Abstract
Hepatocellular carcinoma (HCC), the sixth most common cancer worldwide, is a frequent cause of diagnostic liver imaging [1]. It causes approximately 250,000 deaths per year worldwide [2]. Given the high prevalence of the disease, accurate screening and diagnosis of this tumor are critical for possible early intervention.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Acoustic Radiation Force Impulse (ARFI)
- Additional Major Features
- SPIO Particles
- Background Liver Parenchyma
- Late Arterial Phase
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
7.1 Introduction
Hepatocellular carcinoma (HCC), the sixth most common cancer worldwide, is a frequent cause of diagnostic liver imaging [1]. It causes approximately 250,000 deaths per year worldwide [2]. Given the high prevalence of the disease, accurate screening and diagnosis of this tumor are critical for possible early intervention.
Liver cirrhosis increases the risk of HCC. The annual risk of HCC in patients with cirrhosis is approximately 1–6% [3]. Although there are a myriad of causative factors for cirrhosis including alcohol, autoimmune hepatitis, nonalcoholic fatty liver disease, and specific metabolic disorders, hepatitis B and C are responsible for a majority of causes of cirrhosis and subsequent HCC [4]. There is evidence to suggest that viral hepatitis is responsible for 75% of HCC worldwide. Given that up to 90% of HCC occurs in patients with a background of cirrhotic liver disease, screening for HCC in this patient population is critical [4].
7.2 Screening of HCC
The purpose of screening is to allow early detection of HCC in at-risk, asymptomatic individuals in order to decrease HCC-related mortality. Since the HCC tumor doubling time is 6–12 months, the American Association for the Study of Liver Diseases (AASLD) recommends HCC screening every 6 months. Ultrasound has a sensitivity of 65–80% and a specificity of greater than 90% for screening of HCC [5]. It has replaced serum alpha-fetoprotein (AFP) marker as the predominant screening test for HCC.
Screening recommendations apply to patients with chronic hepatitis C who have developed cirrhosis. Some experts also recommend screening HCV-infected patients who have liver fibrosis but not cirrhosis. HCC surveillance is not recommended in HCV-infected patients without fibrosis or cirrhosis [6].
Hepatitis B is considered more oncogenic compared to hepatitis C, and, therefore, screening recommendations in patients with hepatitis B are more aggressive. In patients with hepatitis B infection, screening for HCC is recommended in patients with cirrhosis, patients with family history of HCC, Asian males >40 years, Asian females >50 years, and Africans >20 years [6].
HCC screening is also recommended in other patients with cirrhosis, including those with stage 4 primary biliary cirrhosis (PBC), genetic hemochromatosis, alpha 1 antitrypsin deficiency, or other causes of cirrhosis [6]. Surveillance is also performed in patients listed for liver transplantation because the development of HCC increases the priority of these patients on the transplant list [5].
Once a small nodule is identified on screening ultrasound, further imaging recommendations are based on the size of the nodule. If the nodule is less than 1 cm, repeat ultrasound in 3 months is recommended to assess for stability. If the nodule increases in size during the 3-month period, further investigation is recommended. If the initial nodule is greater than 1 cm, evaluation with multiphase multidetector CT or dynamic contrast-enhanced MRI is recommended [5].
7.3 Altered Hemodynamics
The physiologic and pathologic alterations that occur in a cirrhotic liver are critical to understanding the imaging findings of HCC. With cirrhosis, the liver becomes more fibrotic. There is hypertrophy of the caudate lobe (segment I) as well as the lateral segments of the left hepatic lobe (segments II and III). The portal blood flow is altered as the portal veins become more tortuous and subsequently diminutive, which may ultimately lead to reversal of portal flow. There are multiple regenerative nodules that emerge as the liver tries to regenerate its parenchyma. The umbilical vein is recanalized, and multiple varices form in an effort to divert portal flow away from the liver tissue, which now has increased parenchymal resistance. With time, dysplastic nodules can emerge, which are precancerous and can serve as precursors to HCC [7]. Stromal invasion and alteration of arterial supply to the nodule then occur, allowing the dysplastic nodule to develop into HCC. While most of the liver is supplied by the portal system (75%) in normal hepatic physiology, the predominant supply to a HCC is from the hepatic arterial system. This altered hemodynamic system is the basis of radiologic imaging as it helps differentiate background liver parenchyma from HCC.
7.4 Ultrasound
As stated previously, ultrasound is the predominant screening tool for HCC. Unenhanced brightness mode (B-mode) ultrasound is most commonly used for HCC screening. Additional sonographic tools that can be used in the detection of HCC are Doppler imaging and contrast-enhanced ultrasound.
HCC does not have a specific appearance on ultrasound (Fig. 7.1). Well-differentiated HCC less than 3 cm usually appears as a well-circumscribed hypoechoic mass [8]. There are studies to suggest that small HCCs that are less than 5 cm are hypoechoic on ultrasound approximately 75% of the time [9]. However, HCC can be hyperechoic or of mixed echogenicity on ultrasound [10]. As the tumor grows, a hypoechoic rim can develop [11]. The HCC can also become more heterogeneous with growth. If there is a fatty component to the tumor, or if there is hemorrhage within the tumor, these can lead to a hyperechoic signal as well.
