Abstract
Polyhydroxyalcanoates (PHAs) are biodegradable polyesters produced by many bacteria that accumulate them as intracellular storage material in the cytoplasm. These polymers are potential candidate for substitution of petrochemical nonrenewable plastics for their biodegradable and nontoxic properties.
Polyhydroxyalkanoate (PHA) can be synthesized by different strategies, such as microbial production by wild type or recombinant microorganisms, in vitro production via PHA synthase-mediated catalysis, or using genetically engineered plants.
PHA accumulation in natural strains is favored by high availability of carbon source and a limited amount of macrocomponents (nitrogen, phosphate, oxygen) or microcomponents (sulfate, magnesium ions, and other trace elements).
PHAs are applied in many fields, such as packaging, medicine, or agriculture, but the extensive application of the bioplastics is constrained by high production costs, especially for raw material, downstream processing, and polymer recovery.
In this chapter, the progresses in production of PHA in natural strains and in engineered E. coli, Pseudomonas spp., Bacillus, Aeromonas, and other bacteria, such as the halotolerant Halomonas spp., are presented.
In addition, the constrains on purification steps and the potential of high value applications are presented.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Introduction
Polyhydroxyalkanoates (PHAs) are biodegradable polyesters produced by bacteria such as Cupriavidus necator (Ralstonia eutropha), as well as other Gram-negative and Gram-positive bacteria. PHAs is accumulated in response to stress conditions. The carbon/nitrogen (C:N) ratio may be determinant for some species, while other bacterial species have growth-associated PHA production which is independent of C:N ratio. Poly(3-hydroxybutyrate) (PHB) is the prototype polymer for biodegradable polymers, appearing a tough, brittle plastic-like material, with good toughness and stiffness, which may be made more fluid by the addition of plasticizers, adapting its mechanical properties by the addition of other fibers and compounding materials. Applications of PHA are various, such as in coating materials and bioplastic components, packaging films and bottling, in drug delivery, and medical devices [1]. PHAs-based bioplastics possess good mechanical properties and are easily molded into various shapes and materials for bottles, inks, sealants, packaging for consumer goods and food films, and agriculture sheets.
Bioderived, biodegradable polymers are suitable for different kind of applications, in electronics, cosmetics, biomedical sector, aerospace, consumer goods, agriculture, packaging industries, and active packaging. It can also act as bioplastic material for soft biocomposition, conductive bioplastics, high-tech electronic devices, disposable tableware, toys, golf tees, bags, automotive components, lightweight structural composites for the buildings industry, textiles, elastomeric plastics, disposable materials and fibers, performance additives, transparent films, high-strength fibers, fertilizer mulches, and pellet for soil application.
Due to its biocompatibility and resorption qualities, PHA polymers have been exploited in devices for minimally invasive delivery, microparticles in drug delivery, for cardiac valves and surgical sutures, in regenerative medicine, artificial skin, tissue engineering such as scaffolds for tendon and fractured bones, artificial organ reconstruction, in matrices for nerve repair as support of cell growth [2, 3]. The FDA has delivered its approval for P4HB applied to clinics and human therapies; therefore, PHA applications in the medical fields will continue to grow.
PHA Industrial Production: Feed Costs
PHAs costs may also vary depending on the type of application, since materials for drug delivery and medical device components have high value [1]. The main problem is the cost of the feedstock, with incidence of 50% of the costs, in addition to biofermentor and personnel costs, the costs for extraction and purification.
As a raw material for the fermentation, in addition to carbohydrates and sugarcane molasses (Nikel et al. 2006; Naheed and Jamil 2014), vegetable oil or glycerol can be used (Kumar et al. 2015). With the aim to improve the sustainable use of feed materials, wastes and nonfood-competing sources have been used in production of PHA polymers. Therefore, methods to exploit by-products as feedstock have been developed (Kumar et al. 2016). High production of PHA in fermentation process has been obtained using C1 carbon sources (CH4, syngas) [4], sugarcane molasses [5, 6, 7, 8, 9], soy molasses, agroindustrial wastes [10,11,12], glycerol from diesel waste [13, 14]; animal and vegetable fats [11, 15, 16, 17], and biorefinery byproducts [9, 14, 18, 19, 20, 132, 137]. New approaches of bioprocessing domestic kitchen waste, municipal solid waste (MSW), and organic biowastes [133, 137], using adapted bacterial strains, with possibility of hydrogen (H2) and methane coproduction, have been shown feasible (Patel et al. 2015; Kumar et al. 2013, 2016), pushing forward the applications in the biorefineries sector [163,164,165].
Global demand for biodegradable plastics has reached the value of 700,000 tonnes. Envisioned trends of production sum up to 270,000 tonn/year. Table 1 describes the companies producing PHB and its copolymers, P2HB4HB, PHBHV, and PHBHHx. In 2016, Metabolix has closed its activities in the PHA field, and patents were acquired by Cheil Jedang, based in Seoul.
Types of PHA
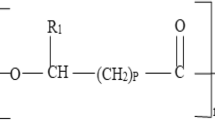
While some species produce mainly 3PHB polymers, other species can synthesize PHAs, depending on availability of intermediate precursors, such as citrate [103]. PHA synthases can polymerize short-chain-length PHAs (scl-3PHAs), or medium-chain-length PHAs (mcl-3PHAs), depending on the class of enzymes and the species and genetic background [21]. Among the short-chain-length PHA (scl-PHA) types produced are copolymers containing hydroxypropionate (3HB-co-HP), 4-hydroxybutyrate (3HB-co-4HB), hydroxyvalerate (3HB-co-HV) [22], and 3-hydroxyhexanoate (3HB-co-HH), depending on the availability of precursors (propionate, valerate, hexanoate); as for the medium-chain-length PHA (mcl-PHA) types produced are copolymers containing hydroxyhexanoate, hydroxyeptanoate, hydroxyoctanoate, hydroxydecanoate, and hydroxydodecanoate [19, 23,24,25,26,27]. The synthesis of polyhydroxyalkanoate copolymers with controlled composition of hydroxyalkanes has been reported [25]. Until today, around 150 different compounds have been identified as the monomeric units within the PHA polymer. Randomly ordered copolymers, such as poly 3-hydroxybutyrate-co-3-hydroxyhexanoate (P3HB3HH) containing functional groups, i.e., olefin groups, branched alkyl chains, halogen atoms, aromatic groups, and cyano groups have been described [28]. New P(LA-co-3HB-co-3HP) terpolyesters incorporating polylactate have also been described [29]. The flexibility of PHA biosynthesis favors the design and production of biopolymers having particular physical properties, such as stiffness, elasticity, durability, resistance, or rubbery properties.
Homopolymers
Several homopolymers for bioplastics have been produced in bacteria, such as polylactic acid (PLA), and, for PHA, the reference polymer P3HB, 3PHB in sizes up to ultrahigh molecular weight (hmw-PHB) [30, 31], poly 4-hydroxybutyrate (P4HB) [143], poly 3-hydroxypropionate (P3HP) [13], poly 3-hydroxyvalerate (PHV) [32]; poly 3-hydroxy-4-pentenoate (P3H4P), poly hydroxyhexanoate (P3HH) [33, 34], poly 3-hydroxyheptanoate (P3HH), poly 3-hydroxyoctanoate (P3HO) [35], poly 3-hydroxydecanoate (P3HD) [36], poly 3-hydroxy-10-undecenoate (3H10U), poly 3-hydroxydodecanoate (P3HDD) [37], poly 3-hydroxytetradecanoate (P3HTD), poly 3-hydroxy-5-phenylvalerate P(3HPhV), 3-hydroxy-6-phenylhexanoate (3H6PhHs), and functionalized mcl-PHA [38, 142]. With the engineering of the β-oxidation pathway, additional homopolymers can be made available [39,40,41].
Random Copolymers
Poly(3HB-co-mcl-3HA) has been produced by P&G on industrial scale, under the NODEX trademark. The wide range of commercially produced PHA as random copolymers include poly(3HP-co-4HB), poly(3HB-co-3HP) [42], poly(3HB-co-3HV) (PHBV) [15, 43,44,45,46], poly(3HB-co-4HB) (P3HB4HB) [47, 143], and poly(3HB-co-3HHx) (PHBHHx) [22, 48,49,50,51]. Using Aeromonas hydrophila expressing phaPCJ synthase, mutations in the enoyl coenzyme A hydratase enhanced the 3-hydroxyhexanoate availability for the synthesis of poly 3-hydroxybutyrate-co-3-hydroxyhexanoate (PHBHHs) [48, 49, 52]. Copolymers of P(3HHx-co-3HO-co-3HD-co-3HDD) can be produced using Pseudomonas spp. but possess a too soft consistence. Mixed polyesters such as poly-lactate-co-glycolate and poly(LA-co-3HB-co-3HP) have also been produced [29, 53]. Copolymers of poly(3HB-co-3MP) and poly(3HB-co-LA) have been produced using recombinant Escherichia coli; these copolymers demonstrated improved characteristics.
Block Copolymers
Block copolymerization allows to modify the thermodynamic property of polymers that resist ageing processes. PHA block copolymers of PHB-b-PHBV, a material more resistant to ageing process, were obtained by feeding alternated carbon sources during fermentation process [54]. Various researchers produced different diblock copolymers, such as PHB-b-P3HVHHp, PHB-b-P4HB [29]), PHB-b-PHHx [55], P3HB-b-P3HP [42], P3HP-b-P4HB, and P3HHx-b-P(3HD-co-3HDD) [37, 39, 40]. The addition in sequence of two carbon substrates can lead to the incorporation into the PHA of the block copolymer [42, 51]. By adding 1,3-propanediol followed by 1,4-butanediol, E. coli cells synthesized P3HP-b-P4HB block copolymers. These copolymers show optimized properties. Various diblock copolymers can be obtained regulating the availability of fed substrates.
Graft Polymers
Graft copolymers are synthesized by chemical modification, introducing a functional group into PHA chains, through insertion of small molecules (double bonds, triple bonds, epoxy groups, carbonyl, cyano, phenyl groups, or halogens) into the PHA side chain, with improved property and characteristics. Presently, PHA derivatives obtained are: poly(styrene peroxide)-g-PHA (PS-g-PHA), poly(methyl methacrylate peroxide)-g-PHA (PMMA-g-PHA), PHA-g-polyacrylic acid (PHA-g-PAA), PHA-g-cellulose, PHB-g-acrylic acid-starch (PHB-g-AA/starch), PHA-g-AA-chitosan (PHA-g-AA-COS), polyethylene glycol-g-PHA (PEG-g-PHA), monoacrylate-polyethylene glycol-g-PHO (PEGMA-g-PHO), polylactic acid-g-PHA (PLA-g-PHA), glycerol-1,3-diglycerol diacrylate-g-PHO (GDD-g-PHO), vinylimidazole-g-PHO (VI-g-PHO), PHBV-g-poly(phenyl vinyl ketone) (PHBV-g-PVK), PHBV-g-polyacrylamide (PHBV-g-PA), among others.
