Abstract
Ebstein’s malformation is a complex lesion whose spectrum varies from the serious, when there is marked valvar dysplasia and deformity, to being relatively benign in nature. It is, therefore, impossible to provide a single option for treatment without understanding both the developmental aspects of this lesion and the fetal and neonatal presentations affecting not only the heart, but also other organs such as the lungs. An echocardiographer approaching this condition must take cognizance of many issues that are of critical importance when approaching the echocardiogram, which, while it cannot determine the repair on its own, is certainly a major determinant in deciding the type of repair needed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Ebstein’s malformation is a complex lesion whose spectrum varies from the serious, when there is marked valvar dysplasia and deformity, to being relatively benign in nature. It is, therefore, impossible to provide a single option for treatment without understanding both the developmental aspects of this lesion and the fetal and neonatal presentations affecting not only the heart, but also other organs such as the lungs. In a previous volume in this series, I described the echocardiographic features of this condition [1].
An echocardiographer approaching this condition must take cognizance of many issues that are of critical importance when approaching the echocardiogram, which, while it cannot determine the repair on its own, is certainly a major determinant in deciding the type of repair needed, as shown below [1,2,3,4,5,6,7,8,9,10,11,12].
Pathological Considerations for Echocardiography
The tricuspid valvar abnormality in Ebstein’s malformation describes the proximal displacement of the tricuspid valve from its proper position at the right atrioventricular junction (Fig. 13.1) This displacement is not uniform. The anterior leaflet is usually attached normally, with progressive displacement of the septal leaflet from its junctional position across the septum to its junction with the mural (or inferior) leaflet, thus creating two divisions within the right ventricle: an “atrialized” portion of the right ventricle and a functional (or true part) of the right ventricle, distal to the tricuspid attachment to the underlying right ventricular myocardium (Fig. 13.2). This displacement comes about because normal apoptosis, which brings the tricuspid valve into its usual position, does not occur during development.
This pathological specimen is cut to simulate and apical four-chamber view with enlarged right atrium (RA) and right ventricle. The anterior leaflet is seen with its normal cordal attachment to the underlying inferior tricuspid wall. The rest of the mural leaflet is seen lying deep within the ventricle toward the apex (arrows) plastered down from that point to the atrioventricular junction posteriorly (A-V J). The displacement of the valve from the atrioventricular junction (arrows), delimit the “atrialized” portion of the right ventricle from the rest of the right ventricle. The tricuspid septal leaflet is on the ventricular septum but is displaced apically as well when compared to the mitral leaflet lying within the left ventricle LV. Other abbreviations; LA left atrium. With permission from Silverman NH. Anatomic Definition and Imaging of Ebstein’s Malformation. In: Reddington AN, Van Arsdell GS, and Anderson RH, eds. Congenital Diseases of the Right Heart. Springer-Verlag, London 2009 [1]
A pathological view of the valve from an 8-year-old boy who died after the atrial septal defect patch (AS) was placed. The right atrium (RA) has been opened and the tricuspid valve is exposed from this chamber. The three leaflets of the tricuspid valve are labeled. The anterior leaflet (AL) is large and sail-like. The arrows show normal attachment of the leaflet to the underlying atrioventricular junction. The septal leaflet (SL) is not delaminated from the underlying ventricular septal myocardium and the hinge point of the valve (arrow becomes progressively more displaced from the atrioventricular junction). At the junction with the mural leaflet (ML), which is difficult to define clearly in this specimen and may indicate fusion of these two leaflets, the displacement reaches a maximum point away from the right atrioventricular junction (compare this to Fig. 13.1) The mural leaflet in this specimen is largely adhering to the underlying myocardium. It then makes its way back to the atrioventricular junction on its lateral surface. On this specimen note also the abnormal distal attachments of the valve via the abnormal chordae tendiniae as well as the thickening of the valve itself. Other abbreviations; CS coronary sinus orifice. With permission from Silverman NH. Anatomic Definition and Imaging of Ebstein’s Malformation. In: Reddington AN, Van Arsdell GS, and Anderson RH, eds. Congenital Diseases of the Right Heart. Springer-Verlag, London 2009 [1]
The degree of displacement is variable, leading to a variety of situations from mild to severe valvar displacement, and even to the obliteration of the right ventricular outflow tract, producing pulmonary atresia (Fig. 13.3).
This figure shows the morbid anatomy from a fetus that died at 38 weeks of gestational age. The heart is viewed from the anterior aspect of the right ventricle. The features of Ebstein’s malformation of the tricuspid valve are noted with a normal anterior leaflet (AL), a moderately sized mural leaflet (ML) and plastered down (non-delaminated) septal leaflet of the tricuspid valve.(SL). At 20 weeks of gestation this patient’s fetal echocardiogram of the pulmonary valve showed the valve to be patent, but in the course of the next 16 weeks when we last examined this fetus echocardiographically, the pulmonary valve was motionless. The fetus died 2 weeks later. On the specimen a probe has been placed in the main pulmonary artery and shows a thin but atretic pulmonary valve, which had no cusp formation noted on it
It is important to understand the condition’s developmental aspects, because the lesion is modified by the secondary consequences of fetal cardiovascular development, particularly by the effects on the developing right ventricle. In addition to the displacement of the valve from its intended proximal attachment, there is also a change in the tricuspid valve that is deformed in many ways. There are often verrucae on the valvar surfaces. The valve appears to be thickened in some regions and thinned in others (Fig. 13.3). The tendinous cords may be visible through some parts of the valve and the edges of the valve are thickened and rolled. Adherence of the valve to the wall of the ventricle, particularly the mural and septal leaflets, is part of the fundamental morphology of this lesion (Fig. 13.1). The distal attachments of the valvar leaflets to the tendinous cords and papillary muscles and, indeed, to the underlying ventricle, is fundamental and important to surgical liberation of the valve. The attachment of the valve to the underlying myocardium or papillary muscle, particularly of the anterior leaflet, may be linear, hyphenated (through a series of abnormally thickened tendinous cords), or through abnormal or normally attached cords. In some specimens the anterior leaflet can be muscularized (Fig. 13.4).
This morbid anatomic e xample of Ebstein’s malformation from a neonate shows an extremely large right atrium (RA). The patent fossa ovalis (FO can be identified. The mural (ML), septal (SL) and anterior leaflets (AL) of the tricuspid valves are all identified. White arrows highlight the atrioventricular junction. The septal leaflet is only minimally delaminated at its leaflet tips. The anterior leaflet is muscularized (yellow arrowheads). The muscular structures of the right ventricle (RV) are abnormal with thinned trabeculations and papillary muscles supporting the leaflets
In addition to the valvar pathology, other defects of morphological concern are important to the surgeon. The papillary muscles are often elongated and attenuated, and the myocardium is often abnormal in several areas (Fig. 13.4). Because of fetal tricuspid regurgitation the atrium is enlarged and surprisingly thinned. The “atrialized” portion of the right ventricle may also be thinned, sometimes demonstrating replacement fibrosis, and the rest of the ventricle may be thinned as well. Due to the lack of propulsive forward flow across the right ventricular outflow tract the outflow area may not develop well and may become narrowed.
