Abstract
Cytokinins are phytohormones that regulate plant growth, development and senescence. Experiments both in vitro and in vivo demonstrate that they can also have diverse effects on animal cells and tissues. Particularly interesting is their ability to protect cells against various forms of stress and prevent some detrimental effects of cell aging. For example, human skin fibroblasts cultured in the presence of kinetin or trans-zeatin retain some characteristics of cells of lower passage. Kinetin is even able to increase the lifespan of invertebrates. In this chapter, we review protective effects of cytokinins in animals at molecular, cellular, tissue and organismal levels. We also discuss potential application of cytokinins for the treatment of age-related diseases, including neurodegenerations, inflammatory diseases and disorders caused by aberrant cell proliferation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Cytokinin
- Kinetin
- Kinetin riboside
- Zeatin
- Benzyl adenine
- Topolin
- Skin
- Anti-aging
- Anti-inflammatory activity
- Neuro protection
1 Introduction
Cytokinins are phytohormones identified originally as substances that promote plant cell division in the presence of another phytohormone, auxin (Skoog et al. 1965). They play important roles in the development and growth of both root and shoot systems. Processes regulated by cytokinins include water and nutrient mobilization, apical dominance, branching, flowering, breaking of bud dormancy and seed germination (Werner and Schmülling 2009). Cytokinins also delay leaf senescence—they prevent the degradation of chlorophyll and outflow of nutrients from the leaf. A good demonstration of this activity occurs in “evergreen” transgenic tobacco plants with the gene encoding ipt (isopentenyltransferase), the protein responsible for cytokinin biosynthesis under the control of a senescence-specific promoter (Gan and Amasino 1995).
Knowledge that cytokinins play key roles in the regulation of plant growth and development has stimulated studies into their potential utility for treating human diseases, especially those involving dysfunctional cell proliferation and/or differentiation. Cytokinin ribosides were even evaluated in patients with diverse malignancies as early as the 1970s (Mittelman et al. 1975). Later on, cytokinins inspired development of inhibitors of cyclin-dependent kinases olomoucine, bohemine, roscovitine and their analogues (Veselý et al. 1994; Havlíček et al. 1997; Vermeulen et al. 2002; Kryštof et al. 2002). The ability of cytokinins to prevent processes associated with plant senescence has attracted research focused on their ability to ameliorate aging traits in animals, including humans. In this article, we review the effects of cytokinins in animals at molecular, cellular, tissue and organismal levels with an emphasis on anti-aging and cytoprotective activity. We also discuss potential application of cytokinins in the treatment of inflammation and neurodegeneration as relevant to various age-related diseases. Finally, we include a section about the antiproliferative activity of cytokinins—they are cytotoxic for malignant cells of diverse histopathological origin but are also able to induce differentiation of some leukemia cells and keratinocytes.
2 Chemical Structure and Occurrence of Cytokinins
Regarding their chemical structure, plant cytokinins are adenine derivatives substituted at the N 6-position with either an isoprenoid or aromatic side chain. Synthetic compounds with cytokinin activity can have other scaffolds, phenylureas being the best known example. Isoprenoid cytokinins include cis- and trans-zeatin (tZ) and their analogues with a saturated side chain (dihydrozeatin) or without an hydroxyl group (N 6-isopentenyladenine, iP). Whereas isoprenoid cytokinins are present in all plants, aromatic cytokinins with N 6-benzyl substituents have only been found in certain taxa (Horgan et al. 1975; Strnad 1997). Besides N 6-benzyladenine (BA) , its hydroxylated derivatives, i.e., topolins , have been described. Kinetin (K), the first identified cytokinin, has an N 6-furfuryl side chain. K was first recognized as the substance responsible for the cytokinin activity of autoclaved herring sperm, which was attributed to thermal DNA damage. Later it was reported to occur naturally in plant material (Ge et al. 2004), as well as human cells and urine (Barciszewski et al. 1996, 2000). However, its natural occurrence is probably very rare and rather mysterious. We have not even been able to unequivocally identify this compound in diverse plant material using sophisticated tandem mass spectrometry analysis. Barciszewski et al. (1997) proposed that endogenous K is a result of oxidative DNA damage. Both isoprenoid and aromatic cytokinins occur as free bases, ribosides, ribotides, N-glucosides and amino acid conjugates. Moreover cytokinins with hydroxylated isoprenoid side chains can also form O-glycosides. N 6-substituted adenine derivatives also occur in certain tRNA species of all organisms with the exception of Archea. For example, in mammals, an N 6-isopentenyladenosine (iPR) moiety forms part of tRNA[Ser]Sec. It facilitates codon-anticodon interactions and contributes to the efficiency of selenoprotein synthesis. Adenines and adenosines with N 6-substitution may be released into the cytosol, and subsequently into body fluids, as a result of tRNA turnover. iPR was detected in human urine many years ago (Chheda and Mittelman 1972) (Fig. 14.1).
3 Cytokinin Signaling in Plants
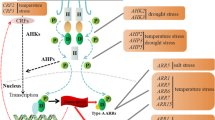
The cytokinin signal in plants is perceived by a His-Asp phosphorelay similar to the two-component systems of bacteria. After recognition of the cytokinin ligand by the extracellular domain of the transmembrane cytokinin receptor (AHK2, AHK3, or AHK4 in Arabidopsis thaliana), the intracellular portion of the receptor phosphorylates histidine phosphotransfer proteins (AHPs). These transmit the signal to nuclear response regulators (ARRs), which can activate or repress transcription of the response genes. Anti-senescence activity of cytokinins is mediated through the activation of AHK3, the type-B response regulator ARR2 and cytokinin response factor CRF6. Increased cell-wall invertase activity in response to cytokinins is both necessary and sufficient for the inhibition of senescence (Zwack and Rashotte 2013).
Although differences in the substrate specificity between individual cytokinin receptors exist, cytokinin bases are consistently the most active cytokinin form in both receptor assays and cytokinin biotests (Mok and Mok 2001; Spíchal et al. 2004). The intensity and duration of the signaling is dependent on the receptor and response regulator composition of the given cell/tissue and the availability of individual cytokinins. The rate-limiting step in cytokinin biosynthesis is catalyzed by isopentenyltransferases (IPTs), which synthesize either free cytokinin nucleotides (adenosine phosphate-IPTs) or modify adenosine in tRNA (tRNA-IPTs). Conversion of cytokinin 5′-monophosphates into their respective free bases is catalyzed by phosphoribohydrolase encoded by the gene LONELY GUY (LOG) (Kurakawa et al. 2007). An alternative pathway where dephosphorylation of riboside-5′-monophosphates precedes the cleavage of the glycoside bond also exists (Chen and Kristopeit 1981), but the genes responsible have not yet been characterized. Cytokinins are degraded by cytokinin oxidase/dehydrogenases (CKXs), which catalyze removal of the side chain. The cytokinin signal is also attenuated by conversion of free bases into less active (ribosides, ribotides) or inactive forms (glucosides, conjugates with alanine). With the exception of N7- and N9-glucosides, cytokinin conjugates can be converted back into free bases and are seen as transport/storage cytokinin forms. The uptake and efflux of cytokinins by cells is facilitated by members of the purine permease family (PUPs) of transmembrane channels (Gillissen et al. 2000) and by equilibrative nucleoside transporters (ENTs) (Hirose et al. 2008). Cytokinins are present in both phloem and xylem fluid and serve as both acropetal and basipetal messengers (Kudo et al. 2010). The first acropetal transporter was described recently (Zhang et al. 2014).
4 Effects of Cytokinins in Animal Systems
4.1 Cytoprotective and Anti-aging Activity of Cytokinins
Interest in the anti-aging activity of cytokinins started in 1994 when Rattan and Clark discovered positive effects of K on several characteristics related to aging in human skin fibroblasts during serial passage in vitro (Rattan and Clark 1994). The size and morphology of fibroblasts passaged in the presence of K resembled those of the cells at lower passage numbers. Treatment with K decreased the number of actin stress fibers and autofluorescence due to accumulated lipofuscin was also less intense. Similar effects of tZ on in vitro aging of a fibroblast population were reported more than 10 years later (Rattan and Sodagam 2005). Optimal anti-aging effects were observed at 80 μM concentration for both compounds. It is important to note that these long-term cultivation experiments were enabled by the remarkably low toxicity of the cytokinins tested. Beneficial effects of several other cytokinin bases on various parameters relevant for aging amelioration and/or age-related disease therapy were reported in the years following the original discovery, and we discuss them below. Active compounds include para-topolin, iP and a K derivative 6-furfurylamino-9-(tetrahydropyran-2-yl)-9H-purine (Pyratine-6, PRK-124) (Walla et al. 2010). K, tZ and Pyratine are the principal ingredients of several marketed cosmeceuticals.
Notably, K effects related to aging are not limited to cells and tissues as dietary K has been shown to increase the life span of Zaprionus paravittiger fruit flies. K prolonged the larval and pupal stages but also reduced the age-specific death rates throughout the adult lifespan (Sharma et al. 1995). The effect was accompanied by enhanced catalase activity (Sharma et al. 1997). We also recently discovered that K and several other cytokinin derivatives are able to increase the lifespan of Caenorhabditis elegans (our unpublished data).
4.2 Anti-oxidant Activity of Cytokinins
Since the discovery of anti-aging activity of K in human fibroblasts , research has primarily focused on its ability to protect macromolecules and cells against oxidative damage and stress. Barciszewski et al. (1997) hypothesized that endogenous K may arise as a consequence of oxidative damage of DNA, thus creating protection near to the site of damage. The proposed mechanism of K formation assumes that hydroxy radical attack at the 5′ carbon of the deoxyribose residue yields furfural. This aldehyde reacts with the amino group of adenine and, after intramolecular rearrangement, the resulting Schiff base is reduced into K (Barciszewski et al. 1997).
