Abstract
Spiders are among the most common animals in diverse terrestrial environments, and display a variety of lifestyles and foraging modes. This chapter represents an overview of our knowledge of spider–plant interactions. Spiders are strongly influenced by plant architecture, rather than being randomly distributed in the vegetation; structures such as rosette-shaped clusters of leaves or glandular trichomes are particularly common in plants that have associations with spiders. Spiders derive benefits from plants such as shelter and access to insect prey. In turn, they can protect plants against herbivory. However, they may also consume or deter pollinators, imposing a cost that can exceed their benefit to the plant. Specific spider–plant associations are mutualistic if spiders provide protective or nutritional benefits, thus improving plant fitness, and if plants provide shelter and suitable foraging sites to spiders. We examine several case studies of spiders living in association with plants, and describe spatial/temporal adaptations in spider–plant relationships.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Plant vegetation represents a heterogeneous complex of environments (Morse et al. 1985; Sugihara and May 1990; Scheuring 1991), and the animals associated with it must have morphological, physiological, and behavioral characteristics that facilitate their feeding, survival, and reproduction in this context, especially predators such as spiders (Foelix 2011). Plants can directly benefit spiders by providing substrates for web building and hunting (Wise 1993), attracting prey (Morse 1999; Schmalhofer 2001), and offering suitable microclimates (Riechert and Tracy 1975; Whitney 2004), whereas spiders can benefit plants by consuming or deterring herbivores and, in some cases, by providing nutritional resources to plants.
Spiders are among the most common animals in terrestrial environments, and they inhabit areas ranging from the hottest, most arid deserts to the deepest caverns and the highest, coldest mountains. They have the seventh largest number of species of any animal group, with 46,650 described species (Coddington and Levi 1991; World Spider Catalog 2017). They present a wide variety of lifestyles and behaviors (Foelix 2011). Many species are dispersed by the wind and can achieve great heights in the atmosphere (Turnbull 1973; Suter 1999). Spiders are among the most common arthropods that inhabit vegetation (e.g., Wise 1993), although they generally do not consume plant tissue, and are useful in studies that investigate how the habitat structure affects the community of arthropod predators (Gunnarsson 1990, 1992; Romero and Vasconcellos-Neto 2005a; Sanders et al. 2008).
Spiders capture prey using a variety of different foraging strategies. Some spiders are active hunters, such as the jumping spiders (Salticidae), which pursue their prey, whereas others remain motionless on vegetation, especially flowers, waiting for an insect to approach. Spiders of the family Thomisidae are typical hunters of the sit-and-wait type. Several other foraging modes exist along a continuum between the active-hunter and sit-and-wait strategies. In addition, many species build webs to capture prey (Romero and Vasconcellos-Neto 2007a, b). Several studies have sought to determine which foraging strategies cause the greatest indirect effects within terrestrial trophic cascades (Preisser et al. 2007).
Because spiders are predators, they can profoundly affect the dynamics of prey populations and the structure of prey communities (Wise 1993; Romero and Vasconcellos-Neto 2007a). In addition, as they often consume insect herbivores, the presence of spiders on plants can decrease herbivory on these plants (review in Romero and Vasconcellos-Neto 2007a). In fact, spiders are excellent biological control agents of pests in agroecosystems (review in Romero 2007). Spiders are among the most abundant and diverse arthropods in vegetation, but studies focusing on their interactions with plants are relatively scarce. In fact, only a few studies have reported specific associations between spiders and plants. Moreover, few studies have used an integrative approach to address the mutualistic relationships between spiders and plants. Spiders are often used as models of predators to answer questions related to the dynamics and structures of food webs (review in Romero and Vasconcellos-Neto 2007a). However, we lack a complete understanding of how morphological and structural aspects of plants can benefit spiders and how changes in the architecture of plants affect the composition and distribution of spiders (Halaj et al. 1998; Souza and Martins 2005; Souza 2007; Diniz 2011). A few studies have reported that spiders benefit from plants by obtaining alternative food resources, such as nectar and pollen (Romero and Vasconcellos-Neto 2007b; Meehan et al. 2009; Nahas et al. 2012; Stefani et al. 2015).
In this chapter we explore the associations between spiders and plants, covering topics such as defense, foraging, and reproduction, as well as providing recent evidence of facultative mutualistic interactions between spiders and plants. Several species of spiders are exclusively associated with plants that have certain types of architecture, which benefit them in many ways. In return for the benefits that they receive, spiders can remove herbivores and even nourish their host plants with feces and prey carrion. Spiders can have mixed effects on flowers: if they capture herbivorous insects that consume parts of the flower or the whole flower, spiders can benefit plants and even increase their reproductive success, but if they capture or expel the insects that pollinate flowers, their presence on the plant can impose a cost.
Guilds of Spiders Associated with Plants
The term guild was applied by plant and animal ecologists to describe trophic groups called Genossenschaften (Schimper 1903) or Syntrophia (Balogh and Loksa 1956). Modern usage of the term guild was formalized in a study of avian niche exploitation patterns as “a group of species that exploit the same class of environmental resources in a similar way” (Root 1967), and this concept was later extended to the arthropod fauna of collards (Root 1973). Thus, a guild comprises potentially competing species and is a fundamental aspect of ecological communities (Uetz et al. 1999). Since the term was coined, the guild concept has been applied to numerous animal and plant communities (Hawkins and MacMahon 1989; Simberloff and Dayan 1991).
Spiders may be classified into guilds according to the different strategies they use to capture their prey. Scientists have proposed different numbers of spider guilds based on their ecological and foraging characteristics: 2 (Uetz 1977), 3 (Nyffeler 1982), 4 (Young and Edwards 1990), 8 (Riechert and Lockley 1984), and 11 (Post and Riechert 1977). A commonly used classification of the different foraging strategies was proposed by Uetz et al. (1999). They performed quantitative analyses of ecological characteristics of families and suggested eight guilds based on hunting strategies: (1) stalkers (e.g., Salticidae and Oxyopidae), (2) ambushers (Thomisidae and Pisauridae), (3) foliage runners (Anyphaenidae and Clubionidae), (4) Ground Runners (Lycosidae and Gnaphosidae), (5) funnel web-builders (Agelenidae and Amaurobiidae), (6) wandering sheet/tangle weavers (Linyphiidae), (7) orb weavers (Araneidae, Tetragnathidae, and Uloboridae), and (8) 3D web builders (Theridiidae and Pholcidae). Höfer and Brescovit (2001) proposed a classification that assigned different families to 12 guilds, and Dias et al. (2010) refined these categories by creating subdivisions within certain families, since different sub-groups or genera of the same family fit better in different guilds, which resulted in 11 groups.
The families of spiders that make up the guilds of the stalkers, ambushers, and foliage runners are generally the most common inhabitants of vegetation (Uetz et al. 1999; Höfer and Brescovit 2001; Romero and Vasconcellos-Neto 2007a; Mohsin et al. 2010; Cardoso et al. 2011). In an extensive study, Nentwig et al. (1993) recorded many spider species associated with flowers, leaves, and trunks of various plant species in Panama, and all the spiders observed belonged to these three guilds. Up to 70% of the spiders found in the flowers of Lantana camara (Verbenaceae) were Thomisidae, and more than 90% of the spiders collected in these flowers hunted by ambushing or stalking. These flowers were also occupied by spiders belonging to the families Salticidae, Anyphaenidae, Oxyopidae, Pisauridae, and Clubionidae. In contrast, 46% of the spiders on Palicourea guianensis (Rubiaceae) flowers belonged to the family Salticidae. In Rhynchospora nervosa (Cyperaceae) flowers, Nentwig et al. (1993) observed a large number of spiders belonging to the families Salticidae, Thomisidae, Oxyopidae, and Clubionidae. They also reported that wandering spiders associated with leaves mainly belonged to the families Salticidae, Pisauridae, and Anyphaenidae; Salticidae and Pisauridae spiders occurred preferentially in flat and xeromorphic leaves, and Anyphaenidae occurred in leaves with trichomes. According to Nentwig et al. (1993), the most common spiders on tree trunks belonged to the family Salticidae, including approximately half of all the spiders sampled.
As the spiders belonging to the stalker, ambush, and foliage runner guilds do not build webs but live in constant contact with the vegetation, they often have closer relationships with this type of substrate than do web builders. In addition to using plants directly for foraging, they use them for shelter and breeding habitat. Therefore, the spiders that belong to these guilds are the main predators in tri-trophic interactions and the main control agents of insect herbivores (Romero and Vasconcellos-Neto 2007b; Romero 2007).
Plant Architecture, Species Richness, and Diversity of Spiders
Understanding the patterns of species richness and abundance, as well as the processes that promote and maintain them, is a central theme in ecological studies (Gonçalves-Souza et al. 2011; Brown 2014). In terrestrial ecosystems, the habitat heterogeneity hypo (MacArthur and MacArthur 1961; Pianka 1966) indicates that complex environments are the predominant determinant of animal diversity (Tews et al. 2004). This hypothesis is supported by several studies of different taxonomic groups and different environments (e.g., Souza 1999; Halaj et al. 2000; Langellotto and Denno 2004; Tews et al. 2004). Vegetation is one element that provides structural diversity to habitat, as different patterns of branching and the modular organization of plants can provide a wide range of architectural arrangements (Bell et al. 1979; Küppers 1989; Bell 1991). Numerous studies have found that the architecture of plants is a major factor in determining the diversity of fauna associated with the vegetation, especially among the arthropod community (e.g., Halaj et al. 2000; Hatley and MacMahon 1980; Lawton 1983; Souza and Martins 2005; Woodcock et al. 2007; Ribas et al. 2011).
For example, the species diversity of birds depends more on the architectural diversity of the vegetation than on the taxonomic diversity of plants (MacArthur and MacArthur 1961). In a shinnery oak ecosystem, lower frequency and abundance of rodent species were recorded in open spaces with no vegetation than in the densely vegetated areas around the oaks (Cramer and Willig 2002). Among arthropods, the diversity of beetles and phytophagous arthropods were explained more by the architectural diversity of vegetation than by the diversity of the plant community (Brose 2003; Woodcock et al. 2007).
Although a strong correlation exists between the increasing architectural complexity of the vegetation and the diversity and abundance of species, studies of the influence of the architectural complexity of plants are biased toward vertebrates, particularly birds, which cover a third of the studies and represent less than 1% of animal diversity (Tews et al. 2004). Moreover, the concept of architectural complexity is difficult to generalize, as the operating variables of architecture, as well as the definition of habitat architecture, vary from author to author (McCoy and Bell 1991; Tews et al. 2004). In contrast, taxonomic groups that represent a large portion of overall animal diversity (e.g., arthropods) have been little studied. For arthropods associated with vegetation, a single plant is the whole habitat, so even small changes in its architecture can have consequences on the community structure and on the foraging efficiency of arthropods (Price et al. 1980; Tews et al. 2004).
Several studies have examined the influence of the architectural characteristics of plants on the abundance and diversity of arthropods, particularly spiders (Riechert and Gillespie 1986; Gunnarsson 1996). This influence is related to the various vegetative parts of the plant (e.g., needles, branches, leaves) and to the presence of reproductive structures that can provide, for example, a large variety of shelters, favorable microclimate conditions, anchoring points for prey capture webs, and opportunities to use different foraging methods (Greenstone 1984; Riechert and Gillespie 1986; Uetz 1991; Dennis et al. 1998).
Inflorescences
Some studies showed a high number of spider species inhabiting plants with inflorescences. These structures provide favorable microclimatic conditions and shelter against possible predators. In addition, inflorescences can attract different types of prey, representing a remarkable benefit for spiders. Structural features in inflorescences, such as number and size of flowers and leaves, arrangement in space, and branch size can vary among plant species and among the inflorescences of the same plant at different phenological stages (e.g., open flowers, flower buds).
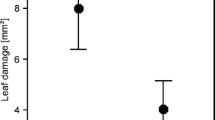
Souza and Módena (2004) compared the differences in abundance and size distribution of ambush spiders (Thomisidae) and active hunting spiders (Salticidae, Oxyopidae, Clubionidae, and Anyphaenidae) in different types of inflorescences in Melanthera latifolia, Conyza bonariensis, and Eupatorium hecatanthum (all belonging to the family Asteraceae). The researchers recorded the architectural features, including the number of inflorescences, the inflorescence branch length, and the size and openness of flowers. M. latifolia had larger (6.5 cm) and more open flowers than the other two species (C. bonariensis = 1.30 cm and E. hecatanthum = 3.47 cm), but it showed fewer flowers per inflorescence. The most spiders were recorded in M. latifolia, followed by E. hecatanthum and then C. bonariensis. Evidently, bigger and more open flowers attract more insects (e.g., Bell 1985; Cohen and Shmida 1993), which become potential prey for spiders. Overall, smaller spiders inhabited M. latifolia than E. hecatanthum and C. bonariensis. Although larger flowers might attract larger insects (Dafni et al. 1997) and thus potentially feed larger spiders, studies indicate that larger spiders on the vegetative branches of plants are more vulnerable to predation by birds (Waldorf 1976; Askenmo et al. 1977). In fact, M. latifolia has the lowest number of flowers, which indicates fewer possible retreats against predators.
In the system Peucetia viridans (Oxyopidae) and Croton ciliatoglandulifer (Euphorbiaceae), Jiménez-Salinas and Corcuera (2008) found that P. viridans is most abundant in plants with the highest number of inflorescences and greater vegetation cover. The researchers experimentally manipulated the architecture of the plants by removing the inflorescences of some of the plants. More spiders were observed in control plants (from which inflorescences were not removed) than in treatment plants (from which all inflorescences were removed). In addition, more spiders were found in plants with the highest number of inflorescences, and more spiders were found in male flowers than in female flowers. The researchers argued that because male flowers produce pollen and, in some cases, more nectar, they attract more insects and provide more resources for the spiders.
Later, in the same spider–plant system, Corcuera et al. (2010) experimentally placed artificial inflorescences on C. ciliatoglandulifer to evaluate their effect on the distribution of the spider P. viridans. The treatments included (1) 15 plants from which all the inflorescences were removed, (2) 15 plants whose natural inflorescences were replaced by artificial ones, and (3) 15 control plants whose natural inflorescences were not changed. More spiders were recorded in plants with natural and artificial inflorescences than in plants from which the inflorescences were removed. However, the abundance of spiders was similar between the control treatment plants and the artificial inflorescence plants. Possibly, P. viridans does not use the scents of flowers as a sign of an available and appropriate microhabitat, but relies only on the inflorescence architecture (e.g., the flower’s shape and size). These studies confirm the positive effect of the inflorescences on the distribution of some groups of spiders. These reproductive structures may provide different resources (e.g., shelter, prey attractants) to the spiders associated with them.
Spacing Between the Branches and Leaf Density
Few experimental studies have manipulated plant architectural complexity, and those that did so have often confused the effects of the architecture with the effects of area. In addition, several studies were restricted to a particular plant species or genus (Hatley and MacMahon 1980), limiting the ability to extend the results related to increasing architectural complexity of the plant and the abundance and diversity of spiders.
Souza and Martins (2005) compared the abundance of spiders in seven species of plants, which were grouped into structural complexity levels. This level of complexity was measured by the leaf density index, which was the number of leaves per branch divided by the estimated volume of the branch. The plant species selected for the study were Baccharis dracunculifolia DC. (Asteraceae), Bidens gardneri Baker (Asteraceae), Chromolaena laevigata (Lam.) King and H. Rob. (Asteraceae), Diplusodon virgatus Pohl (Lythraceae), Microlicia euphorbioides Mart. (Melastomataceae), Microlicia fasciculata Mart. ex Naud. (Melastomataceae), and Vochysia tucanorum Mart. (Vochysiaceae). As expected, the average number of spiders per branch was positively correlated with the structural complexity index. The highest number of spiders was recorded in B. dracunculifolia (which had the highest value complexity index), whereas D. virgatus and B. gardneri had intermediate and low leaf densities, respectively.
Subsequently, to isolate the effects of leaf density from those of biomass, the researchers experimentally manipulated the density of the branches’ leaves, replacing natural branches with artificial ones. Artificial branches were made with wire and plastic models containing 24 leaves per branch. The leaf model was made of cloth to avoid the effects of chemical components and to replicate the texture of natural branches. For this experiment, three plant species were used: B. dracunculifolia, D. virgatus, and M. fasciculata. Two treatments were used per plant species: artificial branches with high leaf density and branches with low leaf density. The average number of spiders that colonized the artificial branches was similar among the three plant species. However, more spiders colonized the branches with high leaf density. The researchers suggest that the architecture of the branches has a significant effect on the abundance of spiders, regardless of biomass, leaf surface area, and branch texture. This effect was also reported by other studies (Hatley and MacMahon 1980; Gunnarsson 1990, 1992). However, in all these studies the effects of biomass and/or surface area available for colonization by spiders were not isolated from the effects of the branches’ architecture.
Diniz (2011 and unpublished data) record changes in the composition of spiders after modifying the spacing of the branches of experimental plants. Closed architectures (closely spaced vegetative branches) favored runner spiders, presumably because they offered a greater number of shelters. This “daytime shelter” resource apparently led runner spiders to accumulate in dense vegetation. Halaj et al. (2000) and Hatley and MacMahon (1980) also reported that closed plant architecture favored non-weaver spiders. In contrast, open architecture (widely spaced branches) strongly favored weaver spiders. Larger open areas are advantageous for the construction of webs, especially orb webs, which need space for expansion and require few attachment points (Shear 1986). Thus, a more open architecture provides adequate space as well as sufficient anchorage points for the web (Diniz 2011 and unpublished data). Hatley and MacMahon (1980) also recorded that these spiders preferred open architecture (greater spacing between the branches and between the leaves of the branches) in Utah, near the entrance to the “Green Canyon.”
