Abstract
The intrinsic anatomic deformities combined with changes related to growth and anatomical modifications and scarring from previous procedures make secondary unilateral cleft rhinoplasty a formidable challenge for the plastic surgeons. A thorough understanding of the deformities and the surgical maneuvers forms the foundation for successful cleft nose repair. In this chapter, we include an overview of secondary unilateral cleft rhinoplasty including a brief history, the anatomy of deformity, and the therapeutic approach.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
20.1 Introduction
Unilateral cleft nose repair is challenging because of the complexity of the deformity. This three-dimensional deformity involves several structures such as the lower lateral cartilage (the medial and lateral crus), the nasal dome, the columella, the nasal septum, and the skeletal platform, which includes the alveolus, maxillary segments, and palate (Fisher et al. 2014; Byrd et al. 2007). Thus, to obtain the realistic treatment goal (normal appearance and function, with better symmetry, balance, and less scarring), both skeletal and soft-tissue structures must be adequately managed. Although the primary cleft rhinoplasty has currently been performed at the time of cleft lip repair, the longitudinal follow-up usually revels a residual (from minor to major) nasal deformity (Freeman et al. 2013; Haddock et al. 2012; Chang et al. 2010; Salyer et al. 2004), regardless of surgeon’s skills. Therefore, secondary (definitive or final) cleft nose repair with a greater number and complexity of maneuvers is needed after the completion of facial growth to correct aesthetic and functional issues (Hwang et al. 2012; Masuoka et al. 2012; Turkaslan et al. 2008; Bashir et al. 2011; Guyuron 2008; Stal and Hollier 2002).
In this chapter, we include an overview of secondary unilateral cleft rhinoplasty including a brief history, the anatomy of deformity, and the surgical approach.
20.2 Brief History of Cleft Nose Repair
In 1932, Gillies and Kilner (1932) introduced a superior advancement of the composite chondrocutaneous hemicolumella flap using a midcolumellar incision. In 1964, Converse (1964) provided the first major modification of this technique by replacing the midcolumellar incision with a marginal incision; the medial crura composite flap was advanced superiorly and sutured to the contralateral dome, and the defect at the base of the columella was repaired with an auricular composite graft. In 1954, Potter (1946) advocated a similar concept but from the opposite direction, using a lateral-to-medial advancement of the lateral crural composite chondrocutaneous flap; the resultant defect created in the lateral vestibular skin was closed in a V-to-Y fashion. In 1977, Tajima and Maruyama (1977) described the reverse-U incision to address two classic cleft problems, namely obliteration of the soft triangle and nostril apex overhang. This incision starts inferomedially at the junction of the columella and membranous septum, and continues superiorly into the depressed dome skin, creating an arc similar in shape to the nostril on the noncleft side, and returning into the mucosa of the nostril. After wide undermining of the nasal skin envelope, the cartilages are repositioned and the excess skin of the nostril apex is rolled into the nostril. Closure of the skin edges creates a soft triangle on the cleft side. In 1982, Dibbell (1982) proposed incisions within the nostril rim and excision of soft tissue to correct medial rotation of the lower lateral cartilage, lateral displacement of the alar base, twisting of the domes, columellar asymmetry, and overhang of the ala. This technique is accomplished through the creation of a double-pedicled composite flap of lower lateral cartilage, mucosa, columella, and nasal floor, followed by superior and medial rotation of the flap, resulting in an anatomical repositioning of the displaced lower lateral cartilage. In 2009, Flores et al. (2009) reported the Cutting’s experience adopting an open rhinoplasty approach using a combination of both the Dibbell and Tajima techniques to correct the nostril apex overhang and reposition the depressed lower lateral cartilage and laterally displaced ala on the cleft side. They reported that avoidance of an upper lip incision with this technique is an advantage, particularly in those patients who have a well-healed lip scar from primary lip repair. Historically, numerous other techniques have been described for cleft nose repair, including suture, flaps, and cartilage grafting techniques (Hwang et al. 2012; Masuoka et al. 2012; Turkaslan et al. 2008; Bashir et al. 2011; Guyuron 2008; Stal and Hollier 2002).
20.3 Unilateral Cleft Nose Deformity
To repair the cleft nose, plastic surgeons should become familiar with the abnormalities and dysmorphology associated with the specific deformity and its effects on nose physiology resulting in nasal dysfunction (Guyuron 2008; Kaufman et al. 2012). It is important to recognize that the nasal deformity at the time of primary cleft repair may vary significantly from the secondary deformity seen in adulthood (Guyuron 2008; Kaufman et al. 2012).
