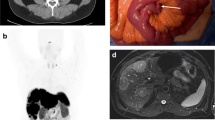
Abstract
Carcinoid tumors can occur anywhere along the gastrointestinal tract and are classified according to their embryological origin as foregut (6%), midgut (62%), and hindgut carcinoids (30%). Thirty-five percent of all carcinoids occur in the appendix which is the most common location, followed by the small bowel and the rectum. Depending on the location of origin, carcinoids can present as bowel obstruction, acute appendicitis, carcinoid syndrome, or can be completely asymptomatic and only found incidentally in pathologic specimens following routine endoscopic polypectomy or appendectomy. The extent of surgical resection indicated for carcinoid tumors depends on tumor location and extent of local and distal spread which is in turn based on tumor size and specific pathological characteristics. Accurate preoperative staging of carcinoid tumors is paramount. When faced with metastatic disease, surgeons need to rule out carcinoid syndrome and determine whether R0 resection can be achieved, in which case en bloc resection of all disease may lead to symptomatic relief and prolonged survival. If an R0 resection is not possible, then cytoreductive debulking surgery in combination with somatostatin analogues and cytotoxic systemic therapies should be considered.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Refer to Algorithm in Fig. 76.1
-
A.
Gastrointestinal carcinoids were first described by Lubarsch in 1888 and in 1907 Oberndorfer coined the term Karzinoide to indicate the carcinoma-like appearance and presumed lack of malignant potential. Carcinoids can occur anywhere along the gastrointestinal tract (GI) and are generally classified according to their embryological origin as foregut (6%); [thymus, bronchopulmonary, stomach, duodenum, and pancreas], midgut (62%); [small intestine, appendix, and the right colon], and hindgut carcinoids (30%); [distal colon and the rectum] (Table 76.1). Thirty-five percent of all carcinoids arise in the appendix which is the most common location followed by the small bowel where 23% of tumors are located within 2 ft of the ileocecal valve, and the rectum which harbors the remaining 20% of all GI carcinoids. Approximately 25% of patients with foregut and midgut carcinoids will develop a synchronous tumor, usually adenocarcinoma of the large bowel, while hindgut carcinoids rarely concur with synchronous tumors. Carcinoid neoplasms can occur as part of inherited neoplastic syndromes such as neurofibromatosis or multiple endocrine neoplasia type I (10%) although the majority occur sporadically.
-
B.
Small bowel carcinoids commonly present as the lead point in bowel obstruction or with chronic mesenteric ischemia from carcinoid-induced idiopathic mesenteric and retroperitoneal fibrosis that progressively encases and obstructs arterial inflow. They are typical multicentric, located in the terminal ileum, and most will present with lymph node or liver metastasis despite their relatively small size (less than 0.5 cm), as tumor size has been shown to correlate poorly with distant spread. Appendiceal carcinoids can present with abdominal pain, bowel obstruction, or as an incidental finding on pathological review following appendectomy. They are rarely multicentric, 95% are less than 2 cm in size and 75% are located in the distal third of the appendix. Tumor size is the strongest predictor of distant metastasis, with smaller tumors (<2 cm) less likely to metastasize to regional lymph nodes than larger tumors (>2 cm), which are more likely to present with metastasis. Hindgut carcinoids including colonic and rectal tumors, are non-secretory tumors and almost always asymptomatic with 50% diagnosed incidentally on pathological review of polypectomy specimens following routine colonoscopy. Occasionally, larger tumors will present with obstructive symptoms, tenesmus and rectal bleeding. Physical examination is typically unremarkable, though a general physical examination should be performed to look for concomitant pathology, adenopathy or comorbid conditions. Similar to appendiceal carcinoids, tumor size correlates with the risk of distant metastasis, where tumors <1 cm have a 5% or less chance to metastasize (Table 76.1)
Table 76.1 Features and characteristics of carcinoid tumors based on embryological origin .
-
C.
