Abstract
The aim of dietary interventions in weight management is to contribute to an energy deficit. A number of studies have demonstrated that interventions that included a dietary component are efficacious in weight loss and are associated with improved cardio-metabolic outcomes. However, the optimal diet for achieving an energy deficit in children and adolescents is unknown. In this chapter we explore conventional and novel dietary approaches including increased-protein, very-low carbohydrate, low glycaemic index and very low-energy diets as well as intermittent fasting. Current evidence suggests adherence to an energy restricted diet, rather than macronutrient content, is the most effective method for long-term weight loss. Differing dietary strategies maybe used to support different outcomes, including improved short-term weight loss and improved cardio-metabolic risk factors.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Paediatric obesity is a major worldwide health issue. Prevention of obesity in childhood and adolescence is important. However, effective treatments for those already affected are needed to reduce the impacts of obesity on their physical, psychological and social development. Obesity management requires a coordinated model of care to deliver a sustained result. Table 16.1 outlines the key principles of obesity management in children and adolescents. The underlying principle of obesity treatment is to focus on changes in behaviours, including diet and physical activity, which influence body weight and adiposity. In this chapter, we will focus on the role of dietary intervention in the treatment of paediatric obesity.
Efficacy of Dietary Interventions
The aim of dietary intervention in weight management is to contribute to an energy deficit. Numerous randomised controlled trials (RCTs) and meta-analyses have demonstrated that interventions that included a dietary component were efficacious in weight loss at least in the short to medium term in children and adolescents [1,2,3]. The latest systematic review and meta-analyses by Ho and colleagues [1], which included 38 studies, estimated that the effect size for lifestyle interventions with a dietary component was a decrease in body mass index (BMI) of 1.30 kg/m2 [95% CI, 1.03–1.58] and a decrease in total body fat of 3.2% [95% CI, 1.39–5.01] at the end of active intervention (range 3–12 months) compared to usual care or minimal intervention. This review also demonstrated that the weight loss effect was sustained after programme completion and that studies with an intervention period longer than 6 months had a greater weight loss than shorter-term interventions [1]. Inclusion of an exercise programme did not improve weight loss, but those who received an exercise intervention in addition to a dietary component had a greater increase in lean body mass and a greater reduction in total fat compared to those who received a dietary component alone [4].
Structured lifestyle intervention programmes incorporating a dietary component have also been associated with improved cardio-metabolic outcomes. A meta-analysis of five RCTs including 440 participants aged between 8 and 16 years showed that structured lifestyle interventions incorporating a dietary component resulted in significant improvements in total cholesterol [weighed mean difference 0.28 mmol/L (10.81 mg/dL); 95% CI, 0.23–0.34] and triglycerides [0.15 mmol/L (13.29 mg/dL); 95% CI, 0.01–0.24] up to 2 years from baseline, as well as improvements in fasting insulin [55.1ρmol/L (7.93 uU/mL); 95% CI, 39.1–71.2] and homeostatic model assessment of insulin resistance (HOMA-IR, 2.32; 95% CI, 1.39–3.25) up to 1 year from baseline [4]. However, the impact of lifestyle interventions on blood pressure is less certain, and long-term data are limited. Importantly, evidence suggests that lifestyle interventions that included a dietary component lead to improvement in cardio-metabolic outcomes in children and adolescents with obesity even in the absence of weight loss or body composition change [1, 2]. Including an exercise component to the intervention can lead to greater improvements in high-density lipoprotein, fasting glucose and fasting insulin levels, but not total cholesterol or triglycerides (Table 16.2) [4]. Nevertheless, partial weight regain and subsequent regression in cardio-metabolic profiles to baseline levels are common in lifestyle intervention programmes at follow-up [4]. Longer-term studies are warranted to examine if the improvements in cardio-metabolic profile that resulted from obesity treatment in childhood will prevent the development of obesity-associated cardiovascular co-morbidities in adulthood.
Dietary Interventions
For many children and adolescents who are overweight, dietary interventions are frequently aimed at weight maintenance that is to slow or prevent weight gain rather than weight loss. However, given the high prevalence of obesity, the increasing prevalence of severe obesity and co-morbidities and the high number of youth who are heavier than their ideal adult body weight, dietary interventions for weight loss are frequently indicated. While the appropriateness of weight loss can be contentious due to proposed physiological and psychological impacts, there is no evidence to suggest that adiposity loss in children and adolescents with obesity causes harm.
The optimal diet for achieving an energy deficit in children and adolescents with obesity is unknown. Intervention trials have examined a number of different dietary strategies with the general agreement that adherence to energy restriction remains the most effective for weight loss [5]. However, achieving long-term adherence to energy restriction is challenging, and it is unlikely that one dietary strategy will fit all. In addition, there is potential for the specific application of certain dietary strategies to lead to greater short-term weight loss and target particular cardio-metabolic risk factors [5]. It is also essential that treatment options are acceptable to both the child/adolescent and their parents/caregivers. Below we explore some conventional and novel dietary approaches.
Conventional Approaches
Currently, the most frequently reported dietary interventions for weight management in dietary intervention trials are healthy eating advice, which is low in fat, based on dietary guidelines or the Traffic Light/Stop Light diet [1, 4, 6].
