Abstract
Pouchitis, especially, chronic antibiotic-refractory pouchitis, is one of the most challenging disease conditions of the ileal pouch. While the majority of pouchitis is idiopathic, secondary causes, such as the use of non-steroidal anti-inflammatory drugs (NSAID), infection of Clostridium difficile and cytomegalovirus, ischemia and obesity, should be evaluated and may be modified. Symptomatology of pouchitis is not specific, as the similar symptoms can be presented in irritable pouch syndrome, cuffitis, Crohn’s disease of the pouch. Pouchitis can be classified into microbiota-associated, autoimmune-associated, and ischemia-associated phenotypes. Pouchoscopy is the most important modality for the diagnosis and differential diagnosis of pouchitis and various pouch disorders. Oral antibiotic therapy is the main stay for the treatment of acute microbiota-associated pouchitis. Oral budesonide and immunomodulators may be effective for the treatment of autoimmune-associated pouchitis. While anti-tumor necrosis factor agents are effective in treating Crohn’s disease of the pouch, anti-integrin agents has demonstrated promising results in CARP.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Refer to Algorithms in Figs. 93.1 and 93.2
-
A.
Pouchitis is the most common long-term complication in patients undergoing restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) for medically refractory ulcerative colitis (UC) or colitis-associated neoplasia (CAN). Patients with familial adenomatous polyposis (FAP) who had the same surgical procedure may occasionally experience symptoms of pouchitis. The predominance of UC-associated pouchitis, as opposed to FAP- associated pouchitis suggests the role of systemic, genetic, and microbiological factors in the development of pouchitis.
-
B.
Etiology and pathogenesis of pouchitis is not entirely clear. Since the majority of patients with initial acute episodes of pouchitis respond to antibiotic therapy, microbiota are believed to play a key role in the etiopathogenesis of pouchitis. The contribution of microbiota to the development of pouchitis are two folds: (1) dysbiosis or alteration in quantity or composition of commensal bacteria; and (2) pathogens, including pathogenic bacteria (e.g. C. difficile, C. perfringens, Campylobacter spp., Group D streptococci (Enterococcus spp.), hemolytic strains of E. coli) viruses (e.g. cytomegalovirus [CMV]), and fungi (e.g. Candida albicans and Histoplasma). However, in our clinical practice, we have seen a growing number of patients with pouchitis develop a refractory course to anti-microbial therapy. A number of patients may develop chronic antibiotic-refractory pouchitis (CARP).
-
C.
Risk factors for pouchitis, especially CARP, have been extensively studied. The presence of extraintestinal manifestations of inflammatory bowel disease (IBD), particularly primary sclerosing cholangitis (PSC), arthralgia, and arthropathy, has been found to be related to pouchitis. In addition, genetic polymorphisms such as those of IL-1 receptor antagonist, NOD2/CARD15 or a combined carriership of TLR9-1237C and CD14-260T alleles may be associated with pouchitis. The modifiable risk factors include the use of non-steroidal anti-inflammatory drugs (NSAID) and surgery associated ischemia. It is interesting to find that smoking has an opposite effect on acute (exacerbating factor) pouchitis and chronic (protective effect) pouchitis. This suggests that etiopathogenetic pathways of acute and chronic pouchitis are different. Recent data showed that weight gain, especially gain of mesenteric fat, is related to the development of pouchitis, suggesting the role of mesenteric fat and surgery-procedure associated ischemia. Furthermore, recent studies also reported that C. difficile-associated pouchitis, CARP, and presacral anastomotic leak or sinus are predominantly seen in male. With the male gender is the common denominator for the three pouch disease conditions, along with the “reach issue” of pouch body to the cuff mainly seen in male, we speculate that mesenteric fat deposition and pouch ischemia are the contributing factors for pouchitis. It is believed that the risk of pouchitis, especially CARP, can be reduced by the use of proper surgical techniques, life style modification (such as weight loss), and avoidance of NSAID use.
