Abstract
In the 21st century, fresh water scarcity is perhaps one of the biggest challenges in many coastal regions worldwide due to the rapid population growth, fast urbanization and unpredictable impacts of global climate change. Given this context, the identification of groundwater status is a crucial task for sustainable groundwater use and management practices in coastal areas around the world. This work, conducted in coastal areas of Soc Trang province, is an effort to assess groundwater quality and its controlling factors in a coastal area of the Mekong Delta, Vietnam. In this study, we investigate groundwater quality based on chemical parameters, stable isotopes (δ18O, δ2H) and saturation indices (SI). The study showed that groundwater in the study area is mainly classified into four groups: Na-Cl, Na-Mg-Ca-HCO3, Na-Mg-Ca-HCO3-SO4 and Na-HCO3-Cl. Groundwater quality might be substantially controlled by the rock-water interaction, particularly by mineral dissolution and ion-exchange process. Further, the stable isotopes and saturation indices depict the origin of salt water presenting in the aquifers because of three factors, including paleo-saline water dissolution at deeper aquifers, seawater intrusion into shallow aquifers and saline water diffusion at middle aquifers. This result suggests that the characteristics of hydrogeology, inappropriate groundwater pumping activities and change of hydrological regimes might be the main driving forces of disturbance groundwater flow systems and expansion of saline boundary in the coastal areas of the Vietnamese Mekong Delta.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
It is widely recognized that fresh water shortage is becoming the most challenge for satisfying domestic, industrial and agricultural water demands in many countries around the world in the 21st century [1, 2]. As groundwater is a largely invisible resource, its dynamic change of quantity and quality is difficult to grasp even for experts [3]. Understanding the groundwater quality and its controlling factors, therefore, is the critical task for groundwater planning and management, ensuring sustainability of safe water use for national and global socio-economic development [4].
In natural conditions, groundwater moves slowly through the aquifer system under controlling by geological characteristics, hydrological and geological processes [5,6,7,8]. However, the human development might disturb this process, resulting in the serious changes of groundwater quality. The groundwater depletion due to excessive extraction and significant land-use changes, for instance, results in many groundwater quality-related problems, especially arsenic release from soils and sediments into groundwater [9,10,11,12,13], heavy metals and nitrate contamination [14,15,16,17,18,19], as well as the adverse seawater intrusion into coastal aquifers worldwide [20,21,22,23,24,25]. Additionally, the unpredictable impacts of climate change and sea level rise in the coastal regions might potentially accelerate the degradation of groundwater quality and put this resource be likely high crisis [3, 26, 27]. This fact, thus, poses the biggest issue to sustainable water management in coastal regions around the world in this century [26, 28].
Over the last several decades, hydrochemistry and stable isotopes have been widely applied to understand hydrological processes and groundwater evolution such as mixing different water sources, mineral weathering and evaporating [29,30,31,32,33]. Recently, by employing hydrochemistry and stable isotopes techniques, many studies [23, 34,35,36,37] has been proven that the deterioration of groundwater quality in many coastal regions is a result of excessive groundwater extraction and significant land-use changes coupled with unpredictable impacts of climate change, and sea level rise. As the intensive human development and natural dynamic might cause the changes of hydrogeological characteristics and groundwater quality, the combination of chemical and stable isotopes signatures, therefore, is a unique tool for investigating groundwater characteristics and suitable groundwater quality for drinking and irrigation [38].
Groundwater is a key resource for socio-economic development in the Vietnamese Mekong Delta region. The long-term exploitation and inappropriate management of groundwater, however, has resulted in many severe issues in the Mekong Delta, especially land subsidence [39] and arsenic contamination related to serious public health problems [40,41,42]. Some previous studies such as Ho et al. [43], Khoi et al. [44] and An et al. [45] employed the stable isotopes and hydrochemistry to understand the groundwater quality in term of salinization in the Mekong Delta. Yet the contribution of geological features and groundwater exploitation activities to hydrochemical characteristics and groundwater quality in the coastal area of the Mekong Delta has remained unknown. Meanwhile, towards sustainability of groundwater use and management in the context of rapid socio-economic development and natural variation requires an in-depth understanding not only groundwater characteristics but also the main driver. The study, therefore, is the first effort to investigate groundwater quality and its controlling factors based on hydrogeochemical and stable isotopes signatures in a coastal area of the Vietnamese Mekong Delta.
2 Study Area
2.1 Location and Climatic Background
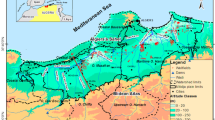
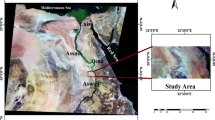
The study area – Soc Trang province is located at a low-lying part of the Mekong River Basin, reaching directly to the East Sea of Vietnam. It covers approximately 2311.76 km2 and accounts for around 0.7% and 5.9% areas of Vietnam and Mekong Delta, respectively with an average population of 1,310,700 people (General Statistics Office of Vietnam 2015). The study site falls in a strong tropical monsoon region with two distinguish seasons, the dry and rainy seasons. In the rainy season, climate condition is strongly affected by the Southwest Monsoon, which brings more than 85% amount of annual rainfall, having a high temperatures and humidity. Meanwhile, the Northeast Monsoon dominates in the dry season from November until April, contributing to 15% of the annual rainfall with a relative low temperature and humidity. The average annual air temperature is 26.8 °C with over 36 °C in the warmest months (April and May) and the lowest mean monthly temperature in January is around 24 °C; average annual relative humidity of 84%; and annual precipitation of 1,772 mm with low rainfall from January to April. As relative low lying inland (0.5–2.5 m above mean sea level) and reaching directly with East Sea, the hydrological regime in the Delta, therefore is strongly controlled upstream discharge, local rainfall and river-marine dynamic interactions. These factors remarkably affect water quality of both surface water and groundwater sources in this region.