Using 8-oxo-2′-deoxyguanosine (8-oxo-dG) as a marker for oxidative damage of DNA, Olsen et al. (1999) showed that K significantly protects DNA against reactive oxygen species (ROS) generated by the Fenton reaction in vitro. The effect was dose dependent, with a maximum of about 50% protection observed at 100 μM K. K has also been shown to protect proteins against oxidative/glycoxidative damage more efficiently than adenine in several experimental systems in vitro (Olsen et al. 1999). Besides decreasing protein carbonylation in an iron/ascorbate system, it also prevented formation of advanced glycation end-product pentosidine and aggregation after incubation of proteins with sugars. Active K concentrations were in the range 50–200 μM (Verbeke et al. 2000). Recently, the antioxidant activities of K, BA, para-topolin and iP were evaluated by fluorimetric and spectrophotometric assays (Brizzolari et al. 2016). With the exception of BA, all the compounds showed significant activity in the oxygen radical absorbance capacity (ORAC) assay at 2.5 and 5 μM concentrations. In the Trolox equivalence antioxidant capacity (TEAC) assay, only para-topolin (0.5–5 μM) was active, probably due to the presence of a phenolic hydroxyl. All the compounds were able to react with hydroxyl radicals generated in the 2-deoxyribose degradation assay. A somewhat higher activity of iP was ascribed to the presence of the double bond in the N 6-side chain. Using electron spin resonance, Hsiao et al. (2003) showed that short pretreatment with K at concentrations of 70 and 150 μM effectively inhibited hydroxyl radical formation in collagen-activated platelets.
Direct comparison of K with a group of other established antioxidants, comprising l-ascorbic acid, dl-alpha-tocopherol, dl-alpha lipoic acid, ubiquinone and idebenone, has been carried out (McDaniel et al. 2005). K quenched radicals generated through the photochemical excitation of water molecules effectively only at a concentration of 1 μM. The effective concentrations of the other compounds, with the exception of dl-alpha lipoic acid, were one or two orders of magnitude lower. K (100 μM) completely prevented oxidation of low density lipoproteins by Cu2+. The other compounds decreased the production of lipid hydroxyperoxides in equimolar concentrations less efficiently (idebenone: 80%, dl-alpha-tocopherol: 50%, ubiquinone: 26%, dl-alpha lipoic acid: 22%, l-ascorbic acid: 15%). However, this exceptional activity of K was not observed in the microsome oxidation (NADPH/ADP/Fe3+) assay, which is considered to be a more realistic model of cell membrane peroxidation. Whereas the activity of K was comparable with that of ubiquinone and l-ascorbic acid (reduction of malonyldialdehyde equivalents by 24–27%), the other test compounds decreased MDA production by 47–55%.
Cytokinins may also form complexes with ions of metals with the ability to quench ROS. Superoxide dismutase-like activity of Cu2+ complexes of K and BA have been reported (Goldstein and Czapski 1991). The ability of Cu2+ complexes of BA derivatives to protect against oxidative damage in vivo (aloxan induced diabetes) has been observed (Štarha et al. 2009).
Besides their direct anti-oxidant activities, cytokinins also activate cellular anti-oxidant defense mechanisms. tZ has been shown to induce hydrogen peroxide decomposing enzymes in human skin fibroblasts. The treatment was also able to protect both proliferating and senescent cells against the hydrogen peroxide induced cell death (Rattan and Sodagam 2005). Induction of antioxidant enzymes may also contribute to the lifespan extension observed in flies after K treatment mentioned above (Sharma et al. 1997) because it enhances the catalase activity in this organism. K also has been demonstrated to exhibit protective effects in the d-galactose model of glycoxidative stress. In cultured rat astrocytes, K partially reversed a decrease in the activities of glutathione peroxidase and superoxide dismutase induced by d-galactose treatment. K treatment also decreased malonyldialdehyde concentration in the cell membranes and increased cell viability. The active concentrations were in the range 50–100 µM. In addition, K was shown to have beneficial effects in rats receiving daily subcutaneous injections of d-galactose at 125 mg/kg for 6 weeks. K (10, 20 and 40 mg/kg) was administered by gastric perfusion for the whole period of galactose exposition. However, no information was provided about K concentrations in plasma or brain tissue. A dose of 10 mg/kg was concluded to have the most promising effect because it was able to attenuate the negative effects of d-galactose on malonyldialdehyde concentration and activities of glutathione peroxidase and superoxide dismutase in brain tissue (Liu et al. 2011).
Although iPR and N 6-benzyladenosine (BAR) have been studied traditionally as anti-cancer agents, they possess cytoprotective activities as well. Dassano et al. (2014) showed that treatment of several cancer cell lines with iPR or BAR induced robust expression of genes regulated by transcription factor Nrf2. Moreover, the transcription of Nrf2 itself was upregulated. These expression changes were not a just a futile response to oxidative stress resulting from drug-induced damage. In fact, the treatment with cytokinin ribosides decreased the basal levels of ROS and increased the resistance of the cells to pro-oxidative insults. Our earlier unpublished observation that not only iPR but also other cytotoxic ribosides, including kinetin riboside (KR), in low micromolar concentrations induce Nrf2-dependent gene expression in multiple cell lines led us to speculate that the cytoprotective activity of K could be mediated by its metabolite KR and/or appropriate ribotides. The conversion of cytokinin bases into their respective riboside 5′-monophosphates by human cells has been reported (Mlejnek and Doležel 2005). However, treatment of several cell lines, including skin fibroblasts and immortalized keratinocytes, with 100 μM K did not have any significant effect on hemeoxygenase expression (unpublished data). Because tissues may differ in expression and/or activity of phosphoribosyltransferase, and thus in the rate of K conversion into its riboside, studies of a wider panel of cell lines could identify tissues where the proposed mechanism is relevant. Nevertheless, Hertz et al. (2013) recently demonstrated that conversion of K into its ribotides may be indeed important for its cytoprotective activity. Mitoprotective PTEN-induced putative kinase 1 (PINK1, PARK6) is able to use kinetin riboside 5′-triphosphate (KRTP) as a donor of a phosphate group more efficiently than ATP. Because of its unusual behavior, Hertz et al. (2013) designated KRTP as a PINK1 neo-substrate. They showed that K is converted into KRTP within the cells and that treatment with K accelerated Parkin recruitment to depolarized mitochondria and suppressed oxidative stress-induced apoptosis in human-derived neural cells in a PINK1-dependent manner.
4.3 Cytokinins in the Therapy of Skin Diseases and Cosmetics
Because the anti-aging activity of K, and later tZ, was demonstrated for the first time in skin fibroblasts, multiple studies evaluating the applicability of cytokinin bases in skin protection both in vitro and in vivo have been conducted. Positive effects include protection against UV-induced damage, improved wound healing and aquaporin induction. Cytokinins also modulate melanogenesis and keratinocyte differentiation. Several clinical trials have shown that they can improve multiple traits of photoaging skin and also some symptoms of acne rosacea.
McDaniel et al. (2005) compared the UV-protective activity of a group of antioxidants, i.e., K, l-ascorbic acid, dl-alpha-tocopherol, dl-alpha lipoic acid, ubiquinone and idebenone. K was shown to protect primary keratinocytes against UVB (single dose, 200 mJ/cm2); it reduced the number of cells stained positively by immunohistochemistry with an antibody against thymine–dimer from 53 to 34%. Whereas idebenone, l-ascorbic acid and dl-alpha-tocopherol offered similar protection levels (29–35% of positive cells), ubiquinone and DL lipoic acid were not active. Subsequently, the UV-protective activity of the compounds was tested in patients, each compound on five subjects. The compounds were applied as ethanolic solutions (0.5% w/w) on mid-back regions once a day for 2 weeks. Sunburn cells (SBC) induced by a 1.5 × minimal erythema dose of UVB were quantified in biopsies obtained 20 h later. K reduced the number of SBC by 20%, compared to about 10% for ubiquinone and dl-lipoic. Whereas idebenone and dl-alpha-tocopherol were more active (reduction by 38 and 30%, respectively), ascorbic acid did not have any protective effect. Unfortunately, no UV protective effects of K were observed in pigs. Four days’ application of 0.1% K cream or 0.5% K solution neither ameliorated UV-induced erythema nor reduced the number of SBC in pigs (Tournas et al. 2006) exposed to a one to five times minimal erythema dose of UV irradiation (~5 mW/cm2 of UVB and ~40 mW/cm2 of UVA).
Cell culture experiments have suggested that tZ could improve skin hydration and wound healing, as well as prevent detrimental effects of photoaging on those processes (Ji et al. 2010). tZ at 40 and 80 μM concentration also induced expression of aquaporin 3 protein (AQP3) in spontaneously immortalized HaCaT keratinocytes. Treatment with tZ was also able to ameliorate a UV-induced decrease in AQP3 concentrations and membrane water permeability to a large extent. Experiments with pharmacological MAPK pathway inhibitors revealed that tZ inhibits UV-induced MEK/ERK activation. tZ has also been reported to promote wound healing in the scratch assay with either irradiated or non-irradiated cell cultures (Ji et al. 2010) and inhibit (tZ at 20–40 μM) UVB-induced MMP-1 expression in skin fibroblasts (Yang et al. 2009).
Cytokinin bases have been shown to promote differentiation of keratinocytes. K at 40–200 μM concentration induced growth arrest and changes of several markers of differentiation (keratin K10 and involucrin) in human keratinocytes in cell culture (Berge et al. 2006). The effect was augmented by the presence of Ca2+ ions. Other markers of differentiation (trans-glutaminase) were unchanged, suggesting that K-induced differentiation might be mediated by pathways different from those activated by other differentiation inducing agents. In a subsequent study, Berge et al. (2008) reported that treatment with K improved the sensitivity of aging keratinocytes to the differentiating effects of Ca2+ ions. The authors mentioned unpublished data indicating that the effect was accompanied by induction of Hsp90, Hsp70 and heme-oxygenase-1. They suggested that the effects were mediated by stress-induced hormesis based on this observation.
A positive effect of K on filaggrin levels, another marker of keratinocyte differentiation, was observed in an in vitro reconstructed skin equivalent (Vičanová et al. 2006). In contrast to 2D culture, K promoted the growth of keratinocytes, as indicated by an increase in the number of Ki67-positive cells.
