Abstract
Aspergillus fumigatus is one of the most ubiquitous opportunistic fungal pathogen, which can cause life-threatening invasive pulmonary infections in immunocompromised populations. Upon the inhalation of the A. fumigatus conidia, the encounter between the fungus and the host presents a complex interplay. This chapter will summarize the host innate immunity against A. fumigatus, and emphasize on the host immune evasion mechanisms of A. fumigatus.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords:
- Aspergillus fumigatus
- Invasive pulmonary aspergillosis
- Innate immunity
- Pattern-recognition receptors
- Pathogen-associated molecular patterns
- Antifungal
4.1 Introduction
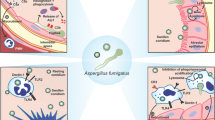
Aspergillus species are saprotrophic thermophilic fungi living in decaying material in the soil. When the fungus is starved, it produces millions of aerial conidia which are responsible for the propagation of the fungus (Fig. 4.1). Some of the Aspergillus species cause clinical manifestations ranging from chronic to invasive pulmonary infections, following the inhalation of the conidia (hundreds per day in normal environments) [1, 2]. Aspergillus fumigatus is the most prevalent etiologic agent of aspergillosis, followed by Aspergillus flavus, A. niger, A. nidulans, A. terreus [3]. In immunocompetent individuals, the inhaled conidia rarely cause infections since the host innate immunity is efficient in the clearance of the fungal pathogen [4,5,6,7,8,9] (Fig. 4.2). In the populations with pre-existing pulmonary cavities who are otherwise immunocompetent, colonization of Aspergillus species will only lead to chronic pulmonary aspergillosis (CPA) [10,11,12]. Unlike CPA, invasive pulmonary aspergillosis (IPA) predominantly affects immunocompromised individuals [13]. Individuals with primary or secondary immunodeficiency, such as chronic granulomatous disease, hematologic malignancy, hematopoietic stem cells transplantation, solid organ transplantation, and neutropenia consecutive to cancer chemotherapy or immunosuppressive treatment are typically at risk for IPA (Fig. 4.2) [2, 14]. The mortality rates of IPA vary among groups of host with different underlying risk factors, but it is generally high [2, 14]. A. fumigatus benefits from immune deficiencies, and it also possesses various well-established and specific strategies which helps the fungus to evade from the host debilitated immune system and colonize the lung parenchyma [15,16,17,18,19,20].
This chapter will dissect both the host innate immunity and the pathogen anti-immune strategies on the early stages of the fungal infection. Since contact between membrane-bound and soluble pattern recognition receptors (PRRs) of the host and surface pathogen-associated molecular patterns of A. fumigatus is the first event leading to the reciprocal recognition of the host and the pathogen (Fig. 4.3). It will especially focus on the host and pathogen molecules favoring their respective recognition, as well as the role of the innate immune cells involved in the anti-A. fumigatus defense. A thorough understanding of this complex “tug of war” between the host and the pathogen is fundamental in providing new insights in developing prophylactic strategies of IPA.
4.2 Molecules Responsible for the “Hide-and-Seek” Between Host and Aspergillus fumigatus
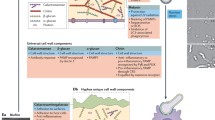
The fungal cell is surrounded by a cell wall with a specific composition which is very different from the phospholipid bilayer of the host cell plasma membrane. The A. fumigatus cell wall which surrounds the fungal cell is mainly composed of polysaccharides which are interlinked alkali insoluble β-1,3-glucans, chitin, and galactomannan and, alkali soluble α-1,3-glucans [21, 22]. These fungal structural polysaccharides are absent in mammalian cells and are therefore pathogen-associated molecular patterns (PAMPs), which are recognized by various membrane-bound and soluble PRRs of the host as foreign objects [21, 23,24,25,26].
In resting conidia, this polysaccharide core which is of similar composition both in the conidium and hyphal cell wall, is covered by a bilayered outer layer composed of hydrophobins (the most external) and melanin. The external hydrophobin rodlet layer, which is responsible for the hydrophobicity of the conidia is exclusively composed of the amyloid hydrophobic RodA proteins [27,28,29]. One way for A. fumigatus to become a pathogen is its capacity to “hide” from the host defensive response immediately after inhalation and to go undetected after entering the respiratory tract [30,31,32]. This is due to the presence of the rodlet and melanin outer layers of the dormant conidia which hide the immunogenic cell wall polysaccharides. By doing so, an immediate strong inflammatory response, which would be detrimental for the fungus, is avoided. Moreover, by delaying the immune response, the initial survival of the fungus is prolonged in the host.
The loss of the rodlet and melanin layer during germination leads to an entire modification of the surface layer, which leads to the apparition of immunogenic polysaccharides on the hyphal surface. These surface molecules are recognized by PRRs that are mostly lectin receptors (carbohydrate-recognizing) involved in the initiation of the antifungal response. Indeed, conidial germination can be considered by the fungus as a form suicide. However, instead of being covered by melanin and RodA protein, the cell wall of the mycelium is covered by a specific hyphal galactosaminogalactan, which is immunosuppressive and favors the vegetative fungal growth. All these strategies for the host to seek for the fungal alien and for the fungus to counteract the host immune response after the immediate contact will be discussed below.
4.2.1 Membrane-Bound Pattern Recognition Receptors
4.2.1.1 Toll-Like Receptors
Toll-like receptors (TLRs) are a family of membrane-bound and soluble receptors on sentinel cells involved in the recognition of specific PAMPs [33]. Although the role of TLR2 and TLR4 in the immune defense against fungal pathogens have been extensively studied in the past decades [34]; so far, their precise functions have not yet been clearly elucidated. Various in vitro studies have demonstrated that TLR2 is involved in recognizing A. fumigatus [35, 36]. Blocking TLR2 led to a reduced phagocytic rate of A. fumigatus conidia, but not of control beads, suggesting that the phagocytic machinery is not impaired by TLR2 blocking [37]. Neutrophil-depleted TLR2-deficient mice had however a lower survival and produced less TNF-α upon stimulation with A. fumigatus [38]. The recruitment of neutrophils was severely attenuated in non-immunosuppressed mice deficient in both TLR2 and TLR4, when compared to that in mice with single deficiency [39]. Furthermore, the production of different cytokines in response to A. fumigatus is individually mediated by TLR2 and TLR4. For instance, the production of IL-12 and IL-6 from monocytes-derived dendritic cells are mediated by TLR2 and TLR4 respectively [40]. TLR4-mediated pro-inflammatory signals were lost during phenotypic switch from conidia to hyphae, contributing to the escape of the pathogens from the host immune defense [36, 41]. The presence of an optimal innate immune response required both TLR2 and TLR4. Although a direct binding has not been shown, polysaccharides of the cell wall seem to be recognized by TLR2 and TLR4. The IL-6 production via TLR2 and TLR4 stimulation in PBMCs (incubated with TLR2 and TLR4 ligands respectively) was attenuated by pre-incubation with α-glucan and galactomannan, while pre-incubation with β-glucan attenuated the IL-6 production via only TLR4 stimulation [42]. However, TLR2- and TLR4-knockout immunocompetent mice were not more susceptible to challenge of invasive aspergillosis, suggesting that these two receptors are not essential in preventing Aspergillus infection in an immunocompetent status [43].
