Abstract
Endogenous neural stem cells (eNSC) in the adult brain mainly reside in two stem cell niches, the subventricular zone (SVZ), and the hippocampal dentate gyrus. Following cerebral insults, they are mobilized from their niches to engage in regeneration and mediate functional recovery. After cerebral ischemia, eNSC generate new neurons in a process called neurogenesis, but also indirectly mediate regeneration via pleiotropic functions including neuroprotection, reduction of neuroinflammation, revascularization, and induction of plasticity. However, the physiological capacity of the brain for self-repair after stroke is insufficient in mammals. Thus, a promising therapeutic approach in stroke constitutes the targeted activation of eNSC by pharmacological substances, e.g. osteopontin or FGL, and by non-pharmacological approaches, such as transcranial direct current stimulation (tDCS). Since treatments based on the transplantation of stem cells harbor several disadvantages including poor long-term cell survival and a lack of integration into the host circuitry, mobilizing the eNSC niche for therapeutic purposes constitutes a most promising approach in stem cell research.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Osteopontin
- FGL
- Ar-tumerone
- Transcranial direct current stimulation (tDCS)
- Recovery
- Neurogenesis
- Neuroprotection
- Plasticity
- Functional recovery
1 Introduction
For a long time it has been assumed that neurogenesis does not occur in the postnatal brain. This paradigm was opposed by Altman et al. [1], who first described the ability of the postnatal mammalian brain to generate new neurons in the postnatal rat hippocampus, and by Kaplan et al., who essentially discovered neurogenesis in the adult dentate gyrus [2]. They found labeled neurons using radioactive thymidine to label all dividing cells and histological examination of postmortem brains. This proved that new neurons in the adult brain are generated following cell division. The extend of neurogenesis declines in adulthood, but remains in a significant steady-state. Subsequent studies showed that in addition to the hippocampal dentate gyrus, immature precursor cells also persist in the subventricular zone of the lateral ventricles [3, 4] and are capable of differentiating into all three cell fates of the central nervous system (CNS): neurons, astrocytes and oligodendrocytes [5,6,7,8,9].
2 Endogenous Neural Stem Cells Under Physiological Conditions
2.1 Neural Stem Cells in Animals Models
Endogenous neural stem cells (eNSC) in the adult brain mainly reside in two stem cell niches: the subventricular zone (SVZ) adjacent to the lateral ventricles, and the subgranular zone of the hippocampus (SGZ). These stem cell niches are part of two distinct neuronal networks: eNSC from the SVZ can migrate via the rostral migratory stream (RMS) to the olfactory bulb. ENSC from the SGZ give rise to neurons and glia cells in the hippocampal dentate gyrus (reviewed by [10, 11]).
Under physiological circumstances, eNSC are mostly in a quiescent state. Interestingly, driven by Sonic hedgehog (Shh) signalling, they are able to self-renew over the course of a year and generate multiple cell types in vivo such as (inter)neurons, astrocytes or oligodendrocytes [12]. Furthermore, neurogenesis seems not be restricted to the SVZ and SGZ. There have been several reports of adult neurogenesis in the neocortex, the striatum, the amygdala, the hypothalamus, the substantia nigra, in the brain-stem, olfactory tubercle and piriform cortex. However, data about neocortical neurogenesis remains conflicting (reviewed by [13]). Inside the stem cell niches, eNSC reside in close connection to blood vessels. Extending this anatomical connection, endothelial vascular cells stimulate the self-renewal of neural stem cells. Even activated eNSC maintain their connection to blood vessels. This microenvironment is referred to as the neurovascular niche [11, 14].
Hippocampal neurogenesis seems to play an important role in learning and memory. Exercise and an enriched environment increase hippocampal neurogenesis and thus improves learning abilities [15,16,17]. Importantly, while eNSC numbers and neurogenesis decline during aging [18, 19], the capacity of the remaining eNSC to respond to cerebral insults seems stable over most of the life-span [20].