20.3.1 Primary Cleft Nose Deformity
The primary unilateral cleft nose deformity is characterized by the following features: the columella is shorter on the cleft side; the base of the columella is deviated to the noncleft side; the lateral crus of the lower lateral cartilage is longer on the cleft side; the nasal tip is displaced in both the frontal and the horizontal planes; the nasal tip is asymmetric; the ala is flattened, resulting in horizontal orientation of the nostril; the nostrils are asymmetric; the entire nostril is retropositioned because of the deficiency in the underlying frame; the base of the ala is displaced laterally and/or posteriorly and sometimes inferiorly; the nasal floor is caudal on the cleft side; a nasolabial fistula could be present; the septum and anterior nasal spine are shifted toward the noncleft vestibule; the nasal septum is deviated, resulting in a varying degree of nasal obstruction; the inferior turbinate on the cleft side is hypertrophic; the maxilla is hypoplastic on the cleft side; and the premaxilla and the maxillary segments are displaced (Bardach and Cutting 1990).
20.3.2 Secondary Cleft Nose Deformity
Features of the primary deformity complicated by the influence of primary rhinoplasty and facial growth eventually determinate a complex and wide spectrum of secondary cleft nasal deformities (Figs. 20.1, and 20.2). The cleft ala lies caudal and lateral to the noncleft side. It rests on an underdeveloped maxilla, which partly accounts for alar base lowering and horizontal nostril seating. The cleft ala may be underdeveloped and weak and exhibit a convoluted shape. This contributes further to dome lowering on the cleft side. Malfunction of the cleft ala external valve is caused by alar base malposition, imbalanced muscular pull, and abnormal attachment of the cheek muscles to the lateral crus. Tip projection is further compromised by a foreshortened columella that lies obliquely with its base directed away from the cleft side. The caudal septum is associated with the anterior nasal spine, which is deviated off facial midline to the noncleft side. The cartilaginous mid-septum and the osseous posterior septum (perpendicular plate of the ethmoid bone) deviate significantly toward the cleft side, resulting in a complex C-shaped deformity both craniocaudally and anteroposteriorly. The deviation of the cartilaginous septum toward the cleft side narrows the cleft-side airway while enlarging the noncleft cross-sectional area. The noncleft-side turbinate hypertrophies to occupy this space on the noncleft side. The nasal bones are frequently widened both at the dorsum and at the frontal process of the maxilla. Deviation may affect the bony and the cartilaginous segments. Generally, midvault curvature is present with collapse on the concave side and fullness on the convex side. Furthermore, smaller airways as demonstrated in rhinometry and external valve malfunction may add to the airway problem (Fisher et al. 2014; Byrd et al. 2007).
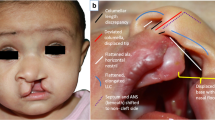
(Left) Full-face front and (right) basal views of a skeletally mature patient with unilateral complete cleft lip and palate illustrating the secondary unilateral cleft lip nasal deformity: nasal tip deviated; alar cartilage displaced caudally; angle between medial and lateral crura more obtuse buckling in lateral crura; the alar base deviated posteriorly, inferiorly, and laterally when compared with the noncleft side; flattened alar facial angle; widened nostril floor; columella and anterior caudal septal border deviated on noncleft side
20.4 Surgical Management
The goals of cleft nose repair include final creation of lasting symmetry, achieving definition of the nasal base and nasal tip, relief of nasal obstruction, and management of nasal scarring and webbing. In the literature (Fisher et al. 2014; Byrd et al. 2007; Guyuron 2008; Stal and Hollier 2002; Wolfe et al. 2016; Sykes et al. 2016), there are an enormous variation in techniques and treatment protocols for the cleft nose. In fact, as the clinical presentation of cleft nose deformities varies widely, each particular cleft patient presents a unique challenge and an arsenal of well-orchestrated maneuvers can be used with slight variations from patient to patient. As complete correction of all of the cleft nose deformities remains a challenge for plastic surgeons, a standardized surgical approach based on the severity of soft-tissue and skeletal deformities as well as previous procedures performed is important to outline the predilection of the results and their limitations.
20.4.1 Timing
Relevant standardized surgical steps (namely, primary rhinocheiloplasty, alveolar bone grafting, and Le Fort I advancement) from the comprehensive rehabilitate longitudinal cleft care are extremely relevant prior to the secondary cleft rhinoplasty as it may directly influence the surgical approach and outcomes.