Density of Needles
The coniferous forests of northern and central Europe are severely affected by air pollution (Schulze 1989). This contamination results in accelerated loss of leaves (needles) and has been interpreted as a symptom of environmental stress (Sundberg and Gunnarsson 1994). The loss of leaves affects the architecture of the trees, making the branches more exposed (lower coverage) and directly affecting the fauna associated with these types of trees. Gunnarsson (1988) compared the abundance and distribution of spider sizes on branches with different needle densities on Picea abies (L.) Karst. (Pinaceae), a predominant conifer in the forests of southwest Sweden. There were greater numbers of large spiders on the branches with high needle density, whereas both the number of small spiders and the total number of spiders did not significantly differ between branches with dense and sparse needles. Two possible explanations are that the microclimate conditions in the branches with low needle density are more favorable, and that predation by birds is lower on branches with high needle density. Predation by birds is one of the highest causes of mortality in spiders associated with fir (P. abies) (Gunnarsson 1983), and branches with more thorns can provide better shelter from potential predators. Another important finding was the positive relationship among families of weaver spiders, such as Linyphiidae, with sparsely needled branches, contrary to the results for raptorial spiders (e.g., Thomisidae), which were reported more frequently in branches with high needle density.
Later, Gunnarsson also conducted experimental field and laboratory studies by manipulating the density of needles on the branches to investigate the effect on the abundance and size distribution of spiders. In both studies (Gunnarsson 1990, 1992), a positive correlation was recorded between the density of needles and the abundance of spiders, supporting the hypothesis that these structures play an important role in the survival of spiders. In several experiments, there was no correlation between the size of spiders (bigger or smaller) and the density of needles on the branches. A positive correlation was found, however, between larger spiders and density of branches (not needles). In a later experiment, Gunnarsson (1996) isolated the effect of predation by birds from the effect of the needle density. Again, spider abundance of spiders was correlated with higher needle density on the branches. In this case, a significant decrease in the abundance of spiders in treatments in which the predation by birds was not isolated indicated that these predators exert strong pressure on spiders. However, the effect of the needle density on the distribution of the spiders’ sizes is not clear, as smaller spiders appear to be affected by the change in density of needles.
As spiders are generalist predators and influence the balance of the populations of other arthropods (Wise 1993), changes in the architectural complexity of vegetation may culminate in top-down effects on the community of spiders, which would affect the populations of phytophagous arthropods and primary productivity as well (Denno et al. 2005; Sanders 2007).
Use of Specific Parts of Plants by Spiders
The different structures of plants (e.g., tree trunks, branches, leaves, flowers), comprise their structural complexity, and, as we have seen, are determinants of community distribution of spiders on vegetation. Most studies that address this topic have focused on the relationship between measures of structural complexity and the diversity of spiders. Only a few studies have examined the specific use of certain parts of plants and specific associations between spider species and plants (Fig. 7.1).
Use of different plant structures by spiders. Flowers: (a) Thomisidae on Rubus rosifolius (Rosaceae). (b) Epicadus heterogaster (Thomisidae) on Trixis antimenorrhoea (Asteraceae). (c) Adult male and female of Anyphaenidae using flowers as refuge. (d) Misumenops argenteus (Thomisidae) feeding on Pseudoscada erruca (Ithomiinae) on Trichogoniopsis adenantha (Asteraceae). Leaves: (e) Refuge of Anyphaenidae, (f) Spiderlings on folded leaves. (g) Salticidae feeding on extrafloral nectary. (h) Adult female of Anyphanidae protecting its eggsac. Camouflage on stems: (i) Ariamnes sp. (Theridiidae). (j) Araneidae. (k–l) Senolocus sp. (Senoculidae) (Photographs: a–c, f–l Yuri Fanchini Messas; d, e João Vasconcellos-Neto)
Spiders of the family Thomisidae are typical sit-and-wait predators, hunting prey that visit flowers (Morse 1981). These spiders choose locations on the flowers so that they are not perceived or even increase insects’ attraction to the flowers (Heiling et al. 2006). The associations between these spiders and flowers are well known, but little is known about specific associations or with specific groups of plants, let alone whether they represent specific examples of mutualism.
Spiders use the leaves on plants, as well as dried leaves on the ground, for foraging, shelter, and reproduction (Foelix 2011). There is little information about the specific use of certain plant species. Some studies have reported that spiders, such as those in the family Anyphaenidae, use leaves with specific formats belonging to certain species of plants for mating, nest shelter, or eggsac protection (Foelix 2011; Zanatta 2013; Zanatta et al. 2016). In some of these cases, leaf characteristics (e.g., the presence of trichomes) are essential to their use as shelter for the ovisac (see examples below).
Certain types of branches or stems can be used for foraging and/or protection, including camouflage (Messas et al. 2014; Souza et al. 2015). The set of characteristics belonging to a plant helps determine the composition of spider species associated with it or not associated with it.
Spiders that Feed on Pollen and Plant Fluids
Most spiders are considered obligatory carnivores that feed almost exclusively on insects and other arthropods (Wise 1993; Foelix 2011). However, recent studies have examined the possibility of vegetation as a direct food source, and some spiders appear to be true omnivores, as they can feed on nectar and/or pollen under certain environmental conditions (e.g. Taylor 2004; Eggs and Sanders 2013; Suetsugu et al. 2014; Nyffeler 2016). In a recent review, Nyffeler et al. (2016) recorded more than 60 species belonging to ten families of spiders that feed directly on plant products (e.g., pollen, nectar, Beltian corpuscles) in natural conditions. These families include non-weaver (Anyphaenidae, Clubionidae, Eutichuridae, Oxyopidae, Salticidae, Thomisidae, and Trachelidae) and weaver (Araneidae, Linyphiidae, and Theridiidae) spiders. A species of the Salticidae family was observed feeding on Mullerian corpuscles in trees of the genus Cecropia in the Serra do Japi, Jundiaí, São Paulo State, Brazil (JVN personal observation). Laboratory studies indicate that other families of spiders can also feed on plant products in nature.
Smith and Mommsen (1984) performed one of the first extensive studies on spiders that feed on pollen. These researchers reported that newly emerged Araneus diadematus (Araneidae) reared in the laboratory had longer life expectancies and produced more silk web when they fed on pollen than did newly emerged spiders that fed on aphids and spore fungi. The consumption of pollen could be adaptive, because during the time when the young spiders emerge (spring), few or no insects are available, but ample pollen is produced by dominant woody plants in temperate regions (e.g., Pinus) and dispersed by wind. Vogelei and Greissl (1989) tested the survival of Thomisus onustus (Thomisidae) spiderlings that were given no food (control), Erigeron annuus pollen, Bellis perennis pollen (Asteraceae), artificial nectar (30% sucrose solution), or Drosophila melanogaster. The control group of spiders survived an average 21 days. The spiders that were fed pollen survived for 35–49 days, depending on the plant species, and those fed artificial nectar survived for 130 days. However, only individuals who were fed with flies were able to develop normally, showing normal molting (ecdysis), and only they survived to the end of the experiment (>250 days).
Some wandering spiders, such as Hibana velox, Hibana similaris (Anyphaenidae) Cheiracanthium mildei (Miturgidae), and Trachelas similis (Corinnidae), were observed by Taylor and Foster (1996) feeding on both floral nectaries and on extra-floral nectaries of various plant species in several locations in Costa Rica and Florida. The researchers reported that there is evidence that Myrmarachne foenisex (Salticidae), a spider associated with ants, feeds on the exudate of coccidia (Coccidae). To test the role of nectar in the longevity of newly emerged H. velox spiders, the researchers provided young spiders with either water or 25% sucrose solution. The spiders in the sucrose group lived twice as long as did those in the control group.
Pollard et al. (1995) observed Misumenoides formosipes (Thomisidae) males feeding on nectar from extra-floral nectaries of some plant species. To determine whether these individuals consumed nectar as a source of water or energy, the researchers developed double-choice experiments, introducing small amounts of water vs 30% sucrose solution in experimental arenas, and found that the spiders preferred the sucrose. Even individuals that had consumed their fill of water ingested the sucrose solution. Males that drank only water died sooner than those that fed on nectar. The researchers suggest that as the males of this spider species are much smaller than females, they can become more dehydrated; therefore, feeding on the nectar of extra-floral nectaries may be an adaptive behavior.
In nature, Jackson et al. (2001) observed 31 species of Salticidae feeding on floral nectar. In the laboratory, they tested the preference of 90 species of Salticidae for distilled water vs 30% sucrose solution. All of the species selected and remained longer on the sucrose solution, indicating that nectarivory must be a common habit in that family. The researchers suggest that feeding on nectar may be advantageous because the fluids are rich in amino acids, lipids, vitamins, and minerals and because feeding on a flower involves no risk of injuries, unlike the capture of prey. The use of nectar by spiders can also benefit the plants. In fact, Ruhren and Handel (1999) showed that the presence of Eris sp. and Metaphidippus sp. (Salticidae) increased the production of fruits and seeds of the plant Chamaecrista nictitans (Caesalpineaceae). As in some species of ants, these spiders feed on the nectar of extra-floral nectaries. Meehan et al. (2009) recorded in the field and in the laboratory, using stable isotopes, that the main diet of the Bagheera kiplingi spider, a Neotropical salticid, is the Beltian corpuscles of the plant Vachellia sp. (Fabaceae). B. kiplingi presented concentrations of 15N and 13C isotopes in an intermediate range between ants that feed on the plant and other spiders that do not feed on plant products, confirming that this salticid consumes plant products.
Taylor and Bradley (2009) also showed the importance of nectar in the diets of the non-weaver spiders C. mildei (Miturgidae) and H. velox (Anyphaenidae). The researchers tested the importance of extrafloral nectaries to the survival, molting, and nighttime activity of these spiders. Tests of survival and molting were conducted in plastic terrariums containing a single spider newly emerged from the ovisac. To test the effects on survival, H. velox spiders were assigned to receive 25% sucrose, nectar from Terminalia catappa (Combretaceae), or water. A second experimental group was assigned to receive 69% sucrose, nectar, or water. To test the effects on molting, a single adult Drosophila was provided to a C. mildei spider on alternate days until the spider molted. T. catappa nectar was provided on the days when the Drosophila was not provided. In a second experiment, nectar was not provided (although water was given as a control). To test the effects on nighttime activity, newly emerged spiders received either water and nectar or water alone (control). To quantify the nocturnal activity, the number of spiders in each group that ran continuously during the night for at least 1 min was recorded. The spiders were filmed at 10-min intervals, for a total of 54 observation periods, and both species of spiders were used.
In the survival test, the spiders that received nectar or either sucrose concentration survived significantly longer than those that received water. There were no differences in survival between nectar and sucrose treatments. In the molting test, the number of spiders that molted was significantly higher in the nectar group. Finally, in the nighttime activity test, spiders ran more when their diets included nectar. These results suggest that nectar can be a source of energy for spiders, especially during periods when prey are scarce, since survival and molt rates were significantly higher when the nectar was provided. The researchers noted that the sugars obtained from the nectar supplied much of the energy demands of locomotion, freeing up the proteins contained in reserves for use in growth and/or new deposition of cuticle (Dalingwater 1987).
These results, however, are not easily generalizable. Carrel et al. (2000) found that Frontinella pyramitela (Linyphiidae) individuals gained weight when fed D. melanogaster but lost weight when fed pine pollen, suggesting that polinivory is restricted to particular groups of spiders and/or conditions of food scarcity.
Non-weaver spiders are not the only species that feed on nectar or pollen. Weaver spiders (Araneidae) may dismantle and eat their webs at regular intervals, which enables them to recycle the silk proteins efficiently. As the webs are not just a trap for potential prey, but also a trap for air plankton, spiders may also feed on adhered spores and pollen when they are recycling the webs. Eggs and Sanders (2013) tested the percentage of pollen in the diet of the orb-weaver spiders Aculepeira ceropegia and Araneus diadematus (Araneidae) in the presence of other food sources (insects). Their experiment included ten spiders that were fed fruit flies (Drosophila) and pollen from Betula pendula (Betulaceae) that was adhered to their webs, and ten spiders that were fed exclusively Drosophila. An analysis of stable isotopes in the body tissues of the spiders was performed. The results indicated that about 25% of the diet of spiders was composed of pollen and the other 75% was composed of flying insects, mainly small Diptera and Hymenoptera. The amount of pollen consumed was similar in laboratory and field observations (10–40% of the diet). Therefore, orb-weaver spiders actively feed on pollen, together with insects, to obtain the essential nutrients they need, at least during the early stages of life. The consumption of pollen by adult spiders decreased in the natural environment when insects became more abundant (during the summer season). The researchers suggested that this group of spiders be classified as omnivores, rather than as strict predators, as both carnivory and herbivory occur during important life stages of these orb-weaver spiders.
Spider–Plant Specific Associations
Arthropod–plant interactions have been studied extensively in some groups, for example, phytophagous insects, which live exclusively on vegetation and show highly specific relationships with their host plants (Schoonhoven et al. 1998). However, few studies have demonstrated this kind of association in spiders, despite the incredible diversity of spiders and their habitats. Some spider species belonging to the families Oxyopidae, Thomisidae, Salticidae, Araneidae, Ctenidae, Corinnidae, Selenopidae, and Theraphosidae have been shown to live strictly associated with a particular plant species or groups of plants that share morphological characteristics (e.g., glandular trichomes, rosettes, tree bark containing specific structures) (see review in Romero 2006; Messas et al. 2014).
Few studies have explored the reasons that spiders have specific associations with plants. Generally speaking, plants can provide suitable sites for protection, foraging, and reproduction. Recently, Hormiga and Scharff (2014) described a new species of Linyphiidae, Laetesia raveni (Araneae) collected in New South Wales and Queensland (Australia). This new linyphiid species seems to build its webs almost exclusively on two plant species, Calamus muelleri Wendland (Arecaceae) and Solanum inaequilaterum Domin (Solanaceae), both densely covered with thorns. The abundant thorns may protect the spiders from certain predators. Another unusual and little explored association involves the thomisid Synema obscuripes and the carnivorous plant Nepenthes madagascariensis (Nepenthaceae) (Rembold et al. 2012). This species spends its entire life cycle within the plant pitchers, structures that provide food (the pitchers attract insects) and shelter against predators (the pitchers secrete a liquid containing digestive enzymes).
In Central America, at least seven species of wandering spiders belonging to the Cupiennius genus (Ctenidae) are known for their intimate associations with certain plant groups (Barth et al. 1988a, review in Romero 2006). These ctenid spiders live exclusively on plants of the families Musaceae and/or Bromeliaceae, sheltering in them during the day and using them for ecdysis, courtship, and foraging at night. For example, Cupiennius salei lives in certain species of bromeliads (Barth and Seyfarth 1979; Barth et al. 1988a) and exchanges vibratory signals through the leaves of these plants (Barth et al. 1988b; Baurecht and Barth 1992). Other spider species, such as Pachistopelma rufonigrum (Theraphosidae; Santos et al. 2002), Nothroctenus fuxico (Ctenidae; Dias and Brescovit 2003, 2004), and various species of jumping spiders (Salticidae; see review in Romero 2006), are exclusively associated with tank bromeliads in several countries. Some corinnid species use bromeliads as habitats, in coastal and inland areas of Brazil (Cotgrave et al. 1993; Dias et al. 2000; Mestre et al. 2001; Araújo et al. 2007; Gonçalves-Souza et al. 2010). According to Gonçalves-Souza et al. (2010), of the five species of Corinnidae found in three types of habitat, four occurred only on bromeliads. However, only a few studies have demonstrated exclusivity with regard to Corinnidae and bromeliads. The first study demonstrating this specific association was Piccoli (2011), who reported that the spider Corinna sp. nov., described later by Rodrigues and Bonaldo (2014) as Corinna demersa, is exclusively associated with Quesnelia arvensis (Bromeliaceae) in restinga forests. The spider constructs a shelter in the axils of leaves or along the central tank and dives into the water when threatened.
Spiders that are associated with plants necessarily have adaptations that facilitate their relationships with host plants. These adaptations are usually related to spiders’ sensory systems, allowing them to discern specific plant species through visual, olfactory, and tactile stimuli (review in Romero and Vasconcellos-Neto 2007b). The spider Misumena vatia (Thomisidae), for example, when presented with a choice of differently colored artificial flowers, more often chose yellow flowers (Greco and Kevan 1994). The morphological characteristics of plants also affect spiders’ selection of habitat. In a field study, Morse (1990) demonstrated that M. vatia lays its eggsacs preferably in plants of the genus Asclepias (Apocynaceae). Leaf characteristics (e.g., flexibility, large size, high density of trichomes) appear to be fundamental factors determining the selection of this plant as an oviposition site. A social species, Diaea (Thomisidae), chooses Eucalyptus leaves, also using morphological leaf characteristics to recognize them (Evans 1997). However, this species selects smaller leaves than does M. vatia, because it is too small to handle the large leaves.