HCC can have variable appearances on ultrasound. ( a ) Arrow points to a HCC lesion, which is hyperechoic relative to liver parenchyma. ( b ) Arrow points to a HCC lesion that is hypoechoic on ultrasound. ( c ) Arrow points to a HCC, which is heterogeneous and large on ultrasound. ( d ) Arrow points to a HCC lesion, which has a target appearance. A target appearance is used to describe a lesion that has a hyperechoic center and a hypoechoic rim
Doppler ultrasound is an adjunct to B-mode ultrasound for detection of HCC. Approximately 75% of HCC tumors demonstrate internal vascularity on Doppler ultrasound. This is in contrast to liver metastatic lesions, which demonstrate internal vascularity only approximately 25% of the time [8]. Invasion of the hepatic or portal veins is also strongly suggestive of HCC.
Contrast-enhanced ultrasound (CEUS) is an emerging technique that can potentially detect HCC. The technique involves administration of intravenous contrast and obtaining images in the arterial, venous, and delayed phases. After administration of contrast, the arterial phase images are obtained at 15–30 s, the venous phase images are obtained at 50–80 s, and the delayed phase images are obtained at 180–240 s [12]. Since HCC obtains most of its blood flow from the hepatic arterial system, contrast flows into the tumor in the arterial phase, and the tumor appears hyperechoic on ultrasound compared to the rest of the liver parenchyma. Subsequently, when the rest of the liver parenchyma enhances with contrast in the venous and delayed phases, the contrast within the HCC lesion washes out. This is because the predominant blood supply to the liver is from the portal veins, while the tumor predominantly gets its blood supply from the hepatic arteries. The lesion therefore becomes isoechoic and subsequently hypoechoic compared to the rest of the liver parenchyma with time.
While this can potentially play an important role in identifying HCC and has an advantage of avoiding the radiation risk that patients obtain with CT, the AASLD excludes CEUS as a diagnostic tool in patients with cirrhosis due to its high false positive rate. There are, however, societies such as the Italian Association for the Study of the Liver (AISF) who maintain a role for CEUS in identifying HCC nodules that are specifically greater than 1 cm [12].
7.5 Ultrasound Surveillance Algorithm
Although any nodule detected on ultrasound in a patient undergoing surveillance could potentially represent HCC, there are guidelines by the AASLD for subsequent management based on size. If a nodule <1 cm is detected on screening ultrasound, it is suggested that the nodule be followed every 3 months with ultrasound. If it increases in size within this time period, further imaging using multiphase contrast-enhanced CT or MRI is recommended. If the nodule initially detected on screening ultrasound is greater than 1 cm in size, multiphase contrast-enhanced CT or MRI are directly recommended as the next step in management [5].
7.6 Computed Tomography (CT)
Multiphase CT is an option used to further characterize liver lesions noted on ultrasound or single-phase CT (Fig. 7.2). After the administration of intravenous contrast, CT scans are usually performed in the late arterial phase, portal venous phase, and delayed phase. The arterial phase is usually performed at 15–30 s [13] and represents the enhancement of the hepatic arteries with some early enhancement of the portal veins. The portal venous phase is performed at approximately 60–80 s and represents enhancement of the entire portal venous system as well as the hepatic veins [14]. A delayed scan is obtained at approximately 3–5 min and represents the equilibrium phase when contrast has mostly washed out of the liver parenchyma.
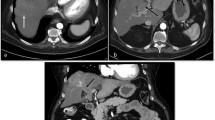
( a ) CT of the liver during the arterial phase demonstrates a lesion in the liver dome that is hyperenhancing relative to the rest of the liver parenchyma. ( b ) Venous phase demonstrates that the lesion is hypoenhancing compared to surrounding liver parenchyma. This is referred to as “washout.” There is a faint area of enhancement around the lesion, which is the pseudocapsule. ( c ) Delayed phase imaging demonstrates persistent washout of the lesion relative to surrounding liver parenchyma. ( d ) Initial single-phase scan obtained during the early venous phase, which better demonstrates the peripherally enhancing pseudocapsule. Collectively, this multiphase exam demonstrates characteristics highly suspicious for HCC, which are arterial enhancement, venous phase washout, and pseudocapsule formation
Hepatocellular carcinoma is an epithelial tumor composed of cells similar to normal hepatocytes. In the process of hepatocarcinogenesis, there is development of increased arterial supply to the tumor [7]. The purpose of the late arterial phase therefore is to identify intrahepatic lesions that demonstrate hypervascularity compared to the rest of the liver parenchyma. Studies have shown that approximately 78% of HCCs demonstrate arterial enhancement [15]. Findings, however, do vary depending on tumor differentiation and size. Although the majority of HCC lesions demonstrate hypervascularity regardless of differentiation, moderately differentiated HCCs are noted to have the highest proportion of arterial enhancement. The number of well-differentiated HCCs demonstrating hyperenhancement is slightly less, and poorly differentiated HCCs are proportionally the least in terms of arterial enhancement [15, 16]. The variation of arterial enhancement is again secondary to the degree of tumoral neoangiogenesis in the various levels of differentiation [15]. Tumoral size also plays a role in the visualization of arterial enhancement. Studies have shown that arterial enhancement is more commonly found in HCCs that are 1–2.9 cm (70–75%) compared to HCCs that are less than 1 cm (52%) [16]. However, when tumors significantly increase in size to greater than 5 cm, arterial flow may diminish, likely secondary to increased cell proliferation causing increased interstitial pressure and regression of neoarteries [7].