Healthy Eating Advice Based on Dietary Guidelines
Food-based dietary guidelines provide advice on foods and food groups to provide the required nutrients to promote overall health and prevent chronic diseases. In many countries, including Australia, the USA and the UK, current guidelines recommend a diet that is high in carbohydrate (45–65% of daily energy) and low in fat (less than 35% of daily energy), with protein contributing approximately 15% of daily energy. This diet is often supported by food guides, in the form of food pyramids and food plates (e.g. Fig. 16.1), which are used for consumer education. It is recommended that these guidelines be adopted at a population level [7, 8], including for the treatment of child and adolescent obesity.
Choose MyPlate . MyPlate was developed by the United States Department of Agriculture to assist individuals create healthier eating styles (Source: https://www.choosemyplate.gov. US Department of Agriculture. Public Domain)
A diet based on the dietary guidelines may also be energy restricted. Determining the appropriate energy targets for weight loss can be challenging, needs to be assessed on an individual basis and will depend on a number of factors including age, sex, physical activity levels, co-morbidities and the speed of weight loss required. Dietary intervention trials involving an energy-restricted diet are often based on either a standardised daily kilocalorie (kcal) deficit of 300–500 kcal, 30% less than the reported energy intake or 15% less than the estimated energy requirement [1]. Some studies impose energy restrictions on snacks and beverages only. However, if energy restriction is indicated, a diet based on dietary guidelines can present challenges in achieving micronutrient adequacy, particularly essential fatty acids, and careful selection of foods is required [9]. This diet is generally successful in achieving weight loss in the short term in children and adolescents [1]. In adults, a 2015 systematic review and meta-analysis suggested that these diets are equally as effective as other dietary interventions, including low carbohydrate and higher fat, of similar intensity in the long term [10].
Traffic Light/Stop Light
The Traffic Light/Stop Light diet is an energy-controlled approach. Foods in each category are colour coded according to their caloric densities per average serving: green for low-calorie foods that can be eaten freely, yellow for moderate-calorie foods that can be eaten occasionally and red for high-calorie foods that should be eaten rarely. This diet was one of the original diets to treat childhood obesity as well as used in intervention trials [6]. Similar to a diet based on dietary guidelines, this diet is successful in achieving modest weight loss in the short term in children and adolescents [1].
Novel Approaches
While there is evidence to support conventional dietary interventions short term, their effectiveness in sustaining long-term changes remains uncertain. Hence, there has been recent interest in exploring different dietary strategies that optimise weight loss as well as improve cardio-metabolic outcomes. These strategies include modifying the macronutrient content and/or the quality of carbohydrate, as well as novel dietary approaches to restrict energy intake.
Increased-Protein Diet
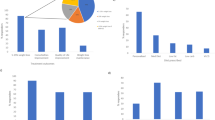
An increased-protein diet typically aims for approximately 26–44% of daily energy as carbohydrate, <35% as fat and 20–40% as protein [11]. These diets are hypothesised to lead to greater weight loss by evoking (a) sustained satiety despite reduced energy intake, (b) sustained energy expenditure despite loss in body mass by sparing loss of fat-free mass and (c) increased dietary-induced thermogenesis, because the thermal effect of protein is greater than that of carbohydrate or fat [12]. In children and adolescents, there have been seven studies, ranging in duration from 1 to 12 months, comparing isocaloric increased-protein (mean 24.4 ± 3.9% of energy) and standard-protein (mean 16.3 ± 2.1% of energy) diets [5, 13]. The studies included boys and girls, aged 7–18 years, with a mean sample size of 110 participants [5]. All studies found improved weight, fasting glucose, fasting insulin, insulin sensitivity, blood lipids and blood pressure irrespective of diet group and irrespective of whether participants were free-living or in highly controlled environments (boarding school or camp). Figure 16.2d shows the 12-month change in glycaemic status and body composition measures from one of the included RCTs undertaken by Garnett and colleagues [14]. This study specifically targeted adolescents with insulin resistance and/or prediabetes and randomised 111 participants to a hypocaloric diet, which was either increased protein (25–30% of energy) or standard protein (15% protein). The authors reported no significant differences between diet groups at any time point. The authors concluded, as did the systematic review and a later similar study by Truby et al. [13], that the protein content of the diet appears to have little effect when given isocalorically. The results emphasise the importance of total energy intake for improved weight status in children and adolescents with obesity.
(a–d) Glycaemic status and body composition measures by dietary group over the 12-month intervention. Estimated marginal means (SE) are presented from linear mixed models for the increased-protein diet group (downward triangle) and the high-carbohydrate diet group (upward triangle). Differences between diet groups were not significant at any time period. (a) Insulin sensitivity index. 1Significance between baseline and 3 months and 12 months as indicated. 2Significance between 3 and 12 months. (b) Total body fat percent (Fat % DXA). 1Significance between baseline and 3 months and 12 months as indicated. 2Significance between 3 and 12 months. (c) Fat-free mass index. 1Significance between baseline and 3 months and 12 months as indicated. 2Significance between 3 and 12 months. (d) BMI%95th centile. 1Significance between baseline and 3 months, 6 months and 12 months as indicated. 3Significance between 3 and 6 months.4Significance between 6 and 12 months (Source: Garnett SP, Gow ML, Ho M, Baur LA, Noakes M, Woodhead HJ, Broderick CR, Chisholm K, Briody J, De S, et al. Improved insulin sensitivity and body composition, irrespective of macronutrient intake, after a 12 month intervention in adolescents with pre-diabetes; RESIST a randomised control trial. BMC Pediatrics. 2014;14:289)
In adults, results from several trials have indicated that increased-protein diets increased fat loss, attenuated reductions in fat-free mass and improved an array of cardio-metabolic factors, including glucose homeostasis and the blood lipid profile compared to standard-protein diets [15]. However, the effects are not consistently reported. A recent systematic review that compared energy-restricted, isocaloric, high-protein (mean 30.5 ± 2.4% of energy) with a standard-protein (mean 17.5 ± 1.5% of energy) diet concluded that the high-protein diet provided modest benefits for reductions in body weight (0.79 kg; 95% CI, 1.50-0.08 kg), fat mass (0.87 kg; 95% CI, 1.26-0.48 kg) and triglycerides [0.23 mmol/L (8.9 mg/dL); 95% CI, 0.33-0.12 mmol/L], with better preservation of lean body mass. Changes in fasting plasma glucose, fasting insulin, blood pressure, total cholesterol and low- and high-density cholesterol were similar across dietary treatments [15].