Moreover, eNSCs secrete trophic factors supporting neuroprotection such as glial-derived neurotrophic factor, vascular endothelial growth factor, or Shh [21, 22]. Additionally, eNSCs promote other regenerative processes including remyelination, angiogenesis, remodeling, and immunomodulation [23, 24].
2.2 Neural Stem Cells in Humans
The knowledge of eNSC derived neurogenesis in humans is restricted by the limited amount of detection methods (reviewed by [25]). The first study reporting about neurogenesis in the human hippocampus examined human brain tissue that was obtained postmortem from patients who had been treated during a cancer treatment with the thymidine analog, bromodeoxyuridine (BrdU), that labels DNA during the S phase [26]. Another method to estimate human neurogenesis was established by Jonas Frisén’s group by measuring the concentration of nuclear bomb test-derived 14C in genomic DNA [27,28,29]. Though, limitations of this technique are the demanding infrastructure and the natural decline of 14C levels [28].
In humans, the rostral migratory stream is organized around a tubular extension of the lateral ventricle that reaches the olfactory bulb [30]. However, neurogenesis in the olfactory bulb in humans seems not to reach relevant levels [31]. On the other hand, relevant human adult hippocampal neurogenesis with an estimated number 700 new neurons are added per day was described [27]. Moreover, integration of newborn neurons into the striatum was observed in humans [32]. Notably, in most cases, the intrinsic response of eNSCs is obviously not sufficient to lead to detectable neocortical neurogenesis after stroke [33]. Of note, all of the latter results were obtained by 14C measuring. This method can only detect larger numbers of new cells that were generated at a given time point with a detectable limit at about 1% of the total population of neurons [29]. Therefore, neurogenesis occurring at low levels, and new neurons that are not permanently integrated into the circuitry, may not be tracked using this method. This may explain contradictory data about neocortical neurogenesis occurring after stroke, which was reported to be present in immunocytochemical analyzes of human postmortem brain slices [34]. Additionally, some reports found cortical neurogenesis in small numbers in stroke animal models [35, 36].
3 Neural Stem Cells After Ischemia
Stroke is one of the major causes of adult disability [37]. To date, re-perfusion treatment is only possible in a narrow time window, and there is no neuroprotective or even regenerative treatment for the subacute or chronic phase after stroke yet. Thus, current treatment in this phase is limited to functional treatment such as physiotherapy. From the pathophysiological point of view, after the initial ischemic damage with disruption of the blood flow that leads to necrotic cell death, brain resident immune cells such as microglia and astrocytes are rapidly activated, and blood-borne immune cells (granulocytes, T-cells, monocytes/macrophages) are recruited from the blood stream to the lesion site [38,39,40,41,42,43]. This process is called neuroinflammation. There are many beneficial effects of neuroinflammation such as containment of necrotic damage, trophic support, support of neurons and mobilization of endogenous stem cells [44]. But on the other hand, persistent neuroinflammation can also cause secondary tissue damage by excessive release of proinflammatory cytokines and reactive oxygen species [45].
The immune cells attract eNSC to the site of the lesion by secretion of various inflammatory cytokines such as stromal cell-derived factor-1, tumor necrosis factor-alpha, and interferon-γ [46,47,48,49]. This attraction of eNSC is also referred to as mobilization of eNSCs. In various models of cerebral ischemia in experimental animals, including transient global ischemia, transient focal ischemia, or permanent focal ischemia, a mobilization of eNSC was demonstrated [50,51,52,53]. However, this eNSC mobilization is not sufficient to provide functional recovery, because the majority of newly generated neuroblasts in ischemic stroke models die by the time they have reached the peri-infarct area [52]. Moreover, in humans, no relevant neocortical neurogenesis in humans was detected after stroke [33].
