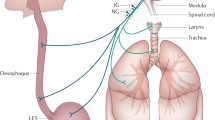
Abstract
Gastroesophageal reflux has been held responsible for a variety of respiratory symptoms including asthma, recurrent pneumonia, and myriad upper airway symptoms in the pediatric population. The focus of much of the early research has been on proving the association between esophageal reflux events and extraesophageal symptoms though recent studies have explored the role of biomarkers as novel diagnostic tests. Because of the lack of sensitive diagnostic tests for extraesophageal reflux disease, many clinicians continue to prescribe or recommend empiric medical and surgical reflux therapies though there is again a lack of convincing data showing benefit to these therapies and some studies even suggesting harm. The field of reflux-related respiratory disorders continues to evolve, however, and the challenge of caring for these pediatric patients requires a multidisciplinary team-based approach.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Gastroesophageal reflux disease
- Extraesophageal reflux
- Reactive airway disease
- Asthma
- Recurrent pneumonia
- Upper airway symptoms
- Multichannel impedance with pH
- Pepsin
- Microbiome
- Proton pump inhibitor
- Fundoplication
Introduction
There is perhaps no other manifestation of reflux that has been subjected to more study and debate than respiratory tract symptoms. Gastroesophageal reflux has been postulated to cause respiratory symptoms in the pediatric population for many years due to the concern that either distal esophageal reflux triggers reflex bronchospasm or, more recently and perhaps more likely, that full-column refluxate reaches the oropharynx and causes direct and/or indirect damage to the larynx, trachea, and/or lungs [1, 2]. Even with improved technology, proving causality is difficult, and many patients undergo a variety of diagnostic tests and empiric therapies which result in significant cost and effect on quality of life. Children who suffer from extraesophageal symptoms of reflux often have decreased quality of life and see multiple specialists at great expense in the evaluation and treatment of their symptoms [3]. Recognizing the difficulty these families face in this experience is essential since children’s symptoms can sometimes be debilitating, highlighting the significance of taking a multidisciplinary team-based approach that combines gastroenterologists with otolaryngologists, pulmonologists, and other supportive team members including speech language pathologists and dieticians. This approach has been shown to decrease both healthcare costs and burden in the pediatric population [4]. This communication is essential to not only coordinate testing and treatments.
Epidemiology of Extraesophageal Reflux Disease
Signs and symptoms of extraesophageal reflux disease are varied and are shown in Table 13.1. Reflux has been implicated as a cause of up to 57% of these signs and symptoms [5]. Multiple cross-sectional studies and systematic reviews in adults and children have shown possible associations between GERD and these respiratory disorders, but causality remains difficult to establish with clarity [5,6,7,8]. Because of the varied signs and symptoms of reflux and the number of specialists involved in the patient’s care, there is often a costly workup for patients; while no pediatric data exists, the cost for diagnosing and managing these patients is upward of 50 billion dollars per year, on par with cancer diagnosis and management. These tremendous costs are driven by testing (on average 5 diagnostic tests per patient) and empiric therapies with proton pump inhibitors.
Respiratory tract symptoms most frequently attributed to reflux include reactive airway disease, recurrent pneumonia, and an assortment of upper airway symptoms. The epidemiology and evidence for these proposed symptoms will be presented briefly followed by a discussion of diagnostic testing and treatment options.
Reactive Airway Disease
As one of the most common chronic medical problems affecting children, asthma is a cause of great morbidity in pediatrics, resulting in more than 20,000 hospitalizations each year [9]. In the current era, asthma is not thought to be a single simple disease entity but rather a complex interplay between multiple individual diseases and pathways [10]. In younger children in particular, it is thought that reflux might be an important mediator or even cause of reactive airway disease in select patients [11].
Reflux has been proposed to play a role in reactive airway disease and asthma for many years, and a recent systematic review of 20 well-designed pediatric studies suggests that the average prevalence of GERD (diagnosed by testing or symptoms) in children with asthma was 22% compared to 4.8% of controls [12, 13]. While acid infusion has been shown in adults to induce bronchospasm in patients with asthma, no comparable pediatric studies have been performed, and more recent studies have suggested the microaspiration may be a more significant mechanism [14,15,16,17]. Studies of children with asthma and subsequent reflux testing have mixed results. In a study of 21 children using oropharyngeal pH monitoring, Banaszkiewicz et al. suggested that pharyngeal pH may correlate with poorer asthma control in children though the technology used in this study may not be reliable [18]. Kilic et al. studied 50 children with controlled and uncontrolled asthma and found no relationship between esophageal acidification and asthma control [19]. Additionally, Condino et al. studied 24 asthmatic children with multichannel impedance with pH and concluded that most asthma symptoms occur in the absence of a reflux event, and Chang et al. used an ambulatory pHmetry-cough logger to analyze 5628 coughs in 20 children with chronic cough and found that 84% of coughs were independent of a reflux event [20, 21]. Despite reports in the adult literature about the impact of nocturnal reflux on asthma symptoms, no similar pediatric association has been found [22]. While case control studies support that patients with asthma may experience asthma improvement after reflux therapies, well-designed randomized controlled studies have failed to show any benefit of reflux therapies in asthma outcomes [23,24,25].