It is not clear why the protein effect is not seen in children and adolescents. Achieving dietary protein targets in studies of free-living children and adolescents is difficult, and in part the lack of difference may be due to the reported lower protein intake in youth [5, 16]. In addition, some will contend that RCTs are not the ideal study design to determine the effect of dietary intervention. The isocaloric nature of experimental diets may blunt the satiating effect of protein thought to contribute to a lower ad libitum total energy intake and consequent improved weight loss as reported in some adult studies [12, 17].
Very Low-Carbohydrate Diet
A popular alternative to the low-fat diet is a very low-carbohydrate diet, typically aiming for <50 g carbohydrate per day with high or ad libitum fat and/or protein intake (e.g. the Atkins diet) (Table 16.3). A recent systematic review suggested that a very low-carbohydrate diet may result in greater weight loss (a mean decrease in BMI of 1.46; 95% CI, 0.44–2.48 and a mean decrease in BMI z-score 0.25; 95% CI, 0.06–0.44) immediately following active treatment (10–26 weeks) compared with a low-fat diet [5]. However, the difference between diet groups was not maintained at the 2-year follow-up, nor was the difference observed in the larger, higher methodological quality studies [18, 19]. The current evidence suggests that a very low-carbohydrate diet may lead to greater short-term weight loss and may be useful when indicated, for example, in severe obesity prior to surgery.
In terms of cardio-metabolic outcomes, very low-carbohydrate diets reportedly improve insulin levels and/or insulin resistance compared with a low-fat diet immediately following active treatment [18, 20] and at follow-up [5]. These findings suggest that a very low-carbohydrate diet as part of obesity treatment may facilitate improvements in hyperinsulinemia compared with a traditional low-fat approach in children and adolescents. Furthermore, a very low-carbohydrate diet may also facilitate improved body composition [21] and/or triglycerides but may increase LDL cholesterol levels [18, 22]. In adults with type 2 diabetes, a very low-carbohydrate diet may improve glucose status and lipid profile when compared to a high-carbohydrate diet (>200 g/day). To date, there is insufficient evidence for use of very low-carbohydrate diets in children and adolescents with diabetes [23].
There have been no reported adverse effects on cardio-metabolic profile in association with following a very low-carbohydrate diet in children and adolescents, suggesting the short-term safety of the diet. The long-term safety of very low-carbohydrate diets in children and adolescents is unknown. One concern is that restricting carbohydrate in the diet, without a sufficient increase in vegetable consumption, reduces the intake of nutrients obtained from high-quality carbohydrates, particularly fibre and phytochemicals [24]. An increased feeling of fatigue has also been a reported side effect of following a very low-carbohydrate diet in adults [25]. This could result in a reduced desire to complete physical activity in youth with overweight or obesity and should be considered in an individual who is, or plans to be, very active as part of their weight loss regimen.
Low Glycaemic Index/Glycaemic Load
A diet that has a lower glycaemic index (GI) generally refers to a balanced diet that incorporates carbohydrate foods that have reduced glycaemic load (GL), i.e. foods/meals that produce a slower rise in blood glucose levels and have lower overall carbohydrate content [26]. However, consumption of lower-GI foods does not necessarily translate to a “healthy” diet, with some discretionary foods such as ice cream, cakes and potato crisps having a low GI. In children and adolescents, a 2015 systematic review that included nine RCTs with a duration between 10 and 96 weeks and a total of 1065 children and adolescents found that lower-GI/GL diets produced greater improvements in insulin resistance (HOMA-IR mean difference 0.70; 95% CI, 0.04–1.37) and triglyceride levels (mean difference 0.17 mmol/L; 95% CI, 0.05–0.30) compared to higher-GI/GL diets [27]. However, there was no beneficial effect for weight loss or cholesterol levels (total, HDL or LDL). Similar findings have been reported in young adults with obesity [28].
