Abstract
A parasite depends, during its entire life or at least part of it, on other organisms, but parasites often “jump” from one host species to another and may be able to colonize new host species. The chances of parasite spillover, the first step in such a host switch, may be influenced by factors such as the local ecosystem, community composition, and modes of transmission, among others. In Galapagos, for example, seabirds show a spatially clustered community, with several species that are related and/or nest in close proximity, a seemingly perfect scenario for host switching. However, only one instance of a straggling ischnoceran louse and larva (indicating successful reproduction on the new host) was found on a different host species, suggesting that the specifics of ectoparasite body size and host feather interbarbular space may prevent lice from readily switching hosts. On the other hand, the haemosporidian parasite, Haemoproteus multipigmentatus, of the Columbiform-specific sub-genus Haemoproteus, was found in significant numbers of Galapagos passerines. The spillover events occur where Galapagos doves (Zenaida galapagoensis), a widespread endemic, are present or abundant enough; however, there is no evidence of parasite development in the passerine birds. Thus, the Galapagos archipelago provides an exceptional host-parasite system to investigate details of parasite spillover and its implications for host health and survivorship.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Spillover
When a parasite finds itself on a host individual that is not of its typical host species, we may call this a host switch. If successful, it could lead to speciation in the parasite lineage, a process that could increase biodiversity in healthy ecosystems (Hudson et al. 2006). When a parasite switches hosts, it changes branches in the tree of life, occupying a different niche and potentially expanding its range. If the isolation from the previous host is relatively strong, it can lead to genetic differentiation and speciation (Ogden and Thorpe 2002; Johnson et al. 2002a, b; Clayton and Johnson 2003; Schluter 2009; Feder et al. 2012). But parasites can jump to other hosts and not establish a viable population. It can be a single individual that jumps and cannot reproduce alone, or the intricacies of host-parasite interaction may hamper establishment of the parasite on the new host; these more temporary relationships are called straggling events (Rozsa 1993; Paterson and Gray 1997; Norton and Carpenter 1998; Ricklefs et al. 2004).
Straggling events may be the starting point of a successful host switch. Parasites that continuously end up in a different host are more likely to end up in enough numbers to establish a population, competing with the native parasites and even evolve to “tweak open” the lock of the host immune system or defense mechanisms (Rozsa 1993; Ricklefs et al. 2004). A major challenge when studying host switching has been to draw a line between a straggling or a host switching event (Rozsa 1993; Whiteman et al. 2004). For the purposes of this chapter, we will define a host switch as having occurred when there is evidence of reproduction (or reproductive stages) in the novel or atypical host.
The chances of parasite spillover, from one host species to another one, are influenced by various ecological and life history traits. Aspects such as niche similarity among host species, modes of transmission, and vector dietary preferences are only a few of the most relevant ones (Rozsa 1993; Johnson et al. 2002a, b; Clayton and Johnson 2003; Whiteman et al. 2004; Bush et al. 2006). We will continue to discuss in detail these and other aspects that may explain the spillover (or straggling) events observed in Galapagos and the ecological and biological factors that explain them. Galapagos is a great laboratory to understand parasite spillover.
1.1 Host Community Structure and Transmission
Host-parasite interactions are present throughout the tree of life. The specifics of those interactions depend on the specific host and parasite species involved (Price et al. 2003; Koh et al. 2004; Whiteman and Parker 2005). For example, avian malaria parasites interact directly with the host immune system and need very specific surface proteins to infect the host red blood cells (Valkiūnas 2004). Moreover, these parasites are vector-borne, so they also need a set of proteins that let them infect the arthropod vector, moving through different organs and reproductive phases. In contrast, ectoparasitic avian lice are directly transmitted and barely interact with the host immune system; what they need to be worried about is host preening, which is the main defense mechanism of the host (Price et al. 2003; Whiteman and Parker 2005).
Community composition , its phylogenetic clustering, and similarity of niches among hosts and potential host species define the chances for spillover (Johnson et al. 2003; McCoy et al. 2005; Whiteman and Parker 2005; Hughes et al. 2007; Whiteman et al. 2007). Communities of species that are very distinct phylogenetically or for which related species have very divergent niches, present lower opportunities for parasites to colonize a novel host (Ricklefs et al. 2004). Galapagos shows a very clustered community, with adaptive radiations in the Darwin finches (Lamichhaney et al. 2015), and several species of seabirds that are related and/or nest in close proximity and have significant ecological and social interactions (Baião and Parker 2012; Rivera-Parra et al. 2014).
Having a clustered community is not the only requirement; there must also be real chances for host switching. For example, ectoparasitic lice cannot survive long off the body of the host (Price et al. 2003), so the typical and potential host species must interact physically for the lice to jump from one to the other (Rivera-Parra et al. 2014). Vector-borne parasites such as Haemoproteus or Plasmodium depend on the dietary preference of the biting insect vector to move across hosts (Valkiūnas 2004; Njabo et al. 2011). Thus, even when there are many potential hosts that have similar niches, there must be opportunities for host switching, through generalist vectors or physical interactions. Depending on the specifics of the transmission mode, there might be even bigger challenges not only for host switching but for parasite survival. For example, if an infected host colonizes a novel environment but there is no competent vector or other competent hosts for the parasite, then the parasite will die off (Telfer and Bown 2012; Inbar et al. 2013; Levin et al. 2013).
Therefore, the way parasites are transmitted across individuals (and potentially across species) is crucial for understanding parasite diversity, specificity, evolutionary history, and chances for spillover (Whiteman and Parker 2005; Rivera-Parra et al. 2015). Roughly, parasites can be classified depending on their transmission as either directly transmitted or vector-borne.
1.1.1 Directly Transmitted
Parasitism is a complicated way of life. Parasites depend, during their entire life cycle or part of it, on another organism (Price et al. 2003; Valkiūnas 2004). This makes them vulnerable to stochasticity (e.g., the death of a host before transmission) and even co-extinction (Koh et al. 2004; Whiteman and Parker 2005). Parasites are said to be directly transmitted when they do not rely on other organisms to be vectored from one host to another (Price et al. 2003). Thus, parasites use their own means or their hosts’ habits to colonize another individual.
