Abstract
Polymers are considered as a versatile class of materials due to their enormous contributions that helped the mankind to reach the comfort level that we enjoy in today’s society. On the other hand, their extensive use creates various threats to the environment in terms of pollution to the atmosphere, various health hazards to humans and other living species, and moreover weakening of nonrenewable natural assets. The last few decades witnessed a paradigm shift toward development and commercialization of polymers from bio-based renewable resources that can suit very well for all those applications achieved by their petroleum-based counterparts. The present chapter analyzes in detail about the various life stages, starting from the sources, for a very important and widely commercialized biopolymer, polyhydroxybutyrate (PHB). Recent developments in blends and composites of PHB with other polymers and fillers are discussed in terms of structure-property relationships and applications. Their contribution toward the preservation of the ecosystem and the sustainability is also described.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Biopolymers and Ecosystem
An ecosystem consists of all the plants, animals, other habitats, and the environment in a particular area along with the multifaceted association that exists between them and their surroundings. A pictorial representation of an ecosystem is shown in Fig. 1. The biodiversity existing in the ecosystem is very much essential for the preservation of a balance in the life cycle of each participating species. Any damage or threat to one of the components of the ecosystem has serious implications on the existence of others and results in total disturbance to the harmony existing between them.
There is a serious concern related to the damages caused by waste plastics to the ecosystem. Littering of used/waste plastics leads to a serious threat to the ecosystem as a whole. The reason for this concern is the growing demand and hence the growth in the production of plastics all over the world. According to a recent data published by Plastics Europe [1], the world plastic production in the year 2015 reached almost 322 metric tons. with an annual growth rate of 8.4%. Interestingly, this figure does not include polyethylene terephthalate (PET), polyamides (PA), and polyacrylic fibers. A serious consequence of this rapid growth in production and consumption of plastics all over the world is the generation of large volumes of used plastic wastes. Another study reports that almost 60% of all those plastic materials ever produced all over the world are discarded and they are accumulated in landfills or end up as litter in the environment [2]. A gargantuan amount of these waste plastics are accumulated in the marine environment and cause a serious threat to the marine ecosystem [3, 4]. A scientific study reports that around 5.25 trillion plastic particles weighing 268,940 tons are currently floating in the sea [5]. If the trend continues like this, then the prediction is that by 2050, the sea will contain more plastic waste than fish.
All these problems point toward the serious damage to the ecosystem. Biopolymers or polymers derived from natural sources came into the market as eco-friendly materials, causing minimal harm to the harmony existing in the ecosystem. Non-fossil fuel-originated polymers that are either biological in their source or susceptible to degradation by microorganisms or chemical breakdown in the environment (such as hydrolysis) are termed as biopolymers [6]. Biomolecules such as proteins, DNA, RNA, peptides, etc. are also considered as biopolymers. Other than the biomolecules, there are mainly four different types of biopolymers. These are cellulose-based biopolymers, starch-based biopolymers, sugar-based biopolymers, and finally synthetic material-based biopolymers. Lactic acid-based polymers are produced mainly by fermentation of sugar (lactose) originated from potatoes, maize, wheat, and sugar beet. It is expected that the world production of bioplastics shall reach 8 metric tons by 2019 [7]. Most of the biopolymers are currently finding their use in the areas of biomedical applications, packaging field, textiles, consumer goods, agriculture, automotive, and transport applications [8,9,10]. The environmental benefits of biopolymers can be briefed as:
-
(a)
First of all, they reduce the dependency on nonrenewable fossil fuels for their synthesis.
-
(b)
Biopolymers are compostable, and hence there is less chance of environmental pollution compared with their petroleum- or fossil fuel-originated counterparts.
-
(c)
Biopolymers are considered as carbon neutral and sustainable as they are originated from renewable sources.
-
(d)
Biopolymers reduce carbon emissions and reduce the carbon dioxide levels in the atmosphere.
PHB as a Biopolymer
Polyhydroxyalkanoate (PHA) is a group of linear polyester biopolymers produced by bacteria during fermentation of sugars and lipids that are accumulated in the cell as a carbon and energy storage body, under circumstances of nutrient deficiency and in the existence of excess carbon source [11] . At first, PHA was discovered in Bacillus megaterium, which are synthesized and stored by both gram-positive and gram-negative bacteria as insoluble biopolymers [12]. Poly(3-hydroxybutyrate) (PHB) is a homopolymer of 3-hydroxybutyrate and the most significant member of the biodegradable thermoplastic polyhydroxyalkanoate family, with characteristics of high melting temperature, a high degree of crystallinity, and low permeability to O2, H2O, and CO2. The structure of PHB is shown in Fig. 2. The average molecular weight of PHB varies from 2 to 4 × 103 KDa. The molecular weight depends on the ability of microbes to accumulate the produced polymer, conditions of growth, and extraction method. Polyhydroxybutyrate can be classified into different monomers based on the carbon chain attached: short length (contains 5 carbon PHB units), medium length (contains 6–14 carbon PHB units), and long length (contains more than 14 carbon PHB) [13]. PHB is a good replacement for synthetic polymer, and its mechanical properties are comparable to that of polypropylene [14]. PHB possesses the characteristics of thermoplasticity and biodegradability in compost which has attracted commercial attention. PHB has great potential in food packaging applications with better water vapor barrier properties than polypropylene and better oxygen barrier properties than both polyethene terephthalate and polypropylene [15]. The typical properties of PHB are compared with polypropylene in Table 1. PHB monomer is a chiral molecule which is insoluble in water and exhibits a high degree of polymerization. The PHB monomers can be used for the synthesis of complex chiral pharmaceutical compounds and also have the potential to be used as chiral precursors [16]. PHB is biocompatible and can be implanted in the human body without any inflammatory response. Degradation of PHB is a slow process inside the body, and therefore PHB can be useful in the slow drug release application as a carrier [17]. The use of PHB can be explored in the field of tissue engineering due to its biocompatible characteristics [18]. PHB in vitro biocompatibility has been established on different cell lines such as fibroblasts, osteoblasts, bone marrow cells, endothelial cells, smooth muscle cells, etc. PHB homopolymer is rigid and brittle in nature [19]. PHB has limited chemical resistance as it is attacked by acids and alkalis and dissolves in chlorinated solvents. PHB’s degradation rate at melt-processing temperature is high; thereby its copolymers and blends are ideal for general applications. Blending of PHB with other polymers or with plasticizers offers opportunities to reduce the brittleness and improve processability by lowering the processing temperature [14, 20].