Bacterial Species Producing PHAs
Many bacteria produce polyhydroxyalkanoates (PHAs) polymers and store them in intracellular organelles. Among the chemolytotrophic bacteria are Cupriavidus necator (Ralstonia eutropha), Cupriavidus metallidurans, and Alcaligenes latus [12, 56, 57, 58]; producing PHB from simple carbon sources. Among Gram-negative bacteria, Pseudomonas spp. [16, 156], has attracted the interest for their metabolism of oil wastes, such as P. oleovorans [15], P. putida [26, 59, 60, 151], P. aeruginosa [17], P. pseudoflava [61], Thermus thermophilus, Azotobacter vinelandii [62] a diazothroph bacterium, Enterobacter spp., Burkholderia spp. [61, 162] and halophilic, alkaliphilic, denitrifying species, such as Halomonas campisalis [43], that, being halophile, can be fed with fish industry wastes, with reduced needs to sterilize the feed and the biofermentors.
Among Gram-positive bacteria are Bacillus spp. [163,164,165], B. subtilis [7], Bacillus thuringiensis [7, 63, 163], and B. cereus [64, 65]. PHAs are produced by bacteria under stress conditions (pressure, N or P limitation) increasing the synthesis of PHAs [60, 66]; the bacteria perceive the signals, such as Guanosyl diphosphoguanosine (GppG) or dicyclic GTP alarmones and produce energy-storing polymers. Bacterial cells respond to environmental stress by PHA production has been found to be growth associated as well as nongrowth associated depending on the bacterial species and culture conditions [141, 146, 147]. In the production of PHA in biofermentors, one-stage culture [53, 67], two-stage batch culture [62, 67], fed-batch [16, 68]; high-cell density cultures [10, 18]; and mixed cultures [6, 134], as well as submerged and solid state fermentation processes [69] have shown potential to be exploited to produce PHAs [70]. Carbohydrates and feed stocks can be added in continuous or at determined time points (fed-batch), thus providing the required substrates for PHA polymers [8, 71, 72, 73].
Various factors (type of feed, aeration) influence the biomass growth, synthesis of PHA and its molecular weight [129, 149, 151, 152, 153]. Some author described higher PHA production in E. coli by increasing the oxygen dissolved into the medium [14], using high rate sparging and aeration [74]. Other authors choose to grow metabolically engineered E. coli in a microaerobic environment, exploiting metabolic pathways associated to anaerobic metabolism [75]. Several bacterial species have been genetically modified through gene engineering, in addition to the E. coli system; Aeromonas hydrophila and Halomonas spp. [76] can be genetically modified; mutants of Pseudomonas putida [77], P. aeruginosa [17], and Bacillus spp. have been obtained using biotechnology approaches [163]. In particular, interest has been linked to ability of bacteria to use as feeds vegetal oils and glycerol from biodiesel industry, or lignocellulose feedstock such as xylose [132], and halophytic species that can be repeatedly grown in high salt medium from one cycle to the next one with saving on the cost for sterilization of feed and biofermentor. Though genetic engineering helped a lot in broadening the substrate range to be metabolized into PHA as well as the polymer composition, it has been of limited success since mostly wild-type strains could produce higher PHA. Moreover, changing the culture conditions may enhance the overall PHA yield.
PHA Synthases and PhaCAB Operons
PHA synthases (PhaC) are grouped into four classes based on the kinetics and mechanisms of reaction. The PhaCs in Alcaligenes eutrophus synthesizes scl-3PHAs with monomer units oxidized at positions other than the third carbon; the Pseudomonas oleovorans PhaCs synthesize mcl-3PHAs with monomer units oxidized at the third position [28], with few exceptions. The grouping of PhaC enzymes into four classes is dependent on substrate specificity, according to the preference in forming scl- or mcl-polymers: class I, class II, class III, and class IV PHA synthases have been characterized, determining catalysis properties, substrate recognition, and affinity [78]. Class I PhaC enzymes accept 3-hydroxyalkanes preferably forming short-chain-length PHAs utilizing CoA thioesters with a limited number of carbons [79]. Class I Cupriavidus necator PHA synthase structure of catalytic domain has been described. Class I phaC enzymes were N-terminally truncated, with different range of truncations, showing how the length of sequences affect the polymer length and product specificity [80, 81]. The enzymes belonging to class III, made of two 40 kDa subunits, PhaC and PhaE, have been recently reviewed [82]. The phaC catalytic site possesses a typical PhaC box sequence ([GS]-X-C-X-[GA]-G). Class III phaC are structured as tetramers, such as phaEC from Allochromatium vinosum, of phaE and phaC monomers of 40 kDa. Trapping of intermediates with substrate analogues showed that class III PHA synthases slow the rate of catalysis depending on cycling of reacylation and hydrolysis [39, 140]. In addition, class IV PHA synthases, in Bacillus spp., B. megaterium and B. cereus [82,83,84], are formed by the subunits PhaC and phaR (similar to phaE) [78].
Furthermore, chemically modified compounds have been developed as inhibitors of phaC, to study the synthesis mechanism and reaction kinetics [3]. The crystal structure of the catalytic domain of PhaC from Chromobacterium sp. USM2, Cs-CAT [83] was recently reported. Chromobacterium USM2 strain phaC(Cs) was found highly active, with fast polymerization rate, and preferring hydroxyvalerate, 3-hydroxyhexanoate (3HHx), in addition to 3HB [85]. The studies on PhaCs from Chromobacterium sp. USM2 were aimed to increase the activity and broaden substrate specificity of PhaC(Cs) [83]. PhaC(Cs) showed utilization of 3HB, 3HV, and 3HH, with high 3-hydroxybutyryl-coenzyme A activity.
Enzymatic activity has been determined on PhaC for PHA synthesis, studying the specificities for medium-chain-length monomers as 3-hydroxyhexanoate, on the enzymes from Chromobacterium sp., Allochromatium vinosum, and Caulobacter crescentus (PhaCCs, PhaCCc, A479S-PhaCCs, PhaECAv) [86], displaying varying preference for the alkyl side-chain length. Several point mutations, in particular at the position 479, are reported to increase PhaCCc substrate preference for 3HHx [86]. The Chromobacterium sp. PhaC synthase having a catalytic site Cs-CAT containing Cys291, Asp447, and His477 was studied, determining that the substrate-binding site is hidden by a partially disordered protein domain [85]. The structure has peculiar properties, differing from the catalytic domain from Cupriavidus necator (PhaC Cn-CAT). PhaC Cn-CAT adopts a partially open form maintaining a narrow substrate access to the active site, that needs PhaM for activation. Recently, studies have been focused on PHB synthases with mutations enabling the enzymes to accelerate the reaction kinetics [87, 88] and the catalytic site to accept bulk substrates as precursors for the production of mcl-PHAs and grafted copolymers. R. euthopha, Aeromonas caviae, and A. punctata phaCs were modified by mutagenesis, for example, in S477X and Q481X, for efficient production of 3HB copolymers [50, 89,90,91].
Class IV phaCs, such as PHA synthase PhaC1 and PhaC2 from Pseudomonas stutzeri [92] has been exploited in polymerization of mcl-PHAs in engineered bacteria [82]. PhaC2P with four point mutations, at E130D, S325T, S477G, and Q481K [93] was used to accommodate substrates with various shapes and structures, to produce mcl-PHAs and block copolymers, and tolerate modifications of side chains, i.e., unsaturated bonds or azide groups [51]. These studies have the potential to enlarge the range of various copolymers and their larger physicochemical properties [27, 88, 94, 95]. Phasins, PhaPs are PHB granule-associated proteins, attached to the surface of PHA granules, with structural and regulatory functions, as reviewed in Ralstonia eutropha H16 [96]. Structure of PhaP from Aeromonas hydrophila has been described, while PhaM, the physiological activator of PHB synthase (PhaC1), has been analyzed in Ralstonia eutropha [97]. PhaM is a natural primer of phaC1 activity, decreasing the time of polymerization and increasing PhaC1 specific activity. Several authors reported that, rearranging the order of integration of PhaCAB operon genes, it is possible to synthesize P3HB-co-4HB [47], P3HP [13], P4HB [98], ultrahigh molecular weight P3HB [30, 163], PHA-containing hydroxyl groups [99], and copolyesters of 3-HB and mcl-3-PHA using an optimized PHA synthase gene [94].
Engineering Bacteria for Optimized PHA Synthesis
Through up-to-date gene engineering systems, researchers showed that PHB content and molecular weight are directly related to PhaC activity. PHA has been produced in E. coli, using genetic engineering [8, 13, 14, 30, 66, 100, 101] and through bacterial cell factories. The E. coli system overcomes fermentation problems [47, 73, 132], can grow rapidly, accumulate PHA up to 60% of dry weight [102, 103], and can be fed with various intermediate compounds [14, 103]. Chromosome integration of PHA synthase genes (phbCAB operon) and expression of metabolism regulating genes in recombinant E. coli was shown to increase the PHA yield [144]; for instance, NAD kinase gene yfiB was shown to improve PHB production for the efficiency to supply NADPH. E. coli engineered to synthesize various types of PHA have been obtained through sleeping beauty mutase, to modulate the synthesis of P(3HB-co-3HV) polymers [135, 161] through expression of β-ketothiolases, to condense acetyl-CoA or acetyl-CoA and propionyl-CoA to form acetoacetyl-CoA and 3-ketovaleryl-CoA, followed by expression of acetoacetyl-CoA reductase (PhaB) for thioester reduction and PHA synthase (PhaC) for copolymer synthesis [44].
E. coli was metabolically engineered to synthesize poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (3HB-co-3 HV) [45] through propionyl-CoA produced from 2-ketobutyrate, which is generated via 2-hydroxy-2-methylbutanedioate through an enzyme involved in isoleucine synthesis from acetyl-CoA and pyruvate. Two approaches have been pursued for changing metabolic pathways to the synthesis of propionyl-CoA. In the first case, 2-ketobutyrate oxidase produces propionic acid, used to form propionyl-CoA. The other pathway relays on the conversion of 2-ketobutyrate into propionyl-CoA through pyruvate formate lyase. E. coli metabolic fluxes were modified to block succinate production and increase the carbon flux towards P4HB biosynthesis. The recombinant and metabolically improved E. coli can produce poly(3HB-co-4HB), and the presence of α-ketoglutarate or citrate can increase the content of 4HB up to 20% [47]. Also, threonine and serine metabolism can increase the availability of acetyl-CoA and utilization of the CO2 derived from pyruvate dehydrogenase reaction [73]. The conversion of threonine into acetyl-CoA and glycine relies on threonine availability. During phosphoenolpyruvate carboxylase (PPC)-mediated carboxylation of phosphoenolpyruvate (PEP) into oxaloacetate (OAA), two Acetyl-CoA are produced through CO2 fixation, with consumption of NADH and ATP.
In the Lin et al. study [73], enzymes for PHB synthesis were coexpressed with enzymes for threonine synthesis and degradation (Fig. 1).