Directly transmitted parasites can take advantage of social interactions to be transmitted (Whiteman et al. 2006). They can be transmitted among independent individuals, which is called horizontal transmission, or they can be transmitted from parents to offspring (vertical transmission; Clayton et al. 1992, Whiteman and Parker 2004). Parasites that are more mobile and/or inhabit social host species or hosts that interact regularly and directly with other potential host species are more likely to spread to novel hosts.
The Galapagos hawk (Buteo galapagoensis) is an endemic and diurnal predator of the Galapagos Archipelago (Fig. 6.1). As predators, they interact intimately with their prey, and there is evidence of parasite spillover from their prey to the hawks. Whiteman et al. (2004) found Galapagos dove (Zenaida galapagoensis) and introduced goat (Capra hircus) ectoparasites on a Galapagos hawk. As the authors suggest, this seems like an example of a parasite straggling. Thus, parasites will survive only for a short period of time and not establish a viable population. The intricacies at play in a host-parasite interaction, such as specific defense mechanisms (like preening) or the host immune system, may prevent a successful colonization, but represent how the host habits create opportunities for parasite spillover.
1.1.2 Indirectly Transmitted
Indirect transmission of parasites usually brings another player into action, the vector. Although this complicates the parasite’s life cycle, it also enhances the possibility of transmission as direct contact between hosts is no longer necessary. In the past few decades most emergent infectious diseases that involved wildlife were exotic to the environment in which the epidemic occurred (Daszak et al. 2000; Dobson and Foufopoulos 2001). Even though we would generally expect host-parasite introductions to be greater for parasites with direct life cycles, various co-introduction studies involve parasites with indirect life cycles, the majority of which resulted in host-switches to native hosts (Lymbery et al. 2014). Once parasites are introduced, the potential for pathogen spillover will depend on the host community structure and the presence or co-introduction of alternative hosts or vectors (e.g., Warner 1968; van Riper et al. 1986; Gaither et al. 2013; Novak and Goater 2013).
Spillover occurs when the disease dynamics in one or multiple host populations are driven by transmission from a reservoir host in which the pathogen is highly prevalent, regardless of the mode of transmission (Daszak et al. 2000; Power and Mitchell 2004). Introduced species are often the reservoirs of these pathogens in naive native communities (Lymbery et al. 2014). For this reason, various research efforts in Galapagos have focused on assessing the risk that the poultry industry or backyard chickens pose to endemic wild birds, as introduced chickens may serve as reservoirs for important infectious diseases (Gottdenker et al. 2005; Soos et al. 2008; Deem et al. 2012).
The first evidence of possible spillover of disease from domestic to wild birds in Galapagos was found during a study that assessed pathogens and parasites in chickens and wild birds on Floreana Island, to determine disease risks prior to a possible re-introduction of the endangered Floreana mockingbird (Mimus trifasciatus, see Fig. 4.4) (Deem et al. 2012). Thirty percent of chickens presented antibodies against paramyxovirus-1 and 11.3% presented antibodies against adenovirus-2, while for wild birds, prevalence was much lower with only 3% presenting antibodies against paraxymovirus-1 and 2.4% against adenovirus-2, suggesting the direction of transmission from chickens to wild birds. Paramyxovirus-1 and adenovirus-2 are viruses that are transmitted via airborne particles (direct) but transmission can also occur from contaminated surfaces or material or even from fecal matter (indirect). Thus, the potential for indirect transmission of these viruses may increase the risk of transmission from introduced chickens to the endemic wildlife.
Another example of possible spillover from an introduced species to the endemic Galapagos avifauna involves the common protozoan , Toxoplasma gondii. Exposure to T. gondii has been shown in Galapagos penguins (Spheniscus mendiculus) and Flightless cormorants (Phalacrocorax harrisi) (Deem et al. 2010). Prior to this study, there had been a single report of a domestic chicken infected with T. gondii (Gottdenker et al. 2005). Introduced cats (Felis catus) are likely the major reservoir for infection as they are the only host in which sexual reproduction of the parasite is known to occur. Domestic cats on Isabela have been found to have an antibody prevalence of 65% (Levy et al. 2008). Furthermore, it appears that the spillover of disease occurs not only on islands where cats are present, like Isabela, but also on Fernandina, one of the most pristine islands in the archipelago where there are no introduced cats (Deem et al. 2010). Plausible explanations for this observation include but are not limited to: widespread movement of Galapagos penguins (Nims et al. 2008) and dispersal of oocysts by ocean currents (Dubey 2004); attempts to evaluate this mode of dispersal in Galapagos have not been conclusive (Verant et al. 2013). Although T. gondii infections are common in many avian species, pigeons and canaries can be severely affected and it can even cause blindness (Dubey 2002). Moreover, Toxoplasma gondii poses a significant threat to isolated island avifauna as it has been associated with mortality in several Hawaiian endemics (Work et al. 2000, 2002).
Native species can also become reservoirs for introduced pathogens (Woodworth et al. 2005). In Galapagos, this appears to be the case of the Haemosporidian parasite Haemoproteus multipigmentatus and the endemic Galapagos dove (Zenaida galapagoensis) (Santiago-Alarcon et al. 2008). H. multipigmentatus belongs to the subgenus Haemoproteus, thought to be transmitted by hippoboscid flies and previously recorded only in columbiform birds (Valkiūnas 2004; Valkiūnas et al. 2010). Two other species within the subgenus Haemoproteus have since been described in Galapagos hosts, H. iwa from frigatebirds and vectored by Olfersia spinifera (Levin et al. 2011), and H. jenniae from swallow-tailed gulls (Levin et al. 2012) (Fig. 6.2); these two species form a deeply divergent sister clade to the hippoboscid-transmitted dove-specific species.