Carcinoid syndrome has been the hallmark of carcinoid neoplasms, however, only 20% of patients with local disease and 50% of patients with advanced disease actually develop this syndrome. Carcinoid syndrome is usually caused by both high levels of biologically active compounds secreted by the tumors into the systemic circulation causing episodic symptoms, including serotonin, hydroxytryptophan, prostaglandins, histamine, and bradykinin, as well as by the propensity of these tumors to metastasize to the liver, hindering the liver ability to degrade these biological active compounds into inactive by-products. Midgut carcinoids are most commonly associated with carcinoid syndrome as midgut tumors tend to produce high levels of serotonin. Foregut carcinoids , on the other hand, lack the enzyme required to convert chemical precursors such as 5-hydroxytryptophan into serotonin, so it is uncommon for them to result in carcinoid syndrome. Hindgut carcinoids are usually non-functional and rarely produce serotonin or the enzymes necessary to produce serotonin, and hence rarely produce the carcinoid syndrome, even when they metastasize to the liver. Symptoms of carcinoid syndrome include abdominal pain, non-bloody diarrhea, flushing, sweating and valvular heart disease (pulmonary stenosis, tricuspid insufficiency, and tricuspid stenosis) (Table 76.2)
Table 76.2 Clinical symptoms of carcinoid syndrome . Carcinoid crisis is a life-threatening condition characterized by severe abdominal pain, flushing, hypotension, or hypertension. It can be precipitated by stress such as anesthesia and surgery. In the setting of a carcinoid crisis, symptoms are usually managed with short-acting octreotide (Section K). Octreotide prevents and treats carcinoid syndrome by activating two out of five know somatostatin receptors identified within carcinoid tumors, subtypes 2 and 5. Activation of these receptors results in the reduction in the synthesis and secretion of serotonin, hydroxytryptophan, and other biologically active compounds.
-
D.
5-Hydroxyindoleacetic acid (5-HIAA) 24-h urine test is the most commonly used diagnostic test for carcinoid tumors with a 29–92% sensitivity and a 79–100% specificity. This compound is excreted in the urine after serotonin metabolism by the liver and lung to a pharmacologically inactive 5-HIAA. Prior to performing this test, patients should avoid serotonin-rich foods such as pineapples, kiwi, nuts, bananas and some medications that may result in falsely elevated urine 5-HIAA levels. Serum Chromogranin A (CgA), which is elevated in in 80% of patients with carcinoids tumors, is another useful marker with a sensitivity of 73% (range, 32–92%) and specificity of 95% (range, 63–100%). Circulating CgA levels reflect tumor load and can provide early diagnosis of persistent or recurrent carcinoid disease, with studies reporting that in over 80% of patients, serum elevation of CgA precedes the clinical diagnosis of recurrence by up to 2 years. This makes this marker valuable for monitoring the extent of disease and for long-term follow-up. The Ki67 antigen is a nuclear protein expressed by proliferating carcinoid cells and is absent in resting cells. Ki67 expression can be tested in resected tumors specimens using anti-Ki67 antibodies. Tumor levels of Ki67 expression can help predict response to chemotherapy. Low values (<2% tumor expression levels) indicate a low likelihood of clinical downstaging with chemotherapy whereas, high values (>2% tumor expression levels) suggest that tumors are more likely to benefit from chemotherapy in conjunction with surgery.
-
E.
Radiologic staging of carcinoid tumors is performed using computerized tomography scans (CT) and magnetic resonance imaging (MRI) of the chest, abdomen and pelvis. The diagnostic sensitivity for carcinoids ranges 57–94% with CT and 85–94% with MRI. Somatostatin receptor scintigraphy (SRS) is a whole body nuclear imaging study that is useful for localizing carcinoid tumors that express somatostatin receptors (SSTR 1–5), with a sensitivity that ranges 57–85% and specificity reported as high as 90% for localizing carcinoid tumors that express somatostatin receptors (SSTR 1–5). It is commonly used to rule out occult metastases when curative resection is intended. Furthermore, it may also be used to direct the choice of therapy (e.g. use of the somatostatin analogues in patients with unresectable tumors). Meta-iodobenzylguanidine scan (MIBG) is another nuclear imaging study that has been increasingly recommended as a diagnostic test for carcinoids tumors. Since about 10% of carcinoids do not express somatostatin receptors, but do occasionally take up Meta-iodobenzylguanidine rather than octreotide, MIBG scan be useful to detect carcinoids lesions that appeared negative on SRS scan. Most recently, whole body positron emission tomography scan (PET) with serotonin precursor 5-hydroxytryptophan, labelled with 11C (5HTP-PET) has been associated with high diagnostic sensitivity, and described as a helpful adjunct to monitor the effects of therapy.
-
F.
On gross examination, carcinoids appear as small submucosal (less than 2 cm in size), multicentric, yellow colored tumors on cut surface due to their high lipid content. However, they may also appear as subtle small white colored plaques on the antimesenteric border of the small or large bowel. They are typically associated with desmoplastic invasion and fibrosis of the mesentery caused by local effects of serotonin, growth factors, and other released substances, which can appear as large mesenteric masses often mistaken for the primary tumor. On microscopic examination, carcinoid tumors appear as round uniform cells packed with various secretary peptides. There are five histologic patterns which include insular, trabecular, glandular, undifferentiated, and mixed types. Histological features of aggressive carcinoid tumors include increased cellular atypia, necrosis and/or high mitotic rate.
-
G.
Upper and lower GI endoscopy should be considered especially in patients with midgut carcinoids as they tend to have high rate of synchronous tumors in regions remote from the primary tumor, particularly adenocarcinoma of the large bowel, where this has been reported in up to 6–15% of patients.