Glycolysis flux and TCS cycle leading to Acetyl-CoA and threonine bypass scheme. Acronyms of metabolites: AKB 2-amino-3-ketobutyrate, AKG 2-oxoglutarate, Asp aspartate, ASA aspartate semialdehyde, A4P aspartyl-4-phosphate, ATP adenine trinucleotide phosphate, DHAP dihydroxyacetone phosphate, F6P fructose 6-phosphate, FBP fructose 1,6-bisphosphate, GAP d-Glyceraldehyde-3-phosphate, Glu glutamate, Gly glycine, Hser homoserine, MTHF methyltetrahydrofolate, NADH nicotinamide adenine dinucleotide, OAA oxaloacetate, PEP phosphoenolpyruvate, PHS phosphorylated homoserine, Pyr pyruvate, Ser serine, Thr threonine, TCA tricarboxylic acids cycle
Bacterial shape is a factor limiting the space and the potential accumulation of high amounts of PHA granules. Several scientists studied the suppression of filamentation to increase the synthesis of PHA. Subsequently, E. coli mutants in genes regulating cell division, FtsL, FtsN, FtsQ, FtsW, FtsZ, or the overexpression of SulA, an FtsZ inhibiting protein, have made possible an increase in yield and dry cell mass with increased recovery of PHA [104]. Multiple, dividing E. coli cells [105] with deleted genes minC and minD, and cell shape controlling, actin-like gene mreB, show formation of several fission rings and the elongated shaped cells divide into multiple daughter cells. The creation of new PHA synthesis pathways has been made possible by recent advancements and genetic modification in various species. Remarkably, the weakening of β-oxidation cycle in Pseudomonas putida and Pseudomonas entomophila has allowed production of different PHA polymers, with varying monomer ratios, forming either random and/or block copolymers when fatty acids (hexanoic, octanoic, and dodecanoic acids) are made available as PHA precursors [40]. When fatty acids containing functional groups are fed and taken up by the bacteria, PHA polymerization occurs with the functional groups incorporated into the PHA. The functional PHA polymer may be then processed with other reactive molecules to allow formation of grafted polymers.
Other factors influencing the heterogeneity of polymers are consequence of controlled synthesis of homopolymers, random copolymers, block copolymers, and grafted polymers.
Engineering Pathways for scl-PHA Synthesis
Metabolic engineering has been applied to microbial synthesis of PHAs. Synthetic biology approaches to regulate metabolic fluxes can be exploited to control PHA composition [130]. The PhaC recombinant E. coli inactivated in the succinate semialdehyde dehydrogenase and expressing a more efficient succinate semialdehyde dehydrogenase, enhancing the carbon flux toward P4HB production.
Poly 3-hydroxypropionate (P3HP) was produced in E. coli containing four heterologous genes, propionyl-CoA ligase, dehydratase, aldehyde dehydrogenase, and 4-hydroxybutyrate-coenzyme A transferase, with bacteria producing 3HP4HB when fed by 1,3-propanediol and 1,4-butanediol added in sequence. The pathways were combined together, implementing the E. coli with glycerol dehydratase, propionaldehyde dehydrogenase, beta-ketothiolase A, and acetoacetyl-CoA reductase, the bacteria synthesized 3HP3HB with various content of 3HP, or block copolymers depending on the feeding method used.
Engineering the β-Oxidation Pathway mcl-PHA Synthesis
Many Pseudomonas spp. utilize fatty acids through β-oxidation to produce energy and feed to sustain their growth. The β-oxidation pathway produces alkanes with shortened carbon chain, that when incorporated into PHA lead to the production of random PHA copolymers. To make available fatty acid substrates of various length, several genes involved in β-oxidation were deleted to make ß-oxidation weakened, engineering a mutated P. putida KTQQ20 strain.
P. putida KTQQ20 strain was able to produce homopolymer PHD as well as the copolymer P(3HD-co-3HDD) by feeding sequentially decanoate and dodecanoate [39]. P. putida KTQQ20 grown on hexanoate plus decanoate, synthesized random copolymers of (HH-HD) with composition depending on hexanoate/decanoate ratio, or diblock copolymer -P(3HD-co-3HDD), when the two substrates were added in succession. The β-oxidation pathway was knocked down also in P. entomophila, to produce mcl-PHA. P. entomophila LAC26 strain produced PHA in high amounts. Homopolymers of 3HDD were synthesized, as well as other types of polymers, depending on the source of fatty acid added. The P. putida KTOY08DGC was able to produce the block copolymer P3HB-b-P4HB.
Recombinant Aeromonas hydrophila 4AK4 fatty acid β-oxidation impaired mutant expressing a bacterial hemoglobin and acyl-CoA synthase, produced PHBHV and PHBHHx copolyesters, using undecanoate as feedant, and supplying 3-hydroxyvalerate through threonine catabolism. Recently, the production of PHA containing 2-hydroxybutyrate and 3-hydroxypropionate was shown feasible. P3HP has been produced starting from 1,3-propandiol or from glycerol [39].
Engineering Pathways for Scl- and mcl-PHA Copolymers
P. putida KTOYO6 was mutated in the fatty acid β-oxidation enzymes 3-ketoacyl-CoA thiolase and 3-hydroxyacyl-CoA dehydrogenase, to increase PHA synthesis. When the strain was transformed with a less specific PHA synthase, the P. putida KTOYO6DC strain synthesized both scl- and mcl-PHAs, such as copolymers of PHB-b-PHVHHp, obtained through feed regulation, at first by addition of sodium butyrate, followed by sodium heptanoate [55]. When mixtures of butyrate and hexanoate were added as feed, bacteria produced P(3HB-co-3HHx) polymers in which the percentage of monomer contents depended on C4/C6 feed [33, 48, 49, 106].
Engineering Pathways for Functional PHA
When P. putida KTQQ20 or P. entomophila LAC23 were cultivated in presence of fatty acids containing functional groups (double or triple bonds, epoxy groups, carbonyl, cyano, phenyl, and halogen groups), the resulting PHA contained the functional groups on side chains, exploited for chemical grafting on the reactive residues. Homopolymers, random copolymers, or a blend of both have been produced containing aromatic groups. PHAs containing alkoxy, acetoxy, or hydroxyl groups are important for their hydrophilicity, high solubility, and versatility of use.
P. entomophila LAC23 accumulated PHA containing phenyl groups on the side chain, while P. putida KT2442 accumulated diblock copolymer PHB-co-PHHx [55]. These strains, grown in presence of 5-phenylvaleric acid, accumulated poly(3-hydroxy-5-phenylvalerate) homopolymer. P. entomophila LAC23 cultured using phenylvaleric acid/dodecanoic acid as feed accumulated the copolymer containing 3-hydroxy-5-phenylvalerate (3HPhV) and 3-hydroxydodecanoate (3HDD). The content of 3HPhV in P(3HPhV-co-3HDD) was regulated by the ratio of dodecanoate/5-phenylvalerate [41, 106].
Fermentation in Biofermentors: Industrial Optimization of Costs/Yield
The principal bottleneck in production costs are the costs of feed substrates, operational cost of fermentors, extraction and purification costs. Therefore, producers have optimized bioreactor use and protocols for scaling up of the processing capacity of fermentors, the use of cheaper feeds, and high bacterial cell density to increase the yield of PHAs [157, 158, 159]. PHA determination in bacteria have relied on spectrophotometric techniques or on chromatographic methods. RAMAN spectra were acquired from marine bacteria mixed cultures [107]. Several dyes have been used, with most of them not specific for PHA, but binding also to membrane lipids. The most common methods are based on Nile Red (λ excitation: 543 nm, λ emission: 560–710) [108] and Nile Blue stain (Spiekermann et al. 1999; Oshiki et al. 2011; Weissgram et al. 2015). New quantitative methods have been based on fluorometry combined with a flow cell to evaluate PHB in bacteria stained with Nile Blue [56] [148, 166, 167], while other methods were based on fluorescence [108] and laser scanner quantification (λexc 460 nm/λem 550 nm) of bacteria stained with Nile Blue, determined end point PHA accumulation [103].
Biosensors and enzymatic methods for the evaluation of feed consumption have been described [109] and their applicability and usefulness validated [103]. Recently, a metabolic modeling system has been applied to control nutritional and aeration conditions for biomass and PHA production optimization. Also, sensors can determine bacterial concentration, and whole-cell bacterial detection has been shown feasible [102, 110]. A second critical point for the scale up of the process is the lysis of cells and the extraction of granules of PHAs. Several approaches have been proposed to make the process economically advantageous, from the single cell protein method, to make fermentation and recovery of granules in a single passage [111], to various lysis systems [112, 113], to alkaline treatment for PHA recovery [114]. All these methods show economical advantage and are environmentally friendly, since they do not require the use of solvents such as chloroform.
Industrial Applications of PHA Polymers
Polyhydroxyalkanoates (PHAs) are a great opportunity for the polymer industry due to their property of high biodegradability and processing versatility, and the potential to replace fossil fuel-based plastics. Presently marketed PHAs originate from microbial cultures fed with renewable feedstocks (i.e., glucose) following sterilization [115]. The main properties of PHAs are: water insolubility and resistance to ultraviolet rays; low tendency to hydrolysis; degraded by acids and bases; solubility in chloroform and chlorinated solvents; biocompatibility with biological fluids and tissues; degradable anaerobically in sediments; nontoxic; and non“sticky” when melted in respect to other polymers. Polyhydroxybutyrate is brittle, fragile, and stiff, with low elongation ability, and a break point below 15%. Main problems from PHB ageing at room temperature is recrystallization and consequently mechanical properties changing with time. mcl-PHAs are elastomers, have low melting point, and a relatively low degree of crystallinity. PHB and other PHAs are degraded by exposure to temperatures above PHA melting point, that is 170 °C.
PHA tendency to thermal degradation is a serious problem in the PHB industry and its applications. An exposure to 180 °C induces PHB degradation with production of crotonic acid and shorter chain polymers. PHA is processed by extrusion, producing various rigid and flexible plastics for goods molded to the various shapes needed, coatings, fabrics, packaging films, films for agriculture, adhesives, additives, and medical applications. Based on the properties of the different types of PHAs, there are aspects related to the processing, commercial availability, challenges, and opportunities that need to be approached.
Compounding PHB
Plasticizers can be added to modify the thermal and mechanical properties of PHAs, to control and retard the crystallization process, and optimize flexibility and elongation ability of polymers.
Blending PHA polymers with plasticizers and nucleating agents modifies the physical properties of polymers, decreasing the processing temperature and lowering the crystallinity, for the formation of small and numerous crystallites.
During the processing, PHB may not tolerate high temperatures; therefore, a lubricant is added to prevent the degradation of the chains, and the process may be carried on at 170–180 °C. This leads to a decrease in the molecular weight and to a reduced melt viscosity. The temperature of crystallization (Tc) decreases and lowers, allowing crystallization to endure for longer times.
The mechanical properties of PHB are improved by its blending with P(3/4HB), increasing the elongation at break.
The plasticizers mostly used are cheap materials easily available on the market, such as glycerol, tributyrin, triacetin, acetyltriethylcitrate, acetyltributylcitrate, oxypropylated glycerol (or laprol), soybean oil, epoxidized soybean oil, fatty alcohols with glycerol fatty esters, triethyl citrate, triacetine, acetyl tributyl citrate, salicylic ester, acetylsalicylic acid ester, dioctyl sebacate (ATBC), polyethylene glycol (PEG), oligohydroxybutyrate, and triethylene glycol-bis-2-ethylhexanoate.