H. multipigmentatus is highly prevalent in Galapagos doves (Santiago-Alarcon et al. 2008) and is transmitted between doves by the endemic hippoboscid fly (Microlynchia galapagoensis) (Valkiūnas et al. 2010). H. multipigmentatus seems to have a wide distribution in the American continent as it has been found in Mexico, Guatemala, and Peru (Valkiūnas et al. 2010). A phylogenetic study of H. multipigmentatus recovered from Galapagos doves and from continental doves suggested that there were multiple events associated with the colonization of the parasite (Santiago-Alarcon et al. 2010, Chap. 7 this volume). The pathogen was likely brought to the Galapagos Islands via domestic rock pigeons (Columba livia) which were repeatedly introduced to the archipelago (Harmon et al. 1987; Padilla et al. 2004). Furthermore, sampling of nine pigeons, before they were completely eradicated in 2002, revealed that several individuals were in fact infected with H. multipigmentatus (Levin and Parker pers. comm.).
The first report of Haemoproteus (Haemoproteus) infection in a passerine bird was by Sari et al. (2013), during an effort to elucidate the origin of parasites infecting Galapagos flycatchers, Myiarchus magnirostris. Five flycatchers from Santa Cruz Island were infected with Haemoproteus multipigmentatus out of a total of 254 Galapagos flycatchers sampled from six different islands in the archipelago. The presence of H. multipigmentatus in these birds was detected by molecular methods and examination of the infected blood smears presented no evidence of parasite development (gametocytes were absent), indicating that Galapagos flycatchers may not be competent hosts. Thus, it appeared that the parasites detected in M. magnirostris were acquired in the Galapagos Islands by spillover from their reservoir host, the Galapagos dove (Sari et al. 2013).
An ongoing large-scale avian disease survey that began in 2001 detected Haemoproteus PCR signals in passerines but they were not reported because the numbers were usually too small and too scattered to determine the cause of infection (Parker and collaborators, unpublished data). Infected species included a small tree finch (Camarhynchus parvulus), a yellow warbler (Setophaga petechia), a large cactus finch (Geospiza conirostris), seven common cactus finches (Geospiza scandens), three small ground finches (Geospiza fuliginosa), two large ground finches (Geospiza magnirostris), four Galapagos flycatchers and a vegetarian finch (Platyspiza crassirostris) on the islands of Santa Cruz, Isabela, Santiago, Floreana, and Pinta in a span of 6 years.
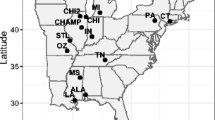
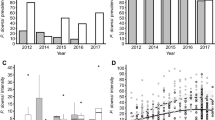
The most recent avian haemosporidian survey in the archipelago sampled 2254 individuals of 19 endemic and three introduced bird species along an altitudinal gradient in the islands of Isabela, Santa Cruz and Santiago (Jaramillo et al. 2017). The survey revealed 90 PCR positive birds in all years (2013–2015), 89 of which occurred on Santiago. Of these, 31 were Galapagos doves, and the other 58 included small ground finches, medium ground finches (G. fortis), large ground finches, a large tree finch (C. psittacula), Galapagos mockingbirds, and yellow warblers. These clusters of PCR-positive birds appeared only in locations where doves were also captured and all captured doves were infected (100% prevalence) (Fig. 6.3). Infection intensity in Galapagos doves was generally high, averaging 357 (±307) gametocytes per 10,000 erythrocytes, whereas Galapagos passerines presented no evidence of intraerythrocytic development. This suggests the role of Galapagos doves as reservoir hosts for Haemoproteus multipigmentatus in multiple spillover events (Jaramillo et al. 2017).
Map of the Galapagos Islands indicating islands (in grey) where a study found Haemoproteus multipigmentatus in 100% of sampled doves, and sites (stars) where it has been found in passerine birds. Galapagos doves are present in all major islands of the archipelago and show high infection at all sampled sites (Adapted from Jaramillo et al.2017)
Although Haemoproteus infections have been considered to be relatively benign to their bird hosts (Bennett et al. 1993) or even positive for their lifetime reproductive success (Zylberberg et al. 2015), numerous field and experimental studies have shown the negative effects these parasites can have on birds’ fitness (Valkiūnas 2004; Marzal et al. 2005; Møller and Nielsen 2007; Atkinson 2008) and have also been found to be lethal in adapted (Earle et al. 1993) and non-adapted birds (Atkinson et al. 1988; Cardona et al. 2002; Donovan et al. 2008; Olias et al. 2011; Cannell et al. 2013).
Some scientists propose that pathogen spillover from single key host species may be the main source of the parasitic fauna in evolutionarily recent bird communities (Hellgren et al. 2011). We have reviewed a few examples in which introduced species are likely to be the source for various pathogenic agents found in wild birds in Galapagos (Deem et al. 2010, 2012), and an example of a vector-borne parasite that was likely brought to Galapagos by an introduced dove and whose current reservoir is a widespread Galapagos endemic (Jaramillo et al. 2017). The presence of native alternative hosts and vectors has enabled the spillover of disease to a native community of susceptible hosts. Spillover is the preceding step to host switching, but even if a host switch never occurs, there still might be important effects for the non-adapted hosts and the possibility that these parasites are in turn shaping their hosts’ population dynamics.
2 Opportunities for Host-Switching
The chances of moving from one host species to another depend on the opportunities the local ecosystem presents. For a successful host-switch to happen there has to be a suitable potential host. This means that the host needs to offer similar “environmental” conditions and similar defense mechanisms (that can be dealt with in a similar way as in the typical host). In addition, there should be enough chances for a parasite to be transmitted across species, so if the parasite is vector-borne, the vector should be more generalist; if the parasite is directly transmitted, the hosts must interact in some way (Whiteman et al. 2004; Whiteman et al. 2005; Rivera-Parra et al. 2015).
Communities that share phylogenetically related species may be more susceptible to host switching, assuming that related hosts maintained similar mechanisms against parasites and share similar niches (Johnson et al. 2003). Niche similarity is relevant because it means more interaction among species. For example, in Galapagos, Darwin’s finches are closely related phylogenetically and share the same ectoparasitic lice species (Brueelia interposita and B. chelydensis; Price et al. 2003). Thus, it seems likely that populations of these two parasites on their hosts have not been sufficiently isolated to allow speciation.