While most of the studies support an association between asthma and GERD, it is not clear if the GERD causes the asthma or rather that asthma triggers the GERD. There is a mechanistic basis for this latter theory. Chronic lung hyperinflation can effectively lower the lower esophageal sphincter pressure and promote the occurrence of reflux events [26, 27]. Additionally, while beta-agonists have not been associated with reflux, oral corticosteroids have been shown to promote reflux in adults though their impact in children is not known [28, 29]. Other asthma medications such as theophylline have been shown to inhibit lower esophageal sphincter pressure in studies utilizing pressure recordings in adults, thus predisposing patients to reflux, but Berquist et al. combined theophylline administration with 24-h pH monitoring in 10 asthmatic children and found no increase in reflux episodes [30, 31].
Recurrent Pneumonia
Reflux has classically been thought to cause recurrent pneumonia by way of gastric aspiration or microaspiration of full-column reflux, typically in patients with impaired airway protective mechanisms [32]. Unfortunately proving that pneumonias are resulting from gastric aspiration is almost impossible as these patients typically also have oropharyngeal dysphagia with salivary aspiration as well. The impact of GERD on pneumonias is largely gleaned from the fundoplication data in which reduction in pneumonia risk after fundoplication has been reported to range from 0 to 83% in neurologically impaired children, but there has been difference seen in hospitalization rates for recurrent pneumonia in these children [33,34,35]. In studies that compared rates of respiratory complications after gastrostomy tube with fundoplication to gastrostomy tube placement alone, there were no differences in pneumonia risk, suggesting reflux is not a significant contributor [33, 36]. In a study by Duncan et al. of 116 children undergoing multichannel intraluminal impedance with pH testing (pH-MII), he found that there was no increased risk of pulmonary hospitalizations in children with pathologic reflux, even after adjustment for aspiration risk, again suggesting gastroesophageal reflux may not be a significant contributor to pulmonary disease [37].
Upper Airway Symptoms
Reflux is typically thought to be a cause of hoarseness, chronic cough, and globus sensation, but the evidence for a clear association with these symptoms is weak [38]. A systematic review by Rosbe et al. found a relationship between reflux and upper airway symptoms in children but noted marked heterogeneity between the studies that were analyzed [39]. There is frequent discussion of upper airway symptoms in the otolaryngology literature, where this clinical entity is frequently referred to as laryngopharyngeal reflux, differentiating it from reflux that does not pass the upper esophageal sphincter [40]. Otolaryngologists frequently cite findings of erythema, edema, and cobblestoning seen on laryngoscopy as evidence of reflux causing upper airway symptoms, but the correlation of these findings with reflux testing is poor, and these findings are therefore generally felt to be unreliable. Most recently, Rosen et al. studied 77 children with pH-MII testing and airway exams blindly scored by otolaryngologists and found no relationship between any of the reflux parameters including the type of reflux (acid/nonacid) or the height of the reflux and the appearance of the airways [41].
Cystic Fibrosis
Studies have shown that the rate of pathologic gastroesophageal reflux in patients with cystic fibrosis is as high as 54% and these patients have been shown to have poor acid clearance and inadequate acid suppression responsiveness [42, 43]. As with all respiratory disease, it is not clear if the pulmonary pathology causes the increased reflux or vice versa. In patients with cystic fibrosis, gastroesophageal reflux could be exacerbated by chronic coughing increasing the intra-abdominal pressure, poor motility due to required high-fat diets, or changes in the role of the diaphragm in reinforcing the lower esophageal sphincter. Furthermore, some studies have even suggested that gastroesophageal reflux may modify the lung microbiome of children with cystic fibrosis which then may result in functional declines [44]. Sometimes even therapies for cystic fibrosis may worsen gastroesophageal reflux; for example, studies vary about the impact of chest physiotherapy on gastroesophageal reflux with the number of reflux events varying depending on the position of the patient during the therapy [45, 46].
While studies have shown a correlation between pathologic gastroesophageal reflux and worse pulmonary function, proving causality is again difficult because both decline in lung function and worsening gastrointestinal function may merely represent that the patient is sicker in general [44, 47]. Studies of fundoplication in patients with cystic fibrosis show no apparent benefit to lung function postoperatively, and similar results are seen in the lung transplant population [48,49,50,51].
Diagnostic Testing
While some of the diagnostic tests for typical symptoms of gastroesophageal reflux are used for the diagnosis of extraesophageal reflux disease, some of the options differ, and there are a number of new potential modalities. Prospective studies have shown a high yield to reflux testing in children presenting with chronic cough and wheezing, but the yield of each test varies depending on the symptom under evaluation. Each of the commonly used approaches to testing will be discussed below.
Impedance Testing
Functional testing utilizing multichannel intraluminal impedance with pH monitoring (pH-MII) has become the test of choice in evaluating patients with both typical and atypical symptoms. In contrast to traditional pH probe studies, pH-MII allows for the measurement of both acid and nonacid reflux and the height of the refluxate. The measurement of nonacid reflux is particularly important in pediatrics since up to 50% of pediatric reflux episodes are nonacid events, and there is some evidence to suggest that respiratory symptoms occur more frequently with nonacid events [52, 53]. The measurement of full-column reflux is also important because the presumed mechanism of many extraesophageal symptoms is full-column reflux causing laryngeal or bronchial inflammation, bronchospasm, or laryngospasm. In a prospective study of 112 children with respiratory symptoms, up to 58% of patients were found to have abnormal reflux testing with the most common pH-MII finding being an abnormal symptom association between cough and reflux [54]. Rosen et al. found, in a study of 28 children with respiratory symptoms, that nonacid reflux events and full-column events were more likely to cause respiratory symptoms than acid reflux or distal esophageal reflux [52]. Jadcherla et al. found, in a study of nine preterm infants, that full-column events were more likely to trigger respiratory symptoms [55]. Borrelli et al. prospectively analyzed 21 children with suspected pulmonary aspiration who underwent pH-MII testing and found a correlation between nonacid reflux and lipid-laden macrophage index, but, as discussed below, this specificity of the lipid-laden macrophage index has been called into question [56]. Finally, studies of the lung microbiome suggest that full-column, nonacid reflux in children may be associated with positive lung cultures which may impact lung function and symptoms [57].