The pulmonary valve plays a unique part in the ongoing development of Ebstein’s malformation (Fig. 13.3). Because tricuspid regurgitation in the fetus is at levels of systemic systolic pressure, and pulmonary arterial pressure transmitted via the arterial duct is also at systemic systolic pressure, the pulmonary valve may be held in a closed position interfering with its development, leading to pulmonary atresia, stenosis or insufficiency (Fig. 13.4). Physiologically this leads to the development of a reversed circuit within the right heart, from left-to-right shunting at arterial duct level through pulmonary regurgitation, tricuspid regurgitation and atrial right-to-left shunting. These features are important both for neonatal management and for surgery. Future fetal therapy may involve administering oxygen to the mother in an attempt to increase fetal oxygen tensions, thereby lowering fetal pulmonary vascular resistance when fetal pulmonary arterioles become sensitive to oxygen in the last trimester. Additionally, prostaglandin synthetase inhibitors such as Indomethacin or ibuprophen can close the ductus in utero, thereby lowering pulmonary arterial pressure, may play a part in allowing affected fetuses to be delivered in a healthier state.
Fetal Echocardiographic Considerations
In a recent collaborative multi-institutional study of a cohort of 264 fetuses, Freud et al. showed a persistently high mortality, both for fetuses and neonates with this condition [13]. This study reported that fetuses have a 40% prenatal demise and neonates have a 35% mortality rate. Of the 72% live-born infants only 68% survived through discharge from the nursery. Although there has been some improvement in the statistics, the prognosis for symptomatic fetal survival still appears poor [14,15,16,17].
Recognition of fetal disorders begins early. We have recorded frank anomalies as early as 16 weeks, and the changes are progressive, relating to developing physiologic abnormalities. It is the degree of tricuspid regurgitation that tends to hold the pulmonary valve in the closed and functionless position (Fig. 13.3). Consequently, pulmonary valvar maldevelopment either causes atresia, stenosis or regurgitation that can be observed by fetal echocardiography (Fig. 13.5; Video 13.1). With progressive increase in systemic pressure and increased afterload the amount of tricuspid regurgitation worsens, because the valve is functionally maldeveloped and the annulus of the valve dilates as right atrial and right ventricular volumes increase (Fig. 13.6; Videos 13.2a and 13.2b). This leads to enlargement of the right heart structures and to the fetal scoring of Roberson and, later, Celermajer and his colleagues, involving the ratio of the area of the right atrium and atrialized right ventricle to the area of the rest of the heart in the four-chamber view (Figs. 13.7, 13.8, and 13.9; Videos 13.3a, 13.3b, 13.3c, and 13.3d) [3, 5, 8].
This fetal echocardiogram was taken from a fetus near term and shows features of the so-called circular shunt. This Doppler color flow superimposition on a cross-sectional image shows tricuspid regurgitation (TR). With ineffective forward flow from the right ventricle, pulmonary blood flow is derived from the ductus shunting left-to-right (L-R Duct). Because the pressure in the pulmonary artery is systemic and the pulmonary valve is not functioning well, there is pulmonary regurgitation (PR). Other abbreviations are (Ao) aorta (RA) right atrium
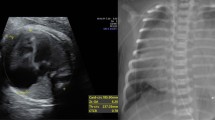
(a) A four-chamber view in a 33-week-old fetus with Ebstein’s malformation and early hydrops fetalis as evidenced by the early skin edema (S Ed). The heart is enlarged, occupying about 70% of the area for the cardiothoracic cavity, due to enlargement of the right heart chambers, the right atrium (RA) and the right ventricle (RV). The lungs (L) are correspondingly small. The left atrium LA and left ventricle (LV) is not enlarged. (b) A corresponding four-chamber view with superimposed Doppler color flow imaging indicates the marked degree of tricuspid regurgitation
This frame is from a fetus with Ebstein’s malformation where the right atrium (RA) and atrialized right ventricle (ARV) are planimitered separately from the left atrium (LA), left ventricle (LV) and the functional right ventricle (FRV). A ratio of >1:1 is a poor prognostic sign. Celermajer and his colleagues expanded this index. With permission from Roberson DA, Silverman NH. Ebstein’s anomaly: echocardiographic and clinical features in the fetus and neonate. J Am Coll Cardiol 1989; 14:1300–7 © Elsevier 1989 [3]
This is a series of four-chamber images in the fetus with varying severities, from the most severe top left) to the mildest (bottom right). The variations on the Celermajer index [7] are shown in white lettering at the bottom part of each frame and the mortality as per these indices are shown in green at the bottom right corner of each image
These are Celermajer plottings of Kaplan-Meier survival curves, cases with fetal or neonatal diagnosis from Arizmendi et al. [5] The left-hand graph shows a dark line representing cases with Celermajer index of Grade 1 or 2 (continuous line), and cases with Grade 3 or 4 (broken line). The right-hand panel represents patients with fetal or neonatal Ebstein’s malformation with cardiothoracic index less than 65% (continuous line) and cases with index equal or greater than 65% (broken line). With permission from Arizmendi AF, Pineda LF, Jiménez, CQ, Azcárate MJM, Sarachaga IH, Urroz E, de León JP, Moya JL, Jiménez MQ. The clinical profile of Ebstein’s malformation as seen from the fetus to the adult in 52 patients. Cardiol Young 2004; 14: 55–63 © Cambridge University Press 2004 [5]
Survival stat istics are available for fetuses and infants with this classification [8]. When pulmonary insufficiency occurs and blood flows backward, this is an ominous sign that my students have termed the “circle of death” (Fig. 13.5). The left heart is also affected, with evidence that the left ventricle contracts poorly because there is no support for the upper ventricular septum against the stress of a systemic right ventricle. Tissue Doppler abnormalities have also been reported [17]. All these features lead to a dilated right heart. The degree of cardiomegaly can be profound, and an increase in the cardiothoracic ratio over 65% leads to an ominous prognosis (Fig. 13.10) [5, 15]. When the circulation is overcome hydrops fetalis develops, because increased permeability of fetal capillaries and low fetal colloid oncotic pressure fail to draw fluid back from the interstitium in the vascular system. As fluid accumulates in the pleural and pericardial spaces lung development is compromised, thereby contributing to postnatal mortality. In some measure hydrops, increased heart size, and the serous cavity component of hydrops all compromise cardiac filling and contribute to diastolic dysfunction.
