatomical imaging modalities play a complementary role in many clinical settings, including the assessment and management of patients with infectious processes.
1.1 Devices
1.1.1 Gamma Camera
The vast majority of all clinical nuclear medicine studies are based on the imaging of the internal distribution of one or more radiopharmaceuticals with a gamma camera. The term gamma camera refers to a device that can image the distribution of a gamma-emitting radionuclide.
For many years, nuclear medicine procedures have been performed using a gamma camera. Originally, multiple planar projections were acquired to provide diagnostic information, but, more recently, the techniques of SPECT (Single Photon Emission Computed Tomography) have been utilized. Conventional planar images generally suffered from poor contrast due to the presence of overlying and underlying activity that interferes with imaging of the region of interest. The resulting planar image is low in contrast due to the effect of the superposition of depth information.
This effect can be reduced by collecting images from multiple positions around the distribution and producing an image of a transverse slice through the distribution.
The resulting tomographic image is of higher contrast than the planar image due to the elimination of contributions of activity above and below the region of interest. This is the goal of SPECT to provide images of slices of radionuclide distributions with image contrast that is higher than that provided by conventional techniques. Currently with the new hybrid tomograph, SPECT/CT images could be performed with a more accurate localization.
1.1.2 PET/CT
Positron emission tomography (PET) is a nuclear medicine functional imaging technique that is used to observe metabolic processes in the body. The system detects pairs of gamma rays emitted indirectly by a positron-emitting radionuclide (tracer), which is introduced into the body on a biologically active molecule. Three-dimensional images of tracer concentration within the body are then constructed by computer analysis. In modern PET/CT scanners (Fig. 13.1), three-dimensional imaging is often accomplished with the aid of a CT performed on the patient during the same session, in the same machine [2]. Currently, it is principally used for oncology purpose (almost 90% of the scan), but there is a continuous rise of the use in the field of neurology, cardiology, and infectious disease. It is clear that the different types of tests that we can do with the PET depend on the type of tracer rather than from the tomograph; it follows that the variation of the tracer will change the function studied. However there are tracers which have a major versatility, compared to others, and can be used for different purpose.
1.2 Tracers
The detection and localization of inflammation and infection with nuclear medicine techniques has been studied using different methods of conventional nuclear medicine since the 1970s. The ideal characteristics of an infection-imaging radiopharmaceutical are well synthesized in Table 13.1.
Currently there are several SPECT and PET tracers in general use. The common tracers are being gallium-67 (67Ga)-, indium-111 (111In)-, and 99mTechnetium (99mTc)-labeled white blood cells (WBCs), labeled antigranulocyte antibody (LeukoScan), and fluorine-18-fluorodeoxyglucose (18F-FDG). The sensitivity and specificity of these tracers depend on the clinical scenario and the organ involved.
1.2.1 67Ga-Citrate
67Ga-citrate (Ga) has been used for imaging infection for almost four decades, since its discovery in the early 1970s [3]. Ga was used for the investigation of fever of unknown origin, acute or chronic osteomyelitis, and the diagnosis of infections in immunocompromised patients. Although Ga scintigraphy shows high sensitivity in detection of both acute and chronic infections, it has several drawbacks that limit its clinical application. Its specificity is relatively low because of early physiologic excretion through the urinary system and delayed excretion through the bowel and because of the normal biodistribution to the liver. Ga is a bone-seeking tracer, accumulating in areas of bone remodeling. It is also a tumor-seeking agent, accumulating in such tumors as lymphoma or hepatoma. Physiological hepatic, bowel, and renal uptake can either mimic or mask foci of infection localized inside or in the vicinity of these organs. Optimal Ga imaging may therefore require delayed imaging of up to a few days after injection, a disadvantage when diagnosing acute infectious processes that may require rapid therapeutic interventions [3, 4].
1.2.2 Radiolabeled Leukocytes
Labeled WBC imaging using either 99mTc or 111In has been shown to perform excellently in detecting acute and chronic infections. This test has become the nuclear medicine modality of choice in a variety of clinical settings when the suspicion of an infectious process exists. Clinical indications for labeled leukocytes scintigraphy include osteomyelitis, especially in cases where infected joint prosthesis or posttraumatic osteomyelitis is suspected.
Scintigraphic assessment, however, lacks anatomical landmarks, rendering the topographic localization of sites of abnormal tracer uptake difficult. In this clinical setting, the precise localization achieved with SPECT/CT in a single imaging step and with greater diagnostic accuracy may provide a tool for guiding invasive tissue sampling procedures and further treatment planning.
A study aimed at investigating the potential advantage of 111In-labeled WBC-SPECT/CT compared with SPECT alone evaluated 14 patients with various sites of suspected infection. The authors concluded that SPECT/CT improved the accuracy of anatomic localization of foci of abnormal WBC uptake and led to modification in clinical management [5].