Unlike TLR2 and TLR4, TLR9 is located intracellularly in the endosome compartment of the immune system cells [44]. TLR9 was originally thought to be activated by unmethylated CpG sequences in DNA of bacterial and viral origins only [45]. It was later found that TLR9 can also be activated by unmethylated CpG sequences in fungal DNA [46]. The A. fumigatus DNA contains unmethylated CpG sequences, and is therefore capable of activating TLR9 and induce the production of pro-inflammatory cytokines [47]. Since TLR9 is an intracellular receptor, it was suggested that the activation would follow the release of DNA content after fungal lysis in the phagosome. TLR9 is recruited to the phagosomes that contain internalized A. fumigatus conidia [48]. However, TLR9 is already recruited to the phagosome merely 1h after phagocytosis of resting conidia [48]. At this stage, the internalized conidia should still be dormant and intact, and the fungal DNA is not exposed. This leaves the underlying recognition mechanism of the fungal DNA and the activation of TLR9 unresolved. Paradoxically, in vivo, TLR9-deficient mice, immunosuppressed by cyclophosphamide, had lower fungal burden than the wild-type mice upon intranasal challenge by A. fumigatus [49]. Consistently, in another in vivo study, the neutrophil-depleted TLR9-deficient mice was less susceptible to challenge of dormant or swollen A. fumigatus conidia by showing delayed mortality [50]. The expression of dectin-1 was also significantly lowered in the bone marrow-derived dendritic cells from TLR9-deficient mice 14 days post-infection following challenge of swollen conidia [50].
Taken together, the role of TLR2, 4 and 9 in host defense against A. fumigatus remains to be further explored.
4.2.1.2 C-Type Lectin Receptors
C-type (Ca2+-dependent) lectin receptors (CLRs), including dectin-1, dectin-2, mannose receptor, Mincle, and DC-SIGN, are important in recognizing the major carbohydrate moieties in the fungal cell wall [51].
Dectin-1 is a major transmembrane receptor for β-1,3-glucan which is found mainly on myeloid cells [52,53,54]. Since β-1,3-glucan is the major constituent of the fungal cell wall, it was expected that dectin-1 plays a crucial role in the control of fungal infection and especially favors recognition and phagocytosis of fungal particles [37, 55]. In the case of A. fumigatus, dectin-1 preferentially recognizes swollen and germinating conidia, as the β-1,3-glucan is exposed on the conidial surface [56,57,58]. Dectin-1 contains an extracellular lectin-like carbohydrate recognition domain and a cytoplasmic tail, which is phosphorylated at the tyrosine, upon binding with β-1,3-glucan. The phosphorylated cytoplasmic tail then can interact with spleen tyrosine kinase (SYK), which induces cellular responses, such as respiratory burst, phagocytosis and production of pro- and anti-inflammatory cytokines [54, 58, 59]. Dectin-1 induces different cytokine responses independent or in conjunction with TLR: for example, dectin-1 alone induces the production of IL-10, but requires the adaptor MyD88 for the induction of IL-8 and IL-12 [54, 56, 60].
The ligands for the other membrane-bound CLRs remained to be fully determined. Another member of the dectin family, dectin-2, has been shown recently to recognize A. fumigatus galactomannan, but its role in the innate response has not been really evaluated [61,62,63]. Mannose receptor, Mincle and DC-SIGN recognize the N-linked mannan and N-acetylglucosamine (GlcNAc) in the fungal cell wall [51, 64]. Being a polymer of GlcNAc, chitin could also be recognized by mannose receptor [65]. However, it was recently found that chitin is mainly recognized by the Fcγ receptor [66]. Further studies should be warranted to better investigate the respective roles of these polysaccharides tested alone or in association, in the immune response.
4.2.1.3 Crosstalk Between TLRs and CLRs
Some PRRs collaborate to produce a synergetic induction of immune response [67]. For instance, dectin-1, which recognizes β-1,3-glucan, interact with TLR2, which recognizes zymosan, but not β-1,3-glucan [68]. Interestingly, crosstalk between PRRs does not always lead to enhanced immune response. The CLR Mincle suppresses the antifungal immune response mediated by dectin-1 and Mincle [69, 70]. This has also been suggested to be an evasion mechanism of fungal pathogen from the host immune defense [70]. However, this has not been studied in A. fumigatus.
4.2.2 Humoral Pattern Recognition Receptors
4.2.2.1 Complement Components
The complement system is often considered an irrelevant immunological weapon against A. fumigatus for the following reasons. First, owing to the presence of the thick fungal cell wall, the membrane attack complex (MAC) of the complement system is ineffective on A. fumigatus [71, 72]. Second, complement deficiency in human was not found to increase the risk of aspergillosis. However, all three of the complement pathways, classical, alternative and the lectin pathway, are indeed involved in the host defense against A. fumigatus, through opsonization and thus, facilitation of phagocytosis [73]. Complement component C3 can directly, or through other components, such as mannose-binding lectin or immunoglobulin, deposit on the surface of the conidia and mycelia [73,74,75,76,77,78]. The exact chemical nature of the ligand(s) of the complement components on the surface of the conidia or mycelia remain unknown. Furthermore, A. fumigatus can evade from the complement attack by scavenging complement regulators, Factor H and C4b binding protein [79, 80], and secreting proteases that degrades complement proteins [81]. Alp1p, a major protease secreted by A. fumigatus, can degrade complement proteins C3, C4 and C5 [81].
4.2.2.2 Collectins and Ficolins
Collectins (Collagen + lectin) are a group of soluble pattern recognition receptors characterized by a collagen-domain and carbohydrate recognition domain (CRD), which is responsible in the binding to the carbohydrate moiety in a Ca2+-dependent manner. Collectins in the lung bind to A. fumigatus conidial surface as opsonins, and facilitate phagocytosis [51, 82, 83].
4.2.2.2.1 Surfactant Proteins
There are four surfactant proteins (SP) in human, SP-A, SP-B, SP-C, and SP-D, that are secreted by alveolar type II cells into the alveolar space [84,85,86]. Both SP-B and SP-C are small hydrophobic proteins that are mainly responsible in reducing surface tension at the air–liquid interface in the lungs, and their role during Aspergillus infection have not been studied [87]. It was only recently found that SP-C binds to bacterial lipopolysaccharides [85, 88]. Apart from that, the immunomodulatory role of SP-C is still uncertain. Meanwhile, SP-A and SP-D, which are hydrophilic proteins, are primarily responsible for the host defense against pulmonary pathogens by facilitating phagocytosis after opsonization [84, 86, 89,90,91]. SP-A and SP-D are not involved in the lectin complement pathway [92].