Table 2 illustrates various PHB-based blends and its application field. Table 3 shows some of the commercially available PHB and their trade names.
Sources of PHB
Poly-3-hydroxybutyrate (PHB) is a linear polyester, which was first discovered in bacteria by Lemoigne in 1925 [21, 22]. The most important factor in production of PHB is that their biological production from non-replenishing sources by bacterial systems occurs in both gram-positive and gram-negative bacteria under the nutrient imbalance condition [23]. More than 20 bacterial species such as Bacillus megaterium, Methylobacterium rhodesianum, Alcaligenes eutrophus, M. extorquens, P. putida, Sphaerotilus natans, Escherichia coli, etc. are known as effective producers of PHB [24].
The chemolithotrophic bacteria like Ralstonia eutropha, Cupriavidus metallidurans, and Alcaligenes latus are capable of producing PHB from simple carbon sources. Among gram-positive bacteria, Bacillus spp., B. subtilis, Bacillus thuringiensis, and B. cereus and, among gram-negative bacteria, Pseudomonas spp. are notable for the production of PHB. A. eutropha is a gram-negative bacteria, capable of accumulating PHB, from simple carbon substrates such as glucose, palm oil, molasses, and cereal grains [25]. The microbial cells store PHB in the form of about 0.5 μm diameter granules, in the cytoplasmic fluid of the producing organism in well-defined subcellular complex sections called carbonosomes [26]. Bacterial species gather intracellular polyhydroxybutarates granules as energy and carbon reserves within their cells. Media play a vital function for the production of PHB. Molasses, corn steep liquor, wheat bran, starch, etc. are some of the cheap sources of media. The other important factors are carbon, nitrogen sources, pH, temperature, pressure, media flow rate, oxygen supply, etc. [14].
Bacterial PHB production belongs to two main groups; the first can be induced by deficiency of essential nutrients such as nitrogen, sulfate, phosphate, potassium, magnesium, etc., and the second group synthesizes PHB by the normal logarithmic growth stage. In the process, microorganisms are unable to synthesize amino acid but produce PHB and accumulate it as discrete granules in the presence of excess carbon. Glucose can be restored by starch, molasses, whey, activated sludge, etc. as the carbon source to enhance PHB yield. Utilization of cheap substrates will eventually decrease the production cost. Among different microorganisms, Alcaligenes eutrophus, a gram-negative bacteria, employs many advantages as it gathers PHB at up to 80% of its dry weight, is safe to handle in large quantities, grows efficiently on glucose as a carbon source, and is able to produce the industrially significant PHBV copolymer when propionic acid is added to the glucose feedstocks. PHBV copolymers with 30 mol% of hydroxyvalerate are formed, the hydroxyvalerate fraction being controlled by varying the ratio of glucose to propionic acid in the feedstock [14, 25, 27].
Life Stages of PHB
During polymerization, a skin is formed around the granule to separate the polymers from the aqueous cytosol. PHB granules are surrounded by a thick proteinaceous layer known as phasing which decides the size of the PHB granule and shields the granule in vivo [28]. After fermentation, bacterial cells containing polymer granules are separated from the medium by centrifugation. The polymers are easily recovered from the cells through solvent extraction. The intracellular PHB granules show amorphous characteristics, but the obtained extracellular PHB granules after solvent extraction show a semicrystalline configuration with an ordered molecular arrangement [29]. PHB granules accumulated in bacterial cells as the amorphous state are easily accessible by PHB depolymerases. PHB depolymerases use the presence of water, which is an integral part of the system. Freezing and storage of polymer granules at low temperature or by treatment with solvents during extraction activate irreversible conversion of PHB to the crystalline form [30]. Electron and X-ray diffraction data on single PHB crystals confirm an orthorhombic crystal system and indicate a fiber repeat of 5.9 Å [31]. Organic solvents like acetone, chloroform, methylene chloride, etc. are involved in the solvent extraction methods to recover the intracellular PHB. The requirement of organic solvents makes the process economically and environmentally disagreeable. But, for medical products, the solvent extraction is a good technique due to obtaining PHBs with a high purity. There are many potential alternative methods to the solvent extraction. Aqueous enzymatic procedures, treatments with ammonia, and digestion with sodium hypochlorites and surfactant are some of the alternative methods of the critical solvent extraction proposed for the recovery of PHBs. A new farming method allows the spontaneous release of up to 95% PHB per cell dry weight from Escherichia coli [14, 32]. The cost of PHB depends on the cost of carbon sources, equipment used, and downstream processing techniques. The scale-up of PHB production builds up recombinant strains capable of metabolizing different carbon sources. E. coli is a generally established recombinant system in terms of PHB production. E. coli has a delicate cell wall which makes it a suitable host for the PHB production. A promising strategy involves genetic engineering of microorganisms for the more efficient production of PHB [25]. Biosynthesis of PHB consists of three structural genes phbC, phbA, and phbB: the phbC codes involved in the polymerization of 3-hydroxybutyryl-CoA monomer to poly-3-hydroxybutyryl-CoA, phbA codes involved in the condensation of two acetyl-CoA molecules to form acetoacetyl-CoA, and phbB codes for the reduction of acetoacetyl-CoA to 3-hydroxybutyryl-CoA [12, 33].
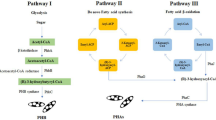
The compounding of PHB involves the incorporation of plasticizers and a nucleating agent to modify the thermal and mechanical properties by controlling the crystallization process and optimizing the flexibility of polymer. The elongation at break of PHB is improved by blending with P(3/4HB). Addition of lubricant helps to prevent the degradation of the PHB during the processing temperature of 170–180 °C. The use of glycerol, tributyrin, triacetin, acetyl triethyl citrate, and acetyltributylcitrate as plasticizers, saccharin as nucleation agent, and glycerol monostearate, various triglycerides, and 12-hydroxystearate as lubricants have been reported. Blending PHB with other polymers improves processability and reduces the brittleness of PHB homopolymer. PHB-co-HV possesses higher crystallization temperature and an increase in the HV fraction to 20 mol% in PHB-co-HV decreases the melting temperature. Vinyl acetate polymer and polyvinyl alcohol have been used to strengthen PHB. Different fillers have been used to improve tensile properties of PHB. PHB can be injection molded, compression molded, and extruded into films and hollow bodies. PHB is processed at a temperature in the range of 160 °C and 170 °C. The viscosity of PHB is similar to that of polypropylene [25].The complete carbon cycle of PHB is shown in Fig. 3.