1.2.3 Labeled Antigranulocyte Antibodies
The preparation of radiolabeled leukocytes is laborious, requires specialized staff and dedicated expensive equipment, and can be hazardous because of blood-product handling.
The advantages of radioimmunoscintigraphy over techniques involving labeled autologous leukocytes for imaging infection have to do mainly with the simplicity of its use due to the fact that there is no need for blood-product handling. Indications for labeled antigranulocyte antibody scintigraphy include the suspicion of osteomyelitis, fever of unknown origin, abdominal infections, and vascular graft infections [6,7,8].
Correlation of results from this scintigraphic technique to anatomical imaging modalities is considered mandatory for accurate diagnosis. False-positive studies may be the result of increased vascular permeability. Increased uptake of labeled antibodies has been observed in perivascular hematomas and contusions, especially in the delayed phase images obtained 24 h after injection. With the additional anatomical information it provides, SPECT/CT can be of value in excluding or confirming the presence of a hematoma as the cause of abnormal tracer uptake. Similar to the performance capabilities of other nuclear medicine procedures, the precise localization of the infected site may be difficult on scintigraphy alone. A study investigating the value of SPECT/CT in chronic osteomyelitis assessed 27 patients with 29 sites of suspected bone infection and compared planar and SPECT/CT imaging after injection of 99mTc-labeled antigranulocyte antibodies [9]. SPECT/CT was able to correctly localize all positive foci detected on planar and SPECT images. SPECT/CT also allowed the differential diagnosis of soft tissue infection, septic arthritis, and osteomyelitis. Furthermore, following the diagnosis of bone involvement, hybrid imaging was also able to differentiate between cortical, corticomedullar, and subperiosteal foci of disease involvement. The authors concluded that combined SPECT/CT imaging improves the accuracy of radioimmunoscintigraphy for diagnosis of soft tissue infection and osteomyelitis [9].
1.2.4 FDG
Fluorodeoxyglucose (FDG) is currently mainly used in oncology, neurology, and cardiology and in the study of infectious and inflammatory disease.
FDG has been approved by US Food and Drug Administration (FDA) and European Medicines Agency (EMEA) and authorized as a diagnostic radiopharmaceutical in the diagnosis of infection. Fluorine-18 (18F) is a cyclotron-produced radioisotope with a half-life of 109.7 min that undergoes positron decay. [18F]FDG is an analogue of glucose, whereby the 2-carbon hydroxyl group of glucose is substituted with a fluorine atom (Fig. 13.1).
Like glucose, [18F]FDG is taken up by cells via the glucose transporter (GLUT1) and phosphorylated by hexokinase II (HKII) to form [18F]FDG-6-PO4; however, unlike glucose, further metabolism is prevented due to the absence of the required 2-carbon hydroxyl, and hence [18F]FDG remains trapped within the cell (Fig. 13.2). [18F]FDG-6-PO4 accumulates in cells over time, leading to signal amplification and making this imaging agent a suitable indicator of hexokinase II activity as well as a cell’s need for glucose [10].
FDG rapidly accumulates at the sites of infection and inflammation with high target-to-background ratio. The mechanism of FDG localization in infection is that cells involved in infection and inflammation, especially neutrophils and the monocyte/macrophage, are able to express high levels of glucose transporters, especially GLUT1. The common indications of FDG-PET/CT in infection and inflammation included the following in descending order of accuracy: sarcoidosis, osteomyelitis, spondylodiscitis, fever of unknown origin, vasculitis, diabetic foot, prosthesis (especially hip), and vascular grafts. Also it is used for assessing the extent of fungal infection and evaluation of therapy in infectious or inflammatory diseases [11].