Both experimental systems mentioned above used human keratinocytes from healthy donors. However, it should be noted that models using keratinocytes from psoriatic lesions might be more appropriate for evaluation of the utility of cytokinins in the therapy of psoriasis.
Other dermatologic/cosmetic applications of cytokinins relate to the therapy of pigmentation disorders. Whereas K has been reported to decrease hyperpigmentation in dogs (Kimura and Doi 2004), BA is a stimulator of melanogenesis (Kim et al. 2009). At concentrations of 50 and 100 μM, it stimulated melanogenesis in B16 mouse melanoma cells through protein kinase A mediated induction of microphthalmia-associated transcription factor (MITF), tyrosinase and tyrosinase-related proteins 1 and 2 (TYRP1, TYRP2). In contrast to alpha-MSH, BA activated protein kinase A in a cAMP independent manner. Also, para-topolin induced tyrosinase expression and melanogenesis in B16 cells (our unpublished data).
4.4 Clinical Examination of Kinetin and Its Relatives
Topical K has been evaluated in multiple open-label single-arm clinical trials, in which effects were compared before and after treatment. K lotion 0.1% (Kinerase) applied twice daily was evaluated as a treatment for mildly to moderately photodamaged facial skin in 32 subjects. Twelve and twenty four week treatments were found to improve significantly skin texture, mottled hyperpigmentation and fine wrinkles according to the both patient’s and physician’s assessment. The treatment also improved transdermal water loss (McCullough and Weinstein 2002). The same regimen was later evaluated in patients (N = 17) with mild to moderate facial rosacea in a 12 week study (Wu et al. 2007). K treatment was found to reduce redness and had also a positive, yet statistically insignificant, effect on telangiectasia. The physician’s rating of overall improvement of rosacea symptoms increased over the study period and by week 12, almost 60% of the subjects showed at least moderate improvement. K was generally well tolerated in those studies. However, in rare cases, acne or a rash was observed after 8 weeks’ treatment. Wanitphakdeedecha et al. (2015) evaluated the effects of applying 0.1% K cream twice daily on the photoaging facial skin of 100 Thai subjects. Twelve weeks’ treatment was accompanied by small but statistically significant improvements in overall skin condition, skin texture, color and wrinkles. K also improved ultraviolet spots and redness. Chiu et al. (2007) evaluated possible synergistic effects of using a combination of K (0.03%) and niacin (4%) in a double-blind clinical study on 52 Taiwanese subjects. Serum containing either K and niacin (27 subjects) or niacin alone (25 subjects) was applied to one half of the face and the vehicle to the other twice daily for 12 weeks. Although the combination of K and niacin had a larger positive effect on most of the evaluated parameters, including corneal hydration and erythema index, than niacin alone when compared to the baseline, the differences between the treatments were not statistically significant. Also, vehicle alone had a positive effect on multiple parameters, yet the effects of K were typically larger.
To the best of our knowledge, no reports of clinical trials with tZ have been published. Several open-label, single-arm clinical trials have evaluated the K derivative 6-furfurylamino-9-(tetrahydropyran-2-yl)-9H-purine (Pyratine-6, PRK-124), which originated from our laboratory (Laboratory of Growth Regulators, Olomouc, Czech Republic) and then was developed by companies Senetek PLC and Pyratine PLC, USA. In a trial evaluating its effects on aging and photodamaged skin of 40 subjects (34 finished the study), Pyratine (0.1%) was reported to improve skin moisturization, roughness, mottled hyperpigmentation, fine wrinkles and facial erythema in comparison with the baseline within 4 weeks (McCullough et al. 2008). Application of the compound (0.125%) twice daily was also found to improve symptoms of acne rosacea compared to the baseline in a 12 week study (Ortiz et al. 2009), with some beneficial effects observed as early as week 4. In a later study, 18 subjects were followed over an extended 48 week trial (Tremaine et al. 2010). A mean 44% reduction in erythema severity and 89% reduction in inflammatory lesions was observed at week 48. The treatment also had a positive effect on telangiectasias, transepidermal water loss and skin dryness. No treatment-induced skin irritation was observed.
5 Neuroprotective Activity of Cytokinins
Multiple reports of cytokinin activities relevant to the treatment of neurodegeneration exist. Notably, such effects are not limited to cytokinin bases only, but cytokinin ribosides have also been shown to possess neuroprotective activity. As discussed above, K protects rat astrocytes in vitro as well as rat brain against glycoxidative damage in the D-galactose model via promotion of anti-oxidant defense (Liu et al. 2011). KRTP increases kinase activity of PTEN-induced putative kinase 1 (PINK1). PINK1 is critical for mitochondrial quality control—it accumulates in the outer membrane of impaired mitochondria and, through the recruitment of Parkin protein, targets them for autophagy. Both PINK1 and Parkin are mutated in the familial forms of recessive Parkinson’s disease. Treatment with K protected SH-SY5Y5 neuroblastoma cells against proteasomal and oxidative stress in PINK1-dependent manner. K is therefore an interesting drug candidate for the treatment of familial form of Parkinson’s disease and possibly also therapy of other diseases with mitochondrial dysfunction. It would be interesting to identify other N 6-substitued adenines with similar type of activity. This may prove difficult because both base and riboside require not only multistep metabolic activation, but they also have to mimic the shape and behavior of ATP in the PINK1 binding pocket (Hertz et al. 2013).
K is also a candidate drug for neurodegenerative disease familial dysautonomia. A clinical study (NCT02274051) is in the process of recruiting patients at the time of writing this text according to web page www.clinicaltrials.gov. Both in vitro and in vivo experiments have demonstrated that K is able to correct aberrant splicing of pre-mRNA originating from the IKBKAP gene (inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase complex-associated protein). The mechanism of K’s action is unknown. However, the very limited number of transcripts with splicing influenced by the treatment suggests that K interacts with regulators or components of specific spliceosome sub-species.
tZ also exhibits multiple activities relevant for the therapy of diseases of the central nervous system, in particular Alzheimer’s disease. It was identified as the substance responsible for inhibition of rat acetylcholinesterase contained in an extract from the traditional Korean edible plant Fiatoua villosa (IC50 1.09 × 10−4 M) (Heo et al. 2002). Follow-up studies showed that tZ protects rat pheocytohroma cells PC12 against toxic effects of an amyloid beta fragment comprising amino acids 25–35. tZ treatment had a positive effect on both ROS production and cell viability (Choi et al. 2009). Also reported was a beneficial effect of long-term treatment with tZ on scopolamine-induced amnesia. Scopolamine is an antagonist of muscarinic acetylcholine receptors, but its administration also influences other neurotransmitter systems. For example, repeated treatment of scopolamine has been shown to decrease levels of monoamines (noradrenaline, dopamine and serotonin) and also increase oxidative stress in brain (Haider et al. 2016). In Choi et al.’s study (2009), animals had ad libitum access to either normal drinking water (control and scopolamine control groups) or tZ solution (0.002, 0.004, and 0.008% w/v) for 21 days. More specific information about dosing (drinking volume by the individual animals) and tZ concentrations in plasma and brain tissues was not reported. After the treatment period, temporal amnesia was induced by a single s.c. injection of scopolamine (1 mg/kg) and behavioral tests were started 30 min later. All three tested concentrations markedly attenuated negative effects of scopolamine on passive avoidance and spontaneous alternation behaviors in the Y-maze test. After finishing the tests, the animals were sacrificed and acetylcholinesterase activity in brain lysates was measured. The two highest tZ concentrations were able to reduce the effects of scopolamine on acetylcholinesterase activity. Similar effects on behavior were observed when mice were provided food mixed with tZ ad libitum for 4 weeks (Choi et al. 2009). However, the mechanism of the observed tZ activity is unclear. The authors provided no rationale for the treatment regime length—much shorter regimes and possibly single tZ injection may have been more appropriate for evaluation of acetylcholinesterase inhibition or direct antioxidant capacity. It would be interesting to assess the effect of tZ on the level of oxidative stress and level/activity of the antioxidant defense system in brain before and after scopolamine treatment.
KR and trans-zeatin ribosides (tZRs) have been shown to have cytoprotective activities. Both of them, but not BAR, can protect PC12 cells against serum starvation induced apoptosis (Lee et al. 2012). The corresponding free bases, tZ and K were inactive in this model. The protective effects of KR were apparent at concentrations as low as 1 μM, whereas tZR did not show any significant activity at this concentration. However, at a concentration of 100 μM, tZR was more effective than equimolar KR and was therefore selected for follow-up experiments. Its protective activity in a serum deprivation model was mediated by activation of the A2A adenosine receptor (A2AR) and the downstream protein kinase A (PKA) as co-treatment with inhibitors of those proteins abolished the protective effect. A2AR has been suggested as a potential therapeutic target in Huntington’s disease because it is highly expressed in the striatum, where mutant huntingtin causes early damage. Moreover, A2AR-selective agonists effectively ameliorate several symptoms of Huntington’s disease in both cell cultures and animal models (Chou et al. 2005; Chiu et al. 2015). Therefore, tZR’s ability to prevent huntingtin aggregation was tested in PC12 cells overexpressing the mutant protein. Indeed, tZR treatment prevented both the mutant huntingtin aggregation and subsequent proteasome dysfunction in an A2AR and PKA dependent manner.