pH-MII testing has also served as the gold standard tool to disprove the role of reflux in extraesophageal reflux disease. For example, there is perhaps no better studied population than infants presenting with apparent life-threatening event (ALTE) or brief resolved unexplained event (BRUE), a cohort of patients who have choking and even cyanotic episodes. Multiple studies using pH-MII testing have failed to show a consistent relationship between reflux events [58,59,60]. Similarly, pH-MII has been used to disprove the relationship between reflux events and airway erythema and proposed extraesophageal reflux disease biomarkers [61].
While pH-MII testing offers significantly more insight into esophageal physiology compared to standard pH probe testing, there are still several limitations to this and all esophageal-based technology. It is not clear that measuring esophageal reflux burden reflects the amount of reflux seen by extraesophageal sites. Second, it is not clear how much reflux is considered pathologic for extraesophageal sites, so the normal values for reflux burden in the esophagus may not apply to extraesophageal sites. Third, extraesophageal symptoms and signs are sporadic, so correlating symptoms with reflux events can be difficult.
Intraesophageal Pressure Recording and Acoustic Cough Recording
It is important to note that symptom recording, an essential component of impedance testing, can be flawed by frequent reporting errors by both parents and patients. In adult studies, patients fail to report up to 61% of symptoms during pH-MII testing [62]. Similar studies have been performed in pediatrics and suggest that up to 60% of cough episodes during pH-MII testing are not reported by parents [63]. To overcome this inaccurate symptom reporting, manometry sensors can be placed in the esophagus alongside the pH-MII catheters. These pressure sensors measure coughs which appear as simultaneous high-pressure spikes. The addition of these pressure sensors increases cough detection by more than 100% and changes the reflux-symptom association in 20% of patients; the manometry catheter detects 94% of coughs compared to only 48% recorded by the family [64]. An example of intraesophageal pressure recording detecting coughs combined with pH-MII is shown in Fig. 13.1. Because the passage of the manometry catheter (cough catheter) in addition to the pH-MII catheter can be uncomfortable, another option for measurement of symptoms is the use of tracheal and chest wall microphones to detect sound and synch respiratory sounds with reflux events. As with the cough catheter, the addition of acoustic sound recording increases cough detection by more than 100% and improved reflux-cough correlation.
Reflux Finding Score
The reflux finding score is a clinical composite based on flexible laryngoscopy findings by otolaryngologists that was initially validated against pH probe results before and after acid suppression treatment for use in adults [65]. The score involves such findings as erythema, edema, and other markers of suspected reflux-related injury in the pharynx and has the benefit of being relatively noninvasive. This approach remains widely used by pediatric otolaryngology providers to guide therapy for aerodigestive patients in clinical practice. However, recent studies have questioned the reliability of this scoring system, showing that none of the airway findings correlate with any reflux parameters by pH-MII testing or endoscopy [66].
Oropharyngeal pH Monitoring
Oropharyngeal pH monitoring is a newer approach that utilizes a small probe placed through the nose into the posterior oropharynx behind the palate. Initial studies in adults showed a high degree of concordance with traditional pH probes and that oropharyngeal probes are perhaps more sensitive for detecting laryngopharyngeal reflux [67, 68]. However, in a definitive pediatric study in which both pH-MII and oropharyngeal probes were placed simultaneously in the same patient, there was no correlation between esophageal events or oropharyngeal drops in pH suggesting that the oropharyngeal probe was not, in fact, measuring esophageal events [69]. Subsequently, adult studies have shown similar findings, and for this reason, oropharyngeal pH monitoring is not recommended for the diagnosis of extraesophageal reflux disease [70, 71].
Esophageal Manometry
High-resolution esophageal manometry (HRM) testing does not have a role in the diagnosis of extraesophageal disease, but it does have a role in the diagnosis of gastroesophageal reflux mimickers. For typical reflux symptoms, HRM with impedance is important in the diagnosis of rumination syndrome [72]. For atypical symptoms, HRM with impedance is important in the diagnosis of causing esophageal stasis (which puts patients at risk for aspiration) and for cricopharyngeal dysfunction which causes oropharyngeal dysphagia (with symptoms of coughing and/or choking with feeds) [73, 74]. In cases where a motility disorder is suspected as a cause of respiratory symptoms, the addition of impedance to HRM is critical to assess the impact of esophageal clearance on symptoms [74].
Biomarkers: Lipid-Laden Macrophage Index, Bile, and Pepsin
Because it is not clear that measuring esophageal reflux burden reflects the impact of reflux beyond the lung, researchers have sought biomarkers in the oropharynx and lung. In the past, lipid-laden macrophage index was thought to be a useful marker of aspirated refluxate, but more recent studies have called this practice into question. Studies comparing bronchoscopy samples from patients undergoing pH-multichannel impedance testing have shown no significant correlation between lipid-laden macrophage index and the number of acid or nonacid reflux events, and therefore this marker is thought to lack the specificity needed to detect reflux-related lung disease [75, 76].