2.1 Mixed Species Colonies of Seabirds and Their Lice
Among the rich seabird fauna of the Galapagos archipelago, there are two frigatebirds, magnificent (Fregata magnificens) and great (F. minor), and three species of boobies, Nazca (Sula grantii), blue-footed (S. nebouxii), and red-footed (S. sula). These five species of seabirds present specific local combinations and degree of spatial overlap. Each seabird species has one specific species of ischnoceran louse, the frigatebirds share an amblyceran louse (Fregatiella aurifasciata) and Nazca and blue-footed boobies share another amblyceran (Eidmaniella albescens) (Fig. 6.4). In this context, where hosts species nest in close proximity and the lice are phylogenetically related or shared, we expected to find a high degree of host switching (Rivera-Parra et al. 2014).
Ischnoceran and Amblyceran lice infecting the three species of boobies and two frigatebirds from the Galapagos Islands . Amblycerans: Colpocephalum spineum (commonly infects Magnificent frigatebirds), Fregatiella aurifasciata (ex. Magnificent and Great frigatebirds), Eidmaniella albescens (ex. Blue-footed and Nazca boobies). Ischnocerans: Pectinopygus fregatiphagus (ex. Magnificent frigatebird), P. annulatus (ex. Nazca booby), P. minor (ex. Blue-footed booby) and P. sulae (ex. Red-footed booby)
Fregatiella aurifasciata, which was thought to be a single species, showed evidence of genetic differentiation, suggesting lineage sorting, even on the islands where great and magnificent frigatebirds nest together (Rivera-Parra et al. 2015). Similarly, Eidmaniella albescens shows two distinct lineages, one in Nazca boobies and the other in blue-footed boobies (Rivera-Parra et al. 2015). Amblyceran lice tend to be highly mobile and transmit horizontally or vertically, but even in this scenario where they could jump from one host to another, they do not seem to do it regularly (Rivera-Parra et al. 2015).
The ischnoceran lice seemed to be extremely specific as well. This system with closely related hosts and parasites seemed perfect for finding host switches, but only a single adult individual and some larvae were found straggling on a different species. Even the effect of neighbor identity did not increase the likelihood of host-switch (Rivera-Parra et al. 2017). It seems plausible that the differences between parasite size (body width) and feather interbarbular space are preventing lice from establishing on a different host. Ischnoceran lice insert themselves in the interbarbular space of the feather as a mechanism of defense against the host’s preening; if the parasite is too big, they do not fit and are more easily dislodged (Bush et al. 2006). Boobies plunge dive to fish (del Hoyo et al. 1992), so their ectoparasitic lice have to withstand not only preening by the host, but the forces exerted during the plunge. Thus, any sub-optimal attachment to the feather may result in the lice falling from the host which would prevent the establishment of a viable population.
3 Implications for Avian Health
3.1 The Immune System of Island Endemics
Biologists frequently believe that isolated island parasite communities are small and impoverished (Wikelski et al. 2004), thus theoretically reducing the number of interactions that occur between parasites and hosts (Hochberg and Møller 2001). The costs associated with maintenance of immune function (Sheldon and Verhulst 1996; Norris and Evans 2000) also suggest that reduced selective pressures, due to low parasite diversity, would result in weakening of the immune system function of hosts through time (Van Riper and Scott 2001; Jarvi et al. 2001). In Hawaii, for example, endemic honeycreepers have been shown to be highly susceptible to introduced pathogens such as Plasmodium relictum. The susceptibility of these birds to avian malaria appears to be related to the low genetic diversity of their major histocompatibility complex (MHC) which in turn may reduce antigen recognition and antibody production by the host’s immune system (Jarvi et al. 2001).
Loss of MHC and neutral genetic diversity is perhaps an inevitable result of genetic drift for small populations (Sutton et al. 2011) like those found on isolated archipelagos. The Galapagos penguin’s (Spheniscus mendiculus) population size, for example, was last estimated at 1,500 individuals and it has undergone repeated bottlenecks of about 50% reduction in size every time there is an El Niño event (Vargas et al. 2006). It exhibits low levels of genetic diversity throughout its entire population in the archipelago and presents a lack of population structure among subpopulations (Nims et al. 2008). This low genetic variability can also be expressed at immunological loci that are fundamental in host resistance to disease. Compared to eight other species of penguins, including the Magellanic penguin (S. magellanicus) and the king penguin (Aptenodytes patagonicus), the Galapagos penguin had the lowest MHC diversity (Bollmer et al. 2007). Hence, the Galapagos penguin has been classified as Endangered (Birdlife International 2016) due to the risks presented by its demographic factors and the genetic monomorphism at loci involved in immune resistance.
Similarly, the endemic Galapagos hawk (Buteo galapagoensis) also presented reduced MHC and neutral genetic diversity related to a founder event and subsequent genetic drift, compared to its closest mainland relative the Swainson’s hawk (B. swainsoni) (Bollmer et al. 2011). Unlike the penguin, the Galapagos hawk exhibits a significant genetic population structure that increases as distance between islands increases (Bollmer et al. 2005; Koop et al. 2014). This structure provided the context for Whiteman et al. (2006) to examine the association between genetic diversity, inbreeding, and disease resistance in the Galapagos hawk. Island populations of hawks with higher degrees of inbreeding presented higher ectoparasite abundance and lower and less variable natural antibody (Nab) levels, demonstrating, for the first time in a wild island endemic, the link between genetic diversity, the innate immune system, and parasitic load.
The relationship between parasite abundance, immunity, and population size has also been investigated for Darwin’s finches. Lindström et al. (2004) compared four island populations of small ground finches (Geospiza fuliginosa) and found that as parasite prevalence and/or intensity increased with island size, concentrations of natural antibodies and the speed of specific antibody responses also increased with island size. However, the strength of the cell-mediated immune response decreased with increasing island size, presenting an opposite pattern that suggested a tradeoff between antibody and cell-mediated immunity. In environments where parasites are more abundant, it may be more cost-effective to combine the presence of natural antibodies and a rapid production of specific antibodies than to invest in cell-mediated immunity.