Performing primary cleft rhinoplasty at the same setting as the cleft lip repair had been accepted worldwide and the traditional concern for disruption of growth centers in the nose has waned (Millard and Morovic 1998; McComb and Coghlan 1996). The principal goal of primary nasal correction has been to produce a more symmetrical nasal form (closure of the nasal floor and sill, repositioning of the alar base, and repositioning of the lower lateral cartilages) and to reduce the stigma that is often experienced during childhood. It may also provide a less complicated secondary revision, which is required by many patients in late adolescence (Byrd et al. 2007; Haddock et al. 2012). At our craniofacial plastic surgery center, primary cleft lip nose repair is typically performed at 3 months of age; we adhere to the conventional rule of 10s, and surgery is deferred until the child is 10 pounds in weight, at or after 10 weeks of age, with a hemoglobin concentration of 10 g/dL. We (Buzzo 2010; Raposo-Amaral et al. 2014, 2012; Raposo-Amaral 2010; Somensi et al. 2012) have particularly adopted two primary cleft lip repairs (namely, modified Göteborg technique and modified Cutting extended Mohler technique according to author’s experience) without presurgical nasoalveolar molding. The treatment of the unilateral cleft nose has been according to McComb primary nasal reconstruction principles (McComb and Coghlan 1996; Buzzo 2010; Raposo-Amaral et al. 2014, 2012; Raposo-Amaral 2010; Somensi et al. 2012): using the existing cleft lip incisions, wide undermining of the nasal cartilages from the nasal skin is undertaken from the nostril rim to the nasion; and the lower lateral cartilages are then supported in proper position with sutures. Further relevant modifications were compiled in the “fifty years of the Millard rotation-advancement” article (Stal et al. 2009).
As the interplay of anatomy variables between maxillary advancement and rhinoplasty is inseparable (Davidson and Kumar 2015), secondary cleft nasal reconstruction should not be performed without first evaluating and correcting any significant problems with the skeletal base under the nose (Cutting 2000). Restoration of the continuity of the maxillary arch with alveolar bone grafting allows closure of oronasal fistulae, proper platform for tooth eruption, and alar base and pyriform aperture augmentation (Alonso et al. 2014; Raposo-Amaral et al. 2015a). In our center, cleft patients have preferably undergone transferring of secondary alveolar bone graft (between 7 and 12 years old) immediately before the cleft-side canine eruption and with previous orthodontic management. Late secondary alveolar bone grafting (>12 years) has been implemented in delayed referral. We adopted well-described principles (Alonso et al. 2014; Raposo-Amaral et al. 2015a; Santiago et al. 2014) including appropriate flap design, wide exposure, nasal floor reconstruction without tension, closure of oronasal fistula, packing bony defect with cancellous bone, and coverage of bone graft with gingival mucoperiosteal flaps. Bone grafts have been harvested from the anterior superior iliac crest by minimal access using two different techniques (Raposo-Amaral et al. 2015b).
Once skeletal growth nears completion, patients with repaired cleft lip and palate often exhibit a characteristic concave facial profile, which requires correction by Le Fort I osteotomy and maxillary advancement (Good et al. 2007). Le Fort I internal distraction presents better dental occlusion, less relapse, and better speech results than conventional orthognathic procedure, particularly in cleft patients with severe maxillary deficiency (Kumar et al. 2006), and the gradual advancement produced by distraction osteogenesis may result in greater facial soft-tissue changes and nasal projection than similar advancements using conventional maxillary advancement (Chua and Cheung 2012). At our center, Le Fort I internal distraction is adopted for surgical correction of the class III malocclusion secondary to maxillary hypoplasia in cleft patients with established severe negative overjet near the time of maxillary growth completion (11–12 years of age) and in cleft patients with maxillary retrusion (10 mm or higher of discrepancy between jaws) who have reached skeletal maturity. On the other side, conventional maxillary advancement (combined or not with mandibular setback) has been adopted in selected skeletal maturity patients with cleft maxillary hypoplasia according to the availability of devices and potential to adhere to the institutional protocol of distraction osteogenesis.
Finally, we perform the secondary cleft rhinoplasty at 14–16 years of age in female patients and at 16–18 years of age in male patients, as it allows the completion of the postpubertal growth spurt in the maxillary and nose (anterior septum and bony dorsum). Rhinoplasty at this time is definitive and more aggressive surgical maneuvers (e.g., septoplasty, cartilage grafting, and osteotomies) may be performed without concerns for affecting maxillary and nasal growth. In selected situations (i.e., severe nasal obstruction due to caudal septal deviation; and severe emotional distress from peer psychological pressure even with all the multidisciplinary support including longitudinal psychological care), an intermediate rhinoplasty (generally more conservative) is performed before the completion of nasal growth. In addition, if a cleft patient with significant dentofacial deformities (typically class III malocclusion) refuses to undergo maxillary reconstruction, their secondary rhinoplasty is delayed until this patient with aid of psychological support accepts the correction of the underlying skeletal base by alveolar bone grafting and/or Le Fort I advancement according to their individual needs.
