Abstract
The phosphodiesterase (PDE) family of enzymes play a role in the degradation of cyclic adenosine monophosphate, an important intracellular second messenger central to multiple signaling pathways. PDE inhibitors have been developed and evaluated for the treatment of a variety of conditions, including asthma, chronic obstructive pulmonary disease, erectile dysfunction, Alzheimer’s disease, and chronic inflammatory skin diseases. Apremilast, an oral PDE4 inhibitor, has FDA approval for the treatment of moderate to severe plaque and psoriatic arthritis. It has also been shown to have efficacy in other skin diseases, such as atopic dermatitis, Bechet’s disease, pityriasis rubra pilaris, alopecia areata, and discoid lupus erythematosus. Topical PDE4 inhibitors (e.g., crisaborole) also show efficacy in the treatment of atopic dermatitis and psoriasis. In this review, we provide an overview of the role of PDE inhibition in the treatment of inflammatory skin disease and discuss ongoing developments for this novel drug class.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Phosphodiesterase inhibitor
- PDE4
- Cyclic adenosine monophosphate
- Inflammatory skin disease
- Psoriasis
- Atopic dermatitis
- Apremilast
- Crisaborole
Introduction
Phosphodiesterases (PDEs) are a family of enzymes that hydrolyze cyclic nucleotides and contribute to the intracellular regulation of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) [1]. cAMP and cGMP are key secondary messengers central to numerous signaling pathways and normal cellular functions, including the neurotransmitter signaling and the intracellular effects of hormones [1]. The regulation of cAMP is also essential for immune cell homeostasis [2]. Therefore, PDE inhibitors represent a novel class of medications with broad therapeutic application [3].
In 2014, apremilast became the first FDA-approved PDE inhibitor for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. The anti-inflammatory properties of apremilast also have efficacy in the treatment of other chronic inflammatory skin diseases, such as atopic dermatitis, alopecia areata, and lupus erythematosus [4,5,6]. In this chapter, we provide a brief overview of the PDE family and their role in the regulation of the immune response. We will also discuss the use of oral and topical PDE inhibitors in the treatment of these conditions.
The PDE Family and Their Mechanism of Action
There are 11 PDE families, each family having a different tissue-expression pattern [7]. Eight of the eleven PDE families have the capacity to degrade intracellular cAMP [8]. The phosphodiesterase-4 (PDE4) family consists of 4 genes (PDE4A-D) that generate >20 different variants [9] and account for much of the cAMP-hydrolyzing activity of epithelial cells, chondrocytes, keratinocytes, dendritic cells, and inflammatory cells [10,11,12,13,14,15].
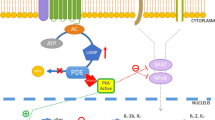
Inhibition of PDE leads to decreased degradation of cAMP, resulting in elevated cAMP levels. Subsequently, cAMP activates protein kinase A (PKA) [16], which phosphorylates a nuclear transcription factor named the cAMP responsive element binding protein (CREB) [17]. This sequence of events results in the inhibition of nuclear factor kappa beta (NF-κB) signaling and the transcription of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) [18]. The mechanism by which activated CREB does this is by competing with the NF-κB p65 subunit for binding of the coactivator CREB-binding protein [19]. In other studies, inhibition of PDE has resulted in decreased levels of other pro-inflammatory cytokines such as interleukin (IL)-2 and interferon-γ (IFN-γ) [13]. Elevation of anti-inflammatory cytokines (e.g., IL-10) with inhibition of PDE has also been shown [20]. Therefore, regulation of cAMP signaling is essential for maintaining appropriate levels of inflammation.
Apremilast: General Information
Before the anti-inflammatory effects of PDE4 inhibitors were discovered, PDE4 inhibitors were being studied for the treatment of depression [21] and chronic obstructive pulmonary disease [22]. In 2014, the FDA approved apremilast for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. Apremilast is an oral, small molecule inhibitor that is highly selective for PDE4 with no appreciable effect on other cell enzymes or cell surface receptors [23]. Apremilast’s specificity for PDE4 is attributed to its dialkoxyphenyl pharmacophore chemical group [24].
Schafer et al. showed that apremilast increases intracellular cAMP levels in peripheral blood monocytes and T cells [23] and inhibits the production of pro-inflammatory cytokines and chemokines, such as IL-2, IL-12, IL-17, IL-23, TNF-α, granulocyte-macrophage colony-stimulating factor (GMCSF), and IFN-γ [23, 25]. It has similar anti-inflammatory effects in dendritic cells, polymorphonuclear cells, natural killer cells, and keratinocytes [23, 25]. Apremilast also results in upregulation of IL-10, which has important anti-inflammatory properties [25]. The foregoing observations support the broad anti-inflammatory effects seen with apremilast [26].
Apremilast is absorbed rapidly and reaches its maximum concentration in the serum in less than 2 h [27]. The major route of elimination is hepatic metabolism with a lesser extent of excretion due to nonenzymatic hydrolysis and elimination of unchanged drug [27]. Its pharmacokinetic properties are affected by severe renal impairment, whereas moderate to severe hepatic impairment does not require dose adjustment. Apremilast is in pregnancy category C and has a similar efficacy in adult and elderly populations. The use of apremilast with strong CYP3A4 inducers (e.g., St. John’s wort, phenytoin, rifampin, and carbamazepine) is not recommended as this combination may result in decreased serum levels of apremilast.
Common adverse events include diarrhea, nausea, and weight loss [28, 29]. While these adverse events affect approximately 20% of patients and often resolve within 1 month of starting apremilast [29], they may negatively affect patient compliance and/or the long-term treatment of chronic inflammatory conditions. In the authors’ experience, antidiarrheal agents (e.g., loperamide or psyllium) seem to mitigate diarrhea symptoms and may improve compliance in patients affected by these symptoms. Less common side effects include upper respiratory infections, headaches, depression, suicidal ideation, and fatigue. The average wholesale acquisition cost for sixty 30 mg tablets is currently estimated to be $2221 [30]. Unfortunately, the high cost of apremilast may limit its use where cheaper medications with comparable efficacy are available, such as methotrexate [30,31,32].
Apremilast for the Treatment of Inflammatory Skin Disease
Apremilast is currently available in the USA, Canada, and Europe for the treatment of psoriasis and psoriatic arthritis. Strong evidence supports the use of apremilast for the treatment of psoriasis, and its potential benefits for the treatment of other chronic inflammatory conditions of the skin are rapidly increasing. Here, we provide a summary of the evidence supporting the use of this medication in the treatment of various inflammatory skin diseases.
Plaque Psoriasis
Psoriasis is a chronic, T-cell-mediated, inflammatory skin condition with several distinct clinical subtypes. The pathogenesis of this inflammatory skin disease is the result of a complex interplay between the skin, immune system, genetics, and environmental triggers. T helper (Th) cell populations (e.g., Th-1 and Th-17) and their respective cytokines (e.g., TNF-α, IFN-γ, IL-17, IL-12/23) are the primary effector cells in psoriasis [33,34,35,36,37].