Human SP-A and SP-D bind directly to surface of dormant conidia, swelling conidia and hyphae of A. fumigatus [83, 93], and the responsible ligand(s) are under investigation by our group. When bound to microorganisms via its CRD region, SP-D binds to the calreticulin/CD91 complex on the surface of macrophages, via its collagenous region, which then mediates the uptake of SP-D opsonized microorganisms by phagocytes and, stimulates inflammatory response by activating NF-κB [94, 95]. Although it was found that the mortality of SP-D-deficient mice was similar to that of the wild-type mice following intranasal challenge of A. fumigatus conidia, under immunosuppression of corticosteroids, shorter survival duration, higher hyphal density and more tissue damage were observed in the SP-D deficient mice in comparison to the wild-type [96, 97]. This observation suggested that SP-D plays a role in immune recognition and killing, which have been however insufficiently investigated.
Compared to SP-D, the role of SP-A in the immune defense against A. fumigatus is less significant. Although human SP-A bind to conidial surface, the binding is reduced in the presence of extracellular alveolar lipids, while that of SP-D remained unchanged [93]. Immunosuppressed SP-A deficient mice are resistance to lethal IPA challenge when compared to wild-type mice [97], which could be explained by the increased level of SP-D in SP-A-deficient mice [98].
At present, no evasion mechanism from the opsonization of collectins has yet been observed in A. fumigatus.
4.2.2.2.2 Mannose-Binding Lectin and Ficolins
Mannose-binding lectin (MBL) binds to the conidial surface of A. fumigatus [99]. Mannose-binding lectin by binding to the MBL-associated serine proteases (MASP1, MASP2, and MASP3), activate the lectin complement pathway [100]. Non-immunosuppressed MBL-knockout mice were less susceptible to systemic invasive aspergillosis [101]. It was suggested that the lack of MBL reduced the recruitment of neutrophils, which in turn leads to less tissue damage from inflammation. In contrast, in human, a deficiency in MBL is associated with chronic and invasive aspergillosis [102, 103]. The role of MBL should be revisited.
Ficolins (fibrinogen + collagen + lectin) are a family of lectins that consist of a collagen-like domain and a fibrinogen-like domain described as recognizing specifically N-acetylglucosamine (GlcNAc) [104]. There are three types of ficolins in human, ficolin-1, 2, and 3, which are secreted into the human plasma and binding to ficolin activate the lectin complement pathway [104, 105]. Ficolin-2 binds to A. fumigatus [74, 106, 107]. The in vitro binding of ficolin-2 to A. fumigatus is inhibited by the presence of GlcNAc and Curdlan (β-1,3-glucan) [107], suggesting that chitin and β-1,3-glucan are the responsible ligands. Previously, ficolin-1 and ficolin-3 were not found to bind to A. fumigatus. However, recently, the role of ficolin-3 in lung immune defense was discovered. Ficolin-3 indeed binds to A. fumigatus resting or swollen conidia in a calcium-independent manner [74, 82], suggesting that the binding does not involve the carbohydrate recognition domain. Unlike ficolin-2, which is produced in the liver, ficolin-3 is produced by type II alveolar cells and secreted into the alveolar space. Moreover, ficolin-A (the orthologue of ficolin-2 in mouse) increases the release of IL-8 (a proinflammatory cytokine and chemokine for neutrophils) [74]. Upon binding to the carbohydrate ligand on the pathogen surface, ficolins facilitate opsonization, phagocytosis and the activation of the lectin complement pathway. However, complement activation was not impaired in ficolin-knockout mice [74, 108]. This could be explained by the similar role of MBL and ficolins, that both trigger the lectin complement pathway.
Although many elements of the immune system in the anti-A. fumigatus response have been discovered or rediscovered in recent years, a comprehensive picture of their roles as well as the ranking of their importance in the immunocompetent, as well as immunocompromised host remains to be elucidated.
4.3 The Cell Actors
4.3.1 Airway Epithelial Cells
Airway epithelial cells (AECs) are the first type of cells to enter in contact with the inhaled conidia [109, 110]. AECs, being nonprofessional phagocytes, are capable of internalizing and killing A. fumigatus conidia [111, 112]. Comparing with murine macrophage cell line (J774), the internalized conidia survive longer in the human airway epithelial cell line (A549) than in the alveolar macrophages [113]. Accordingly, a small portion of the internalized conidia are not killed by the AECs, which then germinate and break the epithelial barrier [113]. The inefficient killing of the internalized conidia by the AECs is probably due to inefficient respiratory burst. In addition, the adherence of A. fumigatus conidia to the A549 cell line inhibits the release of IL-6, IL-8, and TNF-α, which thus inhibits the recruitment of immune cells and apoptosis of the epithelial cells [114, 115].
AECs produce antimicrobial peptides such as human β-defensins, human lactoferrin (hLF), and histatin 5, which possess some antifungal activity against A. fumigatus [4, 6, 109, 110, 116, 117]. The gene expression of human β-defensins hBD2 and hBD9 were found to be induced in A549 cells that are exposed to swollen conidia [116]. hBD2 displayed direct, but low antifungal activity in vitro against A. fumigatus [118] and does not enhance the antifungal killing activity of neutrophils [118]. However, hBD could act as chemoattractant for immune cells and activation of professional antigen-presenting cells [119].
4.3.2 Alveolar Macrophages
The resident alveolar macrophages (AMs), which are responsible of the recognition and phagocytosis of the A. fumigatus conidia, serve as the major immune cells in the defense of A. fumigatus. Upon internalization of A. fumigatus conidia, phagolysosomes were not acidified [18]. Dihydroxynaphthalene-melanin (DHN-melanin) on the outer layer of dormant A. fumigatus conidia was shown to inhibit the acidification of phagolysosome, and thus, prevent intracellular killing [120,121,122]. The mutant of polyketide synthase (ΔpksP), the enzyme responsible for the initial step in DHN-melanin formation is devoid of DHN-melanin and avirulent [123]. Interestingly, the melanin of A. niger, which is different from the melanin in A. fumigatus [124, 125], does not inhibit phagolysosome acidification [18]. The intracellular killing of A. fumigatus conidia in AMs is triggered by the swelling of conidia. The phagolysosome acidification and the increase in the production of reactive oxygen species (ROS) following phagocytosis is essential for conidial killing [126, 127]. Upon swelling, the outer layer of hydrophobins and melanin is shed. In the absence of inhibition by melanin, the phagolysosome acidifies. On the other hand, the absence of the outer layer of rodlet and melanin unmasks the PAMPs in the inner layer. The exposure of PAMPs recruits PRRs to the phagosome, which triggers production of ROS, cytokines and chemokines and the recruitment of autophagy proteins, LC3 II, Atg5 and Atg7 to the phagolysosome [57, 128, 129]. Pyomelanin, another type of melanin that A. fumigatus produces, can also protects the fungus from ROS attack [13, 120, 130]. However, pyomelanin-minus mutant are as pathogenic as their parental strain. In addition, DHN-melanin inhibits LC3-associated phagocytosis (LAP) [123, 131, 132]. Taken together, pyomelanin and DHN-melanin permits the fungus to escape host immune defense by inhibiting phagolysome acidification, quenching ROS and inhibiting LC3-associated phagocytosis.