2.2 Hydrogeology
The geology and geomorphology of Soc Trang province were formed by the glaci-eustatic sea-level change and the ongoing tectonic subsidence of the Mekong River Basin, therefore, its hydrogeology is somewhat complex [46]. In general, hydrogeology consists of seven distinct aquifers namely, Holocene (qh), Upper Pleistocene (qp3), Upper-Middle Pleistocene (qp23), Lower Pleistocene (qp1), Middle Pliocene (n22), Lower Pliocene (n21), Upper Miocene (n13) aquifer layers. Generally, the lithology of each aquifer consists of fine to coarse sand, gravel, and pebbles (Fig. 2).
The Holocene layer (qh) was formed from the coarse-grained rocks sedimentary rocks originating from mainly three types of sediments, including: Lower to Middle Holocene sediments (qh1-2) of alluvial and marine origin composed dominantly of clayey silt and fine sand and are rich organic compositions. Alluvial, marine and eolian sediments (qh2-3) include 1.0–12.0 m below ground level (mbgl) thick remnants of sand dunes from paleo-sea shores which can be found in Long Phu, Vinh Chau, Soc Trang and My Tu districts. These sand dunes are often shaped arc extends parallel with the coast of the northeast - southwest or northwest – southeast, extending from 3.0 to 4.0 km along the coast and distributing around 200–300 m from the shoreline to inland. Upper Holocene sediments (qh3), accumulated in the river valleys and flood plains, consists of clayey silt, silt-mud, and fine sand. Slug tests results of wells in this area point out that groundwater flow rate (Q) ranges approximately 0.20–0.50 L/s (aveg. 0.30 L/), drawdown (S) is 0.30–0.70 m (aveg. 0.53 m) (Fig. 1).
The Upper Pleistocene unit (qp3) is widely distributed over the whole Soc Trang province, mainly overlaying by Holocene sediments. This aquifer was formed by coarse-grained sedimentary rock formations of Long My (mQ13 lm), composed mainly of fine sand, fine gravel and medium and small gray-green shells, gray and white sand with thickness changing 3.0–50.9 m (aveg. 20.50 m). Hydro-geologically, qp3 strata could be divided into two parts: The Lower part of the high permeable aquifer is covered by an upper part of low permeable aquitards, generally consisting of silt to a clay-size fraction. The top of qp3 aquifer distributes heterogeneously with a depth of 24.0 m–95.0 mbgl (aveg. 50.39 mbgl) and its bottom ranges around 30.0–125.0 mbgl (aveg. 70.74 mbgl). There is limited slug test result of the qp3 aquifer, however, based on analyzing the thickness and grain size suggests that groundwater flow rate of this aquifer is 0.185 L/s–0.195 L/s.
Middle Pleistocene aquifer (qp23) was overplayed by Upper Pleistocene (qp3). The lithology is dominantly composed of alluvial sediments, marine alluvial and marine origins from Long Toan formations system. The aquifer covers and distributes widely throughout the province. This aquifer is also divided into a low permeable part, composing silt and clay, which can be encountered in depth from 54.0 mbgl to 137.0 mbgl (aveg. 83.63 mbgl). A confined aquifer represents the low part, consisting of well stored and high permeable fine to coarse sand mixing with gravel sand and thin lenses of clay powder in the depth of 92.0–175.0 mbgl (aveg. 131.47 mbgl). The thickness of this part ranges from 7.0 m to 81.0 m (aveg. 49.75 m). The composition is mainly coarse sand in various sizes containing water. The result of pumping test showed that water absorption is very high in aquifers with groundwater flow rate from 9.05 to 19.10 L/s (aveg. 14.57 l L/s), drawdown (S) is 2.51–18.81 m (aveg. 10.31 m).
Lower Pleistocene aquifer (qp1) is generally formed from the coarse-grained rock under the bottom part of the Binh Minh formation system (m, amQ11bm). Lithology consists of dominate fine to coarse sand and less gravel. The qp1 aquifer is widely distributed over the whole Soc Trang province. The depth of the top part of the qp1 aquifer varies from 110.50 mbgl to 192.0 mbgl (aveg. 145.29 mbgl) while that of bottom part ranges from 146.00 m to 250.0 m (aveg. 187.40 m). The thickness of the aquifer varies from 6.0 m to 79.50 m (aveg. 40.29 m depth). The static groundwater level of this aquifer varies from −0.50 m to −8.78 m above sea level (masl) with an aveg. of −1.78 masl. The slug test result shows that this aquifer has very high groundwater flow rate (Q) distributing from 12.26 to 33.90 L/s (aveg. 17.92 L/s), drawdown (S) ranges 2.571–13.55 m (aveg. 8.48 m).
Since the last several decades, the Middle Pleistocene (qp23), Lower Pleistocene (qp1) have become the most attractive aquifer for groundwater pumping practices as they have high potential groundwater capacity and also good quality compared to remaining aquifers [45]. Recently, however, groundwater degradation has occurred in many parts of these aquifers due to intensive groundwater withdrawal with approximately 200 wells/km2. Consequently, the residents have to access groundwater at deeper aquifers (n21, n22 and n13 aquifers). This fact coupled with the changes of recharge pattern into aquifer system, seawater intrusion obviously poses a big challenge to the sustainable use and effective management practices of water resources in the study area.