Carbon cycle of poly(hydroxybutyrate) (Adapted from [14])
Blends and Composites of PHB
PHB is an environmentally friendly polymer and has a lot of applications in packaging and biomedical fields. However, the phenomenon called embrittlement limits the use of the polymer over a period of time due to the enhanced brittleness of the polymer [34]. In order to curb this issue, blending with other polymer is recommended. Several studies related to PHB blends are reported recently, and a short account of them is described in the following sections.
High-molecular-weight components in polymer blends influence the morphology and thereby the properties. Sharma et al. investigated the effect of ultrahigh-molecular-weight PHB in PHB on the morphology by a semi-continuous shear flow technique [35]. They found that the shishes formed by the said technique is stable for 5–10 min and on blending with different propotions of PHB stable fibers were formed. When the shear was severe (10–20), the formation of fibers became oriented, and stable morphology was obtained.
Generally, the blends of PHB with other polymers are immiscible due to the less interacting species in the component polymers. Similarly, the blends of high-molecular-weight PHB with PCL showed immiscibility in the entire composition range established by techniques such as SEM, DSC, and optical microscopy [36]. When low-molecular-weight modified PCL was used as the blending agent, the partially miscible blends were obtained, and the properties showed improvement. The biodegradation of the blends was also investigated, and it was found that the partially miscible blends showed a reduction in the degradation rate. This is due to the fractionated crystallization and the two-phase morphology seen in the blends. The different spherulitic morphology of the various blends is shown in Fig. 4. The miscibility of the components reflected in the higher degradation rate when the blends were exposed to A. flavus. The much higher degradation rate for the blends is believed to be due to two factors, the large surface area exposed to the surface due to the dispersion of the one component into the second one and the crystallinity changes due to fractionated crystallization and impurity transfer.
Spherulitic morphology obtained for the different blend systems at the indicated crystallization temperatures (reproduced with permission) [36].
Hong et al. investigated the effect of blending a small amount of carboxyl-terminated butadiene acrylonitrile rubber (CTBN) and biocompatible polyvinylpyrrolidone (PVP) with PHB on the crystallization and thermal degradation properties [37]. The blends were analyzed with respect to melting behavior, crystallinity, size of the crystals formed, the rate of crystallization, thermal stability, and degradation kinetics. It was found that the addition of both PVP and CTBN in small amounts greatly affected the degradation reaction. Other properties also showed significant improvements, for example, both thermal stability and crystallization rate were enhanced. 1 wt% of PVP was seen as the optimum and showed the maximum enhancement in crystallization and degradation behaviors.
Kumagai and Doi tried to blend PHB with poly(e-caprolactone) (PCL), poly(1,4-butylene adipate) (PBA), and poly(vinyl acetate) (PVAc) in different proportions [38]. The binary blends were investigated based on miscibility, morphology, and biodegradability. Out of the three systems, only PHB/PAc blends were miscible, and the other two were immiscible as evidenced by glass transition temperatures from DSC. Both the immiscible blends have phase-separated structure which was confirmed from the mechanical properties and SEM images. The enzymatic degradation of the blends was conducted at 37 °C at a pH of 7.4, and it was confirmed that the blend component with PHB has a greater influence on the degradation properties. As the weight percentage of the component increased especially for PVAc, the weight loss decreased gradually, and this behavior was later correlated with the morphology of the blends.
Barham and Organ studied the mechanical properties of PHB blends with hydroxyvalerate prepared by extrusion process [39]. They confirmed that at certain temperature and compositions, these blends form phase separation. The yield and fracture properties were correlated with crystallization aspects of the blends. Also, the effects of ageing at room temperature on the mechanical properties were investigated, and it was found that the properties get deteriorated over the course of time. Solution casting method using chloroform was used to prepare PHB with polyvinyl acetate and polyvinyl acetate-co-vinyl alcohol to get improved materials with physical properties [40]. The miscibility and biological applications of the prepared materials were investigated.
PHB as a brittle material caused a lot of problems for the process engineers in order to prepare suitable materials for different applications. Blending with other polymers is one of the options, and a few examples are described above. A few recent reports are collected in Table 4. Binary blends with polycaprolactone and PHB and their characterization are reported recently [52]. The blends were prepared like 100/0 to 0/100 using co-rotating twin-screw extruder followed by injection molding techniques. The effect of PCL on PHB and vice versa on mechanical, miscibility, and thermal properties were evaluated. The DSC and DMA analyses showed that the blends are immiscible in nature. The impact properties and flexural properties depended on the blend composition. Overall 25% of PCL or PHB showed good results.
Composites of PHB with other heterogeneous materials such as metal powders, nanotubes, fibers, fillers, etc. were prepared to improve the properties. Natural and synthetic fibers were used as potential reinforcements. Rajan et al. investigated the effect of chitosan on the mechanical and thermal properties of PHB up to 40 wt% of filler loading [53]. The composites were prepared by micro-compounding at a temperature of 175 °C, and the pelletized resultant materials were injection molded at appropriate conditions. Young’s modulus of PHB showed significant improvement with respect to the chitosan loading as well as glass transition temperature. Thermal stability of PHB did not improve much.
In order to improve the thermal stability near the melting point of PHB, two different nanoclays were incorporated by D’Amico et al. [54]. Two types of nanocomposites with montmorillonite Cloisite® Na+ and organomodified Cloisite® C15A were prepared by solution casting in chloroform. Mechanical, thermal, and crystallization characteristics were analyzed with appropriate techniques. The organomodified clay improved mechanical and crystallization properties compared to the former one. The more hydrophobic clay showed better dispersion due to chain mobility and catalytic activity.
The centrifugal spinning method was used to prepare PHB pectin composite fibers by Foster and Tighe to examine the in vitro hydrolytic degradation [55]. This method is more sophisticated than the conventional spinning technique. The hydrolytic degradation was conducted at 70 °C and at a pH of 10.6 with varying loadings of pectin in PHB. The degradation profile was greatly influenced by the pectin types used, and the materials were suggested for potential applications in wound dressing.