A different shift in immune defense strategy of insular versus continental birds was suggested by Matson (2006). His comparison of eight indices of immune function between insular and continental species of birds found that island birds had increased innate and inducible immune responses. Insular birds presented higher concentrations of plasma haptoglobin and elevated levels of two innate leukocytes (heterophils and eosinophils) than continental birds but showed no differences in agglutination and lysis titers (acquired responses). However, Matson warns, the increase in innate responses may be a way to compensate for aspects of insular life such as reduced genetic variation and could possibly intensify the disease risks. In whole, it appears that the relationship between the host’s immune system and parasite diversity in island populations is too complex to expect only a simple reduction in immune response in insular birds. Development of the immune system of isolated populations may depend not only on the diversity of parasites present but also on the specific parasites encountered and the stochasticity of mutation and genetic drift (Beadell et al. 2007).
3.2 Mortality
Island bird species have shown high vulnerability to introduced parasites. A clear example of this comes from Hawaii, where endemic honeycreepers experimentally infected with Plasmodium relictum have been shown to be extremely susceptible to the pathogen, with high mortality rates after a single mosquito bite (Jarvi et al. 2001). Other examples from islands include Plasmodium sp. parasites and mortality of native captive birds in New Zealand (Tompkins and Gleeson 2006), and reduced survivorship of endangered pink pigeons (Columba mayeri) infected with Trichomonas gallinae in Mauritius (Bunbury et al. 2008), among others (Wikelski et al. 2004).
In Galapagos wild birds, documented pathogenic causes of mortality include Philornis downsi, avian pox (genus Avipoxvirus: Poxviridae), and schistosomiasis (Gottdenker et al. 2008). An experimental approach attributed 27% of nestling mortality to P. downsi infestation given that pathogen-reduced nests had three times the nesting success of control parasitized nests (Fessl et al. 2006, see Chap. 9 this volume). P. downsi has been found in the nests of 12 introduced, native and endemic species in the archipelago (Fessl and Tebbich 2002) and has been associated with nestling morality in the small (Geospiza fuliginosa) and medium ground finches (Geospiza fortis) and in the critically endangered medium tree finch (Camarhynchus pauper) in Floreana (Fessl et al. 2006; Huber 2008; O’Connor et al. 2010). Avian pox is a prevalent disease affecting a wide variety of Galapagos endemic birds that has been present in Galapagos for at least a century (Parker et al. 2011). High mortality rates had been suggested for young Galapagos mockingbirds (Mimus parvulus) given the low recapture rates exhibited by infected individuals (Vargas 1987). Even though P. downsi and avian pox are highly prevalent pathogens, these examples constitute the only evidence of disease-related mortality in the avifauna of Galapagos.
Until now, no reports of Haemosporidian infection-related mortality have been documented for any Galapagos bird. Mortality associated with blood parasites in Galapagos wild birds may be underreported or hard to find as most of the Galapagos National Park is uninhabited; moreover, passerine carcasses may be rapidly scavenged by raptors or by feral dogs and cats. However, the potential risks that the parasites reported in the archipelago represent are great as these parasites can be lethal in non-adapted hosts (Atkinson et al. 1988; Jarvi et al. 2001; Cardona et al. 2002; Ferrell et al. 2007; Donovan et al. 2008; Olias et al. 2011; Cannell et al. 2013).
4 Concluding Remarks and Future Directions
The Galapagos archipelago provides an exceptional system to investigate the intricacies of parasite spillover. Its simplicity, or low number of host-parasite interactions , compared to continental systems, provides a natural laboratory to determine where the line falls between spillover and host-switching. Future research efforts should focus on determining the effects and risks that each of these events has on host health and survivorship. Furthermore, the link between genetic diversity, the immune system, and disease risk has only been touched and continues to pose very interesting questions about the ecology and evolution of hosts and parasites in isolated ecosystems. The degree of isolation of the archipelago declines with its increasing popularity as a travel destination, which in turn will increase the likelihood for introduced species and pathogens to arrive to the islands and bring ever-increasing opportunities for spillover. Thus, it is of great importance to continue to monitor avian health and pay close attention to ectoparasites and potential vectors of disease.
References
Atkinson CT, Forrester DJ, Greiner EC (1988) Pathogenicity of Haemoproteus meleagridis (Haemosporina: Haemoproteidae) in experimentally infected domestic turkeys. J Parasitol 74:228–239
Atkinson CT (2008) Haemoproteus. In: Atkinson CT, Thomas NJ, Hunter DB (eds) Parasitic diseases of wild birds. Wiley-Blackwell, Ames, IA, pp 11–34
Baião PC, Parker PG (2012) Evolution of the melanocortin-1 receptor (MC1R) in Boobies and Gannets (Aves, Suliformes). J Heredity 3:322–329. doi:10.1093/jhered/esr151
Beadell JS, Atkins C, Cashion E, Jonker M, Fleischer RC (2007) Immunological change in a parasite-impoverished environment: divergent signals from four island taxa. PLoS ONE 2(9):e896. doi:10.1371/journal.pone.0000896