20.4.2 Preoperative Characterization of Deformity
To make an accurate diagnosis of secondary cleft nose deformity, the skin (thickness), the nasal bones (symmetry, length, and distance from the midline; the depth of the radix; and the presence or absence of a dorsal hump), the midvault (upper lateral cartilage collapse and vertical symmetry), the nasal tip (asymmetry or fullness; projection; bulbous, boxy, narrow, or parenthesis deformity), the alar base (width), the alae (thickness, vertical position), the nasal sill (configuration), the nasolabial angle, the internal and external valves (stenosis), the septum (deviation, perforation), and the turbinates (size and shape) should be examined and documented in detail (Fisher et al. 2014; Guyuron 2008). Nasal endoscopy and computed tomographic scans provide visualization beyond that which is visible on anterior rhinoscopy and are useful in surgical planning (Fisher et al. 2014). Some of the cardinal deformities proposed by Lee et al. (2011) and the key points described by Byrd et al. (2007) are extremely useful and complementary in the characterization of the deformity, contributing to elucidation of a specific anatomic pattern, and allow plastic surgeons to perform the most effective and directed correction procedures based on the formulation of a patient-customized surgical plan (Table 20.1).
20.5 Secondary Unilateral Cleft Rhinoplasty
The surgical reconstruction of cleft nose deformity borrows from a large number of historical and innovative surgical principles as stated by Wolfe (2004). We compile the previously described surgical maneuvers (Fisher et al. 2014; Byrd et al. 2007; Guyuron 2008; Stal and Hollier 2002; Potter 1946; Tajima and Maruyama 1977; Flores et al. 2009; Kaufman et al. 2012; Wolfe et al. 2016; Sykes et al. 2016; Cutting 2000; Basta et al. 2014; Chang et al. 2011; Wang 2010; Cho 2007) which our group have adopted in secondary unilateral cleft rhinoplasties.
20.5.1 Inferior Turbinate Hypertrophy
We preferably perform selective submucosal resection of bone combined with lateral out-fracture and lateral displacement on one or both sides depending on the degree of obstruction. Performing this first will avoid trouble with bleeding in the remaining surgical intervention.
20.5.2 Open Approach
An open rhinoplasty approach facilitates nasal correction as it allows maximal visualization for accurate diagnosis, and adequate exposure for placement and suturing of structural grafts. We adopted a standard inverted V-shaped incision or a prior transcolumellar incision. In asymmetric nostrils, we connect the inferior/medial pole of the Tajima inverted-U nostril apex incision with the transcolumellar incision to reposition the alar cartilage and recontour the soft-tissue envelope of the nose on the cleft side. Subsequently, the nasal tip and nasal dorsum are degloved in a supraperichondrial and subperiosteal plane. Next, the septum is approached by dividing the interdomal ligament of the lower lateral cartilages. A submucoperichondrial dissection is performed, beginning at the anterior septal angle. Bilateral mucoperichondrial tunnels are dissected deep to the upper lateral cartilages, and a scalpel is used to separate the upper lateral cartilages from the dorsal septum, taking care not to disrupt the k-area and lose the anchoring point of the upper lateral cartilages.
20.5.3 Dorsum
Dorsal humps are usually not a significant issue for cleft patients, but if a dorsal humpectomy is indicated, conservative excision is advisable at the outset of the surgical procedure because additional excision is always possible. If a bone hump is to be reduced, a subperiosteal pocket should be created; if osteotomies are expected, this dissection should not be carried out laterally to preserve soft-tissue support of the nasal bones. The cartilaginous dorsum is sharply excised with a number 11 blade under direct vision, followed by a series of graded fomon rasps for mild-to-moderate osseous hump reduction. For large dorsal humps, an en bloc bone and cartilaginous dorsal hump reduction is performed with a 10-mm nasal osteotome. Care is taken not to disrupt the upper lateral cartilage attachments to the undersurface of the nasal bones. Nasal rasps can be further used to soften any jagged, asymmetrical, or irregular edges; it should be performed at an oblique angle to again avoid loss of upper lateral cartilage attachment and direct trauma to these cartilages themselves.
If the dorsum is deficient or the nose is short, osseocartilaginous dorsal onlay rib graft is our choice for reconstruction of the dorsum. The harvested rib segment is shaped to span the entire length of the nasal dorsum, from the radix to the septal angle, to minimize the risk for palpable irregularities. In addition, the recipient bed must be made as flat and as smooth as possible to give the greatest surface area for the dorsal onlay graft to contact.
20.5.4 Septum
Having achieved a smooth dorsum, comprehensive treatment of the septum is undertaken. The bowing midportion of the cartilaginous septum is resected, leaving behind a 12–15 mm L-strut; it not only treats the septal deformity but also provides graft material. Deviated portions of the perpendicular plate of the ethmoid bone are carefully resected, avoiding transmission of forces cephalad that can injure the cribriform plate. A typically lengthy spur along the maxillary crest is also resected using a combination of a 2 mm osteotome and Kerrison rongeurs. Next, the caudal portion of the L-strut is disarticulated from the osseocartilaginous junction with the anterior nasal spine and maxillary crest in the noncleft side, the degree of vertical excess is then excised as indicated, and it is finally anchored at the midline into the periosteum of the anterior nasal spine.