Para-Topolin riboside (pTR, 6-(4-hydroxybenzylamino)-9H-purine riboside) is an A2AR agonist as well. It was isolated as a neuroprotective substance from the Chinese medicinal plant Gastrodia elata (Huang et al. 2011a, b). pTR (designated as T1-11 in the study) has been shown to interact with not only A2AR but also equilibrative nucleotide transporter ENT1. ENT1 inhibition can increase the amount of adenosine in extracellular space and possibly contribute to modulation of adenosinergic signaling in synapses where both proteins are present. Treatment of R6/2 mice with pTR administered using a subcutaneous Alzet minipump for 48 h improved motor deterioration. pTR administered in drinking water (0.05 mg/ml ad libitum, plasmatic or brain concentration unknown) improved the rotarod performance already after 2 weeks and the effect persisted until the end of the experiment after 7 weeks. Analysis of the brains showed a lower accumulation of huntingtin, improved activity of proteasome and higher levels of mRNA for brain-derived neurotrophic factor (BDNF). Finally, pTR also binds to A3R, another adenosine receptor with a known role in CNS physiology (Borea et al. 2014). For example, agonists of A3R have been shown to have neuroprotective effects in subarachnoid hemorrhage-induced brain damage (Maria Pugliese et al. 2007). Nevertheless, the therapeutic utility of targeting the brain is somewhat controversial because prolonged A3R activation may be neurotoxic (Luo et al. 2010). Recently, A3R agonistic activity of iPR has been reported (Blad et al. 2011). However, to the best of our knowledge, its effects in models of neurological diseases have not yet been studied.
6 Immunomodulatory Activities of Cytokinins
In the previous section, we discussed interaction of cytokinin ribosides with the adenosine receptor A2A in relation to their neuroprotective activities. However, A2A is also expressed in various immune system cells (T-lymphocytes, macrophages, monocytes and polymorphonuclear leukocytes) and its activation plays an important role in the regulation of both innate and adaptive immunity (Milne and Palmer 2011). Selective A2A receptor agonists have been suggested as drugs for treatment of graft-versus-host disease, colitis and T-lymphocyte mediated ischemia-reperfusion injury (Chhabra et al. 2012; Jones and Kang 2015). The immunomodulatory activity of tZR was demonstrated for the first time by Lappas (2015). It was shown to inhibit production of interferon (IFN)-γ, IL-2 and TNF-α in either CD3+CD4+ or CD3+CD8+ T-lymphocytes incubated with anti-CD3 antibody. In the case of the CD3+CD4+ population, tZR also inhibited production of IL-4. EC50 values were in the range 10–100 μM. Furthermore, tZR inhibited upregulation of CD25, CD69 and CD40L. The inhibitory effects in the latter assays were strongly attenuated by co-treatment with 10 μM of the selective A2A receptor antagonist ZM241385, thereby confirming its involvement. In mice, tZR decreased the number of white-blood cells in intraperitoneal infiltrate in thioglycollate-induced peritonitis. tZR was administered as a 1 mg/kg i.p. bolus at times 0, 1.5 and 3 h after the thioglycollate injection. Overall, the data showed that tZR is not a very potent immunomodulator in terms of the active concentration. However, the absence of cytotoxic effects up to 1000 μM suggest that the low activity could be possibly compensated by the administration of higher doses.
As discussed above, iPR and BAR activate the Nrf2 pathway and protect cells against oxidative stress (Dassano et al. 2014). The employed models also included 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced superoxide production by HL-60 cells differentiated along the neutrophilic lineage. Further, the authors tested the activity of the cytokinins in a mouse ear inflammation model where topical TPA-induced oxidative stress stimulates an inflammatory response. Pretreatment with iPR and BAR attenuated the inflammation and reduced the number of infiltrating neutrophils. The exact mechanism of the protective effect is unclear and other processes besides Nrf2 activation could be involved. The authors discussed possible involvement of glucocorticoid receptor signaling (iPR induced several transcripts in this pathway) and adenosine receptor A3R activation. The A3R agonistic activity of iPR and tZR was reported recently (Blad et al. 2011). A3R regulates various aspects of inflammation, including neutrophile chemotaxis and superoxide production (van der Hoeven et al. 2010). The anti-inflammatory effects of A3R activation are mediated through the inhibition of NF-κB dependent production of cytokines, including TNF-α. A3R agonists have been shown to have robust anti-inflammatory activity in animal models of inflammatory bowel disease, systemic toxemia, pulmonary and liver inflammation as well as rheumatoid arthritis (Borea et al. 2014). Nanomolar A3R agonist CF101 (IB-MECA) structurally related to aromatic cytokinins is currently being evaluated as an antirheumatic and antipsoriatic agent in clinical trials. Recently, promising activity in patients with moderate to severe plaque psoriasis was reported (David et al. 2016).
Another explanation of the anti-inflammatory activity of iPR was offered by studies of its effects on natural killer cells (NK cells) (Ciaglia et al. 2014). iPR at 10 μM concentration inhibited the cytotoxicity of NK cells against leukemia K562 cells. The treatment prevented ERK/MAPK and STAT5 activation in IL-2-activated NK cells, downregulated the expression of activating receptors NKp44 and NKG2D and decreased secretion of cyto/chemokines (RANTES, MIP-1α, TNF-α and IFN-γ). Topical application of iPR significantly reduced ear edema in a mouse model of croton oil-induced ear dermatitis with a potency comparable to that of indomethacin. Histology analysis showed lower leukocyte infiltration and decreased staining of the natural cytotoxicity receptor NKp46, whose expression is typical for NK cells, in irritated mid and papillary dermis.
Finally, cytokinin bases may also possess anti-inflammatory activity. K and Pyratine have been shown to have beneficial effects on acne rosacea, a skin condition with an inflammatory component (Wu et al. 2007; Tremaine et al. 2010).
7 Antiproliferative Effects of Cytokinins and Cytokinin Analogues
Natural cytokinin ribosides iPR, KR, BAR, ortho-topolin riboside (oTR) and N 6-(2-hydroxy-3-methoxybenzyl)adenosine (but not their respective bases) have been reported to exhibit strong cytotoxic effects against a range of human cell lines derived from both hematological malignancies and solid tumors (Doležal et al. 2007; Voller et al. 2010, 2017). Numerous studies with various experimental designs (assay principle, endpoint, length of treatment) have demonstrated that some cytokinin ribosides are active at submicromolar (against some leukemia) or micromolar concentrations (against other leukemia, adherent cells). The toxicity of tZR and cis-zeatin riboside, other natural cytokinins that differ from iPR by hydroxylation of the isoprenoid side-chain, is very limited (Ishii et al. 2002; Rattan and Sodagam 2005; Voller et al. 2010). Further, isomers of oTR with a hydroxy group in either the meta- or para-position of the phenyl ring do not show significant toxicity (Voller et al. 2010).
In leukemia cell lines, cytokinin ribosides induce rapid apoptosis (Mlejnek and Kuglík 2000; Ishii et al. 2002; Voller et al. 2017). Cell death is preceded by ATP depletion. An exception is N 6-(2-hydroxy-3-methoxybenzyl)adenosine, where apoptosis ensues without marked effects on cell energy levels (Voller et al. 2017). It has recently been demonstrated that KR may be a potential drug for the treatment of multiple myelomas. In several cell lines, KR has been found to suppress cyclin D1 and D2 transcription, followed by arrest of the cell-cycle and selective apoptosis in tumor cells (Tiedemann et al. 2008). KR may be also effective against leukemia stem cells (McDermott et al. 2012).
Cytotoxic effects of iPR and KR against mammalian cell lines derived from solid tumors have been reported many times (Meisel et al. 1998; Griffaut et al. 2004; Laezza et al. 2006, 2009, 2010; Spinola et al. 2007; Cheong et al. 2009; Cabello et al. 2009; Colombo et al. 2009; Rajabi et al. 2012; Wang et al. 2012; Ciaglia et al. 2014, 2016). Depending on the cell line and cytokinin used, the treatment resulted in apoptosis, G1 or G2/M block. The spectrum of the effects induced by cytokinin ribosides in the tested cell lines included ATP depletion, genotoxic stress (Cabello et al. 2009), JNK activation (Laezza et al. 2009), inhibition of farnesyl-protein transferase activity (Laezza et al. 2006), inhibition of EGFR signaling (Ciaglia et al. 2016) and changes in levels of mitochondrial proteins (Cheong et al. 2009). Recently, microarray analysis of the effects of iPR (100 μM) on MCF7 and A549 cell lines was published. iPR induced a set of genes involved in stress induced cell cycle arrest, e.g., PPP1R15A, DNAJB9, DDIT3, and HBP1 (Colombo et al. 2009). Cytokinin ribosides may also interfere with neoangiogenesis (Pisanti et al. 2014). Further, cytokinin riboside-5′-monophosphates have been identified as inhibitors of putative oncogene RCL1 (Amiable et al. 2013; Voller et al. 2017).
In vivo anticancer activity of iPR, KR, oTR and BAR has been demonstrated using several animal and xenograft models of cancer (Griffaut et al. 2004; Laezza et al. 2006; Tiedemann et al. 2008; Voller et al. 2010). iPR and BAR have been shown to exhibit limited activity against a diverse range of cancers in a small clinical trial (Mittelman et al. 1975). Micromolar concentrations of both cytokinin ribosides and cytokinin bases can also induce cell death in plant cell cultures, with some traits typical for apoptosis (activation of caspase-like proteases and fragmentation of DNA) (Mlejnek and Procházka 2002; Mlejnek and Doležel 2005). This cell death is preceded by depletion of ATP and the production of ROS.
In contrast to their hormonal activity in plants, which requires interaction with specific membrane-bound receptors, intracellular conversion of cytokinins to 5′-monophosphates is necessary for their cytotoxic effect. The concentrations of cytokinins required to produce cytotoxic effects are higher than those found endogenously in plant tissues, but they do fall within the range used in plant bioassays (Carimi et al. 2003; Mlejnek and Doležel 2005). Phosphorylation of cytokinin ribosides by adenosine kinase (ADK) is a requirement for the cytotoxic effect of cytokinin ribosides in both animal (Mlejnek and Doležel 2005; Voller et al. 2010) and plant cells (Mlejnek and Procházka 2002). Low affinity to ADK (and possibly other nucleoside kinases) may explain the lack of activity of other cytokinin ribosides (Mlejnek and Doležel 2005) and possibly also their analogues with ribose replaced by acyclic polyols (Colombo et al. 2009; Ottria et al. 2009). Contrary to other nucleoside analogues that are converted to nucleoside triphosphates, the dominant metabolites of cytokinin ribosides are their respective riboside monophosphates (Mlejnek and Doležel 2005). This observation suggests that cytokinin ribosides have a different mechanism of action than classical antimetabolites which, after phosphorylation, directly interfere with the synthesis of nucleic acids. In cultured plant cells, the cytoxicity of cytokinin bases and their corresponding ribosides are comparable because, in contrast to human cell lines, plant cells are able to convert both metabolic forms efficiently into riboside-5 ́-monophosphates (Mlejnek and Procházka 2002; Mlejnek and Doležel. 2005).