The DiOGenes study is the largest study conducted to date which examined the effect of varying the GI and protein content of the diet on weight and cardio-metabolic outcomes in children, recruiting families from eight European countries. Eligible parents were randomised as a family unit to one of five ad libitum diets: low-protein and low-GI, low-protein and high-GI, high-protein and low-GI, high-protein and high-GI and control diet (national dietary guidelines, medium protein content and no instructions on GI) [29]. A difference of 15 GI units between the higher-GI and lower-GI diets was targeted. The results of this study showed that neither GI nor protein had an isolated effect on body composition among children following an ad libitum diet. However, the low-protein, high-GI combination increased body fat, whereas the high-protein, low-GI combination was protective against obesity [30]. It is important to recognise that the children in the DiOGenes study were not necessarily overweight; they were the children of parents who were overweight or obese. The children were not given advice on weight loss but were educated on the diet’s ability to regulate appetite [30].
Very Low-Energy Diet
A very low-energy diet (VLED) is a dietary approach that has gained popularity due to its association with rapid weight loss. It is a very strict diet aiming for <800 kcal/day. VLEDs are largely protein based and contain essential fatty acids, vitamins and minerals but very little carbohydrates (typically <50 g) and are aimed at inducing ketosis [31]. They reduce portion size and, consequently, energy intake. Because a VLED is difficult to follow, it is usually implemented short term, aiming for rapid weight loss, and comprised of meal replacement products (e.g. shakes, bars, soups, desserts) to achieve nutritional adequacy.
Studies in adolescents with obesity have demonstrated that a VLED can safely induce rapid weight loss in the short term (4–15 kg over 3–12 weeks) while preserving lean body mass [21, 32,33,34]. Studies have also demonstrated improvements in blood pressure, total cholesterol, LDL and HDL cholesterol, triglycerides, fasting glucose, fasting insulin, HbA1c and insulin sensitivity [21, 32, 33]. One of these studies found that a short-term (10 weeks) daily VLED (600–800 kcal/day) compared with a hypocaloric low-fat diet produced significantly greater reductions in percentage body fat while maintaining lean body mass [21]. In another study comparing a VLED with a hypocaloric low-fat diet, weight loss was greater in the VLED group at 4 months, but this was not sustained at 12 months [32]. A recent pilot study investigated the effects of an 8-week VLED in adolescents with type 2 diabetes [34]. Rapid weight loss was achieved, and 6 months after the VLED intervention, four of the five participants who adhered to the diet had reversal of type 2 diabetes. These results highlight the potential benefit of following a VLED beyond weight loss [34].
Overall VLEDs are tolerated by adolescents and result in rapid weight loss, improvements in body composition and improved metabolic risk profile short term, but long-term outcomes are not clear. In adults the metabolic benefits of bariatric surgery have been reproduced by VLED [35]. The diet, although strict, may be an alternative to pharmacological therapies or surgical interventions to treat adolescents with severe obesity. VLEDs require intensive monitoring by a team of health professionals.
Intermittent Fasting
Intermittent fasting is also known as “intermittent energy restriction” and “alternative day fasting”. This diet has been popularised as the 5:2 diet and has gained much media interest and celebrity endorsement. Intermittent fasting includes 1–4 “fasting” (or VLED) days per week, where energy intake is drastically limited (typically less than 600 kcal), and 3–6 “feeding” days per week, where food is either consumed ad libitum or a diet based on healthy eating guidelines. It is speculated that intermittent fasting comprising of shorter periods of energy restriction coupled with longer periods of habitual energy intake may be more sustainable and promote better adherence than continuous daily energy restriction [36]. To date, studies examining the effectiveness of intermittent fasting have not been conducted in children or adolescents.
In 2016 there were two systematic reviews of adult trials comparing intermittent fasting with daily energy restrictions [37, 38]. While there were conflicting results from individual studies on which group achieved the greatest weight loss, overall both reviews indicated that intermittent fasting is as equally effective as daily energy restriction in the short term (5 weeks to 6 months) and long term (12–18 months) to help individuals with obesity decrease weight (4–8%) and body fat and reduce cardio-metabolic risk [37, 38]. Measures of compliance were limited, but one study indicated that short-term compliance for the intermittent fasting group was effective but not in the long term [39]. A greater number of adverse effects were also experienced in the intermittent fasting group which included headache, lack of energy and difficulty fitting the diet into their daily routine. Trials are ongoing in children and adolescents.
Potential Risks of Dietary Interventions
Disordered Eating and/or Eating Disorders
A concern about using dietary energy restrictions in obesity treatment is the potential risk of developing or exacerbating disordered eating and/or eating disorders. Overweight children are more likely to have dysfunctional eating behaviours, including emotional eating and restrained eating, compared to normal-weight children [40, 41], and overweight adolescents are more likely to engage in disordered eating, such as binge eating [42]. The RESIST study examined the impact of using a prescriptive hypocaloric meal plan (500 kcal less than the recommended daily energy intake) on the psychological dimensions of eating behaviours in over 100 adolescents with obesity [43]. The results of the RESIST study showed that a prescriptive dietary approach led to significant reductions in dysfunctional eating behaviours, particularly external and emotional eating, and the intervention did not elicit any adverse effects on dietary restraint in adolescents with obesity. Evidence from an earlier systematic review also supports the view that professionally administered paediatric weight loss interventions do not increase the risk of eating disorders and may, indeed, improve psychological wellbeing in adolescents with obesity [44]. Furthermore, there is evidence that adolescents seeking weight management have a preference for prescriptive dietary advice, as opposed to unstructured advice [43, 45]. In adults, a recent systematic review of the safety of severe dietary energy restriction (430 to 1200 kcal/day) in overweight and obese adults reported that clinically supervised programmes do not necessarily trigger binge eating in overweight individuals without pre-existing binge eating disorder [46]. On the contrary, severe dietary energy restriction does not exacerbate and may even reduce binge eating in overweight individuals with pre-existing subclinical binge eating or binge eating disorder. These findings indicate that dietary interventions are generally safe when they are run by professional teams. In view of the association between obesity and disordered eating, it is recommended that eating behaviour should be monitored during, and preferably also after, obesity treatment.