-
H.
Prior to any elective resection electrocardiogram and echocardiogram should be performed in both symptomatic and asymptomatic patients to rule out carcinoid valvular disease, a potential complication of carcinoid syndrome.
-
I.
Using the diagnostic tests and imaging modalities discussed earlier, carcinoids tumors are staged using the American Joint Committee on Cancer staging system (Tables 76.3, 76.4 and 76.5) to guide the multidisciplinary treatment and optimize surgical and/or systemic therapies. Furthermore, the world health organization (WHO) grading system, which is used to grade GI pancreatic neuroendocrine and carcinoid tumors and is based on the degree of tumor differentiation, tumor mitotic rate, Ki67 expression and presence or absence of ulceration, can be helpful in assessing tumor aggressiveness, predict tumor response to specific therapies and optimize treatment approach for each individual case (Table 76.6).
Table 76.3 Table American Joint Committee on Cancer (AJCC) 8th edition, small bowel Table 76.4 American Joint Committee on Cancer (AJCC) 8th edition, appendix Table 76.5 Table American Joint Committee on Cancer (AJCC) 8th edition, colon and rectum Table 76.6 Pathological features of carcinoid tumors used in staging and predicting prognosis
Refer to Algorithm in Fig. 76.2
-
J.
The surgical treatment of carcinoid tumors is based on tumor location, local, regional and distant extent of disease. For small bowel carcinoids where tumor size does not correlate with lymph node or distant metastasis, oncologic resection of the small bowel and associated mesenteric lymph nodes is the standard of care. Moreover, the entire small intestine should be carefully examined to exclude possible synchronous tumors. In addition, the proximity and or involvement of the superior mesenteric artery and vein should be assessed during surgery. For appendiceal carcinoids where tumor size correlates with metastatic potential, tumors <1 cm are treated with simple appendectomy while tumors >2 cm, which are associated with a 30–60% rate of positive lymph nodes and distant metastasis, should be treated with radical right hemicolectomy. Tumors 1–2 cm in size, in which the risk of metastasis is 0–1%, should be treated with primary or salvage right hemicolectomy if the base of the appendix is involved, if there is tumor extension to the mesoappendix or subserosal lymphatics, or if goblet cells are present on pathology. Rectal carcinoids <1 cm can be treated with local excision, either endoscopically, using transanal excision (TAE) or transanal endoscopic surgery (TES). Tumors >2 cm are treated with radical proctectomy, either low anterior resection or abdominoperineal resection with total mesorectal excision (TME). For rectal lesions 1–2 cm in size, the risk of lymph node metastasis can be up to 66%, so if the lesion involves the muscularis propria or is associated with suspicious lymph nodes or lymphovascular invasion, it should be treated with radical oncological resection, whereas lesions without high-risk histologic features can be considered for local excision. For colonic carcinoids, radical oncologic colon resection with regional lymphadenectomy is recommended.
-
K.
The cornerstones of systemic therapy for carcinoids tumors consist of octreotide and somatostatin analogues (SSA) . They are used in patients with carcinoid syndrome and for symptom control prior to definitive surgical resection. Octreotide and SSA are also used in asymptomatic patients with disease progression, to treat and prevent carcinoid crisis before, during and after surgical resection and/or liver embolization. Out of five subtypes of somatostatin receptors identified within carcinoid tumors (SSTR 1–5), subtype 2 and 5 are the receptors that mediate most of the beneficial effect of SSA. Activation of these receptors results in reduction in active hormonal synthesis and secretion. SSA has also been shown to be very effective in reducing disease progression in 50–60% of patients with advanced carcinoid tumors. However, its impact on overall survival remains to be proven. Standard dosing of octreotide long acting repeatable (LAR) consist in 20–30 mg intramuscular injection every 4 weeks. However, since therapeutic levels are not achieved for 14 days after the first LAR injection, short acting octreotide (150–250 μg three times daily subcutaneously) can be added to achieve rapid relief of symptoms and for breakthrough of symptoms. One of the major side effects of octreotide and SSA is the risk of biliary complications (gallbladder empyema, acute cholecystitis, acute pancreatitis and biliary colic) with a 5-year incidence of 19%. In patients in whom treatment with octreotide or SSA is anticipated postoperatively, concurrent cholecystectomy is recommended.
Refer to Algorithm in Fig. 76.3
-
L.