Another way that Thomisidae spiders find their foraging sites is through volatile substances. Heiling et al. (2004) offered crab-spiders (Thomisus spectabilis) and floral visitors (Apis mellifera, Hymenoptera) both flowers with natural scents and those from which the scents had been removed. Both species preferred the flowers with the scents. The spider and the bee favored different visual characteristics of flowers (size and reflectance). Krell and Krämer (1998) showed that the volatile eugenol [2-methoxy-4-(2-propenyl) phenol], a component of the floral fragrance found in plants of different families around the world, attracts the cogeneric spiders Thomisus daradioides and T. blandus (Thomisidae). The researchers suggest that the spiders are able to memorize common chemical compounds in flowers and use them as cues to locate their foraging sites, which in turn are highly visited by pollinators.
Lyssomanes viridis is a translucent green jumping spider that perches on the abaxial surface of leaves. This salticid has a chemically mediated preference for, and higher hatching success on, the sweet gum Liquidambar styraciflua L. (Altingiaceae) during the summer. Compared with other sympatric species, the sweet gum leaves presents a potent broad-spectrum antimicrobial volatile compound, notably the monoterpene terpinen-4-ol, a well-studied antimicrobial agent known from tea tree oil. This chemical compound could protect the spider eggs against microbes, promoting higher hatchings (Tedore and Johnsen 2015).
The contact, but not airborne, with chemical cues of this plant species are attractive to L. viridis. These spiders overwinter predominately on leaves of a broadleaf evergreen species, the American holly Ilex opaca Aiton (Aquifoliaceae), so must migrate to American holly in the autumn, and back to sweet gum in the spring once its leaves have re-emerged (Tedore and Johnsen 2015). Posteriorly, these same authors (2016) expected that L. viridis might use leaf shape to find sweet gum, and green coloration to detect American holly. However, their results suggest that L. viridis does not attend to the leaves color or shape, but does have a visually mediated preference for a particular level of ambient illumination and possibly perceived leaf brightness. In experimental conditions, spiders overtake any potential preference for leaf size. Importantly, if they had not controlled for the effect of leaf size on the ambient illumination in the area surrounding the leaf, they would have concluded that L. viridis was capable of judging the relative sizes of leaves using other parameters, like relative area or linear dimension. The authors conclude that ambient illumination was the most important factor in determining which leaf the spiders settled during their experiments.
Some studies have demonstrated intimate associations between spiders and plant species or groups of plants that share features in common. The best-known associations involve interactions between species of the genus Peucetia (Oxyopidae) and plants with grandular trichomes, jumping spiders (Salticidae) and Bromeliaceae plants, and araneids of the genus Eustala and tree species or dry vegetation structures. Some of these studies will be discussed later.
Associations Between Spiders and Plants with Glandular Trichomes
Several plant species from different taxa have glandular trichomes on the surfaces of their leaves and stems. These structures possibly arose as a direct defense against herbivores and pathogens (Duffey 1986). Enhancing the plants’ defense systems, some spiders belonging to the families Thomisidae and Oxyopidae forage and reproduce preferentially on plants containing this type of glandular structure.
Spiders of the genus Peucetia (Oxyopidae) do not construct webs, weaving only silk threads leading to the branches, leaves, or flowers of the plants in which they live. Females lay their eggsacs under leaves and remain near them for several days, until the emergence of the spiderlings. Some species belonging to this genus are commonly found on plants that have glandular trichomes. In a literature review coupled with over 30 years of field observations, Vasconcellos-Neto et al. (2007) showed that ten species of Peucetia occur in association with more than 55 species of plants that have these trichomes. The plant species more frequently used by these spiders belong to the families Solanaceae, Asteraceae, and Melastomataceae. Whereas the species Peucetia flava, Peucetia rubrolineata, Peucetia longipalpis, and P. viridans occur in the Americas (Brazil, Colombia, Panama, Mexico, and the United States), the oxyopids Peucetia arabica, Peucetia crucifera, Peucetia maculifera, Peucetia nicolae, Peucetia transvaalica, and Peucetia viridis occur in the Old World, including Spain and some parts of Africa (Fig. 7.2).
In the municipality of Sumaré, São Paulo (Brazil), P. rubrolineata occurred more frequently on Solanaceae species that contained leaves with a high density of glandular trichomes (Vasconcellos-Neto et al. 2007). In other regions, P. flava and P. rubrolineata were exclusively associated with plants containing these structures (Morais-Filho and Romero 2008, 2009; Vasconcellos-Neto et al. 2007).
Why do several species of the genus Peucetia specialize in plants containing glandular trichomes? Vasconcellos-Neto et al. (2007) suggested that this specialization may have evolved as a result of the adhesive nature of glandular trichomes, which hold small insects captive or hinder the movements of larger arthropods. In fact, many arthropod predators, such as insects belonging to the family Miridae, also have specific associations with plants containing glandular trichomes (Anderson and Midgley 2003; Sugiura and Yamazaki 2006; Romero and Vasconcellos-Neto 2004b) and capture prey that become adhered to these plant structures. Therefore, trichomes make it easier to capture prey, enabling predators to save the energy they would have expended in the capture and subjugation of prey.
Do spiders actively select plants with glandular trichomes, and does this behavior really benefit the spiders? To answer this question, Romero et al. (2008a) conducted field experiments using P. rubrolineata and P. flava in Serra do Japi, in Jundiaí, São Paulo (Brazil). The researchers estimated the residence time of these spiders in plants with glandular trichomes (Trichogoniopsis adenantha, Asteraceae) and without glandular trichomes (Melissa officinalis, Lamiaceae, and Lantana camara, Verbenaceae). Both spider species remained significantly longer on plants with trichomes (approximately 60 h on average) than on plants without these structures (a few minutes). In a second experiment, the researchers placed dead vestigial Drosophila flies (with atrophied wings) on T. adenantha plants (which have glandular trichomes) and on M. officinalis (which do not have trichomes). The plants were placed individually in exclusion cages and a spider was maintained on each plant. The spider’s biomass was estimated at the beginning of the experiment and 6 days later. Spiders on plants with glandular trichomes did not lose or gain biomass, whereas spiders on plants without trichomes lost biomass. The findings indicate that Peucetia spiders consume dead organisms attached to trichomes and therefore act as scavengers on these structures. Detection and recognition of dead prey on vegetation are not common behaviors among spiders. The dead prey adhered to the trichomes probably provide extra nutrients during periods of live food scarcity.
In a complementary study, Morais-Filho and Romero (2010) used razor blades to remove the glandular trichomes from some Rhynchanthera dichotoma (Melastomataceae) plants. As in the study by Romero et al. (2008a), P. flava spiders stayed longer on plants with intact trichomes than on those whose trichomes had been removed. Next, the researchers released 30 vestigial winged Drosophila flies on each plant in a sample that included both plants with intact glandular trichomes and those from which the trichomes had been removed. More flies adhered to plants with intact trichomes. The study confirmed the hypothesis that arthropods can become adhered to these plant structures.
As shown here, Peucetia can consume dead insects that are attached to trichomes. In nature, do these spiders consume more live or dead insects? Morais-Filho and Romero (2010) enriched vestigial Drosophila with large amounts of nitrogen-15 isotope (15N, see the procedure in Romero et al. 2006), to identify how much of the enriched prey was transferred to the spiders. The treatment groups included plants with dead enriched flies and plants with live enriched flies. Spiders were introduced to both treatments, and after a few days were collected for isotopic measurements of their body tissues. The spiders that preyed on dead and live flies showed similar amounts of the nitrogen-15 isotope, indicating that the consumption of live prey and decomposing insects on trichomes occurs in similar proportions.
Among the Thomisidae spiders, Misumenops argenteus was also found on plants with glandular trichomes in Serra do Japi (Romero and Vasconcellos-Neto 2004b). This spider occurred more frequently in T. adenantha and Hyptis suaveolens (Laniaceae), both containing trichomes, than in other plants available in the study area that do not present these structures. T. adenantha blooms all year and can thus attract potential prey throughout the life cycle of the spider. The glandular trichomes hinder the movement of ants and Chironomidae mosquitoes, which constitute up to 21% of the diet of M. argenteus (Romero and Vasconcellos-Neto 2003). According to Romero and Vasconcellos-Neto (2004a), these characteristics of the spider–plant interaction may all contribute to make the relationship beneficial to both species.
Specializations of Spiders for Bromeliads or Similar Plants
A wide diversity of aquatic and terrestrial arthropods inhabit plants belonging to the family Bromeliaceae, especially in Neotropical environments (Benzing 2000). Some spider species occur preferentially on bromeliads, and some present morphological features (e.g., dorsoventrally flat body) that facilitate their colonization of these plants. Associations between spiders and bromeliads and/or similar plants (e.g., plants that have leaves arranged as rosettes) have been described for the spider families Anyphaenidae (Brescovit 1993), Araneidae (Figueira and Vasconcellos-Neto 1991), Corinnidae (Piccoli 2011), Ctenidae (Barth et al. 1988a, b; Dias and Brescovit 2004), Salticidae (Young and Lockley 1989; Maddison 1996; Rossa-Feres et al. 2000; Frank et al. 2004; Romero and Vasconcellos-Neto 2005a, b, c; Romero 2006; Romero et al. 2007), Trechaleidae (Brescovit and do Oliveira 1994), and Theraphosidae (Dias and Brescovit 2004). The jumping spider Pelegrina tillandsiae (Salticidae) was recorded on Spanish moss (Tillandsia usneoides, Bromeliaceae) in the southeastern United States (Romero 2006).
Among these, the most studied associations involve Salticidae species. So far, nine species of jumping spiders associated with bromeliads in South America have been reported. The studies showing those associations were conducted in several countries, including Brazil, Bolivia, Argentina, and Paraguay, in areas containing different vegetation types, including cerrado regions, semi-deciduous forests, vegetation of coastal dunes, restingas, rocky outcrops (inselbergs), chacos, seasonal forests, dense rainforests, and tropical montane forests (Rossa-Feres et al. 2000; Romero and Vasconcellos-Neto 2004c, 2005a, b, c; Romero 2006). These studies showed that spiders use bromeliads as foraging sites and breeding, nursery, and shelter sites where they can avoid predators and adverse weather conditions.
Some of these species are specialists that are associated almost exclusively with one type of bromeliad (e.g., Psecas chapoda and Bromelia balansae) over a large geographical area (Fig. 7.3). In contrast, other species are generalists (other Psecas sp., Coryphasia spp., Eustiromastix nativo, Uspachus sp. new) that have been found inhabiting as many as eight species of bromeliads. The specialists occur in phytogeographical regions such as the cerrado and semi-deciduous forests, where one species of bromeliad (B. balansae) typically dominates, while general spiders usually live in areas with high species richness and diversity of bromeliads, such as in the rain forest (Romero 2006). Bromeliad species that occur in rainforests share morphological characteristics (e.g., broad leaf, presence of a tank) that are attractive to spiders.
So far, the spider–bromeliad association that has been studied the most involves Psecas chapoda (Salticidae) and Bromelia balansae (Bromeliaceae). This spider occurs almost exclusively on B. balansae in various regions of cerrado and semi-deciduous forest in Brazil, Bolivia, and Paraguay (Rossa-Feres et al. 2000; Romero and Vasconcellos-Neto 2005a, b, c). The spider uses the bromeliad throughout its reproductive cycle, from courtship and mating to the deposition of eggsacs and population recruitment of young spiders. Each female can lay up to two eggsacs, always in the middle region and on the concave side of the leaves, covering them with a silk sheet woven over the leaf edges (Rossa-Feres et al. 2000).
Romero and Vasconcellos-Neto (2005c) found that P. chapoda occur more frequently in open areas (fields) than within the forest. The distribution may be related to the blocking of the rosettes’ central base (which are used by the spiders as shelter) by dry leaves that fall from the trees. To test this hypothesis, Romero and Vasconcellos-Neto (2005a) conducted a field experiment in open areas containing two treatments (bromeliads with and without dry leaves in rosettes) and found that plants containing leaves were less often colonized by the spiders. However, in a similar experiment carried out within the forest, the spiders did not occupy bromeliads that lacked dry leaves. As the abundance of prey (insects) was significantly higher in open areas than in the forest, the researchers suggested that both the presence of dry leaves and the availability of prey affect the spatial distribution of P. chapoda.
The spiders of this species rarely occur in flowering plants and may occupy up to 90% of the plants that lack inflorescences (Romero and Vasconcellos-Neto 2005b, c). The leaves of bromeliads close to the ground decline when the plant blooms, and this structural modification exposes the flowers to pollinators (e.g., hummingbirds). Romero and Vasconcellos-Neto (2005a) showed that this change affects spiders by modifying their shelter and the nesting sites available inside the rosette, exposing jumping spiders to abiotic (e.g., severe weather conditions) and biotic (e.g., natural enemies) factors.
The central layer of the rosette is occupied by the majority (approximately 70%) of freshly emerged P. chapoda. The center may be preferred because it offers the best shelter from desiccation and/or cannibalism, which is very common in this species of spider (G. Q. Romero unpublished data). Females are commonly observed in external layers of the plant, but they build their eggsacs and remain with them in the inner layers, where young spiders find shelter. This behavior may indicate the existence of maternal care against cannibalism (i.e., the closer the eggsacs are to the center of the bromeliad, the less the spiderlings must travel to find shelter and the greater their chances of survival) (Romero and Vasconcellos-Neto 2005c).
Romero and Vasconcellos-Neto (2005b) collected spiders in São Paulo (Brazil), using an entomological umbrella, a visual search of vegetation, and pitfall traps in the soil to verify whether P. chapoda lives exclusively on B. balansae. The species did not occur in the soil and was found only in B. balansae among the available plants in the study area. These results, in addition to the previous studies on the behavior and geographic distribution of P. chapoda on B. balansae, were the first evidence that this spider–plant association could be obligatory.
B. balansae is the only bromeliad species found in the habitat range of P. chapoda and is a species that does not accumulate water in its rosette. To determine whether the selection of bromeliads by P. chapoda is species-specific, Omena and Romero (2008) planted blocks of bromeliads in the field, each containing three species: B. balansae, Aechmea distichantha, and Aechmea blanchetiana. A. distichantha has a leaf architecture that is similar to B. balansae, but retains water in its central tank, while A. blanchetiana has much wider leaves than the other two species and also accumulates water in the rosette. Spiders colonized B. balansae and A. distichantha equally, but occupied A. blanchetiana less frequently. Therefore, P. chapoda selects plants according to their architecture (long, narrow leaves) and is not species-specific. When an observer approaches P. chapoda spiders on their host plant (B. balansae), spiders flee to the base of the leaves. However, spiders that colonized bromeliads which accumulate water in the tank could not escape to the base of the rosettes. Interestingly, jumping spiders inhabiting tank bromeliads in other geographic regions (along the Brazilian coast) can dive into the tanks to escape from predators (Romero and Vasconcellos-Neto 2004c).
The study by Omena and Romero (2008) demonstrated that P. chapoda selects plants with specific architectures. However, the mechanisms by which the spiders detect, identify, and evaluate the plants remain unclear. Jumping spiders have good vision and, therefore, may be able to use visual cues to choose plants. To test this hypothesis, Omena and Romero (2010) offered spiders a choice of four plants: Agave augustifolia (which has a rosette similar to a bromeliad), Euterpe oleracea (a palm), Croton floribundus (dicotyledonous with large leaves), and Delonix regia (dicotyledonous with small folioles). Almost all spiders chose the agave, which has similar architecture to a bromeliad, showing that these spiders select plants with similar architectural features by using visual cues. In a similar experiment, Omena and Romero (2010) offered P. chapoda spiders a choice of four bromeliads: B. balansae, A. distichantha, A. blanchetiana, and Aechmea fasciata. The first two species have long and narrow leaves, while the others have short and broad leaves. The spiders more frequently chose the bromeliad with narrow leaves, indicating that they use fine details to choose their microhabitats. In order to eliminate the possible effects of color and scent, the researchers used life-size black-and-white photographs of the bromeliad species used in the previous experiment. Interestingly, spiders still chose photographs of bromeliads with long, narrow leaves. These two studies showed that spiders use visual cues to choose bromeliads, relying mainly on the plant architecture and not on coloration.
Three other salticid species were recorded living in bromeliads in the coastal regions of Brazil (Romero and Vasconcellos-Neto 2004c; Santos and Romero 2004). The jumping spiders E. nativo and Psecas sp. are associated with bromeliads in two different types of vegetation in Linhares (ES): native grasslands (a plant formation similar to restingas) and mussunungas, a low forest growing on sandy soils that is typically found in the northern region of this state. E. nativo was also found on bromeliads in a restinga region in the city of Trancoso (BA). Another species, Uspachus sp., also occurs in native grasslands in Linhares and is more frequent in dune areas in Natal (RN). Romero and Vasconcellos-Neto (2004c) suggest that these three spider species are associated specifically with plants of the Bromeliaceae family, as they were not found on other plants. They also point out that members of this plant family have a highly complex architecture and provide favorable microhabitat for jumping spiders.
As with P. chapoda, characteristics related to the physical structure of bromeliads and the environment can affect habitat selection by jumping spiders. E. nativo occurs preferentially on large bromeliads in two different regions (Linhares and Trancoso). Larger bromeliads have increased sheltering capacity and are more likely to be visited by insects (offering higher availability of prey) due to the larger surface area; thus, they are considered better quality foraging sites (Romero and Vasconcellos-Neto 2004c).
In contrast with E. nativo, which occurred more frequently in bromeliads in open areas (native grassland), Psecas sp. mostly occupied bromeliads from adjacent forests in Linhares. In Trancoso, even in the absence of Psecas sp., E. nativo occurred only in open areas (restingas), indicating that this pattern of distribution reflects the habitat and/or microhabitat choice, rather than being due to interspecific competition between these two species of spiders. Romero and Vasconcellos-Neto (2004c) concluded that E. nativo first selects the habitat and then chooses the microhabitat.