Mechanism of uptake of SPECT and PET | |
|---|---|
SPECT and PET tracer | Mechanism of uptake |
67Ga-citrate and 99mTc-HMPAO migration of leukocytes | Transferrin and lactoferrin receptor binding |
99mTc-HMPAO-WBC | Migration of leukocytes |
111In-oxine-WBC | Cellular migration of leukocytes |
LeukoScan (Tc-anti-NCA-90 FAB antigranulocyte antibody) | Increased vascular permeability and binding and migration of antibody-labeled granulocytes |
18F-FDG | Glucose uptake by activated inflammatory cells |
2 Diagnosis of Cyst Infection
Cyst infection is a diagnostic challenge in patients with autosomal dominant polycystic kidney disease (ADPKD) because of the lack of specific manifestations and limitations of conventional imaging procedures [12]. ADPKD represents the most common inherited kidney disease [13]. It is characterized by the development of fluid-filled cysts in kidney and liver parenchyma, derived from various renal tubular segments and biliary ducts. Cyst growth causes organ enlargement leading to abdominal and/or loin discomfort. Liver cysts are not associated with hepatic dysfunction, whereas kidney cysts cause end-stage renal disease (ESRD) in more than 70% of ADPKD patients. Also, cysts carry significant morbidity, including bleeding and infection. Cyst infection is a serious complication of ADPKD. Its incidence is 0.01 episode/patient/year, according to a retrospective monocentric series of Sallée et al. [14]. Predisposing conditions include age, female gender, and recent instrumentation of the urinary tract. In the chronic hemodialysis population, the prevalence of renal infection is significantly higher in ADPKD patients than in controls [15]. In the renal transplant recipient (RTR) population, the prevalence of urinary tract infections in patients with ADPKD does not appear to be increased [16]. Cyst infection is the cause of hospitalizations for 15% of ADPKD patients [14, 17]. Most common pathogens are enteric flora, Escherichia coli. The retrograde route via the ureters is the presumed mechanism of cyst infection in the kidney. The identification of the germ is lacking in more than half of cases, similar to the rate observed in the general population with severe sepsis. Although the identification of the infectious agent is essential for tailoring the antibiotic therapy, the diagnosis of cyst infection is not easy because of the various, most often nonspecific, clinical manifestations and the limitations of conventional imaging techniques. Proving the presence of cyst infection requires cyst fluid analysis. However, this is not always possible or indicated, so that diagnosis relies practically on a constellation of concurrent clinical, biological, and radiological parameters.
Conventional imaging procedures, such as CT and magnetic resonance imaging (MRI), were largely discussed on the previous chapter.
2.1 WBC
Radiolabeled-leukocyte scintigraphy in the diagnosis of kidney cyst infection is a complement of the radiological procedures in the assessment of infectious site localization. Particularly, 111In leukocyte scanning allowed the identification of renal cyst infection in ADPKD patients in whom other noninvasive imaging procedures had failed. 111In leukocyte scanning requires the handling of blood derivatives and in vitro labeling process, as well as a 24-h delay imaging. 111In scintigraphy is characterized by poor spatial resolution, low sensitivity, high radiation activity, and significant interobserver variability. Hexamethylpropyleneamine oxime (HMPAO) represents an alternative lipophilic chelator for efficient labeling of leukocytes with 99mtechnetium (99mTc). Radiation characteristics of 99mTc-HMPAO are more favorable for imaging than those of 111In, particularly for single-photon emission computed tomography (SPECT). Furthermore, the dual modality technique combining CT with SPECT using radiolabeled WBC has been associated with a diagnostic yield of 85% of cases with abdominal infections. The relevance of SPECT/CT to cyst infection diagnosis in ADPKD patients is currently unknown [12].
2.2 FDG-PET/CT
In the general population, 18FDG-PET/CT imaging represents a reliable tool for the detection of tissue infection on the basis of the high metabolic activity and increased uptake of the radiolabeled glucose analogue, 18FDG, by inflammatory cells [22]. Importantly, 18FDG is not nephro- or hepatotoxic and has been successfully used in patients with renal function ranging from mildly reduced GFR to ESRD [14, 23]. First, 18FDG-PET alone proved helpful in identifying or excluding renal and hepatic cyst infection in case reports and two retrospective series [14, 20, 24]. To further improve the localization of infectious sites, PET was combined with CT to integrate metabolic data from PET with anatomical information from CT [22]. In our series, 18FDG-PET/CT yielded positive results in 87% of cyst infection cases [17]. PET/CT was considered as positive for cyst infection when the uptake of 18FDG was focally increased around at least one cyst in comparison with the physiological accumulation in the parenchyma and was located at distance from the pelvicalyceal excretion. PET/CT yielded two false-negative results in a diabetic RTR during the immediate posttransplantation period and in a 62-year-old nondiabetic woman with stage IV CKD. By contrast, three liver pyocysts could be percutaneously drained only after localization by PET/CT. The median delay between the onset of symptoms and PET/CT imaging was 9 days, and the mean maximal standardized uptake value (SUVmax) reached 5.1 ± 1.7 g/mL. The measurement of SUVmax allows standardized