Nutritional Adequacy of Reduced Energy Diets
Children and adolescents have unique and differing nutrition requirements, including calcium, iron and zinc, and energy-restricted diets reduce the opportunity to meet micronutrient requirements by limiting overall food intake. There is a paucity of data on the effect of energy restriction on nutritional adequacy. There is one recent paper which has examined the nutritional adequacy of three energy-restricted diets which are utilised in clinical practice for adolescents with obesity: a diet based on dietary guidelines, a modified-carbohydrate diet and an intermittent fasting diet [9]. In this paper the authors undertook dietary modelling and demonstrated that these eating patterns can be adapted to achieve nutritional adequacy and energy restriction; however, they did need careful consideration to meet nutritional requirements of adolescents.
Reduced Resting Metabolic Rate and Lean Body Mass
Concerns have been expressed that dietary restrictions used in obesity treatment in children and adolescents may adversely decrease resting metabolic rate and lean body mass [47]. Results from a systematic review which included five RCTs comparing diet-only interventions with a diet-plus-exercise interventions reported that an energy-restricted diet (900 to 1400 kcal/day) along with a moderate protein content (20–30%) did not result in a loss of lean body mass in 6- to 18-year-olds over 4 months [4]. Loss of lean body mass was reported in one small study (n = 38) in which children with obesity (8–12 years old) were prescribed an 1800 kcal high-carbohydrate low-fat diet (65% total energy from carbohydrate,15% from protein and 20% from fat) and participated in supervised exercise training three times/week (60 min/session) [48]. Participants lost 1.3 kg [95% CI, -2.01–0.59] in lean body mass over 4 months. Overall these findings suggest that for lifestyle interventions with an exercise component, diets with an increased protein content may protect against the loss of lean body mass.
Conclusion
The current evidence indicates that an improvement in weight status can be achieved in children and adolescents with obesity, irrespective of the dietary macronutrient profile or dietary pattern, provided the diet is energy reduced. This suggests that the primary objective of dietary interventions should be to reduce total energy intake. However, there is some inconsistent evidence from child and adolescent obesity treatment intervention studies to suggest that very low-carbohydrate diets, VLEDs and lower-GI diets may achieve greater weight loss compared with a more traditional low-fat diet, at least in the short term. These diets also appear to have advantages over a low-fat diet for improving cardio-metabolic risk profile. A common feature of these diets is that they ultimately reduce glycaemic load by modifying diet carbohydrate quality or quantity compared with the traditional low-fat approach. These dietary options give clinicians multiple diet strategies to offer children and adolescents, which may assist in achieving greater weight loss compared with a low-fat approach. Hence, diets may be personalised depending on patient preference and suitability.
Editor’s Comments and Question
Social media and mainline newspapers are awash with testimonials “demonstrating” that obesity and its complications are caused by discrete macronutrients such as sugar, fructose or saturated fat. However, as discussed in Chap. 1, it is the patterns of dietary intake, rather than the intake of single macronutrients, that are the principal determinants of childhood weight gain. Diets that are high in energy density, fat and sugar and low in fibre, fruits and vegetables are associated with higher percent body fat and excess adiposity in childhood and adolescence. Total caloric intake, as you note, is critically important.
It is not surprising, then, that reductions in any single macronutrient have had little or no effect on body mass index in obese childrena,b,c and that long-term weight loss in obese adults is determined by total energy intake, rather than the specific macronutrient content of the dietd,e,f. Moreover, the effect of any single macronutrient on metabolic risk (e.g. type 2 diabetes mellitus) pales in comparison to the effect of obesity itselfg.
Common-sense approaches to diet can reduce weight in obese children and prevent weight gain in children at risk: in my experience, nearly anyone can lose weight by eliminating sugar drinks and reducing intake of fried and fast foods and high-density starches. The trick is actually doing it.
Where do you place your focus in promoting weight loss in obese children?
Authors’ Response
The focus of our approach to weight loss in children is dependent upon a number of factors including the age of the child, the amount of weight loss required and the presence or absence of co-morbidities.
For the younger child, the focus of treatment is the family, and generally we aim at simple changes as you have indicated above. We encourage drinking water and limit the intake of fruit juice, cordial and soft drink. We encourage the use of low-fat dairy food types for children over the age of 2 years instead of full cream and encourage the consumption of fruits and vegetables. We also consider it important to eat breakfast and to sit down and enjoy meals together as a family with the television switched off.
In our adolescent clinic, where many of the adolescents present greater than their ideal adult weight with several co-morbidities, we aim for weight loss. We offer a number of the weight loss strategies as discussed in this chapter. Diets are personalised depending on patient preference and suitability. Adolescents are allowed to change strategy; we are acutely aware that if there is no weight loss in the first 3 months of treatment, a different strategy is indicated h .