The use of glycerol, tributyrin, triacetin, acetyl triethyl citrate, acetyl tributyl citrate as plasticizers has been reported, and saccharin has been used as nucleation agent. Glycerol monostearate, various triglycerids, and 12-hydroxystearate have been used as lubricants.
Acetyl tributyl citrate has been used as PHB plasticizer. It influences PHB thermal properties during melting, while PHB needs to be rapidly cooled to reach the degree of crystallization required.
Blends obtained adding polyethylene glycol (2–5%) are compatible with PHB, with good miscibility, as shown by DSC analysis. PEG 400 is a good PHB plasticizer, able to reduce melting temperature of PHB. The blends of PHB with PEG 400 show that elongation at break is increased, but the tensile strength is reduced. The PEG plasticizing effect is ascribed to weakening of intermolecular force between the PHB chains, which leads to a change in free volume and to a decreased temperature of melting.
In the production of PHB composites with wood fibers, PEG 400 is added by extrusion and injection molding, with a lubricant effect on melted PHB/wood formulations, making the processing easier. However, PEG is not completely blended with PHB and leaches to the outside after some time, with a loss of plasticizing power.
The adhesion of PEG to the natural fibers is the main reason of its plasticizing effect in the manufacture of composites made of PHB and natural fibers. Organo-modified montmorillonite (OMMT) clay is a nanofiller used in PHB/V blends. The nanobiocomposites possess an intercalated/exfoliated structure and show good mechanical properties. The OMMT filler acts as a nucleating agent, enhancing the crystallization, and improves thermal stability of the polymer.
Blending of PHB with Other Polymers
Blending PHB with other polymers improve processability and reduce the brittleness of PHA bioplastics. Several blends containing PHA have been developed and various plasticizers have been used.
PHB-co-HV possesses higher temperature of crystallization (Tc). An increase in the HV fraction to 20 mol% in PHB-co-HV decreases the melting temperature (Tm) of the copolymer to 168.5 °C in respect to the initial value of 175.4 °C. Further increase in the HV fraction shows an isodimorphic relationship. The nucleating agent ULTRATALC 609 was used to obtain higher temperature of crystallization (Tc), while reducing crystallization time required for injection molding [115]. Temperature of decomposition (Tdec) of homopolymer and copolymers mixed with ULTRATALC_609 resulted increased.
Poly(3-hydrobutyrate) mixed with dioctyl sebacate (ATBC) as plasticizer, shows a lowering of glass transition temperature (Tg), with improvement of thermal characteristics, without changes in mechanical properties. PHBHHx and/or P(3/4HB) with ATBC and antioxidant 1010 as stabilizer show improvement of PHB thermal stability and stabilization of the melt flow index (MFI), widening the application range of PHB processing methods.
Vinyl acetate polymer as well as polyvinyl alcohol have been used to strengthen PHAs blends. Different contents of the fillers have been shown to improve PHB tensile properties (modulus and strength).
Mixed PHA Copolymers
One approach to widen the properties of PHA-based bioplastics is the synthesis of copolymers, such as PHBHV or PHBHHx, with different molar ratios of hydroxycarboxylic acids, improving the mechanical properties and lowering the melting point, slowing down the degradation during processing. In addition, blends with other biodegradable polymers and composites are convenient as materials for industrial applications.
A largely used approach in PHA polymers with improved properties is blending the polymer with a second thermoplastic polymer. The degree of crystallinity is modified, and production costs can be lowered.
P(3HB) is miscible with poly(ethylene oxide), poly(epichlorohydrin), poly(vinyl acetate), highly substituted cellulose esters and trisubstituted cellulose butyrate and caprolactone, among others.
The blends with P(3HB) with immiscible polymers are important in the control of biodegradation profile. Binary blends, such as P(3HB)/poly(propiolactone), P(3HB)/poly(ethylene adipate), and P(3HB)/poly(3-hydroxybutyric acid-co-hydroxyvaleric acid) degrade more rapidly, and the acceleration depends on the phase separation of the two structures.
Wood flour and lignin have been used as fillers in composite materials. Fibers derived from renewable resources on conventional reinforcements such as glass and aramid fibers are convenient, cheap, recyclable, and competitive as strength per weight of material.
Processing of PHA Copolymers: Challenges and Opportunities
Depending on the molecular weight of the polymer and on the content of comonomer, different processing techniques have been developed.
Powdered PHB is blended with additives by mixing in a kneader at 170–180 °C in an extruder at temperatures in the range of 160 °C and 170 °C. The thread is cooled in water and a pelletizer allows the cutting into pieces.
Then, the granulates can be compressed and molded in a hydraulic press, heated at temperature of 170–180 °C between sheets of Teflon. After that, samples are cooled to room temperature.
Melting Behaviour of PHB
PHB is a linear polymer with elevated level of crystallinity (60–70%). The crystallization speed is slow below 60 °C and above 130 °C, behaving as an amorphous and sticky material. The rapid fluid/solid transition is exploited to obtain fast speed in the processing. The material is melted in proximity of the filling zone, lowering the temperature during the die-casting. The viscosity of PHB plastics is similar to polypropylene.
Processing Techniques and Conditions
PHAs can be extruded by injection molding and various types of extrusion protocols, into films and hollow bodies. The thermal, rheological, mechanical, and barrier properties of PHBV with different valerate contents and molecular weights have been characterized. The use of copolymers is very frequent, since they improve the plastics flexibility and lower the glass transition temperature (Tg) and the melting temperature (Tm).
The presence of HV in the copolymer enlarges and improves the processing parameters, thanks to higher melt stability at lower processing temperatures. The processing of copolymer below 160 °C is beneficial with low screw speed. PHBV shows good mechanical property, high elastic modulus and flexibility strength, low tensile strength, and low elongation at break. PHBV polymers are unstable at temperatures over 160 °C, possibly because of polymer breakdown due to random chain scission process, which leads to decreased molecular weight and lower viscosity. The PHBV plastics are brittle, elastic and with low tensile strength. P3HB4HB polymers and conventional thermoplastic used for packaging instead show high tensile strength and higher elongation at break. The PHBHV subjected to injection molding at temperatures from 135 to 160 °C, show low degradation and only for a small extent [115].
Although the incorporation of nanoclay improves the properties of PHB and its copolymers, thermo-mechanical degradation of the PHB and PHBV, in the presence of ammonium surfactants, used as clay organo-modifiers, has been reported. The surfactants affect polymer degradation.
The use of surfactants in PHBV processing is optimal. The surfactants improve the characteristics of PHBV-based nanocomposites with organomodified clays, exhibiting good thermo-mechanical properties, high shear rate, good level of exfoliation of layered silicates, and stabilization of the bioplastic products. The PHB blend with poly(vinyl acetate) (PVAc) in the amorphous state was characterized, showing that the two polymers are miscible. Overall, polymer blends containing PHA show good properties and high biodegradability [115].
Industrial Applications of PHAs
The bioplastics have a wide range of applications for the industry [27, 138, 139, 145, 154, 160] PHA is biodegradable, highly deformable, has high heat resistance and good resistance to hydrolysis, balancing both the degree of toughness and the degree of stiffness. PHA shows for many aspects similarity with linear low-density polyethylene (LLDPE). This makes PHA versatile enough to be made into a wide range of molded items, fiber and film. Manufacturers of hard-line consumer goods showed the potential many PHA uses due to its flexibility and its resemblance to LLDPE. In addition to furniture, tools, and sports equipment, PHA can be used in the molded parts of household appliances, such as covers, filters, housings, fasteners, and clips. The bioplastics have been used in cables, connectors, and housings of consumer electronic devices. The developing trend of use of bioplastics has been seen in electronics manufacturers: Samsung, NEC, Sony, Fujitsu, and Nokia make use of bioplastics in their goods. Consumers have shown increasing interest and support for green product content even at a higher, premium price, such as in packaging materials for cosmetics and personal care products. The products are marketed stressing their environmentally friendly qualities. PHA’s flexibility makes it a natural bioplastic material for caps, bottles, blister packs, and other containers for the industry of consumer goods. It can also be used for packaging applications for articles in the food industry, such as bottles, laminated foils, fishnets, flowerpots, sanitary goods, fast foods, disposable cups, agricultural foils, and fibers in textiles.
The properties of various copolymers and block polymers, and their processability, enlarge the potential in their applications. Similarly to PVC and PET, PHA exhibits good barrier properties and can be used in the packaging industry as a bioplastic, contributing to solve environmental pollution problems. Due to these properties, PHB is a good candidate to substitute PP and PE but also PET. Poly(vinyl acetate)-based resins enhance the physical properties of the material containing PHB, which significantly simplifies the processing. This, combined with the high heat resistance of PHB, allows the possible use of this material in applications such as hot filling.
Compounders found PHA having great potential as a modifier for PVC as it can improve toughness and plasticization without affecting transparency or UV stability. As PHA has high miscibility with PVC, it is easy to handle and process in the same conditions as PVC.
Overall, PHA is a promising polymer for a wide range of applications. For example, it has better barrier properties and mechanical strength than other more widespread bioplastics such as polylactic acid. In spite of its intrinsic brittleness, a lot of progress has been made through the formulation of PHAs with tailored additives and blends, leading to greatly improved mechanical profiles, as well as suitable processability via extrusion or injection molding. This makes PHA versatile enough to be made into a wide range of molded items, fibers for textiles and biofilms. Blow-molded bottles and injection-molded hair caps are two main industrial products that benefited from the easiness to process the PHA polymer into a vast range of shapes. These advances will improve its capacity to penetrate markets such as packaging foils for the storage of food products.
PHA is a very versatile polymer, that is well suited in a wide range of applications. For example, it has good barrier properties, good oxygen transmission rate (OTR) and water vapor transmission rate (WVTR), and good mechanical strength, in respect to other bioplastics such as PLA. In spite of its brittleness, a lot of progress has been made through the formulation of PHB with tailored additives and blends leading to greatly improved mechanical profiles, as well as suitable processability via extrusion or injection molding. These advances will improve its capacity to penetrate markets and find new application at industrial level. Finally, further improvements (new block polymers, varying monomer percentage in copolymers, grafted polymers) could allow even more flexible grades of PHAs or transparent ones through the control of its crystallization.
PHA has a great potential for applications in agriculture, being a biodegradable plastic, having a controlled-release mechanism, and as fertilizer. Pellets made of PHA can be placed on field to the soil, gradually degraded and contents released over a prolonged period, reducing fertilizer and labor costs. The use of natural fillers with high availability and low cost will allow the production of biocomposite more suited for their application in packaging, such as consumer goods and in food packaging.
Automotive industry, under the recycle directive of plastic components, are driving the next development in components for cars and buildings. The EU End-of-Life Vehicle Directive requires reuse and recycling content rates of 85% in passenger vehicles and light commercial vehicles from 1 January 2015 onwards. As a consequence, automotive manufacturers are developing applications of bioplastics and other biobased materials in their products.