There are a few lesions in addition to HCC that also may demonstrate hyperenhancement in the arterial phase. These include benign perfusion alterations, small hemangiomas, small focal nodular hyperplasia-type lesions, atypical cirrhotic or dysplastic nodules, atypical focal/confluent fibrosis, and other malignancies such as small intrahepatic cholangiocarcinomas or hypervascular metastases such as neuroendocrine tumors [14]. Additionally, since HCC commonly occurs on a background of cirrhosis or chronic hepatitis where arterioportal shunting is common, a large majority of focal enhancing areas less than 2 cm that are only identified on the arterial phase and are predominantly wedge shaped and subcapsular are actually nonneoplastic [14]. The other phases of multiphase CT therefore are important to further characterize lesions that are hyperenhancing in the arterial phase and well as to identify HCC lesions that may not arterially enhance.
The portal venous phase and delayed phases also play a critical role in the evaluation of HCC. HCC predominantly (approximately 72% of the time) demonstrates a washout pattern on these two phases, which means it is hypoenhancing compared to the rest of the background liver parenchyma [15]. This occurs because, with hepatocarcinogenesis, there is a decrease in the number of portal tracts that contain the portal veins. This leads to a gradual decrease in blood flow to the tumor during the portal venous phase. The reduction in portal flow parallels the progression of HCC such that the more advanced the HCC, the more likely it is to have reduced or absent portal blood flow [15].
As with arterial phase imaging, tumoral differentiation plays a role in the imaging pattern of HCC during the portal venous and delayed phases. Moderately differentiated and poorly differentiated HCCs are more likely to demonstrate washout on these phases (75–76%, respectively). This is in contrast to well-differentiated tumors which were shown to demonstrate washout only 50% of the time according to some studies [15]. There is also evidence to suggest that with progression from well to moderate to poor differentiation of HCC, there is a shift in the timing of washout pattern during the portal venous phase. Well-differentiated HCC tends to washout relatively late compared to poorly differentiated tumors [15]. Additionally, a minority of HCC tumors are iso- or hyperattenuating during the portal venous phase. It is important to note that there are other liver lesions that can be hypoattenuating relative to the rest of the liver parenchyma on the portal venous or delayed phases, making it important to look at the enhancement pattern of the lesion on all three phases of imaging.
The delayed phase is especially important in HCC tumors that demonstrate slow washout, as this may not be apparent on the initial portal venous phase. This is in part because with cirrhosis, the increased hepatic tissue resistance causes delayed background parenchymal enhancement. The delayed phase imaging is also important in differentiating HCC from other tumors like cholangiocarcinoma, which demonstrate progressively increased enhancement on the portal venous and delayed phases.
There are additional characteristics of HCC that can be present on CT, including capsule formation and portal vein invasion. Capsule formation is a feature of HCC that occurs with disease progression and is suggestive of tumor with increased malignant potential compared to early HCC. Progressed HCC tumors are noted to have capsule formation and internal fibrous septa approximately 70% of the time [14]. Capsule enhancement appears as a complete or partial peripheral hyperenhancing rim around the tumor. It is classically seen a few seconds after tumoral enhancement and is best visualized in the late arterial or early portal venous phase. Visualization of a capsule suggests that tumor venous drainage has progressed from the hepatic veins to the portal veins [14].
Invasion into the venous system, predominantly into the portal veins, is another characteristic of HCC that helps differentiate it from other tumors. It is more frequently found in tumors of increased size and histologic grade. Vascular invasion carries a poorer prognosis as it represents a means by which HCC metastasizes to other areas of the liver as well as to different parts of the body [7]. Tumor thrombus within a lumen of a vein can also demonstrate enhancement on the arterial phase and appear hypodense on the portal venous phase. If present, these characteristics help differentiate it from bland venous thrombosis, which can also occur in this patient population.
Intratumoral fat, or hepatosteatosis, is another characteristic feature of HCC. It represents a process by which abnormal hepatocytes accumulate more intracellular fat and is seen in approximately 40% of early HCCs [7]. However, this finding is most commonly seen in HCCs that are approximately 1.5 cm in diameter. It is uncommon with further increase in tumor size and grade and not frequently seen in HCCs larger than 3 cm [7]. Intratumoral fat however is better recognized on MRI as it is difficult to characterize well on CT.