Our primary focus for adolescents with obesity and type 2 diabetes is rapid weight loss. We recognise the severity of the condition including increased morbidity and mortality. The traditional approach has been the management of blood sugar levels, but results from our recent study have indicated that it is possible to reverse the pathology of type 2 diabetes with a very low-energy diet in adolescents as seen in adult studiesi. We see this as a first-line treatment option in newly diagnosed youth.
References for Editor’s Comments and Question and Authors’ Response Sections
-
(a)
Kirk S, Brehm B, Saelens BE, Woo JG, Kissel E, D’Alessio D, Bolling C, Daniels SR. Role of carbohydrate modification in weight management among obese children: a randomized clinical trial. J Pediatr. 2012;161(2):320–7.
-
(b)
Casazza K, Cardel M, Dulin-Keita A, Hanks LJ, Gower BA, Newton AL, Wallace S. Reduced carbohydrate diet to improve metabolic outcomes and decrease adiposity in obese peripubertal African American girls. J Pediatr Gastroenterol Nutr. 2012;54(3):336–42.
-
(c)
Mirza NM, Palmer MG, Sinclair KB, McCarter R, He J, Ebbeling CB, Ludwig DS, Yanovski JA. Effects of a low glycemic load or a low-fat dietary intervention on body weight in obese Hispanic American children and adolescents: a randomized controlled trial. Am J Clin Nutr. 2013;97(2):276–85.
-
(d)
Atallah R, Filion KB, Wakil SM, Genest J, Joseph L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. Long-term effects of 4 popular diets on weight loss and cardiovascular risk factors: a systematic review of randomized controlled trials. Circ Cardiovasc Qual Outcomes. 2014;7(6):815–27.
-
(e)
Naude CE, Schoonees A, Senekal M, Young T, Garner P, Volmink J. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: a systematic review and meta-analysis. PLoS One. 2014;9(7):e100652.
-
(f)
Mancini JG, Filion KB, Atallah R, Eisenberg MJ. Systematic Review of the Mediterranean Diet for Long-Term Weight Loss. Am J Med. 2016;129(4):407–415.
-
(g)
Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–7.
-
(h)
Gow ML, Baur LA, Ho M, Chisholm K, Noakes M, Cowell CT, Garnett SP. Can early weight loss, eating behaviors and socioeconomic factors predict successful weight loss at 12-and 24-months in adolescents with obesity and insulin resistance participating in a randomised controlled trial? Int J Behav Nutr Phys Act. 2016;13(1):43.
-
(i)
Gow ML, Baur LA, Johnson NA, Cowell CT, Garnett SP. Reversal of type 2 diabetes in youth who adhere to a very-low-energy diet: a pilot study. Diabetologia. 2017;60(3):406–415.
References
Ho M, Garnett SP, Baur L, Burrows T, Stewart L, Neve M, Collins CE. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics. 2012;130(6):e1647–e71.
Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury V, O’Malley C, Stolk R, Summerbell C. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;21(1):CD001872.
McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, Erwin PJ, Montori VM. Treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clin Endocrinol Metab. 2008;93(12):4600–5.
Ho M, Garnett SP, Baur LA, Burrows T, Stewart L, Neve M, Collins C. Impact of dietary and exercise interventions on weight change and metabolic outcomes in obese children and adolescents: a systematic review and meta-analysis of randomized trials. JAMA Pediatr. 2013;167(8):759–68.
Gow ML, Ho M, Burrows TL, Baur LA, Stewart L, Hutchesson MJ, Cowell CT, Collins CE, Garnett SP. Impact of dietary macronutrient distribution on BMI and cardiometabolic outcomes in overweight and obese children and adolescents: a systematic review. Nutr Rev. 2014;72(7):453–70.
Epstein LH, Myers MD, Raynor HA, Saelens BE. Treatment of pediatric obesity. Pediatrics. 1998;101(3 Pt 2):554–70.
National Health and Medical Research. Australian dietary guidelines; 2013. https://www.nhmrc.gov.au/guidelines-publications/n55.
Office for Disease Prevention and Health Promotion. 2015–2020 Dietary Guidelines for Americans. https://health.gov/dietaryguidelines/2015/.
Lister NB, Gow ML, Chisholm K, Grunseita A, Garnett SP, Baur LA. Nutritional adequacy of diets for adolescents with overweight and obesity: considerations for dietetic practice. Eur J Clin Nutr. 2017 May;71(5):646–51.
Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(12):968–79.
Gow ML, Garnett SP, Baur LA, Lister NB. The effectiveness of different diet strategies to reduce type 2 diabetes risk in youth. Forum Nutr. 2016;8(8):486.
Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein - its role in satiety, energetics, weight loss and health. Br J Nutr. 2012;108(Suppl 2):S105–12.
Truby H, Baxter K, Ware RS, Jensen DE, Cardinal JW, Warren JM, Daniels L, Davies PS, Barrett P, Blumfield ML, et al. A randomized controlled trial of two different macronutrient profiles on weight, body composition and metabolic parameters in obese adolescents seeking weight loss. PLoS One. 2016;11(3):e0151787.
Garnett SP, Gow ML, Ho M, Baur LA, Noakes M, Woodhead HJ, Broderick CR, Chisholm K, Briody J, De S, et al. Improved insulin sensitivity and body composition, irrespective of macronutrient intake, after a 12 month intervention in adolescents with pre-diabetes; RESIST a randomised control trial. BMC Pediatr. 2014;14:289.
Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(6):1281–98.