20.5.5 Middle Nasal Vault
If the internal valve has collapsed, suturing Sheen’s spreader grafts (contoured in a rectangular shape with variable length, 1–4 mm in width, and no more than 5 mm in height so as not to impinge on the nasal airway) between the dorsal septum (2 mm below the septal border) and the anterior aspect of the upper lateral cartilages reconstructs the midvault (close the open-roof deformity, if present) while improving the internal valve and straightening the dorsal angle. Depending on the amount of deviation and asymmetry, bilateral or asymmetric spreader grafts are applied; a thicker graft can be placed on the cleft side to address concavity, if present. In selected patients, caudally extended bilateral spreader grafts is a very useful technique for nasal lengthening and controlling of tip projection, rotation, and shape; stability is optimized when these grafts are integrated with a columellar strut. We prefer harvesting the spreader grafts from the cartilage of the septum. If the quadrangular cartilage is insufficient, the costal cartilage grafts are harvested from the sixth or seventh ribs. The segment of the rib harvested can be up to 3.5–4 cm in length (it generally provides sufficient cartilaginous tissue for both the dorsal graft and the columellar strut) (Figs. 20.3 and 20.4) and be delivered through an inframammary incision in female (marked approximately 5 mm above the fold) or subcostal incision in male with variable dimensions according to the surgical technique and patients’ characteristics.
(Left) Rib cartilage grafts (strut columellar and spreader grafts). (Central) Basal and (right) lateral intraoperative views of bilateral spreader grafts and strut columellar graft inset. The columellar strut placed between the paired intermediate and medial crura provides structural support to the nasal tip and improves tip projection. Spreader grafts placed along either side of the septum correct internal nasal valve dysfunction. Clinical photographs of the patient in Figs. 20.27–20.31
Spreader flaps, also known as autospreader flaps or turnover flap, entail mucosal elevation as with normal spreader grafts, then use of the medial aspect of the upper lateral cartilages themselves as a spreader; this is accomplished with either complete separation, a partial-thickness incision and hinged placement, or folding of the medial aspect of the cartilage without any incisions. It adjusts the height of the upper lateral cartilages in a precise and safe manner while preserving the function of the internal valve. These flaps are secured in the same fashion as standard spreaders.
20.5.6 Nasal Tip
A variety of surgical maneuvers can be used to enhance or improve the nasal tip. The tongue-in-groove technique allows the nasal tip to be resuspended on the septum (i.e., fixation of the medial crura of the lower lateral cartilages to the caudal end of the nasal septum) to improve tip support and projection; the cleft alar cartilage has to be advanced more than the noncleft side to improve the flattening of the cleft lower lateral cartilage and enhance overall tip symmetry. As an alternative, we prefer to advance and fix the medial crura on the columellar strut cartilage graft to enhance projection and support according to the Anderson’s tripod theory of nasal tip support.
Once the central limb of the tripod is stabilized, attention is directed to its lateral limbs. The cleft lateral crus of the lower lateral cartilage is usually concave and often associated with alar malposition, with the cartilage often being inferiorly displaced in relation to the position of the noncleft lower lateral cartilage. An alar margin (rim) graft (placed inferior to the existing cartilage in a nonanatomic position) or a Gunter’s lateral crural strut graft (placed on the deep surface of the lower lateral cartilage, with the graft sutured to the undersurface of the cartilage, and the lateral extent positioned in a pocket at the pyriform aperture) can be adopted for supporting the alar rim, elevating the level of the alar rim, and repositioning the rim laterally. Importantly, the lateral crural strut graft is well suited to the thin-skinned patient who has a moderate degree of alar collapse and in whom an unfavorable aesthetic result would be expected with alar batten grafting (placed cephalad to the alar rim for correction of external nasal valve collapse; the exact position of the graft is determined by the site of maximal collapse). An alar turn-in flap (the cephalic portion of the lower lateral cartilage is transposed on a pedicle and sutured to the undersurface of the remaining lower lateral cartilage) or the flip-flop technique (dissecting the lateral crura off the underlying vestibular skin, excising this portion, turning it over, and resuturing it to the vestibular lining) can also be adopted to strengthen and support the lower lateral cartilage and to flatten the preexisting concavity. A superomedially based V-Y chondromucosal composite flap of the cleft-side lateral crus of the lower lateral and its attendant nasal mucosa in association with an interdomal suture (to advance the cleft-side lower lateral cartilage flap) and a Tajima-type suture (to suspend the lower lateral cartilage to the contralateral upper lateral cartilage) can also be an option to achieve symmetric tip contour and projection.