-
D.
Pouchitis is not single disease entity. Rather, it represents a spectrum of diseases with ranging risk factors, clinical presentation, disease course, and prognosis. Proper diagnosis and classification of various phenotypes of pouchitis are important for the management and improvement in prognosis. Pouchitis can be classified into acute (<4 weeks) and chronic (≥4 weeks) forms, according to the duration of symptoms; antibiotic-responsive; antibiotic-dependent; and antibiotic-refractory phenotypes, based on the response to and frequency of requirement of antibiotic therapy; idiopathic and secondary (such as NSAID-induced, ischemia-related, and CMV-associated) entities, based on the etiology. The terminology combing the above features may be used to characterize certain type of pouchitis, such as CARP or acute NSAID-induced pouchitis. Of note, the phenotype of pouchitis can change over time, in a more unidirectional way. For example, acute antibiotic-responsive pouchitis may evolve into CARP, but not other way around. Pouchitis may be classified three main categories: (1) microbiota-associated; (2) autoimmune-associated; and (3) ischemia-associated, based on the distribution of mucosal inflammation.
-
E.
Patients with pouchitis often presents with increased bowel frequency, loose or watery bowel movement, urgency, nocturnal seepage, abdominal pain or cramps, and pelvic pressure. Those symptoms are not specific for pouchitis, as they can be presented in those with irritable pouch syndrome, small bowel bacterial overgrowth, cuffitis and CD of the pouch.
-
F.
Fever, chills, leukocytosis, not common in classic pouchitis, may be presenting symptoms of pathogen-associated pouchitis or CD of the pouch with abscess. Hematochezia, typically seen patients with cuffitis, is an uncommon presentation for pouchitis. Low back pain or pain at the tip of the coccyx may suggest a diagnosis of presacral anastomotic leak, abscess, or sinus. Weight loss is not common in classic pouchitis. Significant weight loss may trigger a full evaluation of mechanical complications of the pouch, such as anastomotic leak and afferent limb or efferent limb syndrome, CD of the pouch, and concurrent celiac disease.
-
G.
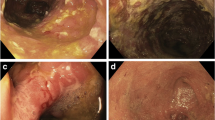
Pouch endoscopy or pouchoscopy is the most valuable diagnostic modality for the diagnosis and differential diagnosis of pouchitis. Pouchoscopy is used to assess the degree and distribution of mucosal inflammation, evaluate structural abnormalities (such as strictures, fistula, and anastomotic leak, bowel angulation, and prolapse), take tissue specimens, and monitor the risk of dysplasia. The distribution pattern of inflammation may provide important clues for diagnosis of various phenotypes of pouchitis, cuffitis, and CD of the pouch. For example, the inflammation of microbiota-associated pouchitis is often limited to the pouch body; the inflammation of autoimmune-associated pouchitis (classic example: PSC-associated pouchitis/enteritis) is extended from pouch body to a long segment of the afferent limb. Mucosal inflammation in ischemic pouchitis is typically asymmetric, involving the distal pouch body, suture line, pouch inlet or afferent limb site of a J pouch body. CD of the pouch often presents with discrete ulcers in the pouch body and afferent limb, often along with strictures and/or fistula.
Symptomatic patients without endoscopic inflammation in any segments of IPAA may be classified as having irritable pouch syndrome or small intestinal bacterial overgrowth. Those patients may respond favorable to antibiotic therapy.
Esophagogastroduodenoscopy (EGD) may be performed in patients with autoimmune-associated pouchitis or CD of the pouch, and those with suspected celiac disease.
-
H.
The role of mucosal biopsy for the evaluation of pouch disease is to identify neoplasia, ischemia, prolapse, granulomas, virus- or fungus-infected mucosa. The accuracy of mucosal biopsy in grading severity of mucosal inflammation is limited. Immunohistochemistry can be performed to evaluate CMV infection.
-
I.