Presently, Hyundai and Toyota carmakers incorporate bioplastics in their vehicles. Because of its physical properties, PHA could serve as a more environmentally friendly material for automotive tubing, seat materials, interior panels, and trim parts.
However, in case the of PHA will be more competitive and affordable at cost level, and PHA will become comparable to the cheaper, nondegradable plastics, and similar in costs to polymers originated from petrochemicals (whose availability is estimated to decrease with time), it is envisaged that also the packaging industry will see an increase of the requests for PHA-based films and molded forms, and in fiber reinforced material for textiles, bioplastic composites for aerospace, automotive industries.
Sustainability of materials is a necessary aspect that need to be kept into account, needing to meet current and future regulatory requirements in many markets. The consumers will support a premium cost for products and services with positive social and environmental impact. Biobased products are set to capture an increasingly large share of the consume market.
PHA in Medical Applications
The most promising field of applications due to the high competitivity of products and high prize of the instruments is in the high-tech electronic devices and in the biomedical sector. PHA polymers, depending on the properties of the copolymer used, have been applied to development of medical devices, surgical sutures, stents, cardiac valves, in tissue repair and regenerative medicine applications [116,117,118], bone and tendon repair [119,120,121], titanium bone implants with enhanced antibacterial power [122], tissue engineering [2, 123], artificial organ reconstruction such as artificial esophagus replacement, in drug delivery for nutritional/therapeutic uses, such as nanoparticles-releasing bioactive drugs. In pharmacology, PHB can be used as microcapsules in therapy or as materials for cell and tablet packaging, for encapsulation of Langerhans cells to restore insulin production and release [124, 125], and for coupling the bioplastics with arginine-glycine-aspartate (RGD) cell adhesion motif [126] or in microbeads for targeted drug release [127].
Antibacterial PHAs coating for titanium implants have been developed through the incorporation of antibiotics into the PHA polymer [122].
In biomedical applications, PHB is compatible with human cells and tissues. The hydroxybutyrate is present in the human body and is metabolized. Since PHB is reabsorbed in the body, it could be incorporated in implants in surgery, in sutures, in seam threads, as wound healing and blood vessel reconstruction. At Massachusetts Institute of Technology, several applications of bioplastics with slow release of chemotherapeutics implanted in the brain have been produced and patented. This, together with the effect of blood-brain barrier, encloses spatially and temporally the chemotherapeutics for a local and prolonged bioactivity.
Biomedical applications have been described in the last 5 years. Scaffold from poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and poly-(3-hydroxybutyrate) mixed to type-I collagen have been tested for tissue engineering [45, 116].
Long bone fractures, bone fragility, and osteoporosis problems have been medicated using bioresorbable materials based on PHAs [128]. Osteoblast cells were shown to grow at high density on nanocomposite scaffold of hydroxyapatite/titania coated with poly hydroxybutyrate [121], making bone implants to be realized at high success rate.
Furthermore, PHA scaffolds for bone tissue engineering and orthotopic bone implants based on a hybrid construction from poly(3-Hydroxybutyrate) have been tested and applied in preclinical studies [3]. There are also studies to apply poly-3-hydroxyoctanoate as peripheral nerve graft. In general, the technological readiness level of these prototypes is at research and development level, so there is a need to push the advancements in this field to a preindustrial and demonstration level.
Conclusions
The PHAs have shown their potential as low-cost biodegradable plastics. Presently, there are still some bottlenecks in their wide use and applications, since the high costs of production, in respect to other nonbiodegradable plastics, and to the problems in controlling the ratio of monomers that influences the PHA properties. In order to lower costs of PHA production, agricultural byproducts, industrial wastes, and biorefinery byproducts have been successfully used as feedstock. One of the main bottlenecks in the application of PHB for the production of single-use items is based on its relatively high cost (7–10 Euro/kg) when compared to other polymers. In this respect, the use of waste feedstock for the culture of the microorganisms accumulating PHAs is a way to lead to their greater economic viability and sustainability. Presently, different research studies are ongoing regarding the improvement of the yield of PHA by genetic modification of the bacteria or the use of waste for their growth. The β-oxidation weakened Pseudomonas strains newly developed have increased the range of polymers and copolymers that can be produced, allowing to obtain PHA with controlled mechanical and thermal properties. The possibility to introduce various functional groups into the PHA side chains and side chain grafting has made possible to enlarge the multiplicity of polymers available for industrial applications, with high value-added functionalities. Further investigations and efforts will allow reducing the production costs of PHA polymers, increasing the industrial sustainability and commercialization of PHAs.
Abbreviations
- PHBHHx:
-
poly-3-hydroxybutyrate-co-hydroxyhexanoate
- P(3/4HB):
-
poly(3-hydroxybutyrate-co-4-hydroxybutyrate)
- PHB-co-HV:
-
poly-3-hydroxybutyrate-co-3-hydroxyvalerate
- PHBHV:
-
poly(3-hydroxybutyrate-cohydroxyvalerate)
References
Chanprateep S (2010) Current trends in biodegradable polyhydroxyalkanoates. J Biosci Bioeng 110:621–632
Lomas AJ, Webb WR, Han JF, Chen G-Q, Sun X, Zhang Z, El Haj AJ, Forsyth NR (2013) Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)/collagen hybrid scaffolds for tissue engineering applications. Tissue Eng Part C Methods 19(8):577–585
Zhang W, Chen C, Cao R, Maurmann L, Li P (2015) Inhibitors of polyhydroxyalkanoate (PHA) synthases: synthesis, molecular docking, and implications. Chembiochem 16(1):156–166
Heinrich D, Raberg M, Fricke P, Kenny ST, Morales-Gamez L, Babu RP, O’Connor KE, Steinbüchel A (2016) Synthesis gas (syngas)-derived medium-chain-length polyhydroxyalkanoate synthesis in engineered Rhodospirillum rubrum. Appl Environ Microbiol 82(20):6132–6140
Albuquerque MGE, Eiroa M, Torres C, Nunes BR, Reis MAM (2007) Strategies for the development of a side stream process for polyhydroxyalkanoates (PHA) production from sugarcane molasses. J Biotechnol 130:411–421
Bengtsson S, Pisco AR, Johansson P, Lemos PC, Reis MAM (2010) Molecular weight and thermal properties of polyhydroxyalkanoates produced from fermented sugar molasses by open mixed cultures. J Biotechnol 147:172–179
Singh G, Kumari A, Mittal A, Yadav A, Aggarwal NK (2013) Poly ß-hydroxybutyrate production by Bacillus subtilis NG220 using sugar industry waste water. Biomed Res Int 2013:952641
Zhou XY, Yuan XX, Shi ZY, Meng DC, Jiang WJ, Wu LP, Chen JC, Chen G-Q (2012) Hyperproduction of poly(4-hydroxybutyrate) from glucose by recombinant Escherichia coli. Microb Cell Factories 11:54
Getachew A, Woldesenbet F (2016) Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res Notes 9(1):509
Cerrone F, Davis R, Kenny ST, Woods T, O’Donovan A, Gupta VK, Tuohy M, Babu RP, O’Kiely P, O’Connor K (2015) Use of a mannitol rich ensiled grass press juice (EGPJ) as a sole carbon source for polyhydroxyalkanoates (PHAs) production through high cell density cultivation. Bioresour Technol 191:45–1952
Follonier S, Goyder MS, Silvestri AC, Crelier S, Kalman F, Riesen R, Zinn M (2014) Fruit pomace and waste frying oil as sustainable resources for the bioproduction of medium-chain-length polyhydroxyalkanoates. Int J Biol Macromol 71:42–52
Haas C, Steinwandter V, Diaz De Apodaca E, Maestro Madurga B, Smerilli M, Dietrich T, Neureiter M (2015) Production of PHB from chicory roots – comparison of three Cupriavidus necator strains. Chem Biochem Eng Q 29:99–112
Andreeßen B, Lange AB, Robenek H, Steinbuchel A (2010) Conversion of glycerol to poly (3-hydroxypropionate) in recombinant Escherichia coli. Appl Environ Microbiol 76:622–626
de Almeida A, Giordano AM, Nikel PI, Pettinari MJ (2010) Effects of aeration on the synthesis of poly(3-hydroxybutyrate) from glycerol and glucose in recombinant Escherichia coli. Appl Environ Microbiol 76:2036–2040
Allen AD, Anderson WA, Ayorinde FO, Eribo BE (2010) Biosynthesis and characterization of copolymer poly(3HB-co-3HV) from saponified Jatropha curcas oil by Pseudomonas oleovorans. J Ind Microbiol Biotechnol 37(8):849–856
Mozejko J, Wilke A, Przybylek G, Ciesielski S (2012) Mcl-PHAs produced by Pseudomonas sp. Gl01 using fed-batch cultivation with waste rapeseed oil as carbon source. J Microbiol Biotechnol 22(3):371–377
Abid S, Raza ZA, Hussain T (2016) Production kinetics of polyhydroxyalkanoates by using Pseudomonas aeruginosa gamma ray mutant strain EBN-8 cultured on soybean oil. 3 Biotech 6(2):142
Ienczak JL, Schmidell W, De Aragão GMF (2013) High-cell-density culture strategies for polyhydroxyalkanoate production: a review. J Ind Microbiol Biotechnol 40:275–286
Peña C, Castillo T, García A, Millán M, Segura D (2014) Biotechnological strategies to improve production of microbial poly-(3-hydroxybutyrate): a review of recent research work. Microb Biotechnol 7(4):278–293
Jiang G, Hill DJ, Kowalczuk M, Johnston B, Adamus G, Irorere V, Radecka I (2016) Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int J Mol Sci 17(7), pii:E1157