In other regions of Brazil and Argentina, five other species of Salticidae (Psecas vellutinus, P. splendidus, Coryphasia sp. 1 and sp. 2, and Asaphibelis physonychus) were observed specifically associated with bromeliads. The biology and natural history of these species remain unknown (Romero 2006).
Associations of Spiders with Arboreal Plants
Arboreal plant species provide a high diversity of microhabitats due to their huge biomass (large surfaces) and high structural complexity (Draney et al. 2014). Among these microhabitats, tree trunks can provide concavities, cracks, epiphytes (e.g., other vegetables, moss, and lichen), fissures, and patches of loose bark (Szinetár and Horváth 2005; Michel and Winter 2009; Messas et al. 2014). Due to this variation, the bark of a particular tree can have its own microclimate (Nicolai 1986, 1989) and that resource can significantly affect the distribution of species in tree trunks (Prinzing 2001, 2005).
Spiders may use the tree trunk as an exclusive, facultative, or occasional habitat (Wunderlich 1982). The spider Neriene radiata (Linyphiidae), for example, sometimes occurs on tree trunks, but it prefers the more stable environment offered by the bark to the understory environment (Herberstein 1998). Species that live exclusively associated with tree trunks generally present behavioral (e.g., seeking shelter under tree bark), morphological (e.g., flattened body), physiological (e.g., camouflage), and phenological adaptations to the environment in which they live (Szinetár and Horváth 2005). Bark-dwelling spiders (e.g., Telaprocera; Harmer 2009, Harmer and Herberstein 2009) and Eustala perfida (Messas et al. 2014) can construct vertically long webs, called ladder-webs, whose shape is probably due to the horizontal space limitation caused by the trunks or to a specialization for specific prey (e.g., moths; Harmer and Herberstein 2010). Bark-dwellers occur more frequently in trunks that exhibit surfaces with specific characteristics (e.g., E. perfida; Messas et al. 2014). These spiders usually select microhabitats containing essential characteristics such as shelter, high prey availability, and anchorage points for web construction (Herberstein 1998; Harmer 2009; Draney et al. 2014; Messas et al. 2014). The structural characteristics of the bark invite a wide variety of potential prey to spiders (Horvath et al. 2005).
Messas et al. (2014) investigated the spatial distribution and habitat selection of E. perfida (Araneidae), a spider that presents chromatic polymorphism, with colors ranging from green, red, white, and black. The study was conducted in Serra do Japi, a semi-deciduous rainforest located in São Paulo state (Brazil), with altitudinal variation from 700 to 1300 m. To verify the spatial distribution of this species, the researchers delimited plots on the edge and in the interior of the forest at different altitudes (basal, intermediate, and high) and performed a visual search for spiders on vegetation. The spiders were not found at the edge or on shrubby and herbaceous vegetation; instead, they occurred strictly on tree trunks inside the forest. Therefore, the species is an exclusively bark-dwelling spider.
Subsequently, Messas et al. (2014) proposed that E. perfida prefers trunks containing specific characteristics. They measured structural attributes of the trunks within the plots, characterizing each trunk according to texture (smooth or rough bark), size (diameter at breast height), and the presence of features such as lichens, mosses, and concavities. The characteristics of over 3000 tree trunks were evaluated in an analysis of use by spiders. E. perfida was found in different tree species, both native and introduced, indicating that the spider did not require a unique host plant species. E. perfida occurred most frequently on trees with rough trunks and mosses, lichens, and/or concavities (Fig. 7.4a). These structures provide insertion points for the construction of orb webs. In addition, more spiders were found on trunks with larger diameters at intermediate and lower regions of the mountain. Larger trunks have more surface area for web construction and are more common in these low-altitude areas. In contrast, the highest region of the mountain consists of a semideciduous rainforest that is typical for the altitude, with thinner trees and, coincidentally, fewer spiders.
(a) Adult female of Eustala perfida (Araneidae). (b) Expected and observed frequencies of E. perfida on trunks with different bark surfaces. (c) Adult female of Selenops cocheleti (Selenopidae) on Myrtaceae. (d) Expected and observed frequencies of Selenops cocheleti on trees with exfoliating and not exfoliating barks in 2014 (Photographs: Yuri Fanchini Messas)
These studies demonstrated that E. perfida occurs in narrowly defined environments, determined by the type of vegetation (large trees) and tree trunks that share the same structural characteristics. Furthermore, the species presents chromatic polymorphism (at least from a human’s point of view) that is similar to the colors found in the bark or in elements of the trunk, such as mosses and lichens. This adaptation is probably due to the pressure exerted by visually oriented predators such as birds and hymenopteran parasitoids. The researchers observed some spiders whose bodies contained larvae of the koinobiont ectoparasitoid Acrotaphus tibialis (Hymenoptera, Ichneumonidae), but the parasitism rate was extremely low (Messas et al. in preparation). In fact, during the study period, few E. perfida predation events by other animals were observed, mostly involving other spiders (e.g., Gelanor sp., Argyrodes sp., and a species of Salticidae). This indicates that the camouflage in this species is effective, but further studies should be conducted to determine how predators perceive the coloration of E. perfida.
In another study conducted in Serra do Japi, Villanueva-Bonilla (2015) investigated habitat selection by the wall crab spider Selenops cocheleti (Selenopidae), which lives on tree trunks and presents a dorsoventrally flattened body. Unlike E. perfida, this selenopid is strongly associated with trees that have desquamative stems and smooth texture. This preference is related to the spiders’ use of cracks as shelter, since the flat body of S. cocheleti enables the spider to shelter in areas between the bark and the tree trunk. Furthermore, this spider species prefers Myrtaceae plants to other plants in the study area (Fig. 7.4b). Nevertheless, as with E. perfida (Messas et al. 2014), the species does not show specificity for a single plant species, but for a set of trees that share structural characteristics.
Associations of Spiders and Dry Structures of Vegetation
Spiders are commonly found living on shrubby and herbaceous vegetation. Within the group of orb-weaver spiders, some species of the Araneidae family are associated with specific plants (Hesselberg and Triana 2010) or with plants that share characteristics in common, such as density and architecture of branches that enable the construction of orb webs (Turnbull 1973).
Souza et al. (2015) investigated the spatial distribution and habitat selection of two sympatric and cogeneric species of orb-weaver spiders, Eustala taquara and Eustala sagana in Serra do Japi. Both species have chromatic polymorphism, with many shades of brown, gray, and green. A remarkable morphological feature that distinguishes the two species is the long, longitudinally striped abdomen of E. sagana, while E. taquara has a subtriangular, slightly lengthened abdomen (Fig. 7.5). This study was conducted in the same environment using a similar methodology to that employed for E. perfida (see Messas et al. 2014), making the results comparable.
To verify the spatial distribution of these two species, the researchers visually searched for spiders within plots inside the forest and on the forest edge. Both species were found living exclusively associated with shrub and herbaceous plants on the forest edge. Both E. taquara and E. sagana have cryptic coloration (from a human point of view) and rest on dry vegetation structures. To show that their distribution was not random, the frequencies of green (live) and dry (dead) vegetation was estimated for the plots on the edge of the forest, and posterior comparison was made with the frequency of sites (green or dry) effectively used by spiders to rest. Again, both species were more abundant in similar environments, with more than 90% of individuals occupying dry vegetation structures.
If both species occur in such similar environments, which factors determine the spatial segregation between E. taquara and E. sagana? The researchers tested the hypothesis that the altitude and the type of vegetation help determine the distribution of these two species. In fact, E. taquara occurred more frequently in the intermediate regions (1000 m above sea level), while most E. sagana individuals were found at lower elevations (750–850 m) of Serra Japi. The authors argued that these differences may be related to biotic (e.g., architecture of vegetation, availability of prey, and the presence of natural enemies) and abiotic factors (e.g., temperature, humidity, and solar radiation) (Turnbull 1973; Brown 1981; Janetos 1986; Lubin et al. 1991; Marshall and Rypstra 1999).
In Ecuador, Purcell and Avilés (2007) observed that the altitude can also affect the distribution of some species of Anelosimus (Theridiidae), mainly in response to biotic factors, such as the prey size and predator pressure. To verify whether spiders show specificity for certain plant species, the researchers estimated the diversity and frequency of plant species in plots on the forest edge (in the altitudes where each spider species shows greater abundance). The relative abundance of plant species (expected frequency) was compared with the relative abundance of plants that are effectively used as sites for web construction (observed frequency). E. taquara were found more frequently in plants belonging to the species Conyza bonariensis (Asteraceae), apparently avoiding web-building in H. suaveolens (Lamiaceae), which is preferably used by E. sagana (Fig. 7.5). Hesselberg and Triana (2010) also studied the specificity of Eustala for certain plant species, showing that the spiders Eustala illicita and Eustala oblonga are associated with the plants Acacia collinsii and Acacia melanoceras (Fabaceae) respectively, which present a complex plant–ant–spider interaction.
The arboreal araneid E. perfida (Messas et al. 2014) and both E. taquara and E. sagana (Souza et al. 2015) are sympatric spider species that are phylogenetically related. In all three species, the cryptic coloration matching the plant substrate seems to play a fundamental role in the history of these animals’ lives. Studies suggest that the camouflage and the color polymorphism may be a result of the selective pressure exerted by visually oriented predators such as birds and hymenopteran parasitoids. Souza et al. (2015) observed that E. taquara and E. sagana rest on specific plant structures, the former preferring dry capitula and the latter dry stems, which are similar in shape to the long body of the spider (Souza et al. unpublished data). Thus, it is likely that the spiders are choosing specific microhabitats that may promote the effectiveness of their cryptic coloration.
Phenological Synchrony and Lags in Plant–Spider Relationships
An interesting question in ecology is how populations of plants and spiders interact to maintain specific phenological associations. For example, do spiders have the same effects on plants throughout the year? Studies have shown that populations of spiders associated with vegetation often suffer directly from climatic factors or indirectly from changes in the availability of foraging sites or prey. However, the populations of these predators do not always show synchronized responses to biotic or abiotic variables.
Arango et al. (2000) studied the relationship between the spider P. viridans and the plant Cnidoscolus aconitifolius (Euphorbiaceae), which attracts floral visitors, including flies, bees, and wasps, in Mexico. There was a clear lag time between events such as the onset of rains, the flowering of the plant, the arrival of floral visitors, and an increase in the spider population. In May, the rains began and the plants flowered. In July, floral visitors increased, and in August, the spiders increased in number. A similar phenological pattern was observed in the system featuring the spider M. argenteus and the plant T. adenantha, which attracts herbivores and floral visitors, in Serra do Japi, Jundiaí (SP). Temporal lag analysis (with up to a 3-month delay) detected a 1-month delay between the start of rains and the flowering period of T. adenantha. An increase in the arthropod population (potential prey for M. argenteus) on the plant occurred in synchrony with the increase in the number of reproductive branches. The population of M. argenteus increased 2 months after the numerical response of arthropods (Romero 2001; Romero and Vasconcellos-Neto 2003).
These results indicate that climatic factors such as rainfall primarily shape the phenological pattern of plants. In response to increased rainfall, plants produce more reproductive branches. These branches, which are used as foraging sites by spiders, provide food resources in the form of several species of herbivores and pollinators (Arango et al. 2000; Romero 2001; Romero and Vasconcellos-Neto 2003, 2004a). If these resources are scarce at a particular time of the year, such as the dry season, the insects that directly depend on them will be scarce too. Consequently, the availability of prey and foraging sites for the spiders also decreases, reducing their populations. These results indicate that the systems studied by Arango et al. (2000) and Romero and Vasconcellos-Neto (2003, 2004a) are strongly influenced by bottom-up effects, when changes in the lower levels of the food chain, such as the producers, affect the levels above (Romero 2007). These studies reveal the importance of interactions between biotic and abiotic forces in determining the community structure of arthropods on plants.
P. rubrolineata and P. flava (Oxyopidae) are two species associated with T. adenantha, and population sizes and age structures of spiders are related to climatic variables, plant phenology, and abundance of prey, which may or may not result in synchrony and time lags in this system of tri-trophic interactions (Villanueva-Bonilla et al. in preparation).
Studies of the phenology of T. adenantha (Romero and Vasconcellos-Neto 2005d) and the natural history of M. argenteus reveal the lifecycle adjustments (phenogram) made by this spider species to climatic conditions, plant phenology, and prey availability (Romero and Vasconcellos-Neto 2003, 2004a). The age structure of the spider population throughout the year expresses the interactions of the spiders’ lifecycle with biotic and abiotic conditions. During colder and drier periods of the year, the juvenile and subadult instars have longer durations. The longer development time for these phases may result from low availability of prey.
Negative Effects of the Presence of Spiders on Plants
Although spiders frequently occur on plants, their role as predators and the cascade effect of their presence on herbivores and plants have not been fully explored. Their effects may be positive or negative for the plant. In some cases, spiders prey on herbivores, favoring the plant’s fitness (Fig. 7.6). These mutualistic relationships will be discussed later.
In other cases, spiders consume or interfere with pollinators, reducing the plant’s fitness. Usually the negative effects of spiders occur through trait-mediated indirect interactions (TMIIs), defined by Abrams et al. (1996) as effects transmitted through changes in traits (e.g., behavioral, morphological, and life history) of affected species. The most studied TMIIs induced by spiders involve deterrence of pollinating insects from plants containing spiders; the insects can detect flowers that contain sit-and-wait spider predators and avoid them. Spiders can also negatively affect digestive mutualistic interactions between insects and plants. Furthermore, spiders are known to repel predators of phytophagous insects and/or consume insects (e.g., ants) that protect the plant against predators, facilitating the presence of herbivores on the plant. Some of these interactions will be discussed individually in the sections that follow.
Negative Effects of Spiders on Plant–Insect Digestive Mutualism
Although spiders frequently contribute to plant nutrition by producing feces, they can also negatively affect digestive mutualism. The heteropteran predator Pameridea roridulae (Miridae), for example, lives exclusively associated with the carnivorous plant Roridula gorgonias (Roridulaceae) in South Africa, and can contribute up to 70% of the total N used by its host plants (Ellis and Midgley 1996). However, in some regions, R. gorgonias is also inhabited by the spider Synaema marlothi (Thomisidae), which often decreases the density of the mutualist P. roridulae on the plant. Spiders do not defecate directly on the plant so they do not contribute N to it. Anderson and Midgley (2002) showed that in the presence of spiders, plants had low density of Heteroptera and were less enriched with nitrogen (15N isotope).
Negative Effects of Spiders on Ant–Plant Mutualism
Gastreich (1999) showed that the spider Dipoena banksii (Theridiidae) exerts TMIIs in a mutualistic association between the ant Pheidole bicornis and the plant Piper obliquum (Piperaceae) in Costa Rica. This ant–plant interaction follows the general pattern of this type of association, wherein the plant provides food for the ant and the ants protect the plant against insect herbivores, reducing folivory and consequently increasing the fitness of the plant. The theridiid D. banksii constructs its web in the base of new leaves of Piper plants, and preys almost exclusively on P. bicornis. The presence of the web helps the spider to capture ants but at the same time allows the ants to detect and avoid the spiders, making it possible to study indirect interactions mediated by behavioral modification.
If TMIIs actually exist in this ant–plant interaction mediated by D. banksii, plants containing spiders would be expected to exhibit lower density of ants (whose standard foraging patterns would be altered by the presence of spiders). Consequently, these plants would exhibit an increased rate of folivory compared with plants lacking spiders. To test these hypotheses, Gastreich (1999) compared folivory rates among plants with and without spiders in the field. Subsequently, she investigated the effect of D. banksii on P. bicornis’ behavior by removal experiments. She compared the numbers of ants patrolling leaves with spiders and on these same leaves after removal of the spider and, finally, after removal of the web. The removal of spiders and webs increased folivory and decreased the number of ants on plants that had contained spiders or webs, supporting the hypothesis that there is a TMII between D. banksii and Piper plants.
Negative Effects of Spiders on Plant–Pollinator Mutualism
Spiders that live on flowers can interfere in the dynamics of plant communities when they mediate the balance between pollination (e.g., by preying on or repelling pollinator insects) and herbivory by insects. Louda (1982b) was the first to investigate the negative effects of spiders on mutualistic relationships between plants and pollinators. Louda reported that the spider P. viridans (Oxyopidae), which lives in Haplopappus venetus (Asteraceae) in California (United States), was responsible for a significant reduction in the number of pollinated flowers and in the average fertility rate of the flowering branches.
Spiders can have strong negative effects on pollinator behavior and plant fitness (Dukas 2001; Dukas and Morse 2003; Suttle 2003; Heiling and Herberstein 2004). For example, Gonçalves-Souza et al. (2008) showed that the presence of artificial spiders designed to mimic species of the Thomisidae family interferes in the visitation behaviors of several species of pollinators of the plant Rubus rosifolius (Rosaceae), especially Hymenoptera (bees). Plants containing spider models produced 42% fewer seeds and the biomass of their fruits was reduced by approximately 50%.