3 Materials and Method
3.1 Data Collection and Analysis
During 2013 and 2014, a hundred and forty-two groundwater and surface samples were collected and stored into 100 ml plastic bottles. The water samples drive from households’ wells, municipal groundwater treatment plants and monitoring boreholes both shallow (qh - Holocene, qp3 - Upper Pleistocene), and deep aquifers (qp23 - Middle Pleistocene, qp1 - Lower Pleistocene, n22 - Middle Pliocene, n21 - Lower Pliocene and n13 - Miocene) with an average depth of wells ranges from 4.5 m to 480 m below ground level (m.bgl). The water chemistry characteristics were on-site measured using HANNA portable instruments, including: pH, Dissolved Oxygen (DO), Electrical Conductivity (EC), and Total dissolved solids (TDS).
All water samples were filtered with a 0.02 µm cellulose ester filter before analyzing chemical and stable isotopes compositions. The bicarbonate (HCO3 −) was deduced by using the titration method with sulfuric acid (0.05 M H2SO4). Major anions (Cl−, SO4 2−, and NO3 −) were analyzed using ion liquid chromatography (Shimadzu Co. Ltd., HIC-SP/VP Super) at the Hydrological sciences laboratory in the University of Tsukuba. The main cations (Na+, K+, Ca2+ and Mg2+) were analyzed using inductively coupled plasma optical emission spectrometer (ICP-OES, PERKIN ELMER, Optima 7300) at the Center of Chemical Analysis, University of Tsukuba, Japan. The stable isotopes of water samples were analyzed with a Finnigan MAT 252. Results were expressed relative to the international standards (V-SMOW for δ18O and δ2H) represented in ‰ the uncertainties were ±0.1‰ for δ18O and ±1‰ for δ2H.
3.2 Graphical and Geochemical Techniques
The Piper diagram was employed to classify water types in the study area, and the geochemical process was analyzed by using inverse-geochemical model. Geochemical modeling is a useful tool to determine the thermodynamic processes, which control groundwater quality [8, 31]. It simulates the mass balance with responses to chemical reactions and geochemical processes such as mineralization, gasses dissolution and precipitation throughout different hydrogeological settings. In this study, the Saturation Indices (SI) of minerals were calculated by using PHREE QC version 3 model [47]. The SI of a mineral is defined as Eq. (1) as followed:
where IAP is the ion activity product of the mineral-water reaction, and Ks is the thermodynamic equilibrium constant adjusted to the temperature of the given sample. The SI values depict three states of saturation including saturation (SI = 0), undersaturation (SI < 0), and supersaturation (SI > 0). The result of SI is useful information to understand the different hydrogeochemical processes that have been occurring in the specific aquifers. In this study, five major saturation indices were calculated to understand hydro-geochemical processes of groundwater in coastal aquifers including saturation index of calcite (SCal), dolomite (SDol), anhydrite (SAn), gypsum (SGyp) and halite (SHa).
4 Results and Discussion
4.1 General Hydrogeochemistry
Table 1 shows the statistical analysis of water chemistry in Soc Trang province. Obviously, the groundwater temperature in all aquifers in study site is relatively different between shallow and deep aquifers ranging from 25.0 °C to 40.3 °C, respectively. The pH values of shallow (qh, qp3), middle (qp23, qp1) and deep aquifers (n21, n22, n13) range from 6.52 to 7.05 and 6.53 to 8.68 with an average of 7.05 and 7.99, respectively. The changes of alkalization processes among these aquifers with high pH values suggest the interaction between soil, rain water and groundwater along flow path [8], while low pH value may be attributed to dilution and reaction between acid sulfate soils and groundwater [48, 49].
The DO varies widely among aquifers, ranging from (1.16–2.37) mg/L to (2.36–3.62) mg/L for shallow and deep groundwater, respectively. The EC values of groundwater samples were significant difference among aquifers with 906–21,200 µS/cm; 116.7–4,760 µS/cm and 204–13,720 µS/cm for (qh, qp3), (qp23, qp1) and (n21, n22, n13) aquifers, respectively. For a century, groundwater from middle aquifers (qp23, qp1) and deeper aquifers (n21, n22, n13) has become an important fresh water source for the Mekong Delta, especially along coastal areas because this is the only freshwater sources with high yields, good quality and cost-effective extraction. Conversely, shallow groundwater has very high salinity (TDS > 1000 mg/L) and is unsuitable for drinking and irrigation purposes. The wide variation of ionic composition reflects the complexity of groundwater evolution processes in different aquifers. Although groundwater samples of the (qh, qp3) and the (n21, n22, n13) aquifers were dominantly occupied by sodium (Na+) and chloride (Cl−), these aquifers showed different trends of salinity concentration. For instance, the salinity concentration in shallow groundwater samples were very high Na+ (266.85–8,535.80 mg/L) and Cl− (345.71–16,970.45 mg/L) compared to deep groundwater samples with concentration of Na+ (320.51–3,512.51 mg/L) and Cl− (84.66–7,286.02 mg/L). Conversely, middle groundwater samples presented relatively low of (Na+) and chloride (Cl−) concentration, ranging from 16.88–51.25 mg/L and 2.85–69.74 mg/L, respectively. Solutes concentration (K+, Ca2+, Mg2+, HCO3 − and NO3 −) varies widely from shallow to deep aquifers, especially deep groundwater samples displayed very high HCO3 − concentration varying from 561.38 to 779.33 mg/L, reflecting the strong influence of mineral calcite dissolution in this aquifer. More noticeably, a high concentration of NO3 − (113.33–264.18 mg/L) was detected in some locations surrounding shrimp farms close to the coastline (Fig. 3a). This fact might be attributed to moving pollutant sources into shallow and deep aquifers via leaking aquitards and unprotected wells as a result of excessive groundwater [50].