quantification of the inflammatory process in addition to the visual evaluation [23]. Repeated measurements of SUVmax may help follow up the inflammatory process over time. Piccoli et al. [18] reported on the clinical management of ten patients with suspected cystic infection, which was tailored upon 18FDG-PET/CT results. PET/CT identified five kidney and one liver cyst infections. The mean SUVmax reached 8.4 ± 5.4 g/mL on initial PET/CT images. The follow-up of four patients included a comparative PET/CT performed 3–6 weeks later, which showed a visual reduction of pathological 18FDG uptake but no significant change of SUVmax. Three patients underwent a third PET/CT 7–9 weeks after the initial imaging, which disclosed no residual 18FDG uptake. Of note, the normalization of serum CRP levels preceded PET/CT normalization. The clinical relevance of persistent altered PET/CT images to treated infectious diseases remains unclear. The literature in oncology supports that the follow-up by 18FDG-PET/CT of therapeutic responses to chemo- or radiotherapy varies from 3 to 12 weeks depending upon the type of cancer and the administered therapy. However, the pathophysiology of infection is intrinsically different from neoplasia, and cyst infection is associated with the additional challenge of antibiotic diffusion into a chronically damaged organ and a cystic cavity. Consequently, 18FDG-PET/CT probably represents an optimal tool for the detection and localization of pyocysts in ADPKD patients, but its role in the follow-up after antibiotic therapy remains uncertain. PET/CT in ADPKD patients with suspected cyst infection offers the additional advantage of entirely scanning the abdominal cavity, thereby occasionally identifying non-cystic inflammatory disorders and adjusting the therapy. PET/CT results significantly changed the management of 26% of cases [17]. Moreover, PET/CT confirmed two kidney cyst infections, although both patients did not meet all four of the standardized criteria [19]. The advantages of 18FDG-PET/CT are rapid imaging, minimal labor intensity, high target-to-background ratio, high interobserver agreement, and a simultaneous coregistration with low-dose CT without administration of contrast medium [23]. Limitations of PET/CT include its cost, restricted availability, and relative inability to reliably distinguish infectious from noninfectious inflammation or malignancy. The differentiation of 18FDG accumulation in residual functional renal parenchyma from that in inflammatory cells lining pyocysts remains debatable. The distinction between cyst infection and pyelonephritis may not be easy. The PET/CT pattern of pyelonephritis usually includes a diffuse 18FDG uptake in an edematous cortex and locoregional hypermetabolic adenopathies, which contrasts with the focally increased uptake of 18FDG lining the pyocyst. Besides infectious processes, 18FDG uptake can be increased in other conditions, such as cancer. The actual risk of malignancy in ADPKD patients does not seem to be increased [25]. Liver cystadenocarcinoma is very uncommon, and most kidney tumors show low-grade malignancy leading to low 18FDG uptake. However, “false-positive” rate of 18FDG-PET/CT in cyst infection diagnosis remains to be prospectively investigated. Finally, PET/CT has not been evaluated in intracystic bleeding, the main differential diagnosis of cyst infection in ADPKD patients. Accumulation of 18FDG has been reported in the setting of extrarenal hematoma [26]. In conclusion the 18FDG-PET is a very useful method for increasing the accuracy of the diagnosis of the infected cyst with a sensitivity of 77%, a specificity of 100%, and a negative predictive value of 77% [27].
2.3 Cases Presentation
2.3.1 Case 1
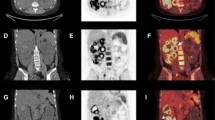
An 81-year-old woman, suffering from polycystic hepatorenal disease, was hospitalized for onset of fever of unknown origin. Following investigations, because of the suspect of left acute multifocal pyelonephritis, the patient was treated with ceftriaxone and amikacin. The abdominal CT examination showed the presence of a renal cyst with diameter of 80 × 90 mm and characterized by homogeneous fluid content and slightly and uniformly thickened walls in the absence of nodular lesions. Then therapy was later stopped due to the persistence of symptoms and high levels of inflammatory markers. The patient was thus treated with ertapenem getting better results but not a complete response. She was therefore aimed at our center to perform an 18F-FDG-PET/CT scan. This functional examination showed the presence of an abnormal FDG uptake at the level of the left kidney cyst walls (Fig. 13.3).
The pathological presence of radiotracer at this level, indicative of an active inflammatory process, suggested the continuation of antibiotic treatments. After 37 days a second PET scan showed a good response to the treatment documented by a significant reduction in the extent and intensity of the abnormal uptake of radiotracer (Fig. 13.4).
During the follow-up a second CT scan showed the persistence of the right renal cysts although dimensionally significantly reduced compared to the previous control. In the absence of a definitive imaging judgment of complete response, a third PET scan was performed. PET showed the complete disappearance of the abnormal uptake of the radiopharmaceutical at the walls of the kidney cysts, still present from a morphological point of view (Fig. 13.5).
In this clinical case, the PET examination, together with clinical parameters, seems to have helped, both in the correct diagnosis and in response assessment to the treatment.