7.7 Magnetic Resonance Imaging (MRI)
Given technical advancements, HCC has now become an imaging diagnosis. The criteria include any nodule greater than 1 cm that demonstrates arterial enhancement and subsequent washout on CT or MRI. Although many meta-analyses found CT and MRI to have comparable specificities for the diagnosis of HCC in a cirrhotic liver, MRI is noted to have a higher sensitivity than CT, with MRI sensitivities ranging from 70 to 85% and CT sensitivities ranging from 50 to 68% [17]. The sensitivity for MRI detection of HCC, however, does vary depending on the size of tumor. A HCC larger than 2 cm was noted to have 100% sensitivity on MRI, while a HCC less than 1 cm had only a 4% sensitivity on one study [17].
The typical MRI protocol for HCC includes multiple T1-weighted imaging sequences and T2-weighted imaging sequences, including diffusion-weighted imaging. The T1 sequences are preferably obtained both pre- and postcontrast if the patient is able to receive intravenous contrast. There are several contrast agents that can be used for liver imaging, the most common being extracellular agents, hepatobiliary agents, or reticuloendothelial agents.
Gadolinium chelates are common extracellular contrast agents used in the MRI detection of HCC. Gadolinium demonstrates pharmacokinetics similar to the iodinated contrast media used in CT. As an extracellular agent, it leaves the vasculature and enters the interstitial space after it is administered intravenously. It causes relaxation of adjacent water protons since it is highly paramagnetic and has seven unpaired electrons. This in turn shortens the T1 and T2 times, leading to enhanced T1 signal and hypointense T2 signal on MRI. A single gadolinium atom has the potential to relax multiple adjacent protons, allowing better visualization of subtle small areas of contrast enhancement, which makes MRI more sensitive than CT [18]. The T1 postcontrast images, therefore, are a primary tool for evaluating HCC. A similar enhancement pattern as CT is used to characterize HCC on MRI, which includes arterial enhancement and subsequent washout on the venous and/or delayed imaging.
Gadoxetate disodium (Eovist) is a hepatobiliary (or hepatocyte-specific) agent that is used for MRI evaluation of liver lesions, including HCC (Fig. 7.3). After intravenous administration, it enters the extracellular space similar to extracellular agents. However, it is unique in that it is subsequently transported into the hepatocyte by an ATP-dependent receptor called organic anion transporting polypeptide (OATP1). Once inside the hepatocyte, it is excreted into the biliary canaliculi by another transporter known as canalicular multispecific organic anion transporter (cMOAT). Excretion of Eovist into the biliary system therefore is dependent on the overall liver function. In patients with normal liver function, approximately 50% of Eovist is excreted via the hepatobiliary system, and the rest is excreted by the kidneys [7]. Since it has a half-life of 56 min, hepatic phase imaging for Eovist is usually done 20 min after intravenous administration of the contrast agent. By 20 min, the contrast is taken up by the hepatocytes where it reversibly interacts with intracellular proteins and leads to increased T1 relaxivity compared to other contrast agents. The 20 min hepatocyte sequence is unique to Eovist and is simply an addition to the usual hepatic imaging sequences. Since HCC has altered hepatocyte function, the tumor cells are not able to take up Eovist. The HCC therefore appears hypointense on the 20 min phase compared to the surrounding liver parenchyma where the hepatocytes are able to uptake Eovist and demonstrate increased signal.
MRI of the liver was performed using Eovist. ( a ) T1-weighted image of a HCC during the arterial phase demonstrates hyperenhancement of the lesion relative to liver parenchyma as demonstrated by the arrow . ( b ) T1-weighted image of the HCC during the delayed phase demonstrates washout relative to liver parenchyma. There is also a visible pseudocapsule around the lesion. ( c ) T2-weighted image of the HCC lesion demonstrates mild hyperintensity relative to the rest of the liver parenchyma. ( d ) 20 min hepatocyte phase demonstrates no uptake of contrast by the tumor, while the rest of the liver parenchyma is able to uptake the Eovist and demonstrate increased signal
A third agent used for imaging HCC is superparamagnetic iron oxide (SPIO) particles. These are iron-based particles designed to target the reticuloendothelial system, specifically the liver and the spleen. Only one of these agents, ferumoxide, is approved for use in the United States. In the liver, SPIO particles are phagocytosed by a type of macrophages known as Kupffer cells which line the sinusoids [18]. Subsequently, the particles cause inhomogeneities in the magnetic field leading to T2 and T2* shortening which is reflected on MRI as hypointense signal. Tissues containing these particles also demonstrate mildly decreased T1 signal. SPIO particles are helpful in the imaging of HCC because while background liver parenchyma contains Kupffer cells and is able to take up these particles, most HCC tumors are deficient in Kupffer cells. HCC tumors, therefore, appear hyperintense relative to the surrounding hypointense liver parenchyma. The degree to which SPIO particles are used is variable. In terms of accuracy, it was reported in one series that gadolinium was better than SPIO particles for the detection of small HCC tumors [18]. SPIO particles are also more expensive and take a longer time to image than gadolinium. However, in patients with significant cirrhosis and alteration in liver perfusion, gadolinium enhancement of HCC may be poor. SPIO particles therefore can be used as an adjunct in such situations to help in the detection of HCC. It has been proposed that SPIO particles are most useful when administered along with gadolinium to increase contrast between HCC and background liver parenchyma and thereby improve the detection of HCC [18].