This is a diagrammatic tracing of the cardiothoracic cavity at the four-chamber level (outer circle) and the outline of the heart (inner circle) from the patient in Fig. 13.6 left. The heart is markedly enlarged and would only fill the area of the thoracic cavity less than twice, whereas it should fill the cavity three times. The cardiac enlargement is due to right heart enlargement
Prenatal echocardiography provides clues as to how the lesion might be handled surgically and which medical and surgical techniques might be considered [18, 19].
Surgical Considerations
As an echocardiographer o ne has to know what the surgical options for this lesion are. There are many options, because of the marked variability in the expression of the lesion (Fig. 13.11). Echocardiography can help in deciding which treatment algorithms are relevant. These algorithms can be divided into the one-ventricle repair option (shunt + tricuspid exclusion and/or pulmonary arterial exclusion), one-and-a-half ventricle repair (with a Glenn shunt) or a two-ventricle repair (tricuspid valvar repair plus tricuspid valvar annuloplasty and atrial septal defect closure). Finally, one has to consider the option of cardiac transplantation. The time of presentation of the anomaly may determine the treatment options. Should the fetus present with a severe form of the disease, for example when hydrops is present, the options are limited. If, for example, there is a small left ventricle that would have difficulty supporting a greater part of the circulation, options are a one-ventricle repair or transplant. The fetal option may, however, be modified by medical management such as early delivery for the severer forms of this malformation (Tworetzky W, personal communication) has used Indomethacin in the hope of decreasing pulmonary pressure by closing the arterial duct, just as Wald et al. [20] achieved in the postnatal situation with a group of neonates presenting with a severe form of the disease. Kohl [18] has suggested, in addition, using maternal hyperoxia in the third trimester fetus in the hope that, should pulmonary vascular resistance fall, right ventricular forward flow may increase (personal communication). These suggestions are currently anecdotal. In the neonate, the treatment algorithm allows several options, as have been espoused by Dearani and his colleagues [21] from the Mayo Clinic (Fig. 13.8) and Bove and his colleagues from the University of Michigan (Fig. 13.9) [22]. If there is antegrade flow in the right heart with a good anterior leaflet as defined echocardiographically, the patient is put on a path toward a two-ventricle repair, which can be delayed to later infancy. If there is anatomic pulmonary atresia and the pulmonary arteries are hypoplastic, their approach is to use the one-ventricle route for repair. If there is functional pulmonary atresia with a hypoplastic left ventricle, or if the left ventricle functions poorly and cannot support the systemic circulation and part or all of the pulmonary circulation, the only option for survival is heart transplantation. If there is functional pulmonary atresia aggressive lowering of the pulmonary arterial pressure by means of oxygen and nitric oxide, and the attempt to close the ductus with indomethacin, as described by Wald and colleagues, should be attempted before surgery is undertaken [20].
This Logic diagram is modified from the method of Joseph Dearani and the Mayo Clinic. With permission from Dearani JA, Said SM, Burkhart HM, Pike RB, O’Leary PW, Cetta F., Strategies for Tricuspid Re-Repair in Ebstein Malformation Using the Cone Technique. Ann Thorac Surg. 2013J;96):202–8 © Elsevier 2013 [21]
The treatment algorithm for single ventricle repair has been successfully used by Starnes et al. and involves an aortopulmonary shunt with closure of the tricuspid and the pulmonary valve [23,24,25,26,27]. These choices depend both on the surgeon’s intuitive operative preferences and on the combined opinion and skill of the medical team. Bove et al. [22] offer an approach in the neonate (Fig. 13.9). In their opinion, repair or replacement of the valve is best avoided in the neonate but may be utilized selectively when the morphology of the valve and the RV function are favorable. If the cyanosis does not abate and there is additional heart failure due to fetal hydrops, the Starnes approach is used [22] When there is cyanosis without heart failure they suggest a shunt alone. If the neonate cannot be weaned from the prostaglandin infusion on the first or second attempt, the patient is on-track for a single ventricle operation, ultimately involving the Fontan procedure , or one-and-a-half ventricle repair. The single ventricle repair track is also chosen if tricuspid closure with a shunt is needed. If the patient can be weaned from pharmacological and ventilation support, no further treatment is offered in the neonatal period.
A decision about a two-ventricle or one-and-a-half ventricle repair, or a total cavopulmonary connection approach can be made at a later date. If there is a later presentation after the neonatal period, or when the neonate successfully recovers from the period of transitional circulation, some form of biventricular repair can be offered.
When there is additional left ventricle compromise in addition to the Ebstein’s malformation, heart transplant remains a plausible and viable option. This option is also indicated if the anterior leaflet of the tricuspid valve is poor or unstable. In some cases where there is left ventricular dysfunction, such as in conditions associated with spongy myocardium, or where there is bad function of the ventricles, heart transplant remains the only viable option. We had a two-day-old neonate with a poor left ventricle due to spongy myocardium with Ebstein’s malformation who was successfully transplanted (Figs. 13.12 and 13.13; Videos 13.4a and 13.4b).
This diagram of the treatment protocol for Ebstein’s anomaly in the neonate is modified from Bove EL, Hirsch JC, Ohye RG, Devaney EJ. How I Manage Neonatal Ebstein’s Anomaly Semin Thorac Cardiovasc Surg Pediatr Card Surg Ann 2009;12:63–65, with permission © Elsevier 2009 [22]
Top. A Four-chamber image in a 36-week-old fetus with marked enlargement of the right atrium (RA) and an atrial septum which is aneurysmal (ASA), bulging into the left atrium (LA) The left ventricle (LV) demonstrates numerous trabeculations characteristic of spongy myocardium. Only the atrialized portion of the right ventricle is seen in this four-chamber cut because the tricuspid valve was displaced into the right ventricular outflow area. The descending aortic (Dao) lies posterior to the left atrium. Bottom: An immediate postnatal echocardiogram demonstrating the spongy trabeculations lying within the apical portion of the left ventricle in this color-compare apical magnified four-chamber cut
There are several operative techniques involved when considering a two-ventricle repair. Carpentier and Chavaud described the first approach [27,28,29,30]. This approach involves repair of the tricuspid valve, atrial reduction and tricuspid annuloplasty. Knott-Craig, and most recently da Silva and Dearani, have reported modification of the approach in what has now been termed the “Cone” procedure (Figs. 12.3 and 13.14) [21, 31,32,33]. In this procedure, the antero-superior and posterior tricuspid valve leaflets are detached from the right ventricle, the cut edge of this complex is rotated clockwise and re-sutured to the septal border of the anterior leaflet (thus creating a cone), the vertex of which remains fixed at the right ventricular apex and the base of which is sutured to the true tricuspid valve annulus level. The septal leaflet is incorporated into the cone of tissue whenever possible, even incorporating a pericardial addition to the circumference of the cone. A folding or excision of the redundant tissue accomplishes reduction in the atrial size and the atrialized portion of the right ventricle, and the atrial septal defect is closed in a “valved” fashion. The results have been excellent and have been successfully completed in very young infants, as young as 5 days of age [33].