2.3.2 Case 2
A 55-year-old patient with hepatorenal polycystic disease went to the hospital for pain in the left lumbar region associated with fever. Biochemical examinations showed a high CRP value (188.3 mg/L), and a possible left renal cyst with hemorrhagic aspects was detected by contrast-enhanced CT in the suspect of an active inflammatory process in this site; the patient was subjected to antibiotic therapy with amoxicillin. One month later the symptoms disappeared almost completely, and the PCR values were significantly reduced although not yet in standard levels, while a second CT scan showed no more signs of active left kidney inflammation. In consideration of the difficult judgment of treatment response, the patient was sent to our clinic for a PET/CT examination. The examination showed an abnormal uptake of radiotracer at the lower portion of the known left renal cyst, with a SUVmax 2.9 (Fig. 13.6).
In the following months, the patient underwent further antibiotic therapy and was monitored with multiple diagnostic tests. It was interesting to note that PET scan was the only imaging examination able to identify the persistent focus of the disease. This finding was further reduced by size and fixation in the subsequent PET control, showing a SUVmax of 2.4 (Fig. 13.7).
A further reduction in this finding was found in the third PET examination, showing a SUVmax of 2.0 (Fig. 13.8).
One last PET scan was performed when the patient showed total normalization of inflammatory indices and a complete resolution of the symptoms. This last examination showed the complete disappearance of the radiotracer uptake (Fig. 13.9).
In this clinical case, PET has proven to be a good diagnostic tool in evaluating minimal persistence of inflammatory disease. PET scan, used during the treatment, has helped clinicians decide on the type and duration of therapy.
3 Future Development
In addition to glucose metabolism, a variety of targets for inflammation imaging are being discovered and utilized, some of which are considered superior to FDG for imaging inflammation. We summarize the potential inflammation imaging targets and corresponding PET tracers and the applications of PET in major inflammatory disease.
18F-FDG-PET imaging of inflammation tends to give false-positive results, especially in patients with cancer. Moreover, the high tracer accumulation in the heart and brain makes it difficult to detect inflammatory foci near those organs or tissues. Consequently, new imaging tracers and targets for more specific inflammation detection and therapy evaluation are under intensive investigation. PET imaging with these new tracers greatly improved our understanding of the mechanism of inflammation and increased the diagnostic specificity and accuracy of inflammatory foci. As summarized in Fig. 13.10, various radiopharmaceuticals have been developed for PET imaging of inflammation, targeting different biomarkers from macrophages to angiogenesis.
A small survey of the new tracer potentially available in the next future.
3.1 Translocator Protein (TSPO)
Formerly known as peripheral benzodiazepine receptor (PBR), TSPO is ubiquitously expressed in peripheral tissues but is only minimally expressed in the healthy human brain. Previous studies found high TSPO expression in macrophages, neutrophils, lymphocytes [31,32,30], activated microglia, and astrocytes [34,35,36,37,35]. PET imaging using TSPO as an inflammation biomarker has also been reported for atherosclerosis detection with promising results [31, 32, 36, 37]. TSPO PET has also been used to image inflamed lung and liver diseases [30, 38, 39].
3.2 Type 2 Cannabinoid Receptor (CB2R)
There are at least two subtypes of CBRs in the endocannabinoid system. The first in vivo PET of brain CB2R was performed in 2010 by Horti and his group [40]. Promising results on CB2R targeted PET imaging warrant further applications in a wide range of neuroinflammatory diseases and evaluation of the therapeutic value of novel CB2R-related drugs. However, the exact role of CB2R in CNS still remains to be fully elucidated, and more in vivo studies using relevant disease models should be conducted to get a better understanding.
3.3 Formyl Peptide Receptor (FPR)
FPR is a type of G-protein-coupled receptor expressed on neutrophils, responsible for the leukocyte migration cascade in the inflammation process. PET using cFLFLFK-PEG-64Cu as FPR-specific ligand could visualize inflammatory foci within the lung in an animal model of lung inflammation induced by Klebsiella pneumoniae [41].
3.4 Cyclooxygenase (COX)
COX is the target of nonsteroidal anti-inflammatory drugs (NSAIDs) [42]. In addition, COX is an integral membrane glycoprotein which can be induced by acute and chronic inflammatory stimulations. Thus far, three COX subtypes (COX-1, 2, and 3) have been identified. Among them, the inducible isoform COX-2 plays a pivotal role in cancer, cardiac/cerebral ischemia, Alzheimer’s/Parkinson’s disease, and response to inflammatory stimuli, especially neuroinflammation [43, 44]. Celecoxib is broadly used as a selective COX-2 inhibitor to treat inflammatory diseases. Imaging tracers have also been developed using celecoxib and some other COX inhibitors by radiolabeling them with either 18F or 11C. They have been used to image neuroinflammations [45], tumors, or experimental skin inflammation [46, 47]. However, most of the tracers showed unsatisfactory ex vivo or in vivo properties due to either nonspecific bindings or low sensitivity in inflammatory foci or both.