Khanna S, Srivastava AK (2005) Recent advances in microbial polyhydroxyalkanoates. Process Biochem 40(2):607–619
Chen G-Q, Zhang G, Park SJ, Lee SY (2001) Industrial scale production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl Microbiol Biotechnol 57:50–55
Anjum A, Zuber M, Zia KM, Noreen A, Anjum MN, Tabasum S (2016) Microbial production of polyhydroxyalkanoates(PHAs) and its copolymers: a review of recent advancements. Int J Biol Macromol 89:161–174
Chen G-Q, Hajnal I (2015) The ‘PHAome’. Trends Biotechnol 33(10):559–564
Dai Y, Lambert L, Yuan Z, Keller J (2008) Characterisation of polyhydroxyalkanoate copolymers with controllable four-monomer composition. J Biotechnol 134:137–145
Le Meur S, Zinn M, Egli T, Thöny-Meyer L, Ren Q (2012) Production of medium-chain-length polyhydroxyalkanoates by sequential feeding of xylose and octanoic acid in engineered Pseudomonas putida KT2440. BMC Biotechnol 12:53
Wang Y, Chen G-Q (2017) Polyhydroxyalkanoates: sustainability, production, and industrialization. In: Tang C, Ryu CY (eds) Sustainable polymers from biomass. Wiley-VCH Verlag, Amsterdam, The Netherlands
Ojumu TJ, Solomon BO (2004) Production of polyhydroxyalcanoates, a bacterial biodegradable polymer. Afr J Biotechnol 3(1):18–24
Ren Y, Meng D, Wu L, Chen J, Wu Q, Chen G-Q (2017) Microbial synthesis of a novel terpolyester P(LA-co-3HB-co-3HP) from low-cost substrates. Microb Biotechnol 10(2):371–380
Hiroe A, Tsuge K, Nomura CT, Itaya M, Tsuge T (2012) Rearrangement of gene order in the phaCAB operon leads to effective production of ultrahigh-molecular-weight poly[(R)-3-hydroxybutyrate] in genetically engineered Escherichia coli. Appl Environ Microbiol 78:3177–3184
Kabe T, Tsuge T, Kasuya K-I, Takemura A, Hikima T, Takata M, Iwata T (2012) Physical and structural effects of adding ultrahigh-molecular-weight poly[(R)-3-hydroxybutyrate] to wild-type poly[(R)-3-hydroxybutyrate]. Macromolecules 45:1858–1865
Wang S, Chen W, Xiang H, Yang J, Zhou Z, Zhu M (2016a) Modification and potential application of short-chain-length polyhydroxyalkanoate (SCL-PHA). Polymers 8:273
Jian J, Li ZJ, Ye HM, Yuan MQ, Chen G-Q (2010) Metabolic engineering for microbial production of polyhydroxyalkanoates consisting of high 3-hydroxyhexanoate content by recombinant Aeromonas hydrophila. Bioresour Technol 101(15):6096–6102
Zhang HF, Ma L, Wang ZH, Chen G-Q (2009) Biosynthesis and characterization of 3-hydroxyalkanoate terpolyesters with adjustable properties by Aeromonas hydrophila. Biotechnol Bioeng 104(3):582–589
Rai R, Yunos DM, Boccaccini AR, Knowles JC, Barker IA, Howdle SM, Tredwell GD, Keshavarz T, Roy I (2011) Poly-3-hydroxyoctanoate P(3HO), a medium chain length polyhydroxyalkanoate homopolymer from Pseudomonas mendocina. Biomacromolecules 12(6):2126–2136
Gao J, Ramsay JA, Ramsay BA (2016) Fed-batch production of poly-3-hydroxydecanoate from decanoic acid. J Biotechnol 218:102–107
Chung AL, Jin H-L, Huang L-J, Ye H-M, Chen J-C, Wu Q, Chen G-Q (2011) Biosynthesis and characterization of poly(3-hydroxydodecanoate) by β-oxidation inhibited mutant of Pseudomonas entomophila L48. Biomacromolecules 12:3559–3566
Tortajada M, da Silva LF, Prieto MA (2013) Second-generation functionalized medium-chain-length polyhydroxyalkanoates: the gateway to high-value bioplastic applications. Int Microbiol 16(1):1–15
Chen G-Q, Hajnal I, Wu H, Lv L, Ye J (2015) Engineering biosynthesis mechanisms for diversifying polyhydroxyalkanoates. Trends Biotechnol 33:565–574
Meng DC, Shen R, Yao H, Chen JC, Wu Q, Chen G-Q (2014) Engineering the diversity of polyesters. Curr Opin Biotechnol 29:24–33
Poblete-Castro I, Binger D, Oehlert R, Rohde M (2014) Comparison of mcl-poly(3-hydroxyalkanoates) synthesis by different Pseudomonas putida strains from crude glycerol: citrate accumulates at high titer under PHA-producing conditions. BMC Biotechnol 14:962
Wang Q, Yang P, Xian M, Yang Y, Liu C, Xue Y, Zhao G (2013) Biosynthesis of poly(3-hydroxypropionate-co-3-hydroxybutyrate) with fully controllable structures from glycerol. Bioresour Technol 142:741–744
Kulkarni SO, Kanekar PP, Jog JP, Patil PA, Nilegaonkar SS, Sarnaik SS, Kshirsagar PR (2011) Characterisation of copolymer, poly (hydroxybutyrate-co-hydroxyvalerate) (PHB-co-PHV) produced by Halomonas campisalis (MCM B-1027), its biodegradability and potential application. Bioresour Technol 102(11):6625–6628
Srirangan K, Liu X, Tran TT, Charles TC, Moo-Young M, Chou CP (2016) Engineering of Escherichia coli for direct and modulated biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer using unrelated carbon sources. Sci Rep 6:36470
Yang JE, Choi YJ, Lee SJ, Kang KH, Lee H, Oh YH, Lee SH, Park SJ, Lee SY (2014) Metabolic engineering of Escherichia coli for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from glucose. Appl Microbiol Biotechnol 98(1):95–104
Zhu C, Nomura CT, Perrotta JA, Stipanovic AJ, Nakas JP (2012) The effect of nucleating agents on physical properties of poly-3-hydroxybutyrate (PHB) and poly-3-hydroxybutyrate-o-3-hydroxyvalerate (PHB-co-HV) produced by Burkholderia cepacia ATCC 17759. Polym Test 31:579–585
Li Z-J, Shi Z-Y, Jian J, Guo Y-Y, Wu Q, Chen G-Q (2010) Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli. Metab Eng 12:352–359
Hu F, Cao Y, Xiao F, Zhang J, Li H (2007) Site-directed mutagenesis of Aeromonas hydrophila enoyl coenzyme A hydratase enhancing 3-hydroxyhexanoate fractions of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). Curr Microbiol 55(1):20–24
Lee SH, Oh DH, Ahn WS, Lee Y, Choi J, Lee SY (2000) Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by high-cell-density cultivation of Aeromonas hydrophila. Biotechnol Bioeng 67:240–244
Tsuge T, Saito Y, Kikkawa Y, Hiraishi T (2004) Y. Biosynthesis and compositional regulation of poly(3-hydroxybutyrate)-co-(3-hydroxyhexanoate) in recombinant Ralstonia eutropha expressing mutated polyhydroxyalkanoate synthase genes. Macromol Biosci 4(3):238–242
Wang Y, Chung A, Chen G-Q (2017a) Synthesis of medium-chain-length polyhydroxyalkanoate homopolymers, random copolymers, and block copolymers by an engineered strain of Pseudomonas entomophila. Adv Healthc Mater 6:1601017
Xie WP, Chen G-Q (2008) Production and characterization of terpolyester poly(3-hydroxybutyrate- co-4-hydroxybutyrate-co-3-hydroxyhexanoate) by recombinant Aeromonas hydrophila 4AK4 harboring genes phaPCJ. Biochem Eng J 38:384–389
Choi SY, Park SJ, Kim WJ, Yang JE, Lee H, Shin J, Sang Y (2016) One-step fermentative production of poly(lactate-co-glycolate) from carbohydrates in Escherichia coli. Nat Biotechnol 34:435–440
Pederson EN, McChalicher CWJ, Srienc F (2006) Bacterial synthesis of PHA block copolymers. Biomacromolecules 7:1904–1911
Tripathi L, Wu L-P, Chen J, Chen G-Q (2012) Synthesis of diblock copolymer poly-3-hydroxybutyrate -block-poly-3-hydroxyhexanoate [PHB-b-PHHx] by a β-oxidation weakened Pseudomonas putida KT2442. Microb Cell Factories 11:44
Kacmar J, Carlson R, Balogh SJ, Srienc F (2005) Staining and quantification of poly-3-hydroxybutyrate in Saccharomyces cerevisiae and Cupriavidus necator cell populations using automated flow cytometry. Cytometry A 69A:27–35
Heinrich D, Madkour MH, Al-Ghamdi MA, Shabbaj II, Steinbüchel A (2012) Large scale extraction of poly(3-hydroxybutyrate) from Ralstonia eutropha H16 using sodium hypochlorite. AMB Express 2:59
Volova T, Kiselev E, Vinogradova O, Nikolaeva E, Chistyakov A, Sukovatiy A, Shishatskaya E (2014) A glucose-utilizing strain, Cupriavidus euthrophus B-10646: growth kinetics, characterization and synthesis of multicomponent PHAs. PLoS One 9(2):e87551
Agrawal T, Kotasthane AS, Kushwah R (2015) Genotypic and phenotypic diversity of polyhydroxybutyrate (PHB) producing Pseudomonas putida isolates of Chhattisgarh region and assessment of its phosphate solubilizing ability. 3 Biotech 5(1):45–60
Follonier S, Henes B, Panke S, Zinn M (2012) Putting cells under pressure: a simple and efficient way to enhance the productivity of medium-chain-length polyhydroxyalkanoate in processes with Pseudomonas putida KT2440. Biotechnol Bioeng 109(2):451–461
Dietrich D, Illman B, Crooks C (2013) Differential sensitivity of polyhydroxyalkanoate producing bacteria to fermentation inhibitors and comparison of polyhydroxybutyrate production from Burkholderia cepacia and Pseudomonas pseudoflava. BMC Res Notes 6:1–4
Chen G-Q, Page WJ (1997) Production of poly-b-hydroxybutyrate by Azotobacter vinelandii in a two-stage fermentation process. Biotechnol Tech 11:347–350
Wang X, Li Z, Li X, Qian H, Cai X, Li X, He J (2016b) Poly-β-hydroxybutyrate metabolism is unrelated to the sporulation and parasporal crystal protein formation in Bacillus thuringiensis. Front Microbiol 7:836
Bhagowati P, Pradhan S, Dash HR, Das S (2015) Production, optimization and characterization of polyhydroxybutyrate, a biodegradable plastic by Bacillus spp. Biosci Biotechnol Biochem 79(9):1454–1463
Sharma P, Bajaj BK (2015) Cost-effective-substrates for production of poly-ß-hydroxybutyrate by a newly isolated Bacillus cereus PS-10. J Environ Biol 36(6):1297–1304
Guevara-Martínez M, Sjöberg Gällnö K, Sjöberg G, Jarmander J, Perez-Zabaleta M, Quillaguamán J, Larsson G (2015) Regulating the production of (R)-3-hydroxybutyrate in Escherichia coli by N or P limitation. Front Microbiol 6:844
El-sayed AA, Abdel Hafez AM, Hemmat Abdelhady M, Khodair TA (2009) Production of polyhydroxybutyrate (PHB) using batch and two-stage batch culture strategies. Austr J Basic Appl Sci 3:617–627
Wang F, Lee SY (1997) Production of poly ß(3-hydroxybutyrate) by fed-batch culture of filamentation-suppressed recombinant Escherichia coli. Appl Environ Microbiol 63:4765–4769
Castilho LR, Mitchell DA, Freire DMG (2009) Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour Technol 100:5996–6009