4.2 Hydrogeochemical Facies
In this study, Piper diagram [51] was employed to classify the characteristics of groundwater and surface between 2013 and 2014. As can be seen in Figs. 3b, c and d, groundwater quality shows a distinguish trend from aquifers. Although groundwater chemistry of most groundwater samples at qp23 and qp1 aquifers was stable in both season, it represented relatively a different trend with other aquifers (qh,qh3, n21, n22 and n13). Groundwater samples from these aquifers were characterized by fresh water (Ca-Mg-HCO3) and were suitable for water supply system. Shallow groundwater aquifers (qh, qh3), however, was classified into saline water (Na-Cl) type, ranging from moderate to very heavy salinity and were unusable for drinking and irrigation purposes. The remaining groundwater samples of deeper aquifers (n21, n22, n13) were grouped into brackish water (Na-Cl, Ca-Mg-Cl) type and mixed water type (Na-HCO3-Cl) which limited to fresh water demands.
The quality of surface water showed a seasonal variation, indicating the impacts of changing water flow between dry and rainy season as well as seawater intrusion on the surface water system of the Mekong Delta. Although almost all groundwater samples from qp23 and qp1 aquifers show seasonal stability in chemistry and stable isotopes, some of these aquifers closing to estuary have a relative high chloride and isotopic concentration, indicating effects of heterogeneous stratigraphy, salinization and freshening processes on groundwater quality. Similarly, groundwater samples from deeper aquifers were classified by Na-HCO3-Cl type with high salinity (around 750 mg/L), compared with other aquifers, demonstrating the different magnitudes of calcite and dolomite minerals dissolution processes. The difference of groundwater quality between shallow and deep aquifers reflects the complexity of stratigraphic formation processes of the Delta as a result of marine transgression and regression processes [52, 53], and the possibility human-induced activities resulting in modern seawater intrusion [54].
The spatial distribution of stiff diagrams and isotopic concentration of oxygen-18 was shown in the Figs. 4, 5 and 6. The chemical and isotopic compositions of middle and deep aquifers show a stable trend while those of shallow groundwater presented a similar trend to river water closed to river estuary, indicating the possibility of mixing between shallow groundwater and river water in this area. The spatial and seasonal variation of chemical and isotopic concentration of river water reveals effects of seawater intrusion in coastal river system with very high salinity along the coast in the dry season. The stability of groundwater chemistry and stable isotopes of (qp1, qp23) and (n21, n22, n13) aquifers might indicate a low-hydraulic connection between these aquifers and river water in this area.
4.3 Stable Isotopes Signatures
Stable isotopes of δ18O and δD have been widely used as useful signatures to understand hydrogeological processes such recharge, evaporation and mixing with different water sources that might significantly control groundwater quality [31, 55]. The δ18O, δD values of groundwater and surface samples were plotted in the conventional diagram (Fig. 7) with respect to the Meteoric Global Water Line [56] and the Local Meteoric Water Lines(LMWLs) of Cambodia [57] and An Long, Dong Thap province in the Mekong Delta. The isotopic values of groundwater samples among aquifers vary widely, ranging −7.92‰–−1.73‰ for δ18O, and −53.53‰–−8.66‰ for δD, which can be divided into three groups (G1, G2 and G3). Most of the groundwater samples from deep aquifers classifying into group G1 that is depleted in stable isotopes of oxygen-18 and detium, closing to river water in rainy season. The depletion of isotopic values in these deep aquifers can be explained by effects of ultrafiltration process on groundwater during passage via compacted clayey sediment layers [58] for a long period of time. This also suggests the complex processes of mixing between paleo-groundwater with different water sources during the period of aquifer formation. The variation in δ18O and δD values of surface water was larger than that of groundwater, indicating effects of seasonal variation on surface water than groundwater in this area with relatively light isotopic values of surface water (aveg. δ18O = −7.06‰, δD = −50.20‰) in the rainy season and high isotopic enrichments (aveg.δ18O = −5.40‰, δD = −40.15‰) in the dry season.
More surprisingly, most of the groundwater samples (group G1, G2 and G3) distributed consistently under the Local Meteoric Water Line of Cambodia, illustrating groundwater resource in the study might originate from Cambodia or upstream parts of the Mekong River Basin. This also suggests that local recharge may less contribute to these aquifers. These results were supported by recent research [45, 59].
Effect of evaporation on the physico-chemical characteristics of groundwater water is also confirmed by decreasing d-excess [60]. In general, groundwater has experienced to evaporation process but in different magnitudes. As shown in Fig. 8, groundwater at qp23 and qp1 aquifers show a strong impact of evaporation process while the shallow groundwater (qh, qp3 aquifers) presents a wide variation trend and have high isotopic values, suggesting the effects of both evaporation and seawater intrusion. The groundwater samples from deeper aquifers (n22, n21, n13) have low isotopic values but show a wide variation indicating different states of evaporation affecting on these aquifers.
4.4 Hydrogeochemical Processes
Mineral Dissolution Processes.
In the coastal aquifers, the mineral dissolution processes are frequently controlled groundwater quality [8, 61], therefore the PHREEQC version 3 model was used to calculate the Saturation Indices (SI). As shown in the Fig. 9a, most of the groundwater samples showed the trend of sub-saturation to oversaturation of calcite, dominating Ca2+ concentration, while some deep groundwater samples were only under saturated by dolomite dissolution. An increasing Ca2+ concentration, however, might result in calcite precipitation these aquifers. Additionally, groundwater quality in coastal aquifer could be strongly affected by gypsum and anhydrite dissolution due to the undersaturated status of gypsum and anhydrite dissolution (Fig. 9b). Deep groundwater samples show only undersaturation of gypsum dissolution, which suggests the fact that groundwater quality of these aquifers is also influenced by gypsum mineral dissolution. These processes might increasingly accelerate in the context of intensive groundwater extraction from these aquifers.