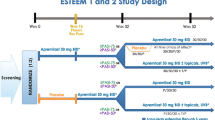
Early phase clinical trials demonstrated a clear treatment response in psoriatic patients treated with apremilast (20–30 mg twice daily) [38,39,40,41]. In two of these early studies, 46.7–57% of patients experienced a >50% improvement in their Psoriasis Area and Severity Index (PASI) scores after 12 weeks of treatment [38, 41]. In two studies by Gottlieb et al., one demonstrated a 34% median reduction in epidermal thickness of psoriatic lesions at 12 weeks, and both had significant reductions of infiltrating inflammatory cells of psoriatic lesions [40, 41]. Two phase 3, randomized, controlled trials entitled the “Efficacy and Safety Trials Evaluating the Effects of Apremilast in Psoriasis ” (e.g., ESTEEM 1 and 2) have evaluated the benefit of apremilast for moderate to severe plaque psoriasis [28, 42]. After 16 weeks, 28.8–33.1% of the 836 patients treated with apremilast 30 mg twice daily versus 5.3–5.8% of 419 patients on placebo achieved a PASI-75. Additionally, ~20% of patients achieved a Static Physician’s Global Assessment (PGA) score of 0 or 1 (clear or almost clear) at week 16, and pruritus and skin discomfort were decreased by ~50% in the apremilast group by week 16. A decrease of ≥5 points in the Dermatology Life Quality Index (DLQI) was also seen in ~70% of patients with a baseline of DLQI >5 in the apremilast group [28, 42]. a phase 4 trial looking at apremilast for the treatment of moderate plaque psoriasis reported the mean percentage change in the product of sPGA and BSA scores (PGAxBSA) was -48.1% for apremilast versus only -10.2% for placebo Efficacy and Safety of Apremilast in Patients With Moderate Plaque Psoriasis With Lower BSA: Week 16 Results from the UNVEIL Study. J Drugs Dermatol. 2017 Aug 1;16(8):801-808. PMID 28809995].
Several studies have assessed the efficacy of apremilast in combination with other psoriatic therapies. In patients with chronic plaque psoriasis on narrowband ultraviolet B (NB-UVB), systemic medications (i.e., methotrexate, cyclosporine), and/or biologics for at least 16 weeks (i.e., etanercept, adalimumab, infliximab, ustekinumab), the addition of apremilast 30 mg twice daily resulted in 51 of 63 patients (81%) achieving PASI-75 after 12 weeks [43]. Additionally, two recent case reports describe recalcitrant psoriatic patients who failed treatment with secukinumab and adalimumab but experienced dramatic clinical improvement following the addition of apremilast [44, 45].
A recent meta-analysis of 13 studies comparing the effectiveness of apremilast with other systemic anti-psoriatic medications found apremilast to have the lowest response rates (18.7%) and maintenance of response in initial responders (61%) at 1 year [46]. A different meta-analysis compared methotrexate (7.5 mg weekly increased to 25 mg as tolerated or needed) and apremilast 30 mg twice daily. In this study, there was no statistically significant difference in PASI-75 between apremilast (36.6%) and methotrexate (36.4%) at week 16 [30]. Another study compared the efficacy and safety of apremilast 30 mg twice daily (n = 83) to etanercept 50 mg once a week (n = 83) or placebo (n = 84). Although the study was not designed to compare apremilast with etanercept, 39.8% of patients taking apremilast achieved PASI-75 in comparison to 48.2% of patients taking etanercept at week 16 [47]. Both groups showed significant efficacy when compared to placebo. At week 16, the patients originally started on etanercept were switched to apremilast and had no significant adverse events [47].
Importantly, one case report demonstrated that apremilast 30 mg twice daily was effective in a 14-year-old patient. The patient achieved a meaningful improvement in his psoriasis at 6 months of treatment and experienced decreased plaque thickness and reductions in pruritus and scale as early as 1 month after treatment [48]. No significant adverse events were noted. This case report suggests that apremilast may be a safe systemic treatment for pediatric psoriasis. There is currently a phase 2 trial looking at apremilast in the treatment of moderate to severe plaque psoriasis in ages 6–17 years (ClinicalTrials.gov Identifier: NCT02576678).
Psoriatic Arthritis
The pathophysiological mechanisms leading to plaque psoriasis and psoriatic arthritis are largely shared, making apremilast a potential therapeutic option for both disease variants. The psoriatic arthritis long-term assessment of clinical efficacy (PALACE) clinical trial program was designed to further evaluate the safety and effectiveness of apremilast in psoriatic arthritis and consists of four phase 3 randomized, placebo-controlled clinical trials [49]. The PALACE 1–3 trials included psoriatic arthritis patients previously treated with disease-modifying antirheumatic drugs (DMARD) as well as those taking concomitant therapies like methotrexate [29, 50, 51]. In contrast, the PALACE 4 was designed to evaluate the efficacy of apremilast in DMARD-naïve patients [52].
In the PALACE 1–3 trials, the proportion of patients that achieved the American College of Rheumatology criteria for 20% improvement (ACR20) at week 16 ranged from 28– -37.4% for those taking apremilast 20 mg twice daily, 32.1–41% for apremilast 30 mg twice daily, and 18–19% for placebo [29, 50, 51]. For PALACE 4, ACR20 at week 16 was 29.2% for apremilast 20 mg twice daily, 32.3% for apremilast 30 mg twice daily, and 16.9% for placebo [52]. In all of the PALACE trials, ACR20 was achieved in a statistically significant number of psoriatic arthritis patients compared to placebo at week 16. At week 52, the PALACE 1–3 trials demonstrated that 52.6–63% of patients taking apremilast 30 mg twice daily met ACR20 [29, 50, 51]. Improvement was also seen with the number of swollen and tender joints at both 16 and 52 weeks with apremilast 30 mg twice daily. The mean percent change for the number of swollen joints ranged from -24.5– -42.2 at 16 weeks and -66.8– -73.6 at 52 weeks, and the number of tender joints ranged from -18.6– -32.1 at 16 weeks and -51.8– -53.5 at 52 weeks [29, 51]. Lastly, the proportion of patients in the PALACE 3 trial that reached the minimal clinically important difference in quality of life as measured by the Health Assessment Questionnaire Disability Index was 32% at week 16 and 52% at week 52 [29].
Long-term data for the PALACE 1 revealed that 65.3% of patients taking apremilast 30 mg twice daily and 60.9% of patients taking apremilast 20 mg twice daily achieved ACR20 at week 104 [53]. For the PALACE 4 trial at 104 weeks, 64.8% taking apremilast 20 mg twice daily and 57.3% taking apremilast 30 mg twice daily achieved ACR20 [54]. Interestingly, diarrhea and nausea occurred at lower rates after week 52 compared to week 52, and there were no significant differences in the type or severity of adverse events with apremilast exposure beyond 52 weeks [54].