Dukas and Morse (2003) showed that in Maine (United States), the bumblebee Bombus ternarius (Hymenoptera) visited Asclepias syriaca (Apocynaceae) less frequently when the plant contained Thomisidae spiders. The honeybee Apis mellifera showed a similar trend in behavior, although it was not significant. This decrease in visitation rate by pollinators can be explained both by direct effects (e.g., predation of pollinators by spiders) and indirect effects (e.g., avoidance of plants containing spiders by pollinators). Robertson and Maguire (2005) also showed a reduction in insect visitation of flowers of the plant Lepidium papilliferum L. (Brassicaceae), which housed the crab spider Misumena vatia. Flower visitors increased significantly after the spiders were removed.
In a later experiment, Dukas and Morse (2005) tested whether plants with crab spiders had fewer bee visitors than the plants without spiders, and verified whether the pollinia removal rate (indicating male fitness) and seed production rate (indicating female fitness) were lower in plants containing spiders than in those without spiders. In contrast with their earlier findings (Dukas and Morse 2003), the researchers found that the presence of spiders had no effect on the visitation of two species of bumblebee, B. ternarius and Bombus vagans, but A. mellifera visited significantly fewer plants containing spiders. This difference may be related to the higher rate of honeybee predation by spiders (as the honeybees are smaller and easier to capture) compared with bumblebee predation by spiders. Male and female plant fitness were not affected by the presence of M. vatia spiders in A. syriaca. Dukas and Morse (2005) hypothesized that the lack of an effect on plant fitness may be due to the low predation rate by spiders; in addition, spiders consume their prey slowly and other insects have a lengthy opportunity to visit and pollinate the plant while this is occurring.
These studies showed strong evidence that the presence of sit-and-wait spiders on flowers can negatively affect plant–pollinator mutualism. However, the components of this system and the mechanisms that affect it require clarification. Can the top-down effects of these spiders cascade to affect plant fitness, or are effects on fitness derived from TMIIs or density-mediated indirect interactions (DMIIs)? If the latter is true, there are probably adaptations related to traits of predators (e.g., foraging mode and morphology) and/or visual components that enable pollinators to recognize and avoid predation.
Gonçalves-Souza et al. (2008) tested these hypotheses through a series of experiments in the Atlantic Forest of southeastern Brazil. Artificial spiders were placed on flowers of the plant R. rosifolius (Rosaceae) (see details in Gonçalves-Souza et al. 2008), and randomized block experiments were conducted to test the effects of predator presence on pollinators and the power of the TMIIs over components of plant fitness (e.g., individual seed set and fruit biomass). The results showed that, in fact, some floral-visiting insects (e.g., Hymenoptera) can use visual cues to evaluate and avoid flowers containing objects that are similar to spiders or that mimic different morphological traits of spiders (e.g., abdomen and front legs). Thus, morphological traits, but not coloration, are responsible for the avoidance shown by insects. In addition, plants containing artificial spiders showed a considerable reduction in fitness, producing only about half of the individual seed set and fruit biomass. These findings showed that a reduction in the plant fitness is due to TMII related to the presence of spiders on flowers. Subsequently, Brechbühl et al. (2010) reported that different types of pollinators react differently to the presence of spiders (only solitary bees and syrphid flies avoided plants with spiders) and that these effects may also differ between plant species. They hypothesized that top-down effects of predators on plants via pollination depend on the degree of specialization of pollinators and the strength of their tendency to avoid spiders.
Gonçalves-Souza et al. (2008) used conspicuous spider models, and the coloration of crab spiders apparently had no effect on TMII mediated by the spiders. However, some species of Thomisidae, such as M. vatia, have similar coloration to the flowers of their hosts. Through two complementary studies, Ings and Chittka (2008, 2009) showed that this cryptic coloration can increase the TMII of bees in this system. In their 2009 study, these researchers exposed bees to predation risk experiences by placing cryptic robotic crab spiders on yellow flowers. After being exposed to the spiders, the bees were released and avoided yellow flowers even if they lacked spiders. Thus, it was demonstrated that when spider cryptic coloration causes avoidance by bees, it can negatively affect the reproductive success of plants containing cryptic spiders.
Some species of Thomisidae spiders, which appear cryptic from the human point of view, reflect ultraviolet wavelengths of light and thus attract their prey (Heiling et al. 2003, 2005a, b, Herberstein et al. 2009, see detailed review in Théry et al. 2011, see also Welti et al. 2016). Therefore, it is necessary to evaluate the function of the spiders’ coloration from the point of view of their prey. Llandres and Rodríguez-Gironés (2011) conducted a study in Queensland, Australia that studied the response of A. mellifera to the presence of T. spectabilis spiders (which have chromatic white and yellow polymorphism) in inflorescences of the plant Bidens alba (Asteraceae). The authors used spectrophotometry to collect the data of reflectance from the spiders and inflorescences to determine how they are perceived by A. mellifera. Subsequently, they conducted a series of experiments to determine which traits of spiders (e.g., size, cryptic coloration, UV reflectance, and movement) result in higher rates of avoidance by bees. Unlike the results reported by Ings and Chittka (2009), the cryptic coloration did not play a strong role in avoidance behavior. However, spider size, movement, and UV reflectance did affect TMII by the spiders.
Arango et al. (2012) studied the system composed by the plant Cnidoscolus multilobus (Euphorbiaceae), its floral visitors, and the predatory spider P. viridans (Oxyopidae). The researchers evaluated the effect of spider presence on the plant on seed production during the whole year and showed that spiders may indirectly reduce the fitness (i.e., number of seeds) of plants, especially in months with few floral visitors.
However, according to Ribas and Raizer (2013), when spiders are rare on the plant and/or pollinators are very abundant, these predators have very small effects on the fitness of the plant, either low or neutral, and their presence does not significantly affect the production of seeds. Through two meta-analyses, Romero et al. (2011) and Romero and Koricheva (2011) synthesized the available literature regarding the risk effects of predation on the behavior of pollinators and the cascade effects of spiders on the fitness of plants, respectively. Romero et al. (2011) showed that different methods of foraging by spiders (e.g., sit-and-wait predation vs. active hunting) both caused avoidance behavior in pollinators. Furthermore, the effect of repelling pollinators was stronger in pollinators of smaller size. Romero and Koricheva (2011) reported that even though some studies show that spiders negatively affect pollination and the quantity of plant seeds, this has little effect on the global fitness of the plant. It is important to note that spiders can cause simultaneous positive and negative effects on plant fitness, and these effects are complementary and not mutually exclusive. Recent advances in our knowledge of these interactions have opened new perspectives for understanding the mechanisms of co-evolution in plant–pollinator–predator tri-trophic systems.
Negative Effects of Spider–Floral Herbivore Interactions on Plants
The plant–pollinator system can be affected by other aspects of the trophic chain, such as herbivore–predator interactions. These interactions can have direct effects (e.g., plant damage) or indirect effects (e.g., interruptions in pollination). Herbivory, specifically florivory, may influence plant breeding and plant population growth (Louda 1983; Marquis 1984). Florivory can directly reduce plant fitness by destroying reproductive tissues such as petals and sepals, which attract pollinators (Cardel and Koptur 2010; Botto-Mahan et al. 2011). The damage to these tissues can change the appearance of flowers and inflorescences, preventing pollinator visits (Møller and Sorci 1998). Predators on flowers can also cause indirect effects by reducing pollinator visits and time spent pollinating flowers (Romero et al. 2011), affecting the fitness of the plant. However, hardly any studies have evaluated the combined risk effect of floral herbivory and predation on the behavior of pollinators and the reproductive success of plants.
Antiqueira and Romero (2016) manipulated the floral symmetry and the presence of predators (artificial Thomisidae spiders) on flowers on the shrub R. rosifolius (Rosaceae) to evaluate the effect of these factors and the additive or interactive effects on the visitation of pollinators and the reproductive success of the plant. Their study randomly assigned flowers on 112 plants to the following groups: addition of artificial spider, manipulation of flower to produce asymmetry and addition of spider, asymmetry without spider, and control (no treatment). The artificial spiders simulated a thomisid that usually occurs in the flowers of R. rosifolius. Both asymmetry and the presence of a predator reduced the number of visits from pollinators (mostly Hymenoptera). The effects were additive, rather than interacting. Interestingly, the risk effect of predation was 62% greater than the effect of flower asymmetry on the avoidance behavior of pollinators. In addition, only the risk of predation significantly decreased the biomass of the fruits (by 33%) and the number of seeds (by 28%). It appears that although the asymmetry caused by herbivory can alter the quality of resources, this effect does not carry the same evolutionary pressure as do interactions between predators and prey.
Positive Effects of the Presence of Spiders on Plants
Several studies have reported the positive effects of spiders on plants (Louda 1982b; Carter and Rypstra 1995; Ruhren and Handel 1999; Whitney 2004; Romero and Vasconcellos-Neto 2011) due to their predation on herbivores. Spiders can affect herbivory even if they do not consume herbivores directly, which can have important implications for biological control programs. Signs of their presence, such as draglines, feces, or chemotactile cues, can alter the foraging behavior of insect herbivores and thereby reduce the damage to plants. Several studies have tested this hypothesis experimentally. Pest insects of soybean leaves reduced their foraging activity in the presence of spiders or spider cues (e.g., silk draglines and feces) under laboratory conditions (Hlivkro and Rypstra 2003). In another study, Rypstra and Buddle (2012) treated entire plants in the field with silkworm or spider silk, and compared the amount of herbivory they experienced. Herbivory was lowest in plants that received spider silk treatments, intermediate in plants treated with silkworm silk, and highest in control plants (which received no treatment). These results suggest that silk might be a mechanism for trait-mediated impacts of spiders and might be used in integrated pest management programs.
Bucher et al. (2015) also performed a field experiment to determine the extent of spiders’ effects that are distinct from herbivore consumption, by enclosing Urtica dioica plants and removing all arthropods from them, then repeatedly placing Pisaura mirabilis spiders on them so that they could deposit cues. Control plants were enclosed in the same way, but did not have spiders. After cue deposition, the enclosures were removed to allow arthropods to colonize the plants and feed on them. The presence of chemotactile spider cues reduced leaf damage by 50% and also led to changes in the arthropod community. Smaller spiders avoided plants with spider cues. In contrast, the aphid-tending ant Myrmica rubra showed higher recruitment of workers on cue-bearing plants, possibly because the presence of more ant workers could protect aphids.
Work by Romero and Koricheva (2011) also supported the prediction that the strength and direction of terrestrial trophic cascades are strongly influenced by the relative effects of carnivores on pollinators vs herbivores, predator hunting mode, carnivore habitat domain and taxonomy, and presence and type of plant attractors. The net positive effect of carnivores on plant fitness suggests that carnivore effects on herbivores were stronger than their effects on pollinators.
Multitrophic Interactions and Mutualism
Although spiders are often involved in complex food webs or in direct or indirect interactions with other arthropods and plants (review in Romero and Vasconcellos-Neto 2007a), few studies have shown evidence of mutualism between plants and spiders (Louda 1982b; Ruhren and Handel 1999; Whitney 2004; Romero and Vasconcellos-Neto 2004a; Romero et al. 2008a; Morais-Filho and Romero 2010). Spider–plant mutualistic interactions fall into two categories: defensive/protective, in which spiders increase the fitness of plants by removing phytophagous insects, and digestive, in which spiders contribute to the nutrition of their host plants.
Protective Mutualism and Glandular Trichomes
Protective mutualism occurs when a symbiont reduces the negative effects of another symbiont or of a natural enemy (e.g., a predator) in a common host (Golubski and Abrams 2011). According to Krimmel and Pearse (2012), plants that produce sticky substances are common and often entrap and kill small insects, which can increase predator densities and potentially affect the plants’ indirect defenses. The common tarweed (Madia elegans, Asteraceae) is an annual flowering plant that produces abundant glandular trichomes. Common predators on tarweed include the assassin bug Pselliopus spinicollis, the two stilt bugs Hoplinus echinatus and Jalysus wickhami, the green lynx spider Peucetia sp., and the crab spider Mecaphesa schlingeri. The researchers manipulated the abundance of insects’ carrion entrapped on tarweed plants under natural field conditions, and found that carrion augmentation increased the abundance of a set of predators, decreased herbivory, and increased plant fitness. The carrion of entrapped insects may function broadly as food provided by the plant for predators.
Mutualism between spiders and plants with glandular trichomes was first investigated by Louda (1982b), who studied the interaction between P. viridans and the plant H. venetus (Asteraceae). The presence of the spider on the plant inflorescences reduced the number of fertilized ovules, indicating that its occurrence can harm the plant by disrupting plant–pollinator interactions. However, the presence of the spider also reduced the number of dry fruits (achenes) damaged by endophagous insects of the capitula, compared with inflorescences that did not have spiders. According to Louda (1982b), the benefits to the plant outweighed the costs. However, Romero and Koricheva (2011) used a meta-analysis metrics (log response ratio) which concluded that the positive and negative effects were similar in magnitude.
To test the effects of the spiders P. flava and P. rubrolineata on T. adenantha, a plant with glandular trichomes, Romero et al. (2008a) developed field experiments that compared plants with and without spiders. The plants without spiders showed a higher abundance of insects that are harmful to plants, such as leafhoppers, Lepidoptera larvae, and endophagous insects that feed on seeds. Plants with spiders experienced less damage from most of these insects and from leafminers. Moreover, seed damage by Geometridae sp. (Lepidoptera) larvae, a sessile insect, was 16 times higher in plants that lacked the presence of Peucetia. The most common species of endophagous insects were Melanagromyza spp. (Diptera, Agromyzidae) and Trupanea sp. (Diptera, Tephritidae). Spiders decreased the damage caused by Trupanea but did not affect Melanagromyza. Romero et al. (2008a) attributed these results to the different behavior of these two flies. Whereas Trupanea adult females remain on the plant for a long time to lay eggs (~30 min) and travel relatively long distances among the leaves to perform oviposition (18.8 cm on average), Melanagromyza females laid their eggs faster (~16 min) and moved much less on the plant (2.9 cm on average). Therefore, it is likely that Trupanea is more vulnerable to Peucetia spiders than Melanagromyza. In contrast with the data obtained by Louda (1982b), the two species of Peucetia studied by Romero et al. (2008a) tended (p = 0.067) to decrease the fitness of the plant T. adenantha via the effect on pollinators (Fig. 7.7).
Another species of spider (M. argenteus, Thomisidae) lives on the same plant, and also captures insect herbivores and floral visitors. Romero and Vasconcellos-Neto (2004a) tested whether these crab spiders increase or decrease plant fitness, and obtained results similar to those obtained by Romero et al. (2008a). For example, while the presence of Misumenops decreased seed damage caused by Geometridae, Trupanea and Melanagromyza were not affected. Herbivore vulnerability, as discussed above, may be valid here as well. Therefore, the spiders P. flava, P. rubrolineata, and M. argenteus all affected communities of phytophagous insects similarly. Furthermore, the trophic cascade of these predators affecting plant fitness was similar.
Interestingly, in the study that evaluated the effects of M. argenteus via pollinators, no decrease in the number of fertilized ovules was found in the capitula of the plants that were not previously damaged by phytophages. Yet fertilized ovules on damaged capitula were more frequent in plants with spiders. How could this happen? When the spiders forage on the capitula buds, they capture the endophagous insects that usually cause damage to the eggs. Therefore, the presence of spiders reduces the floral damage, resulting in capitula with more flowers, which are more attractive to flower visitors. In the absence of spiders, plants had many damaged capitula that were less attractive to pollinators. Although the spiders feed on floral visitors, capitula with flowers are widely spaced and spiders cannot forage on all of them at the same time (Romero and Vasconcellos-Neto 2003). Spiders may remain on one of the capitula, leaving the others free from predators. Thus, the spiders exert a dual beneficial effect on the plants in this system: they reduce herbivores in the capitula attacked by endophages and help attract pollinators.
The spider P. flava also occurs on R. dichotoma (Melastomataceae), a plant with glandular trichomes, in the northwest of São Paulo state (Brazil). Through field experiments, Morais-Filho and Romero (2010) showed a decline of the abundance of several guilds of insects in plants with spiders. To test whether spiders decrease leaf damage, the authors compared plants with and without spiders during different seasons. They found that the spiders do not affect leaf herbivory rates during the rainy season. In contrast, their presence reduced leaf damage by herbivores by 74% during the post-rain period. In this system, the role of spiders as bodyguards was temporally conditional. During the rainy season, the plants invest in growth by producing a huge amount of vegetative biomass, a phenomenon triggered by bottom-up forces (i.e., by the presence of rain and addition of nutrients in the system). This vegetative productivity supports a great quantity of herbivores, which exceed the capacity of the leaves, affecting the top-down effects of spiders on leaf herbivory. In contrast, during the post-rain period the plants do not grow, investing instead in reproductive tissues (inflorescences). This allows the spiders and herbivores to remain exposed on the leaves for a longer time, possibly strengthening the top-down effects of spiders in this system (Morais-Filho and Romero 2010). The presence of P. flava decreased the number of damaged flower buds, increasing plant fitness via herbivory. However, plants with and without spiders produced a similar number of seeds per fruit, indicating that spiders do not negatively affect the plants’ fitness via pollinator inhibition.
It is intriguing that the effect of Peucetia spiders is strongly negative in some plants (Louda 1982b), less negative in other systems (Romero et al. 2008a), and exclusively positive in others (Morais-Filho and Romero 2010). The system studied by Louda (1982a, b) attracts many small pollinators that are appropriate prey for spiders. In addition, the capitula of H. venetus are very close to each other, forming a flat platform where the spiders forage. This type of architecture favors the capture of pollinators by spiders. In contrast, in the system studied by Romero et al. (2008a), the T. adenantha capitula are more spaced and spiders could not forage on all of them, although several Peucetia spiders can group the capitula and unite them with silk threads to forage. The plants studied by Morais-Filho and Romero (2010) present bigger flowers in great quantities and are spaced apart, attracting larger pollinators (Bombus spp., Hesperiidae butterflies) whose capture is difficult for the spiders. Therefore, apparently the architecture of flowers or inflorescences and the type of floral visitors affect the direction and intensity of trophic cascades via pollinators.