Seawater Intrusion.
Groundwater salinization is perhaps one of the most concern issues in many coastal regions in the world since it causes degradation of groundwater quality and threat to sustainability of groundwater use and management [62, 63]. In fact, the origin of salinity in coastal aquifers may be driven from different sources such as paleo-saline [64], seawater intrusion due to over pumping [65], wastewater and irrigation return flow [66]. In the Mekong delta, salt intrusion into aquifers is being major concerned due to excessive practices of groundwater extraction for drinking and irrigation. The earliest study on seawater intrusion in the Mekong Delta aquifers was conducted by Ho et al. [43]. They argue that shallow groundwater (qh, qp3 aquifers) was directly recharged by surface water and local precipitation and were intruded by seawater while deep aquifers had isolated each other and recharged by meteoric water from different altitudes. This was also confirmed by the recent research conducting in Dong Thap Province – a flooded plain of the Mekong Delta [59], in which canals and river water contributes dominantly to shallow groundwater but widely spatial variation while deep groundwater might be recharged by water driving from upstream of the Mekong River Basin. Until recently, however saline water in the Mekong Delta’s aquifer system is still poorly known [45].
In Soc Trang province, groundwater at shallow (qh, qp3) and some parts of deep aquifers (n22, n21, n13) has very high salinity (TDS > 1500 mg/L) that is unsuitable for both drinking and irrigation purposes. Meanwhile, groundwater samples from qp23 and qp1 aquifers are relatively high yield and low salinity concentration (TDS < 1000 mg/L), becoming the only freshwater choice for water supply system along the coast. However, some parts of these fresh aquifers close to the estuarial and coastal areas have relatively high salinity such as in the locations of T9 N, T10, T12, T14 samples, indicating the possibility of salt intrusion (see Fig. 10). An increase of salinity in these locations exhibits the impacts of saline diffusion from saline layers into fresh groundwater aquifers and/or an increase of halite dissolution due to over groundwater extraction for a long time. To classify groundwater salinization processes in coastal aquifers of the study area, a plot of stable isotopes and chlorite concentration was created shown in Fig. 10. It is obvious that groundwater samples from different aquifers represent variable hydrogeological processes. Most of the shallow groundwater and some deep groundwater samples, for example, distributes around mixing fresh-seawater line might indicate the influence of seawater intrusion, while the remaining groundwater samples from deeper aquifers (n21, n22, n13) display stably in the stable isotopes and relative increase of the Cl− values, exhibiting effects of paleo-saline water intrusion and halite rocks dissolution. More specifically, groundwater samples at qp23 and qp1 aquifers reveal two main tendencies. On the one hand, an increase of stable isotopes composition associated with a stability of Cl− values in almost all the groundwater samples might suggest the impacts of evaporation process during the paleo-recharge period or/and during groundwater flow paths. On the other hand, an increase of both stable isotopic oxygen-18 and Cl− concentration might attribute to saline water diffusion and halite-dissolution. These processes might be the main factors controlling the quality of groundwater responding to salinity.
5 Conclusion
The combination of chemical parameters, stable isotopes and geochemical reaction modeling was successfully applied to assess groundwater quality and its controlling factors in the coastal area of the Vietnamese Mekong Delta. The major finding of this study can be summarized as follows:
-
(1)
Groundwater in the study area is mainly classified into four groups: Na-Cl, Na-Mg-Ca-HCO3, Na-Mg-Ca-HCO3-SO4 and Na-HCO3-Cl.
-
(2)
Groundwater quality was strongly influenced by the rock-water interaction, particularly by calcite, dolomite, and gypsum and anhydrite dissolution.
-
(3)
The stable isotopes and saturation indices depict the origin of salt water presenting in the aquifers because of paleo-saline water dissolution at deeper aquifers, seawater intrusion into shallow aquifers and saltwater diffusion at Middle aquifers;
-
(4)
Groundwater in the study area might be mainly originated from upstream parts of the Mekong River Basin and experienced effects of evaporation before recharging into coastal aquifer system of the Mekong Delta;
-
(5)
An increase of salinity in some locations close to the coast might indicate the fact of modern seawater intrusion into coastal aquifers of the study site.
These findings suggest that its hydrogeological features might mainly control groundwater quality in the coastal aquifer system of the Mekong Delta. Additionally, the intensive groundwater extraction, uncontrolled drilling and unprotected unusable wells coupled with the severe seawater intrusion might potentially accelerate the deterioration of groundwater quality in the coastal aquifers of the Mekong Delta. Further study, therefore is needed to understand impacts of human activities and natural dynamic on the coastal aquifers of the Mekong Delta in both quality and quantity.