Palmoplantar Psoriasis
Palmoplantar psoriasis has a spectrum of clinical phenotypes that can include pustular lesions and/or thick, hyperkeratotic plaques. This disease variant is often severe and difficult to manage. In a retrospective review of 150 patients with palmoplantar psoriasis, 48% of patients were categorized as having moderate psoriatic disease, whereas 34% had severe disease [55]. Another retrospective analysis of 114 patients with palmoplantar psoriasis demonstrated that less than one-third of patients had marked clinical improvement with topical therapies and the remaining patients required systemic therapy [56]. In the authors’ experience, the quality of life for patients with palmoplantar disease is often equal to or lower than other disease variants. These observations underscore challenges associated with the management of this psoriasis and the need for better treatments.
Bissonnette et al. [57] performed a post hoc analysis of patients enrolled in the phase 2 and ESTEEM trials for chronic plaque psoriasis. A total of 427 patients were found to have palmoplantar psoriasis with a total of 274 patients in the apremilast 30 mg twice daily group and 153 patients in the placebo group. A significant number of the patients in the apremilast group with moderate to severe palmoplantar psoriasis, defined by a baseline Palmoplantar Psoriasis Physician Global Assessment (PPPGA) score ≥3, experienced significant improvement in the PPPGA score with 48% of these patients achieving a clear or almost clear score at 16 weeks compared to 27% of patients taking placebo (P = 0.021) [28, 39, 42, 57]. Apremilast was generally well tolerated, and most adverse events were mild in severity [28, 39, 42, 57]. There is currently a phase 4 trial looking at apremilast in the treatment of palmoplantar psoriasis (ClinicalTrials.gov Identifier: NCT02400749).
Nail and Scalp Psoriasis
Approximately two-thirds of patients in the ESTEEM 1 and 2 trials had moderate to severe scalp psoriasis and nail disease. In these patients, a significant proportion of patients taking apremilast 30 mg twice daily achieved a ≥50% reduction in their baseline Nail Psoriasis Severity Index (NAPSI -50) score at week 16 compared to baseline (33.3–44.6% vs. 14.9–18.7%, respectively). They also achieved a score of 0 (clear) or 1 (minimal) in the Scalp Physician Global Assessment compared to baseline (40.9–46.5% vs. 17.2–17.5%, respectively). Additionally, the apremilast group demonstrated a mean decrease of 0.7–1.3 nails involved at week 16. At week 32, those achieving NAPSI-50 in the apremilast group was as high as 55.4%, and the number of nails and nail bed/matrix scores continued to decrease. The improvements seen in nail and scalp psoriasis were maintained through 52 weeks [28, 42, 58]. Taken together, this clinical trial data suggests that apremilast has the ability to reverse the systemic effects of psoriasis including the inflammation at distant skin sites.
Atopic Dermatitis
Like psoriasis, atopic dermatitis (or eczema) is a common, chronic, inflammatory skin disease. Atopic dermatitis is mediated by pathogenic T-cell populations and the increased expression of Th-2, Th-17, and Th-22 cytokines [59, 60]. Two small studies have been performed to look at the efficacy of apremilast in adults with atopic dermatitis, and the results are conflicting [4, 61]. In one study, ten patients with atopic dermatitis received apremilast 30 mg twice daily. At 3 months, these patients experienced a 39% reduction in their Eczema Area and Severity Index (EASI) scores, a 25% reduction in itch as measured by a Visual Analog Scale, and a 58% improvement in quality of life scores as measured by the DLQI. Statistically significant clinical improvement in atopic dermatitis was seen within the first 2 weeks of the study, and improvements in quality of life, itch, and EASI scores remained statistically significant at 6 months [4]. In a separate study, ten adult patients with atopic dermatitis or allergic contact dermatitis received apremilast 20 mg twice daily. At 12 weeks, one patient achieved a 75% reduction in EASI, and two achieved a 50% reduction in EASI. The mean EASI score only decreased by 5% at 12 weeks. There was no statistically significant reduction in itch or improvement in quality of life in this specific study [61]. The majority of adverse events in these two studies were mild and were generally well tolerated [4, 61].
With regard to the treatment of atopic dermatitis in children, one case report describes an 8-year-old male with a history of severe and recalcitrant atopic dermatitis that was treated with apremilast 30 mg daily. The patient saw a drastic improvement in symptoms such as pruritus in as little as 2 weeks [62]. Given the limited number of atopic dermatitis patients treated with apremilast and the lack of randomized clinical trials, it is difficult to assess the efficacy of apremilast for this condition. Nevertheless, additional systematic studies are warranted and needed as PDE inhibitors may represent a safe alternative for atopic dermatitis patients who fail to respond to topical therapies and/or traditional immunosuppressant medications. A phase 2 trial is underway and is further investigating apremilast in the treatment of moderate to severe atopic dermatitis (ClinicalTrials.gov Identifier: NCT02087943).
Alopecia Areata
Alopecia areata is an autoimmune disorder characterized by the immune destruction of hair follicles and non-scarring alopecia. Lesional skin biopsies from the scalp of alopecia areata patients reveal robust activation of Th-1, Th-2, and IL-23 cytokine pathways as well as increased PDE4 levels [63]. Interestingly, atopic dermatitis is two to three times more likely to be found in patients with alopecia areata [64]. The overlapping cytokine profile of alopecia areata with other inflammatory skin disorders, its co-occurrence with atopic dermatitis, and the increased PDE levels in areas of hair loss support the notion that apremilast may represent an effective treatment modality for alopecia areata.
This hypothesis has been studied in a preclinical mouse model of alopecia areata. Using a humanized alopecia areata model where normal human scalp skin is transplanted onto mice with severe combined immunodeficiency, hair loss is induced in mice by injecting IL-2-stimulated peripheral blood mononuclear cells [65]. Oral apremilast abrogates this hair loss phenotype and is associated with reduced IFN-α, TNF-γ, and perifollicular inflammatory cells [5]. There is currently a randomized controlled trial looking at the treatment of apremilast in moderate to severe alopecia areata (ClinicalTrials.gov Identifier: NCT02684123).
Rosacea
Rosacea is a pleomorphic, inflammatory skin disease affecting the face. Common clinical manifestations include flushing, erythema, telangiectasia, and papules/pustules. The etiology of this condition is poorly understood and involves a complex interaction between the innate immune response, cutaneous microbiota, environmental factors, and adnexal structures of the skin. Traditional treatments are aimed at the prevention of symptoms or clinical manifestations (e.g., erythema or telangiectasia) by targeting the pilosebaceous units and blood vessels [66]. However, the clinical symptoms of rosacea are bothersome to patients, and management of this condition can be challenging.
In a recent phase 2 study for moderate to severe erythematotelangiectatic and papulopustular rosacea, ten adult patients were treated with apremilast 20 mg twice daily for 12 weeks. While the primary endpoint of papule and pustule count did not reach statistical significance during the study, statistically significant improvements were seen in the following outcomes at the end of 12 weeks: the Physician Global 7-Point Assessment, Physician Overall Erythema Severity, the erythematotelangiectatic rating, and nontransient erythema. Affirmation of these findings in a larger controlled study is needed to determine the efficacy of apremilast for rosacea [67].