Garnett SP, Gow M, Ho M, Baur LA, Noakes M, Woodhead HJ, Broderick CR, Burrell S, Chisholm K, Halim J, et al. Optimal macronutrient content of the diet for adolescents with prediabetes; RESIST a randomised control trial. J Clin Endocrinol Metab. 2013;98(5):2116–25.
Astrup A, Raben A, Geiker N. The role of higher protein diets in weight control and obesity-related comorbidities. Int J Obes. 2015;39(5):721–6.
Krebs NF, Gao D, Gralla J, Collins JS, Johnson SL. Efficacy and safety of a high protein, low carbohydrate diet for weight loss in severely obese adolescents. J Pediatr. 2010;157(2):252–8.
Partsalaki I, Karvela A, Spiliotis BE. Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J Pediatr Endocrinol Metab. 2012;25(7–8):697–704.
Kirk S, Brehm B, Saelens BE, Woo JG, Kissel E, D’Alessio D, Bolling C, Daniels SR. Role of carbohydrate modification in weight management among obese children: a randomized clinical trial. J Pediatr. 2012;161(2):320–7.e1.
Figueroa-Colon R, von Almen TK, Franklin FA, Schuftan C, Suskind RM. Comparison of two hypocaloric diets in obese children. Am J Dis Child. 1993;147(2):160–6.
Sondike SB, Copperman N, Jacobson MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factor in overweight adolescents. J Pediatr. 2003;142(3):253–8.
Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, Yancy WS, Brinkworth GD. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: a randomized trial. Am J Clin Nutr. 2015;102(4):780–90.
Meckling KA, O’Sullivan C, Saari D. Comparison of a low-fat diet to a low-carbohydrate diet on weight loss, body composition, and risk factors for diabetes and cardiovascular disease in free-living, overweight men and women. J Clin Endocrinol Metab. 2004;89(6):2717–23.
White AM, Johnston CS, Swan PD, Tjonn SL, Sears B. Blood ketones are directly related to fatigue and perceived effort during exercise in overweight adults adhering to low-carbohydrate diets for weight loss: a pilot study. J Am Diet Assoc. 2007;107(10):1792–6.
Brand-Miller J, McMillan-Price J, Steinbeck K, Caterson I. Dietary glycemic index: health implications. J Am Coll Nutr. 2009;28(Suppl):446S–9S.
Schwingshackl L, Hobl LP, Hoffmann G. Effects of low glycaemic index/low glycaemic load vs. high glycaemic index/high glycaemic load diets on overweight/obesity and associated risk factors in children and adolescents: a systematic review and meta-analysis. Nutr J. 2015;14(87)
Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297(19):2092–102.
Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, Martinez JA, Handjieva-Darlenska T, Kunesova M, Pihlsgard M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. NEJM. 2010;363(22):2102–13.
Papadaki A, Linardakis M, Larsen TM, van Baak MA, Lindroos AK, Pfeiffer AFH, Martinez JA, Handjieva-Darlenska T, Kunesova M, Holst C, et al. The effect of protein and glycemic index on children’s body composition: the DiOGenes randomized study. Pediatrics. 2010;126(5):E1143–E52.
National Health and Medical Research. Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia; 2013. https://www.nhmrc.gov.au/guidelines-publications/n57.
Berkowitz RI, Wadden TA, Gehrman CA, Bishop-Gilyard CT, Moore RH, Womble LG, Cronquist JL, Trumpikas NL, Levitt Katz LE, Xanthopoulos MS. Meal replacements in the treatment of adolescent obesity: a randomized controlled trial. Obesity (Silver Spring). 2011;19(6):1193–9.
Willi SM, Martin K, Datko FM, Brant BP. Treatment of type 2 diabetes in childhood using a very-low-calorie diet. Diabetes Care. 2004;27(2):348–53.
Gow ML, Baur LA, Johnson NA, Cowell CT, Garnett SP. Reversal of type 2 diabetes in youth who adhere to a very-low-energy diet: a pilot study. Diabetologia. 2016:1–10.
Leslie WS, Taylor R, Harris L, Lean MEJ. Weight losses with low-energy formula diets in obese patients with and without type 2 diabetes; systematic review and meta-analysis. Int J Obes. 2017;41:96.
Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90(5):1138–43.
Davis CS, Clarke RE, Coulter SN, Rounsefell KN, Walker RE, Rauch CE, Huggins CE, Ryan L. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70(3):292–9.
Headland M, Clifton PM, Carter S, Keogh JB. Weight-loss outcomes: a systematic review and meta-analysis of intermittent energy restriction trials lasting a minimum of 6 months. Forum Nutr. 2016;8(6):354.
Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes. 2011;35(5):714–27.
Braet C, Claus L, Goossens L, Moens E, Van Vlierberghe L, Soetens B. Differences in eating style between overweight and normal-weight youngsters. J Health Psychol. 2008;13(6):733–43.
Birch LL, Fisher JO, Davison KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls’ eating in the absence of hunger. Am J Clin Nutr. 2003;78(2):215–20.
Loth K, Wall M, Larson N, Neumark-Sztainer D. Disordered eating and psychological well-being in overweight and nonoverweight adolescents: secular trends from 1999 to 2010. Int J Eat Disord. 2015;48(3):323–7.