3.5 Interleukin-2 (IL-2)
IL-2 is a small single-chain glycoprotein synthesized and secreted by activated T lymphocytes seen in many types of inflammatory diseases, such as inflammatory degenerative diseases, graft rejection, tumor inflammation, organ-specific autoimmune diseases, and adipose inflammatory insulin resistance [48]. Previously, 123I and 99mTc-labeled IL-2 have been used in many chronic inflammatory diseases, such as autoimmune diseases [49], celiac disease [50], and vulnerable atherosclerotic plaques [51] via SPECT imaging. However, routine application of this technique was limited because the labeling procedures are complex and the spatial resolution of SPECT is not high enough. Recently, Gialleonardo et al. reported the labeling of IL-2 with N-succinimidyl 4-18F-fluorobenzoate (18F-SFB) for the synthesis of 18F-FB-IL-2 to detect activated T lymphocytes in inflammation [52].
3.6 Tumor Necrosis Factor-α (TNF-α)
TNF-α is a cytokine that can contribute to cell apoptosis and organ dysfunction [53]. Many studies show that TNF-α is important in acute immune response to infection, injury, autoimmune, and chronic inflammatory disorders such as rheumatoid arthritis [54] and psoriasis [55]. Previously, some group used a PET tracer 64Cu-DOTA-etanercept, to image acute inflammatory process induced by 12-O-tetradecanoylphorbol-13-acetate (tetradecanoylphorbol acetate, TPA) [56].
So far, many inflammation-related biomarkers have been identified and investigated as imaging or therapy targets, including inflammatory cell metabolism, membrane markers, cytokines, and vascular changes during inflammation. After intensive preclinical studies, some of these targets have been tested in humans. However, very few of them are considered to be inflammation specific. With better understanding of the inflammatory reaction in each disease type, more sensitive and specific biomarkers will be identified, and potential new imaging probes may be developed to target these biomarkers. Moreover, multiplexed imaging with tracers targeting different biomarkers and multimodal imaging by incorporating PET with other imaging modalities will also contribute to improved visualization and quantification of the inflammatory diseases.
References
Jamar F, Buscombe J, Chiti A, Christian PE, Delbeke D, Donohoe KJ, Israel O, Martin-Comin J, Alberto S (2013) EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J Nucl Med 54(4):647–658
Bailey DL et al (2005) Positron emission tomography: basic sciences. Springer-Verlag, Secaucus
Boerman OC, Rennen H, Oyen WJ et al (2001) Radiopharmaceuticals to image infection and inflammation. Semin Nucl Med 31:286–295
Love C, Palestro CJ (2004) Radionuclide imaging of infection. J Nucl Med Technol 32:47–57
Mirtcheva RM, Kostakoglu SJ, Goldsmith SJ (2003) SPECT/CT fusion imaging in 111In WBC scintigraphy. J Nucl Med 44:341
Becker W, Meller J (2001) The role of nuclear medicine in infection and inflammation. Lancet Infect Dis 1:326–333
Hakki S, Harwood SJ, Morrissey MA et al (1997) Comparative study of monoclonal antibody scan in diagnosing orthopaedic infection. Clin Orthop 335:275–285
Palestro CJ, Kipper SL, Weiland FL et al (2002) Osteomyelitis: diagnosis with (99m)Tc-labeled antigranulocyte antibodies compared with diagnosis with (111)In-labeled leukocytes––initial experience. Radiology 223(3):758–764
Horger M, Eschmann SM, Pfannenberg C et al (2003) The value of SPET/CT in chronic osteomyelitis. Eur J Nucl Med Mol Imaging 30(12):1665–1673
James ML, Gambhir SS (2012) A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev 92(2):897–965
Farghaly H, Nasr H, Al Qarni A (2015) Role of FDG PET/CT in infection and inflammation. J Nucl Med 56(supplement 3):1954
Jouret F, Lhommel R, Devuyst O, Annet L, Hassoun Z, Kanaan N (2012) Diagnosis of cyst infection in patients with autosomal dominant polycystic kidney disease: attributes and limitations of the current modalities. Nephrol Dial Transplant 27(10):3746–3751
Torres VE, Harris PC, Pirson Y (2007) Autosomal dominant polycystic kidney disease. Lancet 369(9569):1287–1301
Sallée M, Rafat C, Zahar JR et al (2009) Cyst infections in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 4(7):1183–1189