Measurement of bile acid in the oropharynx or in bronchoalveolar lavage has also been proposed as a marker of reflux-related disease. The idea of using bile stems from the lung transplant literature in which bile in BAL fluid was correlated with weakly acidic reflux by pH-MII testing, and patients with bile in BAL had a worse prognosis in terms of both survival and the presence of bronchiolitis obliterans [77]. There is some pediatric data about bile as a biomarker in the neonatal population showing that infants with bile aspiration have issues with surfactant and may have more severe bronchopulmonary dysplasia [78]. One of the limitations of bile is that it may not be present in all refluxate and therefore might be less generalizable [40]. Furthermore, measurement of bile is difficult, requiring mass spectrometry for accurate identification and quantification of bile acids.
Several research groups have attempted to validate pepsin, a protein produced solely in the stomach, as a biomarker of extraesophageal reflux disease [40]. Pepsin has been measured in saliva, BAL fluid, middle ear fluid, and sinus washings, and depending on the fluid source, pepsin has been found in 13–88% of extraesophageal sites in symptomatic patients and 0–30% of sites in control subjects [79,80,81]. In children, pepsin has been found in 35–56% of BAL fluid and in 42–86% of saliva from symptomatic patients [37, 81,82,83]. Some groups have shown a correlation between bronchoalveolar lavage pepsin and reflux symptoms but not with pH-MII results [79, 81]. However, other studies have shown correlation between nonacid reflux and pepsin positivity, and this pepsin positivity does seem to be correlated with lung inflammation, suggesting that pepsin might be a useful marker of reflux-related lung disease [84]. Research from the intensive care unit has also suggested that a measure of pepsin in tracheal aspirates might be a useful marker of microaspiration in ventilated patients though [16, 80]. Because of the variability of these study results, the sensitivity of BAL pepsin positivity for predicting extraesophageal reflux has been estimated at 57–80% and the specificity has been estimated at 56–100% [81, 84, 85].
More recently, groups have attempted to validate salivary pepsin as a less invasive marker of extraesophageal reflux disease, but studies have shown mixed results, and at this point salivary pepsin remains of unclear clinical utility. In a study of 50 patients undergoing pH-MII for GERD, Dy et al. showed significant difference in the distribution of acid, nonacid, total reflux episodes and full-column reflux between those who were salivary pepsin positive or negative and also no correlation between number of reflux episodes and salivary pepsin concentration [83]. However, Fortunato et al. collected multiple salivary pepsin samples from subjects and found variability in these measurements throughout the day, with the highest correlation found soon after reflux events measured by 24-h impedance, suggesting that perhaps defining a specific regimen for measurement will be needed to validate salivary pepsin as a marker of extraesophageal reflux [82]. Lastly, it is also important to consider that reflux of pepsin into the oropharynx does not always necessarily lead to aspiration and lung disease [86].
The analysis of exhaled breath condensate is another recent approach to measuring pH, pepsin, and other molecules as a means of noninvasively evaluating for reflux disease. Various groups have attempted to correlate condensate values with the occurrence of cough, nocturnal reflux, and response to acid-suppressing medications [17, 87, 88]. This method represents an intriguing and still emerging approach to the diagnosis of reflux disease, but current published studies do not include adequate control and comparison with pH-MII, and a more recent study of children with asthma and reflux based on 24-h pH monitoring concluded that exhaled breath testing did not provide useful information for discriminating between asymptomatic children and those with poorly controlled asthma [89].
Therapies
Potential therapies for reflux-related respiratory symptoms are varied. These include non-pharmacologic therapies such as dietary and lifestyle changes, pharmacologic therapies with the mainstay of pharmacologic therapy being acid suppression, and surgical approaches.
Non-Pharmacologic Therapies
The mainstay for reflux therapy in pediatrics remains dietary and lifestyle changes, especially with the recent publication of multiple studies highlighting the potential risks of anti-reflux medications [90,91,92]. Non-pharmacologic therapies for reflux include upright positioning, thickening of feeds, change to hypoallergenic formula, and modification of meal frequencies [38]. While these modifications have been studied in the infant population with classical symptoms of reflux such as fussiness, arching, and colic, unfortunately there is limited data to suggest any of these approaches reliably help with the extraesophageal manifestations of reflux [5]. In one study of a potential approach to preventing respiratory symptoms from reflux, Garland et al. evaluated tracheal pepsin samples from intubated neonates and found lower rates of pepsin detection with head-of-bed elevation in this patient population, suggesting that at least this potential marker of extraesophageal reflux can be modulated by position changes [93].
Pharmacologic Therapies
Significant controversy surrounds the use of acid-suppressing medications such as proton pump inhibitors for extraesophageal reflux symptoms [94]. Initial studies of proton pump inhibitor (PPI) for laryngopharyngeal reflux in adults were encouraging but not well-controlled [95, 96]. More recent randomized trials, however, showed no evidence for benefit of PPI for laryngopharyngeal reflux in adults [97, 98]. Both meta-analyses and two randomized controlled trials also showed no benefit in a comparison of PPI vs placebo for chronic cough in adults [99, 100]. A small randomized controlled trial of 38 children randomized to omeprazole or placebo showed no improvement in asthma symptoms, quality of life, lung function, or use of beta-agonists in children with asthma and GERD [101]. A well-powered randomized, placebo-controlled trial of lansoprazole in 306 children aged 6–17 years with poorly controlled asthma also showed absolutely no benefit compared to placebo in improving asthma control or pulmonary function, even when looking at subgroups of patients with pathologic reflux [23]. Another double-blind placebo-controlled study showed no difference in the frequency of cough, hoarseness, or wheezing in infants treated with lansoprazole compared to placebo [102]. There is also good evidence that acid suppression only increases the burden of nonacid reflux, which may worsen symptoms especially since nonacid reflux might be the primary driver of respiratory symptoms in these patients [52, 92].
