Abstract
The knowledge of the natural history of the breast carcinoma and its origin, the use of all diagnostic methods, and the good preoperatory control of the suspect zone in the lobe allow us in many cases to carry out a limited operation which is rationally and oncologically correct and to always give the greatest importance to the woman’s image, for her well-being. This chapter points out the need to correlate the radial echographic technique introduced by Michel Teboul and conservative breast surgery.
We always closely connect this methodology to the preoperative ultrasound evaluation and the subsequent reevaluation of the removed lobe with the pathologist.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Ductal echography
- Breast ultrasound
- Lobectomy
- Early breast cancer
- Anatomopathological examination
- Presurgical evaluation
-
Breast cancer is not a lump but a lobar disease.
-
Involvement of the breast lobe is often patchy or diffuse (multifocal or extensive DCIS).
-
Breast conservative surgery to be radical must excise the whole lobe (lobar surgical approach).
-
Lobar ultrasound is a useful tool to assist and guide breast surgeon.
16.1 Introduction
Breast-conserving surgery is the treatment of choice for women with relatively small breast cancers. The long-term survival rate among women who undergo breast-conserving surgery is the same as that among women who undergo radical mastectomy [1].
In the era of breast-conserving surgery, the problem is local recurrence. Conservative surgery with radiotherapy has been widely accepted as an alternative to mastectomy in the management of early-stage breast cancer, with a long-term local recurrence rate of approximately 10–20%. This number is much higher if postoperative irradiation is omitted. The cumulative incidence of recurrent tumor in the ipsilateral breast was 14.3% in the women who underwent lumpectomy followed by breast irradiation compared with 39.2% in the women who underwent lumpectomy without irradiation (P < 0.001) [2].
A considerable number of patients still experience local recurrence, even in some of the cases when the surgical margins of the resection have been judged to be cancer free.
Although several studies have shown no significant difference in distant disease-free survival (DFS) between women who did and did not have a breast recurrence after conservation therapy, a local failure can destabilize the patient’s psychological balance. Besides, a re-excision after lumpectomy often causes an unsatisfactory cosmetic outcome.
Working together with other colleagues has proved to be particularly interesting and stimulating, insofar as each of us has come to the same conclusion via different routes: that, in order to battle against breast tumors, we all have to recognize, from within his/her own field, that the functional anatomical unity of the breast is in fact the lobe.
For several decades, Dolfin [3] has performed breast surgery and concurrently utilized ultrasound diagnosis, following directives used in the school led by his friend Michel Teboul [4]. The pathological contribution has always also proved indispensable; indeed, Drs. Botta and Dolfin have always compared correspondences between data as outlined either by microscope, by ultrasound, or by surgical evidence.
Botta has scrutinized such correspondences between ultrasound data and anatomical pathology by utilizing lobar dissection with macrosection, a technique introduced by Dr. Tibor Tot [5 ], a contributor to the present volume.
Other surgical groups—and Prof. Durante [6] at the University of Ferrara in particular—have used the lobar approach in their surgical interventions in order to obtain better oncological and aesthetic outcomes. Like us, these other circles have obtained a dramatic reduction in the rate of recurrence in women suffering from this pathology, limiting the extension of surgical interventions, whenever allowed by the pathological criteria. Throughout Europe, courses have been offered based on the experiences accrued from the collaboration of diverse specialists in senology. Among the most enthusiastic organizers of these courses, we would like to mention in particular our recently deceased Spanish friend, Javier Amoros Oliveros [7].
Surgery should achieve both acceptable cosmetic results and negative margins [8], which require a preoperative study and localization of lesions.
The surgeon must have a thorough knowledge of the anatomical and functional changes of the mammary gland, the neoplastic and preneoplastic modifications, their origin, and their location; the pathologist must check that the surgeon has removed all the suspected areas, included in a halo of healthy tissue.
Today we know that the disease is not limited to an identified breast node but is extended to the whole breast lobe where that node arose [9].
The “ sick lobe” is born genetically misshapen, during embryonic development, carrying some kind of genetic instability that implies an increased sensitivity to endogenic and exogenic oncogenic stimuli.
The number of the transformed cells, their location within the sick lobe, and the time difference in their transformation determines the morphology of the individual cancer and its biological potential [10].
The following parameters could influence the surgical approach and the patient’s prognosis:
-
1.
Multiple localization (either in situ or invasive)
-
2.
Tumor extension
-
3.
Lobe extension
-
4.
Skin/fascia/areola/nipple involvement
16.2 Multiple Localization
In their early phase, almost all breast carcinomas seem to affect only one sick lobe [11]. Breast carcinoma is a lobar disease in that the tumor foci (appearing simultaneously or asynchronously, in situ or invasively) develop within a single sick lobe and the cancerous structures are confined to the area of the sick lobe at the early stage of the disease.
For this reason, most breast carcinomas exhibit complex subgross morphology and frequently multiple locations.
The prevalence of multicentric (MC) and multifocal (MF) tumors varies from 5% to 44% in published series [12], depending on the definition used, the method of histological examination of mastectomy specimens, and the type of imaging used for diagnosis.
Different diagnostic criteria for multifocality have deeply influenced the various studies, causing these results to be hardly comparable to each other.
MF carcinomas are usually defined by the presence of several invasive tumors in the same quadrant of the breast within a distance inferior to 5 cm.
MC carcinomas are defined by the presence of at least two invasive tumors in two different quadrants of the breast or in the same quadrant but at least 5 cm apart [13].
Related to lobar breast anatomy and physiology, multifocality means the presence of several tumor foci within a single lobe and multicentricity the presence of two or more sick lobes within the same breast.
Most of these definitions focus on invasive tumor foci, ignoring the in situ component of the tumor [12, 14,15,16].
Mammography and ultrasound are the standard imaging tools for the diagnosis of breast cancer and are also used to determine the extent of the disease within the affected breast.
Magnetic resonance imaging is increasingly used. However, the impact of breast MRI on breast cancer management is debated, due to a large number of additional benign lesions that could be detected and could incorrectly influence clinical decisions [17, 18].
The histopathologic method used is also an important factor influencing the results in studies of multifocality and everyday diagnostic routine.
Large-section histopathology substantially increases both the proportion of detected multiple tumor foci and their number for each case [19]. Regular use of large sections in diagnostic pathology results in 25–37% multifocality regarding the invasive component [20].