References
Wichelns, D.: Volumetric water footprints, applied in a global context, do not provide insight regarding water scarcity or water quality degradation. Ecol. Ind. 74, 420–426 (2017)
Liu, J., Liu, Q., Yang, H.: Assessing water scarcity by simultaneously considering environmental flow requirements, water quantity, and water quality. Ecol. Ind. 60, 434–441 (2016)
Aeschbach-Hertig, W., Gleeson, T.: Regional strategies for the accelerating global problem of groundwater depletion. Nat. Geosci. 5, 853–861 (2012)
Alaya, M.B., Saidi, S., Zemni, T., Zargouni, F.: Suitability assessment of deep groundwater for drinking and irrigation use in the Djeffara aquifers (Northern Gabes, south-eastern Tunisia). Environ. Earth Sci. 71, 3387–3421 (2014)
Zhang, R., Hu, S., Zhang, X., Yu, W.: Dissolution kinetics of dolomite in water at elevated temperatures. Aquat. Geochem. 13, 309–338 (2007)
Rosenthal, E., Zilberbrand, M., Livshitz, Y.: The hydrochemical evolution of brackish groundwater in central and northern Sinai (Egypt) and in the western Negev (Israel). J. Hydrol. 337, 294–314 (2007)
Wen, X., Diao, M., Wang, D., Gao, M.: Hydrochemical characteristics and salinization processes of groundwater in the shallow aquifer of Eastern Laizhou Bay, China. Hydrol. Process. 26, 2322–2332 (2012)
Singh, C.K., Kumar, A., Shashtri, S., Kumar, A., Kumar, P., Mallick, J.: Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. J. Geochem. Explor. 175, 59–71 (2017)
Kaltreider, R.C., Davis, A.M., Lariviere, J.P., Hamilton, J.W.: Arsenic alters the function of the glucocorticoid receptor as a transcription factor. Environ. Health Perspect. 109, 245–251 (2001)
Mandal, B.K., Suzuki, K.T.: Arsenic round the world: a review. Talanta 58, 201–235 (2002)
Bui Huy, T., Tuyet-Hanh, T.T., Johnston, R., Nguyen-Viet, H.: Assessing health risk due to exposure to arsenic in drinking water in Hanam Province, Vietnam. Int. J. Environ. Res. Public Health 11, 7575–7591 (2014)
Shankar, S., Shanker, U., Shikha: Arsenic contamination of groundwater: a review of sources, prevalence, health risks, and strategies for mitigation. Sci. World J. 2014, 18 (2014)
Jiang, J.-Q., Ashekuzzaman, S.M., Jiang, A., Sharifuzzaman, S.M., Chowdhury, S.R.: Arsenic contaminated groundwater and its treatment options in Bangladesh. Int. J. Environ. Res. Public Health 10, 18–46 (2013)
Singh, B., Sekhon, G.S.: Nitrate pollution of groundwater from nitrogen fertilizers and animal wastes in the Punjab, India. Agric. Environ. 3, 57–67 (1976)
Zhang, W.L., Tian, Z.X., Zhang, N., Li, X.Q.: Nitrate pollution of groundwater in northern China. Agr. Ecosyst. Environ. 59, 223–231 (1996)
Almasri, M.N.: Nitrate contamination of groundwater: a conceptual management framework. Environ. Impact Assess. Rev. 27, 220–242 (2007)
Vithanage, M., Mikunthan, T., Pathmarajah, S., Arasalingam, S., Manthrithilake, H.: Assessment of nitrate-N contamination in the Chunnakam aquifer system, Jaffna Peninsula, Sri Lanka. SpringerPlus 3, 271 (2014)
Zhang, Q., Sun, J., Liu, J., Huang, G., Lu, C., Zhang, Y.: Driving mechanism and sources of groundwater nitrate contamination in the rapidly urbanized region of south China. J. Contam. Hydrol. 182, 221–230 (2015)
Zhai, Y., Zhao, X., Teng, Y., Li, X., Zhang, J., Wu, J., Zuo, R.: Groundwater nitrate pollution and human health risk assessment by using HHRA model in an agricultural area, NE China. Ecotoxicol. Environ. Saf. 137, 130–142 (2017)
Vengosh, A.: 9.09 - Salinization and Saline Environments A2 - Holland, Heinrich D. In: Turekian, K.K. (ed.) Treatise on Geochemistry, pp. 1–35. Pergamon, Oxford (2003)
Abd-Elhamid, H.F., Javadi, A.A.: Impact of sea level rise and over-pumping on seawater intrusion in coastal aquifers. J. Water Clim. Chang. 2, 19–28 (2011)
Park, H.-Y., Jang, K., Ju, J.W., Yeo, I.W.: Hydrogeological characterization of seawater intrusion in tidally-forced coastal fractured bedrock aquifer. J. Hydrol. 446–447, 77–89 (2012)
Werner, A.D., Bakker, M., Post, V.E.A., Vandenbohede, A., Lu, C., Ataie-Ashtiani, B., Simmons, C.T., Barry, D.A.: Seawater intrusion processes, investigation and management: recent advances and future challenges. Adv. Water Resour. 51, 3–26 (2013)
De Filippis, G., Foglia, L., Giudici, M., Mehl, S., Margiotta, S., Negri, S.L.: Seawater intrusion in karstic, coastal aquifers: Current challenges and future scenarios in the Taranto area (southern Italy). Sci. Total Environ. 573, 1340–1351 (2016)
Mahlknecht, J., Merchán, D., Rosner, M., Meixner, A., Ledesma-Ruiz, R.: Assessing seawater intrusion in an arid coastal aquifer under high anthropogenic influence using major constituents, Sr and B isotopes in groundwater. Sci. Total Environ. 587–588, 282–295 (2017)
Ferguson, G., Gleeson, T.: Vulnerability of coastal aquifers to groundwater use and climate change. Nature Clim. Change 2, 342–345 (2012)