Little work has been done to look at any potential role for pro-motility medications in this patient population, but this area needs more investigation. At the present time, the adverse effects of currently available prokinetic medications are thought to outweigh potential benefits in children [103]. Data from studies in adults, however, suggest that a not insignificant proportion of these patients might have esophageal motility disorders that might benefit from manometric testing and therapeutics if motility disorders are diagnosed [104]. Intriguing studies in both animal models and humans have shown that macrolides can play an anti-inflammatory role by way of inactivation of NF-kappaB in a rat model and that azithromycin treatment can decrease both reflux as measured by impedance and also aspiration events in human lung transplant recipients [105,106,107].
More research is needed regarding potential effective pharmacologic therapies since despite multiple studies showing limited benefit of currently available pharmacologic options, there remains a large clinical and economic burden for patients with extraesophageal symptoms. Prescription costs, primarily in the form of proton pump inhibitors, remain the single largest contributor to the cost of extraesophageal reflux management in adults with expenditures on PPIs constituting 52% of the total cost of care [3]. After such significant expenditure on the evaluation and treatment of their symptoms, only 54% of patients had improvement in their symptoms [3].
An additional consideration in the current use of proton pump inhibitors in these patients is the increased risk of adverse effects, including respiratory tract infections and pharyngitis, which could paradoxically lead to worsened symptoms in children already suffering from respiratory complaints [90]. In a study of children undergoing combined endoscopy and bronchoscopy for cough, we found that patients on acid suppression had increased gastric bacterial overgrowth of both staphylococcus and streptococcus and that full-column nonacid reflux was associated with increased bacteria concentrations in the lung [108]. For these reasons, any potential benefit of acid suppression in this patient population must be weighed carefully against clearly reported risks. At the current time, initiation of pharmacologic therapy for suspected reflux-related lung disease must involve a thorough discussion between clinicians and patient families, and if no benefit is seen, then such therapeutic trials must be time-limited.
Surgical Therapies
If both acid and nonacid reflux are proposed to cause respiratory problems by direct interaction with the pulmonary system, then it would seem reasonable to utilize anti-reflux surgeries to prevent this interaction. Fundoplication has been the primary surgical approach for medically refractory reflux disease in adults and children. The use of anti-reflux surgery has declined in recent years, but there remains a great deal of variability in the utilization of this surgical procedure between institutions throughout the country [109].
A number of studies have evaluated the effectiveness of surgery in treating respiratory tract symptoms, and the results overall have not been encouraging. Tannuri et al. found in a prospective single-center study of 151 children that only 45% had relief from bronchospasm following fundoplication and concluded that the surgical approach had better results for digestive compared to respiratory symptoms with a median follow-up time of 11 months [110]. In contrast, Frongia et al. reported respiratory symptom resolution in 68% of children for a median duration of 3.6 years follow-up after fundoplication [111]. Another study showed that patients had decreased use of anti-reflux medications but either no change or even increased use of asthma medications following anti-reflux surgery [112]. These studies were limited, however, by the lack of a control group, making it difficult to draw firm conclusions from their results.
As another proxy for reflux-related lung disease, several studies have looked at reflux-related hospitalization rates following anti-reflux surgery. Lee et al. retrospectively reviewed the records of 342 pediatric patients and found no improvement in hospital admission rates for aspiration, pneumonia, and respiratory distress following Nissen fundoplication [34]. In an administrative database study of 1142 children who underwent anti-reflux procedures, Goldin et al. showed a modest decline in reflux-related hospitalizations in younger children but less benefit in children above 4 years of age [113]. In contrast, Barnhart found that reflux-related hospitalizations did not differ in the year following surgery in a cohort of neurologically impaired children undergoing gastrostomy tube placement, regardless of whether patients had anti-reflux surgery or not [33].
Therefore, studies of a surgical approach for reflux-related lung disease do not suggest a strong benefit in respiratory outcomes. It is important to note that dysphagia and associated retching can be a frequent side effect of fundoplication [114]. Additionally, children with significant lung disease are necessarily placed at higher risk when undergoing anesthesia, further tipping the calculus of potential options away from the surgical approach. Unfortunately, this leaves limited options for patients with reflux-related lung disease and no strong evidence base for any clear approach.
Economic Impact
Lack of definitive and standardized testing and treatment approaches leads to a great economic burden in caring for patients with suspected extraesophageal manifestations of reflux. Patients with respiratory symptoms suspected of being reflux-related in particular often undergo an extensive workup. Typically, the care of these patients involves multiple subspecialists along with multiple procedures, medication trials, and diagnostic tests, all of which contribute to great expense. A study of the expenditures involved in caring for adults with extraesophageal symptoms revealed that the cost for the first year of workup and treatment was 5.6 times that of adults with typical GERD [3]. The expenditures involved in caring for these patients can become quite high, but the actual benefits to the patients remain limited, and perhaps these should be taken into account and balanced in the approach to caring for these patients [115].
Conclusions
Respiratory tract symptoms due to gastroesophageal reflux disease represent an important and controversial category of extraesophageal reflux symptoms and an area of active research in pediatric gastroenterology. At this point, multichannel impedance with pH monitoring appears to be the diagnostic test of choice in order to best prove an association between respiratory symptoms and reflux events, but many other diagnostic approaches are currently under active investigation. There are no clear consistent benefits to non-pharmacologic, pharmacologic, and surgical therapies for extraesophageal symptoms, and larger, randomized controlled trials are critically needed in pediatrics. In a field with more questions than answers, a multidisciplinary approach is essential.