A characteristic trait of leukemia cells is blockade of their differentiation into functional mature cells. Various chemicals acting by diverse mechanisms are able to force malignant cells to undergo terminal differentiation. Such differentiation therapy could be much safer than regimens based on cytotoxic effects. Cytokinin bases K, iP, BA and, to lesser degree, also tZ have been shown to induce granulocytic differentiation of human myeloid leukemia cell line HL-60 (Ishii et al. 2002) derived from the peripheral blood leukocytes of a patient with acute myeloid leukemia. The activity was mediated by phosphorylation of ERK1/2, expression of CEBPD and S100P (Ishii et al. 2005a, b). Whereas cytokinin bases induced differentiation at rather high concentrations (25–100 μM), their ribosides caused rapid apoptosis at low micromolar levels. Treatment with caspase inhibitors shifted the activity of iPR in HL-60 from pro-apoptotic to growth inhibitory and differentiating activity (Ishii et al. 2002). Cytokinins are also able to promote differentiation of keratinocytes, as discussed above.
8 Conclusions
Cytokinins regulate a wide range of plant processes, including growth, differentiation and organ senescence. This fascinating activity together with reports that some cytokinins occur in animals, including humans, has inspired interest in studying their effects in mammalian cell cultures and animal models. However, current knowledge of cytokinin signaling in plants suggests that the diverse effects of cytokinins in animal systems are relayed by mechanisms and pathways different from those that mediate hormonal activity in plants.
In plants, cytokinins are recognized by receptor histidine kinases, which are part of the His-Asp phosphorelay resembling the two-component environmental sensors of bacteria. No signaling system with similar organization exists in animals. In addition, plant leaf senescence, an active and highly regulated process, is a phenomenon completely different from animal aging (stochastic accumulation of damage) and cell senescence (irreversible growth arrest that protects the body against tumor development).
Nevertheless, studies of cytokinins in mammalian cell cultures and animals have led to discoveries of a range of responsive pathways and processes, as well as numerous prospective therapeutic applications. The propensity of cytokinins, which are adenine and adenosine derivatives, to influence diverse processes in animal cells is probably a consequence of their ability to interact with various components of the animal purinome.
The anti-proliferative activity of cytokinin ribosides (through induction of cell cycle block or/and cell death) and bases (through induction of cell differentiation) has prompted studies into their potential utility for the therapy of proliferative diseases like leukemias, cancers and psoriasis. Although anti-cancer cytokinin ribosides were evaluated in patients with diverse malignancies as early as the 1970s (Mittelman et al. 1975), limited activity and problems with stability led to a loss of interest in further development at that time. Recent interest in cytotoxic anti-cancer ribosides has been stimulated by molecular studies of their mechanism of action, e.g., by microarray analyses (Colombo et al. 2009) and discovery of the high anticancer activity of BARs hydroxylated on the phenyl ring (Doležal et al. 2007; Voller et al. 2010). Further, inhibitors of cyclin-dependent kinases olomoucine, bohemine, roscovitine, developed in our laboratory, were inspired by cytokinins (for a review see Jorda et al. 2012).
Another fertile research area was spurred on by the finding that the cytokinin bases K and tZ delay the onset of several characteristics related to aging in human skin fibroblasts during serial passage in vitro (Rattan and Clark 1994; Rattan and Sodagam 2005). Some cytokinin bases even extend the lifespan of invertebrates such as drosophila (Sharma et al. 1995) and C. elegans (our unpublished data). Even today, when tZ, K and its analogue Pyratine are the principal components of cosmetics used for the treatment of aging skin, their mechanism of action is still not fully understood. Since the original discovery, research has mainly focused on the ability of cytokinins to protect macromolecules and cells against oxidative damage and stress. Numerous studies have reported the ability of K to quench radicals directly and this activity has also been observed for several other cytokinin bases. However, K and tZ also induce cellular antioxidant defense by an as yet unidentified mechanism. Moreover, K modulates splicing of certain pre-RNAs (Slaugenhaupt et al. 2004). Therefore, it is tempting to speculate that it may also modulate splicing of transcripts of some genes related to cytoprotection.
Recently, Hertz et al. (2013) described an unusual mechanism of cytoprotective activity of K, including its intracellular conversion into respective ribotides. KRTP increases the activity of the mitoprotective kinase PINK1 by acting as a more active ATP analogue (neosubstrate). Other mechanisms of action including the ribosylation of cytokinin bases cannot be ruled out either. Cytokinin ribosides induce Nrf2-dependent transcription (Dassano et al. 2014, our unpublished data), inhibit proteosynthesis (as indicated by a decrease of activating phosphorylation of p70 S6 kinase and S6 protein) and activate autophagy (increase in levels of LC3-II) (our unpublished data). All these pathways have been implicated in cytoprotection and aging prevention (Bruns et al. 2015; Madeo et al. 2015). If cytokinin bases are metabolized into respective ribosides/ribotides in quantities sufficient to activate those stress response pathways, they could act as hormetin precursors, i.e., prohormetins.
In the last few years, cytokinin ribosides have been studied in connection with their anti-inflammatory and neuroprotective activities. Activation of A2A or A3 adenosine receptors has been implicated in the effect. Because much more active agonists have already reached clinical trials, purinergic activity alone may not warrant future development of such compounds into drugs. However, other pathways could possibly be involved in immunomodulatory activity, including activation of an Nrf2 response and inhibition of farnesyl diphosphate synthase. Notably, inhibition of protein farnesylation by iPR mediates not only its anti-cancer and immunomodulatory activities (Ciaglia et al. 2014; Laezza et al. 2006) but also its ability to decrease the accumulation of abnormal lamin A (progerin) in the nuclear envelope of cells originating from patients with Hutchinson-Gilford progeria (Bifulco et al. 2013). Progerin resulting from aberrant processing of wild type lamin A transcripts accumulates in tissues including skin of elderly and was therefore suggested as a biomarker of aging (McClintock et al. 2007). Hence, topical application of iPR (or possibly iP) could have multiple benefits.
A major obstacle in the development of cytokinin ribosides into anticancer drugs has been their pharmacokinetics. Clinical trials in the 1970s showed that they have a short plasmatic half-life (Mittelman et al. 1975). Our studies indicate that cytokinin ribosides may have problems with crossing biological barriers (Voller et al. 2010). However, studies in rodents reviewed above suggest that some cytokinin ribosides can be absorbed from the gut and even reach the brain, whereas others are able to penetrate the skin, as indicated by their therapeutic effects. Unfortunatelly, no information about plasma and/or tissue concentration has been reported. Such observations do not guarantee similar behavior in humans because of differences in physiology. For example, human skin has not only less hair follicles but also a thicker epidermis and dermis, which pose a much higher barrier to the penetration of drugs, especially hydrophilic ones. Prodrugs may be necessary to allow the active compound to reach target deep layers of (intact) skin. Another option could be administration of cytokinin bases that could be converted into active ribosides within the tissue, as discussed above. Once a cytokinin riboside is present in target skin layers, its pharmacokinetic properties may be seen as an advantage as they will prevent it from reaching the systemic circulation, and thus limit possible side effects (e.g., vasodilatation in the case of A2AR agonists).
K, tZ and Pyratine are principle components of cosmetics products (Kinerase and Pyratine-6 lines). Clinical evaluation reports have been published for K and Pyratine. Both compounds showed beneficial effects on photoaging skin and also ameliorated some symptoms of acne rosacea. Most of the studies were open-label and single-arm and effects were compared to the state before the treatment. The reported positive results together with multiyear experience of their safe usage in cosmetics could encourage testing of these compounds in clinical trials with a better design that may eventually support their approval in acne rosacea or possibly other inflammatory skin conditions. Recent discoveries of the unique activities of K relevant for the treatment of Parkinson’s disease (PINK1 activation) or familial dysautonomia (correction of aberrant splicing of IKBKAP transcripts) may result in clinical trials in those indications. Recruitment of patients for a study of familial dysautonomia is currently underway. Because the therapy would require chronic administration of K, we may also learn about its anti-aging activities not yet described from those studies.