Combining the distribution of the in situ and invasive components results in up to 60% to 65% nonunifocal subgross distribution of the lesions. Such a high level of multifocality seems to be independent of tumor size and histologic type and was observed in a series of smaller than 15-mm invasive carcinomas [10, 11, 21,22,23].
Approximately half the invasive cases are unifocal (only one invasive focus could be observed in the large sections, which may or may not have contained an in situ component close to the invasive focus.
A quarter of the cases are characterized by the presence of a multifocal invasive lesion characterized by the presence of multiple, well-delineated invasive tumor foci separated from each other by uninvolved breast tissue containing normal tissue, benign lesions, or in situ carcinoma.
The final quarter of the cases exhibit tumors dispersed over a large-section area with no distinct tumor mass, like a spider’s web. In situ or invasive breast carcinomas of diffuse type often represent extensive disease, limiting the success of breast-conserving surgery [11, 23].
16.3 Tumor and Lobar Extension
Lobar ultrasound examination allows us to study the ductal tree of the sick lobe and map out the diseased part(s) which is essential in guiding adequate surgical intervention. Breast carcinomas of limited extent (<4 cm), whether unifocal or multifocal, are proper candidates for breast-conserving surgery. Adequacy of breast conservation in more extensive tumors should be carefully judged preoperatively in every individual case.
The dimensions of the breast lobes are very different within the same breast and also individually [24, 25]. The largest lobe appearing in one of the very few related studies comprised 25% of the breast volume, the smallest only 1% of the breast volume. Lobes are larger in the upper outer quadrant of the breast than in the medial parts. In addition, the dimensions of the lobes are also age related; they are larger in younger women and undergo involution around and after the menopause. The lobes in the medial quadrants of the breast develop later and undergo involution earlier than the lobes in the lateral quadrants. During the malignant transformation of the structures of the sick lobe, new cancerous TDLUs and ducts may develop and increase the dimension of the involved lobe [11].
Young age strongly correlates with a high risk of local recurrence after breast-conserving surgery, whether or not radiotherapy is given. This relationship is associated with the dimensions of the sick lobe, which is an important factor in determining the success of breast-conserving surgery.
16.4 Skin/Fascia Involvement
Equally important, both from the oncological point of view and for the surgical planning, is the distance of the tumor from the skin. We need a careful study of the subcutaneous tissue and the areas corresponding to Cooper’s ligaments. Through these ligaments, in fact, it has been demonstrated that tumor cells can spread to upper areas. This migration can increase the relapses [24].
16.4.1 Presurgical Cytohistological Examination of Breast Lesions
Presurgical cytohistological examination of breast lesions integrated by palpatory exam and diagnostic imaging (ultrasound and radiological) would minimize the proportion of excision biopsies of benign lesions for diagnostic purposes and would maximize the preoperative diagnosis of cancer (Table 16.1 )
Cytological investigation is applicable:
-
On secretion from the nipple: The examination is indicated when the secretion is the only clinical sign and especially if it is hematic. The prevalence of cancer in the presence of each other type of secretion and in the absence of other clinical findings is irrelevant.
-
On liquid inside a cyst: The examination is indicated in the presence of bloody or siero-hematic liquid. The prevalence of cancer in the presence of another type of content is irrelevant.
-
On material obtained by abrasion of erosive lesions of the nipple: The examination is indicated whenever it poses the minimal suspicious for cases of the nipple Paget unaccompanied by mass clinically or radiologically appreciable, a very rare lesion.
-
On material obtained by fine needle aspiration of solid palpable or non-palpable breast lesion (FNA) [26, 27].
16.4.1.1 Cytopathological Report (Secretion, Scraping, or FNA)
The descriptive diagnosis is optional. In this case, the cytopathological report must be clear and succinct and, if possible, have reference to the corresponding histopathology. The diagnostic conclusion is obligatory according to one of the following formulas:
C1: Inadequate
Reason must be indicated:
-
Cellularity poor or absent
-
Artefactual cellular distortions by unsuitable sample
-
Cellularity obscured by blood and/or inflammation
The presence of only fat tissue cannot be considered in any case as an “inadequate diagnosis” because in some cases may be the expected finding.
C2: Benign. No evidence of malignancy
If there is sufficient cytological features, a specific diagnosis may be indicated (e.g., fibroadenoma).
C3: Atypical/probably benign
The basic framework of cytology is benign, but there are one or more of the following characteristics:
-
Increased cellularity
-
Polymorphism cyto-nuclear
-
Loss of initial or focal cell cohesion
C4: Suspicious/probably malignant
The cytological features are suggestive but not diagnostic of malignancy. In this category may fall a number of lesions “borderline” or ductal low-G (cribriform, papillary, tubular) when there is insufficient cytologists criteria for belonging to the next category or the presence of atypical elements indicative of malignant lesion in a small amount for the “application of the higher category”.
C5: Malignant
The cytological features are diagnostic of malignancy, when it is possible it is preferable to mention the nuclear grade (G) and to specify the presence or absence of microcalcifications.
16.4.1.2 Diagnostic Performance Indices of Cytological Investigation (FNA)
In major centers, the sensitivity for cancer (positive + suspicious, inadequate excluded) is 90–95%. The specificity of a positive outcome is less than 1% and its predictive value greater than 99%. The rate of inadequate in the case of cancer is less than 10%. In the presence of a positive report, verified the high predictability, intraoperative biopsy can be omitted. In the presence of a suspicious report, verified his prediction that ranges in literature between 40% and 80%, surgical biopsy is mandatory, regardless of the clinical evidence. In the presence of a negative report, given the possible false negative, the opportunity to suggest a biopsy indicated by other diagnostic tests is not denied. Rates of sensitivity, specificity, and predictive value not compatible with those observations call for a critical review of the sequence collection/treatment/reading and possibly a comparison with an experienced center. The overall sensitivity and specificity of fine needle aspiration depend on variables intrinsic to the technique as well as related to radiological/clinical and histological features [28].
16.4.1.3 Sampling Techniques for FNA Cytology
According to the guide line of the Royal College of Pathologist of Australia (www.nbcc.org.au) [29], we describe a recommended approach to taking the tissue sample using FNA cytology under clinical guidance (palpable lesion):
-
The woman is placed in supine position.