Macedo et al. did an interesting work with coir dust as the filler for PHB [56]. Coir dust is a biowaste generated during the processing of coir, and it contains short fiber and powder. The samples were prepared using an internal mixer and followed by hot pressing at suitable temperature and pressure. The loading of coir dust was varied from 0 to 30 wt%. From the different analytical techniques, it was confirmed that the 10 wt% loading has the optimum properties. Even though the matrix polymer is hydrophobic in nature and the filler is hydrophilic, a certain degree of interaction between them is visible at low loading due to surface features in the filler.
In order to utilize PHB as a hard tissue replacement owing to its biocompatibility, a composite with particulate hydroxyapatite (HA) was prepared [57]. The composites with varying weight percentages of HA up to 30 were prepared via compounding, milling, and molding techniques. In vitro tests in simulated body fluid (SBF) and other conventional tests were done to examine the suitability of the composites. The resultant composites showed high in vitro bioactivity in accordance with the volume percentage of HA. Initially, HA forms a layer on the composite surface as evidenced from SEM images, and prolonged immersion in SBF degrades it. Thus, the prepared composites are recommended for use as biodegradable materials for hard tissue replacement and regeneration.
Krishnaprasad et al. used bamboo microfibrils extracted from bamboo as reinforcements for PHB for different loadings [58]. The composites were prepared by compounding, pelletization, and microinjection molding at a temperature of 175 °C. The microfibrils showed irregular surface morphology as evidenced by SEM, thereby improving the mechanical properties due to the better adhesion between the polymer and fibrils. The optimum concentration of microfibrils was 10%. The optimum loading was again confirmed from impact strength analysis [59].
Cabedo et al. analyzed the degradation of PHB during processing with different nanoclays [60]. Processing styles such as internal mixing, extrusion, and solvent casting were employed. The effect of processing time, clay type, and clay content was systematically studied. When processed with montmorillonite-type clays, moecular weight of PHB showed decrease with respect to the loading. The release of water molecules present on the clay surfaces during processing might have reduced the molecular weight. Also, the modifier present on clay can catalyze the degradation of PHB. This was proved by different experimental techniques especially XRD studies which indicated intercalated morphology for the composites.
An interesting study with biocomposites of PHB and potato peel waste fermentation residue fiber is reported recently [61]. The by-product fibers were recovered from the fermentation process of potato peel waste. The biocomposites were prepared by premixing the components first, followed by internal mixing/compounding using extruder and finally injection molding at 180 °C. The biocomposites were investigated with respect to mechanical, thermal, dynamic mechanical properties, water absorption, and biodegradability. The biodegradability was tested periodically by keeping the composites under soil for 8 months. The fiber content was varied up to 50% and has a profound effect on the surface hydrophobicity and water absorption of the biocomposites. The mechanical properties showed a decrease compared to PHB. However, the biodegradability showed significant improvements for the composites having a higher loading of fibers. The composites having 25 and 50 wt% fibers showed more cracks and lengthy pits on the microscopic investigation. After 8 months the biocomposites with 50 wt% showed complete degradation as shown in Fig. 5.
Reflective optical micrographs (20×) of PHB and biocomposites exposed to soil biodegradation for 0–8 months (reproduced with permission) [61]
PHB-based materials are recently recommendded for use as biomedical devices and applicators [62]. A very recent study to utilize composite nanofiber membrane as tissue engineering scaffold is promising. Electrospinning technology was used to prepare a composite nanofiber scaffold by mixing PHB with carboxyl multiwalled carbon nanotubes grafted PHB. The grafting of the carbon nanotubes to PHB was achieved by condensation reactions of carboxyl groups and hydroxyl groups of the components. The physical and chemical properties of the composite nanofibers were examined by various techniques. The static mechanical properties showed greater improvement than the parent polymer. The composite nanofiber membrane with large surface area, high porosity, better biodegradability, cytocompatibility and appreciable mechanical properties could be useful as a tissue engineering scaffold in the future.
Biocomposites of PHB with natural fibers is another important field of study as both are biodegradable in nature. Hosokawa et al. prepared composites of PHB with random mats of sisal and coconut fibers using compression molding technique at 180 °C by varying the fiber content between 10 and 15% [63]. Thermal properties, water absorption, and mechanical properties of the composites were investigated before and after conditioning in a climate chamber. The composites showed appreciable thermal stability and flexural and tensile properties even after conditioning. The composites are suggested to be used as plastic bags for planting seedlings of vegetables and fruits in agriculture and disposable type of packaging trays instead of expanded polystyrene.
PHB can also be used for drug delivery applications. A series of compounds were investigated recently in this regard [64]. The crystal growth rates of supercooled liquids were tested with the presence of a small amount of PHB. The compounds were selected according to the glass transition temperature (Tg) which was much higher than PHB (around 50 °C). They showed an acceleration in crystal growth indicating that the presence of PHB enhanced the molecular movement of the crystallizing species. However, in the compounds which have similar Tg to PHB, the polymer acted as crystal growth inhibitor. This specificity like inhibitor or accelerator to crystal growth in organic compounds is significant for applications such as drug delivery.
Drug delivery applications and biomedical devices of PHB are getting significant attention in recent years. Scaffolds, films, and membranes based on PHB are being prepared using different techniques. A highly porous structured PHB films were synthesized by Tan et al. recently [65]. Solvent evaporation technique was used to prepare metal chloride/methanol/PHB/chloroform (MCl2/CH3OH/PHB/CHCl3) films, and the films were characterized by SEM and other techniques which confirmed the porosity. The change in concentration of metal chloride increased the average pore size. Also, they acted as nucleation sites, thereby increasing crystallinity as well as spherulitic growth.
Barouti et al. very recently reviewed the drug delivery applications of PHB in different forms [66]. The basis of the study is that the degradation of PHB leads to the compound present in humans, 3-hydroxybutyric acid, and the PHB-based systems generally show no cytotoxicity. High hydrophobicity and crystallinity of the microbial PHB prevent its use as biomedical devices due to poor compatibility with therapeutic agents, limited encapsulation effects, and inferior drug delivery rates. The chemical modification of PHB and many synthetic routes to prepare it have increased its usage as drug delivery systems. The review detailed the different synthesis approaches, the physicochemical characteristics of the systems from nanoscale to macroscale. The comparison with PLA- and PLGA-based self-assembly systems, the details regarding the in vivo and in vitro experiments, and the steps taken for the use of PHB materials as drug delivery systems are detailed carefully. It is believed that after the systematic clinical trials based on the advances in research from past years, nanoscale PHB-based composite materials will become a valuable platform for current drug delivery systems.