4.3.3 Neutrophils
Neutrophils act as a strong second line of innate immune defense against A. fumigatus [133]. Upon recruitment to the alveolar space, neutrophils constitute the majority of phagocytes to eliminate the conidia [4, 134]. Even though AMs by themselves are able to eradicate resting conidia in a mouse deprived of neutrophils, neutrophils are more important than AMs in the pathogen clearance [127, 135]. Indeed, low number of neutrophils in the host (neutropenia) renders patients at great risk of IPA [2]. Moreover, molecules of A. fumigatus which impairs the neutrophil action are true virulence factors. Galactosaminogalactan is one of them. This polysaccharide, which is secreted by A. fumigatus hyphae, suppresses the recruitment of neutrophils, which then favors the fungal survival in the host [136, 137]. In addition, it induces anti-inflammatory response by the induction of interleukin-1 receptor antagonist (IL-1Ra) and reduces neutrophil recruitment [136, 138].
An important function unique to neutrophils is its capacity of attacking A. fumigatus hyphae, which are too large to be phagocytosed by immune cells. Neutrophils attack the fungal hyphae extracellularly by the secretion of enzymes, which degrade and permeabilize the cell wall and makes the fungus more sensitive to the neutrophil granular toxic molecules. Recently, neutrophil extracellular traps (NETs) which are mainly composed of nuclear DNA and antimicrobial proteins, have been mentioned as playing a major role against microbial pathogens [139]. Although the production of NETs is stimulated by Aspergillus hyphae [140], the hyphae are reported to be only slightly susceptible to the killing by NETs [141]. It is suggested that NETs only has a fungistatic effect on the hyphae and prevent further spreading of the disease [141]. This finding is in contrast to the one regarding Candida albicans, which is completely susceptible to the killing by NETs [142]. The fungistatic activity of NETs towards A. fumigatus hyphae is attributed by calprotectin, a Zn2+ chelator, [143]. Interestingly, it was shown recently that A. fumigatus hyphae generate hyphal branching upon interaction with neutrophils, which allows lower host immune interference and increases invasive growth [144].
Moreover, A. fumigatus seems to have other mechanisms to evade the neutrophil antagonistic action since A. fumigatus is less sensitive than A. niger for which the germination and hyphal length are more significantly reduced by neutrophils [18]. For example, fumagillin, secreted by the A. fumigatus hyphae, inhibits the antifungal function of neutrophils by inhibiting the formation of NADPH oxidase and reduces the degranulation [145]. Neutrophils exposed to fumagillin in vitro also demonstrated reduced rate of phagocytosis of A. fumigatus conidia [145]. An analysis of the differences between A. fumigatus and other non- or poorly pathogenic Aspergillus species, such as melanin structure and cell wall composition, should definitely reveal more information of the success of A. fumigatus as pathogen.
4.4 Perspectives
The current arsenal of antifungal agents available for the treatment of IA is mainly limited to azoles, echinocandins, and polyenes [146]. However, there are major drawbacks regarding the drug resistance, efficiency, and toxicity of these current agents [147, 148]. Furthermore, the pipeline of antifungal development has not been introducing any new classes of antifungal agents, especially since there is not a market for drugs for rare diseases such as aspergillosis. Can immunotherapy lead to the development of specific drugs or antibodies that are able to block virulence factors that allow the evasion of A. fumigatus from the host immune system? The past decade has witnessed a shift in paradigm of antifungal development, in which the virulence factors are targeted for drug candidates instead of essential genes [149,150,151]. Analysis of the transcriptomic and proteomic changes occurring after internalization of the pathogen and especially in the phagolysosome, where the fungus is inhibited in the immunocompetent host but not in the immunocompromised host, may lead to the identification of essential virulence factors in vivo. Such approach focused on the inhibition of virulence factors would exert less selective pressure on the pathogens, and thus, the chance of resistance development is lower [152]. However, such immunotherapeutic approach has not been yet undertaken with A. fumigatus but the situation may be difficult in the case of pulmonary aspergillosis, which occurs mainly among immunocompromised patients. The identification of monoclonal antibodies able to block hyphal emergence could be also possible. Such strategy has been proposed a few years ago [153, 154], however it has not been pursued yet. Although a lot of progresses have been obtained in recent years, the overall picture to fully understand the innate immune defense against A. fumigatus is missing. For example, the respective role of the different PRRs and the lack of definition of all the ligands binding to complement proteins, collectins and other PRRs are essential gaps to fill. The reasons for the ineffectiveness of NETs to kill A. fumigatus hyphae should also be investigated. Comparative characterization of major Aspergillus species may also provide valuable understanding of how A. fumigatus took the “throne” and became the most predominant and successful pathogen among the Aspergillus species. Finally, it has been reported that many cytokines and chemokines plays a major role in the defense reactions (not discussed here but see for review [155, 156]. However, the interweaving network between all these chemokines, cytokines and the defense against A. fumigatus remains obscure. In conclusion, the early events “when A. fumigatus meets the man” remain enigmatic and this chapter paves the way for the direction of future exploration.