-
First disinfect the skin over the lesion.
-
The lesion is immobilized between two fingers of one hand, and the needle is introduced with the other hand. Depending on operator preference, the needle may be introduced on its own or directly with the syringe (and its holder) attached. We suggest to use needles from 22G to 27G (2–4 cm long), however, giving preference to small caliber needles.
-
When the needle tip is felt to be at the edge of the lesion, negative pressure is applied while entering the lesion.
-
Rapid multiple passes are made through the lesion, varying the angling of the sampling.
-
If blood is seen in the hub, sampling should be ceased as excessive blood reduces the quality of the sample.
-
The negative pressure is released while the needle is still in the lesion. The needle is then withdrawn.
-
The material is expelled from the needle onto a labeled glass slide using the syringe
-
Using another clean glass slide, smear the material to obtain a thin/uniformed distributed sample followed by a rapid fixation (alcohol 95 or methanol). This is a critical point to obtain a high-quality sample (an appropriate and well-fixed smear).
-
If a pathologist is available an immediate examination of the slides after a rapid staining (Blue Toluidine) allow to recognize if aspirated material is sufficient. If an immediate examination is not possible, at least three passes are taken. Typically, any further sampling has little additional yield.
The cells can be also sampled without aspiration (cytopunction) taking advantages of spontaneous rising capillary action. This method offer qualitative advantages (less cell distortion) but usually scant material compare to aspiration technique.
It is preferable to perform FNA by ultrasound guide (this approach it is mandatory in a non-palpable lesion) [28]. The operator holds the ultrasound probe with one hand and introduces the needle with the other. The needle should be introduced so its long axis is in line with the long axis of the probe face. With this approach, the full length of the needle, including the tip, is visualized at all times. The needle angle should be kept as parallel to the probe face and chest wall as possible, so as to aid visualization and reduce the risk of accidental pneumothorax. A hard copy image may be taken to record the position of the tip of the needle inside the lesion. It is also advisable that in the palpable lesions, especially if large and inhomogeneous, an ultrasound examination is preliminarily performed in order to be sure that the needle has reached the diagnostic component of the lesion (Fig. 16.1 ).
In particular, in the lesions of extension of greater dimensions than or equal to 20 mm, mammographically connoted by hypodense central core, the sampling should be carried out on the marginal areas of the lesion, the more likely mobile and free from phenomena of sclerosis (Fig. 16.2 ).
FNA cytology requires training in the preparation of quality smears and a considerable cytology expertise for sample interpretation.
Liquid-based cytology applied to FNA of breast lesions allows to reduce unsatisfactory samples giving increased attention to nuclear details [30].
16.5 Percutaneous Microbiopsy (Core Biopsy) (CB): Sampling Procedure
According to the guideline of the Royal College of Pathologist of Australia [29], the following description represents one approach to taking the tissue sample using core biopsy under ultrasound guidance (www.nbcc.org.au):
-
Patient positioning, skin cleansing, and lesion fixation are the same as for a cytology procedure. However, local anesthesia is always used in the skin and in immediate subcutaneous tissue.
-
Using a scalpel blade, a small cut is made at the selected entry point, and the needle is introduced through this entry point.
-
The needle needs to be introduced so its long axis is in line with the long axis of the probe and as parallel to the chest wall as possible. The area beyond the needle tip should be visualized prior to firing to reduce the risk of a pneumothorax, because the needle is typically thrown forward 15–22 mm.
-
When the tip of the needle is felt to be at the leading edge of the lesion, the firing mechanism is released and the sample taken.
-
The needle is removed and the sample extracted.
-
The procedure is then repeated. Typically three to five samples are taken through different parts of the lesion to ensure adequacy of sampling.
-
The number of cores will be the result of various issues, including lesion characteristics, imaging findings, ability to localize, guidance modality, patient tolerance, and the confidence in the adequacy of the sample. Generally, between three and five cores will be taken.
-
The sample must be fixed immediately; the formaldehyde-based fixatives are the most used but may lead to the dissolution of microcalcifications when the fixing is prolonged beyond 24 h; alcoholic fixatives do not have this problem but cause greater tissue coarctation; a good one is given by the Carnoy’s fixative, which does not dissolve the calcifications, narrows slightly the fabric, and allows good preservation of tissue antigens by immunocytochemical investigations; however, it does not allow the radiography of the sample fixed to the radiopacity of its components. After inclusion, it is appropriate to set up immediately additional sections for the possible biological characterization of the tumor, to avoid losing the fabric during the cutting operations.
-
The use of snap needles 18G provides frustules usually of 5 mm long with a volume of tissue removed (per withdrawal) of about 6 mm3 while needles from 14G for taking cores of length up to 10 mm with a volume of tissue that varies from 20 to 35 mm3. Currently, most of the published series refers to the use of needles from 14G.
16.5.1 Histopathological Report
It is recommended that the classification is divided into five diagnostic categories, with a pattern similar to that used for the needle aspiration cytology; however, it is stressed that the categories do not have the same meaning of the cytological and possess different clinical application outcomes. It is essential to compare the histological picture with the X-ray to ensure the representativeness of the biopsy.
The diagnostic conclusion is obligatory according to one of the following formulas:
-
B1: (a) normal tissue, (b) only stroma, or (c) cannot be interpreted for artifacts. The presence of dystrophic calcification in the absence of stromal epithelium, where compatible with the radiological picture, must be classified as BENIGN.
-
B2: benign lesion. It is recommended a concise text description.
-
B3: benign lesion, but of uncertain biological behavior. It indicates lesions that are associated with increased risk of developing a carcinoma or are often associated with carcinomas in situ or invasive, for example, papillary lesions and scleroelastosis lesions. It is recommended that, where possible, a concise text description.
-
B4: suspect. The category should be used when observing with compatible carcinoma in situ lesions or invasive, but when the final diagnosis is not formulated for artifacts for the presence of atypia or borderline. It is recommended that, where possible, a concise text description.
-
B5: malignant. It indicates the undeniable presence of malignancy or invasive carcinoma in situ. In the case of diagnosis of carcinoma (B5), must indicate the presence or absence of infiltrating carcinoma or if the invasion is possible but not certain. If you suspect the presence of metastatic cancer, the data needs to be specified with a text description.
In case of carcinoma in situ, histological type (ductal or lobular), nuclear grade and presence of associated calcifications must be indicated, if possible.