Russo, T.A., Lall, U.: Depletion and response of deep groundwater to climate-induced pumping variability. Nature Geosci 10, 105–108 (2017)
Gleeson, T., Wada, Y., Bierkens, M.F.P., van Beek, L.P.H.: Water balance of global aquifers revealed by groundwater footprint. Nature 488, 197–200 (2012)
Chae, G.-T., Yun, S.-T., Kim, K., Mayer, B.: Hydrogeochemistry of sodium-bicarbonate type bedrock groundwater in the Pocheon spa area, South Korea: water–rock interaction and hydrologic mixing. J. Hydrol. 321, 326–343 (2006)
Lorenzen, G., Sprenger, C., Baudron, P., Gupta, D., Pekdeger, A.: Origin and dynamics of groundwater salinity in the alluvial plains of western Delhi and adjacent territories of Haryana State, India. Hydrol. Process. 26, 2333–2345 (2012)
Slimani, R., Guendouz, A., Trolard, F., Moulla, A.S., Hamdi-Aïssa, B., Bourrié, G.: Identification of dominant hydrogeochemical processes for groundwaters in the Algerian Sahara supported by inverse modeling of chemical and isotopic data. Hydrol. Earth Syst. Sci. 21, 1669–1691 (2017)
Ben Moussa, A., Mzali, H., Zouari, K., Hezzi, H.: Hydrochemical and isotopic assessment of groundwater quality in the Quaternary shallow aquifer, Tazoghrane region, north-eastern Tunisia. Quatern. Int. 338, 51–58 (2014)
Mohammed, N., Celle-Jeanton, H., Huneau, F., Le Coustumer, P., Lavastre, V., Bertrand, G., Charrier, G., Clauzet, M.L.: Isotopic and geochemical identification of main groundwater supply sources to an alluvial aquifer, the Allier River valley (France). J. Hydrol. 508, 181–196 (2014)
Boschetti, T., González-Hernández, P., Hernández-Díaz, R., Naclerio, G., Celico, F.: Seawater intrusion in the Guanahacabibes Peninsula (Pinar del Rio Province, western Cuba): effects on karst development and water isotope composition. Environ. Earth Sci. 73, 5703–5719 (2015)
Lu, C., Xin, P., Li, L., Luo, J.: Seawater intrusion in response to sea-level rise in a coastal aquifer with a general-head inland boundary. J. Hydrol. 522, 135–140 (2015)
Arfib, B., Charlier, J.-B.: Insights into saline intrusion and freshwater resources in coastal karstic aquifers using a lumped Rainfall–Discharge–Salinity model (the Port-Miou brackish spring, SE France). J. Hydrol. 540, 148–161 (2016)
Mehdizadeh, S.S., Karamalipour, S.E., Asoodeh, R.: Sea level rise effect on seawater intrusion into layered coastal aquifers (simulation using dispersive and sharp-interface approaches). Ocean Coast. Manag. 138, 11–18 (2017)
Hornero, J., Manzano, M., Ortega, L., Custodio, E.: Integrating soil water and tracer balances, numerical modelling and GIS tools to estimate regional groundwater recharge: application to the Alcadozo aquifer system (SE Spain). Sci. Total Environ. 568, 415–432 (2016)
Minderhoud, P.S.J., Erkens, G., Pham, V.H., Bui, V.T., Erban, L., Kooi, H., Stouthamer, E.: Impacts of 25 years of groundwater extraction on subsidence in the Mekong Delta, Vietnam. Environ. Res. Lett. 12, 064006 (2017)
Benner, S.G., Polizzotto, M.L., Kocar, B.D., Ganguly, S., Phan, K., Ouch, K., Sampson, M., Fendorf, S.: Groundwater flow in an arsenic-contaminated aquifer, Mekong Delta, Cambodia. Appl. Geochem. 23, 3072–3087 (2008)
Merola, R.B., Hien, T.T., Quyen, D.T.T., Vengosh, A.: Arsenic exposure to drinking water in the Mekong Delta. Sci. Total Environ. 511, 544–552 (2015)
Stuckey, J.W., Schaefer, M.V., Kocar, B.D., Benner, S.G., Fendorf, S.: Arsenic release metabolically limited to permanently water-saturated soil in Mekong Delta. Nat. Geosci. 9, 70–76 (2016)
Ho, H.D., Aramyossy, J.F., Louvat, D., Huu, M.Q., Nguyen, T.V., Nguyen, K.C.: Environmental isotopes study related to the origin, salinization and movement of groundwater in the Mekong Delta (Vietnam). IAEA, UNESCO (1991)
Khoi, L.V., Chinh, N.K., Hung, D.T.: Groundwater salinity study in the Mekong Delta using isotope techniques. Commun. Phys. 1, 30–35 (2002)
An, T.D., Tsujimura, M., Le Phu, V., Kawachi, A., Ha, D.T.: Chemical characteristics of surface water and groundwater in coastal watershed, Mekong Delta, Vietnam. Procedia Environ. Sci. 20, 712–721 (2014)
Wagner, F., Tran, V.B., Renaud, F.G.: Groundwater resources in the Mekong Delta: availability, utilization and risks. In: Renaud, F., Kuenzer, C. (eds.) The Mekong Delta System: Interdisciplinary Analyses of a River Delta. Springer, Dordrecht (2010). Chap. 7
Slimani, R., Guendouz, A., Trolard, F., Moulla, A.S., Hamdi-Aissa, B., Bourrié, G.: Geochemical inverse modeling of chemical and isotopic data from groundwaters in Sahara (Ouargla Basin, Algeria). Hydrol. Earth Syst. Sci. Discuss. 2016, 1–49 (2016)
Indraratna, B., Sullivan, J., Nethery, A.: Effect of groundwater table on the formation of acid sulphate soils. Mine Water Environ. 14, 71–83 (1995)
Vahedian, A., Aghdaei, S.A., Mahini, S.: Acid sulphate soil interaction with groundwater: a remediation case study in East Trinity. APCBEE Procedia 9, 274–279 (2014)