A view of the Cone reconstruction of Ebstein anomaly. The main principle includes complete surgical delamination of the tricuspid valve septal leaflet, which is mobilized and then rotated and reattached at the true atrioventricular junction, followed by leaflet-to-leaflet attachment, creating a “leaflet cone.” Fig. 12.3 also depicts the Cone operation as defined by Da Silva [32]
There is a preference by some surgeons for performing a bidirectional Glenn when operating on age-appropriate babies in order to assist the right ventricle in its recovery and pulmonary perfusion. This is often done when there is an enlarged and dysfunctional right ventricle, when the right atrial pressure is 1.5 times greater than the left atrial pressure after separation from cardiopulmonary bypass, and when the repaired tricuspid orifice is small. It also has the advantage of reducing tension on the repair, facilitating a tighter tricuspid annuloplasty [34, 35].
Still in favor is the atrial reduction and valve annuloplasty described by Danielson [30, 35] a very successful procedure with long-lasting favorable results. These authors also perform a moderate apical ventricular reduction and an aggressive annular reduction down to an area of 2.5 cm2/m2 BSA [35].
Other options for the worst tricuspid pathology include replacement of the tricuspid valve with a prosthesis.
Attention to associated disease such as ventricular septal defects, or right ventricular outflow problems such as subpulmonary obstruction, pulmonary valvar repair or replacement, repair of supravalvar stenosis, atrial septal defect and partial vein anomaly repair, or electro-physiological operations, are all part of the repair strategy [35].
Echocardiography
What can ultrasound do to define the lesion for the surgeon? As is the case with atrioventricular canal defects, the surgeon who deals with Ebstein’s malformation has to define what can best be done by assessing the valve visually. Ultrasound certainly plays a part in this assessment, but visual inspection of the valve is of the utmost importance. We described the echocardiographic features of this malformation in a previous volume from this symposium so will concentrate on the key features of what echocardiography can currently provide for the surgical repair [1].
The Four-Chamber View
The apical four-chamber view first demonstrated the downward displacement of the septal leaflet of the tricuspid valve and the area of the atrialized right ventricle (Fig. 13.15; Videos 13.5a and 13.5b) [2]. The degree of displacement roughly parallels the degree of tricuspid regurgitation and, therefore, the severity of the disease. Because the valve in this condition is displaced anteriorly there may well be patients with more severe forms of this malformation in whom the valve is not seen in the classical four-chamber view at all. A craniad tilt of the transducer on the cardiac apex brings the imaging plane of the transducer to lie antero-superiorly, defining the tricuspid valve lying in the outflow tract.
This series of apical four-chamber images shows the progressive displacement of the tricuspid septal leaflet with progressive severity of the disease. In this view the anterior and septal leaflets are demonstrated. Top Frame: The right atrium (RA) in the top frame is minimally enlarged, as is the right ventricle. Note the attachment of the anterior leaflet is normal in all of these frames. The lateral arrows indicate the chordae tendiniae, attached to the right ventricular wall. The medial arrows indicate the septal attachment of the tricuspid valve. The mitral valve for comparison lies between the left atrium (LA) and left ventricle (LV). Middle Frame: This shows a moderated degree of displacement of the septal leaflet of the tricuspid valve (Medial arrow). The lateral arrow indicates thin attachment of the tendinous cords underneath the anterior leaflet. The medial arrow shows the septal leaflet of the tricuspid valve, which is thickened and rolled. The right atrium is moderately enlarged and encroaches on the left atrium, which is small. The right ventricle is also much larger than its fellow. Bottom Frame: This shows a severe degree of displacement of the septal leaflet of the tricuspid valve (medial arrow). The lateral arrows again demonstrate tendinous cordal attachment of the anterior leaflet of the tricuspid valve to the underlying right ventricle. Compared to the middle frame, the right heart chambers are proportionally larger. When these images were seen with Doppler color flow the degree of tricuspid regurgitation paralleled the displacement of the valve and the chamber enlargement too
It is important to recognize attachments of the anterior leaflet, which can be identified in this view, to the underlying myocardium (Figs. 13.15 and 13.16; Video 13.6). Indeed, it is possible using this view to define distal attachment of the valve to the underlying myocardium. The attachment may be normal, hyphenated or linear to the underlying myocardium, and these features are important in planning the type of procedure contemplated. The valve leaflets may be thickened in parts, thinned in other parts and a degree of tethering with tendinous cords or attachment to the underlying myocardium can be assessed in this view. Systolic prolapse of the leaflets may be observed on real-time imaging. The displacement of the septal leaflet can be defined, and has been used to define this disorder echocardiographically [2, 11]. The four-chamber view has also demonstrated the mobility of the septal leaflet and how much redundancy of the valvar leaflets is present, which is important for the modern Cone procedure (Fig. 13.16).
This four-chamber image shows an infant with severe Ebstein’s malformation without systolic cooptation of a thickened and restrictive tricuspid valve (arrows). The electrocardiogram indicates the frame is taken in late systole. The right atrium is enlarged far beyond the descending aorta (Dao), which usually delimits the posterior extent of the heart in this view. The functional right ventricle (RV) is small. The left atrium (LA) and left ventricle (LV) appear small
The degree of tricuspid regurgitation may be determined echocardiographically, and the index described by Celermajer may be used to define the outcome of this disorder (Fig. 13.17) [7]. Modern techniques of management, both medical and surgical, have improved the outcomes and blurred the distinctions of Celermajer’s original description [24].
This figures shows the Kaplan-Meier Survival curves for 28 patients with echocardiographic grading of the severity of their disorder as reported by Celermajer et al. [7]. The ratio of the atrialized right ventricle and the right atrium to the rest of the heart in the four-chamber view was shown previously in Fig. 13.8. With permission from Celermajer DS, Dodd SM, Greenwald SE, et al. Morbid Anatomy in Neonates with Ebstein’s anomaly of the Tricuspid Valve: Pathophysiological and Clinical Implications. J Am Coll Cardiol 1992;19: 1049–53 © Elsevier 1992 [7]
From the apical four-chamber view the associated size of the mitral and tricuspid annuli may be measured. It is well known that annular dilation may be a poor prognostic sign, which, at direct surgical inspection of the valve, may be as large as 6 cm/m2 of body surface area [35]. Marked dilation, as well as multiple jets of tricuspid regurgitation, are poor prognostic signs of successful repair.