In case of invasive cancer, histological type, histological grade, the presence of in situ component, and eventually the presence of associated calcifications must be specified.
In cases of locally advanced tumors that will be submitted to neoadjuvant chemotherapy, it is often required to assess the state receptor and the expression of c-erb (Fig. 16.3 ).
16.5.2 Core Biopsy (CB) Diagnostic Accuracy
According to the literature, the sensitivity and specificity of CB are, respectively, 85–98% and 96–100%, with differences attributable to the experience of the pickup, and the type of breast lesions is investigated. CB produces an inadequate rate less than the FNA (0–17% vs. 5–24%) especially if multiple samples are done and if it is performed with 14G needle. CB can reduce 50–64% of surgical biopsies of benign lesions and increases the diagnosis of invasive carcinoma preoperative up to 92% (only with multiple samples) [27, 31].
Core biopsy (CB) compared to fine needle aspiration (FNA) has higher sensitivity and specificity and a lower rate of samples reported as unsatisfactory particularly for image-detected lesions. Most importantly, core biopsy but not FNA cytology differentiate invasive cancer from in situ lesion and distinguish in a high proportion of cases atypical ductal hyperplasia from low-grade in situ carcinoma. However, core biopsy requires local anesthesia and may result in more discomfort post-procedure, and its results usually take longer to be obtained. Disposables and equipment required to perform FNA are less expensive than for core biopsy. Taking into account the benefits and limitations of both techniques (Tables 16.2 and 16.3 ), we argue that CB is to be preferred over FNA for the diagnosis of breast lesions [27, 31,32,33] (Fig. 16.4 ) (Tables 16.2 and 16.3 ).
Vacuum-assisted breast biopsy (VABB) is a more recent technique. VABB has proven clinical value and can be used under sonographic, mammographic, and magnetic resonance imaging guidance. The main indication for the use of VABB is for biopsies of clustered microcalcifications, which are usually performed under stereotactic guidance. This method has been proven reliable and should replace surgical biopsies. For masses that are likely benign or indeterminate, we attempt to completely remove the lesion to eliminate uncertainty on later follow-up images. VABB offers the best possible histological sampling and aids avoidance of unnecessary operations. VABB complications include bleeding or pain during the procedure, as well as postoperative pain, hemorrhaging, and hematomas. But, these hemorrhaging could be controlled by the post-procedural compression and bed resting. Overall, VABB is a reliable sampling technique with few complications, is relatively easy to use, and is well-tolerated by patients. The larger amount of extracted tissue reduces sampling error [34].
16.6 A Lobar Surgical Approach
Surgery is only a part of the therapeutic process in the fight against breast cancer. The set of diagnostic tools, the different associated therapies (medical and surgical), and the ultimate control of the removed area by the pathologist allow us to have a good chance against breast cancer and to have the best therapy with the best aesthetic results and the minimum percentage of tumor recurrence.
The problem of breast cancer is old. The first historical references to the treatment of this pathology [7] are found in the Egyptian papyri, in the schedules of Nineveh (2250 BC) and in an Indian treaty of 2000 BC (Yajiur Veda), where it is recommended to burn with acid rather remove with a blade. Starting from destructive surgery as they did with the intervention of William Stewart Halsted (who describes his technique on his series of patients with breast cancer, operated at Johns Hopkins Hospital from 1889 to 1894) or Urban, passing through less destructive surgery according to Patey or Madden, only in recent decades, thanks to the Milan school (Veronesi) [1], we reach a conservative surgical approach with quadrantectomy.
Equally important is the attempt to overcome the problem of scars, immediately aroused by the operated women, mutilated by such destructive therapies. Good results were obtained by various techniques, including the very old but valid and still relevant one described by Tansini in 1896.
Today, where possible, we can perform a much more limited intervention, lobectomy, thanks to the knowledge and histopathologic confirmation of the concept of lobar disease, now almost universally acknowledged [21, 35].
We know for sure that the tumor propagation can occur in many cases along the milk ducts, so we have strong doubts about the type of intervention called “lumpectomy” [36]. According to us, most recurrences would be eliminated by careful removal of the most central part of the milk duct, which drains the pathological lobe [16].
As early as 1988, Durante introduced the concept of “sectoriectomy”: in other words, conservative surgery is based on the concept of integration of image diagnosis and excision of a breast sector, according to the anatomical and pathological knowledge of the mammary gland (Fig. 16.5a). He makes an incision following Langer’s lines (Fig. 16.5b, c); he carries on by severing the glandular tissue both from the subcutaneous plane and from the fascia (removed only when it is likely to be affected), with resection of healthy tissue at least 10 mm away from the tumor [37] (Fig. 16.5d).
The result is that relapses are drastically reduced in percentage, under 1% of the operated women (Durante’s percentage is 0.6%, the testimony of a perfect radical surgery) [9].
Sometimes with this method you can have a strain of the nipple-areola complex, with the possible presence of annoying scars.
From the experience gained by Durante, we mediated a technique to minimize the scarring consequences and to reconstruct the shape and volume of the operated breast, so that it can be as similar as possible to the contralateral breast, even on oncological request.
In 1975, Hinderer introduced the “periareolar access to the mammary gland” in breast surgery, and, in 1989, Benelli made a further improvement to the previous methodology.
With the introduction of the technique, called “round block,” it has become much more possible to perform a skin reduction while maintaining the same shape and the size of the areola and, at the same time, remodeling the breast cone. Such a contrivance makes use of a “bag of tobacco” simple suture around the areola level, after removal of a circular crown of the periareola epidermis, sufficiently wide to compensate for the area corresponding to the removed glandular tissue, so as to reduce the tension on margins and prevent a gross enlargement of the nipple due to excessive tissue stretching.
This method, modified for this particular indication, seems the most suitable to obtain an excellent surgical field, so as to allow a wide vision, an easy excision of the tumor (and the surrounding tissue), and the recovery of the breast cone after suitable remodeling of the remaining glandular tissue. Now about 60–80% of breast cancers are treated conservatively. To summarize these concepts, here are some key points for a correct approach to the problem [38].