Erban, L.E., Gorelick, S.M., Zebker, H.A., Fendorf, S.: Release of arsenic to deep groundwater in the Mekong Delta, Vietnam, linked to pumping-induced land subsidence. Proc. Natl. Acad. Sci. 110, 13751–13756 (2013)
Piper, A.M.: A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans. Am. Geophys. Union 25, 914–928 (1944)
Hoang, T.M., van Lap, N., Oanh, T.T.K., Jiro, T.: The influence of delta formation mechanism on geotechnical property sequence of the late Pleistocene-Holocene sediments in the Mekong River Delta. Heliyon 2, e00165 (2016)
Delsman, J.R., Hu-a-ng, K.R.M., Vos, P.C., de Louw, P.G.B., Oude Essink, G.H.P., Stuyfzand, P.J., Bierkens, M.F.P.: Paleo-modeling of coastal saltwater intrusion during the Holocene: an application to the Netherlands. Hydrol. Earth Syst. Sci. 18, 3891–3905 (2014)
Robinson, G., Ahmed, A.A., Hamill, G.A.: Experimental saltwater intrusion in coastal aquifers using automated image analysis: Applications to homogeneous aquifers. J. Hydrol. 538, 304–313 (2016)
West, A.G., February, E.C., Bowen, G.J.: Spatial analysis of hydrogen and oxygen stable isotopes (“isoscapes”) in ground water and tap water across South Africa. J. Geochem. Explor. 145, 213–222 (2014)
Craig, H.: Isotopic variations in meteoric waters. Science 133, 1702–1703 (1961)
Kabeya, N., Shimizu, A., Chann, S., Tsuboyama, Y., Nobuhiro, T., Keth, N., Tamai, K.: Stable isotope studies of rainfall and stream water in forest watersheds in Kampong Thom, Cambodia. In: Sawada, H., Araki, M., Chappell, N.A., LaFrankie, J.V., Shimizu, A. (eds.) Forest Environments in the Mekong River Basin, pp. 125–134. Springer, Tokyo (2007)
Coplen, T.B., Hanshaw, B.B.: Ultrafiltration by a compacted clay membrane—I. Oxygen and hydrogen isotopic fractionation. Geochim. Cosmochim. Acta 37, 2295–2310 (1973)
Thu, N.T.: Groundwater and surface water cycle system in Mekong Delta, Vietnam. Life and Environmental Sciences, p. 171. University of Tsukuba, Tsukuba (2017)
Tsujimura, M., Abe, Y., Tanaka, T., Shimada, J., Higuchi, S., Yamanaka, T., Davaa, G., Oyunbaatar, D.: Stable isotopic and geochemical characteristics of groundwater in Kherlen River Basin, a semi-arid region in eastern Mongolia. J. Hydrol. 333, 47–57 (2007)
Senthilkumar, S., Balasubramanian, N., Gowtham, B., Lawrence, J.F.: Geochemical signatures of groundwater in the coastal aquifers of Thiruvallur district, south India. Appl. Water Sci. 7, 263–274 (2017)
Wang, Y., Jiao, J.J.: Origin of groundwater salinity and hydrogeochemical processes in the confined Quaternary aquifer of the Pearl River Delta, China. J. Hydrol. 438–439, 112–124 (2012)
Li, C., Liu, T., Xu, S., Gao, X., Wang, Y.: Groundwater salinization in shallow aquifers adjacent to a low-altitude inland salt lake: a case study at Yuncheng Basin, northern China. Environ. Earth Sci. 75, 370 (2016)
Tijani, M.N.: Evolution of saline waters and brines in the Benue-Trough, Nigeria. Appl. Geochem. 19, 1355–1365 (2004)
Kim, Y., Lee, K.-S., Koh, D.-C., Lee, D.-H., Lee, S.-G., Park, W.-B., Koh, G.-W., Woo, N.-C.: Hydrogeochemical and isotopic evidence of groundwater salinization in a coastal aquifer: a case study in Jeju volcanic island, Korea. J. Hydrol. 270, 282–294 (2003)
Ghabayen, S.M.S., McKee, M., Kemblowski, M.: Ionic and isotopic ratios for identification of salinity sources and missing data in the Gaza aquifer. J. Hydrol. 318, 360–373 (2006)
Acknowledgments
The authors would like to express their gratefulness to University of Tsukuba, Japan for providing necessary facilities for this research. We also would like to thank the Thuyloi University, Department of Natural Resources and Environment of Soc Trang Province, Vietnam for their support during the field surveys between 2013 and 2014. Our thankfulness also is extended to Dr. Bui Tran Vuong, Vice Director of the Division for Water Resources and Planning for South Vietnam, and Mr. Nguyen Van Chanh, Mr. Thach Hoang Linh the hydro-geological specialists in Soc Trang province for their kind supports. Particularly, we would like to address special thanks to Japanese Grant Aid for Human Resources Development Scholarship (JDS program) and MEXT scholarship for supporting successful completion of this study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
An, T.D., Tsujimura, M., Phu, V.L., Ha, D.T., Hai, N.V. (2018). Isotopic and Hydrogeochemical Signatures in Evaluating Groundwater Quality in the Coastal Area of the Mekong Delta, Vietnam. In: Tien Bui, D., Ngoc Do, A., Bui, HB., Hoang, ND. (eds) Advances and Applications in Geospatial Technology and Earth Resources. GTER 2017. Springer, Cham. https://doi.org/10.1007/978-3-319-68240-2_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-68240-2_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68239-6
Online ISBN: 978-3-319-68240-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)