In addition to the three-phase enhancement pattern that characterizes HCC on CT, MRI offers additional tools to aid in the diagnosis of HCC. T2-weighted imaging sequences are an important part of HCC diagnosis on MRI. These are fluid sensitive sequences. So, lesions with high intracellular or extracellular water content demonstrate increased signal, while lesions with low water content appear hypointense. Although many liver lesions can demonstrate increased signal on T2-weighted images, mild-to-moderate T2 hyperintensity is typical of HCC [14]. Most HCCs that demonstrate these findings are of advanced grade. It has been shown that 77% of HCC lesions greater than 3 cm demonstrate this characteristic signal intensity [14].
A specific combination of imaging sequences known as T1 gradient echo in-phase and out-of-phase imaging is a useful adjunct for the diagnosis of HCC on MRI. These sequences are based on the premise that HCC tumors, especially during early development, often contain intralesional fat (Fig. 7.4). Since intralesional fat is a more characteristic of early HCC than progressed HCC, if detected, it can serve as a good prognostic feature. The imaging sequences are based on the principle that in the presence of intralesional fat, lipid and water protons occupy the same voxel. Lipid and water protons, however, inherently precess at different frequencies. In-phase imaging is obtained when lipid and water protons are precessing at a similar frequency. At this time, which occurs approximately every 4.2 ms on a 1.5 T magnet, their signal is additive. When lipid and water proton signals are out of phase with each other, there is cancellation of signal resulting in signal loss. Therefore, loss of intralesional signal on out-of-phase images compared to in-phase images is helpful for diagnosis of intralesional fat when there is a lesion suspicious for HCC, especially during the early stage [19].
Diffusion-weighted imaging (DWI) is another tool that is being increasingly used in liver imaging to diagnose HCC (Fig. 7.5). It is based on the phenomenon of random movement of water molecules driven by their internal thermal energy, a concept known as Brownian motion. DWI is governed by inherent tissue properties, which can allow relatively free movement of water in certain areas and impede diffusion of water molecules in other areas. DWI is a T2-based imaging sequence. Tissues with high cellularity restrict the motion of water molecules within them, while tissues with lower cellularity cause less impedance to the movement of water molecules. Imaging is obtained using two strong gradients, one of which dephases the protons and the other rephases the protons. In tissues with restricted motion, water protons experience both the dephasing and rephasing gradients, thereby producing a hyperintense T2 signal. If there is movement of water molecules between the dephasing and rephasing gradients, there is a reduction in overall T2 signal intensity on imaging. Apparent diffusion coefficient (ADC) is a quantitative expression of diffusion, which is automatically calculated by the software. Low ADC values represent diffusion restriction, whereas high ADC values reflect relatively free diffusion of water molecules [20].
HCC can have a variable appearance on diffusion-weighted imaging depending on histologic makeup. Moderate to poorly differentiated HCC tumors are often hyperintense on DWI, whereas well-differentiated tumors often appear isointense on diffusion-weighted imaging [21]. Diffusion-weighted imaging has been shown to be especially helpful in HCCs measuring less than 2 cm. In a study by Zech et al., conventional MRI demonstrated a sensitivity of 67.6% and a positive predictive value of 59%, while diffusion-weighted imaging had a sensitivity of 91.2% and a positive predictive value of 81.6% in HCC tumors less than 2 cm. In HCC lesions greater than 2 cm, DWI did not appear to be significantly better than conventional MRI [20]. A limitation of DWI is that in cirrhotic livers, the value of ADC might be limited as both cirrhotic liver and HCCs can have low ADC values.
7.8 Emerging Imaging Techniques
7.8.1 Elastography
MR elastography is becoming increasingly utilized for the assessment of liver fibrosis. The concept involves applying a stress to tissue and measuring the resultant response. The first step is causing tissue vibration, which is most commonly done using an audio source located outside the scanner room. These tissue vibrations produce low-frequency shear waves. Typically, a frequency of 60 Hz is used. A motion-sensitive dynamic MRI sequence is then used to image the liver. Spatial information is reflected in quantitative shear stiffness maps using an inversion algorithm. Mechanical shear waves travel more slowly in softer tissues and have a shorter wavelength. Conversely, in stiffer tissues, shear waves travel faster and have a longer wavelength. Since the measured stiffness depends on frequency, the imaging can be done on a 1.5 or 3 T magnet strength given that the frequency is similar [22].