If the tip cartilages have been damaged in the previous rhinoplasty procedures, we adopt the “Golden Arch” procedure described by Wolfe. A whole new alar structure (septal or costal cartilage) is sutured to the tip of the columellar strut and folded over to make a new ala, ignoring the native cartilage still tethered below. Instead, one-half of the arch can be sutured to the columellar strut and the underlying native ala (Figs. 20.5, 20.6, and 20.7).
(Left) Intraoperative basal photograph of a skeletally mature patient with unilateral complete cleft lip and palate illustrating the commitment of the cleft-side alar cartilage. (Right) Intraoperative basal photograph demonstrating a new cleft-side alar cartilage framework fabricated overlying the native alar cartilage, a septal cartilage shield tip graft, and rib cartilage strut columellar graft. Clinical photographs of the patient in Figs. 20.24–20.26
In addition, intradomal, interdomal, and/or transdomal sutures can be used for improvement of alar contour as indicated. Imbrication of the cleft-side scroll area can also be executed by placing mattress sutures internally to raise the lateral crus cephalad, if needed. The glabella, dorsum, tip, and/or infratip lobule can be filled with diced cartilage to camouflage irregularities, especially in cleft patients with thin or inelastic skin. Further cartilaginous tip graft can be added to camouflage irregularities and improve tip definition or according to specific diagnosis, individual needs and/or prevent postoperative abnormalities. Overall, placement of tip grafts over the tip-defining points will increase tip projection and definition, whereas placement of these grafts at and below the tip-defining points will increase projection and add volume to the infratip lobule. The Sheen’s shield graft (or infra-lobular graft) may be inserted at the tip-columellar junction (anterior to the intermediate crura) to define the “double-break” columellar profile; beveling of edges is important to avoid a visible “tombstone” appearance through skin. The anchor graft, a modified infratip shield graft, may be adopted to enhance tip projection, improve alar rim position, and augment the infratip region. Peck’s onlay graft may be placed on the domal area to increase of tip projection in occasion of a thick fatty skin and this graft also permits variation of tip rotation, in relation to its more cranial or caudal placement. In a different maneuver, the cephalic trim portion of lower lateral cartilages can be left attached medially and then be used as an onlay tip graft. Another option is the umbrella graft which integrates an onlay tip graft with a columellar strut. The columella is sutured first with deep 5–0 mononylon (or polypropylene) then 6–0 mononylon in the skin; the intranasal incisions are closed with 5–0 catgut.
20.5.7 Nasal Bone Osteotomies
Nasal osteotomies are performed to straighten and narrow the nasal bridge and align the nasal profile; in cleft patients, abnormalities in the bony vault typically include a deviation to the noncleft side and a broad and flattened dorsum. We preferentially perform lateral osteotomies via an intranasal approach as a final surgical maneuver in our surgical rationale. A high-to-low lateral osteotomy (begins 3–4 mm anteriorly on the aperture and is continued in a posterocephalic direction up to the level of the medial canthus) is generally followed by a digital compression to produce a transverse greenstick fracture. If greater movement is required, we adopt a transverse percutaneous osteotomy (to insure that the thick frontal process of the maxilla breaks at the desired level) followed by a low-to-low lateral osteotomy (begins at the junction of the pyriform aperture and frontal process of the maxilla and is continued cephalically as close to the maxilla as possible up to the medial canthus), which results in a continuous osteotomy and a complete movement. If an open roof deformity is present after dorsal humpectomy, it needs to be corrected by low-to-low lateral osteotomies. At the end of the surgical procedure, home-customized internal paraseptal splints are sutured in position using transseptal nonabsorbable sutures and maintained for 1 month to coapt the mucosal flaps, keep the repositioned septal structures in the midline, and prevent synechiae. Packing (gauze with antibiotic ointment in the nasal cavity for 24 to 48 h), external taping, and dorsal nasal splinting (for 1–2 weeks) are also placed. Finally, we provide a wide spectrum of clinical examples (Figs. 20.8, 20.9, 20.10, 20.11, 20.12, 20.13, 20.14, 20.15, 20.16, 20.17, 20.18, 20.19, 20.20, 20.21, 20.22, 20.23, 20.24, 20.25, 20.26, 20.27, 20.28, 20.29, 20.30 and 20.31) surgically treated with a combination of surgical principles and maneuvers detailed in this chapter.
(Left) Preoperative and (right) postoperative right profile photographs of the patient in Fig. 20.8
(Left) Preoperative and (right) postoperative close-up right profile photographs of the patient in Fig. 20.11
(Left) Preoperative and (right) postoperative close-up right profile photographs of the patient in Fig. 20.14
(Left) Preoperative and (right) postoperative right profile photographs of the patient in Fig. 20.17
(Left) Preoperative and (right) postoperative right profile photographs of the patient in Fig. 20.21
(Left) Preoperative and (right) postoperative right profile photographs of the patient in Fig. 20.24
(Left) Preoperative and (right) postoperative right oblique photographs of the patient in Fig. 20.27
References
Alonso N, Risso GH, Denadai R, Raposo-Amaral CE. Effect of maxillary alveolar reconstruction on nasal symmetry of cleft lip and palate patients: a study comparing iliac crest bone graft and recombinant human bone morphogenetic protein-2. J Plast Reconstr Aesthet Surg. 2014;67:1201–8.