All the studies cited in this section involve sticky plants with glandular trichomes that entrap and kill small insects. These trichomes may provide an important pathway in the evolution of relationships between the Peucetia genus and these plants, helping to develop protective mutualism between spiders and plants.
Digestive Mutualism
Plants are exposed to selective pressure from insect herbivores, and have developed several defense mechanisms: intrinsic (chemical or mechanical), and extrinsic (including the protection of predators and parasitoids) (e.g., Lawton and McNeil 1979; Price et al. 1980; Crawley 1989; Fritz and Simms 1992; Coley and Barone 1996; Marquis and Whelan 1996; Lucas et al. 2000; Del-Claro and Torezan-Silingardi 2011; Marquis 2011). Mechanical defenses include the hardness of leaves, the presence of thorns, and uncinate and glandular trichomes that can trap insects (Levin 1973; Johnson 1975; Fernandes 1994; Fordyce and Agrawal 2001; Medeiros et al. 2004; Medeiros and Boligon 2007; Cardoso 2008).
A limiting factor for plants is the availability of nutrients, especially in poor soils. Although some plants can trap insects on the surfaces of leaves and stems, they are not necessarily able to absorb the nutrients from their decomposition (Anderson et al. 2012). Carnivory in plants seems to be an efficient way to obtain nutrients, particularly during adverse environmental conditions (Adamec 1997). This strategy arose independently in more than 600 species of plants and at least six different subclasses of angiosperms around the world (Albert et al. 1992; Ellison and Gotelli 2001). According to Givnish et al. (1984), carnivorous plants are defined as plants that are able to absorb nutrients from dead animals next to their surfaces and that possess morphological, physiological, or behavioral features whose primary effect is attraction, capture, or digestion of prey. Givnish et al. note that “plants capable of absorbing nutrients from dead animals, but which lack active means of prey attraction and prey digestion, and possess neither motile traps nor passive structures such as one-way passages whose primary result is immobilization of animals near plant surfaces must be considered saprophytes and not carnivorous plants.” Chase et al. (2009) expanded this definition to include the ability to absorb the products of decomposition from organic matter by any tissue. According to this definition, it does not matter whether the decomposition is performed by the individual or whether the plant relies on species-specific mutualism to perform the decomposition (e.g., putrefactive bacteria).
If plants have some way to absorb the feces of the animals living in association with them, they can benefit nutritionally. These additional nutrients may allow plants to store energy reserves and grow more (e.g., Romero et al. 2006). According to Anderson and Midgley (2003), digestive mutualism, in which animals provide nutrients for plants, may represent a step toward the evolution of carnivory in plants. Mutualism involving the provisioning of plants with nutrients by animals (i.e., digestive mutualism) was documented in ant–plant systems, Heteroptera predators (Pameridea spp., Miridae) and plants, amphibians and bromeliads (Romero et al. 2010), and carnivorous plants (Roridula spp.) (Anderson and Midgley 2002, 2003). Only recently has this kind of mutualism been demonstrated in spider–plant associations.
Romero et al. (2006) were the first to show that spiders contribute to the nutrition of Bromeliaceae. These plants’ leaves contain structures that are specialized to absorb water and nutrients (especially nitrogen). The researchers used isotopic techniques (stable isotope 15N) to verify that the spider P. chapoda nourishes the bromeliad B. balansae with its feces and prey carrion. To enrich the feces of spiders with 15N isotope, the authors first enriched yeast with a salt (ammonium sulfate) that had previously been enriched with nitrogen isotope. Then they mixed the yeast in a culture medium to feed D. melanogaster, which became enriched after consuming the yeast. After spiders consumed the flies, the spiders produced enriched feces. The feces and enriched flies were placed in the center of the rosette of B. balansae plants, whose leaves were then analyzed isotopically. The results showed that 15% of the total nitrogen of plants was derived from the spiders, and only 3% of the plants’ nitrogen came from the flies. In another experiment, the authors kept plants with and without spiders for over a year, and showed that those containing spiders grew 15% more than did plants without spiders. As these bromeliads live in regions where the soil is very poor (e.g., cerrado vegetation), an association with spiders can allow the plants to grow faster.
The intensity of the digestive mutualism in this system can vary depending on the density of spiders in different areas. The isotopic nitrogen 15N is 2–4‰ more positive at each trophic level. Therefore, plants inhabited by many spiders can absorb more nitrogen from these predators and present higher values of 15N. In addition, since P. chapoda prefers bromeliads living in open areas, presumably such bromeliads derive more nitrogen from spiders than do bromeliads in the interior of the forest. These hypotheses were confirmed by Romero et al. (2008b), using forest fragments with varying numbers of spiders on bromeliads. There was a positive relationship between the density of spiders and the isotopic values of bromeliads. In addition, the nitrogen derived from animals was much higher in the bromeliads in open areas than in bromeliads in the forest interior. However, the bromeliads in the forest presented total nitrogen concentrations (14N + 15 N) similar to those of plants from open areas. The forest plants may have absorbed nutrients from dry leaves that fell from the trees (plant litter), which have lower isotopic values than those from spider feces (Romero et al. 2008b). These results suggest that bromeliads in the forest derive more of their nutrition from leaf litter, while those in open areas derive more from spider feces.
In another study, Gonçalves et al. (2011) evaluated the role of feces from the spider P. chapoda and from D. melanogaster flies on the nutrition and growth of the host bromeliads B. balansae, Ananas comosus (pineapple), and A. distichantha, as well as the seasonal variation in the importance of this digestive mutualism. The researchers performed isotopic and physiological analyses using the isotope 15N. Spiders contributed from 0.6% (dry season) to 2.7% (wet season) of the total nitrogen in B. balansae, 2.4% (dry) to 4.1% (wet) of the total in A. comosus, and 3.8% (dry) to 5% (wet) of the total in A. distichantha. Flies did not contribute to the nutrition of these bromeliads. Chlorophyll and carotenoid concentrations did not differ among treatments. Plants that received feces had higher soluble protein concentrations and showed leaf growth only during the wet season. These results indicate that the mutualism between spiders and bromeliads is seasonally restricted. Interspecific variation in nutrient uptake occurred, probably related to the performance of each species and to photosynthetic pathways. Whereas B. balansae seems to use nitrogen for growth, A. distichantha apparently stores nitrogen to balance out stressful nutritional conditions.
In southeastern Brazil (Serra do Cipó - Minas Gerais), Alpaida quadrilorata (Araneidae) inhabits almost exclusively Paepalanthus bromelioides (Eriocaulaceae), a plant with rosette-shaped leaves that has similar architecture to bromeliads. The spiders build their webs above the central tank of the plant and, when disturbed, weave a guide wire and dive into the liquid accumulated inside the rosette, using this strategy as defensive behavior against their predators (Figueira and Vasconcellos-Neto 1991; Vasconcellos-Neto et al. unpublished data). Few studies have demonstrated that specific associations of spiders with plants provide the spiders with protection from predators. P. bromelioides leaves are associated with multiple partners, such as spiders and termites, and the plant is considered a protocarnivorous species (Figueira et al. 1994). Nishi et al. (2013) used analysis of 15N to show that the isotopic signature of P. bromelioides is similar to that of carnivorous plants, and is higher than that of the non-carnivorous plants in the study area. They showed that the presence of spiders on the rosettes of P. bromelioides resulted in overall nitrogen contributions of 26.5% (a top-down flux) due to spider feces and prey carrion. Although nitrogen flux was not detected from termites to plants via decomposition of labeled cardboard, the data on 15N in natural nitrogen abundance indicated that 67% of nitrogen from P. bromelioides is derived from termites (a bottom-up flux). Bacteria did not affect nutrient cycling or nitrogen uptake from spider feces and prey carrion. The results suggest that the nitrogen used by P. bromelioides derives from associated predators and termites, despite differences in the rate of nitrogen cycling, which was higher in nitrogen derived from predators (leaves) than in nitrogen derived from termites (roots). This is the first study that demonstrates partitioning effects from multiple partners in a digestion-based mutualistic system. Although most of its nitrogen is absorbed through the roots (via termites), P. bromelioides has all the attributes of a carnivorous plant in the context of digestive mutualism. All these studies reinforce the beneficial role played by spiders in digestive mutualism.
Concluding Remarks
Spider families that actively hunt on vegetation were long thought to be wandering through the plant rather than specifically associated with it. Specific associations and adaptations, and examples of mutualism involving spiders and plants, were not known. Researchers reported that spiders which hunt by ambush (e.g., Thomisidae) chose specific substrates according to the optimal foraging theory (i.e., prey availability).
Recent evidence, however, suggests that the physical structure of the habitat may be a more important factor for spider communities, and that microhabitat selection is mostly influenced by plant architecture per se, not by microclimatic factors or prey availability. Although many spider families live on vegetation, it remains true that very few specific spider–plant associations are known, and it is not known which plant traits attract and facilitate spider populations.
Here, we have reported specific associations between spiders and certain plant species or plants that share similar traits, such as glandular trichomes (Vasconcellos-Neto et al. 2007), spines (Hormiga and Scharff 2014), rosette shape (Romero 2006), tree bark (Messas et al. 2014), and dry structures (Souza et al. 2015). Specific plant traits, such as rosette shape and presence of glandular trichomes, can mediate spider–plant mutualism in which spiders contribute to plant nutrition and growth or protect plants against herbivores. Very little is known about how associations between spiders and plants evolve toward mutualism. Most of the associations reported are occasional, and to achieve a better understanding of their evolution, it is necessary to investigate them while considering spatio-temporal variations.
As the order Araneae presents great diversity on vegetation and a variety of behaviors and lifestyles, we believe that many other specific associations and examples of spider–plant mutualism are waiting to be reported.
References
Abrams P, Menge BA, Mittelbach GG, Spiller D, Yodzis P (1996) The role of indirect effects in food webs. In: Polis GA, Winemiller KO (eds) Food webs: integration of patterns and dynamics. Chapman and Hall, New York
Adamec L (1997) Mineral nutrition of carnivorous plants: a review. Bot Rev 63:273–299
Albert VA, Williams SE, Chase MW (1992) Carnivorous plants: phylogeny and structural evolution. Science 257:1491–1495
Anderson B, Midgley JJ (2002) It takes two to tango but three is a tangle: mutualists and cheaters on the carnivorous plant Roridula. Oecologia 132:369–373
Anderson B, Midgley JJ (2003) Digestive mutualism, an alternate pathway in plant carnivory. Oikos 102:221–224
Anderson B, Kawakita A, Tayasu I (2012) Sticky plant captures prey for symbiotic bug: is this digestive mutualism? Plant Biol 14:888–893
Antiqueira PAP, Romero GQ (2016) Floral asymmetry and predation risk modify pollinator behavior, but only predation risk decreases plant fitness. Oecologia 181:475–485
Arango AM, Rico-Gray V, Parra-Tabla V (2000) Population structure, seasonality, and habitat use by the green lynx spider Peucetia viridans (Oxiopidae) inhabiting Cnidoscolus aconitifolius (Euphorbiaceae). J Arachnol 28:185–194
Arango AM, Portillo JL, Parra-Tabla V, Salazar LTH, Mávil JEM, Rico-Gray V (2012) Effect of the spider Peucetia viridans (Oxyopidae) on floral visitors and seed set of Cnidoscolus multilobus (Euphorbiaceae). Acta Bot Mex 100:1–14
Araújo VA, Melo SK, Araújo APA, Gomes MLM, Carneiro MAA (2007) Relationship between invertebrate fauna and bromeliad size. Braz J Biol 67:611–617
Askenmo C, von Bromssen A, Ekman J, Jansson C (1977) Impact of some wintering birds on spider abundance in spruce. Oikos 28:90–94
Balogh J, Loksa J (1956) Untersuchungen über die Zoozönose des Luzernefeldes. Acta Zool Acad Sci Hung 2:17–114
Barth FG, Seyfarth EA (1979) Cupiennius salei Keyserling (Araneae) in the highlands of central Guatemala. J Arachnol 7:255–263
Barth FG, Seyfarth EA, Bleckmann H, Schüch W (1988a) Spiders of the genus Cupiennius Simon 1891 (Araneae, Ctenidae). I. Range distribution, dwelling plants, and climatic characteristics of the habitats. Oecologia 77:187–193
Barth FG, Bleckmann H, Bohnenberger J, Seyfarth EA (1988b) Spiders of the genus Cupiennius Simon 1891 (Araneae, Ctenidae). II. On the vibratory environment of a wandering spider. Oecologia 77:194–201
Baurecht D, Barth FG (1992) Vibratory communication in spiders. I. Representation of male courtship signals by female vibration receptor. J Comp Physiol 171:231–243
Bell G (1985) On the function of flowers. P Roy Soc Lond B Bio 224:223–265
Bell AD (1991) Plant form: an illustrated guide to flowering plant morphology. Oxford University Press, New York
Bell AD, Roberts D, Smith A (1979) Branching patterns: the simulation of the plant architecture. J Theor Biol 81:351–375
Benzing DH (2000) Bromeliaceae: profile of an adaptive radiation. Cambridge University Press, Cambridge
Botto-Mahan C, Ramírez PA, Ossa CG, Medel R, Ojeda-Camacho M, González AV (2011) Floral herbivory affects female reproductive success and pollinator visitation in the perennial herb Alstroemerialigtu (Alstroemeriaceae). Int J Plant Sci 172:1130–1136
Brechbühl R, Kropf C, Bacher S (2010) Impact of flower-dwelling crab spiders on plant–pollinator mutualisms. Basic Appl Ecol 11:76–82
Brescovit AD (1993) Thaloe e Bromelina, novos gêneros de aranhas neotropicais da família Anyphaenidae (Arachnida, Araneae). Rev Bras Entomol 37:693–703
Brescovit AD, do Oliveira MEES (1994) Rhoicinus urucu, uma espécie nova de Rhoicininae para a Região Amazônica (Araneae, Trechaleidae). Biociencias 2:63–69
Brose U (2003) Bottom-up control of carabid beetle communities in early successional wetlands: mediated by vegetation structure or plant diversity? Oecologia 135:407–413
Brown KM (1981) Foraging ecology and niche partitioning in orb-weaving spiders. Oecologia 50:380–385
Brown JH (2014) Why are there so many species in the tropics? J Biogeogr 41:8–22
Bucher R, Menzel F, Entling MH (2015) Risk of spider predation alters food web structure and reduces local herbivory in the field. Oecologia 178:571–577
Cardel YJ, Koptur S (2010) Effects of florivory on the pollination of flowers: an experimental field study with a perennial plant. Int J Plant Sci 171:283–292
Cardoso MZ (2008) Herbivore handling of a plant’s trichome: the case of Heliconius charithonia (L.) (Lepidoptera: Nymphalidae) and Passiflora lobata (Killip) Hutch. (Passifloraceae). Neotrop Entomol 37:247–252
Cardoso P, Pekár S, Jocqué R, Coddington JA (2011) Global patterns of guild composition and functional diversity of spiders. PLoS One 6(6):e21710
Carrel JE, Burgess HK, Shoemaker DM (2000) A test of pollen feeding by a linyphiid spider. J Arachnol 28:243–244