References
Orenstein SR, Orenstein DM. Gastroesophageal reflux and respiratory disease in children. J Pediatr. 1988;112(6):847–58.
Lasser MS, Liao JG, Burd RS. National trends in the use of antireflux procedures for children. Pediatrics. 2006;118(5):1828–35.
Francis DO, Rymer JA, Slaughter JC, Choksi Y, Jiramongkolchai P, Ogbeide E, et al. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol. 2013;108(6):905–11.
Collaco JM, Aherrera AD, Au Yeung KJ, Lefton-Greif MA, Hoch J, Skinner ML. Interdisciplinary pediatric aerodigestive care and reduction in health care costs and burden. JAMA Otolaryngol Head Neck Surg. 2015;141(2):101–5.
Tolia V, Vandenplas Y. Systematic review: the extra-oesophageal symptoms of gastro-oesophageal reflux disease in children. Aliment Pharmacol Ther. 2009;29(3):258–72.
Havemann BD, Henderson CA, El-Serag HB. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut. 2007;56(12):1654–64.
Gislason T, Janson C, Vermeire P, Plaschke P, Bjornsson E, Gislason D, et al. Respiratory symptoms and nocturnal gastroesophageal reflux: a population-based study of young adults in three European countries. Chest. 2002;121(1):158–63.
Lightdale JR, Gremse DA. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131(5):e1684–95.
Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma--United States, 1980–1999. MMWR Surveil summ. 2002;51(1):1–13.
Potter PC. Current guidelines for the management of asthma in young children. Allergy Asthma Immunol Res. 2010;2(1):1–13.
Gold BD. Asthma and gastroesophageal reflux disease in children: exploring the relationship. J Pediatr. 2005;146(3, Supplement):S13–20.
Thakkar K, Boatright RO, Gilger MA, El-Serag HB. Gastroesophageal reflux and asthma in children: a systematic review. Pediatrics. 2010;125(4):e925–30.
Debley JS, Redding GJ, Critchlow CW. Impact of adolescence and gender on asthma hospitalization: a population-based birth cohort study. Pediatr Pulmonol. 2004;38(6):443–50.
Ravelli AM, Panarotto MB, Verdoni L, Consolati V, Bolognini S. Pulmonary aspiration shown by scintigraphy in gastroesophageal reflux-related respiratory disease. Chest. 2006;130(5):1520–6.
Starosta V, Kitz R, Hartl D, Marcos V, Reinhardt D, Griese M. Bronchoalveolar pepsin, bile acids, oxidation, and inflammation in children with gastroesophageal reflux disease. Chest. 2007;132(5):1557–64.
Gopalareddy V, He Z, Soundar S, Bolling L, Shah M, Penfil S, et al. Assessment of the prevalence of microaspiration by gastric pepsin in the airway of ventilated children. Acta Paediatr. 2008;97(1):55–60.
Lee AL, Button BM, Denehy L, Roberts S, Bamford T, FT M, et al. Exhaled breath condensate pepsin: potential noninvasive test for gastroesophageal reflux in COPD and bronchiectasis. Respir Care. 2015;60(2):244–50.
Banaszkiewicz A, Dembinski L, Zawadzka-Krajewska A, Dziekiewicz M, Albrecht P, Kulus M, et al. Evaluation of laryngopharyngeal reflux in pediatric patients with asthma using a new technique of pharyngeal pH-monitoring. Adv Exp Med Biol. 2013;755:89–95.
Kilic M, Ozturk F, Kirmemis O, Atmaca S, Guner SN, Caltepe G, et al. Impact of laryngopharyngeal and gastroesophageal reflux on asthma control in children. Int J Pediatr Otorhinolaryngol. 2013;77(3):341–5.
Condino AA, Sondheimer J, Pan Z, Gralla J, Perry D, O'Connor JA. Evaluation of gastroesophageal reflux in pediatric patients with asthma using impedance-pH monitoring. J Pediatr. 2006;149(2):216–9.
Chang AB, Connor FL, Petsky HL, Eastburn MM, Lewindon PJ, Hall C, et al. An objective study of acid reflux and cough in children using an ambulatory pHmetry-cough logger. Arch Dis Child. 2011;96(5):468–72.
Molle LD, Goldani HA, Fagondes SC, Vieira VG, Barros SG, Silva PS, et al. Nocturnal reflux in children and adolescents with persistent asthma and gastroesophageal reflux. J Asthma. 2009;46(4):347–50.
Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, Castro M, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA. 2012;307(4):373–81.
Khoshoo V, Haydel R Jr. Effect of antireflux treatment on asthma exacerbations in nonatopic children. J Pediatr Gastroenterol Nutr. 2007;44(3):331–5.
Sopo SM, Radzik D, Calvani M. Does treatment with proton pump inhibitors for gastroesophageal reflux disease (GERD) improve asthma symptoms in children with asthma and GERD? a systematic review. J Investig Allergol Clin Immunol. 2009;19(1):1–5.
Lee AL, Goldstein RS. Gastroesophageal reflux disease in COPD: links and risks. Int J Chron Obstruct Pulmon Dis. 2015;10:1935–49.
Gadel AA, Mostafa M, Younis A, Haleem M. Esophageal motility pattern and gastro-esophageal reflux in chronic obstructive pulmonary disease. Hepato-Gastroenterology. 2012;59(120):2498–502.