Tissue stiffness in vivo depends on tissue components, structural organization, and blood perfusion. Pathology in the liver therefore alters tissue structure causing the abnormal tissue to respond differently under stress than normal tissue. At 60 Hz, normal liver tissue has a mean stiffness of 1.54–2.87 kPa [22]. In chronic liver disease, collagen is deposited in the extracellular matrix causing liver fibrosis. Given that liver fibrosis demonstrates a linear increase in liver stiffness, MR elastography is a great tool for staging liver fibrosis. It has been shown to have a high accuracy in differentiating liver fibrosis from normal liver and/or liver with inflammation but no fibrosis. Preliminary studies have also shown that malignant liver tumors such as HCC have a higher liver stiffness compared to benign tumors and normal liver [23]. Using a cutoff value of 5 kPa, one study demonstrated a 100% accuracy of MR elastography in differentiating malignant tumors from benign tumors [22].
Ultrasound elastography is another method used to evaluate liver fibrosis. It can be performed using different techniques such as transient elastography (TE), real-time/static elastography (RTE), acoustic radiation force impulse (ARFI), or real-time shear wave elastography (SWE). TE is performed by using a mechanical actuator to cause skin vibrations which induces low-frequency mechanical waves to propagate through the liver. The velocity of these waves is measured with ultrasound and used to calculate liver stiffness, which is expressed in kilopascals (kPa). ARFI and SWE are shear wave techniques that use acoustic radiation force to cause microscopic tissue movements and thereby produce shear waves. The waves are studied to estimate tissue stiffness and shear wave velocity.
Both TE and SWE appear promising for the diagnosis of cirrhosis. In a large multicenter study, ARFI-based SWE showed a sensitivity of 69.1% and a specificity of 79.8% to diagnose fibrosis greater than METAVIR F2 stage (defined as moderate liver damage) [24]. TE was also shown to be better than ARFI for predicting the presence of cirrhosis and fibrosis at the F1 stage or greater. Another study demonstrated TE as an ideal method to diagnose cirrhosis in patients with hepatitis C virus as it could potentially decrease the number of liver biopsies [25]. In patients with hepatitis B virus, ARFI and TE had similar diagnostic accuracies of diagnosing stage two fibrosis or greater, with areas under the curve of 0.75 and 0.83, respectively [25]. Several studies have been performed to evaluate the ability of ultrasound elastography to differentiate between benign and malignant lesions. A meta-analysis of RTE and ARFI in 2013 showed a sensitivity of 85% and specificity of 84% of these modalities to distinguish benign from malignant lesions [25]. Other studies, however, have shown no statistically significant difference in differentiating benign and malignant liver lesions using ultrasound elastography [25].
7.8.2 Dual-Energy CT
Dual-energy CT (DECT) is an emerging technique that can be used to characterize liver lesions. While conventional CT uses a single polychromatic x-ray beam ranging from 70 to 140 kVp (standard of 120 kVp), dual-energy CT uses two energy levels, typically 80 and 140 kVp [26]. Dual-energy CT allows for improved conspicuity/enhancement of iodine in parenchymal tissue. Given its high atomic number of 53, iodine attenuates differently when exposed to a lower-energy beam compared to normal soft tissues such as the liver, which are made up of substances with low atomic numbers [27]. The low-energy acquisition from the 80 kVp energy datasets is noted to be more sensitive in detection of hypervascular liver lesions such as HCC due to improved contrast-to-noise ratio [28].
7.9 Imaging Implications on Patient Care Including Transplant Eligibility
In order to have a systematic way of reporting imaging findings on CT and MRI in patients at risk for HCC, the Liver Imaging Reporting and Data System (LI-RADS) was formulated in 2011. It was developed by a committee of international experts in medicine, surgery, and radiology with the ultimate goal of providing an estimated probability of a liver nodule representing a HCC. In patients at high risk for developing HCC, it categorizes liver lesions noted on CT or MRI into LI-RADS category 1–5. These categories represent benign, probably benign, intermediate probability of being HCC, probably HCC, and definitively HCC respectively. The four major imaging features used to assign a LI-RADS category include arterial phase hyperenhancement, washout appearance following hyperenhancement, capsule enhancement, and threshold growth compared to previous imaging [29]. A category of LR-M is reserved for a mass thought to be a malignancy other than HCC. LR-5V is reserved for tumor in a vein.