Carter PE, Rypstra AL (1995) Top-down effects in soybean agroecosystems: spider density affects herbivore damage. Oikos 72:433–439
Chase MW, Christenhusz MJM, Sanders D, Fay MF (2009) Murderous plants: Victorian Gothic, Darwin and modern insights into vegetable carnivory. Bot J Linn Soc 161:329–356
Coddington JA, Levi HW (1991) Systematics and evolution of spiders (Araneae). Ann Rev Ecol Syst 22:565–592
Cohen D, Shmida A (1993) The evolution of flower display and reward. Evol Biol 27:197–243
Coley PD, Barone JA (1996) Herbivory and plant defenses in tropical forests. Ann Rev Ecol Syst 27:305–335
Corcuera P, Valverde PL, Jiménez-Salinas E, Vite F, López-Ortega G, Pérez-Hernández MA (2010) Distribution of Peucetia viridans (Araneae: Oxyopidae) on Croton ciliatoglandulifer. Environ Entomol 39:320–327
Cotgrave P, Hill MJ, Middleton JAG (1993) The relationship between body size and population size in tank bromeliad fauna. Biol J Linn Soc 49:367–380
Cramer MJ, Willig MR (2002) Habitat heterogeneity, habitat associations, and rodent species diversity in a sand–shinnery-oak landscape. J Mammal 83:743–753
Crawley MJ (1989) Insect herbivores and plant population dynamics. Annu Rev Entomol 34:531–564
Dafni A, Lehrer M, Kevan PG (1997) Spatial flower parameters and insect spatial vision. Biol Rev 72:239–282
Dalingwater JE (1987) Chelicerate cuticle structure. In: Nentwig W (ed) Ecophysiology of spiders. Springer, Berlin
de Piccoli GCO (2011) História natural da aranha Corinna sp. nov. (Corinnidae): interações com bromélias e comportamento de submersão de fitotelmatas. Master thesis, Universidade Estadual Paulista, Instituto de Biociências, Letras e Ciências Exatas. São José do Rio Preto – SP
Del-Claro K, Torezan-Silingardi HM (2011) Ecologia das interações plantas–animais: uma abordagem ecológico-evolutiva. Technical Books editora, Rio de Janeiro
Dennis P, Young MR, Gordon IJ (1998) Distribution and abundance of small insects and arachnids in relation to structural heterogeneity of grazed, indigenous grasslands. Ecol Entomol 23:253–264
Denno RF, Finke DL, Langellotto GA (2005) Direct and indirect effects of vegetation structure and habitat complexity on predator–prey and predator–predator interactions. In: Barbosa P, Castellanos I (eds) Ecology of predator-prey interactions. Oxford University Press, Oxford
Dias SC, Brescovit AD (2003) Notes on the behavior of Pachistopelma rufonigrum Pocock (Araneae, Theraphosidae, Aviculariinae). Rev Bras Zool 20:13–17
Dias SC, Brescovit AD (2004) Microhabitat selection and co-occurrence of Pachistopelma rufonigrum Pocock (Araneae, Theraphosidae) and Nothroctenus fuxico sp. nov. (Araneae, Ctenidae) in tank bromeliads from Serra de Itabaiana, Sergipe, Brazil. Rev Bras Zool 21:789–796
Dias SC, Brescovit AD, Santos LT, Couto ECG (2000) Aranhas em bromélias de duas restingas do estado de Sergipe, Brasil. Biol Geral Exp 1:22–24
Dias SC, Carvalho LS, Bonaldo AB, Brescovit AD (2010) Refining the establishment of guilds in Neotropical spiders (Arachnida: Araneae). J Nat Hist 44:219–239
Diniz S (2011) Influência da complexidade arquitetural de ramos vegetativos na diversidade e abundância de aranhas e outros artrópodes. Master thesis, Universidade Estadual de Campinas
Draney ML, Hegnet JA, Johnson AL, Porter BC, Justmann CK, Forsythe PS (2014) Microhabitat distribution of Drapetisca alteranda, a tree trunk specialist sheet web weaver (Araneae: Linyphiidae). J Arachnol 42:195–198
Duffey SS (1986) Plant glandular trichomes: their partial role in defence against insects. In: Juniper B, Southwood R (eds) Insects and the plant surface. Edward Arnold, London
Dukas R (2001) Effects of perceived danger on flower choice by bees. Ecol Lett 4:327–333
Dukas R, Morse DH (2003) Crab spiders affect patch visitation by bees. Oikos 101:157–163
Dukas R, Morse DH (2005) Crab spiders show mixed effects on flower-visiting bees and no effect on plant fitness components. Ecoscience 12:244–247
Eggs B, Sanders D (2013) Herbivory in spiders: the importance of pollen for orb-weavers. PLoS One 8(11):e82637
Ellis AG, Midgley JJ (1996) A new plant–animal mutualism involving a plant with sticky leaves and a resident hemipteran. Oecologia 106:478–481
Ellison AM, Gotelli NJ (2001) Evolutionary ecology of the carnivorous plants. Trends Ecol Evol 16:623–629
Evans TA (1997) Distribution of social crab spiders in eucalypt forests. Aust J Ecol 22:107–111
Fernandes GW (1994) Plant mechanical defenses against insect herbivory. Rev Bras Entomol 32:421–433
Figueira JEC, Vasconcellos-Neto J (1991) Paepalanthus, cupins e aranhas. Ciência Hoje 13:20–26
Figueira JEC, Vasconcellos-Neto J, Jolivet P (1994) Une nouvelle plante protocarnivore Paepalanthus bromelioides Silv. (Eriocaulaceae) du Brésil. Rev Écol 49:3–9
Foelix RF (2011) Biology of the spiders, 3rd edn. Oxford University Press, Inc, New York
Fordyce JA, Agrawal AG (2001) The role of plant trichomes and caterpillar group size on growth and defence of the pipevine swallowtail Battus philenor. J Anim Ecol 70:997–1005
Frank JH, Sreenivasan S, Benshoff PJ, Deyrup MA, Edwards GB, Halbert SE, Hamon AB, Lowman MD, Mockford EL, Scheffrahn RH, Steck GJ, Thomas MC, Walker TJ, Welbourn WC (2004) Invertebrate animals extracted from native Tillandsia (Bromeliales: Bromeliaceae) in Sarasota County, Florida. Fla Entomol 87:176–185
Fritz RS, Simms EL (1992) Plant resistance to herbivores and pathogens: ecology, evolution, and genetics. The University of Chicago Press, Chicago
Gastreich KR (1999) Trait-mediated indirect effects of a theridiid spider on an ant-plant mutualism. Ecology 80:1066–1070
Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD (1984) Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. Am Nat 124:479–497
Golubski AJ, Abrams PA (2011) Modifying modifiers: what happens when interspecific interactions interact? J Anim Ecol 80:1097–1108
Gonçalves AZ, Mercier H, Mazzafera P, Romero GQ (2011) Spider-fed bromeliads: seasonal and interspecific variation in plant performance. Ann Bot London 107:1047–1055
Gonçalves-Souza T, Omena PM, Souza JC, Romero GQ (2008) Trait-mediated effects on flowers: artificial spiders deceive pollinators and decrease plant fitness. Ecology 89:2407–2413
Gonçalves-Souza T, Brescovit AD, Rossa-Feres DC, Romero GQ (2010) Bromeliads as biodiversity amplifiers and habitat segregation of spider communities in a Neotropical rainforest. J Arachnol 38:270–279
Gonçalves-Souza T, Almeida-Neto M, Romero GQ (2011) Bromeliad architectural complexity and vertical distribution predict spider abundance and richness. Austral Ecol 36:476–484
Greco CF, Kevan PG (1994) Contrasting patch choosing by anthophilous ambush predators: vegetation and floral cues for decisions by a crab spider (Misumena vatia) and males and females of an ambush bug (Phymata americana). Can J Zool 72:1583–1588
Greenstone MH (1984) Determinants of web spider species diversity: vegetation structural diversity vs. prey availability. Oecologia 62:299–304
Gunnarsson B (1983) Winter mortality of spruce-living spiders: effect of spider interactions and bird predation. Oikos 40:226–233
Gunnarsson B (1988) Spruce-living spiders and forest decline; the importance of needle-loss. Biol Conserv 43:309–319
Gunnarsson B (1990) Vegetation structure and the abundance and size distribution of spruce-living spiders. J Anim Ecol 59:743–752
Gunnarsson B (1992) Fractal dimension of plant and body size distribution in spiders. Funct Ecol 6:636–641
Gunnarsson B (1996) Bird predation and vegetation structure affecting spruce-living arthropods in a temperate forest. J Anim Ecol 65:389–397
Halaj J, Ross DW, Moldenke AR (1998) Habitat structure and prey availability as predictors of the abundance and community organization of spiders in western Oregon forest canopies. J Arachnol 26:203–220
Halaj J, Ross DW, Moldenke AR (2000) Importance of habitat structure of the arthropod food-web in Douglas-fir canopies. Oikos 90:139–152
Harmer AMT (2009) Elongated orb-webs of Australian ladder-web spiders (Araneidae: Telaprocera) and the significance of orb-web elongation. J Ethol 27:453–460
Harmer AMT, Herberstein ME (2009) Taking it to extremes: what drives extreme web elongation in Australian ladder web spiders (Araneidae: Telaprocera maudae)? Anim Behav 78:499–504
Harmer AMT, Herberstein ME (2010) Functional diversity of ladder-webs: moth specialization or optimal area use? J Arachnol 38:119–122
Hatley CL, MacMahon JA (1980) Spider community organization: seasonal variation and the role of vegetation architecture. Environ Entomol 9:632–639
Hawkins CP, MacMahon JA (1989) Guilds: the multiple meanings of a concept. Annu Rev Entomol 34:423–451
Heiling AM, Herberstein ME (2004) Predator–prey coevolution: Australian native bees avoid their spider predators. P Roy Soc Lond B Bio 271:S196–S198
Heiling AM, Herberstein ME, Chittka L (2003) Crab spiders manipulate flower signals. Nature 421:334
Heiling AM, Cheng K, Herberstein ME (2004) Exploitation of floral signals by crab spiders (Thomisus spectabilis, Thomisidae). Behav Ecol 15:321–326
Heiling AM, Chittka L, Cheng K, Herberstein ME (2005a) Colouration in crab spiders: substrate choice and prey attraction. J Exp Biol 208:1785–1792
Heiling AM, Cheng K, Chittka L, Goeth A, Herberstein ME (2005b) The role of UV in crab spider signals: effects on perception by prey and predators. J Exp Biol 208:3925–3931
Heiling AM, Cheng K, Herberstein ME (2006) Picking the right spot: crab spiders position themselves on flowers to maximize prey attraction. Behaviour 143:957–968
Herberstein ME (1998) Implications of microhabitat selection on prey capture for the web spider Neriene radiata (Walckenaer) (Araneae: Linyphiidae). In: Selden PA (ed) P European Coll Arachnol, vol 17. Burnham Beeches, Bucks, UK, pp 197–202
Herberstein ME, Heiling AM, Cheng K (2009) Evidence for UV-based sensory exploitation in Australian but not European crab spiders. Evol Ecol 23:621–634
Hesselberg T, Triana E (2010) The web of the acacia orb-spider Eustala illicita (Araneae: Araneidae) with notes on its natural history. J Arachnol 38:21–26
Hlivkro JT, Rypstra AL (2003) Spiders reduce herbivory: nonlethal effects of spiders on the consumption of soybean leaves by beetle pests. Ann Entomol Soc Am 96:914–919
Höfer H, Brescovit AD (2001) Species and guild structure of a Neotropical spider assemblage (Araneae) from Reserva Ducke, Amazonas, Brazil. Andrias 15:99–119
Hormiga G, Scharff N (2014) The strange case of Laetesia raveni n. sp., a green linyphiid spider from Eastern Australia with a preference for thorny plants (Araneae, Linyphiidae). Zootaxa 3811:83–94
Horvath R, Lengyel S, Szinetar C, Jakab L (2005) The effect of prey availability on spider assemblages on European black pine (Pinus nigra) bark: spatial patterns and guild structure. Can J Zool 83:324–335
Ings TC, Chittka L (2008) Speed-accuracy tradeoffs and false alarms in bee responses to cryptic predators. Curr Biol 18:1520–1524
Ings TC, Chittka L (2009) Predator crypsis enhances behaviourally mediated indirect effects on plants by altering bumblebee foraging preferences. P Roy Soc Lond B Bio 276:2031–2036
Jackson RR, Pollard SD, Nelson XJ, Edwards GB, Barrion AT (2001) Jumping spiders (Araneae: Salticidae) that feed on nectar. J Zool 255:25–29
Janetos AC (1986) Web-site selection: are we asking the right questions? In: Shear WA (ed) Spiders: webs, behavior, and evolution. Stanford University Press, Stanford, pp 9–48
Jiménez-Salinas E, Corcuera P (2008) Inflorescences and plant selection by the green lynx spider Peucetia viridans (Hentz) in a dry forest of western Mexico. Rev Iber Aracnol 15:63–66
Johnson HB (1975) Plant pubescence: an ecological perspective. Bot Rev 41:233–258
Krell FT, Krämer F (1998) Chemical attraction of crab spiders (Araneae, Thomisidae) to a flower fragrance component. J Arachnol 26:117–119
Krimmel BA, Pearse IS (2012) Sticky plant traps insects to enhance indirect defence. Ecol Lett 2012:1–6. https://doi.org/10.1111/ele.12032
Küppers M (1989) Ecological significance of above-ground architectural pattern in woody plants: a question of cost–benefit relationships. Trends Ecol Evol 4:375–379
Langellotto GA, Denno RF (2004) Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis. Oecologia 139:1–10
Lawton JH (1983) Plant architecture and the diversity of phytophagous insects. Annu Rev Entomol 28:23–39
Lawton JH, McNeil S (1979) Between the devil and the deep blue sea: on the problem of being a herbivore. In: Anderson RM, Turner BD, Taylor LR (eds) Population dynamics. Blackwell, Oxford, pp 223–244
Levin DA (1973) The role of thichomes in plant defense. Q Rev Biol 48:3–15
Llandres AL, Rodríguez-Gironés MA (2011) Spider movement, UV reflectance and size, but not spider crypsis, affect the response of honeybees to Australian crab spiders. PLoS One 6(2):e17136
Louda SM (1982a) Limitation of the recruitment of the shrub Haplopappus squarrosus (Asteraceae) by flower- and seed-feeding insects. J Ecol 70:43–53
Louda SM (1982b) Inflorescence spiders: a cost/benefit analysis for the host plant, Haplopappus venetus Blake (Asteraceae). Oecologia 55:185–191
Louda SM (1983) Seed predation and seedling mortality in the recruitment of a shrub, Haplopappusvenetus (Asteraceae), along a climatic gradient. Ecology 64:511–521
Lubin Y, Kotzman M, Ellner S (1991) Ontogenetic and seasonal changes in webs and websites of a desert widow spider. J Arachnol 19:40–48
Lucas PW, Turner IM, Dominy NJ, Yamashita N (2000) Mechanical defences to herbivory. Ann Bot 86:913–920
Macarthur RH, Macarthur JW (1961) On bird species diversity. Ecology 42:594–598
Maddison WP (1996) Pelegrina Franganillo and other jumping spiders formerly placed in the genus Metaphidippus (Araneae: Salticidae). Bull Mus Comp Zool 154:215–368
Marquis RJ (1984) Leaf herbivores decrease fitness of a tropical plant. Science 226:537–539
Marquis RJ (2011) Uma abordagem geral das defesas das plantas contra a ação dos herbívoros. In: Del-Claro K, Torezan-Silingardi HM (eds) Ecologia das interações plantas–animais: uma abordagem ecológico-evolutiva. Technical Books editora, Rio de Janeiro
Marquis RJ, Whelan C (1996) Plant morphology and recruitment of the third trophic level: subtle and little recognized defenses? Oikos 75:330–334
Marshall S, Rypstra A (1999) Spider competition in structurally simple ecosystems. J Arachnol 27:343–351
McCoy ED, Bell SS (1991) Habitat structure: the evolution and diversifications of a complex topic. In: Bell SS, McCoy ED, Mushinsky HR (eds) Habitat structure: the physical arrangement of objects in space. Chapman and Hall, New York
Medeiros L, Boligon DS (2007) Adaptations of two specialist herbivores to movement on the hairy leaf surface of their host, Solanum guaraniticum Hassl (Solanaceae). Rev Bras Entomol 51:210–216
Medeiros L, Boligon DS, Moreira GRP (2004) Morphological and behavioral adaptations to movement on different leaf surfaces: studies with cassidine larvae. In: Jolivet PH, Santiago-Blay JA, Schmidt M (eds) New contributions to the biology of Chrysomelidae. SPB Academic Publishers, Kugler Publications, Amsterdam
Meehan CJ, Olson EJ, Reudink MW, Kyser TK, Curry RL (2009) Herbivory in a spider through exploitation of an ant–plant mutualism. Curr Biol 19:892–893
Messas YF, Souza HS, Gonzaga MO, Vasconcellos-Neto J (2014) Spatial distribution and substrate selection by the orb-weaver spider Eustala perfida Mello-Leitão, 1947 (Araneae: Araneidae). J Nat Hist 48:2645–2660
Mestre LAM, Aranha JMR, Esper MLP (2001) Macroinvertebrate fauna associated to the bromeliad Vriesea inflata of the Atlantic Forest (Paraná State, Southern Brazil). Braz Arch Biol Technol 44:89–94
Michel AK, Winter S (2009) Tree microhabitat structures as indicators of biodiversity in Douglas-fir forests of different stand ages and management histories in the Pacific Northwest, U.S.A. For Ecol Manag 257:1453–1464
Mohsin M, Sulehria AQK, Yousuf I, Ejaz M, Yousuf MJ, Hussain A (2010) Comparison of spider guilds found in various oilseed crops of Pakistan. Biologia (Pakistan) 56:69–76
Møller AP, Sorci G (1998) Insect preference for symmetrical artificial flowers. Oecologia 114:37–42
Morais-Filho JC, Romero GQ (2008) Microhabitat use by Peucetia flava (Oxyopidae) on the glandular plant Rhyncanthera dichotoma (Melastomataceae). J Arachnol 36:374–378
Morais-Filho JC, Romero GQ (2009) Natural history of Peucetia flava (Araneae, Oxyopidae): seasonal density fluctuation, phenology and sex ratio on the glandular plant Rhyncanthera dichotoma (Melastomataceae). J Nat Hist 43:701–711