4 Mobilizing the Endogenous NSC Niche
Since the endogenous neural stem cell response after stroke is not strong enough for sufficient repair processes, boosting the eNSC response by pharmacological or non-pharmacological methods constitutes a promising therapeutic approach for stroke. In contrast, the transplantation approach of “exogenous” cells is associated with certain disadvantages like poor long-term cell survival, a lack of integration into the host circuitry, immune reactions against the transplants, and limited availability of appropriate cells (reviewed by [54]). Mobilizing the endogenous neural stem cell niche overcomes those difficulties and is additionally less invasive.
4.1 Pharmacological Mobilization of Endogenous Neural Stem Cells
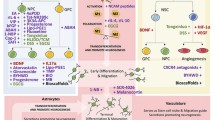
ENSCs can be mobilized for therapeutic purposes by different types of drugs. One group of substances consists of stem cell growth- and regulation factors that target specific intracellular signaling pathways: Early studies reported that intraventricular treatment with fibroblast growth factor 2 (FGF 2) and epithelial growth factor (EGF) stimulates the proliferation of eNSCs in vivo [55, 56]. Intraventricular co-treatment with FGF 2 and EGF increases the number of hippocampal pyramidal neurons after cerebral ischemia by enhancing eNSC proliferation, and their differentiation into neurons [57]. Likewise, augmenting long-term FGF2 expression in rats after stroke increases SVZ and cortical neurogenesis and behavioral outcome [35]. Notch signaling is an important signaling pathway in eNSC and evokes pleiotropic effects in stem cells. Notch receptor activation promotes the survival of neural stem cells. Transient administration of Notch ligands to the brain of adult rats increases the numbers of newly generated precursor cells and improves motor skills after ischemic injury [21]. Moreover, angiopoietins are significant regulators of endothelial and hematopoietic stem cells. Angiopoietin2 rescues injured dopamine neurons with motor behavioral improvement in an experimental model of neurodgeneration [58]. The neural cell adhesion molecule (NCAM) enhances neurite outgrowth, synaptogenesis, and neuronal differentiation. Its mimetic peptide FG Loop (FGL) induces NSC mobilization in vitro and in vivo, and supports oligodendroglial differentiation [59]. After focal cerebral ischemia, FGL mobilizes eNSC from the niches and enhances regeneration by amplifying remyelination and modulating neuroinflammation via affecting microglia [60]. Another important eNSC signaling pathway is initiated by the ligand sonic hedgehog. Jin et al. showed that oral administration of a sonic hedgehog agonist increased functional recovery, neurogenesis and angiogenesis after experimental stroke [61].
In a second pharmacological approach, eNSC mobilization can also be induced by certain nutrition ingredients: Curcumin and ar-turmerone are the major bioactive compounds of the herb Curcuma longa. Ar-turmerone induces NSC proliferation in vitro and promotes neuronal differentiation of eNSC. Concordantly, there was also increased proliferation and mobilization of eNSC in vivo as shown by Positron-Emission-Tomography (PET) [62].
A third group of drugs mobilizing eNSC are endogenous or exogenous factors that are involved in (neuro-)inflammation: Osteopontin (OPN) is an endogenous phosphoglycoprotein with important roles in tissue homeostasis, wound healing, immune regulation, and stress responses. OPN increases survival, proliferation, migration, and neuronal differentiation of eNSC. Increased survival and migration are mediated via the chemokine receptor CXCR4. After cerebral ischemia, OPN increases neurogenesis [63] (Fig. 5.1). Additionally, OPN seems to polarize microglia to a neuroprotective subtype in an inflammation setting [64]. The tetracycline antibiotic minocycline is commonly used to treat bacterial infections. Additionally, it has pleotropic effects on immune processes [65, 66]. In stem cells, minocycline enhances cell survival in vitro, and increases eNSC activity in both the SVZ as well as the hippocampus in animals after experimental stroke [67]. Additionally, minocycline antagonizes the rapid glial differentiation induced by proinflammatory cytokines in vitro, and restores the neurogenic and oligodendrogenic potential [68].