Bennett GF, Peirce MA, Ashford RW (1993) Avian haematozoa: mortality and pathogenicity. J Nat Hist 27:993–1001
BirdLife International (2016) Species factsheet: Spheniscus mendiculus. http://www.birdlife.org. Accessed May 2 2016
Bollmer JL, Whiteman NK, Donaghy Cannon M, Bednarz JC, de Vries TJ, Parker PG (2005) Population genetics of the Galapagos hawk (Buteo galapagoensis): genetic monomorphism within isolated populations. Auk 122:1210–1224
Bollmer JL, Vargas FH, Parker PG (2007) Low MHC variation in the endangered Galapagos penguin (Spheniscus mendiculus). Immunogenetics 59:593–602
Bollmer JL, Hull JM, Ernest HB, Sarasola JH, Parker PG (2011) Reduced MHC and neutral variation in the Galápagos hawk, an island endemic. BMC Evol Biol 11(1):143
Bunbury N, Jones C, Greenwood A, Bell D (2008) Epidemiology and conservation implications of Trichomonas gallinae infection in the endangered Mauritian pink pigeon. Biol Cons 141:153161
Bush S, Sohn E, Clayton D (2006) Ecomorphology of parasite attachment: experiments with feather lice. J Parasitol 92:25–31
Cannell B, Krasnec K, Campbell K, Jones H, Miller R, Stephens N (2013) The pathology and pathogenicity of a novel Haemoproteus spp. infection in wild Little Penguins (Eudyptula minor). Vet Parasitol 197:74–84
Cardona CJ, Ihejirika A, McClellan L (2002) Haemoproteus lophortyx infection in bobwhite quail. Avian Dis 46:249–255
Clayton D, Gregory R, Price R (1992) Comparative ecology of Neotropical bird lice (Insecta: Phthiraptera). J Anim Ecol 61:781–795
Clayton D, Johnson K (2003) Linking coevolutionary history to ecological process: doves and lice. Evolution 57:2335–2341
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife--threats to biodiversity and human health. Science 287:443–449
Deem SL, Merkel J, Ballweber L, Vargas FH, Cruz MB, Parker PG (2010) Exposure to Toxoplasma gondii in Galapagos penguins (Spheniscus mendiculus) and flightless cormorants (Phalacrocorax harrisi) in the Galapagos Islands, Ecuador. J Wildl Dis 46:1005–1011
Deem SL, Cruz MB, Higashiguchi JM, Parker PG (2012) Diseases of poultry and endemic birds in Galapagos: implications for the reintroduction of native species. Anim Cons 15:73–82
Del Hoyo J, Elliott A, Sargatal J (1992) Handbook of the birds of the world, vol 1. Lynx Editions, Barcelona
Dobson A, Foufopoulos J (2001) Emerging infectious pathogens of wildlife. Proc R Soc Lond Biol Sci 356:1001–1012
Donovan TA, Schrenzel M, Tucker TA, Pessier AP, Stalis IH (2008) Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: eleven cases. J Vet Diagn Invest 20:304–313
Dubey JP (2002) A review of toxoplasmosis in wild birds. Vet Parasitol 106:121–153
Dubey JP (2004) Toxoplasmosis–a waterborne zoonosis. Vet Parasitol 126:57–72
Earle RA, Bastianello SS, Bennett GF, Krecek RC (1993) Histopathology and morphology of the tissue stages of Haemoproteus columbae causing mortality in Columbiformes. Avian Pathol 22:67–80
Feder JL, Egan SP, Forbes AA (2012) Ecological adaptation and speciation: the evolutionary significance of habitat avoidance as a postzygotic reproductive barrier to gene flow. Int J Ecol. 2012:456374. doi:10.1155/2012/456374
Ferrell ST, Snowden K, Marlar AB, Garner M, Lung NP (2007) Fatal hemoprotozoal infections in multiple avian species in a zoological park. J Zoo Wildl Med 38:309–316
Fessl B, Tebbich S (2002) Philornis downsi– a recently discovered parasite on the Galápagos archipelago – a threat for Darwin’s finches? Ibis 144:445–451
Fessl B, Kleindorfer S, Tebbich S (2006) An experimental study on the effects of an introduced parasite in Darwin’s finches. Biol Cons 127:55–61
Gaither MR, Aeby G, Vignon M, Meguro YI, Rigby M, Runyon C, Toonen RJ, Wood CL, Bowen BW (2013) An invasive fish and the time-lagged spread of its parasite across the Hawaiian archipelago. PLoS One 8:e56940
Gottdenker NL, Walsh T, Vargas H, Merkel J, Jiménez GU, Miller RE, Dailey M, Parker PG (2005) Assessing the risks of introduced chickens and their pathogens to native birds in the Galápagos archipelago. Biol Cons 126:429–439
Gottdenker NL, Walsh T, Jiménez-Uzcátegui G, Betancourt F, Cruz M, Soos C, Miller RC, Parker PG (2008) Causes of mortality of wild birds submitted to the Charles Darwin Research Station, Santa Cruz, Galapagos, Ecuador from 2002-2004. J Wildl Dis 44:1024–1031
Harmon WM, Clark WA, Hawbecker AC, Stafford M (1987) Trichomonas gallinae in columbiform birds from the Galapagos Islands. J Wildl Dis 23:492–494
Hellgren O, Krizanauskiene A, Hasselquist D, Bensch S (2011) Low haemosporidian diversity and one key-host species in a bird malaria community on a mid-Atlantic island (São Miguel, Azores). J Wildl Dis 47:849–859
Hochberg ME, Møller AP (2001) Insularity and adaptation in coupled victim–enemy associations. J Evol Biol 14:539–551
Huber SK (2008) Effects of the introduced parasite Philornis downsi on nestling growth and mortality in the medium ground finch (Geospiza fortis). Biol Cons 141:601–609
Hudson PJ, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? TREE 21:381–385
Hughes J, Kennedy M, Johnson KP, Palma RL, Page RD (2007) Multiple cophylogenetic analyses reveal frequent cospeciation between pelecaniform birds and Pectinopygus lice. Syst Biol 56:232–251
Inbar E, Akopyants NS, Charmoy M, Romano A, Lawyer P, Elnaiem DEA, Kauffmann F, Barhoumi M, Grigg M, Owens K, Fay M (2013) The mating competence of geographically diverse Leishmania major strains in their natural and unnatural sand fly vectors. PLoS Genet 9:e1003672
Jaramillo MC, Rohrer S, Parker PG (2017) From Galapagos doves to passerines: spillover of Haemoproteus multipigmentatus. Int J Parasitol Parasites Wildl 6:155
Jarvi SI, Atkinson CT, Fleischer RC (2001) Immunogenetics and resistance to avian malaria in Hawaiian honeycreepers (Drepanidinae). Stud Avian Biol 22:54–263