Laboratory tests should be routinely checked. Commonly ordered tests include complete blood counts, comprehensive metabolic panel, and C-reactive protein. Fecal lactoferrin or calprotectin may be used as surrogate markers for mucosal inflammation. We routinely check C. difficile test. In patients suspected of autoimmune-associated pouchitis, we routinely check serum anti-nuclear antigen, IgG4, microsomal antibodies. For patients suspected pathogen-associated pouchitis, serum CMV DNA and fungal battery may be assayed.
-
J.
Abdominal and pelvic imaging is mainly used for the differential diagnosis of pouchitis. Ischemic pouchitis may occasionally demonstrate non-hyperenhancement of mucosa in contrasted CT or MRI. CT and MRI have been routinely used to assess the presence of stricture, fistula, abscess, or anastomotic leaks. Gastrograffin enema and barium defecography have been very useful to evaluate stricture, anastomotic leak or sinus, prolapse, angulation of bowel lumen.
-
K.
It is important to follow the disease course of pouchitis. If a patient develop “pouchitis” immediately after ileostomy closure, concurrent mechanical diseases, such as stricture and anastomotic leaks, should be evaluated. If a patient has a normal pouch for many years and gradually develops pouchitis, particularly CARP, triggering factors such as weight gain and abdominal surgery with mesh placement, should be investigated.
-
L.
Treatment of pouchitis is largely based on the underlying risk factors and disease phenotype. For pathogen-associated pouchitis, we are able to choose proper agents for the targeted therapy. For example, C. difficile-associated pouchitis may be treated oral vancomycin or fidaxomicin. For patients with recurrent or refractory C. difficile pouchitis, fecal microbiota transplant (FMT) may be attempted. For patients with dysbiosis-associated pouchitis, broad spectrum oral antibiotics, such as ciprofloxacin, metronidazole, and tinidazole, may be used.
-
M.
For patients with antibiotic-dependent pouchitis , probiotic agents, such as Lactobacillus GG and VSL#3, or a low dose of antibiotics, such as luminal active rifaximin, may be tried.
-
N.
Treatment of CARP can be challenging. It is important to modify exacerbating factors, such as NSAID use. Prolonged courses of dual oral antibiotics, topical mesalamines, and topical corticosteroids may be tried. For patients with autoimmune associated pouchitis, often in the form of CARP, oral budesonide (9 mg/day for treatment and 3–6 mg/day for maintenance can be helpful.
-
O.
The role of immunomodulators, such as azathioprine, 6-mercaptopurine, and methotrexate in the treatment of pouchitis is not well defined. This author has found that low dose of 6-mercaptopurine (i.e. 50 mg/day PO) or methotrexate (12.5 mg QW SQ) has been effective in some patients with autoimmune-associated pouchitis.
-
P.
While anti-tumor necrosis factor (TNF) agents have been effective in treating CD of the pouch, their use in treating CARP warrants further investigation. In the published case series in the literature, infliximab and adalimumab are effective in some patients with CARP, particularly in those with concurrent fistula. The results raise a question on whether those patients had CD of the pouch, rather than CARP. Vedolizumab, a gut-selective anti-integrin monoclonal antibody, has been shown promising results in the treatment of CARP.
-
Q.
Currently, there are no established medical treatment for ischemia-associated pouchitis. This author found that hyperbaric oxygen therapy may be beneficial.
Suggested Reading
Abdelrazeq AS, Kelly SM, Lund JN, et al. Rifaximin–ciprofloxacin combination therapy is effective in chronic active refractory pouchitis. Color Dis. 2005;7:182–6.
Achkar JP, Al-Haddad M, Lashner B, et al. Differentiating risk factors for acute and chronic pouchitis. Clin Gastroenterol Hepatol. 2005;3:60–6.