The highlights of the LI-RADS classification system as outlined by the ACR are discussed below [29, 30]. A LI-RADS 1 is assigned to a lesion that either has diagnostic benign imaging features or resolves without treatment. LI-RADS 1 is benign and LI-RADS 5 has a 100% certainty of a lesion being HCC. A LI-RADS 2 is assigned to a lesion that has imaging features suggestive of a benign entity; the imaging features remain stable for ≥2 years or if the lesion likely disappeared without treatment. LI-RADS 3, LI-RADS 4, and LI-RADS 5 are further subdivided based on size and presence of additional major features which include hypoenhancement during portal venous or delayed phase or increase in diameter of at least 1 cm in 1 year. In a mass-like lesion less than 2 cm, mass-like configuration, and arterial hyperenhancement, a LI-RADS 3 is assigned if there are no additional major features, a LI-RADS 4 is assigned if there is one additional major feature, and LI-RADS 5 if there are two additional major features. In a mass-like lesion less than 2 cm, mass-like configuration, and arterial hypoenhancement, a LI-RADS 3 is assigned if there are zero or one additional major features and a LI-RADS 4 is assigned if there are two additional major features. A LI-RADS 5 does not include arterially iso- or hypoenhancing lesions. In a mass-like lesion ≥2 cm, a LI-RADS 3 is assigned if it is hypoenhancing and a LI-RADS 4 if it is arterially enhancing with no additional major features or if it is arterially hypo- or isoenhancing with one or two major features. In such a lesion, a LI-RADS 5 is assigned if it demonstrates arterial hyperenhancement with one or two major features. If there is probable tumor within a vein, the lesion is assigned a LI-RADS 4, and if there is definite tumor within a vein, the lesion is assigned a LI-RADS 5 [29]. The LI-RADS categories 2, 3, and 4 are not definitely benign and not definitely HCC, and further evaluation may be needed to characterize these lesions [30].
In 2011, the United Network for Organ Sharing and Organ Procurement and Transplant Network (UNOS-OPTN) established imaging criteria to diagnose HCC using dynamic CT and MRI. These criteria were used to determine liver transplantation eligibility of patients with HCC who did not have extrahepatic spread and/or macrovascular involvement of tumor on imaging. The UNOS-OPTN classification system is as follows: 5A, 5A-g, 5B, 5X, and 5T. A 5A lesion measures 10–20 mm, demonstrates hypervascularity during the late arterial phase, and demonstrates both portal venous or delayed washout and capsule formation. A 5A-g lesion measures 10–20 mm, demonstrates hypervascularity during the late arterial phase, and has ≥50% diameter growth on serial MRI or CT ≤6 months apart. A 5B lesion measures 20–50 mm, is hypervascular during the late arterial phase, and has one of the following: portal venous or delayed washout, late capsule or pseudocapsule enhancement, ≥50% diameter growth on serial MRI or CT ≤6 months apart, or biopsy-proven HCC. A 5T lesion includes a biopsy-proven HCC, a class 5 lesion treated with locoregional therapy, or persistent/recurrent HCC at a prior treatment site. A class 5X lesion is one that meets radiologic criteria for HCC but is outside stage T2, including a lesion greater than 5 cm in diameter or more than two lesions, each of which are greater than 3 cm in diameter [31]. The UNOS-OPTN criteria do not include lesions less than 1 cm and those that do not demonstrate arterial hyperenhancement. The UNOS-OPTN criteria also defer to the LI-RADS for categorizing nodules that are not included within its imaging criteria for HCC [31].
Conclusion
In summary, various imaging techniques are currently being utilized for the noninvasive diagnosis of HCC including ultrasound, CT, and MRI. Many novel variations of these modalities are emerging for potential of increased utility in the future, including contrast-enhanced ultrasound, ultrasound elastography, dual-energy CT, and MRI elastography, which has already shown promising results. These imaging modalities, along with imaging criteria developed by expert panels using a multidisciplinary approach, share the ultimate goal of identifying patients with HCC at an earlier stage and thereby allowing for early intervention.
References
Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262(1):43–58.
Clark HP, Forrest Carson W, Kavanagh PV, Ho CPH, Shen P, Zagoria RJ. Staging and current treatment of hepatocellular carcinoma. RadioGraphics. 2005;25(Suppl 1):S3–S23.
Miller JC, et al. Screening for hepatocellular carcinoma in cirrhotic patients. J Am Coll Radiol. 2008;5(9):1012–4.
Hussain SM, Reinhold C, Mitchell DG. Cirrhosis and lesion characterization at MR imaging. RadioGraphics. 2009;29(6):1637–52.
Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134(6):1752–63. https://doi.org/10.1053/j.gastro.2008.02.090. Review.
Choi J-Y, Lee J-M, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272(3):635–54.
Bhosale P, Szklaruk J, Silverman PM. Current staging of hepatocellular carcinoma: imaging implications. Cancer Imaging. 2006;6(1):83–94. https://doi.org/10.1102/1470-7330.2006.0014.
Takayasu K, Moriyama N, Muramatsu Y, et al. The diagnosis of small hepatocellular carcinomas: efficacy of various imaging procedures in 100 patients. AJR Am J Roentgenol. 1990;155:49–54.
McEvoy SH, McCarthy CJ, Lavelle LP, Moran DE, Cantwell CP, Skehan SJ, Gibney RG, Malone DE. Hepatocellular carcinoma: illustrated guide to systematic radiologic diagnosis and staging according to guidelines of the American Association for the Study of Liver Diseases. RadioGraphics. 2013;33(6):1653–68.
Tchelepi H, Ralls PW, Radin R, Grant E. Sonography of diffuse liver disease. J Ultrasound Med. 2002;21:1023–32.