Johnson KP, Williams BL, Drown DM, Adams RJ, Clayton DH (2002b) The population genetics of host specificity: genetic differentiation in dove lice (Insecta: Phthiraptera). Mol Ecol 11:25–38
Johnson KP, Weckstein JD, Witt CC, Faucett RC, Moyle RG (2002a) The perils of using host relationships in parasite taxonomy: phylogeny of the Degeeriella complex. Mol Phylogen Evol 23:150–157
Johnson KP, Adams RJ, Page RD, Clayton DH (2003) When do parasites fail to speciate in response to host speciation? Syst Biol 52:37–47
Koh LP, Dunn RD, Sodhi NS, Colwell RK, Proctor HC, Smith VS (2004) Species co-extinctions and the biodiversity crisis. Science 305:1632–1634
Koop JA, DeMatteo KE, Parker PG, Whiteman NK (2014) Birds are islands for parasites. Biol Lett 10:20140255
Lamichhaney S, Berglund J, Almén MS, Maqbool K, Grabherr M, Martinez-Barrio A, Promerová M, Rubin CJ, Wang C, Zamani N, Grant BR (2015) Evolution of Darwin’s finches and their beaks revealed by genome sequencing. Nature 518:371–375
Levin II, Valkiūnas G, Santiago-Alarcon D, Cruz LL, Iezhova TA, O’Brien SL, Hailer F, Dearborn D, Schreiber EA, Fleischer RC, Ricklefs RE, Parker PG (2011) Hippoboscid-transmitted Haemoproteus parasites (Haemosporida) infect Galapagos Pelecaniform birds: evidence from molecular and morphological studies, with a description of Haemoproteus iwa. I J Parasitol 41:1019–1027
Levin II, Valkiūnas G, Iezhova TA, O’Brien SL, Parker PG (2012) Novel Haemoproteus species (Haemospirida:Haemoproteidae) from the swallow-tailed gull (Lariidae), with remarks on the host range of hippoboscid-transmitted avian hemoproteids. J Parasitol 98:847–854
Levin II, Zwiers P, Deem SL, Geest EA, Higashiguchi JM, Iezhova TA, Jimenez-Uzcategui G, Kim DH, Morton JP, Perlut NG, Renfrew RB, Sari EHR, Valkiūnas G, Parker PG (2013) Multiple lineages of avian malaria parasites (Plasmodium) in the Galapagos Islands and evidence for arrival via migratory birds. Cons Biol 27:1366–1377
Levy JK, Crawford PC, Lappin MR, Dubovi EJ, Levy MG, Alleman R, Tucker SJ, Clifford EL (2008) Infectious diseases of dogs and cats on Isabela Island, Galapagos. J Vet Intern Med 22:60–65
Lindström KM, Foufopoulos J, Pärn H, Wikelski M (2004) Immunological investments reflect parasite abundance in island populations of Darwin’s finches. P R Soc Lond [Biol] 271:1513–1519
Lymbery AJ, Morine M, Kanani HG, Beatty SJ, Morgan DL (2014) Co-invaders: the effects of alien parasites on native hosts. Int J Parasitol Parasites Wildl 3:171–177
Matson KD (2006) Are there differences in immune function between continental and insular birds? P R Soc Lond [Biol] 273:2267–2274
Marzal A, De Lope F, Navarro C, Møller AP (2005) Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142(4):541–545
McCoy KD, Chapuis E, Tirard C, Boulinier T, Michalakis Y, Le Bohec C, Le Maho Y, Gauthier-Clerc M (2005) Recurrent evolution of host specialized races in a globally distributed parasite. P R Soc Lond [Biol] 272:2389–2395
Møller AP, Nielsen JT (2007) Malaria and risk of predation: a comparative study of birds. Ecology 88:871–881
Nims BD, Vargas FH, Merkel J, Parker PG (2008) Low genetic diversity and lack of population structure in the endangered Galapagos penguin (Spheniscus mendiculus). Conserv Genet 9(6):1413–1420
Njabo K, Cornel A, Bonneaud C, Toffelmier E, Sehgal RN, Valkiūnas G, Russell AF, Smith TB (2011) Non-specific patterns of vector, host and avian malaria parasite associations in a central African rainforest. Mol Eco 20:1049–1061
Norris K, Evans MR (2000) Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol 11:19–26
Norton DA, Carpenter MA (1998) Mistletoes as parasites: host specificity and speciation. TREE 13:101–105
Novak CW, Goater TM (2013) Introduced bullfrogs and their parasites: Haematoloechus longiplexus (Trematoda) exploits diverse damselfly intermediate hosts on Vancouver Island. J Parasitol 99:59–63
O’Connor JA, Sulloway FJ, Robertson J, Kleindorfer S (2010) Philornis downsi parasitism is the primary cause of nestling mortality in the critically endangered Darwin’s medium tree finch (Camarhynchus pauper). Biodivers Conserv 19:853–866
Ogden R, Thorpe RS (2002) Molecular evidence for ecological speciation in tropical habitats. PNAS 99:13612–13615
Olias P, Wegelin M, Zenker W, Freter S, Gruber AD, Klopfleisch R (2011) Avian malaria deaths in parrots, Europe. Emerg Infect Dis 17:950
Padilla LR, Santiago-Alarcon D, Merkel J, Miller RE, Parker PG (2004) Survey for Haemoproteus spp., Trichomonas gallinae, Chlamydophila psittaci, and Salmonella spp. in Galapagos Islands columbiformes. J Zoo Wildl Med 35:60–64
Paterson AM, Gray RD (1997) Host–parasite cospeciation, host switching, and missing the boat. In: Clayton DH, Moore J (eds) Host–parasite evolution: general principles and avian models. Oxford University Press, Oxford, pp 236–250
Parker PG, Buckles EL, Farrington H, Petren K, Whiteman NK, Ricklefs RE, Bollmer JL, Jiménez-Uzcátegui G (2011) 110 years of Avipoxvirus in the Galapagos Islands. PloS One 6:e15989
Power AG, Mitchell CE (2004) Pathogen spillover in disease epidemics. Ame Nat 164:S79–S89
Price RD, Hellenthal RA, Palma RL, Johnson KP, Clayton DH (2003) The chewing lice: world checklist and biological overview. Illinois Natural History Survey Special Publication, Champaign, IL
Ricklefs RE, Fallon SM, Bermingham E (2004) Evolutionary relationships, cospeciation, and host switching in avian malaria parasites. Syst Biol 53:111–119
Rivera-Parra JL, Levin II, Parker PG (2014) Comparative ectoparasite loads of five seabird species in the galapagos islands. J Parasitol 100:569–577
Rivera-Parra JL, Levin II, Johnson KP, Parker PG (2015) Lineage sorting in multihost parasites: Eidmanniella albescens and Fregatiella aurifasciata on seabirds from the Galapagos Islands. Ecol Evol 5:3264–3271