Although one has to concede that echocardiography is limited in its ability to predict right ventricular function, it does provide a visual index of how poor the function is and where thinning of the myocardium exists. At the same time, from the four-chamber view, the left ventricle can be assessed for function and dyskinetic contraction. These techniques have now been enhanced by speckle tracking and Tissue Doppler indices for assessing cardiac function [36].
The Subcostal View
This transducer location provides a most important complement to the four-chamber view as can be seen from the diagram popularized by Chauvaud and Carpentier, which can be matched exactly with that seen by echocardiography (Figs. 13.18 and 13.19; Videos 13.7a, 13.7b, 13.7c, and 13.7d) [28]. Even in the adult it is possible to obtain views in this location or they may be obtained by transgastric imaging using transesophageal echocardiography.
This figure is a diagrammatic classification of Ebstein’s malformation using the classification of types. [27] Type A shows minimal displacement of the attachment of the septal leaflet to the underlying myocardium and the small size of the atrialized right ventricle. Type B shows moderate displacement of the septal leaflet with a large atrialized right ventricle. Type C shows important displacement of the septal and postero-inferior leaflet associated with a non-or dyskinetic atrialized right ventricle, and restricted anterior leaflet motion with short cords. Note also the thinning of the inferior wall of the atrialized portion of the right ventricle at its acute margin. Type D is termed the tricuspid sack. Based on this classification various treatment options can be planned
These subcostal coronal images demonstrate the same leaflet pathology as shown in Fig. 13.18 and indicate how echocardiography can fit into a surgical classification for treatment. The lesions are mild in the top left frame, more severe in the top right frame, worse in the bottom left frame and the worst in the bottom right frame. The rotation of the inferior leaflet (arrow) shows progressive displacement from the functional tricuspid annulus. AAo ascending aorta, LA left atrium, RA right atrium, RV right ventricle. These images were matched with the images show in Fig. 13.18
In the subcostal coronal view the anterior and mural leaflets are seen, permitting all the valve leaflets to be assessed. The subcostal coronal view provides the echocardiographic equivalent of what Carpentier and Chauvaud describe in their classification of patients for surgical repair (Figs. 13.18, 13.19, 13.20, and 13.21) [27,28,29]. The view, which is shown diagrammatically (Fig. 13.18), defines another important aspect of leaflet pathology that can be seen on the subcostal coronal echocardiographic cut, which shows mural leaflet displacement from its usual position. Normally, the Eustachian valve and the entrance of the inferior vena cava into the right atrium mark the position of the mural leaflet. In Ebstein’s malformation the mural leaflet is markedly displaced (Fig. 13.19). The position of the anterior leaflet is also defined in this lesion. In the mildest forms the leaflet is minimally displaced and the atrialized portion of the right ventricle can be gauged to be small whereas, when the mural leaflets are maximally displaced, the leaflets can be seen in the right ventricular outflow tract. The direction of the jet of tricuspid regurgitation as directed with Doppler color flow is altered depending on the place where apposition of the valvar leaflets occurs, varying from mild displacement inferiorly from the atrial posterior wall toward the diaphragm in the milder forms of this anomaly, to a position directed toward the inferior wall of the atrialized right ventricle (Fig. 13.20). This occurs because the plane of the valvar closure plane is rotated into the right ventricular outflow, as shown in the diagrammatic images of Schreiber et al. (Fig. 13.21). As the direction of the jet is most frequently axial to that plane, the direction of the tricuspid regurgitation jet is altered, as shown in Fig. 13.20. With progressive valvar rotation toward the outflow tract, the direction of the jet moves perpendicular to the place of the valve indicating the plane of functional apposition of the valvar leaflets.
This figure is the color information for Fig. 13.18 (bottom left). The tricuspid regurgitation from the diminutive right ventricle (RV) is directed inferiorly toward the diaphragm and the apex of the right ventricle, which is substantially “atrialized” (ARV). The right atrium (RA) is also enlarged
This is a stylized representation of the angle of tilt of the effective valvar orifice (ellipses) as estimated from the 23 morbid anatomical specimens from the work of Schreiber et al. [4] which also mirrors our experience using echocardiography. The solid lines represent the cases with linear attachment; the ellipses with dotted lines and broken lines represent hearts with focal and hyphenated attachment of the antero-superior leaflet, respectively. The vertical ellipse represents the solitary case with the orifice parallel to the atrioventricular junction. The scale represents the maximal width of the orifice as a percentage of the distance between the atrioventricular junction and the apex of the right ventricle measured along the acute margin. With permission from Schreiber, C, Cook A, Ho S-Y, Augustin N,. Anderson RH, Morphologic Spectrum of Ebstein’s malformation: Revisitation Relative to Surgical Repair. J Thorac Cardiovasc Surg 1999;117: 148–55 © Elsevier [4]
Mobility and thickening of the leaflets, as well as lack of apposition in systole, can be defined from this view. Cordal attachments of the anterior and mural leaflets of the valve to the underlying myocardium can also be defined from this view (Figs. 13.22, 13.23, and 13.24; Videos 13.8, 13.9, and 13.10).
This subcostal sagittal-cut image series shows gray scale images above with Doppler color flow superimposition below. These are the orthogonal images, taken from Fig. 13.18 top right, and show how small the true right ventricle can be when imaged together from these orthogonal views. Anterior to the right ventricle is a pericardial effusion. The tricuspid leaflets are thickened and rolled, and divide the right ventricle into an atrialized portion (ARV) and a functional portion (RV). The pulmonary valvar leaflets are also thickened and were held closed on the real-time image with blood flow supplied to the pulmonary arteries exclusively from the arterial duct. The bottom color frame shows two jets of tricuspid regurgitation (TR and arrows) arising from different coaptation points of the tricuspid valve
This subcostal sagittal cut shows a series of images in gray scale above with Doppler color flow superimposition below in a milder form of this anomaly. In this example the functional right ventricle (FRV) is larger and the pulmonary artery (PA) more prominent. The atrialized right ventricle (ARV) lies on the diaphragmatic or acute marginal surface of the heart. The tricuspid jet in the bottom frame is directed toward the diaphragm. LV left ventricle
In this example of severe neonatal Ebstein’s malformation taken in the subcostal coronal view in gray scale above and with color below, there is no coaptation of the abnormally thickened and deformed tricuspid valvar leaflets in systole as indicated by arrows on the electrocardiogram. The functional right ventricle is small, as are the main (MPA) and right pulmonary arteries (RPA). The point of junction between the atrialized right ventricle and the right atrium is not shown in this example that concentrates on the valvar leaflets and the tricuspid regurgitation. The arrow shows the direction of the jet on the color flow image
Using this view, and its orthogonal equivalent the subcostal sagittal view, a volume assessment of the right ventricular size may be made by biplane two-dimensional echocardiography. The orthogonal subcostal view and the sagittal view are invaluable for defining the degree of right ventricular outflow narrowing, the quality of contraction of the functional right ventricle and the degree of pulmonary regurgitation (Figs. 13.22, 13.23, and 13.24). In the neonate these subcostal views define the presence of a ductus and, most importantly, the degree of pulmonary and tricuspid regurgitation and the sites of origin of these jets. The two subcostal views in the coronal and sagittal planes are used together to define the size of the tricuspid valve and to provide a much better appreciation of the valvar function, as well as the size of the right atrioventricular junction and the true right ventricle.