Bardach J, Cutting C. Anatomy of unilateral and bilateral cleft lip and nose. In: Bardach J, Morris HL, editors. Multidisciplinary Management of Cleft lip and Palate. Philadelphia: Saunders; 1990. p. 154–8.
Bashir M, Malik A, Khan FA. Comparison of suture and graft techniques in secondary unilateral cleft rhinoplasty. J Craniofac Surg. 2011;22:2172–5.
Basta MN, Goldstein JA, Wilson AJ, Taylor JA. A modified V-Y chondromucosal composite flap for correction of secondary cleft nasal deformity: photogrammetric analysis of a case-control study. Plast Reconstr Surg. 2014;134:94–101.
Buzzo CL. Surgical treatment of the cleft lip by Göteborg technique: 7 years follow up. Rev Bras Cir Plást. 2010;25:251–9.
Byrd HS, El-Musa KA, Yazdani A. Definitive repair of the unilateral cleft lip nasal deformity. Plast Reconstr Surg. 2007;120:1348–56.
Chang CS, Por YC, Liou EJ, Chang CJ, Chen PK, Noordhoff MS. Long-term comparison of four techniques for obtaining nasalsymmetry in unilateral complete cleft lip patients: a single surgeon's experience. Plast Reconstr Surg. 2010;126:1276–84.
Chang CS, Bergeron L, Chen PK. Diced cartilage rhinoplasty technique for cleft lip patients. Cleft Palate Craniofac J. 2011;48:663–9.
Cho BC. Correction of unilateral cleft lip nasal deformity in preschool and school-aged children with refined reverse-U incision and V-Y plasty: long-term follow-up results. Plast Reconstr Surg. 2007;119:267–27.
Chua HD, Cheung LK. Soft tissue changes from maxillary distraction osteogenesis versus orthognathic surgery in patients with cleft lip and palate-a randomized controlled clinical trial. J Oral Maxillofac Surg. 2012;70:1648–58.
Converse JM. Reconstructive plastic surgery, vol. 1. Philadelphia, PA: WB Saunders; 1964.
Cutting CB. Secondary cleft lip nasal reconstruction: state of the art. Cleft Palate Craniofac J. 2000;37:538–41.
Davidson E, Kumar AR. A preliminary three-dimensional analysis of nasal aesthetics following le fort I advancement in patients with cleft lip and palate. J Craniofac Surg. 2015;26:e629–33.
Dibbell DG. Cleft lip nasal reconstruction: correcting the classic unilateral defect. Plast Reconstr Surg. 1982;69:264–71.
Fisher MD, Fisher DM, Marcus JR. Correction of the cleft nasal deformity: from infancy to maturity. Clin Plast Surg. 2014;41:283–99.
Flores RL, Sailon AM, Cutting CB. A novel cleft rhinoplasty procedure combining an open rhinoplasty with the Dibbell and Tajima techniques: a 10-year review. Plast Reconstr Surg. 2009;124:2041–7.
Freeman AK, Mercer NS, Roberts LM. Nasal asymmetry in unilateral cleft lip and palate. J Plast Reconstr Aesthet Surg. 2013;66:506–12.
Gillies H, Kilner TP. Hare-lip: operations for the correction of secondary deformities. Lancet. 1932;220:1369–75.
Good PM, Mulliken JB, Padwa BL. Frequency of le fort I osteotomy after repaired cleft lip and palate or cleft palate. Cleft Palate Craniofac J. 2007;44:396–401.
Guyuron B. MOC-PS(SM) CME article: late cleft lip nasal deformity. Plast Reconstr Surg. 2008;121:1–11.
Haddock NT, McRae MH, Cutting CB. Long-term effect of primary cleft rhinoplasty on secondary cleftrhinoplasty in patients with unilateral cleft lip-cleft palate. Plast Reconstr Surg. 2012;129:740–8.
Hwang K, Kim HJ, Paik MH. Unilateral cleft nasal deformity correction using conchal cartilage lily flower graft. J Craniofac Surg. 2012;23:1770–2.
Kaufman Y, Buchanan EP, Wolfswinkel EM, Weathers WM, Stal S. Cleft nasal deformity and rhinoplasty. Semin Plast Surg. 2012;26:184–90.
Kumar A, Gabbay JS, Nikjoo R, Heller JB, O'Hara CM, Sisodia M, Garri JI, Wilson LS, Kawamoto HK Jr, Bradley JP. Improved outcomes in cleft patients with severe maxillary deficiency after le fort I internal distraction. Plast Reconstr Surg. 2006;117:1499–509.