Pityriasis Rubra Pilaris
Pityriasis rubra pilaris (PRP) is a papulosquamous skin disease that is commonly mistaken for psoriasis. Clinical features of this disease may include follicular hyperkeratosis, palmoplantar keratoderma, and/or reddish-orange-colored scaling patches. The etiology of this disease is not clear; however, studies have shown increased neutrophils and lymphocytes [68] as well as increased TNF-α and CXCL-10 in the lesional skin of individuals with PRP [69].
A potential role for apremilast in the treatment of PRP is supported by one case report involving an elderly male with leukemia and refractory PRP [70]. This patient’s disease was not responsive to acitretin, methotrexate, cyclosporine, or infliximab. His PRP worsened following chemotherapy, and apremilast 30 mg twice daily was started. Within 4 weeks, improvement was observed, and a near complete resolution was noted within 6–8 months of treatment; he remained disease-free at 12 months. The only adverse event reported by the patient was mild gastrointestinal upset [70].
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic autoimmune condition characterized by scaly, disklike plaques commonly on the head and neck. Lesional biopsies have demonstrated increased levels of Th-1 cytokines (IFN-γ and IL-2) [71]. The presence of these cytokines and an associated inflammatory infiltrate in the biopsies of lesional skin make DLE a good target for apremilast. In a study of eight patients with active DLE, apremilast 20 mg twice daily was taken for 85 days [6]. The CLE Disease Area and Severity Index (CLASI) was used to evaluate treatment response and incorporates assessments of erythema, scale/hypertrophy, dyspigmentation, scarring/atrophy/panniculitis, location, mucous membrane involvement, and alopecia [72]. There was a statistically significant decrease in their CLASI scores after 85 days of treatment [6]. Two patients had complete regression of their scalp lesions following treatment. The most common side effects experienced were nausea, diarrhea, and headache.
Bechet’s Disease
Similar to psoriasis, Bechet’s disease has an immunologic and genetic basis, and response to apremilast has been assessed [73]. The disease is a systemic vasculitis with an unknown etiology and is characterized by mouth and genital ulcers [74]. TNF-α, IL-6, IL-1, and IL-8 have been shown to be increased in Bechet’s disease [75]. A phase 2 study was conducted to assess the use of apremilast for the treatment of Bechet’s syndrome. In this study, 111 patients were enrolled and randomized to apremilast 30 mg twice a day or placebo for 12 weeks. At week 12 (the primary endpoint), the mean number of ulcers for each patient was significantly lower in the apremilast group versus placebo (0.5 ulcers vs. 2.1). Clinical responses to apremilast were reported as early as 2 weeks. The mean change in pain from oral ulcers from baseline to week 12, measured by a 100 mm Visual Analog Scale, was −44.7 mm for the apremilast cohort versus -16.0 mm for placebo. All ten patients in the apremilast group that had genital ulcers at baseline were free of genital ulcers by week 12. Improvements in quality of life, as measured by the Bechet’s Disease Quality of Life at week 12, were also statistically significant for the treatment group. There were no unique adverse events different from those commonly found with apremilast [76]. There is currently a phase 3 trial looking at apremilast in the treatment of Bechet’s disease (ClinicalTrials.gov Identifier: NCT02307513).
Lichen Planus
Lichen planus is a T-cell-mediated process that results in painful, pruritic lesions of the skin or mucosal surfaces. The etiology of this condition is not entirely clear [77], though elevated levels of CD8+ cells, TNF-α, and IFN-γ are present in lesional tissues [78]. In one study, ten patients that either had moderate to severe cutaneous lichen planus, lichen planus with severe itching and/or pain that significantly interfered with activities of daily living, or lichen planus that was refractory to topical corticosteroids were treated with apremilast 20 mg twice daily for 12 weeks [79]. At 12 weeks, 30% of patients had a ≥2 grade improvement and a significant decrease in lesion count from 35 at baseline to 20.5. A decrease in pruritus score from 67 to 18.5 at the end of 12 weeks was also noted, and two patients had complete clearance of their lesions at 12 weeks. Additionally, one patient with 40% involvement of her bilateral buccal mucosa at baseline improved to 12% involvement at the end of the study. No significant adverse effects were noted. This study demonstrates a potential role for apremilast in the treatment of lichen planus and might be considered for other related disease variants such as oral lichen planus or lichen planopilaris.
Sarcoidosis
Sarcoidosis , a systemic inflammatory disease characterized by noncaseating granulomas can be associated with a pleomorphic number of skin lesions [80]. Cutaneous sarcoidosis was found to have increased levels of IL-12 and upregulation of the IFN pathway [81]. The efficacy of apremilast 20 mg twice daily for 12 weeks was evaluated in a study of 15 patients with persistent, chronic cutaneous sarcoidosis [82]. For each patient, an index lesion was determined at baseline. Lesion induration was measured by the Sarcoidosis Activity and Severity Index (SASI) induration score. At weeks 4 and 12 of treatment, there were statistically significant decreases in index lesion induration compared to baseline with a median decrease of 1 point in the SASI score for both time points. Paired pre- and post-treatment photographs also supported a beneficial role for apremilast in this patient cohort. Interestingly, one patient required apremilast dosage reduction of 20 mg once daily due to “jitteriness.” No mechanism for this adverse effect has been suggested, and additional studies are necessary to determine its validity.
Other PDE Inhibitors
Introduction
There are other formulations of PDE4 inhibitors aside from oral medications like apremilast that have been developed and studied. For example, inhaled PDE4 inhibitors have been studied in asthma [83], one of the components of the atopic triad. It is not known how the inhaled PDE4 inhibitors affect skin disease. However, topical PDE4 inhibitors have been developed and studied in skin disease.
Topical PDE Inhibitors
The development and study of topical PDE inhibitors are currently under way. In cell culture, benzoxaborole PDE4 inhibitors have been shown to inhibit the release of cytokines like TNF-α, IFN-g, IL-12, IL-23, and Th2 cytokines (e.g., IL-4, IL-5, IL-13) in human peripheral blood mononuclear cells and human monocytes [84]. This is similar to systemic PDE4 inhibitors such as apremilast; however, crisaborole is more active in inhibiting IL-4 release, while apremilast has better inhibition of TNF-α, IL-23, and IL-17 secretion. Apremilast and benzoxaborole PDE4 inhibitors have high infinity for the PDE4 isoforms and are not selective among PDE4 isozymes . However, unlike apremilast, the benzoxaborole PDE4 inhibitors showed moderate inhibitory activity on PDE enzymes outside the PDE4 family and were less selective for PDE4. It is thought that the inhibition of other PDE families in addition to PDE4 may lead to an enhanced anti-inflammatory effect [84].
Using a mouse model of atopic dermatitis, one study demonstrated that a single application of a topical PDE4 inhibitor (E6005) relieved dermatitis-associated pruritus. Hind-paw scratching of the rostral back was used as an index of itching, and the firing activity of the cutaneous nerves was electrophysiologically recorded to assess pruritus/itching. Additionally, cAMP concentration in the involved skin of these mice was markedly decreased and reversed by application of the topical PDE4 inhibitor [85]. Further, a study of Japanese children with atopic dermatitis reported decreased pruritus, erythema, immune cell infiltration, excoriation, and lichenification following topical application of E6005 for 2 weeks compared to vehicle alone [86].