Parasternal Short Axis View
The parasternal short axis view is particularly valuable for surgical decision in older patient as it provides complementary views of the tricuspid valve and the atrial septum as well as pulmonary valvar stenosis, atresia or regurgitation, and the presence of a patent arterial duct (Fig. 13.25; Video 13.11). The view is also used for assessing the atrial septum, and permits important assessment of the sizes of the main and branch pulmonary arteries. It also permits assessment of ventricular “pancaking ” and the attachment of the tricuspid valvar leaflets and their attachment to the underlying myocardium (Fig. 13.26; Videos 13.12a and 13.12b).
This figure, taken in the parasternal short axis cut at the level of the great arteries, is a gray scale and Doppler color flow simultaneous recording in late diastole in an infant. The main (MPA) and left and right branch pulmonary arteries (LAP, RPA) are small and there is some regurgitation into the right ventricular outflow indicating pulmonary insufficiency. There is also a ductus left-to-right shunt noted by the red and yellow stream coming toward the pulmonary valve in the main pulmonary artery, and the majority of this flow is reflected off the pulmonary valve and returns back into the main pulmonary artery indicated by the blue signal, also seen entering the small pulmonary arterial branches. The Doppler color flow also indicates disturbed flow from the descending aorta (DAO). Other abbreviations: Ao Aortic root, RA right atrium
This parasternal short axis view taken at the level of the ventricles shows the Doppler color flow image above and the gray scale image below. The right atrium (RA) right and left ventricles (RV and LV) are shown. The enlargement of the right ventricle pushes the ventricular septum backward, diminishing the size of the left ventricle. In the top frame, the direction of the tricuspid regurgitant jet is from the displaced apposition of the valve as shown by a highlighted arrow. In the bottom frame, small arrows show the attachments of the chordae tendiniae to the tricuspid valve
Right Ventricular Long Axis View
In many adult patients it may be difficult to identify the mural leaflet. Parasternal imaging is helpful in this regard as, when one angulates the transducer medially on the other side of the ventricular septum, one can define the coronary sinus and the mural leaflet of the tricuspid valve (Fig. 13.27; Video 13.13). This view provides the surgeon with the opportunity to assess cordal attachments to the underlying myocardium on the anterior leaflet and a similar view can also be obtained by transesophageal echocardiography.
This parasternal long axis cut with medial angulation brings the right heart into view. The plane passes through the right ventricle (RV) anteriorly and the right atrium (RA) posteriorly. The coronary sinus (CS, highlighted arrow) is identified. The leaflets seen in this view are the anterior (ATL) and the mural (ML) tricuspid leaflets. The small arrows indicate the attachments of the tendinous cords in the anterior leaflet. The mural leaflet is displaced from the coronary sinus orifice demarcating the length of the displacement for the atrialized portion of the right ventricle
The Suprasternal Views
Suprasternal views provide information about the patency of the arterial duct (Fig. 13.28; Video 13.14). Arch morphology, as well as information about the size of the branch pulmonary arteries, is also available from these views.
This suprasternal sagittal plane cut in the plane of the aortic arch in an infant shows the presence of a patent arterial duct (Duct) shunting left to right on this Doppler color flow image. The flow disturbance carries out into the right pulmonary artery (RPA). Other abbreviations: AAo ascending aorta, INN Vn innominate vein, IA innominate artery, DAo descending aorta
Additional Defects
Additional defects of surgical importance may also be present in this anomaly, and a thorough search for these defects needs to be made. We have found a number of patients with ventricular septal defects (Fig. 13.29; Video 13.15). Bicuspid aortic valves and, more important for the patient who requires urgent therapy in the neonatal period, aortic coarctation has also been noted.
This four-chamber cut in an older child shows the presence of severe Ebstein’s malformation with only a small part of the anterior leaflet of the valve identified (AL). The tricuspid valve has become plastered down to the underlying myocardium of the right ventricle. A small ventricular septal defect is identified (VSD, arrow) to the functional right ventricle as the only source of pulmonary blood flow. Other Abbreviations: D Ao descending aorta, LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
Three-Dimensional Echocardiography
Three-dimensional echocardiography affords a tremendous opportunity for defining issues related to surgical repair, including a true picture of leaflet mobility that the surgeon will not be able to emulate, as well as orifice size and points of origin of valvar insufficiency (Figs. 13.30 and 13.31; Videos 13.16 and 13.17). The technique provides useful definition in adult patients with tricuspid valvar dysplasia as opposed to Ebstein’s malformation [37]. The technique provides a view of the tricuspid leaflets in their entirety and images of the leaflets, which cannot be seen surgically because they are viewed from the right ventricular outflow tract (Fig. 13.30). Currently three-dimensional imaging is not able to reproduce the fine attachment of the tendinous cords to the underlying valvar commissures or to the papillary muscles.
This 3-dimensional full volume rendering of a child with Ebstein’s malformation is cut from the anterior aspect of the right ventricle and shows the displaced valvar leaflets at its apex with the anterior (A), septal (S), and mural leaflet (M) seen. The right ventricular anterior (RVAW) and inferior (RVIW) walls are noted
This transesophageal example was made using a full volume technique. The image has been cut from the atrial aspect. The aorta (Ao) is also sliced medially in the image. The anterior (A) septal (S) and mural leaflets (M), are shown and the right ventricular inferior wall (RVIW) is noted. This image is courtesy of Dr. David Roberson
In imaging the tricuspid valve from the three-dimensional dataset it is important to make the viewpoint from the inferior right ventricular wall toward the displaced valve especially if the displacement is marked, where it cannot be seen from the conventional right atrial viewpoint. It is also important to image the valve from the right ventricular apex or anterior wall, looking backward to image the three leaflets and their distal attachments (Fig. 13.30).