Management of metastatic carcinoid disease is dependent on whether carcinoid syndrome is present and whether R0 resection can be achieved. If there are no contraindications to surgery and an R0 resection can be achieved, then en-bloc resection of all disease may achieve long-lasting symptomatic relief and prolong survival. If R0 resection is not possible, then cytoreductive debulking surgery should be considered. Tumor debulking involve resection of the primary tumor for palliative alleviation of local and systemic symptoms with or without metastasectomy, most commonly for liver metastases. Liver metastasectomy commonly involve anatomical or non-anatomical hepatic resection. Non-surgical treatment options used in conjunction with hepatic resection for large hepatic metastases, for unresectable liver disease, and for patients who are not surgical candidates include hepatic artery embolization, hepatic cryoablation, or hepatic radiofrequency ablation (RFA). Hepatic artery embolization is highly effective in debulking liver metastases. The duration of response is usually short ranging from 7 months for hepatic artery embolization alone to 20 months if hepatic artery occlusion was followed by chemotherapy. In Selected patients, embolization can be repeated up to four times every 2–3 months.
-
M.
Chemotherapy has been largely ineffective in the management of advanced unresectable carcinoid tumors. Cisplatin-based chemotherapy has been used in aggressive carcinoids with high proliferative rates. Some studies have shown response rate as high as 67%, but much less in less aggressive indolent tumors. Interferon alpha, has been used in cases refractory to somatostatin and shown to achieve symptomatic relief in over third of cases. It is associated with a median biochemical response rate of 44% (range 0–71%) and a median tumor response rate of 11% (range 0–27%). However, its use is limited by side effects which include anorexia, fatigue, fever and weight loss.
-
N.
Targeted therapy with radiolabeled somatostatin analogues is one of the new developments currently under investigation and used for locally advanced unresectable metastatic carcinoids with positive SRS scan. The peptide receptor radionuclide therapy (PRRT) strategy involves the use of a carrier molecule (octreotide derivate) attached to a variety of different radionuclides including indium-111 (in), 90 Y, and lutetium-177 (177 Lu). The major advantage of PRRT is the ability of these molecules to identify and quantify the target, the somatostatin receptors, before starting treatment. PRRT is well tolerated with low to moderate toxicity. The use of PRRT has been associated with tumor regression in 14–19% of cases with stage IV disease and progression free survival in 4–70% of patients with advanced disease. Finally, external beam radiation is another frequently used option for palliative symptomatic control of bone and central nervous metastasis.
-
O.
With respect to long-term follow-up, in the first year following resection, patients should be monitored every 3–12 months with history and physical exam, CT scans or MRI, serum CgA and urinary 5-HIAA levels. If the baseline SRS scan was positive preoperatively, yearly scans should be performed. Following local excision (endoscopic or transanal excision) of rectal carcinoids for lesion 1–2 cm in size, surveillance endoscopy with rectal MRI should be performed at 6 and 12 months, with subsequent evaluation performed if clinically indicated. For resected rectal lesions <1 cm in size with negative margins and no evidence of high-risk histopathological features, no specific follow-up is recommended. Overall, follow-up should be continued for up to 10 years post-resection. With respect to prognosis, 5-year survival rate for localized carcinoid disease approaches 100% after complete R0 resection, 45–68% for resectable metastatic disease, and 38–58% for unresectable disease.
Suggested Reading
Akerström G, Hellman P, Hessman O. Gastrointestinal carcinoids. In: Lennard TWJ, editor. A companion to specialist surgical practice: endocrine surgery. 3rd ed. Edinburgh: Elsevier Ltd; 2005.
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR, editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2017.
Ganim RB, Norton JA. Recent advances in carcinoid pathogenesis, diagnosis and management. Surg Oncol. 2000;9:173–9.
Guidelines for the diagnosis and management of carcinoid tumours. Part 1: The gastrointestinal tract. A statement from a Canadian National Carcinoid Expert Group. Curr Oncol. 2006;13(2): 67–76.
National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology (NCCN guidelines). Neuroendocrine tumors. Version 1; 2019.
Virgolini I, Britton K, Buscombe J, Moncayo R, Paganelli G, Riva P. In- and Y-DOTA-lanreotide: results and implications of the MAURITIUS trial. Semin Nucl Med. 2002;32(2):148–55.
Welin S, et al. Elevated plasma chromogranin A is the first indication of recurrence in radically operated midgut carcinoid tumors. Neuroendocrinology. 2009;89(3):302–7.
Yang X, Yang Y, Li Z, et al. Diagnostic value of circulating chromogranin a for neuroendocrine tumors: a systematic review and meta-analysis. PLoS One. 2015;10:e0124884.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
AL-Khamis, A., Sylla, P. (2020). Small Bowel Conditions: Carcinoid. In: Steele, S., Maykel, J., Wexner, S. (eds) Clinical Decision Making in Colorectal Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-65942-8_76
Download citation
DOI: https://doi.org/10.1007/978-3-319-65942-8_76
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65941-1
Online ISBN: 978-3-319-65942-8
eBook Packages: MedicineMedicine (R0)