Conservative treatment needs the combination of surgical therapy with radiotherapy. The distance of the tumor from the margins of resection should be more than 10 mm. Mobilization and detachment of the glandular flaps adjacent to the removed lobe, both under the skin and in the prefascial plane, should be carried out by limiting the damage to the vasculature of the flaps, to aim at a structural and functional satisfactory reconstruction. We always make a careful hemostasis, and we limit the use of drainages, so as not to affect the cosmetic result. The use of absorbable materials, the realization of a valid reinforcement under skin, and a dressing with a particular bandaging improve the quality of the scar area.
The possibility of removing the tumor while at the same time maintaining the normal conformation of the breast with scarring reduced to the minimum or almost invisible determines, in addition to the reduction of the negative impact of mutilation, a better tropism in the tissues for radiotherapy, a better therapeutic approach, a better quality of life, and a more active participation of the patient.
After the above assumptions, in this careful and surgically aware planning context, we have tried to make an intervention that associates radical surgery to a minimal mutilating impact. We started from the idea that, for a complete removal of the suspected area, you must always resort to excision of a “slice” of the gland including the ducts of the affected lobe, in order to reduce the possibilities of recurrence, reaching just below the nipple. The rationale for this chapter is to arrive, after a careful selection of the patients, to a valid oncological result, minimizing the aesthetic and psychological negative impact on the modification of the patient’s own body image. This allows a better acceptance of the problematic cancer and its indispensable complementary therapies.
Choosing the surgical treatment, you must take into account the following elements:
-
Size of the neoplasm
-
If it is a single node or if it is multifocal or multicentric [15]
-
Measure of the lesion diameters, its distance from the skin, from the fascia, and from the nipple
-
Evaluation of the lymph node condition
-
Localization of the lesions
-
Relations with the surrounding tissues, with the exclusion of the presence of not previously assessed abnormal areas
Here is the reason why in patients with early breast cancer affecting a single lobe radical surgery must be associated with a good aesthetic and functional result.
In palpable tumors, surgeons usually perform blind surgery trusting preoperative mammography and their tactile skills, which can be problematic, especially in dense breasts. This surgical approach implies a high incidence of pathological surgical margins or an over-excision of healthy tissue in the effort to obtain adequate margins.
Lobar structures, usually invisible to the clinician and to mammographic control, become highly visible with ultrasound. Using the ductal method, cancerous structural irregularities, including indirect surrounding signs, highlight the suspected areas.
Due to the development of imaging techniques and screening programs, the incidence of non-palpable breast cancer has increased with up to one third of the diagnosed breast cancers being non-palpable. In this group, DCIS represents a challenging problem for breast-conserving surgery given that it is typically non-palpable and non-contiguous. Different management procedures are used to remove the non-palpable tumor with optimal resection margins: wire-guided localization, radio-guided localization, or intraoperative ultrasound-guided surgery. We suggest the use of ultrasound-guided surgery in order to localize tumors in their lobar context. Only a few reports have been published of the use of ultrasound-guided surgery in breast cancer but with good results.
Ultrasound-guided surgery is an accurate, simpler, and less invasive procedure compared to wire-guided localization. The breast lesion can be easily visualized on ultrasound. The only problem is that ultrasound-guided surgery is not very accurate for lesions appearing as a cluster of microcalcifications. The surgeon needs an ultrasound training; otherwise, a preoperative assisting radiologist is mandatory in performing ultrasound-guided surgery.
This echographic control helps the surgeon to localize the lesions and to visualize the whole lobe around. As the sick lobe may represent the risk tissue for cancer development within the breast, excision of whole lobe before development of malignant lesions within it reduces the local recurrence incidence.
The integration of all the different diagnostic methods (mammography, MRI in some cases, lobar echography) enables the surgeon to make a highly precise resection based also on his knowledge (anatomical, genetic, endocrinological, etc.).
We know very well that the breast is related to the sexual and relational life and to well-being: always in the foreground of advertising spots (on papers, on TV, etc.), the breast has become an organ with specific aesthetic standards in form and volume.
Current knowledge allows us to limit the surgical treatment, avoiding the large mammary mutilations of a few decades ago.
Preoperative ultrasound study allows us to confirm the location of the pathological area with the possibility of removing only the functional unit affected by the disease. An immediate initial assessment of the anatomo-pathologist will provide us with further confirmatory elements of the complete removal of all the pathological functional units.
We remember that all progress and every improvement that you can get in medicine are nothing but the sum of the experiences of the previous study groups, the technical equipment used for the diagnosis and in the operating theater.
Thus with regard to our surgical technique in case of localized and limited breast cancer, we have to be aware of the progress in the individual fields and the importance of their final combination.
The ultrasonic techniques are particularly refined and have reached very advanced diagnostic levels, as demonstrated by many doctors all around the world.
The study of lobar morphology, started by Michel Teboul, is now integrated with the use of elastography (evaluation of the rigidity of the structures) and Doppler (evaluation of vascularity by Doppler method).
16.7 Casuistry
From 1990 to 2010, we carried out 425 operations of lobectomy or quadrantectomy in women with diagnosis of breast carcinoma, with a previous echographic mapping of the lesions.
Five years on, only five patients had a new diagnosis of carcinoma (probably recurrences) in the same operated breast.
In literature we find a much higher recurrence percentage, as previously reported.
The reduction of the problems of cancer recurrence in the same breast seems to be correlated with the understanding that cancer is a lobar disease and that lumpectomy is not, in the light of current knowledge, ontologically correct, because it doesn’t take into account either the anatomy or the physiology or the natural history of the tumor.
No doubt radiotherapy and chemotherapy performed after surgery may have perfectly sterilized the mammary gland, but this is also true for what concerns the rates reported in the literature with any other technique.
On the other hand, we never know if a new neoplastic disease in the same area is due to a new disease beginning in another lobe of the same breast or if it is due to a focus asynchronously appearing from the same lobe.
In our opinion, a very close collaboration with the pathologist is essential to improve the surgical discourse as required. The possibility of evaluating the microscopic framework through immediate acquisitions and insights executed confirms the validity and usefulness of what we propose. Today, where possible, you carry out a much more limited intervention, lobectomy, thanks to the knowledge and histopathologic confirmation of the concept of lobar disease now almost universally acknowledged.