Conclusion
Echocardiography provides a unique and important addition to the pre-operative assessment of Ebstein’s malformation. The ability to see valve motion, attachment to the wall of the ventricle, cordal attachments, attachments to the underlying myocardium and the degree of thickening and mal-cooptation of the valve are unrivalled by other imaging modalities. Echocardiography also provides an assessment of the areas of tricuspid valve leakage, as well as functional capacity of the right and left ventricles. These attributes persist in the operating room, immediately after repair and in post-operative assessment when the patient has had an operative repair. This view is second only to the exquisite view available to the experienced surgeon performing the repair.
References
Silverman NH. Anatomic definition and imaging of Ebstein’s malformation. In: Reddington AN, Van Arsdell GS, Anderson RH, editors. Congenital diseases of the right heart. London: Springer; 2009.
Ports TA, Silverman NH, Schiller NB. Two-dimensional assessment of Ebstein’s anomaly. Circulation. 1978;58:336.
Roberson DA, Silverman NH. Ebstein’s anomaly: echocardiographic and clinical features in the fetus and neonate. J Am Coll Cardiol. 1989;14:1300–7.
Schreiber C, Cook A, Ho S-Y, Augustin N, Anderson RH. Morphologic spectrum of Ebstein’s malformation: revisitation relative to surgical repair. J Thorac Cardiovasc Surg. 1999;117:148–55.
Arizmendi AF, Pineda LF, Jiménez CQ, Azcárate MJM, Sarachaga IH, Urroz E, de León JP, Moya JL, Jiménez MQ. The clinical profile of Ebstein’s malformation as seen from the fetus to the adult in 52 patients. Cardiol Young. 2004;14:55–63.
Attenhofer-Jost CJ, Connolly HM, Edwards WD, Hayes D, Warnes CA, Danielson GK. Ebstein’s anomaly- review of a multifaceted congenital cardiac lesion. Swiss Med Weekly. 2005;135:269–81.
Celermajer DS, Dodd SM, Greenwald SE, et al. Morbid anatomy in neonates with Ebstein’s anomaly of the tricuspid valve: pathophysiological and clinical implications. J Am Coll Cardiol. 1992;19:1049–53.
Celermajer DS, Cullen S, Sullivan ID, Spiegelhalter DJ, Wyae KH, Deanfield JE. Outcome in neonates with Ebstein’s anomaly. J Am Coll Cardiol. 1992;19:1041–6.
Celermajer DS, Bull C, Till JA, Cullen S, Vassillikos VP, Sullivan ID, Allan L, N Annopoulos P, Sommerville J, Deanfield JE. Ebstein’s anomaly: presentation and outcome from fetus to adult. J Am Coll Cardiol. 1994;23(1):170–6.
Hornberger LK, Sahn DJ, Kleinman CS, Copel JA, Reed KL. Tricuspid valve disease with significant tricuspid insufficiency in the fetus: diagnosis and outcome. J Am Coll Cardiol. 1991;17:167–73.
Shiina A, Seward JB, Edwards WD, Hagler DJ, Tajik AJ. Two-dimensional echocardiographic spectrum of Ebstein’s anomaly: detailed anatomic assessment. J Am Coll Cardiol. 1984;3:356–70.
Shiina A, Seward JB, Tajik AJ, Hagler DJ, Danielson GK. Two dimensional echocardiographic-surgical correlation in Ebstein’s malformation: preoperative determination of patients requiring tricuspid valve application vs. replacement. Circulation. 1983;68:534–44.
Freud LR, Escobar-Diaz M, Kalish B, et al. Perinatal outcomes after fetal diagnosis of Ebstein anomaly or tricuspid valve dysplasia in the current era: a multi-center study. J Am Coll Cardiol. 2014;12(63):A473.
Lee AHS, Moore IE, Nuala LK, Fagg NLK, Cook AC, Kakadekar AP, Allan LD, Keeton BL, Anderson RH. Histological changes in the left and right ventricle in hearts with Ebstein’s malformation and tricuspid valvar dysplasia: a morphometric study of patients dying in the fetal and perinatal periods. Cardiovascu Path. 1995;4:19–24.
Andrews RE, Tibby SM Sharland G Simpson JM. Prediction of outcome of tricuspid valve malformations diagnosed during fetal life. Cardiol Young. 2006;16(Suppl):6–19.
McElhinney DB, Salvin JA, Colan SD, Thiagarajan R, Crawford EC, Marcus EN, del Nido Pedro J, Tworetzky W. Improving outcomes in fetuses and neonates with congenital displacement (Ebstein’s malformation) or dysplasia of the tricuspid valve. Am J Cardiol. 2005;96:582–6.
Inamura N, Taketazu M, Smallhorn JF, Hornberger LK. Left ventricular myocardial performance in the fetus with severe tricuspid valve disease and tricuspid insufficiency. Am J Perinatol. 2005;22:91–7.
Kohl T. Chronic intermittent materno-fetal hyperoxygenation in late gestation may improve on hypoplastic cardiovascular structures associated with cardiac malformations in human fetuses. Pediatr Cardiol. 2010;31:250–63.
Rasanen J, Wood DC, Debbs RH, Cohen J, Weiner S, James C, Huhta JC. Reactivity of the human fetal pulmonary circulation to maternal hyperoxygenation increases during the second half of pregnancy. a randomized study. Circulation. 1998;97:257–62.
Wald RM, Adatia I, Glen S, Van Arsdell Glen S, Hornberger LK. Relation of limiting ductal patency to survival in neonatal Ebstein’s anomaly. Am J Cardiol. 2005;96:851–6.
Dearani JA, Said SM, Burkhart HM, Pike RB, O’Leary PW, Cetta F. Strategies for tricuspid re-repair in Ebstein malformation using the cone technique. Ann Thorac Surg. 2013;96:202–8.
Bove EL, Hirsch JC, Ohye RG, Devaney EJ. How I manage neonatal Ebstein’s anomaly. Semin Thorac Cardiovasc Surg Pediatr Card Surg Ann. 2009;12:63–5.
Starnes VA, Pitlick PT, Bernstein D, Griffin ML, Choy M, Shumway NE. Ebstein’s malformation appearing in the neonate. A new surgical approach. J Thorac Cardiovasc Surg. 1991;101:1082–7.
Reemtsen BL, Fagan BT, Wells WJ, Starnes VA, Current MD. surgical therapy for Ebstein anomaly in neonates. J Thorac Cardiovasc Surg. 2006;132:1285–90.