Osteopontin (OPN) increases survival, proliferation and neurogenesis of neural stem cells (NSC). (a) Adult male rats injected with a single dose of 500 μg OPN i.c.v. displayed a significantly higher number of proliferating NSC in the SVZ as the major NSC niche, corroborating the effects of OPN on NSC proliferation in vivo (values displayed as means ± SEM; **p < 0.01). Representative images from the SVZ of rats treated with either placebo (left) or OPN (right; scale bar represents 200 μm). (b) NSC cultures were exposed to oxidative stress by H2O2 (300 nM for 24 h), increasing cell death as assessed by propidium iodide staining. Pre-treatment of NSC with 6.25 μg/ml OPN 24 h prior to oxidative stress completely rescued NSC from this toxicity, while simultaneous addition of OPN and H2O2 prevented about half the cells from dying (values displayed as means ± SEM; *p < 0.05). (c) Generation of TuJ1-positive neurons (green) during differentiation was increased by OPN treatment (lower row) as compared to control (upper row) at days 7, 10, and 14 after mitogen withdrawal. During that period, the axon length grew notably and neurons began to form networks; both observations were more pronounced in OPN-treated cells. By day 14, mature MAP2+-positive neurons had formed (right column; scale bars represent 100 μm). (d) Osteopontin (OPN) promoted neurogenesis after stroke in vivo. In adults rats that underwent photothrombosis, a single i.c.v. injection of 500 μg OPN significantly increased the area covered by DCX-positive neuroblasts in the SVZ (values are displayed as means ± SEM, **p < 0.01). Representative, DCX-stained images from the SVZ of rats subjected to cerebral ischemia, treated with either placebo (left) or OPN (right). OPN treatment led to an increase of neuroblasts in the SVZ (scale bar represents 100 μm). Adapted from Rabenstein et al. [63] with permission
4.2 Non-pharmacological Mobilization of Endogenous Neural Stem Cells
Clinical data suggest that transcranial direct current stimulation (tDCS) may facilitate rehabilitation after stroke [69, 70]. However, the neurobiological mechanisms underlying tDCS remain poorly explored. TDCS can be applied with either with an anodal or a cathodal current polarity, and with various current densities. In the healthy rat brain, certain polarities and current densities of tDCS increase neural stem cell migration and activate microglia [71, 72]. Under specific conditions, tDCS accelerates functional recovery in animals after experimental stroke. Moreover, both anodal and cathodal tDCS at different current densities induce neurogenesis (Fig. 5.2). Only cathodal tDCS recruits oligodendrocyte precursors towards the lesion, but also supports a proinflammatory M1-polarization of microglia. In contrast, anodal tDCS leads to downregulation of the constitutive expression of Iba1 by microglia [73, 74]. In conclusion, the different tDCS polarities seem to exert different effects on eNSC as well as on migroglia. TDCS acts through multifaceted mechanisms that far exceed its primary neurophysiological effects, encompassing proliferation and migration of stem cells, their neuronal differentiation, and modulation of microglia responses.
Multisession tDCS induced neurogensis in the subventricular zone (SVZ). (a) Representative, DCX-stained images from the SVZ of mice, treated with either sham (left), cathodal tDCS (middle) or anodal tDCS (right). Multisession cathodal or anodal tDCS at 99 kC/m2 increased DCX immunoreactivity in the SVZ (scale bar represents 100 μm). Multisession cathodal or anodal tDCS increased the number of DCX+ neuroblasts in the SVZ of control animals (b) and rats subjected to cerebral ischemia (c) (values are displayed as means ± SEM, *p < 0.05). Adapted from Pikhovych et al. [74] and Braun et al. [73] with permission
5 Future Perspectives
ENSC can be targeted by pharmacologial or non-pharmacological approaches. Thereby, enhancement of eNSC proliferation, migration and differentiation to neurons and oligodendrocytes is possible in order to promote neuroregeneration and functional recovery. As a second step, a translational approach to establish these therapies in clinical treatment for humans is needed. Such clinical trials could include osteopontin (OPN) that modulates eNSC as well as immune cells, and can be applied via a nose spray [75]. As for non-pharmacological approaches, tDCS is already applied experimentally in the clinical setting [69, 70]. With more knowledge about the neurobiological and polarity-dependent effects, a better targeted use of tDCS in the clinic could be archived. Currently, in an animal model of experimental autoimmune encephalomyelitis (EAE), inhibition of Gli1—a transcriptional effector of the sonic hedgehood pathway—improves the functional outcome and offers neuroprotection. This inhibition can be achieved by intraventricular application of GANT61, a small molecule inhibitor of Gli12 [76]. This pathway might be interesting for targeted eNSC activation after stroke as well. However, for clinical application a less invasive application methods needs to be found.