Barreiro-de Acosta M, García-Bosch O, Gordillo J, Mañosa M, Menchén L, Souto R, Marin-Jimenez I, Grupo Joven GETECCU. Efficacy of adalimumab rescue therapy in patients with chronic refractory pouchitis previously treated with infliximab: a case series. Eur J Gastroenterol Hepatol. 2012;24:756–8.
Belluzzi A, Serrani M, Roda G, Bianchi ML, Castellani L, Grazia M, Rosati G, Ugolini G, Roda E. Pilot study: the use of sulfasalazine for the treatment of acute pouchitis. Aliment Pharmacol Ther. 2010;31:228–32.
Ferrante M, Declerck S, De Hertogh G, et al. Outcome after proctocolectomy with ileal pouch–anal anastomosis for ulcerative colitis. Inflamm Bowel Dis. 2008;14:20–8.
Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–9.
Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003a;124:1202–9.
Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003b;124:1202–9.
Gionchetti P, Rizzello F, Poggioli G, et al. Oral budesonide in the treatment of chronic refractory pouchitis. Aliment Pharmacol Ther. 2007;15(25):1231–6.
Gosselink MP, Schouten WR, van Lieshout LM, et al. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis Colon Rectum. 2004;47:876–84.
Kelly OB, Rosenberg M, Tyler AD, Stempak JM, Steinhart AH, Cohen Z, Greenberg GR, Silverberg MS. Infliximab to treat refractory inflammation after pelvic pouch surgery for ulcerative colitis. J Crohns Colitis. 2016;10:410–7.
Madden MV, McIntyre AS, Nicholls RJ. Double-blind crossover trial of metronidazole versus placebo in chronic unremitting pouchitis. Dig Dis Sci. 1994;39:1193–6.
McLaughlin SD, Clark SK, Shafi S, et al. Fecal coliform testing to identify effective antibiotic therapies for patients with antibiotic-resistant pouchitis. Clin Gastroenterol Hepatol. 2009;7:545–8.
Mimura T, Rizzello F, Helwig U, et al. Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment Pharmacol Ther. 2002;16:909–17.
Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL#3®) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–14.
Navaneethan U, Venkatesh PGK, Bennett AE, et al. Impact of budesonide on liver function tests and gut inflammation in patients with primary sclerosing cholangitis and ileal pouch-anal anastomosis. J Crohns Colitis. 2012;6:536–42.
Philpott J, Ashburn J, Shen B. Efficacy of vedolizumab in patients with antibiotic and anti-tumor necrosis alpha refractory pouchitis. Inflamm Bowel Dis. 2017;23:E5–6.
Shen B, Fazio VW, Remzi FH, et al. Combined ciprofloxacin and tinidazole in the treatment of chronic refractory pouchitis. Dis Colon Rectum. 2007;50:498–508.
Shen B, Remzi FH, Lopez AR, Queener E. Rifaximin for maintenance therapy in antibiotic-dependent pouchitis. BMC Gastroenterol. 2008a;8:26.
Shen B, Remzi FH, Lavery IC, Lashner BA, Fazio VW. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clin Gastroenterol Hepatol. 2008b;6:145–58.
Shen B, Remzi FH, Nutter B, et al. Association between immune-associated disorders and adverse outcomes of ileal pouch-anal anastomosis. Am J Gastroenterol. 2009;104:655–64.
Shen B, Plesec TP, Remer E, et al. Asymmetric inflammation of ileal pouch: a sign for ischemic pouchitis? Inflamm Bowel Dis. 2010;16:836–46.
Acknowledgements
Disclosure: The author has received honoraria from Abbvie, Janssen, Salix, and research grant from Takeda.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shen, B. (2020). Complications: Pouchitis. In: Steele, S., Maykel, J., Wexner, S. (eds) Clinical Decision Making in Colorectal Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-65942-8_93
Download citation
DOI: https://doi.org/10.1007/978-3-319-65942-8_93
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65941-1
Online ISBN: 978-3-319-65942-8
eBook Packages: MedicineMedicine (R0)