Abbreviations
- ADK:
-
Adenosine kinase
- A2AR:
-
A2A adenosine receptor
- A3R:
-
A3R adenosine receptor
- BA:
-
N 6-benzyladenine
- BAR:
-
N 6-benzyladenosine
- ENT:
-
Equilibrative nucleoside transporter
- tZ:
-
trans-zeatin
- tZR:
-
trans-zeatin riboside
- iP:
-
N 6-isopentenyladenine
- iPR:
-
N 6-isopentenyladenosine
- ipt:
-
Isopentenyltransferase
- K:
-
Kinetin
- KR:
-
Kinetin riboside
- KRTP:
-
Kinetin riboside-5′-triphosphate
- oTR:
-
ortho-topolin riboside
- pTR:
-
para-topolin riboside
- ROS:
-
Reactive oxygen species
References
Amiable C, Pochet S, Padilla A, Labesse G, Kaminski PA (2013) N6-substituted AMPs inhibit mammalian deoxynucleotide N-hydrolase DNPH1. PLoS ONE 8:e80755. doi:10.1371/journal.pone.0080755
Barciszewski J, Siboska GE, Pedersen BO, Clark BFC, Rattan SIS (1996) Evidence for the presence of kinetin in DNA and cell extracts. FEBS 393:197–200
Barciszewski J, Siboska GE, Pedersen BO, Clark BFC, Rattan SIS (1997) A mechanism for the in vivo formation of N6-furfuryladenine, kinetin, as a secondary oxidative damage product of DNA. FEBS Lett 414:457–460. doi:10.1016/S0014-5793(97)01037-5
Barciszewski J, Mielcarek M, Stobiecki M, Siboska G, Clark BFC (2000) Identification of 6-furfuryladenine (kinetin) in human urine. Biochem Biophys Res Commun 279:69–73. doi:10.1006/bbrc.2000.3928
Berge U, Kristensen P, Rattan SIS (2006) Kinetin-induced differentiation of normal human keratinocytes undergoing aging in vitro. Ann NY Acad Sci 1067:332–336. doi:10.1196/annals.1354.045
Berge U, Kristensen P, Rattan SIS (2008) Hormetic modulation of differentiation of normal human epidermal keratinocytes undergoing replicative senescence in vitro. Exp Gerontol 43:658–662. doi:10.1016/j.exger.2007.12.009
Bifulco M, D’Alessandro A, Paladino S, Malfitano AM, Notarnicola M, Caruso MG, Laezza C (2013) N6-isopentenyladenosine improves nuclear shape in fibroblasts from humans with progeroid syndromes by inhibiting the farnesylation of prelamin A. FEBS J 280:6223–6232. doi:10.1111/febs.12544
Blad CC, von Frijtag Drabbe Künzel JK, de Vries H, Mulder-Krieger T, Bar-Yehuda S, Fishman P, Ijzerman AP (2011) Putative role of the adenosine A(3) receptor in the antiproliferative action of N (6)-(2-isopentenyl)adenosine. Purinergic Signal 7:453–462. doi:10.1007/s11302-011-9244-9
Borea PA, Varani K, Vincenzi F, Baraldi PG, Tabrizi MA, Merighi S, Gessi S (2014) The A3 adenosine receptor: history and perspectives. Pharmacol Rev 67:74–102. doi:10.1124/pr.113.008540
Brizzolari A, Marinello C, Carini M, Santaniello E, Biondi PA (2016) Evaluation of the antioxidant activity and capacity of some natural N6-substituted adenine derivatives (cytokinins) by fluorimetric and spectrophotometric assays. J Chromatogr B: Anal Technol Biomed Life Sci 1019:164–168. doi:10.1016/j.jchromb.2015.12.047
Bruns DR, Drake JC, Biela LM, Peelor FF, Miller BF, Hamilton KL (2015) Nrf2 signaling and the slowed aging phenotype: evidence from long-lived models. Oxid Med Cell Longev 2015:1–15. doi:10.1155/2015/732596
Cabello CM, Bair WB 3rd, Ley S, Lamore SD, Azimian S, Wondrak GT (2009) The experimental chemotherapeutic N6-furfuryladenosine (kinetin-riboside) induces rapid ATP depletion, genotoxic stress, and CDKN1A(p21) upregulation in human cancer cell lines. Biochem Pharmacol 77:1125–1138. doi:10.1016/j.bcp.2008.12.002
Carimi F, Zottini M, Formentin E, Terzi M, Lo Schiavo F (2003) Cytokinins: new apoptotic inducers in plants. Planta 216:413–421. doi:10.1007/s00425-002-0862-x
Chen C, Kristopeit SM (1981) Metabolism of cytokinin: deribosylation of cytokinin ribonucleoside by adenosine nucleosidase from wheat germ cells. Plant Physiol 68:1020–1023. doi:10.1104/pp.68.5.1020
Cheong J, Goh D, Yong JWH, Tan SN, Ong ES (2009) Inhibitory effect of kinetin riboside in human heptamoa, HepG2. Mol BioSyst 5:91–98. doi:10.1039/b712807j
Chhabra P, Linden J, Lobo P, Okusa MD, Brayman KL (2012) The immunosuppressive role of adenosine A2A receptors in ischemia reperfusion injury and islet transplantation. Curr Diabetes Rev 8:419–433
Chheda G, Mittelman A (1972) N6-(Δ2-isopentenyl) adenosine metabolism in man. Biochem Pharmacol 21:27–37
Chiu P-C, Chan C-C, Lin H-M, Chiu H-C (2007) The clinical anti-aging effects of topical kinetin and niacinamide in Asians: a randomized, double-blind, placebo-controlled, split-face comparative trial. J Cosmet Dermatol 6:243–249. doi:10.1111/j.1473-2165.2007.00342.x
Chiu F-L, Lin J-T, Chuang C-Y, Chien T, Chen C-M, Chen K-H, Hsiao H-Y, Lin Y-S, Chern Y, Kuo H-C (2015) Elucidating the role of the A2A adenosine receptor in neurodegeneration using neurons derived from Huntington’s disease iPSCs. Hum Mol Genet 24:6066–6079. doi:10.1093/hmg/ddv318
Choi SJ, Jeong C-H, Choi S-G, Chun J-Y, Kim YJ, Lee J, Shin D-H, Heo HJ (2009) Zeatin prevents amyloid beta-induced neurotoxicity and scopolamine-induced cognitive deficits. J Med Food 12:271–277. doi:10.1089/jmf.2007.0678
Chou S-Y, Lee Y-C, Chen H-M, Chiang M-C, Lai H-L, Chang H-H, Wu Y-C, Sun C-N, Chien C-L, Lin Y-S, Wang S-C, Tung Y-Y, Chang C, Chern Y (2005) CGS21680 attenuates symptoms of Huntington’s disease in a transgenic mouse model. J Neurochem 93:310–320. doi:10.1111/j.1471-4159.2005.03029.x
Ciaglia E, Pisanti S, Picardi P, Laezza C, Sosa S, Tubaro A, Vitale M, Gazzerro P, Malfitano AM, Bifulco M (2014) N6-isopentenyladenosine affects cytotoxic activity and cytokines production by IL-2 activated NK cells and exerts topical anti-inflammatory activity in mice. Pharmacol Res 89:1–10. doi:10.1016/j.phrs.2014.07.003
Ciaglia E, Abate M, Laezza C, Pisanti S, Vitale M, Seneca V, Torelli G, Franceschelli S, Catapano G, Gazzerro P, Bifulco M (2016) Antiglioma effects of N6-isopentenyladenosine, an endogenous isoprenoid end product, through the downregulation of epidermal growth factor receptor. Int J Cancer. doi:10.1002/ijc.30505
Colombo F, Falvella FS, De Cecco L, Tortoreto M, Pratesi G, Ciuffreda P, Ottria R, Santaniello E, Cicatiello L, Weisz A, Dragani TA (2009) Pharmacogenomics and analogues of the antitumour agent N6-isopentenyladenosine. Int J Cancer 124:2179–2185. doi:10.1002/ijc.24168
Dassano A, Mancuso M, Giardullo P, De Cecco L, Ciuffreda P, Santaniello E, Saran A, Dragani TA, Colombo F (2014) N(6)-isopentenyladenosine and analogs activate the NRF2-mediated antioxidant response. Redox Biol 2:580–589. doi:10.1016/j.redox.2014.03.001
David M, Gospodinov DK, Gheorghe N, Mateev GS, Rusinova MV, Hristakieva E, Solovastru LG, Patel RV, Giurcaneanu C, Hitova MC, Purcaru AI, Horia B, Tsingov II, Yankova RK, Kadurina MI, Ramon M, Rotaru M, Simionescu O, Benea V, Demerdjieva ZV, Cosgarea MR, Morariu HS, Michael Z, Cristodor P, Nica C, Silverman MH, Bristol DR, Harpaz Z, Farbstein M, Cohen S, Fishman P (2016) Treatment of plaque-type psoriasis with oral CF101: data from a phase II/III multicenter, randomized, controlled trial. J Drugs Dermatol 15:931–938
Doležal K, Popa I, Hauserová E, Spíchal L, Chakrabarty K, Novák O, Kryštof V, Voller J, Holub J, Strnad M (2007) Preparation, biological activity and endogenous occurrence of N6-benzyladenosines. Bioorg Med Chem 15:3737–3747. doi:10.1016/j.bmc.2007.03.038
Gan S, Amasino RM (1995) Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270:1986–1988. doi:10.1126/science.270.5244.1986
Ge L, Yong JWH, Tan SN, Yang XH, Ong ES (2004) Analysis of some cytokinins in coconut (Cocos nucifera L.) water by micellar electrokinetic capillary chromatography after solid-phase extraction. J Chromatogr A 1048:119–126
Gillissen B, Burkle L, Andre B, Kuhn C, Rentsch D, Brandl B, Frommer WB (2000) A new family of high-affinity transporters for adenine, cytosine, and purine derivatives in Arabidopsis. Plant Cell 12:291–300. doi:10.1105/tpc.12.2.291
Goldstein S, Czapski G (1991) SOD-like activity studies of cytokinin-copper(II) complexes. Free Radic Res Commun 12–13(Pt 1):173–177. doi:10.3109/10715769109145783
Griffaut B, Bos R, Maurizis J-CC, Madelmont J-CC, Ledoigt G (2004) Cytotoxic effects of kinetin riboside on mouse, human and plant tumour cells. Int J Biol Macromol 34:271–275. doi:10.1016/j.ijbiomac.2004.06.004
Haider S, Tabassum S, Perveen T (2016) Scopolamine-induced greater alterations in neurochemical profile and increased oxidative stress demonstrated a better model of dementia: a comparative study. Brain Res Bull 127:234–247. doi:10.1016/j.brainresbull.2016.10.002