Rivera-Parra JL, Levin II, Johnson KP, Parker PG (2017) Host sympatry and body size influence parasite straggling rate in a highly connected multihost, multiparasite system. Ecol Evol 7:3724–3731
Rozsa L (1993) Speciation patterns of ectoparasites and ‘straggling’ lice. Int J Parasitol 23:859–864v
Santiago-Alarcon D, Whiteman NK, Parker PG, Ricklefs RE, Valkiūnas G (2008) Patterns of parasite abundance and distribution in island populations of Galapagos endemic birds. J Parasitol 94:584–590
Santiago-Alarcon D, Outlaw DC, Ricklefs RE, Parker PG (2010) Phylogenetic relationships of haemosporidian parasites in New World Columbiformes, with emphasis on the endemic Galapagos dove. Int J Parasitol 40:463–470
Sari EH, Klompen H, Parker PG (2013) Tracking the origins of lice, haemosporidian parasites and feather mites of the Galapagos flycatcher (Myiarchus magnirostris). J Biogeogr 40:1082–1093
Schluter D (2009) Evidence for ecological speciation and its alternative. Science 323:737–741
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defenses and trade-offs in evolutionary ecology. TREE 11:317–321
Soos C, Padilla L, Iglesias A, Gottdenker N, Bédon MC, Rios A, Parker PG (2008) Comparison of pathogens in broiler and backyard chickens on the Galápagos Islands: implications for transmission to wildlife. Auk 125:445–455
Sutton JT, Nakagawa S, Robertson BC, Jamieson IG (2011) Disentangling the roles of natural selection and genetic drift in shaping variation at MHC immunity genes. Mol Ecol 20:4408–4420
Telfer S, Bown K (2012) The effects of invasion on parasite dynamics and communities. Funct Ecol 26:1288–1299
Tompkins DM, Gleeson DM (2006) Relationship between avian malaria distribution and an exotic invasive mosquito in New Zealand. J R Soc New Zeal 36:51–62
Valkiūnas G (2004) Avian malaria parasites and other haemosporidia. CRC press, Boca Raton, FL
Valkiūnas G, Santiago-Alarcon D, Levin II, Iezhova TA, Parker PG (2010) A new Haemoproteus species (Haemosporida: Haemoproteidae) from the endemic Galapagos dove Zenaida galapagoensis, with remarks on the parasite distribution, vectors, and molecular diagnostics. J Parasitol 96:783–792
van Riper IIIC, van Riper SG, Goff ML, Laird M (1986) The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol Monogr 56:327–344
van Riper IIIC, Scott JM (2001) Limiting factors affecting Hawaiian native birds. Studies in Avian Biol 22:221–233
Vargas H (1987) Frequency and effect of pox-like lesions in Galapagos mockingbirds. J Field Ornitho 158:101–102
Vargas FH, Harrison S, Rea S, Macdonald DW (2006) Biological effects of El Niño on the Galapagos penguin. Biol Conserv 127:107–114
Verant ML, d’Ozouville N, Parker PG, Shapiro K, VanWormer E, Deem SL (2013) Attempted detection of Toxoplasma gondii oocysts in environmental waters using a simple approach to evaluate the potential for waterborne transmission in the Galapagos Islands, Ecuador. EcoHealth 11:207
Warner RE (1968) The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor 70:101–120
Whiteman NK, Parker PG (2004) Effects of host sociality on ectoparasite population biology. J. Parasitol 90:939–947
Whiteman NK, Santiago-Alarcon D, Johnson KP, Parker PG (2004) Differences in straggling rates between two genera of dove lice (Insecta: Phthiraptera) reinforce population genetic and cophylogenetic patterns. Int J Parasitol 34:1113–1119
Whiteman NK, Parker PG (2005) Using parasites to infer host population history: a new rationale for parasite conservation. Anim Conserv 8:175–181
Whiteman NK, Goodman SJ, Sinclair BJ, Walsh TIM, Cunningham AA, Kramer LD, Parker PG (2005) Establishment of the avian disease vector Culex quinquefasciatus Say, 1823 (Diptera: Culicidae) on the Galapagos Islands, Ecuador. Ibis 147:843–847
Whiteman NK, Matson KD, Bollmer JL, Parker PG (2006) Disease ecology in the Galapagos Hawk (Buteo galapagoensis): host genetic diversity, parasite load and natural antibodies. P R Soc Lond [Biol] 273:797–804
Whiteman NK, Kimball RT, Parker PG (2007) Co-phylogeography and comparative population genetics of the Galápagos Hawk and three co-occurring ectoparasite species: natural history shapes population histories within a parasite community. Mol Ecol 16:4759–4773
Wikelski M, Foufopoulos J, Vargas H, Snell H (2004) Galápagos birds and diseases: invasive pathogens as threats for island species. Ecol Soc 9:5
Woodworth BL, Atkinson CT, LaPointe DA, Hart PJ, Spiegel CS, Tweed EJ, Henneman C, LeBrun J, Denette T, DeMots R, Kozar KL (2005) Host population persistence in the face of introduced vector-borne diseases: Hawaii amakihi and avian malaria. PNAS 102(5):1531–1536
Work TM, Massey JG, Rideout BA, Gardiner CH, Ledig DB, Kwok OCH, Dubey JP (2000) Fatal toxoplasmosis in free-ranging endangered ‘Alala from Hawaii. J Wildl Dis 36:205–212
Work TM, Massey JG, Lindsay DS, Dubey JP (2002) Toxoplasmosis in three species of native and introduced Hawaiian birds. J Parasitol 88:1040–1042
Zylberberg M, Derryberry EP, Breuner CW, Macdougall-Shackleton EA, Cornelius JM, Hahn TP (2015) Haemoproteus infected birds have increased lifetime reproductive success. Parasitol 142:1033–1043
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Jaramillo, M., Rivera-Parra, J.L. (2018). Host-Switching: How It Starts. In: Parker, P. (eds) Disease Ecology. Social and Ecological Interactions in the Galapagos Islands. Springer, Cham. https://doi.org/10.1007/978-3-319-65909-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-65909-1_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65908-4
Online ISBN: 978-3-319-65909-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)