Palmieri VO, Santovito D, Marano G, Minerva F, Ricci L, D’Alitto F, Angelelli G, Palasciano G. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. Radiol Med. 2015;120(7):627–33. https://doi.org/10.1007/s11547-014-0494-9. Epub 20 Jan 2015.
Hwang GJ, Kim MJ, Yoo HS, Lee JT. Nodular hepatocellular carcinomas: detection with arterial-, portal-, and delayed-phase images at spiral CT. Radiology. 1997;202:383–8. https://doi.org/10.1148/radiology.202.2.9015062.
Choi J-Y, Lee J-M, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273(1):30–50.
Lee JH, Lee JM, Kim SJ, et al. Enhancement patterns of hepatocellular carcinomas on multiphasic multidetector row CT: comparison with pathological differentiation. Br J Radiol. 2012;85(1017):e573–83. https://doi.org/10.1259/bjr/86767895.
Yoon SH, Lee JM, So YH, Hong SH, Kim SJ, Han JK, Choi BI. Multiphasic MDCT enhancement pattern of hepatocellular carcinoma smaller than 3 cm in diameter: tumor size and cellular differentiation. Am J Roentgenol. 2009;193(6):W482–9.
Parente DB, Perez RM, Eiras-Araujo A, Oliveira Neto JA, Marchiori E, Constantino CP, Amorim VB, Rodrigues RS. MR imaging of hypervascular lesions in the cirrhotic liver: a diagnostic dilemma. RadioGraphics. 2012;32(3):767–87.
Gandhi SN, Brown MA, Wong JG, Aguirre DA, Sirlin CB. MR contrast agents for liver imaging: what, when, how. RadioGraphics. 2006;26(6):1621–36.
Prasad SR, Wang H, Rosas H, Menias CO, Narra VR, Middleton WD, Heiken JP. Fat-containing lesions of the liver: radiologic-pathologic correlation. RadioGraphics. 2005;25(2):321–31.
Kele PG, van der Jagt EJ. Diffusion weighted imaging in the liver. World J Gastroenterol. 2010;16(13):1567–76.
Silva AC, Evans JM, McCullough AE, Jatoi MA, Vargas HE, Hara AK. MR imaging of hypervascular liver masses: a review of current techniques. RadioGraphics. 2009;29(2):385–402.
Venkatesh SK, Ehman RL. Magnetic resonance elastography of liver. Magn Reson Imaging Clin N Am. 2014;22:433–46.
Venkatesh SK, Yin M, Glockner JF, Takahashi N, Araoz PA, Talwalkar JA, Ehman RL. MR elastography of liver tumors: preliminary results. AJR Am J Roentgenol. 2008;190(6):1534–40. https://doi.org/10.2214/AJR.07.3123.
Sporea I, Bota S, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, Badea R, Lupsor M, Fierbinteanu-Braticevici C, Petrisor A, Saito H, Ebinuma H, Friedrich-Rust M, Sarrazin C, Takahashi H, Ono N, Piscaglia F, Borghi A, D’Onofrio M, Gallotti A, Ferlitsch A, Popescu A, Danila M. Acoustic Radiation Force Impulse elastography for fibrosis evaluation in patients with chronic hepatitis C: an international multicenter study. Eur J Radiol. 2012;81(12):4112–8. https://doi.org/10.1016/j.ejrad.2012.08.018. Epub 20 Sept 2012.
Dhyani M, Anvari A, Samir AE. Ultrasound elastography: liver. Abdom Imaging. 2015;40:698–708.
Grajo JR, Patino M, Prochowski A, Sahani DV. Dual energy in practice: basic principles and applications. Radiographics. 2016;36(4):1087–105.
Agrawal MD, Pinho DF, Kulkarni NM, Hahn PF, Guimaraes AR, Sahani DV. Oncologic applications of dual-energy CT in the abdomen. RadioGraphics. 2014;34(3):589–612.
Altenbernd J, Heusner TA, Ringelstein A, Ladd SC, Forsting M, Antoch G. Dual-energy-CT of hypervascular liver lesions in patients with HCC: investigation of image quality and sensitivity. Eur Radiol. 2011;21(4):738–43. https://doi.org/10.1007/s00330-010-1964-7. Epub 10 Oct 2010.
Purysko AS, Remer EM, Coppa CP, Leão Filho HM, Thupili CR, Veniero JC. LI-RADS: a case-based review of the new categorization of liver findings in patients with end-stage liver disease. Radiographics. 2012;32(7):1977–95. https://doi.org/10.1148/rg.327125026.
Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61(3):1056–65.
Cruite I, Tang A, Sirlin CB. Imaging-based diagnostic systems for hepatocellular carcinoma. Am J Roentgenol. 2013;201(1):41–55.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Samreen, N., Grajo, J.R. (2018). Imaging of Hepatocellular Carcinoma. In: Liu, C. (eds) Precision Molecular Pathology of Liver Cancer. Molecular Pathology Library. Springer, Cham. https://doi.org/10.1007/978-3-319-68082-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-68082-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68080-4
Online ISBN: 978-3-319-68082-8
eBook Packages: MedicineMedicine (R0)