Watanabe M, Harada Y, Takeuchi T, Satomi G, Yasukouchi S. Modified starnes operation for neonatal Ebstein’s anomaly. Ann Thorac Surg. 2002;74:917–9.
Pflaumer A, Eicken A, Augustin N, Hess J. Symptomatic neonates with Ebstein anomaly. J Thorac Cardiovasc Surg. 2004;127:1208–9.
Carpentier A, Chauvaud S, Mace L, et al. A new reconstructive operation for Ebstein anomaly of the tricuspid valve. J Thorac Cardiovasc Surg. 1988;96:92–101.
Chavaud S, Fuzellier JF, Berrebi A, et al. Bi-directional cavopulmonary shunt associated with ventriculo and valvuloplasty in Ebstein’s malformation: benefits in high risk patients. Eur J Cardiothorac Surg. 1998;13:514–9.
Chauvaud SM, Mihaileanu SA, Gaer JAR, Carpentier AC. Surgical treatment of Ebstein’s malformation—the “Hôpital Broussais” experience. Cardiol Young. 1996;6:4–11.
Brown ML, Dearani JA, Danielson GK, Cetta F, Connolly HM, Warnes CA, Li Z, Hodge DO, Driscoll DJ. The outcomes of operations for 539 patients with Ebstein anomaly. J Thorac Cardiovasc Surg. 2008;135(5):1120–36.
Knott-Craig CJ, Overholt ED, Ward KE, Ringewald JM, Baker SS, Razook JD. Repair of Ebstein’s malformation in the symptomatic neonate: an evolution of technique with 7-year follow-up. Ann Thorac Surg. 2002;73:1786–92.
da Silva JP, José Francisco Baumgratz JF, da Fonseca L, Franchi SM, Lopes LM, Tavares GMP, Soares AM, Moreira LF, Barbero-Marcial MD. The cone reconstruction of the tricuspid valve in Ebstein’s anomaly. The operation: early and midterm results. J Thorac Cardiovasc Surg. 2007;133:215–23.
Anderson HN, Dearani JA, Said SM, Norris MD, Pundi KN, Miller AR, Cetta ML, Eidem BW, O’Leary PW, Cetta F. Cone reconstruction in children with Ebstein anomaly: the mayo clinic experience. Congenit Heart Dis. 2014;9(3):266–71.
Marianeschi SM, McElhinney DB, Reddy VM, Silverman NH, Hanley FL. Alternative approach to the repair of Ebstein’s malformation: intracardiac repair with ventricular unloading. Ann Thorac Surg. 1998;66:1546–50.
Malhotra SP, Petrosian E, Reddy VM, Qui M, Maeda K, Suleman S, McDonald M, Reinhartz O, Hanley F. Selective right ventricular unloading and novel concepts in Ebtein’s anomaly. Ann Thorac Surg. 2009;88:1975–81.
Eidem BW, Tei C, O’Leary PW, Cetta F, Seward JB. Non-geometric quantitative assessment of right and left ventricular function: myocardial performance index in normal children and patients with Ebstein’s anomaly. J Am Soc Echocardiogr. 1998;11:849–56.
Bharucha T, Anderson RH, Lim ZS, Vettukatti JJ. Multiplanar review of three-dimensional echocardiography gives new insights into the morphology of Ebstein’s Malformation. Cardiol Young. 2010;20:49–53.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Electronic Supplementary Material
This Doppler color flow superimposition on a cross-sectional image shows tricuspid regurgitation (TR) (MOV 923 kb)
Left. Pulmonary valvar maldevelopment either causes atresia, stenosis or regurgitation that can be observed by fetal echocardiography (MOV 1894 kb)
Right. Pulmonary valvar maldevelopment either causes atresia, stenosis or regurgitation that can be observed by fetal echocardiography (MOV 954 kb)
Bottom left. Fetus with varying severities (MOV 628 kb)
Bottom right. Fetus with varying severities (MOV 1740 kb)
Top left. Fetus with varying severities (MOV 1497 kb)
Top right. Fetus with varying severities (MOV 840 kb)
Bottom. A 36-week-old fetus with marked enlargement of the right atrium (RA) and an atrial septum which is aneurysmal (ASA), bulging into the left atrium (LA) (MOV 556 kb)
Top. A 36-week-old fetus with marked enlargement of the right atrium (RA) and an atrial septum which is aneurysmal (ASA), bulging into the left atrium (LA) (MOV 554 kb)
Top. Progressive displacement of the tricuspid septal leaflet with progressive severity of the disease (MOV 904 kb)
Middle. Progressive displacement of the tricuspid septal leaflet with progressive severity of the disease (MOV 260 kb)
Infant with severe Ebstein’s malformation without systolic cooptation of a thickened and restrictive tricuspid valve (MOV 233 kb)
Top left. Leaflet pathology (MOV 649 kb)
Top right. Leaflet pathology (MOV 672 kb)
Bottom left. Leaflet pathology (MOV 283 kb)
RLP. Leaflet pathology (MOV 306 kb)
Cordal attachments of the anterior and mural leaflets of the valve to the underlying myocardium (MOV 133 kb)
Cordal attachments of the anterior and mural leaflets of the valve to the underlying myocardium (MOV 1542 kb)
Cordal attachments of the anterior and mural leaflets of the valve to the underlying myocardium (MOV 592 kb)
Late diastole in an infant (MOV 774 kb)
Top. Ventricular “pancaking” and the attachment of the tricuspid valvar leaflets and their attachment to the underlying myocardium (MOV 384 kb)
Bottom Ventricular “pancaking” and the attachment of the tricuspid valvar leaflets and their attachment to the underlying myocardium (MOV 476 kb)
Coronary sinus and the mural leaflet of the tricuspid valve (MOV 345 kb)
Patency of the arterial duct (MOV 401 kb)
Ventricular septal defect (MOV 3502 kb)
Three-dimensional echocardiography affords a tremendous opportunity for defining issues related to surgical repair, including a true picture of leaflet mobility that the surgeon will not be able to emulate, as well as orifice size and points of origin of valvar insufficiency (MOV 1158 kb)
Three-dimensional echocardiography affords a tremendous opportunity for defining issues related to surgical repair, including a true picture of leaflet mobility that the surgeon will not be able to emulate, as well as orifice size and points of origin of valvar insufficiency (MOV 1483 kb)
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Silverman, N.H. (2018). Ebstein’s Malformation: Does Echocardiographic Assessment Determine Surgical Repair?. In: Friedberg, M., Redington, A. (eds) Right Ventricular Physiology, Adaptation and Failure in Congenital and Acquired Heart Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-67096-6_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-67096-6_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67094-2
Online ISBN: 978-3-319-67096-6
eBook Packages: MedicineMedicine (R0)