Sontag SJ, O’Connell S, Khandelwal S, Miller T, Nemchausky B, Schnell TG, et al. Effect of positions, eating, and bronchodilators on gastroesophageal reflux in asthmatics. Dig Dis Sci. 1990;35(7):849–56.
Lazenby JP, Guzzo MR, Harding SM, Patterson PE, Johnson LF, Bradley LA. Oral corticosteroids increase esophageal acid contact times in patients with stable asthma. Chest. 2002;121(2):625–34.
Berquist WE, Rachelefsky GS, Rowshan N, Siegel S, Katz R, Welch M. Quantitative gastroesophageal reflux and pulmonary function in asthmatic children and normal adults receiving placebo, theophylline, and metaproterenol sulfate therapy. J Allergy Clin Immunol. 1984;73(2):253–8.
Berquist WE, Rachelefsky GS, Kadden M, Siegel SC, Katz RM, Mickey MR, et al. Effect of theophylline on gastroesophageal reflux in normal adults. J Allergy Clin Immunol. 1981;67(5):407–11.
Danus O, Casar C, Larrain A, Pope CE 2nd. Esophageal reflux--an unrecognized cause of recurrent obstructive bronchitis in children. J Pediatr. 1976;89(2):220–4.
Barnhart DC, Hall M, Mahant S, Goldin AB, Berry JG, Faix RG, et al. Effectiveness of fundoplication at the time of gastrostomy in infants with neurological impairment. JAMA Pediatr. 2013;167(10):911–8.
Lee SL, Shabatian H, Hsu JW, Applebaum H, Haigh PI. Hospital admissions for respiratory symptoms and failure to thrive before and after Nissen fundoplication. J Pediatr Surg. 2008;43(1):59–63. discussion -5
Wong KK, Liu XL. Perioperative and late outcomes of laparoscopic fundoplication for neurologically impaired children with gastro-esophageal reflux disease. Chin Med J. 2012;125(21):3905–8.
Livingston MH, Shawyer AC, Rosenbaum PL, Jones SA, Walton JM. Fundoplication and gastrostomy versus percutaneous gastrojejunostomy for gastroesophageal reflux in children with neurologic impairment: a systematic review and meta-analysis. J Pediatr Surg. 2015;50(5):707–14.
Duncan DR, Amirault J, Johnston N, Mitchell P, Larson K, Rosen RL. gastroesophageal reflux burden, even in children that aspirate, does not increase pediatric hospitalization. J Pediatr Gastroenterol Nutr. 2016;63(2):210–7.
Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498–547.
Rosbe KW, Kenna MA, Auerbach AD. Extraesophageal reflux in pediatric patients with upper respiratory symptoms. Arch Otolaryngol Head Neck Surg. 2003;129(11):1213–20.
Johnston N, Ondrey F, Rosen R, Hurley BP, Gould J, Allen J, et al. Airway reflux. Ann N Y Acad Sci. 2016;1381:5–13.
Rosen R, Mitchell PD, Amirault J, Amin M, Watters K, Rahbar R. The edematous and erythematous airway does not denote pathological gastroesophageal reflux. J Peds. 2017;183:127–31.
Dziekiewicz MA, Banaszkiewicz A, Urzykowska A, Lisowska A, Rachel M, Sands D, et al. Gastroesophageal reflux disease in children with cystic fibrosis. Adv Exp Med Biol. 2015;873:1–7.
Woodley FW, Machado RS, Hayes D Jr, Di Lorenzo C, Kaul A, Skaggs B, et al. Children with cystic fibrosis have prolonged chemical clearance of acid reflux compared to symptomatic children without cystic fibrosis. Dig Dis Sci. 2014;59(3):623–30.
van der Doef HP, Arets HG, Froeling SP, Westers P, Houwen RH. Gastric acid inhibition for fat malabsorption or gastroesophageal reflux disease in cystic fibrosis: longitudinal effect on bacterial colonization and pulmonary function. J Pediatr. 2009;155(5):629–33.
Doumit M, Krishnan U, Jaffe A, Belessis Y. Acid and non-acid reflux during physiotherapy in young children with cystic fibrosis. Pediatr Pulmonol. 2012;47(2):119–24.
Button BM, Heine RG, Catto-Smith AG, Phelan PD, Olinsky A. Chest physiotherapy, gastro-oesophageal reflux, and arousal in infants with cystic fibrosis. Arch Dis Child. 2004;89(5):435–9.
Button BM, Roberts S, Kotsimbos TC, Levvey BJ, Williams TJ, Bailey M, et al. Gastroesophageal reflux (symptomatic and silent): a potentially significant problem in patients with cystic fibrosis before and after lung transplantation. J Heart Lung Transplant. 2005;24(10):1522–9.
Palm K, Sawicki G, Rosen R. The impact of reflux burden on Pseudomonas positivity in children with Cystic Fibrosis. Pediatr Pulmonol. 2011;47:582–7.
Boesch RP, Acton JD. Outcomes of fundoplication in children with cystic fibrosis. J Pediatr Surg. 2007;42(8):1341–4.
Pegna V, Mickevicius A, Tsang C. How useful is antireflux surgery in lung transplant patients with gastroesophageal reflux? Medicina. 2014;50(6):318–22.
Dy FJ, Freiberger D, Liu E, Boyer D, Visner G, Rosen R. The impact of gastroesophageal reflux and delayed gastric emptying on pediatric lung transplant outcomes. J Heart Lung Transplant. 2017; doi:10.1016/j.healun.2017.01.005.