Contribution of PHB-Based Materials for the Preservation of Ecosystem and Sustainability
Sustainability is defined as the study of how natural systems function, remain diverse, and produce everything it needs for the ecology to remain in balance. It also acknowledges that human civilization takes resources to sustain our modern way of life [67]. There are various human and social factors that contribute to a healthy preservation of the sustainability. Economic development, social development, and environmental protection are considered as three pillars of sustainability. Public awareness and concern about the growing environmental pollution due to the enhanced usage of fuels and materials based on fossil origin is one of the major driving forces for the development of biopolymers. The frequent fluctuations in the petroleum prices and technological breakthrough in the field of biotechnology for the synthesis of value-added materials from low-cost natural materials are adding flavors to the development of polymers based on renewable resources. The major benefit of using bio-based polymers for various applications is their prospective contribution toward greenhouse gas balances, preservation of the ecosystem, and other positive environmental impacts over their whole life cycles.
PHB is degraded upon exposure to soil, compost, or marine sediment. The percentage of PHB polymer degrading microbes in the environment was likely to be 0.5–9.6% of the entire colonies. Most of the PHB-degrading microorganisms are isolated at ambient temperatures, and very few of them are capable of degrading PHB at a higher temperature. Biodegradation of PHB takes place in both aerobic and anaerobic atmospheres, without developing any toxic products [20, 68]. Aerobic degradation entails the transfer of PHB ultimately to CO2 and H2O. Anaerobic degradation of PHB can be evaluated by the analysis of methane emission. Many bacteria flourish in anaerobic environments and degrade PHB and its blends. PHB biodegradation is dependent on microbial activity, moisture, temperature, environment pH, molecular weight, crystallinity, etc. Copolymers having PHB monomer units are degraded rapidly than either PHB homopolymer or its copolymers. The studies showed that PHB can be degraded by 39 bacterial strains of the classes Firmicutes and Proteobacteria [69]. During biodegradation stage, bacteria develop extracellular hydrolases that are capable of altering the polymers into the respective hydroxyl acid monomers. PHB products are hydrolysed into R-3-hydroxybutyric acid monomers, and they are metabolized by β-oxidation and the tricarboxylic acid cycle to form carbon dioxide and water under aerobic conditions [15].
Understanding the mechanism of degradation and analysis of the degradation products is very much essential to understand the impact of degradation of a biopolymer to its surrounding environment. Several studies were conducted to analyze the degradation profile of PHB under various conditions. Bacterial degradation of PHB in natural environments such as water soil and compost yield water and carbon dioxide [70]. The enzymatic degradation of PHB films was reported by Kumagai et al. [71] at a temperature of 37 °C and pH of 7.4 using aqueous solutions of an extracellular PHB depolymerase obtained from Alcaligenes faecalis T1. It was reported that as the degree of crystallinity of PHB increased, the enzymatic degradation rate of PHB films showed a decreasing trend. The degradation started with hydrolysis of PHB chains on the surface of the film in the amorphous state and proceeded further to the crystalline phase. The same research group compared the degradation behavior of a blend of PHB with poly (ethylene oxide) (PEO) under similar conditions [72]. It was observed that the enzymatic degradation rate of the film prepared from the blend was more rapid than that of films obtained from pure PHB. The surface morphology of the blend films after degradation revealed many holes which were due to the elution of the PEO component of the blend into the aqueous buffer. The enzymatic degradation of the PHB/PEO blend films was improved by the permeation of PHB depolymerase into the films through these holes. The fungal degradation of PHB was reported by Lee et al. [73]. It was shown that the types of fungi present in the pond, soils, compost, sea water, hay, horse dung, etc. are capable of degrading PHB. A very interesting review on the degradation of microbial polyesters including PHB was published by Tokiwa et al. [74].
A general mechanism for the degradation of plastics under aerobic conditions is shown in Fig. 6.
Mechanism of plastics biodegradation under aerobic conditions (Reproduced with permission from [75])
The degradation products of PHB can be analyzed by various analytical techniques such as HPLC, GC-MS, CZE, and NMR [76,77,78,79,80,81,82]. Vapor pressure analysis using HPLC as a technique to monitor degradation of PHB was reported by Polyak and co-workers [83]. Generally, the microorganisms utilize oxygen to oxidize carbon in the polymer to carbon dioxide as one of the degradation products. Hence, consumption of oxygen and formation of carbon dioxide are good indications for the degradation of PHB. The carbon dioxide released from the degradation of PHB is used by the plants for their photosynthesis. A pictorial representation of the life cycle of PHB is shown in Fig. 3. In this manner, the life cycle of a healthy ecosystem is preserved.
The sustainability of the ecosystem can be ensured only by preserving the participating species in the ecosystem. The littering of waste plastic products can pose a serious threat to one or other components of the ecosystem, which in turn affects others and so on. The use of biopolymers including PHB helps in reducing the problems associated with the non-biodegradability of conventional petroleum-based plastics. The current world trend of development and commercialization of more and more bio-based plastics is noteworthy. In the present scenario, the contribution of PHB toward the sustainability of the ecosystem is very much valuable.
Conclusion
Polyhydroxybutyrate is a biopolymer which has unique properties. The sources, inherent properties, and their blends and composites were elaborately discussed in the chapter. This environment-friendly polymer has been utilized widely by various researchers to establish the degradation mechanism of a typical biopolymer. The contribution of PHB and their derivatives to the sustainability of ecosystem has been reviewed. The recent works related to PHB as a biomedical material as scaffolds, tissue engineering material, and drug delivery device were carefully analyzed. It is to be emphasized that the advancement of technology of PHB will lead it into a standout polymer in the near future itself as a wonderful commercial as well as biomedical biopolymer.