Crisaborole 2% ointment is another topical benzoxaborole PDE4 inhibitor that has been studied in the treatment of atopic dermatitis and psoriasis. In December 2016, Crisaborole was approved by the FDA to be used in the treatment of mild to moderate atopic dermatitis. Phase 1b and 2a trials showed promising results for crisaborole 2% ointment applied twice daily to affected areas for 28 days in adolescents with atopic dermatitis [87, 88]. 35–47.1% of these patients achieved a clear or almost clear Investigator Static Global Assessment (ISGA) score with a ≥2 grade improvement in the score compared to baseline [87, 88].
Two phase 3 trials enrolled patients 2 years and older and assigned patients to crisaborole 2% ointment twice daily versus placebo vehicle twice daily for 28 days with a 2:1 randomization [89]. A combined total of 1522 patients were analyzed in these studies. The proportion of individuals that achieved an ISGA score of 1 or less (clear or almost clear) with ≥ 2 grade improvement versus baseline was 32.8% (vs. 25.4% for placebo) for the first trial and 31.4% (vs. 18.0% for placebo) for the second, demonstrating a significant improvement when compared to the vehicle group at day 29. Statistically significant reductions in mean severity at day 29 when compared to baseline were seen in erythema (-41%), exudation (-65%), excoriation (-52%), induration/papulation (-37%), and lichenification (-42%) in a pooled analysis of the two trials. Disease severity improvement was seen as early as 8 days after the start of treatment. Additionally, the early and sustained improvement in pruritus was also noted with no significant adverse effects. Pain or burning/stinging at the site of application were the most common reported adverse effects.
The use of topical PDE inhibitors for the treatment of chronic inflammatory skin diseases shows tremendous promise. According to information obtained from clinicaltrials.org, the efficacy of crisaborole is currently being investigated in other inflammatory conditions, such as psoriasis. The results of these studies have not yet been published.
Conclusion and Future Directions
PDE4 inhibitors have been shown to be efficacious in a number of inflammatory skin diseases. It is interesting to note the mechanism by which these inhibitors work (i.e., inhibition of inflammatory pathways further upstream and within target cells). This is quite different than traditional immunosuppressants and biological agents (e.g., TNF-α inhibitors act primarily within the extracellular compartment). Additionally, the most common reasons for the discontinuation of conventional systemic and biological therapies include the safety concerns/contraindications, fear of injections, cost, loss of effectiveness, and need for routine lab monitoring [90,91,92]. It will be interesting to see whether the availability of oral and/or topical PDE inhibitors, which have fewer contraindications and require less monitoring, will displace the use of traditional systemic and biologic agents in specific subsets of patients and/or diseases.
Long-term safety data for PDE inhibitors, such as apremilast, is not yet available and will require the treatment of thousands of patients over the next 10–15 years. A 5-year extension study of the ESTEEM trial is currently ongoing and offers insight into the long-term safety of apremilast. However, the safety data that we do have indicates that this class of medication is safe and well tolerated, other than those affected by mild gastrointestinal complaints. Unfortunately, the high cost and low efficacy rates of apremilast compared to standard traditional systemic therapies and biologics will likely limit its use in psoriasis and possibly other inflammatory diseases. Randomized controlled trials in diseases other than psoriasis and psoriatic arthritis represent an unmet need, and the safety and efficacy of apremilast in the pediatric population are desperately needed. One clear use for apremilast in dermatology is in the treatment of palmoplantar psoriasis. For many clinicians, apremilast offers the potential of becoming the first-line therapy in this specific patient population. A careful evaluation of apremilast in specific subtypes of diseases is also needed and will offer additional insights into the role of PDE inhibitors in inflammatory skin disease [57].
Case Presentation
A 75-year-old Caucasian male presents to the dermatology clinic with a more than 10-year history of recalcitrant plaque and pustular palmoplantar psoriasis. He notes that he has been treated with multiple topical and systemic agents but with little success. He endorses intermittent joint pains, morning stiffness, and swelling/redness of his fingers or toes. Associated symptoms included decreased sleep, itch, pain, skin tightness, fissures, and bleeding.
Past Medical History
-
Hypertension
-
Hyperlipidemia
-
Obesity
Social and Family History
-
Married
-
35-pack-year history of tobacco use, quit smoking 18 years ago
-
Mother, father, and other first-degree relatives with a history of psoriasis
Previous Therapies
-
High-potency topical steroids, PUVA, NBUVB, and excimer laser
-
Acitretin, cyclosporine, and methotrexate
-
Infliximab, etanercept, ustekinumab, and efalizumab
Physical Examination
-
Thick, well-demarcated, erythematous, scaly plaques with prominent scale on the bilateral palms, soles, scalp, elbows, trunk, lower extremities, and gluteal cleft
-
Thick, scaly, plaques with pustules and fissures on the palms and soles
-
Pitting of the nail plate noted on multiple nails of the bilateral hands
-
No recent dactylitis, tender or swollen joints, or enthesitis
-
Body surface area involvement of approximately 13%
Management
Given the patient’s failure to respond to multiple biologic therapies and the prominent involvement of the palms and soles, apremilast 30 mg twice daily in combination with acitretin 25 mg once daily was started. Within several weeks, the patient experienced a dramatic improvement in his skin lesions and rated his disease severity as a 3. His body surface area involvement at 4 months was less than 1%, and the patient denied any joint symptoms. Adverse events included diarrhea that was problematic for the first 3 weeks of treatment but improved gradually thereafter. He denied any other significant adverse effects other than skin dryness. This particular case highlights the utility of apremilast for the treatment of palmoplantar psoriasis. It also demonstrates its usefulness when combined with other treatment modalities, such as acitretin or phototherapy.
Abbreviations
- cAMP:
-
Cyclic adenosine monophosphate
- cGMP:
-
Cyclic guanosine monophosphate
- CREB:
-
Camp responsive element binding protein
- ESTEEM:
-
Efficacy and safety trials evaluating the effects of apremilast in psoriasis
- IFN-γ:
-
Interferon-gamma
- IL:
-
Interleukin
- NF-κB:
-
Nuclear factor kappa beta
- PASI:
-
Psoriasis Area and Severity Index
- PDE:
-
Phosphodiesterase
- PDE4:
-
Phosphodiesterase-4
- PKA:
-
Protein kinase A
- Th:
-
T helper
- TNF-α:
-
Tumor necrosis factor-alpha
- PASI-75:
-
75% Improvement in PASI scores
- DLQI:
-
Dermatology Life Quality Index
- NB-UVB:
-
Narrowband-ultraviolet B
- PALACE:
-
Psoriatic arthritis long-term assessment of clinical efficacy
- DMARD:
-
Disease-modifying antirheumatic drugs
- ACR20:
-
American College of Rheumatology criteria for 20% improvement
- PPPGA:
-
Palmoplantar Psoriasis Physician Global Assessment
- NAPSI-50:
-
50% Reduction in baseline Nail Psoriasis Severity Index
- EASI:
-
Eczema area and severity index
- PRP:
-
Pityriasis rubra pilaris
- DLE:
-
Discoid lupus erythematosus
- CLASI:
-
CLE Disease area and severity
- SASI:
-
Sarcoidosis Activity and Severity Index
- ISGA:
-
Investigator Static Global Assessment
References
Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147(Suppl 1):S252–7. https://doi.org/10.1038/sj.bjp.0706495.