References
Kwon-Chung KJ, Sugui JA (2013) Aspergillus fumigatus – what makes the species a ubiquitous human fungal pathogen? PLoS Pathog 9:1–4. doi:10.1371/journal.ppat.1003743
Latgé JP (1999) Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 12:310–350
Sugui JA, Kwon-Chung KJ, Juvvadi PR et al (2014) Aspergillus fumigatus and related species. Cold Spring Harb Perspect Med. doi:10.1101/cshperspect.a019786
Balloy V, Chignard M (2009) The innate immune response to Aspergillus fumigatus. Microbes Infect 11:919–927. doi:10.1016/j.micinf.2009.07.002
Cramer RA, Rivera A, Hohl TM (2011) Immune responses against Aspergillus fumigatus: what have we learned? Curr Opin Infect Dis 24:315–322. doi:10.1097/QCO.0b013e328348b159
Espinosa V, Rivera A (2016) First line of defense: innate cell-mediated control of pulmonary Aspergillosis. Front Microbiol 7:1–12. doi:10.3389/fmicb.2016.00272
Lass-Flörl C, Roilides E, Löffler J et al (2013) Minireview: host defence in invasive aspergillosis. Mycoses 56:403–413. doi:10.1111/myc.12052
Margalit A, Kavanagh K (2015) The innate immune response to Aspergillus fumigatus at the alveolar surface. FEMS Microbiol Rev 39:670–687. doi:10.1093/femsre/fuv018
Romani L (2004) Immunity to fungal infections. Nat Rev Immunol 11:275–288
Denning DW, Pleuvry A, Cole DC (2011) Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ 89:864–872. doi:10.2471/BLT.11.089441
Hayes GE, Novak-Frazer L (2016) Chronic pulmonary aspergillosis—where are we? and where are we going? J Fungi 2:18. doi:10.3390/jof2020018
Schweer KE, Bangard C, Hekmat K, Cornely OA (2014) Chronic pulmonary aspergillosis. Mycoses 57:257–270. doi:10.1111/myc.12152
Dagenais TRT, Keller NP (2009) Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev 22:447–465
Steinbach WJ, Marr KA, Anaissie EJ et al (2012) Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect 65:453–464. doi:10.1016/j.jinf.2012.08.003
Chai L, Netea MG, Vonk AG, Kullberg B-J (2009a) Fungal strategies for overcoming host innate immune response. Med Mycol 47:227–236. doi:10.1080/13693780802209082
Chotirmall SH, Mirkovic B, Lavelle GM, McElvaney NG (2014) Immunoevasive aspergillus virulence factors. Mycopathologia 178:363–370. doi:10.1007/s11046-014-9768-y
Collette JR, Lorenz MC (2011) Mechanisms of immune evasion in fungal pathogens. Curr Opin Microbiol 14:668–675. doi:10.1016/j.mib.2011.09.007
Escobar N, Ordonez SR, Wösten HAB et al (2016) Hide, keep quiet, and keep low: Properties that make Aspergillus fumigatus a successful lung pathogen. Front Microbiol 7:1–13. doi:10.3389/fmicb.2016.00438
Krappmann S (2016) How to invade a susceptible host: cellular aspects of aspergillosis. Curr Opin Microbiol 34:136–146
Marcos CM, de Oliveira HC, de Melo W, da Silva JF et al (2016) Anti-immune strategies of pathogenic fungi. Front Cell Infect Microbiol 6:1–22. doi:10.3389/fcimb.2016.00142
Latgé JP (2010) Tasting the fungal cell wall. Cell Microbiol 12:863–872. doi:10.1111/j.1462-5822.2010.01474.x
Mouyna I, Fontaine T (2009) Cell wall of Aspergillus fumigatus: a dynamic structure. In: Latgé J, Steinbach W (eds) Aspergillus fumigatus and Aspergillosis. American Society of Microbiology, Washington, DC, pp 169–183
Bidula S, Schelenz S (2016) A sweet response to a sour situation: the role of soluble pattern recognition receptors in the innate immune response to invasive Aspergillus fumigatus infections. PLoS Pathog 12:1–6. doi:10.1371/journal.ppat.1005637
Latgé JP, Calderone R (2002) Host-microbe interactions: fungi invasive human fungal opportunistic infections. Curr Opin Microbiol 5:355–358
Levitz SM (2010) Innate recognition of fungal cell walls. PLoS Pathog 6:e1000758. doi:10.1371/journal.ppat.1000758
van de Veerdonk FL, Kullberg BJ, van der Meer JW et al (2008) Host-microbe interactions: innate pattern recognition of fungal pathogens. Curr Opin Microbiol 11:305–312. doi:10.1016/j.mib.2008.06.002
Beauvais A, Fontaine T, Aimanianda V, Latgé JP (2014) Aspergillus cell wall and biofilm. Mycopathologia 178:371–377. doi:10.1007/s11046-014-9766-0
Paris S, Debeaupuis JP, Crameri R et al (2003) Conidial hydrophobins of Aspergillus fumigatus. Appl Environ Microbiol 69:1581–1588
Pihet M, Vandeputte P, Tronchin G et al (2009) Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol 11:1–11. doi:10.1186/1471-2180-9-177
Aimanianda V, Bayry J, Bozza S et al (2009) Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460:1117–1121. doi:10.1038/nature08264
Aimanianda V, Latgé JP (2010) Fungal hydrophobins form a sheath preventing immune recognition of airborne conidia. Virulence 1:185–187. doi:10.4161/viru.1.3.11317
Rappleye CA, Goldman WE (2008) Fungal stealth technology. Trends Immunol 29:18–24. doi:10.1016/j.it.2007.10.001
Akira S, Takeda K (2004) Toll-like receptor signalling. Nature 4:88–88. doi:10.1038/nri1391
Underhill DM, Ozinsky A, Hajjar AM et al (1999) The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811–815. doi:10.1038/44605
Mambula SS, Sau K, Henneke P et al (2002) Toll-like receptor (TLR) signaling in response to Aspergillus fumigatus. J Biol Chem 277:39320–39326. doi:10.1074/jbc.M201683200
Netea MG, Warris A, Van der Meer JW et al (2003) Aspergillus fumigatus evades immune recognition during germination through loss of toll-like receptor-4-mediated signal transduction. J Infect Dis 188:320–326. doi:10.1086/376456
Luther K, Torosantucci A, Brakhage AA et al (2007) Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the dectin-1 beta-glucan receptor and Toll-like receptor 2. Cell Microbiol 9:368–381. doi:10.1111/j.1462-5822.2006.00796.x
Balloy V, Si-Tahar M, Takeuchi O et al (2005) Involvement of toll-like receptor 2 in experimental invasive pulmonary aspergillosis. Infect Immun 73:5420–5425. doi:10.1128/iai.73.9.5420-5425.2005
Meier A, Kirschning CJ, Nikolaus T et al (2003) Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell Microbiol 5:561–570. doi:10.1046/j.1462-5822.2003.00301.x
Braedel S, Radsak M, Einsele H et al (2004) Aspergillus fumigatus antigens activate innate immune cells via toll-like receptors 2 and 4. Br J Haematol 125:392–399. doi:10.1111/j.1365-2141.2004.04922.x
Chai L, Kullberg BJ, Vonk AG et al (2009b) Modulation of Toll-like receptor 2 (TLR2) and TLR4 responses by Aspergillus fumigatus. Infect Immun 77:2184–2192. doi:10.1128/iai.01455-08
Chai L, Vonk AG, Kullberg BJ et al (2011) Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes Infect 13:151–159. doi:10.1016/j.micinf.2010.10.005
Dubourdeau M, Athman R, Balloy V et al (2006) Aspergillus fumigatus induces innate immune responses in alveolar macrophages through the MAPK pathway independently of TLR2 and TLR4. J Immunol 177:3994–4001
Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650. doi:10.1016/j.immuni.2011.05.006
Hemmi H, Takeuchi O, Kawai T et al (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408:740–745. doi:10.1038/35047123
Kasperkovitz PV, Khan NS, Tam JM et al (2011) Toll-like receptor 9 modulates macrophage antifungal effector function during innate recognition of Candida albicans and Saccharomyces cerevisiae. Infect Immun 79:4858–4867. doi:10.1128/IAI.05626-11
Ramirez-Ortiz ZG, Specht CA, Wang JP et al (2008) Toll-like receptor 9-dependent immune activation by unmethylated CpG motifs in Aspergillus fumigatus DNA. Infect Immun 76:2123–2129. doi:10.1128/iai.00047-08
Kasperkovitz PV, Cardenas ML, Vyas JM (2010) TLR9 is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking. J Immunol 185:7614–7622. doi:10.4049/jimmunol.1002760
Bellocchio S, Montagnoli C, Bozza S et al (2004) The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol 172:3059–3069. doi:10.4049/jimmunol.172.5.3059
Ramaprakash H, Ito T, Standiford TJ et al (2009) Toll-like receptor 9 modulates immune responses to Aspergillus fumigatus conidia in immunodeficient and allergic mice. Infect Immun 77:108–119
Hardison SE, Brown GD (2012) C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. doi:10.1016/j.tim.2007.10.012
Brown GD, Taylor PR, Reid DM et al (2002) Dectin-1 is a major-glucan receptor on macrophages. J Exp Med 196:407–412. doi:10.1084/jem.20020470
Brown GD, Herre J, Williams DL et al (2003) Dectin-1 mediates the biological effects of beta-glucans. J Exp Med 197:1119–1124. doi:10.1084/jem.20021890
Brown GD (2006) Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol 6:33–43. doi:10.1038/nri1745
Taylor PR, Tsoni SV, Willment JA et al (2007) Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 8:31–38. doi:10.1038/ni1408
Gersuk GM, Underhill DM, Zhu L, Marr KA (2006) Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J Immunol 176:3717–3724. doi:10.4049/jimmunol.176.6.3717
Hohl TM, Van Epps HL, Rivera A et al (2005) Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog 1:e30. doi:10.1371/journal.ppat.0010030
Steele C, Rapaka RR, Metz A et al (2005) The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog 1:e42
Underhill DM, Rossnagle E, Lowell CA et al (2005) Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106:2543–2550. doi:10.1182/blood-2005-03-1239
Rogers NC, Slack EC, Edwards AD et al (2005) Syk-dependent cytokine induction by dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22:507–517. doi:10.1016/j.immuni.2005.03.004
Reedy J, Wuethrich M, Latgé J, Vyas J (2016) Dectin-2 is a receptor for galactomannan. In: 7th Advances Against Aspergillosis. Manchester, United Kingdom
Saijo S, Ikeda S, Yamabe K et al (2010) Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32:681–691. doi:10.1016/j.immuni.2010.05.001
Sun H, Xu XY, Shao HT et al (2013) Dectin-2 is predominately macrophage restricted and exhibits conspicuous expression during Aspergillus fumigatus invasion in human lung. Cell Immunol 284:60–67. doi:10.1016/j.cellimm.2013.06.013
Serrano-Gómez D, Domínguez-Soto A, Ancochea J et al (2004) Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin mediates binding and internalization of Aspergillus fumigatus conidia by dendritic cells and macrophages. J Immunol 173:5635–5643. doi:10.4049/jimmunol.173.9.5635
Shibata Y, Metzger WJ, Myrvik QN (1997) Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J Immunol 159:2462–2467
Becker K, Aimanianda V, Wang X et al (2016) Cytokines in human PBMCs via the Fc-gamm receptor/Syk/PI3K pathway. MBio 7:1–11. doi:10.1128/mBio.01823-15.Editor
Hontelez S, Sanecka A, Netea MG et al (2012) Molecular view on PRR cross-talk in antifungal immunity. Cell Microbiol 14:467–474. doi:10.1111/j.1462-5822.2012.01748.x
Gantner BN, Simmons RM, Canavera SJ et al (2003) Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med 197:1107–1117. doi:10.1084/jem.20021787
Rivera A (2014) When PRRs collide: Mincle meddles with dectin and toll. Cell Host Microbe 15:397–399. doi:10.1016/j.chom.2014.03.013
Wevers BA, Kaptein TM, Zijlstra-Willems EM et al (2014) Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host Microbe 15:494–505. doi:10.1016/j.chom.2014.03.008
Speth C, Rambach G, Lass-Flörl C et al (2004) The role of complement in invasive fungal infections. Mycoses 47:93–103
Speth C, Rambach G (2012) Complement attack against Aspergillus and corresponding evasion mechanisms. Interdiscip Perspect Infect Dis. doi:10.1155/2012/463794
Kozel TR, Wilson MA, Farrell TP, Levitz SM (1989) Activation of C3 and binding to Aspergillus fumigatus conidia and hyphae. Infect Immun 57:3412–3417
Bidula S, Kenawy H, Ali YM et al (2013) Role of ficolin-A and lectin complement pathway in the innate defense against pathogenic aspergillus species. Infect Immun 81:1730–1740. doi:10.1128/IAI.00032-13
Braem SGE, Rooijakkers SHM, van Kessel KPM et al (2015) Effective neutrophil phagocytosis of Aspergillus fumigatus is mediated by classical pathway complement activation. J Innate Immun 7:364–374. doi:10.1159/000369493
Dumestre-Perard C, Lamy B, Aldebert D et al (2008) Aspergillus conidia activate the complement by the mannan-binding lectin C2 bypass mechanism. J Immunol 181:7100–7105. doi:10.4049/jimmunol.181.10.7100
Rosbjerg A, Genster N, Pilely K et al (2016) Complementary roles of the classical and lectin complement pathways in the defense against Aspergillus fumigatus. Front Immunol 7:1–10. doi:10.3389/fimmu.2016.00473
Sturtevant J, Latgé JP (1992) Participation of complement in the phagocytosis of the conidia of Aspergillus fumigatus by human polymorphonuclear cells. J Infect Dis 166:580–586
Behnsen J, Hartmann A, Schmaler J et al (2008) The opportunistic human pathogenic fungus Aspergillus fumigatus evades the host complement system. Infect Immun 76:820–827
Vogl G, Lesiak I, Jensen DB et al (2008) Immune evasion by acquisition of complement inhibitors: the mould Aspergillus binds both factor H and C4b binding protein. Mol Immunol 45:1485–1493. doi:10.1016/j.molimm.2007.08.011
Behnsen J, Lessing F, Schindler S et al (2010) Secreted Aspergillus fumigatus protease Alp1 degrades human complement proteins C3, C4, and C5. Infect Immun 78:3585–3594. doi:10.1128/iai.01353-09
Bidula S, Sexton DW, Yates M et al (2015) H-ficolin binds Aspergillus fumigatus leading to activation of the lectin complement pathway and modulation of lung epithelial immune responses. Immunology 146:281–291. doi:10.1111/imm.12501
Madan T, Eggleton P, Kishore U et al (1997) Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect Immun 65:3171–3179