Chen G-Q (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38(8):2434–2446
Jiang XJ, Sun Z, Ramsay JA, Ramsay BA (2013) Fed-batch production of MCL-PHA with elevated 3-hydroxynonanoate content. AMB Express 3(1):50
Mahishi LH, Tripathi G, Rawal SK (2003) Poly (3-hydroxybutyrate) (PHB) synthesis by recombinant Escherichia coli harbouring Streptomyces aureofaciens PHB biosynthesis genes: effect of various carbon and nitrogen sources. Microbiol Res 158:19–27
Lin Z, Zhang Y, Yuan Q, Liu Q, Li Y, Wang Z, Ma H, Chen T, Zhao X (2015) Metabolic engineering of Escherichia coli for poly(3-hydroxybutyrate) production via threonine bypass. Microb Cell Factories 14:185
Inan K, Sal FA, Rahman A, Putman RJ, Agblevor FA, Miller CD (2016) Microbubble assisted polyhydroxybutyrate production in Escherichia coli. BMC Res Notes 9:338
Wei XX, Zheng WT, Hou X, Liang J, Li ZJ (2015) Metabolic engineering of Escherichia coli for poly(3-hydroxybutyrate) production under microaerobic condition. Biomed Res Int 2015:789315
Tao W, Lv L, Chen G-Q (2017) Engineering Halomonas species TD01 for enhanced polyhydroxyalkanoates synthesis via CRISPRi. Microb Cell Factories 16(1):48
Mozejko-Ciesielska J, Dabrowska D, Szalewska-Palasz A, Ciesielski S (2017) Medium-chain-length polyhydroxyalkanoates synthesis by Pseudomonas putida KT2440 relA/spoT mutant: bioprocess characterization and transcriptome analysis. AMB Express 7(1):92
Tsuge T, Hyakutake M, Mizuno K (2015) Class IV polyhydroxyalkanoate (PHA) synthases and PHA-producing Bacillus. Appl Microbiol Biotechnol 99(15):6231–6240
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Wang Q, Xia Y, Chen Q, Qi Q (2012) Incremental truncation of PHA synthases results in altered product specificity. Enzyme Microb Technol 50:293–297
Zheng Z, Li M, Xue XJ, Tian HL, Li Z, Chen GQ (2006) Mutation on N-terminus of poly-3-hydroxyalkanoate (PHA) synthase enhanced PHA accumulation. Appl Microbiol Biotechnol 72:896–905
Jia K, Cao R, Hua DH, Li P (2016) Study of class I and class III polyhydroxyalkanoate (PHA) synthases with substrates containing a modified side chain. Biomacromolecules 17(4):1477–1485
Chek MF, Kim SY, Mori T, Arsad H, Samian MR, Sudesh K, Hakoshima T (2017) Structure of polyhydroxyalkanoate (PHA) synthase PhaC from Chromobacterium sp. USM2, producing biodegradable plastics. Sci Rep 7(1):5312
Wittenborn EC, Jost M, Wei Y, Stubbe J, Drennan CL (2016) Structure of the catalytic domain of the class I polyhydroxybutyrate synthase from Cupriavidus necator. J Biol Chem 291(48):25264–25277
Bhubalan K, Chuah JA, Shozui F, Brigham CJ, Taguchi S, Sinskey AJ, Rha C, Sudesh K (2011) Characterization of the highly active polyhydroxyalkanoate synthase of Chromobacterium sp. strain USM2. Appl Environ Microbiol 77(9):2926–2933
Chuah JA, Tomizawa S, Yamada M, Tsuge T, Doi Y, Sudesh K, Numata K (2013) Characterization of site-specific mutations in a short-chain-length/medium-chain-length polyhydroxyalkanoate synthase: in vivo and in vitro studies of enzymatic activity and substrate specificity. Appl Environ Microbiol 79(12):3813–3821
Sheu DS, Chen WM, Lai YW, Chang RC (2012a) Mutations derived from the thermophilic polyhydroxyalkanoate synthase PhaC enhance the thermostability and activity of PhaC from Cupriavidus necator H16. J Bacteriol 194(10):2620–2629
Takase K, Matsumoto K, Taguchi S, Doi Y (2004) Alteration of substrate chain-length specificity of type II synthase for polyhydroxyalkanoate biosynthesis by in vitro evolution: in vivo and in vitro enzyme assays. Biomacromolecules 5:480–485
Amara AA, Steinbüchel A, Rehm BH (2002) In vivo evolution of the Aeromonas punctata polyhydroxyalkanoate (PHA) synthase: isolation and characterization of modified PHA synthases with enhanced activity. Appl Microbiol Biotechnol 59:477–482
Shozui F, Matsumoto K, Sasaki T, Taguchi S (2009) Engineering of polyhydroxyallanoate synthase by Ser477X/Gln481X saturation mutagenesis for efficient production of 3-hydroxybutyrate-based copolyesters. Appl Microbiol Biotechnol 84:1117–1124
Tsuge T, Watanabe S, Shimada D, Abe H, Doi Y, Taguchi S (2007) Combination of N149S and D171G mutations in Aeromonas caviae polyhydroxyalkanoate synthase and impacton polyhydroxyalkanoate biosynthesis. FEMS Microbiol Lett 277:217–222
Chen JY, Liu T, Zheng Z, Chen JC, Chen G-Q (2004) Polyhydroxyalkanoate synthases PhaC1 and PhaC2 from Pseudomonas stutzeri 1317 had different substrate specificities. FEMS Microbiol Lett 234:231–237
Shen XW, Shi ZY, Song G, Li ZJ, Chen G-Q (2011) Engineering of polyhydroxyalkanoate (PHA) synthase PhaC2Ps of Pseudomonas stutzeri via site-specific mutation for efficient production of PHA copolymers. Appl Microbiol Biotechnol 91(3):655–665
Gao X, Yuan XX, Shi ZY, Guo YY, Shen XW, Chen JC, Wu Q, Chen GQ (2012) Production of copolyesters of 3-hydroxybutyrate and medium-chain-length 3-hydroxyalkanoates by E. coli containing an optimized PHA synthase gene. Microb Cell Factories 11:130
Nomura CT, Taguchi S (2007) PHA synthase engineering toward superbiocatalysts for custom-made biopolymers. Appl Microbiol Biotechnol 73:969–979
Sznajder A, Pfeiffer D, Jendrossek D (2015) Comparative proteome analysis reveals four novel polyhydroxybutyrate (PHB) granule-associated proteins in Ralstonia eutropha H16. Appl Environ Microbiol 81(5):1847–1858
Pfeiffer D, Jendrossek D (2014) PhaM is the physiological activator of poly(3-hydroxybutyrate) (PHB) synthase (PhaC1) in Ralstonia eutropha. Appl Environ Microbiol 80(2):555–563
Le Meur S, Zinn M, Egli T, Thöny-Meyer L, Ren Q (2014) Improved productivity of poly (4-hydroxybutyrate) (P4HB) in recombinant Escherichia coli using glycerol as the growth substrate with fed-batch culture. Microb Cell Factories 13:131
Insomphun C, Kobayashi S, Fujiki T, Numata K (2016) Biosynthesis of polyhydroxyalkanoates containing hydroxyl group from glycolate in Escherichia coli. AMB Express 6(1):29
Ahn WS, Park SJ, Lee SY (2000) Production of poly(3-hydroxybutyrate) by fed-batch culture of recombinant Escherichia coli with a highly concentrated whey solution. Appl Environ Microbiol 66:3624–3627
Agus J, Kahar P, Abe H, Doi Y, Tsuge T (2006) Molecular weight characterization of poly(R)-3-hydroxybutyrate synthesized by genetically engineered strains of Escherichia coli. Polym Degrad Stab 91:1138–1146
Ahmed A, Rushworth JV, Hirst NA, Millner PA (2014) Biosensors for whole-cell bacterial detection. Clin Microbiol Rev 27:631–646
Poltronieri P, Mezzolla V, D’Urso OF (2017) PHB production in biofermentors assisted through biosensor applications. Proceedings (MDPI, Baseline) 1(2):4
Wu H, Fan Z, Jiang X, Chen J, Chen G-Q (2016) Enhanced production of polyhydroxybutyrate by multiple dividing E. coli. Microb Cell Factories 15:128
Chen G-Q, Jiang X-R, Guo Y (2016) Synthetic biology of microbes synthesizing polyhydroxyalkanoates (PHA). Synthetic Systems Biotechnol 1:236–242
Borrero-de Acuña JM, Bielecka A, Häussler S, Schobert M, Jahn M, Wittmann C, Jahn D, Poblete-Castro I (2014) Production of medium chain length polyhydroxyalkanoate in metabolic flux optimized Pseudomonas putida. Microb Cell Factories 13:88
Tao Z, Peng L, Zhang P, Li YQ, Wang G (2016) Probing the kinetic anabolism of poly-beta-hydroxybutyrate in Cupriavidus necator H16 using single-cell Raman spectroscopy. Sensors (Basel) 16(8):1257
Elain A, Le Fellic M, Corre YM, Le Grand A, Le Tilly V, Audic JL, Bruzaud S (2015) Rapid and qualitative fluorescence-based method for the assessment of PHA production in marine bacteria during batch culture. World J Microbiol Biotechnol 31(10):1555–1563
Cui Y, Barford JP, Renneberg R (2006) Determination of poly(3-hydroxybutyrate) using a combination of enzyme-based biosensor and alkaline hydrolysis. Anal Sci 22(10):1323–1326
Ghoshdastider U, Wu R, Trzaskowski B, Mlynarczyk K, Miszta P, Gurusaran M, Viswanathan S, Renugopalakrishnan V, Filipek S (2015) Nano-encapsulation of glucose oxidase dimer by graphene. RSC Adv 5(18):13570–13578
Kunasundari B, Murugaiyah V, Kaur G, Maurer FH, Sudesh K (2013) Revisiting the single cell protein application of Cupriavidus necator H16 and recovering bioplastic granules simultaneously. PLoS One 8(10):e78528
Hajnal I, Chen X, Chen G-Q (2016) A novel cell autolysis system for cost-competitive downstream processing. Appl Microbiol Biotechnol 100:9103–9110
Martínez V, Herencias C, Jurkevitch E, Prieto MA (2016) Engineering a predatory bacterium as a proficient killer agent for intracellular bio-products recovery: the case of the polyhydroxyalkanoates. Sci Rep 6:24381
Jiang Y, Mikova G, Kleerebezem R, van der Wielen LA, Cuellar MC (2015) Feasibility study of an alkaline-based chemical treatment for the purification of polyhydroxybutyrate produced by a mixed enriched culture. AMB Express 5(1):5
Bugnicourt E, Cinelli P, Lazzeri A, Alvarez V (2014) Polyhydroxyalkanoate (PHA): review of synthesis, characteristics, processing and potential applications in packaging. Express Polym Lett 8(11):791–808
Chen W, Tong YW (2012) PHBV microspheres as neural tissue engineering scaffold support neuronal cell growth and axon dendrite polarization. Acta Biomater 8:540–548
Lomas AJ, Chen GGQ, El Haj AJ, Forsyth NR (2012) Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) supports adhesion and migration of mesenchymal stem cells and tenocytes. World J Stem Cells 4(9):94–100
Oryan A, Alidadi S, Moshiri A, Maffulli N (2014) Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res 9:18
Liu H, Pancholi M, Stubbs J, Raghavan D (2010) Influence of hydroxyvalerate composition of polyhydroxybutyrate valerate (PHBV) copolymer on bone cell viability and in vitro degradation. J Appl Polym Sci 116:3225–3231
Webb WR, Dale TP, Lomas AJ, Zeng G, Wimpenny I, El Haj AJ, Forsyth NR, Chen G-Q (2013) The application of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds for tendon repair in the rat model. Biomaterials 34:6683–6694