Havlíček L, Hanuš J, Veselý J, Leclerc S, Meijer L, Shaw G, Strnad M (1997) Cytokinin-derived cyclizn-dependent kinase inhibitors: synthesis and cdc2 inhibitory activity of olomoucine and related compounds. J Med Chem 40:408–412. doi:10.1021/jm960666x
Heo H-J, Hong S-C, Cho H-Y, Hong B, Kim H-K, Kim E-K, Shin D-H (2002) Inhibitory effect of zeatin, isolated from Fiatoua villosa, on acetylcholinesterase activity from PC12 cells. Mol Cells 13:113–117
Hertz NT, Berthet A, Sos ML, Thorn KS, Burlingame AL, Nakamura K, Shokat KM (2013) A neo-substrate that amplifies catalytic activity of Parkinson’s-disease-related kinase PINK1. Cell 154:737–747. doi:10.1016/j.cell.2013.07.030
Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H (2008) Regulation of cytokinin biosynthesis, compartmentalization and translocation. J Exp Bot 59:75–83. doi:10.1093/jxb/erm157
Horgan R, Hewett EW, Horgan JM, Purse J, Wareing PF (1975) A new cytokinin from Populus x robusta. Phytochemistry 14:1005–1008. doi:10.1016/0031-9422(75)85176-4
Hsiao G, Shen M-Y, Lin K-H, Chou C-Y, Tzu N-H, Lin C-H, Chou D-S, Chen T-F, Sheu J-R (2003) Inhibitory activity of kinetin on free radical formation of activated platelets in vitro and on thrombus formation in vivo. Eur J Pharmacol 465:281–287. doi:10.1016/S0014-2999(03)01528-0
Huang C-L, Yang J-M, Wang K-C, Lee Y-C, Lin Y-L, Yang Y-C, Huang N-K (2011a) Gastrodia elata prevents huntingtin aggregations through activation of the adenosine A2A receptor and ubiquitin proteasome system. J Ethnopharmacol 138:162–168. doi:10.1016/j.jep.2011.08.075
Huang N-K, Lin J-H, Lin J-T, Lin C-I, Liu EM, Lin C-J, Chen W-P, Shen Y-C, Chen H-M, Chen J-B, Lai H-L, Yang C-W, Chiang M-C, Wu Y-S, Chang C, Chen J-F, Fang J-M, Lin Y-L, Chern Y (2011b) A new drug design targeting the adenosinergic system for Huntington’s disease. PLoS ONE 6:e20934. doi:10.1371/journal.pone.0020934
Ishii Y, Hori Y, Sakai S, Honma Y (2002) Control of differentiation and apoptosis of human myeloid leukemia cells by cytokinins and cytokinin nucleosides, plant redifferentiation-inducing hormones. Cell Growth Differ 13:19–26
Ishii Y, Kasukabe T, Honma Y (2005a) Induction of CCAAT/enhancer binding protein-δ by cytokinins, but not by retinoic acid, during granulocytic differentiation of human myeloid leukaemia cells. Br J Haematol 128:540–547. doi:10.1111/j.1365-2141.2004.05326.x
Ishii Y, Kasukabe T, Honma Y (2005b) Immediate up-regulation of the calcium-binding protein S100P and its involvement in the cytokinin-induced differentiation of human myeloid leukemia cells. Biochim Biophys Acta Mol Cell Res 1745:156–165. doi:10.1016/j.bbamcr.2005.01.005
Ji C, Yang Y, Yang B, Xia J, Sun W, Su Z, Yu L, Shan S, He S, Cheng L, Wan Y, Bi Z (2010) Trans-Zeatin attenuates ultraviolet induced down-regulation of aquaporin-3 in cultured human skin keratinocytes. Int J Mol Med 26:257–263. doi:10.3892/ijmm-00000460
Jones KR, Kang EM (2015) Graft versus host disease: new insights into A2A receptor agonist therapy. Comput Struct Biotechnol J 13:101–105. doi:10.1016/j.csbj.2014.12.003
Jorda R, Paruch K, Krystof V (2012) Cyclin-dependent kinase inhibitors inspired by roscovitine: purine bioisosteres. Curr Pharm Des 18:2974–2980
Kim S, Lee JJ, Jung E, Lee JJ, Huh S, Hwang H, Kim Y, Park D (2009) 6-Benzylaminopurine stimulates melanogenesis via cAMP-independent activation of protein kinase A. Arch Dermatol Res 301:253–258. doi:10.1007/s00403-008-0924-4
Kimura T, Doi K (2004) Depigmentation and rejuvenation effects of kinetin on the aged skin of hairless descendants of Mexican hairless dogs. Rejuvenation Res 7:32–39. doi:10.1089/154916804323105062
Kryštof V, Lenobel R, Havlíček L, Kuzma M, Strnad M (2002) Synthesis and biological activity of olomoucine II. Bioorg Med Chem Lett 12:3283–3286
Kudo T, Kiba T, Sakakibara H (2010) Metabolism and long-distance translocation of cytokinins. J Integr Plant Biol 52:53–60. doi:10.1111/j.1744-7909.2010.00898.x
Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J (2007) Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445:652–655. doi:10.1038/nature05504
Laezza C, Notarnicola M, Caruso MG, Messa C, Macchia M, Bertini S, Minutolo F, Portella G, Fiorentino L, Stingo S, Bifulco M (2006) N6-isopentenyladenosine arrests tumor cell proliferation by inhibiting farnesyl diphosphate synthase and protein prenylation. FASEB J Off Publ Fed Am Soc Exp Biol 20:412–418. doi:10.1096/fj.05-4044lsf
Laezza C, Caruso MG, Gentile T, Notarnicola M, Malfitano AM, Di Matola T, Messa C, Gazzerro P, Bifulco M (2009) N6-isopentenyladenosine inhibits cell proliferation and induces apoptosis in a human colon cancer cell line DLD1. Int J Cancer 124:1322–1329. doi:10.1002/ijc.24056
Laezza C, Malfitano AM, Di Matola T, Ricchi P, Bifulco M (2010) Involvement of Akt/NF-κB pathway in N6-isopentenyladenosine-induced apoptosis in human breast cancer cells. Mol Carcinog 49:892–901. doi:10.1002/mc.20666
Lappas CM (2015) The plant hormone zeatin riboside inhibits T lymphocyte activity via adenosine A2A receptor activation. Cell Mol Immunol 12:107–112. doi:10.1038/cmi.2014.33
Lee Y-C, Yang Y-C, Huang C-L, Kuo T-Y, Lin J-H, Yang D-M, Huang N-K (2012) When cytokinin, a plant hormone, meets the adenosine A2A receptor: a novel neuroprotectant and lead for treating neurodegenerative disorders? PLoS ONE 7:e38865. doi:10.1371/journal.pone.0038865
Liu Y, Zhang Z, Yang X (2011) Kinetin protects against lipid peroxidation and improves antioxidant status in cultured astrocytes and mouse brain exposed to d-galactose. Afric J Biotech 10:11721–11727. doi:10.5897/AJB11.289
Luo C, Yi B, Tao G, Li M, Chen Z, Tang W, Zhang JH, Feng H (2010) Adenosine A3 receptor agonist reduces early brain injury in subarachnoid haemorrhage. NeuroReport 21:892–896. doi:10.1097/WNR.0b013e32833dbd13
Madeo F, Zimmermann A, Maiuri MC, Kroemer G (2015) Essential role for autophagy in life span extension. J Clin Invest 125:85–93. doi:10.1172/JCI73946
Maria Pugliese A, Coppi E, Volpini R, Cristalli G, Corradetti R, Jeong LS, Jacobson KA, Pedata F (2007) Role of adenosine A3 receptors on CA1 hippocampal neurotransmission during oxygen–glucose deprivation episodes of different duration. Biochem Pharmacol 74:768–779. doi:10.1016/j.bcp.2007.06.003
McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, Collins FS, Djabali K (2007) The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS ONE 2:e1269. doi:10.1371/journal.pone.0001269
McCullough JL, Weinstein GW (2002) Clinical study of safety and efficacy of using topical kinetin 0.10% (Kinerase) to treat photodamaged skin. Cosmet Dermatol 15(9):29–32
McCullough JL, Garcia RL, Reece B (2008) A clinical study of topical Pyratine 6 for improving the appearance of photodamaged skin. J Drugs Dermatol JDD 7:131–135
McCullough JL, Weinstein GW (2002) Clinical study to assess the safety and efficacy of topical kinetin 0.10% (Kinerase®) for photodamaged skin. Cosmet Dermatol 15:29–32
McDaniel DH, Neudecker BA, DiNardo JC, Lewis JA 2nd, Maibach HI (2005) Idebenone: a new antioxidant—Part I. Relative assessment of oxidative stress protection capacity compared to commonly known antioxidants. J Cosmet Dermatol 4:10–17. doi:10.1111/j.1473-2165.2005.00152.x
Mcdermott SP, Eppert K, Notta F, Isaac M, Datti A, Al-awar R, Minden MD, Dick JE, Dc W, Wrana J (2012) A small molecule screening strategy with validation on human leukemia stem cells uncovers the therapeutic efficacy of kinetin riboside A small molecule screening strategy with validation on human leukemia stem cells uncovers the therapeutic efficacy of ki. Blood 119:1200–1207. doi:10.1182/blood-2011-01-330019
Meisel H, Günther S, Martin D, Schlimme E (1998) Apoptosis induced by modified ribonucleosides in human cell culture systems. FEBS Lett 433:265–268
Milne GR, Palmer TM (2011) Anti-inflammatory and immunosuppressive effects of the A2A adenosine receptor. Sci World J 11:320–339. doi:10.1100/tsw.2011.22
Mittelman A, Evans JT, Chheda GB (1975) Cytokinins as chemotherapeutic agents. Ann NY Acad Sci 255:225–234
Mlejnek P, Doležel P (2005) Apoptosis induced by N6-substituted derivatives of adenosine is related to intracellular accumulation of corresponding mononucleotides in HL-60 cells. Toxicol In Vitro 19:985–990. doi:10.1016/j.tiv.2005.06.023
Mlejnek P, Kuglík P (2000) Induction of apoptosis in HL-60 cells by N6-benzyladenosine. J Cell Biochem 77:6–17. doi:10.1002/(SICI)1097-4644(20000401)77:1<6:AID-JCB2>3.0.CO;2-3
Mlejnek P, Procházka S (2002) Activation of caspase-like proteases and induction of apoptosis by isopentenyladenosine in tobacco BY-2 cells. Planta 215:158–166. doi:10.1007/s00425-002-0733-5