Mosenden R, Tasken K. Cyclic AMP-mediated immune regulation—overview of mechanisms of action in T cells. Cell Signal. 2011;23(6):1009–16. https://doi.org/10.1016/j.cellsig.2010.11.018.
Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC. Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov. 2014;13(4):290–314. https://doi.org/10.1038/nrd4228.
Samrao A, Berry TM, Goreshi R, Simpson EL. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148(8):890–7. https://doi.org/10.1001/archdermatol.2012.812.
Keren A, Shemer A, Ullmann Y, Paus R, Gilhar A. The PDE4 inhibitor, apremilast, suppresses experimentally induced alopecia areata in human skin in vivo. J Dermatol Sci. 2015;77(1):74–6. https://doi.org/10.1016/j.jdermsci.2014.11.009.
De Souza A, Strober BE, Merola JF, Oliver S, Franks AG Jr. Apremilast for discoid lupus erythematosus: results of a phase 2, open-label, single-arm, pilot study. J Drugs Dermatol. 2012;11(10):1224–6.
Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100(3):309–27. https://doi.org/10.1161/01.RES.0000256354.95791.f1.
Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10(22):1503–19. https://doi.org/10.1016/S1359-6446(05)03622-6.
Halpin DMABCD. Of the phosphodiesterase family: interaction and differential activity in COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(4):543–61.
Wright LC, Seybold J, Robichaud A, Adcock IM, Barnes PJ. Phosphodiesterase expression in human epithelial cells. Am J Phys. 1998;275(4 Pt 1):L694–700.
Shepherd MC, Baillie GS, Stirling DI, Houslay MD. Remodelling of the PDE4 cAMP phosphodiesterase isoform profile upon monocyte-macrophage differentiation of human U937 cells. Br J Pharmacol. 2004;142(2):339–51. https://doi.org/10.1038/sj.bjp.0705770.
Heystek HC, Thierry AC, Soulard P, Moulon C. Phosphodiesterase 4 inhibitors reduce human dendritic cell inflammatory cytokine production and Th1-polarizing capacity. Int Immunol. 2003;15(7):827–35.
Claveau D, Chen SL, O’Keefe S, Zaller DM, Styhler A, Liu S, et al. Preferential inhibition of T helper 1, but not T helper 2, cytokines in vitro by L-826,141 [4-[2-(3,4-Bisdifluromethoxyphenyl)-2-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan- 2-yl)-phenyl]-ethyl]3-methylpyridine-1-oxide], a potent and selective phosphodiesterase 4 inhibitor. J Pharmacol Exp Ther. 2004;310(2):752–60. https://doi.org/10.1124/jpet.103.064691.
Tenor H, Hedbom E, Hauselmann HJ, Schudt C, Hatzelmann A. Phosphodiesterase isoenzyme families in human osteoarthritis chondrocytes—functional importance of phosphodiesterase 4. Br J Pharmacol. 2002;135(3):609–18. https://doi.org/10.1038/sj.bjp.0704480.
Tenor H, Hatzelmann A, Wendel A, Schudt C. Identification of phosphodiesterase IV activity and its cyclic adenosine monophosphate-dependent up-regulation in a human keratinocyte cell line (HaCaT). J Invest Dermatol. 1995;105(1):70–4.
Gill GN, Garren LD. A cyclic-3′,5′-adenosine monophosphate dependent protein kinase from the adrenal cortex: comparison with a cyclic AMP binding protein. Biochem Biophys Res Commun. 1970;39(3):335–43.
Montminy MR, Bilezikjian LM. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987;328(6126):175–8. https://doi.org/10.1038/328175a0.
Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem. 1996;271(34):20828–35.
Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappa B-mediated transcription. J Immunol. 1997;159(11):5450–6.
Eigler A, Siegmund B, Emmerich U, Baumann KH, Hartmann G, Endres S. Anti-inflammatory activities of cAMP-elevating agents: enhancement of IL-10 synthesis and concurrent suppression of TNF production. J Leukoc Biol. 1998;63(1):101–7.
Wachtel H. Potential antidepressant activity of rolipram and other selective cyclic adenosine 3′,5′-monophosphate phosphodiesterase inhibitors. Neuropharmacology. 1983;22(3):267–72.
Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374(9691):695–703. https://doi.org/10.1016/S0140-6736(09)61252-6.
Schafer PH, Parton A, Capone L, Cedzik D, Brady H, Evans JF, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26(9):2016–29. https://doi.org/10.1016/j.cellsig.2014.05.014.
Man HW, Schafer P, Wong LM, Patterson RT, Corral LG, Raymon H, et al. Discovery of (S)-N-[2-[1-(3-ethoxy-4-methoxyphenyl)-2-methanesulfonylethyl]-1,3-dioxo-2,3-dihy dro-1H-isoindol-4-yl] acetamide (apremilast), a potent and orally active phosphodiesterase 4 and tumor necrosis factor-alpha inhibitor. J Med Chem. 2009;52(6):1522–4. https://doi.org/10.1021/jm900210d.
Schafer PH, Parton A, Gandhi AK, Capone L, Adams M, Wu L, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159(4):842–55. https://doi.org/10.1111/j.1476-5381.2009.00559.x.
Schett G, Sloan VS, Stevens RM, Schafer P. Apremilast: a novel PDE4 inhibitor in the treatment of autoimmune and inflammatory diseases. Ther Adv Musculoskelet Dis. 2010;2(5):271–8. https://doi.org/10.1177/1759720X10381432.
Hoffmann M, Kumar G, Schafer P, Cedzik D, Capone L, Fong KL, et al. Disposition, metabolism and mass balance of [(14)C]apremilast following oral administration. Xenobiotica. 2011;41(12):1063–75. https://doi.org/10.3109/00498254.2011.604745.
Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (efficacy and safety trial evaluating the effects of Apremilast in psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49. https://doi.org/10.1016/j.jaad.2015.03.049.
Edwards CJ, Blanco FJ, Crowley J, Birbara CA, Jaworski J, Aelion J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75(6):1065–73. https://doi.org/10.1136/annrheumdis-2015-207963.
Armstrong AW, Betts KA, Sundaram M, Thomason D, Signorovitch JE. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naive patients with psoriasis. J Am Acad Dermatol. 2016;75(4):740–6. https://doi.org/10.1016/j.jaad.2016.05.040.
Hinde S, Wade R, Palmer S, Woolacott N, Spackman E. Apremilast for the treatment of moderate to severe plaque psoriasis: a critique of the evidence. PharmacoEconomics. 2016;34(6):587–96. https://doi.org/10.1007/s40273-016-0382-3.
Sideris E, Corbett M, Palmer S, Woolacott N, Bojke L. The clinical and cost effectiveness of apremilast for treating active psoriatic arthritis: a critique of the evidence. PharmacoEconomics. 2016. https://doi.org/10.1007/s40273-016-0419-7.
Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, Gallati H, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994;102(2):145–9.
Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128(5):1207–11. https://doi.org/10.1038/sj.jid.5701213.
Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113(5):752–9. https://doi.org/10.1046/j.1523-1747.1999.00749.x.
Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediat Inflamm. 2005;2005(5):273–9. https://doi.org/10.1155/MI.2005.273.
Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappa B pathways. Nat Genet. 2009;41(2):199–204. https://doi.org/10.1038/ng.311.
Papp KA, Kaufmann R, Thaci D, Hu C, Sutherland D, Rohane P. Efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: results from a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison study. J Eur Acad Dermatol Venereol. 2013;27(3):e376–83. https://doi.org/10.1111/j.1468-3083.2012.04716.x.
Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012;380(9843):738–46. https://doi.org/10.1016/S0140-6736(12)60642-4.
Gottlieb AB, Strober B, Krueger JG, Rohane P, Zeldis JB, CC H, et al. An open-label, single-arm pilot study in patients with severe plaque-type psoriasis treated with an oral anti-inflammatory agent, apremilast. Curr Med Res Opin. 2008;24(5):1529–38. https://doi.org/10.1185/030079908X301866.
Gottlieb AB, Matheson RT, Menter A, Leonardi CL, Day RM, Hu C, et al. Efficacy, tolerability, and pharmacodynamics of apremilast in recalcitrant plaque psoriasis: a phase II open-label study. J Drugs Dermatol. 2013;12(8):888–97.
Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387–99. https://doi.org/10.1111/bjd.14164.
AbuHilal M, Walsh S, Shear N. Use of Apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016. https://doi.org/10.1177/1203475416631328.
Danesh MJ, Beroukhim K, Nguyen C, Levin E, Koo J. Apremilast and adalimumab: a novel combination therapy for recalcitrant psoriasis. Dermatol Online J. 2015;21(6)
Rothstein BE, McQuade B, Greb JE, Goldminz AM, Gottlieb AB. Apremilast and secukinumab combined therapy in a patient with recalcitrant plaque psoriasis. J Drugs Dermatol. 2016;15(5):648–9.
Bartos S, Hill D, Feldman SR. Review of maintenance of response to psoriasis treatments. J Dermatolog Treat. 2016;27(4):293–7. https://doi.org/10.1080/09546634.2016.1177158.
Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept, and placebo, in patients with moderate to severe plaque psoriasis: 52-week results from a phase 3b, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2016. https://doi.org/10.1111/jdv.14015.
Smith RL. Pediatric psoriasis treated with apremilast. JAAD Case Rep. 2016;2(1):89–91. https://doi.org/10.1016/j.jdcr.2015.12.005.
Schett G, Wollenhaupt J, Papp K, Joos R, Rodrigues JF, Vessey AR, et al. Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64(10):3156–67. https://doi.org/10.1002/art.34627.
Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42(3):479–88. https://doi.org/10.3899/jrheum.140647.
Cutolo M, Myerson GE, Fleischmann RM, Liote F, Diaz-Gonzalez F, Van den Bosch F, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016. https://doi.org/10.3899/jrheum.151376.
Wells A, Edwards C, Adebajo A, Kivitz A. Apremilast in the treatment of DMARD-Naïve psoriatic arthritis patients: results of a phase 3 randomized, controlled trial (PALACE 4). 2013. http://acrabstracts.org/abstract/apremilast-in-the-treatment-of-dmard-naive-psoriatic-arthritis-patients-results-of-a-phase-3-randomized-controlled-trial-palace-4/. Accessed 26 July 2016.
Kavanaugh A, Adebajo AO, Gladman DD, Gomez-Reino JJ, Hall S, Lespessailles E. Long-term (156-week) efficacy and safety profile of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis: results from a phase III, randomized, controlled trial and open-label extension (PALACE 1). American College of Rheumatology. 2015. http://acrabstracts.org/abstract/long-term-156-week-efficacy-and-safety-profile-of-apremilast-an-oral-phosphodiesterase-4-inhibitor-in-patients-with-psoriatic-arthritis-results-from-a-phase-iii-randomized-controlled-trial-and/. Accessed 29 July 2016.
Wells A, Edwards C, Adebajo A, Kivitz A. Long-term (104-week) safety and efficacy of monotherapy with apremilast in dmard-naïve patients with psoriatic arthritis: a phase 3, randomized, controlled trial and open-label extension (PALACE 4). American College of Rheumatology. 2014. http://acrabstracts.org/abstract/long-term-104-week-safety-and-efficacy-of-monotherapy-with-apremilast-in-dmard-naive-patients-with-psoriatic-arthritis-a-phase-3-randomized-controlled-trial-and-open-label-extension-palace-4/. Accessed 26 July 2016.
Farley E, Masrour S, McKey J, Menter A. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60(6):1024–31. https://doi.org/10.1016/j.jaad.2008.11.910.
Adisen E, Tekin O, Gulekon A, Gurer MA. A retrospective analysis of treatment responses of palmoplantar psoriasis in 114 patients. J Eur Acad Dermatol Venereol. 2009;23(7):814–9. https://doi.org/10.1111/j.1468-3083.2009.03197.x.
Bissonnette R, Pariser DM, Wasel NR, Goncalves J, Day RM, Chen R, et al. Apremilast, an oral phosphodiesterase-4 inhibitor, in the treatment of palmoplantar psoriasis: results of a pooled analysis from phase II PSOR-005 and phase III efficacy and safety trial evaluating the effects of apremilast in psoriasis (ESTEEM) clinical trials in patients with moderate to severe psoriasis. J Am Acad Dermatol. 2016;75(1):99–105. https://doi.org/10.1016/j.jaad.2016.02.1164.
Rich P, Gooderham M, Bachelez H, Goncalves J, Day RM, Chen R, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). J Am Acad Dermatol. 2016;74(1):134–42. https://doi.org/10.1016/j.jaad.2015.09.001.
Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130(6):1344–54. https://doi.org/10.1016/j.jaci.2012.07.012.
Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128(11):2625–30. https://doi.org/10.1038/jid.2008.111.
Volf EM, SC A, Dumont N, Scheinman P, Gottlieb ABA. Phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11(3):341–6.
Saporito RC, Cohen DJ. Apremilast use for moderate-to-severe atopic dermatitis in pediatric patients. Case Rep Dermatol. 2016;8(2):179–84. https://doi.org/10.1159/000446836.
Suarez-Farinas M, Ungar B, Noda S, Shroff A, Mansouri Y, Fuentes-Duculan J, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136(5):1277–87. https://doi.org/10.1016/j.jaci.2015.06.032.
Huang KP, Mullangi S, Guo Y, Qureshi AA. Autoimmune, atopic, and mental health comorbid conditions associated with alopecia areata in the United States. JAMA Dermatol. 2013;149(7):789–94. https://doi.org/10.1001/jamadermatol.2013.3049.
Gilhar A, Keren A, Shemer A, d’Ovidio R, Ullmann Y, Paus R. Autoimmune disease induction in a healthy human organ: a humanized mouse model of alopecia areata. J Invest Dermatol. 2013;133(3):844–7. https://doi.org/10.1038/jid.2012.365.