Pourmollaabbassi B, Karbasi S, Hashemibeni B (2016) Evaluate the growth and adhesion of osteoblast cells on nanocomposite scaffold of hydroxyapatite/titania coated with poly hydroxybutyrate. Adv Biomed Res 5:156
Rodríguez-Contreras A, García Y, Manero JM, Rupérez E (2017) Antibacterial PHAs coating for titanium implants. Eur Polym J 90:66–78
Collins MN, Birkinshaw C (2013) Hyaluronic acid based scaffolds for tissue engineering – a review. Carbohydr Polym 92:1262–1279
Peng Q, Zhang ZR, Gong T, Chen GQ, Sun X (2012) A rapid-acting, long-acting insulin formulation based on a phospholipid complex loaded PHBHHx nanoparticles. Biomaterials 33:1583–1588
Peng Q, Sun X, Gong T, Wu CY, Zhang T, Tan J et al (2013) Injectable and biodegradable thermosensitive hydrogels loaded with PHBHHx nanoparticles for the sustained and controlled release of insulin. Acta Biomater 9:5063–5069
Dong Y, Li P, Chen CB, Wang ZH, Ma P, Chen G-Q (2010) The improvement of fibroblast growth on hydrophobic biopolyesters by coating with polyhydroxyalkanoate granule binding protein PhaP fused with cell adhesion motif RGD. Biomaterials 31:8921–8930
Heathman TR, Webb WR, Han J, Dan Z, Chen GQ, Forsyth NR, El Haj AJ, Zhang ZR, Sun X (2014) Controlled production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) nanoparticles for targeted and sustained drug delivery. J Pharm Sci 103(8):2498–2508
Nishizuka T, Kurahashi T, Hara T, Hirata H, Kasuga T (2014) Novel intramedullary-fixation technique for long bone fragility fractures using bioresorbable materials. PLoS One 9(8):e104603
Ashby RD, Solaiman DK, Strahan GD, Levine AC, Nomura CT (2015) Methanol-induced chain termination in poly(3-hydroxybutyrate) biopolymers: molecular weight control. Int J Biol Macromol 74:195–201
Beckers V, Poblete-Castro I, Tomasch J, Wittmann C (2016) Integrated analysis of gene expression and metabolic fluxes in PHA-producing Pseudomonas putida grown on glycerol. Microb Cell Factories 15:73
Bidart GN, Ruiz JA, de Almeida A, Méndez BS, Nikel PI (2012) Manipulation of the anoxic metabolism in Escherichia coli by ArcB deletion variants in the ArcBA two-component system. Appl Environ Microbiol 78(24):8784–8794
Cesário MT, Raposo RS, de Almeida MCMD, van Keulen F, Ferreira BS, da Fonseca MMR (2014) Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol 31:104–113
Hamieh A, Olama Z, Holail H (2010) Microbial production of polyhydroxybutyrate, a biodegradable plastic using agro-industrial waste products. Global Adv Res J Microbiol 2:054–064
Higuchi-Takeuchi M, Morisaki K, Toyooka K, Numata K (2016) Synthesis of high-molecular-weight polyhydroxyalkanoates by marine photosynthetic purple bacteria. PLoS One 11(8):e0160981
Jeon JM, Kim HJ, Bhatia SK, Sung C, Seo HM, Kim JH, Park HY, Lee D, Brigham CJ, Yang YH (2017) Application of acetyl-CoA acetyltransferase (AtoAD) in Escherichia coli to increase 3-hydroxyvalerate fraction in poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioprocess Biosyst Eng 40(5):781–789
Koller M, Dias MMS, Rodríguez-Contreras A, Kunaver M, Žagar E, Kržan A, Braunegg G (2015) Liquefied wood as inexpensive precursor-feedstock for bio-mediated incorporation of (.)-3-Hydroxyvalerate into polyhydroxyalkanoates. Materials 8:6543–6557
Koutinas AA, Xu Y, Wang R, Webb C (2007) Polyhydroxybutyrate production from a novel feedstock derived from a wheat-based biorefinery. Enzym Microb Technol 40(5):1035–1044
Kushwah BS, Kushwah AV, Singh V (2016) Towards understanding polyhydroxyalkanoates and their use. J Polym Res 23:1–14
Lee GN, Na J (2013) Future of microbial polyesters. Microb Cell Factories 12:54
Li P, Chakraborty S, Stubbe J (2009) Detection of covalent and noncovalent intermediates in the polymerization reaction catalyzed by a C149S class III polyhydroxybutyrate synthase. Biochemistry 48(39):9202–9211
Liu XJ, Zhang J, Hong PH, Li ZJ (2016) Microbial production and characterization of poly-3-hydroxybutyrate by Neptunomonas antarctica. PeerJ 4:e2291
Lütke-Eversloh T, Bergander K, Luftmann H, Steinbüchel A (2001) Identification of a new class of biopolymer: bacterial synthesis of a sulfur-containing polymer with thioester linkages. Microbiology 147(Pt 1):11–19
Madden LA, Anderson AJ, Asrar J, Berger P, Garrett P (2000) Production and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-4-hydroxybutyrate) synthesized by Ralstonia eutropha in fed-batch cultures. Polymer 41:3499–3505
Meng DC, Shi ZY, Wu LP, Zhou Q, Wu Q, Chen JC, Chen G-Q (2012) Production and characterization of poly(3-hydroxypropionate-co-4-hydroxybutyrate) with fully controllable structures by recombinant Escherichia coli containing an engineered pathway. Metab Eng 14:317–324
Muhammadi S, Afzal M, Hameed S (2015) Bacterial polyhydroxyalkanoates eco-friendly next generation plastic: production, biocompatibility, biodegradation, physical properties and applications. Green Chem Lett Rev 8:56–77
Myung J, Galega WM, Van Nostrand JD, Yuan T, Zhou J, Criddle CS (2015) Long-term cultivation of a stable Methylocystis-dominated methanotrophic enrichment enabling tailored production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioresour Technol 198:811–818
Myung J, Flanagan JCA, Waymouth RM, Criddle CS (2017) Expanding the range of polyhydroxyalkanoates synthesized by methanotrophic bacteria through the utilization of omega-hydroxyalkanoate co-substrates. AMB Express 7(1):118
Ostle AG, Holt JG (1982) Nile blue A as a fluorescent stain for poly ß-hydroxybutyrate. Appl Environ Microbiol 44:238–241
Pagliano G, Ventorino V, Panico A, Pepe O (2017) Integrated systems for biopolymers and bioenergy production from organic waste and by-products: a review of microbial processes. Biotechnol Biofuels 10:113
Pandey A, Negi S, Soccol CR (eds) (2017) Current developments in biotechnology and bioengineering. Production, isolation and purification of industrial products. Elsevier, Amsterdam, The Netherlands
Poblete-Castro I, Binger D, Rodrigues A, Becker J, Martins Dos Santos VA, Wittmann C (2013) In-silico-driven metabolic engineering of Pseudomonas putida for enhanced production of polyhydroxyalkanoates. Metab Eng 15:113–123
Ramachandran H, Iqbal NM, Sipaut CS, Amirul A-AA (2011) Biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-4-hydroxybutyrate) terpolymer with various monomer compositions by Cupriavidus sp. USMAA2-4. Appl Biochem Biotechnol 164:867–877
Ruiz JA, de Almeida A, Godoy MS, Mezzina MP, Bidart GN, Méndez BS, Pettinari MJ, Nikel PI (2013) Escherichia coli redox mutants as microbial cell factories for the synthesis of reduced biochemicals. Comput Struct Biotechnol J 3:e201210019
Rydz J, Sikorska W, Kyulavska M, Christova D (2014) Polyester-based (bio)degradable polymers as environmentally friendly materials for sustainable development. Int J Mol Sci 16(1):564–596
Scheel RA, Ji L, Lundgren BR, Nomura CT (2016) Enhancing poly(3-hydroxyalkanoate) production in Escherichia coli by the removal of the regulatory gene arcA. AMB Express 6(1):120
Simon-Colin C, Raguénès G, Costa B, Guezennec J (2008) Biosynthesis of medium chain length poly-3-hydroxyalkanoates by Pseudomonas guezennei from various carbon sources. React Funct Polym 68:1534–1541
Tan G-YA, Chen C-L, Li L, Ge L, Wang L, Razaad IMN, Li Y, Zhao L, Mo Y, Wang J-Y (2014) Start a research on biopolymer polyhydroxyalkanoate (PHA): a review. Polymers 6:706–754
Tan D, Yin J, Chen G-Q (2017) Production of polyhydroxyalkanoates. In: Pandey A, Negi S, Soccol CR (eds) Current developments in biotechnology and bioengineering. Production, isolation and purification of industrial products. Elsevier, Amsterdam, pp 655–692
Vadlja D, Koller M, Novak M, Braunegg G, Horvat P (2016) Footprint area analysis of binary imaged Cupriavidus necator cells to study PHB production at balanced, transient, and limited growth conditions in a cascade process. Appl Microbiol Biotechnol 100(23):10065–10080
Verlinden RA, Hill DJ, Kenward MA, Williams CD, Radecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102:1437–1449
Yang YH, Brigham CJ, Song E, Jeon JM, Rha CK, Sinskey AJ (2012) Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) containing a predominant amount of 3-hydroxyvalerate by engineered Escherichia coli expressing propionate-CoA transferase. J Appl Microbiol 113(4):815–823
Wang Y, Liu S (2014) Production of (R)-3-hydroxybutyric acid by Burkholderia cepacia from wood extract hydrolysates. AMB Express 4:28
Kumar P, Patel SKS, Lee JK, Kalia VC (2013) Extending the limits of Bacillus for novel biotechnological applications. Biotechnol Adv 31(8):1543–1561
Kumar P, Ray S, Kalia VC (2016) Production of co-polymers of polyhydroxyalkanoates by regulating the hydrolysis of biowastes. Bioresour Technol 200:413–419
Patel SKS, Kumar P, Singh M, Lee JK, Kalia VC (2015) Integrative approach to produce hydrogen and polyhydroxybutyrate from biowaste using defined bacterial cultures. Bioresour Technol 176:136–141
Oshiki M, Satoh H, Mino T (2011) Rapid quantification of polyhydroxyalkanoates (PHA) concentration in activated sludge with the fluorescent dye Nile blue A. Water Sci Technol 64(3):747–753
Spiekermann P, Rehm BHA, Kalscheuer R, Baumeister D, Steinbüchel A (1999) A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. 171:73–80
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Poltronieri, P., Kumar, P. (2019). Polyhydroxyalkanoates (PHAs) in Industrial Applications. In: Martínez, L., Kharissova, O., Kharisov, B. (eds) Handbook of Ecomaterials. Springer, Cham. https://doi.org/10.1007/978-3-319-68255-6_70
Download citation
DOI: https://doi.org/10.1007/978-3-319-68255-6_70
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68254-9
Online ISBN: 978-3-319-68255-6
eBook Packages: EngineeringReference Module Computer Science and Engineering