Evaluation of your patient, in the same position as in the operating theater with lobar echography, is essential. The main duct should be identified since it corresponds to the longitudinal axis of the lobe; the duct should be followed from the nipple to the end of the lobe. The localization of the cancer and the evaluation of the nearby tissues are performed with radial and antiradial scans (Fig. 16.6 ). The design of the ductal axis and the associated abnormalities is then drawn on the overlying skin with a marker. The duct is traced from the nipple-areola border to the distal end.
A transverse evaluation of the lobe is then performed. This marks the lateral borders of the resection, inclusive of a 10-mm security distance from the suspect area. The glandular area to remove is assimilated to a triangular area. It is important to remember that one should remove an area of periareolar epidermis (circular sector) corresponding to the area above the lobe to be removed. Mark the border of the areola. Evaluate the skin area to be removed around the areola. It must be equal to an annulus of epidermis with the inner circumference already marked (see note). This area of redundant skin must be removed in order to balance the deficit of glandular tissue removed in the lobar resection, so you can keep a normal morphology of the breast cone (Fig. 16.7a).
Explanatory notes:
Triangle area = outer circle area − internal circle area.
Triangle area (half of base × height or 1/2 B × H) + internal circle area (PI Greek × r 2) = outer circle area (PI Greek × R 2).
R = square root of (triangle area + internal circle area) divided by PI Greek.
Surgical steps:
After another control on the operating bed, an axillary evaluation is generally required.
The nipple and the breast cone must be kept in tension to easily remove the epithelial strip in the marked zone between the two circles, keeping the vascular plexus undamaged (Fig. 16.7b).
This is essential for a good feeding of the areola-nipple complex. The dermis is incised on the outside perimeter along the outlined length to expose the breast parenchyma in order to proceed with the resection of the sick lobe. A subcutaneous dissection of the whole lobe and of the nearest areas is then carried out. Grasp the zone 2–3 mm under the nipple with a forceps, to mark the central point of resection (Fig. 16.7c ).
Cut the gland as far as the muscular fascia following the marked lines.
Proceed to cut the segment from the surface deep toward the fascia (Fig. 16.7d).
Pulling up on the top of the lobe, you carry on cutting as far as the end of the lobe, and then you remove it (Fig. 16.8a).
If the tumor is close to the muscular fascia, this must be removed with the specimen. A perfect hemostasis must be always achieved.
Real-time ultrasound is now used to study the dissected specimen at both longitudinal and transverse orientation, in order to evaluate the margin around the cancer [36, 40,40,41] (Fig. 16.8b).
Compare the resected images with the preoperative images.
You can place one or two titanium clips on the fascial surface, at the precise location of the cancer, to facilitate the work of the radiotherapist.
An anatomic reconstruction of the breast cone must be performed by the mobilization and detachment of the gland borders close to the resected specimen, using oncoplastic technique. Then proceed with the periareolar “tobacco pouch” suture to remodel the skin and suture the areola mucosal border to epidermis (Fig. 16.8c).
Drains should be avoided if possible to assure better functional and aesthetic results. Absorbable materials, attention to skin closure, and compression bandages are primary factors for an enhanced cosmetic result.
16.8 Surgical Margins Evaluation
The primary goal of breast-conserving surgery is to remove the whole tumor mass with minimal surrounding healthy tissue in order to obtain tumor-free resection margins. Margins positive or focally positive for tumor cells are associated with a high risk of local recurrence, and in these cases, re-excision after lumpectomy or even mastectomy are sometimes needed to achieve definite clear margins.
16.8.1 Intraoperatory Tumor-Free Surgical Margins Diagnosis
The immediate goal of specimen examination is to confirm that the lesion has been completely excised. Ideally, this should be determined intraoperatively. If the lesion is not present in the specimen or if the cut margin is too close to the tumor, the surgeon may immediately re-excide the surgical margins on one or both sides of the specimen.
If the lesion was ultrasound undetectable, you must use RX to mark the zone with a wire guide. After the surgical removal, an RX examination of the whole surgical specimen must be made comparing with the corresponding mammogram. The presence of calcifications associated with a lesion provides an intrinsic marker that can be visualized in clinical and specimen radiographs.
Pathological margin assessment requires an inking of the specimen and multiple section perpendicular to its long axis.
Frozen sections of margins are not indicated unless the tumor appears grossly close to a margin, and confirming this intraoperatively will have an immediate impact on treatment [24].
It is recommended that non-palpable mammographically detected lesions be processed solely for permanent sections and that frozen sections be performed only in exceptional situations.
Despite improved intraoperative techniques, no assessment can ensure clear lumpectomy margins during surgery.
A positive margin is associated with increased risk of local recurrences after BCT for invasive breast cancer and DCIS. There is no cutoff for the margin width, and the significance of a close margin remains controversial. The surgeon needs to balance the risk of local recurrence against cosmetics in planning BCT so that prognosis is not compromised.
16.8.2 Postsurgical Specimen Examination to Confirm Tumor-Free Margins
Ideally, the intact excisional biopsy specimen should be promptly delivered unfixed to the pathology laboratory. It is not possible for a pathologist to accurately orient the margins of an excisional biopsy specimen without appropriate guidance from the surgeon (it is preferable to mark with a suture at the apex of the lobe toward the nipple and with a different suture at the deep margin toward fascia).
Because the contours and orientation of tissue slices may be altered in the course of preparing histologic sections, it is necessary to mark surfaces corresponding to the margin so that they can be identified microscopically. Various colors can be used to designate specified margins. If applied carefully, the ink does not strain into crevices in the tissue surface.
There is no standard method of margin evaluation for breast specimens, and there is no standard number of histologic sections examined from each margin surface. Margins can be evaluated (a) by a radial method, (b) by a shaved method, or (c) by shaving the walls of the lumpectomy cavity.
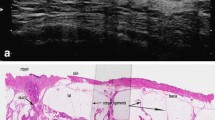
All specimens were prepared using large-section histopathology (Fig. 16.9a). The accurate method accurate for assessing the extent of carcinoma and feasible for routine diagnosis is to use large-format (10 × 8 cm) contiguous histology sections. This kind of histological approach enhances mammographic pathologic correlation, documents the lesion for adequate and reproducible analysis of the extent and distribution of the disease, and preserves the relation of the lesions to each other and to the circumferential surgical margin [42] (Fig. 16.9b).