Culp B, Scheinfeld N. Rosacea: a review. P T. 2009;34(1):38–45.
Thompson BJ, Furniss M, Zhao W, Chakraborty B, Mackay-Wiggan J. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150(9):1013–4. https://doi.org/10.1001/jamadermatol.2013.10526.
Magro CM, Crowson AN. The clinical and histomorphological features of pityriasis rubra pilaris. A comparative analysis with psoriasis. J Cutan Pathol. 1997;24(7):416–24.
Adnot-Desanlis L, Antonicelli F, Tabary T, Bernard P, Reguiai Z. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226(1):41–6. https://doi.org/10.1159/000346640.
Krase IZ, Cavanaugh K, Curiel-Lewandrowski C. Treatment of refractory pityriasis rubra pilaris with novel phosphodiesterase 4 (PDE4) inhibitor apremilast. JAMA Dermatol. 2016;152(3):348–50. https://doi.org/10.1001/jamadermatol.2015.3405.
Toro JR, Finlay D, Dou X, Zheng SC, LeBoit PE, Connolly MK. Detection of type 1 cytokines in discoid lupus erythematosus. Arch Dermatol. 2000;136(12):1497–501.
Albrecht J, Taylor L, Berlin JA, Dulay S, Ang G, Fakharzadeh S, et al. The CLASI (cutaneous lupus Erythematosus disease area and severity index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005;125(5):889–94. https://doi.org/10.1111/j.0022-202X.2005.23889.x.
Zierhut M, Mizuki N, Ohno S, Inoko H, Gul A, Onoe K, et al. Immunology and functional genomics of Behcet’s disease. Cell Mol Life Sci. 2003;60(9):1903–22. https://doi.org/10.1007/s00018-003-2333-3.
Zhou ZY, Chen SL, Shen N, Lu Y. Cytokines and Behcet’s disease. Autoimmun Rev. 2012;11(10):699–704. https://doi.org/10.1016/j.autrev.2011.12.005.
Mege JL, Dilsen N, Sanguedolce V, Gul A, Bongrand P, Roux H, et al. Overproduction of monocyte derived tumor necrosis factor alpha, interleukin (IL) 6, IL-8 and increased neutrophil superoxide generation in Behcet’s disease. A comparative study with familial Mediterranean fever and healthy subjects. J Rheumatol. 1993;20(9):1544–9.
Hatemi G, Melikoglu M, Tunc R, Korkmaz C, Turgut Ozturk B, Mat C, et al. Apremilast for Behcet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372(16):1510–8. https://doi.org/10.1056/NEJMoa1408684.
Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhou XJ, Khan A, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13(4):350–65.
Khan A, Farah CS, Savage NW, Walsh LJ, Harbrow DJ, Sugerman PB. Th1 cytokines in oral lichen planus. J Oral Pathol Med. 2003;32(2):77–83.
Paul J, Foss CE, Hirano SA, Cunningham TD, Pariser DM. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68(2):255–61. https://doi.org/10.1016/j.jaad.2012.07.014.
Haimovic A, Sanchez M, Judson MA, Prystowsky S. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. Cutaneous disease. J Am Acad Dermatol. 2012;66(5):699.e1–718.e1. https://doi.org/10.1016/j.jaad.2011.11.965; quiz 717–8.
Judson MA, Marchell RM, Mascelli M, Piantone A, Barnathan ES, Petty KJ, et al. Molecular profiling and gene expression analysis in cutaneous sarcoidosis: the role of interleukin-12, interleukin-23, and the T-helper 17 pathway. J Am Acad Dermatol. 2012;66(6):901–10. https://doi.org/10.1016/j.jaad.2011.06.017, 10.e1–2.
Baughman RP, Judson MA, Ingledue R, Craft NL, Lower EE. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148(2):262–4. https://doi.org/10.1001/archdermatol.2011.301.
Chapman RW, House A, Richard J, Prelusky D, Lamca J, Wang P, et al. Pharmacology of a potent and selective inhibitor of PDE4 for inhaled administration. Eur J Pharmacol. 2010;643(2–3):274–81. https://doi.org/10.1016/j.ejphar.2010.06.054.
Dong C, Virtucio C, Zemska O, Baltazar G, Zhou Y, Baia D, et al. Treatment of skin inflammation with benzoxaborole PDE inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016. https://doi.org/10.1124/jpet.116.232819.
Andoh T, Yoshida T, Kuraishi Y. Topical E6005, a novel phosphodiesterase 4 inhibitor, attenuates spontaneous itch-related responses in mice with chronic atopy-like dermatitis. Exp Dermatol. 2014;23(5):359–61. https://doi.org/10.1111/exd.12377.
Nemoto O, Hayashi N, Kitahara Y, Furue M, Hojo S, Nomoto M, et al. Effect of topical phosphodiesterase 4 inhibitor E6005 on Japanese children with atopic dermatitis: results from a randomized, vehicle-controlled exploratory trial. J Dermatol. 2015. https://doi.org/10.1111/1346-8138.13231.
Zane LT, Kircik L, Call R, Tschen E, Draelos ZD, Chanda S, et al. Crisaborole topical ointment, 2% in patients ages 2 to 17 years with atopic dermatitis: a phase 1b, open-label, maximal-use systemic exposure study. Pediatr Dermatol. 2016. https://doi.org/10.1111/pde.12872.
Tom WL, Van Syoc M, Chanda S, Zane LT. Pharmacokinetic profile, safety, and tolerability of crisaborole topical ointment, 2% in adolescents with atopic dermatitis: an open-label phase 2a study. Pediatr Dermatol. 2016;33(2):150–9. https://doi.org/10.1111/pde.12780.
Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016. https://doi.org/10.1016/j.jaad.2016.05.046.
Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013;149(10):1180–5. https://doi.org/10.1001/jamadermatol.2013.5264.
Lebwohl MG, Bachelez H, Barker J, Girolomoni G, Kavanaugh A, Langley RG, et al. Patient perspectives in the management of psoriasis: results from the population-based multinational assessment of psoriasis and psoriatic arthritis survey. J Am Acad Dermatol. 2014;70(5):871–881.e1–30. https://doi.org/10.1016/j.jaad.2013.12.018.
Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Am J Clin Dermatol. 2016;17(1):87–97. https://doi.org/10.1007/s40257-015-0169-x.
Conflicts of Interest
JH and JEH have no conflicts of interest to declare. JEH is supported in part by The Rockefeller University CTSA award grant # UL1TR001866 and # KL2TR001865 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. GGK has received honoraria and has served as a consultant for Celgene.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Huber, J., Krueger, G.G., Hawkes, J.E. (2018). Phosphodiesterase (PDE) Inhibitors for the Treatment of Inflammatory Skin Conditions. In: Yamauchi, P. (eds) Biologic and Systemic Agents in Dermatology. Springer, Cham. https://doi.org/10.1007/978-3-319-66884-0_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-66884-0_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-66883-3
Online ISBN: 978-3-319-66884-0
eBook Packages: MedicineMedicine (R0)