Most importantly, in order to translate experimental findings into the clinical setting, translational read-outs need to be advanced to non-invasively monitor treatment effects. Moreover, it is crucial to learn more about physiological human neurogenesis, to then evaluate the treatment efficacy of eNSC mobilization in humans. In this, MRI detection methods require invasive labelling to specifically detect eNSC: This can be achieved either by direct intraventricular injection of labels or viral- or antibody-coupled labels, thus all of these methods are not applicable in humans. Another option is PET-imaging with the radiotracer 3′-deoxy-3′-[18F]fluoro-l-thymidine that labels proliferating cells. This approach offers a promising method to noninvasively quantify eNSC in the live brain [77, 78].
Taken together, targeted activation of eNSC by pharmacological substances, e.g. stem cell regulating factors or osteopontin, and by non pharmacological approaches such as transcranial direct current stimulation (tDCS), constitute a promising approach to facilitate regeneration and enhance recovery after stroke.
Abbreviations
- BrDU:
-
Bromodeoxyuridine
- CNS:
-
central nervous system
- DCX:
-
Doublecortin
- EAE:
-
Experimental autoimmune encephalomyelitis
- EGF:
-
Epithelial growth factor
- eNSC:
-
Endogenous neural stem cells
- FGF 2:
-
Fibroblast growth factor 2
- FGL:
-
Neural cell adhesion molecule FG Loop
- NCAM:
-
Neural cell adhesion molecule
- OPN:
-
Osteopontin
- PET:
-
Positron-Emission-Tomography
- RMS:
-
Rostral migratory stream
- SGZ:
-
Subgranular zone of the hippocampus
- Shh:
-
Sonic hedgehog
- SVZ:
-
Subventricular zone
- tDCS:
-
Transcranial direct current stimulation
References
Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–35.
Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4(6):1429–41.
Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11(1):173–89.
Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13(5):1071–82.
Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–16.
Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–10.
Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5(12):3310–28.
Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90(5):2074–7.
Temple S. Division and differentiation of isolated CNS blast cells in microculture. Nature. 1989;340(6233):471–3.
Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702.
Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 2013;23(6):935–42.
Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437(7060):894–7.
Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8(6):481–8.
Masjkur J, Rueger MA, Bornstein SR, McKay R, Androutsellis-Theotokis A. Neurovascular signals suggest a propagation mechanism for endogenous stem cell activation along blood vessels. CNS Neurol Disord Drug Targets. 2012;11(7):805–17.
van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–70.
Garthe A, Roeder I, Kempermann G. Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis. Hippocampus. 2016;26(2):261–71.
Gonçalves JT, Schafer ST, Gage FH. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell. 2016;167(4):897–914.
Bouab M, Paliouras GN, Aumont A, Forest-Bérard K, Fernandes KJ. Aging of the subventricular zone neural stem cell niche: evidence for quiescence-associated changes between early and mid-adulthood. Neuroscience. 2011;173:135–49.
Signer RA, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12(2):152–65.
Adamczak J, Aswendt M, Kreutzer C, Rotheneichner P, Riou A, Selt M, et al. Neurogenesis upregulation on the healthy hemisphere after stroke enhances compensation for age-dependent decrease of basal neurogenesis. Neurobiol Dis. 2017;99:47–57.
Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442(7104):823–6.
Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20(11):1103–10.
Chopp M, Li Y, Zhang ZG. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke. 2009;40(3 Suppl):S143–5.
Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Arch Neurol. 2008;65(4):452–6.
Jessberger S, Gage FH. Adult neurogenesis: bridging the gap between mice and humans. Trends Cell Biol. 2014;24(10):558–63.
Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–7.
Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–27.
Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisén J. Retrospective birth dating of cells in humans. Cell. 2005;122(1):133–43.
Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Björk-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103(33):12564–8.
Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–9.
Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MS, Steier P, et al. The age of olfactory bulb neurons in humans. Neuron. 2012;74(4):634–9.
Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156(5):1072–83.
Huttner HB, Bergmann O, Salehpour M, Rácz A, Tatarishvili J, Lindgren E, et al. The age and genomic integrity of neurons after cortical stroke in humans. Nat Neurosci. 2014;17(6):801–3.
Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103(35):13198–202.
Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, McKay RD. Long-lasting regeneration after ischemia in the cerebral cortex. Stroke. 2007;38(1):153–61.
Kreuzberg M, Kanov E, Timofeev O, Schwaninger M, Monyer H, Khodosevich K. Increased subventricular zone-derived cortical neurogenesis after ischemic lesion. Exp Neurol. 2010;226(1):90–9.
Mackay J, Mensah G. Atlas of heart disease and stroke. Geneva: World Health Organization; 2004.
Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55(2):195–203.
Schroeter M, Franke C, Stoll G, Hoehn M. Dynamic changes of magnetic resonance imaging abnormalities in relation to inflammation and glial responses after photothrombotic cerebral infarction in the rat brain. Acta Neuropathol. 2001;101(2):114–22.
Schroeter M, Jander S, Witte OW, Stoll G. Heterogeneity of the microglial response in photochemically induced focal ischemia of the rat cerebral cortex. Neuroscience. 1999;89(4):1367–77.
Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184(1-2):53–68.
Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, et al. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31(7):1735–43.
Hallenbeck JM, Dutka AJ, Tanishima T, Kochanek PM, Kumaroo KK, Thompson CB, et al. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986;17(2):246–53.
Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci. 2006;26(12):3182–91.
Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87–113.
Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101(52):18117–22.
Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26(1):125–34.
Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24(3):739–47.
Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006;7:64.
Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98(8):4710–5.
Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18(19):7768–78.
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–70.
Schroeter M, Dennin MA, Walberer M, Backes H, Neumaier B, Fink GR, et al. Neuroinflammation extends brain tissue at risk to vital peri-infarct tissue: a double tracer [11C]PK11195- and [18F]FDG-PET study. J Cereb Blood Flow Metab. 2009;29(6):1216–25.
Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120(1):29–40.
Martens DJ, Seaberg RM, van der Kooy D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and the central canal of the spinal cord. Eur J Neurosci. 2002;16(6):1045–57.
Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17(15):5820–9.
Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110(4):429–41.
Androutsellis-Theotokis A, Rueger MA, Park DM, Mkhikian H, Korb E, Poser SW, et al. Targeting neural precursors in the adult brain rescues injured dopamine neurons. Proc Natl Acad Sci U S A. 2009;106(32):13570–5.
Klein R, Blaschke S, Neumaier B, Endepols H, Graf R, Keuters M, et al. The synthetic NCAM mimetic peptide FGL mobilizes neural stem cells in vitro and in vivo. Stem Cell Rev. 2014;10(4):539–47.
Klein R, Mahlberg N, Ohren M, Ladwig A, Neumaier B, Graf R, et al. The neural cell adhesion molecule-derived (NCAM)-peptide FG loop (FGL) mobilizes endogenous neural stem cells and promotes endogenous regenerative capacity after stroke. J Neuroimmune Pharmacol. 2016;11(4):708–20.
Jin Y, Barnett A, Zhang Y, Yu X, Luo Y. Poststroke sonic hedgehog agonist treatment improves functional recovery by enhancing neurogenesis and angiogenesis. Stroke. 2017;48(6):1636–45.