Kishore U, Greenhough TJ, Waters P et al (2006) Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol 43:1293–1315. doi:10.1016/j.molimm.2005.08.004
Mulugeta S, Beers MF (2006) Surfactant protein C: its unique properties and emerging immunomodulatory role in the lung. Microbes Infect 8:2317–2323. doi:10.1016/j.micinf.2006.04.009
Wright JR (2005) Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5:58–68. doi:10.1038/nri1528
Tafel O, Latzin P, Paul K et al (2008) Surfactant proteins SP-B and SP-C and their precursors in bronchoalveolar lavages from children with acute and chronic inflammatory airway disease. BMC Pulm Med 8:6. doi:10.1186/1471-2466-8-6
Augusto L, Synguelakis M, Johansson J et al (2003) Interaction of pulmonary surfactant protein C with CD14 and lipopolysaccharide. Infect Immun 71:61–67
Carreto-Binaghi LE, Aliouat EM, Taylor ML (2016) Surfactant proteins, SP-A and SP-D, in respiratory fungal infections: their role in the inflammatory response. Respir Res 17:66. doi:10.1186/s12931-016-0385-9
Kishore U, Madan T, Sarma PU et al (2002) Protective roles of pulmonary surfactant proteins, SP-A and SP-D, against lung allergy and infection caused by Aspergillus fumigatus. Immunobiology 205:610–618. doi:10.1078/0171-2985-00158
Sano H, Kuroki Y (2005) The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Mol Immunol 42:279–287. doi:10.1016/j.molimm.2004.07.014
Willment JA, Brown GD (2008) C-type lectin receptors in antifungal immunity. Trends Microbiol 16:27–32. doi:10.1016/j.tim.2007.10.012
Allen MJ, Harbeck R, Smith B et al (1999) Binding of rat and human surfactant proteins A and D to Aspergillus fumigatus conidia. Infect Immun 67:4563–4569
Mehrad B, Strieter RM, Standiford TJ (1999) Role of TNF-α in pulmonary host defense in murine invasive aspergillosis. J Immunol 162:1633–1640
Vandivier RW, Ogden CA, Fadok VA et al (2002) Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol 169:3978–3986. doi:10.4049/jimmunol.169.7.3978
Madan T, Kishore U, Singh M et al (2001) Protective role of lung surfactant protein D in a murine model of invasive pulmonary aspergillosis. Infect Immun 69:2728–2731. doi:10.1128/IAI.69.4.2728
Madan T, Reid KBM, Clark H et al (2010) Susceptibility of mice genetically deficient in SP-A or SP-D gene to invasive pulmonary aspergillosis. Mol Immunol 47:1923–1930. doi:10.1016/j.molimm.2010.02.027
LeVine AM, Hartshorn K, Elliott J et al (2002) Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am J Physiol Lung Cell Mol Physiol 282:L563–L572. doi:10.1152/ajplung.00280.2001
Neth O, Jack DL, Dodds AW et al (2000) Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun 68:688–693. doi:10.1128/iai.68.2.688-693.2000
Oikonomopoulou K, Edimara S, Lambris JD (2012) Complement system and its role in immune responses. Encycl life Sci. doi:10.1002/9780470015902.a0000508.pub3
Clemons KV, Martinez M, Tong AJ, Stevens DA (2010) Resistance of MBL gene-knockout mice to experimental systemic aspergillosis. Immunol Lett 128:105–107. doi:10.1016/j.imlet.2009.12.021
Crosdale DJ, Poulton K V, Ollier WE, et al (2001) Mannose-binding lectin gene polymorphisms as a susceptibility factor for chronic necrotizing pulmonary aspergillosis. J Infect Dis 184:653–656. doi:10.1086/322791. JID001096 [pii]
Lambourne J, Agranoff D, Herbrecht R et al (2009) Association of mannose-binding lectin deficiency with acute invasive aspergillosis in immunocompromised patients. Clin Infect Dis 49:1486–1491. doi:10.1086/644619
Matsushita M (2009) Ficolins: complement-activating lectins involved in innate immunity. J Innate Immun 2:24–32. doi:10.1159/000228160
Endo Y, Matsushita M, Fujita T (2011) The role of ficolins in the lectin pathway of innate immunity. Int J Biochem Cell Biol 43:705–712. doi:10.1016/j.biocel.2011.02.003
Hummelshøj T, Ma YJ, Munthe-Fog L et al (2012) The interaction pattern of murine serum ficolin-A with microorganisms. PLoS One 7:1–12. doi:10.1371/journal.pone.0038196
Ma YJ, Doni A, Hummelshøj T et al (2009) Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J Biol Chem 284:28263–28275. doi:10.1074/jbc.M109.009225
Genster N, Præstekjær Cramer E, Rosbjerg A et al (2016) Ficolins promote fungal clearance in vivo and modulate the inflammatory cytokine response in host defense against Aspergillus fumigatus. J Innate Immun. doi:10.1159/000447714
Bals R, Hiemstra PS (2004) Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J 23:327–333. doi:10.1183/09031936.03.00098803
Croft CA, Culibrk L, Moore MM, Tebbutt SJ (2016) Interactions of Aspergillus fumigatus conidia with airway epithelial cells: a critical review. Front Microbiol 7:1–15. doi:10.3389/fmicb.2016.00472
Botterel F, Gross K, Ibrahim-Granet O et al (2008) Phagocytosis of Aspergillus fumigatus conidia by primary nasal epithelial cells in vitro. BMC Microbiol 8:97. doi:10.1186/1471-2180-8-97
Paris S, Boisvieux-Ulrich E, Crestani B et al (1997) Internalization of Aspergillus fumigatus conidia by epithelial and endothelial cells. Infect Immun 65:1510–1514
Wasylnka JA, Moore MM (2003) Aspergillus fumigatus conidia survive and germinate in acidic organelles of A549 epithelial cells. J Sell Sci 116:1579–1587. doi:10.1242/jcs.00329
Berkova N, Lair-Fulleringer S, Femenia F et al (2006) Aspergillus fumigatus conidia inhibit tumour necrosis factor- or staurosporine-induced apoptosis in epithelial cells. Int Immunol 18:139–150. doi:10.1093/intimm/dxh356
Zhang Z, Liu R, Noordhoek JA, Kauffman HF (2005) Interaction of airway epithelial cells (A549) with spores and mycelium of Aspergillus fumigatus. J Infect 51:375–382. doi:10.1016/j.jinf.2004.12.012
Alekseeva L, Huet D, Femenia F et al (2009) Inducible expression of beta defensins by human respiratory epithelial cells exposed to Aspergillus fumigatus organisms. BMC Microbiol 9:33. doi:10.1186/1471-2180-9-33
Lupetti A, van Dissel J, Brouwer C, Nibbering P (2008) Human antimicrobial peptides’ antifungal activity against Aspergillus fumigatus. Eur J Clin Microbiol Infect Dis 27:1125–1129. doi:10.1007/s10096-008-0553-z
Okamoto T, Tanida T, Wei B et al (2004) Regulation of fungal infection by a combination of amphotericin B and peptide 2, a lactoferrin peptide that activates neutrophils. Clin Diagn Lab Immunol 11:1111–1119. doi:10.1128/CDLI.11.6.1111
Soruri A, Grigat J, Forssmann U et al (2007) β-Defensins chemoattact macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. Eur J Immunol 37:2474–2486. doi:10.1002/eji.200737292
Heinekamp T, Thywißen A, Macheleidt J et al (2012) Aspergillus fumigatus melanins: Interference with the host endocytosis pathway and impact on virulence. Front Microbiol 3:1–7. doi:10.3389/fmicb.2012.00440