Ho M, Gow M, Halim J, Chisholm K, Baur L, Noakes M, Steinbeck K, Kohn M, Cowell C, Garnett S. Effect of a prescriptive dietary intervention on psychological dimensions of eating behavior in obese adolescents. Int J Behav Nutr Phys Act. 2013;10(1):119.
Butryn ML, Wadden TA. Treatment of overweight in children and adolescents: does dieting increase the risk of eating disorders? Int J Eat Disord. 2005;37(4):285–93.
Truby H, Baxter K, Elliott S, Warren J, Davies P, Batch J. Adolescents seeking weight management: who is putting their hand up and what are they looking for? J Paediatr Child Health. 2011;47(1–2):2–4.
Luz F, Hay P, Gibson A, Touyz S, Swinbourne J, Roekenes J, Sainsbury A. Does severe dietary energy restriction increase binge eating in overweight or obese individuals? A systematic review. Obes Rev. 2015;16(8):652–65.
Gutin B. Diet vs exercise for the prevention of pediatric obesity: the role of exercise. Int J Obes. 2011;35(1):29–32.
Prado DM, Silva AG, Trombetta IC, Ribeiro MM, Nicolau CM, Guazzelli IC, Matos LN, Negrao CE, Villares SM. Weight loss associated with exercise training restores ventilatory efficiency in obese children. Int J Sports Med. 2009;30(11):821–6.
Baur LA, Hazelton B, Shrewsbury VA. Assessment and management of obesity in childhood and adolescence. Nat Rev Gastroenterol Hepatol. 2011;8(11):635–45.
Becque MD, Katch VL, Rocchini AP, Marks CR, Moorehead C. Coronary risk incidence of obese adolescents: reduction by exercise plus diet intervention. Pediatrics. 1988;81(5):605–12.
Rocchini A, Katch V, Schork A, Kelch R. Insulin and blood pressure during weight loss in obese adolescents. Hypertension. 1987;10(3):267–73.
Rocchini AP, Katch V, Anderson J, Hinderliter J, Becque D, Martin M, Marks C. Blood-pressure in obese adolescents - effect of weight-loss. Pediatrics. 1988;82(1):16–23.
Sung RYT, CW Y, Chang SKY, Mo SW, Woo KS, Lam CWK. Effects of dietary intervention and strength training on blood lipid level in obese children. Arch Dis Child. 2002;86(6):407–10.
Woo KS, Chook P, CW Y, Sung RYT, Qiao M, Leung SSF, Lam CWK, Metreweli C, Celermajer DS. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 2004;109(16):1981–6.
Yu CCW, Sung RYT, So RCH, Lui K, Lau W, Lam PKW, Lau EMC. Effects of strength training on body composition and bone mineral content in children who are obese. J Strength Cond Res. 2005;19(3):667–72.
CCW Y, Sung RYT, Hau KT, Lam PKW, Nelson EAS, RCH S. The effect of diet and strength training on obese children’s physical self-concept. J Sports Med Phys Fitness. 2008;48(1):76–82.
Ribeiro MM, Silva AG, Santos NS, Guazzelle I, Matos LNJ, Trombetta IC, Halpern A, Negrao CE, Villares SMF. Diet and exercise training restore blood pressure and vasodilatory responses during physiological maneuvers in obese children. Circulation. 2005;111(15):1915–23.
Davis JN, Tung A, Chak SS, Ventura EE, Byrd-Williams CE, Alexander KE, Lane CJ, Weigensberg MJ, Spruijt-Metz D, Goran MI. Aerobic and strength training reduces adiposity in overweight Latina adolescents. Med Sci Sports Exerc. 2009;41(7):1494–503.
Shalitin S, Ashkenazi-Hoffnung L, Yackobovitch-Gavan M, Nagelberg N, Karni Y, Hershkovitz E, Loewenthal N, Shtaif B, Gat-Yablonski G, Phillip M. Effects of a twelve-week randomized intervention of exercise and/or diet on weight loss and weight maintenance, and other metabolic parameters in obese preadolescent children. Horm Res. 2009;72(5):287–301.
Okely AD, Collins CE, Morgan PJ, Jones RA, Warren JM, Cliff DP, Burrows TL, Colyvas K, Steele JR, Baur LA. Multi-site randomized controlled trial of a child-centered physical activity program, a parent-centered dietary-modification program, or both in overweight children: the HIKCUPS study. J Pediatr. 2010;157(3):388–94.e1.
Burrows T, Warren JM, Baur LA, Collins CE. Impact of a child obesity intervention on dietary intake and behaviors. Int J Obes. 2008;32(10):1481–8.
Burrows T, Warren JM, Collins CE. The impact of a child obesity treatment intervention on parent child-feeding practices. Int J Pediatr Obes. 2010;5(1):43–50.
Kelishadi R, Hashemipour M, Mohammadifard N, Alikhassy H, Adeli K. Short- and long-term relationships of serum ghrelin with changes in body composition and the metabolic syndrome in prepubescent obese children following two different weight loss programmes. Clin Endocrinol. 2008;69(5):721–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Gow, M.L., Ho, M., Lister, N.B., Garnett, S.P. (2018). Dietary Interventions in the Treatment of Paediatric Obesity. In: Freemark, M. (eds) Pediatric Obesity. Contemporary Endocrinology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-68192-4_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-68192-4_16
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-68191-7
Online ISBN: 978-3-319-68192-4
eBook Packages: MedicineMedicine (R0)