References
PlasticsEurope (2016) Plastics—the Facts 2016: An analysis of European plastics production, demand and waste data
Geyer R, Jambeck JR, Law KL (2017) Production, use, and the fate of all plastics ever made. Sci Adv 3(7). https://doi.org/10.1126/sciadv.1700782
Derraik JG (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44(9):842–852
Moore CJ (2008) Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ Res 108(2):131–139. https://doi.org/10.1016/j.envres.2008.07.025
Eriksen M, Lebreton LC, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J (2014) Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS One 9(12):e111913
Johnson R, Mwaikambo L, Tucker N (2003) Rapra review reports. Biopolymers 14:3–26
Biocomposites IfBa (2015) Biopolymers—facts and statistics
Kaplan DL (1998) Introduction to biopolymers from renewable resources. In: Biopolymers from renewable resources. Springer, Heidelberg, pp 1–29
Cornell DD (2007) Biopolymers in the existing postconsumer plastics recycling stream. J Polym Environ 15(4):295–299
Niaounakis M (2015) Biopolymers: applications and trends. William Andrew, Oxford
Grousseau E, Blanchet E, Déléris S, Albuquerque MG, Paul E, Uribelarrea J-L (2013) Impact of sustaining a controlled residual growth on polyhydroxybutyrate yield and production kinetics in Cupriavidus necator. Bioresour Technol 148:30–38
Sreedevi S, Unni KN, Sajith S, Priji P, Josh MS, Benjamin S (2014) Bioplastics: advances in polyhydroxybutyrate research
Saharan B, Ankita SD (2012) Bioplastics-for sustainable development: a review. Int J Microbial Res Technol 1:11–23
Verlinden RA, Hill DJ, Kenward M, Williams CD, Radecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102(6):1437–1449
Hankermeyer CR, Tjeerdema RS (1999) Polyhydroxybutyrate: plastic made and degraded by microorganisms. Rev Environ Contam Toxicol 159:1–24
Ren Q, Grubelnik A, Hoerler M, Ruth K, Hartmann R, Felber H, Zinn M (2005) Bacterial poly (hydroxyalkanoates) as a source of chiral hydroxy alkanoic acids. Biomacromolecules 6(4):2290–2298
Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Prog Polym Sci 32(8):762–798
Zhao K, Yang X, Chen G-Q, Chen J-C (2002) Effect of lipase treatment on the biocompatibility of microbial polyhydroxyalkanoates. J Mater Sci Mater Med 13(9):849–854
Chan RT, Russell RA, Marçal H, Lee TH, Holden PJ, Foster LJR (2013) BioPEGylation of polyhydroxybutyrate promotes nerve cell health and migration. Biomacromolecules 15(1):339–349
Bugnicourt E, Cinelli P, Lazzeri A, Alvarez VA (2014) Polyhydroxyalkanoate (PHA): review of synthesis, characteristics, processing and potential applications in packaging. Express Polym Lett 8:791–808
Lenz RW, Marchessault RH (2005) Bacterial polyesters: biosynthesis, biodegradable plastics and biotechnology. Biomacromolecules 6(1):1–8
Lemoigne M (1926) Products of dehydration and of polymerization of β-hydroxybutyric acid. Bull Soc Chem Biol 8:770–782
Luengo JM, Garcıa B, Sandoval A, Naharro G, Olivera ER (2003) Bioplastics from microorganisms. Curr Opin Microbiol 6(3):251–260
Heo K, Yoon J, Jin KS, Jin S, Sato H, Ozaki Y, Satkowski MM, Noda I, Ree M (2008) Structural evolution in microbial polyesters. J Phys Chem B 112(15):4571–4582
Poltronieri P, Kumar P (2017) Polyhydroxyalkanoates (PHAs) in industrial applications. In: Martínez LMT, Kharissova OV, Kharisov BI (eds) Handbook of Ecomaterials. Springer, Cham, pp 1–30. https://doi.org/10.1007/978-3-319-48281-1_70-1
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54(4):450–472
Gassner F, Owen A (1996) Some properties of poly (3-hydroxybutyrate)–poly (3-hydroxyvalerate) blends. Polym Int 39(3):215–219
Cai S, Cai L, Liu H, Liu X, Han J, Zhou J, Xiang H (2012) Identification of the haloarchaeal phasin (PhaP) that functions in polyhydroxyalkanoate accumulation and granule formation in Haloferax mediterranei. Appl Environ Microbiol 78(6):1946–1952
Jendrossek D (2007) Peculiarities of PHA granules preparation and PHA depolymerase activity determination. Appl Microbiol Biotechnol 74(6):1186
Zhang M, Thomas NL (2011) Blending polylactic acid with polyhydroxybutyrate: the effect on thermal, mechanical, and biodegradation properties. Adv Polym Technol 30(2):67–79. https://doi.org/10.1002/adv.20235
Iwata T, Aoyagi Y, Tanaka T, Fujita M, Takeuchi A, Suzuki Y, Uesugi K (2006) Microbeam X-ray diffraction and enzymatic degradation of poly [(R)-3-hydroxybutyrate] fibres with two kinds of molecular conformations. Macromolecules 39(17):5789–5795
Jung IL, Phyo KH, Kim KC, Park HK, Kim IG (2005) Spontaneous liberation of intracellular polyhydroxybutyrate granules in Escherichia coli. Res Microbiol 156(8):865–873
Paganelli FL, de Macedo Lemos EG, Alves LMC (2011) Polyhydroxybutyrate in rhizobium and Bradyrhizobium: quantification and phbC gene expression. World J Microbiol Biotechnol 27(4):773–778
de Koning GJM, Lemstra PJ (1993) Crystallization phenomena in bacterial poly[(R)-3-hydroxybutyrate]: 2. Embrittlement and rejuvenation. Polymer 34(19):4089–4094. https://doi.org/10.1016/0032-3861(93)90671-V
Sharma L, Ogino Y, Kanaya T, Iwata T, Doi Y (2006) A new shear technique: semi-continuous shear flow (SCSF) in medium and ultra high molecular weight polyhydroxybutyrate blends. J Mater Sci 41(17):5687–5695. https://doi.org/10.1007/s10853-006-0330-2