Christophe JL, van Ypersele de Strihou C, Pirson Y (1996) Complications of autosomal dominant polycystic kidney disease in 50 haemodialysed patients. A case-control study. The U.C.L. collaborative group. Nephrol Dial Transplant 11(7):1271–1276
Jacquet A, Pallet N, Kessler M et al (2011) Outcomes of renal transplantation in patients with autosomal dominant polycystic kidney disease: a nationwide longitudinal study. Transpl Int 24(6):582–587
Jouret F, Lhommel R, Beguin C et al (2011) Positron-emission computed tomography in cyst infection diagnosis in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6(7):1644–1650
Piccoli GB, Arena V, Consiglio V et al (2011) Positron emission tomography in the diagnostic pathway for intracystic infection in ADPKD and ‘cystic’ kidneys. A case series. BMC Nephrol 12:48
Telenti A, Torres VE, Gross JB Jr et al (1990) Hepatic cyst infection in autosomal dominant polycystic kidney disease. Mayo Clin Proc 65(7):933–942
Migali G, Annet L, Lonneux M et al (2008) Renal cyst infection in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 23(1):404–405
Ichioka K, Saito R, Matsui Y et al (2007) Diffusion-weighted magnetic resonance imaging of infected renal cysts in a patient with polycystic kidney disease. Urology 25(1):1219
Keidar Z, Gurman-Balbir A, Gaitini D et al (2008) Fever of unknown origin: the role of 18F-FDG PET/CT. J Nucl Med 49(12):1980–1985
Boellaard R, O’Doherty MJ, Weber WA et al (2010) FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging. Eur J Nucl Med Mol Imaging 42:328–354
Bleeker-Rovers CP, de Sevaux RG, van Hamersvelt HW et al (2003) Diagnosis of renal and hepatic cyst infections by 18-F-fluorodeoxyglucose positron emission tomography in autosomal dominant polycystic kidney disease. Am J Kidney Dis 41(6):E18–E21
Bonsib SM (2009) Renal cystic diseases and renal neoplasms: a mini review. Clin J Am Soc Nephrol 4(12):1998–2007
Repko BM, Tulchinsky M (2008) Increased F-18 FDG uptake in resolving atraumatic bilateral adrenal hemorrhage (hematoma) on PET/CT. Clin Nucl Med 33(9):651–653
Bobot M, Ghez C, Gondouin B, Sallée M, Fournier PE, Burtey S, Legris T, Dussol B, Berland Y, Souteyrand P, Tessonnier L, Cammilleri S, Jourde-Chiche N (2016) Diagnostic performance of [(18)F]fluorodeoxyglucose positron emission tomography-computed tomography in cyst infection in patients with autosomal dominant polycystic kidney disease. Clin Microbiol Infect 22(1):71–77
Bird JL, Izquierdo-Garcia D, Davies JR, Rudd JH, Probst KC, Figg N et al (2010) Evaluation of translocator protein quantification as a tool for characterising macrophage burden in human carotid atherosclerosis. Atherosclerosis 210(2):388–391
Gaemperli O, Shalhoub J, Owen DR, Lamare F, Johansson S, Fouladi N et al (2012) Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J 33(15):1902–1910
Hatori A, Yui J, Yamasaki T, Xie L, Kumata K, Fujinaga M et al (2012) PET imaging of lung inflammation with [18F]FEDAC, a radioligand for translocator protein (18 kDa). PLoS One 7(9):e4506
Hannestad J, Gallezot JD, Schafbauer T, Lim K, Kloczynski T, Morris ED et al (2012) Endotoxin-induced systemic inflammation activates microglia:[11C]PBR28 positron emission tomography in nonhuman primates. NeuroImage 63(1):232–239
Roeda D, Kuhnast B, Damont A, Dollé F (2012) Synthesis of fluorine-18-labelled TSPO ligands for imaging neuroinflammation with positron emission tomography. J Fluor Chem 134:107–114
Ching AS, Kuhnast B, Damont A, Roeda D, Tavitian B, Dolle F (2012) Current paradigm of the 18-kDa translocator protein (TSPO) as a molecular target for PET imaging in neuroinflammation and neurodegenerative diseases. Insights Imaging 3(1):111–1119
Oh U, Fujita M, Ikonomidou VN, Evangelou IE, Matsuura E, Harberts E et al (2011) Translocator protein PET imaging for glial activation in multiple sclerosis. J Neuroimmune Pharmacol 6(3):354–361
Papadopoulos V, Lecanu L (2009) Translocator protein (18 kDa) TSPO: an emerging therapeutic target in neurotrauma. 2009. Exp Neurol 219:53–57