The possible scenarios of margin assessment encountered at the microscope are broadly positive margin, focally positive margin, close margin, and negative margin. As previously mentioned, there is a lack of standardization in the pathological methods of margin evaluation, which yields little consensus regarding what constitutes an adequate negative margin. Patient management varies widely based on the threshold that surgeons accept for adequate margins and the subsequent need for re-excision.
Adoption of no ink on tumor as the standard negative margin definition has clear potential to decrease the use of both re-excision and large quadrantectomy-type resections which often necessitate additional surgery on the affected breast for reshaping and on the contralateral breast for symmetry [43,44,45,48] (Table 16.4 ).
Conclusion
Oncological evaluation must be based on the anatomical, endocrinological, and functional knowledge of breast, taking into account the embryological development as well as the natural means of presentation of breast cancer. A full imaging analysis of the breast in question, including ductal echography, is requisite prior to any surgical decision. Because of the use of ductal echography, a better anatomopathological assessment of the extent of disease is now available.
A positive surgical margin after breast-conserving surgery correlate closely to local recurrence. Nevertheless, there are other risk factors for local recurrence after breast-conserving surgery, such as tumor growing pattern (unifocal, multifocal, or diffuse) (Fig. 16.10 ).
Considering that breast cancer is frequently a multifocal/diffuse disease, the finding of negative margins does not necessarily mean that we did not leave in situ or invasive tumor foci in the residue breast. These residual tumor foci are usually localized in the “sick lobe.” Breast cancer develops not as a single tumor but as a lobar disease.
Only a lobar surgical approach allows to excise the whole “sick lobe” which may include any other tumor lesions or in any case the risk tissue for future cancer development.
Lobar resection of the breast is a readily mastered surgical technique that can be performed by every breast surgeon with the appropriate sonographic skills. In this way we will obtain a great (the best?) oncological radicality, with a lower percentage of recurrences, according to Durante and other surgeons’ experience. We also obtain a reduction of scars, with a good aesthetic and functional result (Fig. 16.11 ). This surgical approach needs lobar ultrasound as a useful tool to assist and guide the breast surgeon at each step of the operation [36, 40,40,41] preoperatively. The radial imaging approach provides better display of the anatomy and the lesions within the ductal system, as well as their relation to fascia and skin during surgery. Intraoperative radial ultrasound examination guides the surgeon to delineate the “sick lobe” during postoperative examination of the excised surgical specimen. Ultrasound examination of the removed specimen enables the surgeon and the pathologist to control the presence of the lesion within the specimen and its distance from the surgical margins.
References
Veronesi U, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32.
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41.
Dolfin G, Tagliabue P, Dolfin AM, Indelicato S. Chirurgia conservativa: cosa possiamo fare per evitare la mutilazione? Riv It Ost Gin. 2007;14:66370.
Teboul M. Practical ductal echography. Madrid, Spain: Medgen. S.A; 2004.
Tot T. The clinical relevance of the distribution of the lesions in 500 consecutive breast cancer cases documented in large-format histological sections. Cancer. 2007;110:2551–60.
Durante E. Multimodality imaging and interventional techniques. Ferrara, Italy: IBUS Course Abstracts; 2006.
Amoros J, Dolfin G, Teboul M. Atlas de Ecografia de la Mama. Torino: Ananke; 2009.
Hunt KK, Sahin AA. Too much, too little, or just right? Tumor margins in women undergoing breast-conserving surgery. J Clin Oncol. 2014;32:14–8.
Amy D, Durante E, Tot T. The lobar approach to breast ultrasound imaging and surgery. J Med Ultrasound. 2015;42(3):331–9.
Tot T. The theory of the sick breast lobe and the possible consequences. Int J Surg Pathol. 2007;15:369.
Tot T, Gere M, Pekár G, Tarján M, Hofmeyer S, Hellberg D, Lindquist D, Chen TH-H, Yen AM-F, Chiu SY-H, Tabár L. Breast cancer multifocality, disease extent, and survival. Hum Pathol. 2011;42:1761–9.
Coombs NJ, Boyages J. Multifocal and multicentric breast cancer: does each focus matter? J Clin Oncol. 2005;23:7497–502.
La Parra RF, De Roos WK, Contant CM, Bavelaar-Croon CD, Barneveld PC, Bosscha K. A prospective validation study of sentinel lymph node biopsy in multicentric breast cancer: SMMaC trial. Eur J Surg Oncol. 2014;40:1250–5.
Donker M, Straver ME, van Tienhoven G, van de Velde CJ, Mansel RE, Litière S, Werutsky G, Duez NJ, Orzalesi L, Bouma WH, van der Mijle H, Nieuwenhuijzen GA, Veltkamp SC, Helen Westenberg A, Rutgers EJ. Comparison of the sentinel node procedure between patients with multifocal and unifocal breast cancer in the EORTC 10981-22023 AMAROS Trial: identification rate and nodal outcome. Eur J Cancer. 2013;49:2093.
Yerushalmi R, Tyldesley S, Woods R, Kennecke HF, Speers C, Gelmon KA. Is breast-conserving therapy a safe option for patients with tumor multicentricity and multifocality? Ann Oncol. 2012;23:876–81.
Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer. 1985;56:979–90.
Turnbull L, Brown S, Harvey I, Olivier C, Drew P, Napp V, Hanby A, Brown J. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010;375:563–71.
Peters NH, van Esser S, van den Bosch MA, Storm RK, Plaisier PW, van Dalen T, Diepstraten SC, Weits T, Westenend PJ, Stapper G, Fernandez-Gallardo MA, Borel Rinkes IH, van Hillegersberg R, Mali WP, Peeters PH. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET - randomised controlled trial. Eur J Cancer. 2011;47:879–88627.
Biesemier KW, Alexander C. Enhancement of mammographic-pathologic correlation utilizing large format histology for malignant breast disease. Semin Breast Dis. 2005;8:152–62.
Tot T. The role of large-format histopathology in assessing subgross morphological prognostic parameters: a single institution report of 1000 consecutive breast cancer cases. Int J Breast Cancer. 2012;2012:395–415.
Foschini MP, Flamminio F, Miglio R, et al. The impact of large sections on the study of in situ and invasive duct carcinoma of the breast. Hum Pathol. 2007;38:1736–43.
Tot T, Pekár G, Hofmeyer S, et al. The distribution of lesions in 1-14-mm invasive breast carcinomas and its relation to metastatic potential. Virchows Arch. 2009;455:109–15.