Lovera D, Márquez L, Balsamo V, Taddei A, Castelli C, Müller AJ (2007) Crystallization, morphology, and enzymatic degradation of polyhydroxybutyrate/polycaprolactone (PHB/PCL) blends. Macromol Chem Phys 208(9):924–937. https://doi.org/10.1002/macp.200700011
Hong S-G, Gau T-K, Huang S-C (2011) Enhancement of the crystallization and thermal stability of polyhydroxybutyrate by polymeric additives. J Therm Anal Calorim 103(3):967–975. https://doi.org/10.1007/s10973-010-1180-3
Kumagai Y, Doi Y (1992) Enzymatic degradation and morphologies of binary blends of microbial poly(3-hydroxybutyrate) with poly(ε-caprolactone), poly(1,4-butylene adipate) and poly(vinyl acetate). Polym Degrad Stab 36(3):241–248. https://doi.org/10.1016/0141-3910(92)90062-A
Barham PJ, Organ SJ (1994) Mechanical properties of polyhydroxybutyrate-hydroxybutyrate-hydroxyvalerate copolymer blends. J Mater Sci 29(6):1676–1679. https://doi.org/10.1007/bf00368945
Abou-Aiad TH, El-Sabee MZ, Abd-El-Nour KN, Saad GR, El-Sayed E-SA, Gaafar EA (2002) Miscibility and the specific interaction of polyhydroxybutyrate blended with polyvinyl acetate and poly(vinyl acetate-co-vinyl alcohol) with some biological applications. J Appl Polym Sci 86(9):2363–2374. https://doi.org/10.1002/app.11137
Pankova YN, Shchegolikhin AN, Iordanskii AL, Zhulkina AL, Ol'khov AA, Zaikov GE (2010) The characterization of novel biodegradable blends based on polyhydroxybutyrate: the role of water transport. J Mol Liq 156(1):65–69. https://doi.org/10.1016/j.molliq.2010.04.018
Arrieta MP, López J, Hernández A, Rayón E (2014) Ternary PLA–PHB–limonene blends intended for biodegradable food packaging applications. Eur Polym J 50(Supplement C):255–270. https://doi.org/10.1016/j.eurpolymj.2013.11.009
Nair NR, Nampoothiri KM, Pandey A (2012) Preparation of poly(l-lactide) blends and biodegradation by Lentzea waywayandensis. Biotechnol Lett 34(11):2031–2035. https://doi.org/10.1007/s10529-012-1005-5
Zhang M, Thomas NL (2010) Preparation and properties of polyhydroxybutyrate blended with different types of starch. J Appl Polym Sci 116(2):688–694. https://doi.org/10.1002/app.30991
de Carvalho FP, Quental AC, Felisberti MI (2008) Polyhydroxybutyrate/acrylonitrile-g-(ethylene-co- propylene-co-diene)-g-styrene blends: their morphology and thermal and mechanical behavior. J Appl Polym Sci 110(2):880–889. https://doi.org/10.1002/app.28557
Lin K-W, Lan C-H, Sun Y-M (2016) Poly[(R)3-hydroxybutyrate] (PHB)/poly(l-lactic acid) (PLLA) blends with poly(PHB/PLLA urethane) as a compatibilizer. Polym Degrad Stab 134(Supplement C):30–40. https://doi.org/10.1016/j.polymdegradstab.2016.09.017
Arrieta MP, Fortunati E, Dominici F, Rayón E, López J, Kenny JM (2014) PLA-PHB/cellulose based films: mechanical, barrier and disintegration properties. Polym Degrad Stab 107(Supplement C):139–149. https://doi.org/10.1016/j.polymdegradstab.2014.05.010
Horowitz DM, Sanders JKM (1994) Phase separation within artificial granules from a blend of polyhydroxybutyrate and polyhydroxyoctanoate: biological implications. Polymer 35(23):5079–5083. https://doi.org/10.1016/0032-3861(94)90668-8
Asran AS, Razghandi K, Aggarwal N, Michler GH, Groth T (2010) Nanofibers from blends of polyvinyl alcohol and polyhydroxy butyrate as potential scaffold material for tissue engineering of skin. Biomacromolecules 11(12):3413–3421. https://doi.org/10.1021/bm100912v
Arrieta MP, López J, Rayón E, Jiménez A (2014) Disintegrability under composting conditions of plasticized PLA–PHB blends. Polym Degrad Stab 108(Supplement C):307–318. https://doi.org/10.1016/j.polymdegradstab.2014.01.034
Reis KC, Pereira J, Smith AC, Carvalho CWP, Wellner N, Yakimets I (2008) Characterization of polyhydroxybutyrate-hydroxyvalerate (PHB-HV)/maize starch blend films. J Food Eng 89(4):361–369. https://doi.org/10.1016/j.jfoodeng.2008.04.022
Garcia-Garcia D, Ferri JM, Boronat T, Lopez-Martinez J, Balart R (2016) Processing and characterization of binary poly(hydroxybutyrate) (PHB) and poly(caprolactone) (PCL) blends with improved impact properties. Polym Bull 73(12):3333–3350. https://doi.org/10.1007/s00289-016-1659-6
Rajan R, Sreekumar PA, Joseph K, Skrifvars M (2012) Thermal and mechanical properties of chitosan reinforced polyhydroxybutyrate composites. J Appl Polym Sci 124(4):3357–3362. https://doi.org/10.1002/app.35341
D'Amico DA, Manfredi LB, Cyras VP (2012) Relationship between thermal properties, morphology, and crystallinity of nanocomposites based on polyhydroxybutyrate. J Appl Polym Sci 123(1):200–208. https://doi.org/10.1002/app.34457
Foster LJR, Tighe BJ (2009) In vitro hydrolytic degradation of centrifugally spun polyhydroxybutyrate–pectin composite fibres. Polym Int 58(12):1442–1451. https://doi.org/10.1002/pi.2682
Macedo JS, Costa MF, Tavares MIB, Thiré RMSM (2010) Preparation and characterization of composites based on polyhydroxybutyrate and waste powder from coconut fibers processing. Polym Eng Sci 50(7):1466–1475. https://doi.org/10.1002/pen.21669
Ni J, Wang M (2002) In vitro evaluation of hydroxyapatite reinforced polyhydroxybutyrate composite. Mater Sci Eng C 20(1):101–109. https://doi.org/10.1016/S0928-4931(02)00019-X
Krishnaprasad R, Veena NR, Maria HJ, Rajan R, Skrifvars M, Joseph K (2009) Mechanical and thermal properties of bamboo microfibril reinforced polyhydroxybutyrate biocomposites. J Polym Environ 17(2):109. https://doi.org/10.1007/s10924-009-0127-x