Pugliese F, Gaemperli O, Kinderlerer AR, Lamare F, Shalhoub J, Davies H et al (2010) Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. J Am Coll Cardiol 56(8):653–661
Lamare F, Hinz R, Gaemperli O, Pugliese F, Mason JC, Spinks T et al (2011) Detection and quantification of large-vessel inflammation with 11C-(R)-PK11195 PET/CT. J Nucl Med 52(1):33–39
Hardwick MJ, Chen MK, Baidoo K, Pomper MG, Guilarte TR (2005) In vivo imaging of peripheral benzodiazepine receptors in mouse lungs: a biomarker of inflammation. Mol Imaging 4(4):432–438
Xie L, Yui J, Hatori A, Yamasaki T, Kumata K, Wakizaka H et al (2012) Translocator protein (18kDa), a potential molecular imaging biomarker for non-invasively distinguishing non-alcoholic fatty liver disease. J Hepatol 57(5):1076–1082
Horti AG, Gao Y, Ravert HT, Finley P, Valentine H, Wong DF et al (2010) Synthesis and biodistribution of [11C]A-836339, a new potential radioligand for PET imaging of cannabinoid type 2 receptors (CB2). Bioorg Med Chem 18(14):5202–5207
Locke LW, Chordia MD, Zhang Y, Kundu B, Kennedy D, Landseadel J et al (2009) A novel neutrophil-specific PET imaging agent: cFLFLFK-PEG-64Cu. J Nucl Med 50(5):790–797
Hawkey CJ (1999) COX-2 inhibitors. Lancet 353(9149):307–314
Katori M, Majima M (2000) Cyclooxygenase-2: its rich diversity of roles and possible application of its selective inhibitors. Inflamm Res 49(8):367–392
Minghetti L (2004) Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol 63(9):901–910
de Vries EFJ, Doorduin J, Dierckx RA, van Waarde A (2008) Evaluation of [11C]rofecoxib as PET tracer for cyclooxygenase 2 overexpression in rat. Nucl Med Biol 35(1):35–42
Uddin MJ, Crews BC, Ghebreselasie K, Huda I, Kingsley PJ, Ansari MS et al (2011) Fluorinated COX-2 inhibitors as agents in PET imaging of inflammation and cancer. Cancer Prev Res (Phila) 4(10):1536–1545
Wuest F, Kniess T, Bergmann R, Pietzsch J (2008) Synthesis and evaluation in vitro and in vivo of a 11C-labeled cyclooxygenase-2 (COX-2) inhibitor. Bioorg Med Chem 16(16):7662–7670
Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M et al (2008) T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 28(7):1304–1310
Signore A, Picarelli A, Annovazzi A, Britton KE, Grossman AB, Bonanno E et al (2003) 123I-Interleukin-2: biochemical characterization and in vivo use for imaging autoimmune diseases. Nucl Med Commun 24(3):305–316
Signore A, Chianelli M, Annovazzi A, Rossi M, Maiuri L, Greco M et al (2000) Imaging active lymphocytic infiltration in coeliac disease with iodine-123-interleukin-2 and the response to diet. Eur J Nucl Med 27(1):18–24
Annovazzi A, Bonanno E, Arca M, D’Alessandria C, Marcoccia A, Spagnoli LG et al (2006) 99mTc-interleukin-2 scintigraphy for the in vivo imaging of vulnerable atherosclerotic plaques. Eur J Nucl Med Mol Imaging 33(2):117–126
Di Gialleonardo V, Signore A, Glaudemans AW, Dierckx RA, De Vries EF (2012) N-(4-18F-fluorobenzoyl)interleukin-2 for PET of human-activated T lymphocytes. J Nucl Med 53(5):679–686
Cairns CB, Panacek EA, Harken AH, Banerjee A (2000) Bench to bedside:tumor necrosis factor-alpha: from inflammation to resuscitation. Acad Emerg Med 7(8):930–941
Maki-Petaja KM, Elkhawad M, Cheriyan J, Joshi FR, Ostor AJ, Hall FC et al (2012) Anti-tumor necrosis factor-alpha therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 126(21):2473–2480
Bissonnette R, Tardif JC, Harel F, Pressacco J, Bolduc C, Guertin MC (2013) Effects of the TNF alpha antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging 6(1):83–90
Cao Q, Cai W, Li ZB, Chen K, He L, Li HC et al (2007) PET imaging of acute and chronic inflammation in living mice. Eur J Nucl Med Mol Imaging 34(11):1832–1842
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Penna, D., Militano, V., Arena, V., Cistaro, A., Pelosi, E. (2018). Nuclear Medicine in the Management of Patient with Kidneys Intracystic Infection. In: Tonolini, M. (eds) Imaging and Intervention in Urinary Tract Infections and Urosepsis. Springer, Cham. https://doi.org/10.1007/978-3-319-68276-1_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-68276-1_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68275-4
Online ISBN: 978-3-319-68276-1
eBook Packages: MedicineMedicine (R0)