Morais-Filho JC, Romero GQ (2010) Plant glandular trichomes mediate protective mutualism in a spider–plant system. Ecol Entomol 35:485–494
Morse DH (1981) Prey capture by the crab spider Misumena vatia (Clerck) (Thomisidae) on three common native flowers. Am Midl Nat 105:358–367
Morse DH (1990) Leaf choices of nest-building crab spiders (Misumena vatia). Behav Ecol Sociobiol 27:265–267
Morse DH (1999) Choice of hunting site as a consequence of experience in late-instar crab spiders. Oecologia 120:252–257
Morse RD, Lawton JH, Dodson MM, Williamson MH (1985) Fractal dimension of vegetation and the distribution of arthropod body lengths. Nature 314:731–733
Nahas L, Gonzaga MO, Del-Claro K (2012) Emergent impacts of ant and spiders interactions: herbivory reduction in a tropical savanna tree. Biotropica 44:498–505
Nentwig W, Cutler B, Heimer S (1993) Spiders of Panama: biogeography, investigation, phenology, check list, key and bibliography of tropical spiders fauna. Sandihill Crane Press, Gainesville
Nicolai V (1986) The bark of trees: thermal properties, microclimate and fauna. Oecologia 69:148–160
Nicolai V (1989) Thermal properties and fauna on the bark of trees in two different African ecosystems. Oecologia 80:421–430
Nishi AH, Vasconcellos-Neto J, Romero GQ (2013) The role of multiple partners in a digestive mutualism with a protocarnivorous plant. Ann Bot 111:143–150
Nyffeler M (1982) Field studies on the ecological role of spiders as insect predators in agroecosystems (abandoned grassland, meadows, and cereal fields). PhD thesis, Swiss Federal Institute of Technology, Zurich
Nyffeler M (2016) Phytophagy in jumping spiders: the vegetarian side of a group of insectivorous predators. Peckhamia 137.1:1–17
Nyffeler M, Olson EJ, Symondson WO (2016) Plant-eating by spiders. J Arachnol 44:15–27
Omena PM, Romero GQ (2008) Fine-scale microhabitat selection in a bromeliad-dwelling jumping spider (Salticidae). Biol J Linn Soc 94:653–662
Omena PM, Romero GQ (2010) Using visual cues of microhabitat traits to find home: the case study of a bromeliad-living jumping spider (Salticidae). Behav Ecol 21:690–695
Pianka ER (1966) Convexity, desert lizards, and spatial heterogeneity. Ecology 47:1055–1059
Pollard SD, Beck MW, Dodson GN (1995) Why do male crab spiders drink nectar? Anim Behav 49:1443–1448
Post WM, Riechert SE (1977) Initial investigation into the structure of spider communities. J Anim Ecol 46:729–749
Preisser EL, Orrock JL, Schmitz OJ (2007) Predator hunting mode and habitat domain alter nonconsumptive effects in predator–prey interactions. Ecology 88:2744–2751
Price PW, Bouton CE, Gross P, Mcpheron BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
Prinzing AJ (2001) Use of shifting microclimatic mosaics by arthropods on exposed tree trunks. Ann Entomol Soc Am 94:210–218
Prinzing AJ (2005) Corticolous arthropods under climatic fluctuations: Compensation is more important than migration. Ecography 28:17–28
Purcell J, Avilés L (2007) Smaller colonies and more solitary living mark higher altitude populations of a social spider. J Anim Ecol 76:590–597
Rembold K, Fischer E, Striffler BF, Barthlott W (2012) Crab spider association with the Malagasy pitcher plant Nepenthes madagascariensis. Afr J Ecol 51:188–191
Ribas ACA, Raizer J (2013) Spiders do not affect fruit set in Byrsonima intermedia (Malpighiaceae). JNR EEB 10:1–5
Ribas ACA, Brescovit AD, Raizer J (2011) The composition of spider assemblages varies along reproductive and architectural gradients in shrubs Byrsonima intermedia (Malpighiaceae). J Arachnol 39:537–540
Riechert SE, Gillespie RG (1986) Habitat choice and utilization in web-building spiders. In: Shear W (ed) Spiders: webs, behavior and evolution. Stanford University Press, Stanford
Riechert SE, Lockley T (1984) Spiders as biological control agents. Annu Rev Entomol 29:299–320
Riechert SE, Tracy CR (1975) Thermal balance and prey availability: bases for a model relating web-site characteristics to spider reproductive success. Ecology 56:265–284
Robertson IC, Maguire DK (2005) Crab spiders deter insect visitations to slickspot peppergrass flowers. Oikos 109:577–582
Rodrigues BV, Bonaldo AB (2014) Taxonomic revision of the species group rubripes of Corinna Koch, 1842 (Araneae; Corinnidae). Zootaxa 17(3815):451–493
Romero GQ (2001) Estudo experimental da associação de Runcinioides argenteus (Araneae, Thomisidae) em Trichogoniopsis adenantha (DC) (Asteraceae). Tese de Mestrado. Universidade Estadual de Campinas, Campinas
Romero GQ (2006) Geographic range, habitats and host plants of bromeliad-living jumping spider (Salticidae). Biotropica 38:522–530
Romero GQ (2007) Papel das aranhas como agentes de controle biológico em agroecossistemas. In: Gonzaga MO, Santos AJ, Japyassú HF (eds) Ecologia e comportamento de aranhas. Interciência, Rio de Janeiro
Romero GQ, Koricheva J (2011) Contrasting cascade effects of carnivores on plant fitness: a meta-analysis. J Anim Ecol 80:696–704
Romero GQ, Vasconcellos-Neto J (2003) Natural history of Misumenops argenteus (Thomisidae): seasonality and diet on Trichogoniopsis adenantha (Asteraceae). J Arachnol 31:297–304
Romero GQ, Vasconcellos-Neto J (2004a) Beneficial effects of flower-dwelling predators on their host plant. Ecology 85:446–457
Romero GQ, Vasconcellos-Neto J (2004b) Foraging by the flower-dwelling spider, Misumenops argenteus (Thomisidae), at high prey density sites. J Nat Hist 38:1287–1296
Romero GQ, Vasconcellos-Neto J (2004c) Spatial distribution patterns of jumping spiders associated with terrestrial bromeliads. Biotropica 36:596–601
Romero GQ, Vasconcellos-Neto J (2005a) The effects of plant structure on the spatial and microspatial distribution of a bromeliad-living jumping spider (Salticidae). J Anim Ecol 74:12–21
Romero GQ, Vasconcellos-Neto J (2005b) Population dynamics, age structure and sex ratio of the bromeliad-dwelling jumping spider, Psecas chapoda (Salticidae). J Nat Hist 39:153–163
Romero GQ, Vasconcellos-Neto J (2005c) Spatial distribution and microhabitat preference of Psecas chapoda (Peckham and Peckham) (Araneae, Salticidae). J Arachnol 33:124–134
Romero GQ, Vasconcellos-Neto J (2005d) Flowering phenology, seed set and arthropod guilds in Trichogoniopsis adenantha (Asteraceae) in south-east Brazil. Rev Bras Bot 28:171–178
Romero GQ, Vasconcellos-Neto J (2007a) Aranhas sobre plantas: dos comportamentos de forrageamento às associações específicas. In: Gonzaga MO, Santos AJ, Japyassú HF (eds) Ecologia e comportamento de aranhas, 1st edn. Interciência, Rio de Janeiro
Romero GQ, Vasconcellos-Neto J (2007b) Interações bióticas entre plantas, herbívoros e aranhas. In: Gonzaga MO, Santos AJ, Japyassú HF (eds) Ecologia e comportamento de aranhas, 1st edn. Interciência, Rio de Janeiro
Romero GQ, Vasconcellos-Neto J (2011) Interações entre aranhas e plantas: associações específicas e mutualismos. In: Del-Claro K, Torezan-Silingardi HM (eds) Ecologia de Interações. Technical Books, Rio de Janeiro
Romero GQ, Mazzafera P, Vasconcellos-Neto J, Trivelin PCO (2006) Bromeliad-living spiders improve host plant nutrition and growth. Ecology 87:803–808
Romero GQ, Santos AJ, Wienskoski EH, Vasconcellos-Neto J (2007) Association of two Coryphasia species (Araneae, Salticidae) with tank-bromeliads in southeastern Brazil: habitats and patterns of host plant use. J Arachnol 35:181–192
Romero GQ, Souza JC, Vasconcellos-Neto J (2008a) Anti-herbivore protection by mutualistic spiders and the role of plant glandular trichomes. Ecology 89:3105–3115
Romero GQ, Vasconcellos-Neto J, Trivelin PO (2008b) Spatial variation in the strength of mutualism between a jumping spider and a terrestrial bromeliad: evidence from the stable isotope 15N. Acta Oecol 33:380–386
Romero GQ, Nomura F, Gonçalves AZ, Dias NYN, Mercier H, Conforto EC, Rossa-Feres DC (2010) Nitrogen fluxes from treefrogs to tank epiphytic bromeliads: an isotopic and physiological approach. Oecologia 162:941–949
Romero GQ, Antiqueira PA, Koricheva J (2011) A meta-analysis of predation risk effects on pollinator behaviour. PLoS One 6(6):e20689
Root RB (1967) The exploitation pattern of the blue-grey gnatcatcher. Ecol Monogr 37:317–350
Root RB (1973) Organization of a plant–arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecol Monogr 43:95–124
Rossa-Feres DC, Romero GQ, Gonçalves-de-Freitas E, Feres RJF (2000) Reproductive behavior and seasonal occurrence of Psecas viridipurpureus (Salticidae, Araneae). Rev Bras Biol 60:221–228
Ruhren S, Handel SN (1999) Jumping spiders (Salticidae) enhance the seed production of a plant with extrafloral nectaries. Oecologia 119:227–230
Rypstra AL, Buddle CM (2012) Spider silk reduces insect herbivory. Biol Lett 9(1). https://doi.org/10.1098/rsbl.2012.0948
Sanders D (2007) Ants and spiders in grassland food webs: top-down control and Intraguild interactions. Dissertação de Doutorado em Ciência Naturais e Matemáticas. Universidade Georg August, Göttingen
Sanders D, Nickel H, Ghützner T, Platner C (2008) Habitat structure mediates top-down effects of spiders and ants on herbivores. Basic Appl Ecol 9:152–160
Santos AJ, Romero GQ (2004) A new bromeliad-dwelling jumping spider (Araneae: Salticidae) from Brazil. J Arachnol 32:188–190
Santos RL, Almeida MG, Nunes JV (2002) Notes on the association of Pachistopelma rufonigrum Pocock 1901 (Theraphosidae) with phytotelm bromeliads in eastern Rio Grande do Norte State, NE-Brazil. J Bromeliad Soc 52:122–124
Scheuring I (1991) The fractal nature of vegetation and the species–area relation. Theor Popul Biol 39:170–177
Schimper AFW (1903) Plant-geography upon a physiological basis. Clarendon Press, Oxford
Schmalhofer VR (2001) Tritrophic interactions in a pollination system: impacts of species composition and size of flower patches on the hunting success of a flower-dwelling spider. Oecologia 129:292–303
Schoonhoven LM, Jermy T, van Loon JJA (1998) Insect-plant biology. Chapman and Hall, London
Schulze ED (1989) Air pollution and forest decline in a spruce (Piceaabies) forest. Science 244:776–783
Shear WA (1986) Spiders: webs, behavior, and evolution. Stanford University Press, Stanford
Simberloff D, Dayan T (1991) The guild concept and the structure of ecological communities. Annu Rev Ecol Syst 22:115–143
Smith RB, Mommsen TP (1984) Pollen feeding in an orb-weaving spider. Science 226(4680):1330–1332
Souza ALT (1999) Influência da arquitetura de ramos vegetativos e inflorescências na distribuição de aranhas em plantas. PhD thesis, Universidade Estadual de Campinas, Campinas
Souza ALT (2007) Influência da estrutura do habitat na abundância e diversidade de aranhas. In: Gonzaga MO, Santos AJ, Japyassú HF (eds) Ecologia e comportamento de aranhas. Editora Interciência, Rio de Janeiro
Souza ALT, Martins RP (2005) Foliage density of branches and distribution of plant-dwelling spiders. Biotropica 37:416–420
Souza ALT, Módena EDS (2004) Distribution of spiders on different types of inflorescences in the Brazilian pantanal. J Arachnol 32:345–348
Souza HS, Messas YF, Gonzaga MO, Vasconcellos-Neto J (2015) Substrate selection and spatial segregation by two congeneric species of Eustala (Araneae: Araneidae) in southeastern Brazil. J Arachnol 43:59–66
Stefani V, Pires TL, Torezan-Silingardi HM, Del-Claro K (2015) Beneficial effects of ants and spiders on the reproductive value of Eriotheca gracilipes (Malvaceae) in a Tropical Savanna. PLoS One 10(7):e0131843
Suetsugu K, Hayamizu M, Koike N (2014) Clubiona spider (Araneae: Clubionidae) visiting flowers of nectariferous orchid Neottianthe cucullata. Entomol Sci 17:262–264
Sugihara G, May RM (1990) Applications of fractals in ecology. Trends Ecol Evol 5:79–86
Sugiura S, Yamazaki K (2006) Consequences of scavenging behaviour in a plant bug associated with a glandular plant. Biol J Linn Soc 88:593–602
Sundberg I, Gunnarsson B (1994) Spider abundance in relation to needle density in spruce. J Arachnol 22:190–194
Suter RB (1999) An aerial lottery: the physics of ballooning in a chaotic atmosphere. J Arachnol 27:281–293
Suttle KB (2003) Pollinators as mediators of top-down effects on plants. Ecol Lett 6:688–694
Szinetár C, Horváth R (2005) A review of spiders on tree trunks in Europe (Araneae). Acta Zool Bulgar 1:221–257
Taylor RM (2004) Plant nectar contributes to the survival, activity, growth, and fecundity of the nectar-feeding wandering spider Cheiracanthiuminclusum(Hentz) (Araneae: Miturgidae). PhD dissertation, Ohio State University, Columbus
Taylor RM, Bradley RA (2009) Plant nectar increases survival, molting, and foraging in two foliage wandering spiders. J Arachnol 37:232–237
Taylor RM, Foster WA (1996) Spider nectarivory. Am Entomol 42:82–86
Tedore C, Johnsen S (2015) Immunological dependence of plant-dwelling animals on the medicinal properties of their plant substrates: a preliminary test of a novel evolutionary hypothesis. Arthropod Plant Interact 9:437–446
Tedore C, Johnsen S (2016) Disentangling the visual cues used by a jumping spider to locate its microhabitat. J Exp Biol. https://doi.org/10.1242/jeb.129122
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92
Théry M, Insausti TC, Defrize J, Casas J (2011) The multiple disguises of spiders. In: Stevens M, Merilaita S (eds) Animal camouflage: mechanisms and function. Cambridge University Press, Cambridge
Turnbull AL (1973) Ecology of the true spiders (Araneomorphae). Annu Rev Entomol 18:305–348
Uetz GW (1977) Coexistence in a guild of wandering spiders. J Anim Ecol 46:531–541
Uetz GW (1991) Habitat structure and spider foraging. In: Bell SS, ED MC, Mushinsky HR (eds) Habitat structure: the physical arrangement of objects in space. Chapman and Hall, New York
Uetz GW, Halaj J, Cady AB (1999) Guild structure of spiders in major crops. J Arachnol 27:270–280
Vasconcellos-Neto J, Romero GQ, Santos AJ, Dippenaar-Schoeman AS (2007) Associations of spiders of the genus Peucetia (Oxyopidae) with plants bearing glandular hairs. Biotropica 39:221–226
Villanueva-Bonilla GA (2015) Dinâmica populacional e seleção de habitat por Selenops cocheleti Simon, 1880 (Araneae: Sselenopidae) na Serra do Japi, São Paulo, Brasil. Master thesis, Universidade Estadual de Campinas
Vogelei A, Greissl R (1989) Survival strategies of the crab spider Thomisusonustus Walckenaer 1806 (Chelicerata, Arachnida, Thomisidae). Oecologia 80:513–515
Waldorf ES (1976) Spider size, microhabitat selection, and use of food. Am Midl Nat 96:76–87
Welti EAR, Putnam S, Joern A (2016) Crab spiders (Thomisidae) attract insect flower-visitors without UV signalling. Ecol Entomol 41:611–617
Whitney KD (2004) Experimental evidence that both parties benefit in a facultative plant–spider mutualism. Ecology 85:1642–1650
Wise DH (1993) Spiders in ecological webs. Cambridge University Press, Cambridge
Woodcock BA, Potts SG, Westbury DB, Ramsay AJ, Lambert M, Harris SJ, Brown VK (2007) The importance of sward architectural complexity is structuring predatory and phytophagous invertebrate assemblages. Ecol Entomol 32:302–311
World Spider Catalog (2017) World Spider Catalog. Natural History Museum Bern, version 18.0. http://wsc.nmbe.ch. Accessed 8 Apr 2017
Wunderlich J (1982) Mitteleuropäische Spinnen (Araneae) der Baumrinde. Z Angew Entomol 94:9–21
Young OP, Edwards GB (1990) Spiders in United States field crops and their potential effect on crop pests. J Arachnol 18:1–27
Young OP, Lockley TC (1989) Spiders of Spanish moss in the Delta of Mississippi. J Arachnol 17:143–148
Zanatta MF (2013) História natural, seleção de folhas e locais para nidificação e efeito do cuidado materno em Aysha piassaguera Brescovit, 1992 (Araneae: Anyphanidae) na Serra do Japi, Jundiaí-SP, Brasil. Master thesis, Universidade Estadual de Campinas
Zanatta MF, Romero GQ, Vasconcellos-Neto J (2016) Effect of maternal care on egg survival in Aysha piassaguera (Araneae: Anyphaenidae). Insect Soc 63:439–445
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Vasconcellos-Neto, J., Messas, Y.F., da Silva Souza, H., Villanueva-Bonila, G.A., Romero, G.Q. (2017). Spider–Plant Interactions: An Ecological Approach. In: Viera, C., Gonzaga, M. (eds) Behaviour and Ecology of Spiders. Springer, Cham. https://doi.org/10.1007/978-3-319-65717-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-65717-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65716-5
Online ISBN: 978-3-319-65717-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)