Rajan KP, Veena NR, Maria HJ, Rajan R, Skrifvars M, Joseph K (2011) Extraction of bamboo microfibrils and development of biocomposites based on polyhydroxybutyrate and bamboo microfibrils. J Compos Mater 45(12):1325–1329. https://doi.org/10.1177/0021998310381543
Cabedo L, Plackett D, Giménez E, Lagarón JM (2009) Studying the degradation of polyhydroxybutyrate-co-valerate during processing with clay-based nanofillers. J Appl Polym Sci 112(6):3669–3676. https://doi.org/10.1002/app.29945
Wei L, Liang S, McDonald AG (2015) Thermophysical properties and biodegradation behavior of green composites made from polyhydroxybutyrate and potato peel waste fermentation residue. Ind Crop Prod 69:91–103. https://doi.org/10.1016/j.indcrop.2015.02.011
Zhijiang C, Cong Z, Jie G, Qing Z, Kongyin Z (2018) Electrospun carboxyl multi-walled carbon nanotubes grafted polyhydroxybutyrate composite nanofibers membrane scaffolds: preparation, characterization and cytocompatibility. Mater Sci Eng C 82(Supplement C):29–40. https://doi.org/10.1016/j.msec.2017.08.005
Hosokawa MN, Darros AB, VAdS M, JMFd P (2017) Polyhydroxybutyrate composites with random Mats of sisal and coconut fibers. Mater Res 20:279–290
Sato T, Taylor LS (2017) Acceleration of the crystal growth rate of low molecular weight organic compounds in supercooled liquids in the presence of polyhydroxybutyrate. CrystEngComm 19(1):80–87. https://doi.org/10.1039/C6CE02177H
Tan WL, Yaakob NN, Abidin AZ, Bakar MA, Bakar NHHA (2016) Metal chloride induced formation of porous Polyhydroxybutyrate (PHB) films: morphology, thermal properties and crystallinity. IOP Conference Series: Materials Science and Engineering 133(1):012012
Barouti G, Jaffredo CG, Guillaume SM (2017) Advances in drug delivery systems based on synthetic poly(hydroxybutyrate) (co)polymers. Prog Polym Sci 73(Supplement C):1–31. https://doi.org/10.1016/j.progpolymsci.2017.05.002
Sustainability, http://www.epa.gov/sustainability/basicinfo.htm
Ong SY, Chee JY, Sudesh K (2017) Degradation of Polyhydroxyalkanoate (PHA): a review. Journal of Siberian Federal University Biology 10(2):211
Suyama T, Tokiwa Y, Ouichanpagdee P, Kanagawa T, Kamagata Y (1998) Phylogenetic affiliation of soil bacteria that degrade aliphatic polyesters available commercially as biodegradable plastics. Appl Environ Microbiol 64(12):5008–5011
Swift G (1993) Directions for environmentally biodegradable polymer research. Acc Chem Res 26(3):105–110
Kumagai Y, Kanesawa Y, Doi Y (1992) Enzymatic degradation of microbial poly(3-hydroxybutyrate) films. Die Makromolekulare Chemie 193(1):53–57. https://doi.org/10.1002/macp.1992.021930105
Kumagai Y, Doi Y (1992) Enzymatic degradation of poly (3-hydroxybutyrate)-based blends: poly (3-hydroxybutyrate)/poly (ethylene oxide) blend. Polym Degrad Stab 35(1):87–93
Lee K-M, Gimore D, Huss M (2005) Fungal degradation of the bioplastic PHB (Poly-3-hydroxy-butyric acid). J Polym Environ 13(3):213–219
Tokiwa Y, Calabia BP (2004) Review degradation of microbial polyesters. Biotechnol Lett 26(15):1181–1189
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26(3):246–265. https://doi.org/10.1016/j.biotechadv.2007.12.005
Kanesawa Y, Tanahashi N, Doi Y, Saito T (1994) Enzymatic degradation of microbial poly (3-hydroxyalkanoates). Polym Degrad Stab 45(2):179–185
Eldsäter C, Albertsson AC, Karlsson S (1997) Impact of degradation mechanisms on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) during composting. Acta Polym 48(11):478–483
Braunegg G, Sonnleitner B, Lafferty R (1978) A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Appl Microbiol Biotechnol 6(1):29–37
Scandola M, Focarete ML, Adamus G, Sikorska W, Baranowska I, Świerczek S, Gnatowski M, Kowalczuk M, Jedliński Z (1997) Polymer blends of natural poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and a synthetic atactic poly (3-hydroxybutyrate). Characterization and biodegradation studies. Macromolecules 30(9):2568–2574
Athlan A, Braud C, Vert M (1997) Abiotic aging of water-soluble 3-hydroxybutyric acid oligomers as monitored by capillary zone electrophoresis. J Polym Environ 5(4):243–247
Abe H, Doi Y, Kumagai Y (1994) 3-hydroxybutyrate-β-6-hydroxyhexanoate as a compatibilizer for a biodegradable blend of poly [(R)-3-hydroxybutyrate] and poly (6-hydroxyhexanoate). Macromolecules 27:6012–6017
Abe H, Doi Y, Aoki H, Akehata T, Hori Y, Yamaguchi A (1995) Physical properties and enzymic degradability of copolymers of (R)-3-hydroxybutyric and 6-hydroxyhexanoic acids. Macromolecules 28(23):7630–7637
Polyák P, Rácz P, Rózsa P, Nagy GN, Vértessy BG, Pukánszky B (2017) The novel technique of vapor pressure analysis to monitor the enzymatic degradation of PHB by HPLC chromatography. Anal Biochem 521:20–27
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Rajan, K.P., Thomas, S.P., Gopanna, A., Chavali, M. (2019). Polyhydroxybutyrate (PHB): A Standout Biopolymer for Environmental Sustainability. In: Martínez, L., Kharissova, O., Kharisov, B. (eds) Handbook of Ecomaterials. Springer, Cham. https://doi.org/10.1007/978-3-319-68255-6_92
Download citation
DOI: https://doi.org/10.1007/978-3-319-68255-6_92
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68254-9
Online ISBN: 978-3-319-68255-6
eBook Packages: EngineeringReference Module Computer Science and Engineering