Tot T. DCIS, cytokeratins, and the theory of the sick lobe. Virchows Arch. 2005;447:1–8.
Osen R, et al. Rosen’s breast pathology. 4th ed. Philadelphia: Wolters Kluwer Health; 2015.
Lobar AD. Ultrasound of the breast. In: Tot T, editor. Breast cancer. London: Springer; 2011. p. 153–62.
Lavoué V, Fritel X, Antoine M, Beltjens F, Bendifallah S, Boisserie-Lacroix M, Boulanger L, Canlorbe G, Catteau-Jonard S, Chabbert-Buffet N, Chamming’s F, Chéreau E, Chopier J, Coutant C, Demetz J, Guilhen N, Fauvet R, Kerdraon O, Laas E, Legendre G, Mathelin C, Nadeau C, Naggara IT, Ngô C, Ouldamer L, Rafii A, Roedlich MN, Seror J, Séror JY, Touboul C, Uzan C, Daraï E, French College of Gynecologists and Obstetricians (CNGOF). Clinical practice guidelines from the French College of Gynecologists and Obstetricians (CNGOF): benign breast tumors - short text. Eur J Obstet Gynecol Reprod Biol. 2016;200:16–23.
Mitra S, Dey P. Fine-needle aspiration and core biopsy in the diagnosis of breast lesions: a comparison and review of the literature. Cytojournal. 2016;13:18.
Wesoła M, Jeleń M. The diagnostic efficiency of fine needle aspiration biopsy in breast cancers - review. Adv Clin Exp Med. 2013;22(6):887–92.
National Breast Cancer Centre. Breast fine needle aspiration cytology and core biopsy: a guide for practice. 2004. This book can also be downloaded from the National Breast Cancer Centre website www.nbcc.org.au .
Feoli F, Ameye L, Van Eeckhout P, Paesmans M, Marra V, Arisio R. Liquid-based cytology of the breast: pitfalls unrecognized before specific liquid-based cytology training - proposal for a modification of the diagnostic criteria. Acta Cytol. 2013;57(4):369–76.
Willems SM, van Deurzen CH, van Diest PJ. Diagnosis of breast lesions: fine-needle aspiration cytology or core needle biopsy? A review. J Clin Pathol. 2012;65(4):287–92.
Nassar A. Core needle biopsy versus fine needle aspiration biopsy in breast--a historical perspective and opportunities in the modern era. Diagn Cytopathol. 2011;39(5):380–8.
Rageth CJ, O’Flynn EA, Comstock C, Kurtz C, Kubik R, Madjar H, Lepori D, Kampmann G, Mundinger A, Baege A, Decker T, Hosch S, Tausch C, Delaloye JF, Morris E, Varga Z. First International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res Treat. 2016;159(2):203–13.
Park HL, Hong J. Vacuum-assisted breast biopsy for breast cancer. Gland Surg. 2014;3(2):120–728.
Tot T, Ibarra JA. Examination of specimens from patients with ductal carcinoma in situ of the breast using large-format histology sections. Arch Pathol Lab Med. 2009;133(9):1361.
Fisher CS, Mushawah FA, Cyr AE, Gao F, Margenthaler JA. Ultrasound-guided lumpectomy for palpable breast cancers. Ann Surg Oncol. 2011;18:3198–203.
Luini A, Gatti G, Zurrida S, Caldarella P, Viale G, Rosali dos Santos G, Frasson A. The surgical margin status after breast-conserving surgery: discussion of an open issue. Breast Cancer Res Treat. 2009;113(2):397–402.
Dolfin G, Chebib A, Amy D, Tagliabue P. Carcinoma mammarie et Chirurgie Conservatrice. 30° Seminare Franco-Syrien d’Imagerie Médicale. Tartous, Syrie; 2008.
Volders JH, Haloua MH, Krekel NM, Meijer S, van den Tol PM. Current status of ultrasound-guided surgery in the treatment of breast cancer. World J Clin Oncol. 2016;7(1):44–53.
Krekel N, Zonderhuis B, Muller S, Bril H, van Slooten HJ, de Lange de Klerk E, van den Tol P, Meijer S. Excessive resections in breast-conserving surgery: a retrospective multicentre study. Breast J. 2011;17:602–9.
Pan H, Wu N, Ding H, Ding Q, Dai J, Ling L, Chen L, Zha X, Liu X, Zhou W, et al. Intraoperative ultrasound guidance is associated with clear lumpectomy margins for breast cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e74028.
Tot T, Tabár L. Mammographic pathologic correlation of ductal carcinoma in situ of the breast using two- and three-dimensional large histologic sections. Semin Breast Dis. 2005;8:144–51.
Tomoka H, Masataka S, Junko I, et al. Impact of intraoperative specimen mammography on margins in breast-conserving surgery. Mol Clin Oncol. 2016;5:269–72.
Chiappa C, Rovera F, Corben AD, Fachinetti A, De Berardinis V, Marchionini V, Rausei S, Boni L, Dionigi G, Dionigi R. Surgical margins in breast conservation. Int J Surg. 2013;11(Suppl 1):S69–72.
Houssami N, Morrow M. Margins in breast conservation: a clinician’s perspective and what the literature tells us. J Surg Oncol. 2014;110(1):2–7.
Moehrle M, Breuninger H, Röcken M. A confusing world: what to call histology of three-dimensional tumour margins? J Eur Acad Dermatol Venereol. 2007;21(5):591–5.
Dolfin G, et al. The surgical approach to the “sick lobe” in breast cancer: a new era in management. New York: Springer; 2014.
Tot T. Subgross morphology, the sick lobe hypothesis, and the success of breast conservation. Int J Breast Cancer 2011;2011: Article ID 634021.
Acknowledgment
Thanks to doctors Anna Maria Dolfin for providing the images and for the help in writing this chapter; Paolo Tagliabue, fellow surgeon; and Riccardo Arisio, pathologist. Thanks to Silvia Botta for pathological drawings.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Dolfin, G., Botta, G. (2018). Lobar Surgery and Pathological Correlations. In: Amy, D. (eds) Lobar Approach to Breast Ultrasound. Springer, Cham. https://doi.org/10.1007/978-3-319-61681-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-61681-0_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-61680-3
Online ISBN: 978-3-319-61681-0
eBook Packages: MedicineMedicine (R0)