Jahn B, Langfelder K, Schneider U et al (2002) PKSP-dependent reduction of phagolysosome fusion and intracellular kill of Aspergillus fumigatus conidia by human monocyte-derived macrophages. Cell Microbiol 4:793–803. doi:10.1046/j.1462-5822.2002.00228.x
Thywißen A, Heinekamp T, Dahse HM et al (2011) Conidial dihydroxynaphthalene melanin of the human pathogenic fungus Aspergillus fumigatus interferes with the host endocytosis pathway. Front Microbiol 2:1–12. doi:10.3389/fmicb.2011.00096
Akoumianaki T, Kyrmizi I, Valsecchi I et al (2016) Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity. Cell Host Microbe 19:79–90. doi:10.1016/j.chom.2015.12.002
Eisenman HC, Casadevall A (2012) Synthesis and assembly of fungal melanin. Appl Microbiol Biotechnol 93:931–940. doi:10.1007/s00253-011-3777-2
Pal AK, Gajjar DU, Vasavada AR (2014) DOPA and DHN pathway orchestrate melanin synthesis in Aspergillus species. Med Mycol 52:10–18. doi:10.3109/13693786.2013.826879
Ibrahim-Granet O, Philippe B, Boleti H et al (2003) Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect Immun 71:891–903
Philippe B, Ibrahim-Granet O, Prevost MC et al (2003) Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun 71:3034–3042
Chai L, Netea MG, Sugui J et al (2010) Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology 215:915–920. doi:10.1016/j.imbio.2009.10.002
Kyrmizi I, Gresnigt MS, Akoumianaki T et al (2013) Corticosteroids block autophagy protein recruitment in Aspergillus fumigatus phagosomes via targeting Dectin-1/Syk kinase signaling. J Immunol 191:1287–1299. doi:10.4049/jimmunol.1300132
Jacobson ES (2000) Pathogenic roles for fungal melanins. Clin Microbiol Rev 13:708–717. doi:10.1128/CMR.13.4.708-717.2000
Chamilos G, Akoumianaki T, Kyrmizi I et al (2016) Melanin targets LC3-associated phagocytosis (LAP): a novel pathogenetic mechanism in fungal disease. Autophagy 12:888–889. doi:10.1080/15548627.2016.1157242
Sprenkeler EGG, Gresnigt MS, van de Veerdonk FL (2016) LC3-associated phagocytosis: a crucial mechanism for antifungal host defence against Aspergillus fumigatus. Cell Microbiol 18:1208–1216. doi:10.1111/cmi.12616
Cunha C, Kurzai O, Löffler J et al (2014) Neutrophil responses to aspergillosis: new roles for old players. Mycopathologia. doi:10.1007/s11046-014-9796-7
Levitz SM, Diamond RD (1985) Mechanisms of resistance of Aspergillus fumigatus conidia to killing by neutrophils in vitro. J Infect Dis 152:33–42. doi:10.1093/infdis/152.1.33
Mircescu MM, Lipuma L, van Rooijen N et al (2009) Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect Dis 200:647–656. doi:10.1086/600380
Fontaine T, Delangle A, Simenel C et al (2011) Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. doi:10.1371/journal.ppat.1002372
Robinet P, Baychelier F, Fontaine T et al (2014) A polysaccharide virulence factor of a human fungal pathogen induces neutrophil apoptosis via NK cells. J Immunol 192:5332–5342. doi:10.4049/jimmunol.1303180
Gresnigt MS, Bozza S, Becker KL et al (2014) A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of Interleukin-1 receptor antagonist. PLoS Pathog 10:e1003936. doi:10.1371/journal.ppat.1003936
Gazendam R, van Hamme JL, Tool ATJ et al (2016) Human neutrophils use different mechanisms to kill Aspergillus fumigatus conidia and hyphae: evidence from phagocyte defects. J Immunol. doi:10.4049/jimmunol.1501811
Brinkmann V, Reichard U, Goosmann C et al (2004) Neutrophil extracellular traps and kill bacteria. Science 303:1532–1535. doi:10.1126/science.1092385
Bruns S, Kniemeyer O, Hasenberg M et al (2010) Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog 6:e1000873. doi:10.1371/journal.ppat.1000873
Urban CF, Reichard U, Brinkmann V, Zychlinsky A (2006) Neutrophil extracellular traps capture and kill Candida albicans and hyphal forms. Cell Microbiol 8:668–676. doi:10.1111/j.1462-5822.2005.00659.x
McCormick A, Heesemann L, Wagener J et al (2010) NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect 12:928–936. doi:10.1016/j.micinf.2010.06.009
Ellett F, Jorgensen J, Frydman GH et al (2017) Neutrophil interactions stimulate evasive hyphal branching by Aspergillus fumigatus. PLoS Pathog 13:e1006154. doi:10.1371/journal.ppat.1006154
Fallon JP, Reeves EP, Kavanagh K (2010) Inhibition of neutrophil function following exposure to the Aspergillus fumigatus toxin fumagillin. J Med Microbiol 59:625–633. doi:10.1099/jmm.0.018192-0
Bassetti M, Pecori D, Della Siega P et al (2014) Current and future therapies for invasive aspergillosis. Pulm Pharmacol Ther 32:1–11. doi:10.1016/j.pupt.2014.06.002
Arendrup MC (2014) Update on antifungal resistance in Aspergillus and Candida. Clin Microbiol Infect 8:42–48. doi:10.1111/1469-0691.12513
Hadrich I, Makni F, Neji S et al (2012) Invasive aspergillosis: resistance to antifungal drugs. Mycopathologia 174:131–141. doi:10.1007/s11046-012-9526-y
Barczak AK, Hung DT (2009) Productive steps toward an antimicrobial targeting virulence. Curr Opin Microbiol 12:490–496. doi:10.1016/j.mib.2009.06.012
Clatworthy AE, Pierson E, Hung DT (2007) Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol 3:541–548. doi:10.1038/nchembio.2007.24
Gauwerky K, Borelli C, Korting HC (2009) Targeting virulence: a new paradigm for antifungals. Drug Discov Today 14:214–222. doi:10.1016/j.drudis.2008.11.013
Allen RC, Popat R, Diggle SP, Brown SP (2014) Targeting virulence: can we make evolution-proof drugs? Nat Rev Microbiol 12:300–308. doi:10.1038/nrmicro3232
Bromuro C, Romano M, Chiani P et al (2010) Beta-glucan-CRM197 conjugates as candidates antifungal vaccines. Vaccine 28:2615–2623. doi:10.1016/j.vaccine.2010.01.012
Torosantucci A, Bromuro C, Chiani P et al (2005) A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 202:597–606. doi:10.1084/jem.20050749
Chotirmall SH, Al-Alawi M, Mirkovic B et al (2013) Aspergillus-associated airway disease, inflammation, and the innate immune response. Biomed Res Int 2013:723129. doi:10.1155/2013/723129
Phadke AP, Mehrad B (2005) Cytokines in host defense against Aspergillus: recent advances. Med Mycol 43:173–176. doi:10.1080/13693780500052099
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Wong, S.S.W., Latgé, JP. (2017). When Aspergillus fumigatus Meets the Man. In: Mora-Montes, H., Lopes-Bezerra, L. (eds) Current Progress in Medical Mycology. Springer, Cham. https://doi.org/10.1007/978-3-319-64113-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-64113-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-64112-6
Online ISBN: 978-3-319-64113-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)