Lee DW, Choi BK, Park BY. Seven fundamental procedures for definitive correction of unilateral secondary cleft lip nasal deformity in soft tissue aspects. J Oral Maxillofac Surg. 2011;69:e420–30.
Masuoka H, Kawai K, Morimoto N, Yamawaki S, Suzuki S. Open rhinoplasty using conchal cartilage during childhood to correct unilateral cleft-lip nasal deformities. J Plast Reconstr Aesthet Surg. 2012;65:857–63.
McComb HK, Coghlan BA. Primary repair of the unilateral cleft lip nose: completion of a longitudinal study. Cleft Palate Craniofac J. 1996;33:23–30.
Millard DR Jr, Morovic CG. Primary unilateral cleft nose correction: a 10-year follow-up. Plast Reconstr Surg. 1998;102:1331–8.
Potter J. Some nasal tip deformities due to alar cartilage abnormalities. Plast Reconstr Surg. 1946;13:358–66.
Raposo-Amaral C. Assessment of lip and nasal asymmetry after primary cleft lip repair. Rev Bras Cir Plast. 2010;25:38–48.
Raposo-Amaral CE, Giancolli AP, Denadai R, Marques FF, Somensi RS, Raposo-Amaral CA, Alonso N. Lip height improvement during the first year of unilateral complete cleft lip repair using cutting extended Mohler technique. Plast Surg Int. 2012;2012:206481.
Raposo-Amaral CE, Giancolli AP, Denadai R, Somensi RS, Raposo-Amaral CA. Late cutaneous lip height in unilateral incomplete cleft lip patients does not differ from the normative data. J Craniofac Surg. 2014;25:308–13.
Raposo-Amaral CE, Denadai R, Alonso N. Three-dimensional changes of maxilla after secondary alveolar cleft repair: differences between rhBMP-2 and autologous iliac crest bone grafting. Plast Reconstr Surg Glob Open. 2015a;3:e451.
Raposo-Amaral CA, Denadai R, Chammas DZ, Marques FF, Pinho AS, Roberto WM, Buzzo CL, Raposo-Amaral CE. Cleft patient-reported postoperative donor site pain following alveolar autologous iliac crest bone grafting: comparing two minimally invasive harvesting techniques. J Craniofac Surg. 2015b;26:2099–103.
Salyer KE, Genecov ER, Genecov DG. Unilateral cleft lip-nose repair--long-term outcome. Clin Plast Surg. 2004;31:191–208.
Santiago PE, Schuster LA, Levy-Bercowski D. Management of the alveolar cleft. Clin Plast Surg. 2014;41:219–32.
Somensi R, Giancolli A, Almeida F, Bento DF, Raposo-do-Amaral CA, Buzzo CA, Raposo-do-Amaral CE. Assessment of nasal anthropometric parameters after primary cleft lip repair using the Mohler technique. Rev Bras Cir Plast. 2012;27:14–21.
Stal S, Hollier L. Correction of secondary deformities of the cleft lip nose. Plast Reconstr Surg. 2002;109:1386–92.
Stal S, Brown RH, Higuera S, Hollier LH Jr, Byrd HS, Cutting CB, Mulliken JB. Fifty years of the Millard rotation-advancement: looking back and moving forward. Plast Reconstr Surg. 2009;123:1364–77.
Sykes JM, Tasman AJ, Suárez GA. Cleft lip nose. Clin Plast Surg. 2016;43:223–35.
Tajima S, Maruyama M. Reverse-U incision for secondary repair of cleft lip nose. Plast Reconstr Surg. 1977;60:256–61.
Turkaslan T, Turan A, Yogun N, Ozsoy Z. A novel approach to cleft lip nose deformity: posterior dome graft technique. J Craniofac Surg. 2008;19:1359–63.
Wang TD. Secondary rhinoplasty in unilateral cleft nasal deformity. Clin Plast Surg. 2010;37:383–7.
Wolfe SA. A pastiche for the cleft lip nose. Plast Reconstr Surg. 2004;114:1–9.
Wolfe SA, Nathan NR, MacArthur IR. The cleft lip nose: primary and secondary treatment. Clin Plast Surg. 2016;43:213–21.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Raposo-Amaral, C.A., Denadai, R., Raposo-Amaral, C.E., Buzzo, C.L. (2018). Secondary Unilateral Cleft Rhinoplasty. In: Alonso, N., Raposo-Amaral, C. (eds) Cleft Lip and Palate Treatment. Springer, Cham. https://doi.org/10.1007/978-3-319-63290-2_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-63290-2_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-63289-6
Online ISBN: 978-3-319-63290-2
eBook Packages: MedicineMedicine (R0)