Mok DWS, Mok MC (2001) Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol 52:89–118
Olsen A, Siboska GE, Clark BF, Rattan SI (1999) N(6)-Furfuryladenine, kinetin, protects against Fenton reaction-mediated oxidative damage to DNA. Biochem Biophys Res Commun 265:499–502. doi:10.1006/bbrc.1999.1669
Ortiz A, Elkeeb L, Truitt A, Hindiyeh R, Aquino L, Tran M, Weinstein G (2009) Topical PRK 124 (0.125%) lotion for improving the signs and symptoms of rosacea. J Drugs Dermatol JDD 8:459–462
Ottria R, Casati S, Maier JAM, Mariotti M, Ciuffreda P (2009) Novel isopentenyladenosine analogues: synthesis, characterization, and evaluation of antiproliferative activity on bladder carcinoma cells. nucleosides, nucleotides and nucleic acids 28:736–751. doi:10.1080/15257770903155550
Pisanti S, Picardi P, Ciaglia E, Margarucci L, Ronca R, Giacomini A, Malfitano AM, Casapullo A, Laezza C, Gazzerro P, Bifulco M (2014) Antiangiogenic effects of N6-isopentenyladenosine, an endogenous isoprenoid end product, mediated by AMPK activation. FASEB J 28:1132–1144. doi:10.1096/fj.13-238238
Rajabi M, Gorincioi E, Santaniello E (2012) Antiproliferative activity of kinetin riboside on HCT-15 colon cancer cell line. Nucleosides Nucleotides Nucleic Acids 31:474–481. doi:10.1080/15257770.2012.681825
Rattan SI, Clark BF (1994) Kinetin delays the onset of ageing characteristics in human fibroblasts. Biochem Biophys Res Commun 201:665–672
Rattan SIS, Sodagam L (2005) Gerontomodulatory and youth-preserving effects of zeatin on human skin fibroblasts undergoing aging in vitro. Rejuvenation Res 8:46–57. doi:10.1089/rej.2005.8.46
Sharma SP, Kaur P, Rattan SI (1995) Plant growth hormone kinetin delays ageing, prolongs the lifespan and slows down development of the fruitfly Zaprionus paravittiger. Biochem Biophys Res Commun 216:1067–1071. doi:10.1006/bbrc.1995.2729
Sharma SP, Kaur J, Rattan SI (1997) Increased longevity of kinetin-fed Zaprionus fruitflies is accompanied by their reduced fecundity and enhanced catalase activity. Biochem Mol Biol Int 41:869–875. doi:10.1080/15216549700201911
Skoog F, Strong FM, Miller CO (1965) Cytokinins. Science 148:532–533. doi:10.1126/science.148.3669.532-a
Slaugenhaupt SA, Mull J, Leyne M, Cuajungco MP, Gill SP, Hims MM, Quintero F, Axelrod FB, Gusella JF (2004) Rescue of a human mRNA splicing defect by the plant cytokinin kinetin. Hum Mol Genet 13:429–436. doi:10.1093/hmg/ddh046
Spíchal L, Rakova NY, Riefler M, Mizuno T, Romanov GA, Strnad M, Schmülling T (2004) Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant Cell Physiol 45:1299–1305. doi:10.1093/pcp/pch132
Spinola M, Colombo F, Falvella FS, Dragani TA (2007) N6-isopentenyladenosine: a potential therapeutic agent for a variety of epithelial cancers. Int J Cancer 120:2744–2748. doi:10.1002/ijc.22601
Štarha P, Trávníček Z, Herchel R, Popa I, Suchý P, Vančo J (2009) Dinuclear copper(II) complexes containing 6-(benzylamino)purines as bridging ligands: synthesis, characterization, and in vitro and in vivo antioxidant activities. J Inorg Biochem 103:432–440. doi:10.1016/j.jinorgbio.2008.12.009
Strnad M (1997) The aromatic cytokinins. Physiol Plant 101:674–688. doi:10.1111/j.1399-3054.1997.tb01052.x
Tiedemann RE, Mao X, Shi C-X, Zhu YX, Palmer SE, Sebag M, Marler R, Chesi M, Fonseca R, Bergsagel PL, Schimmer AD, Stewart AK (2008) Identification of kinetin riboside as a repressor of CCND1 and CCND2 with preclinical antimyeloma activity. J Clin Invest 118:1750–1764. doi:10.1172/JCI34149
Tournas JA, Lin F-H, Burch JA, Selim MA, Monteiro-Riviere NA, Zielinski JE, Pinnell SR (2006) Ubiquinone, idebenone, and kinetin provide ineffective photoprotection to skin when compared to a topical antioxidant combination of vitamins C and E with ferulic acid. J Invest Dermatol 126:1185–1187. doi:10.1038/sj.jid.5700232
Tremaine AM, Ortiz A, Elkeeb L, Tran M, Weinstein G (2010) Long-term efficacy and safety of topical PRK 124 (0.125%) lotion (Pyratine-XR) in the treatment of mild-to-moderate rosacea. J drugs dermatology JDD 9:647–650
van der Hoeven D, Gizewski ET, Auchampach JA (2010) Activation of the A3 adenosine receptor inhibits fMLP-induced Rac activation in mouse bone marrow neutrophils. Biochem Pharmacol 79:1667–1673. doi:10.1016/j.bcp.2010.02.002
Verbeke P, Siboska GE, Clark BF, Rattan SI (2000) Kinetin inhibits protein oxidation and glycoxidation in vitro. Biochem Biophys Res Commun 276:1265–1270. doi:10.1006/bbrc.2000.3616
Vermeulen K, Strnad M, Kryštof V, Havlíček L, Van der Aa A, Lenjou M, Nijs G, Rodrigus I, Stockman B, van Onckelen H, Van Bockstaele DR, Berneman ZN (2002) Antiproliferative effect of plant cytokinin analogues with an inhibitory activity on cyclin-dependent kinases. Leuk Off J Leuk Soc Am Leuk Res Fund UK 16:299–305. doi:10.1038/sj.leu.2402378
Veselý J, Havlíček L, Strnad M, Blow JJ, Donella-Deana A, Pinna L, Letham DS, Kato J, Detivaud L, Leclerc S (1994) Inhibition of cyclin-dependent kinases by purine analogues. Eur J Biochem 224:771–786. doi:10.1111/j.1432-1033.1994.00771.x
Vičanová J, Bouez C, Lacroix S, Lindmark L, Damour O (2006) Epidermal and dermal characteristics in skin equivalent after systemic and topical application of skin care ingredients. Ann NY Acad Sci 1067:337–342. doi:10.1196/annals.1354.046
Voller J, Zatloukal M, Lenobel R, Doležal K, Béres T, Kryštof V, Spíchal L, Niemann P, Džubák P, Hajdúch M, Strnad M (2010) Anticancer activity of natural cytokinins: a structure-activity relationship study. Phytochemistry 71:1350–1359. doi:10.1016/j.phytochem.2010.04.018
Voller J, Béres T, Zatloukal M, Kaminski PA, Niemann P, Doležal K, Džubák P, Hajdúch M, Strnad M (2017) The natural cytokinin 2OH3MeOBAR induces cell death by a mechanism that is different from that of the “classical” cytokinin ribosides. Phytochemistry. doi:10.1016/j.phytochem.2017.01.004
Walla J, Szücová L, Císařová I, Gucký T, Zatloukal M, Doležal K, Greplová J, Massino FJ, Strnad M (2010) X-ray structure, NMR and stability-in-solution study of 6-(furfurylamino)-9-(tetrahydropyran-2-yl)purine—a new active compound for cosmetology. J Mol Struct 975(1–3):376–380
Wang L, Sun C, Wang Z-H, Guo G-Q (2012) Mechanism of apoptotosis induced by ortho-topolin riboside in human hepatoma cell line SMMC-7721. Food Chem Toxicol 50:1962–1968. doi:10.1016/j.fct.2012.03.053
Wanitphakdeedecha R, Meeprathom W, Manuskiatti W (2015) Efficacy and safety of 0.1% kinetin cream in the treatment of photoaging skin. Indian J Dermatol Venereol Leprol 81:547. doi:10.4103/0378-6323.157446
Werner T, Schmülling T (2009) Cytokinin action in plant development. Curr Opin Plant Biol 12(5):527–538. doi:10.1016/j.pbi.2009.07.002
Wu JJ, Weinstein GD, Kricorian GJ, Kormeili T, McCullough JL (2007) Topical kinetin 0.1% lotion for improving the signs and symptoms of rosacea. Clin Exp Dermatol 32:693–695. doi:10.1111/j.1365-2230.2007.02513.x
Yang B, Ji C, Kang J, Chen W, Bi Z, Wan Y (2009) Trans-zeatin inhibits UVB-induced matrix metalloproteinase-1 expression via MAP kinase signaling in human skin fibroblasts. Int J Mol Med 23:555–560
Zhang K, Novak O, Wei Z, Gou M, Zhang X, Yu Y, Yang H, Cai Y, Strnad M, Liu C-J (2014) Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins. Nat Commun 5:3274. doi:10.1038/ncomms4274
Zwack PJ, Rashotte AM (2013) Cytokinin inhibition of leaf senescence. Plant Signal Behav 8:e24737. doi:10.4161/psb.24737
Acknowledgements
The study was partly supported by the Internal Grant Agency of Palacký University (IGA_PrF_2017_013), Endowment Fund of Palacký University and by the Grant Agency of the Czech Republic (7-14007S). This work was also funded by the Ministry of Education, Youth and Sports of the Czech Republic (the National Program for Sustainability I: Nr. LO1204).
The authors also would like to thank Sees-editing Ltd. (UK) for the English editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Voller, J., Maková, B., Kadlecová, A., Gonzalez, G., Strnad, M. (2017). Plant Hormone Cytokinins for Modulating Human Aging and Age-Related Diseases. In: Rattan, S., Sharma, R. (eds) Hormones in Ageing and Longevity. Healthy Ageing and Longevity, vol 6. Springer, Cham. https://doi.org/10.1007/978-3-319-63001-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-63001-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-63000-7
Online ISBN: 978-3-319-63001-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)