Hucklenbroich J, Klein R, Neumaier B, Graf R, Fink GR, Schroeter M, et al. Aromatic-turmerone induces neural stem cell proliferation in vitro and in vivo. Stem Cell Res Ther. 2014;5(4):100.
Rabenstein M, Hucklenbroich J, Willuweit A, Ladwig A, Fink GR, Schroeter M, et al. Osteopontin mediates survival, proliferation and migration of neural stem cells through the chemokine receptor CXCR4. Stem Cell Res Ther. 2015;6:99.
Rabenstein M, Vay SU, Flitsch LJ, Fink GR, Schroeter M, Rueger MA. Osteopontin directly modulates cytokine expression of primary microglia and increases their survival. J Neuroimmunol. 2016;299:130–8.
Brown A. Osteopontin: a key link between immunity, inflammation and the central nervous system. Transl Neurosci. 2012;3(3):288–93.
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107(9):1055–61.
Rueger MA, Muesken S, Walberer M, Jantzen SU, Schnakenburg K, Backes H, et al. Effects of minocycline on endogenous neural stem cells after experimental stroke. Neuroscience. 2012;215:174–83.
Vay SU, Blaschke S, Klein R, Fink GR, Schroeter M, Rueger MA. Minocycline mitigates the gliogenic effects of proinflammatory cytokines on neural stem cells. J Neurosci Res. 2016;94(2):149–60.
Sparing R, Thimm M, Hesse MD, Küst J, Karbe H, Fink GR. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain. 2009;132(Pt 11):3011–20.
Hummel F, Celnik P, Giraux P, Floel A, WH W, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128(Pt 3):490–9.
Rueger MA, Keuters MH, Walberer M, Braun R, Klein R, Sparing R, et al. Multi-session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PLoS One. 2012;7(8):e43776.
Keuters MH, Aswendt M, Tennstaedt A, Wiedermann D, Pikhovych A, Rotthues S, et al. Transcranial direct current stimulation promotes the mobility of engrafted NSCs in the rat brain. NMR Biomed. 2015;28(2):231–9.
Braun R, Klein R, Walter HL, Ohren M, Freudenmacher L, Getachew K, et al. Transcranial direct current stimulation accelerates recovery of function, induces neurogenesis and recruits oligodendrocyte precursors in a rat model of stroke. Exp Neurol. 2016;279:127–36.
Pikhovych A, Stolberg NP, Jessica Flitsch L, Walter HL, Graf R, Fink GR, et al. Transcranial direct current stimulation modulates neurogenesis and microglia activation in the mouse brain. Stem Cells Int. 2016;2016:2715196.
Topkoru BC, Altay O, Duris K, Krafft PR, Yan J, Zhang JH. Nasal administration of recombinant osteopontin attenuates early brain injury after subarachnoid hemorrhage. Stroke. 2013;44(11):3189–94.
Samanta J, Grund EM, Silva HM, Lafaille JJ, Fishell G, Salzer JL. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature. 2015;526(7573):448–52.
Rueger MA, Backes H, Walberer M, Neumaier B, Ullrich R, Simard ML, et al. Noninvasive imaging of endogenous neural stem cell mobilization in vivo using positron emission tomography. J Neurosci. 2010;30(18):6454–60.
Rueger MA, Schroeter M. In vivo imaging of endogenous neural stem cells in the adult brain. World J Stem Cells. 2015;7(1):75–83.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Rabenstein, M., Rueger, M.A. (2018). Mobilization of Endogenous Neural Stem Cells to Promote Regeneration After Stroke. In: Lapchak, P., Zhang, J. (eds) Cellular and Molecular Approaches to Regeneration and Repair. Springer Series in Translational Stroke Research. Springer, Cham. https://doi.org/10.1007/978-3-319-66679-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-66679-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-66678-5
Online ISBN: 978-3-319-66679-2
eBook Packages: MedicineMedicine (R0)