Rosen R, Nurko S. The importance of multichannel intraluminal impedance in the evaluation of children with persistent respiratory symptoms. Am J Gastroenterol. 2004;99(12):2452–8.
Ghezzi M, Guida E, Ullmann N, Sacco O, Mattioli G, Jasonni V, et al. Weakly acidic gastroesophageal refluxes are frequently triggers in young children with chronic cough. Pediatr Pulmonol. 2012;48:295–302.
Rosen R, Amirault J, Johnston N, Haver K, Khatwa U, Rubinstein E, et al. The utility of endoscopy and multichannel intraluminal impedance testing in children with cough and wheezing. Pediatr Pulmonol. 2013;49:1090–6.
Jadcherla SR, Gupta A, Fernandez S, Nelin LD, Castile R, Gest AL, et al. Spatiotemporal characteristics of acid refluxate and relationship to symptoms in premature and term infants with chronic lung disease. Am J Gastroenterol. 2008;103(3):720–8.
Borrelli O, Battaglia M, Galos F, Aloi M, De Angelis D, Moretti C, et al. Non-acid gastro-oesophageal reflux in children with suspected pulmonary aspiration. Dig Liver Dis. 2010;42(2):115–21.
Rosen R, Johnston N, Hart K, Khatwa U, Katz E, Nurko S. Higher rate of bronchoalveolar lavage culture positivity in children with nonacid reflux and respiratory disorders. J Pediatr. 2011;159(3):504–6.
Mousa H, Woodley FW, Metheney M, Hayes J. Testing the association between gastroesophageal reflux and apnea in infants. J Pediatr Gastroenterol Nutr. 2005;41(2):169–77.
Zimbric G, Bonkowsky JL, Jackson WD, Maloney CG, Srivastava R. Adverse outcomes associated with gastroesophageal reflux disease are rare following an apparent life-threatening event. J Hosp Med. 2012;7(6):476–81.
Mittal MK, Donda K, Baren JM. Role of pneumography and esophageal pH monitoring in the evaluation of infants with apparent life-threatening event: a prospective observational study. Clin Pediatr. 2013;52(4):338–43.
McMurray JS, Gerber M, Stern Y, Walner D, Rudolph C, Willging JP, et al. Role of laryngoscopy, dual pH probe monitoring, and laryngeal mucosal biopsy in the diagnosis of pharyngoesophageal reflux. Ann Otol Rhinol Laryngol. 2001;110(4):299–304.
Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut. 2005;54(4):449–54.
Blondeau K, Mertens V, Dupont L, Pauwels A, Farre R, Malfroot A, et al. The relationship between gastroesophageal reflux and cough in children with chronic unexplained cough using combined impedance-pH-manometry recordings. Pediatr Pulmonol. 2011;46(3):286–94.
Rosen R, Amirault J, Giligan E, Khatwa U, Nurko S. Intraesophageal pressure recording improves the detection of cough during multichannel intraluminal impedance testing in children. J Pediatr Gastroenterol Nutr. 2014;58(1):22–6.
Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope. 2001;111(8):1313–7.
van der Pol RJ, Singendonk MM, Konig AM, Hoeve H, Kammeijer Q, Pullens B, et al. Development of the reflux finding score for infants and its observer agreement. J Pediatr. 2014;165(3):479–84.
Yuksel ES, Slaughter JC, Mukhtar N, Ochieng M, Sun G, Goutte M, et al. An oropharyngeal pH monitoring device to evaluate patients with chronic laryngitis. Neurogastroenterol Motil. 2013;25(5):e315–23.
Andrews TM, Orobello N. Histologic versus pH probe results in pediatric laryngopharyngeal reflux. Int J Pediatr Otorhinolaryngol. 2013;77(5):813–6.
Chiou E, Rosen R, Jiang H, Nurko S. Diagnosis of supra-esophageal gastric reflux: correlation of oropharyngeal pH with esophageal impedance monitoring for gastro-esophageal reflux. Neurogastroenterol Motil. 2011;23(8):717–e326.
Ummarino D, Vandermeulen L, Roosens B, Urbain D, Hauser B, Vandenplas Y. Gastroesophageal reflux evaluation in patients affected by chronic cough: restech versus multichannel intraluminal impedance/pH metry. Laryngoscope. 2013;123(4):980–4.
Patel DA, Harb AH, Vaezi MF. Oropharyngeal reflux monitoring and atypical gastroesophageal reflux disease. Curr Gastroenterol Rep. 2016;18(3):12.
Rosen R, Rodriguez L, Nurko S. Pediatric rumination subtypes: a study using high resolution esophageal manometry with impedance. Neurogastroenterol Motil. 2016;29:e12998.
Singendonk MM, Smits MJ, Heijting IE, van Wijk MP, Nurko S, Rosen R, et al. Inter- and intrarater reliability of the Chicago Classification in pediatric high-resolution esophageal manometry recordings. Neurogastroenterol Motil. 2015;27(2):269–76.
Rommel N, Omari TI, Selleslagh M, Kritas S, Cock C, Rosan R, et al. High-resolution manometry combined with impedance measurements discriminates the cause of dysphagia in children. Eur J Pediatr. 2015;174(12):1629–37.
Rosen R, Fritz J, Nurko A, Simon D, Nurko S. Lipid-laden macrophage index is not an indicator of gastroesophageal reflux-related respiratory disease in children. Pediatrics. 2008;121(4):e879–84.
Krishnan U, Mitchell JD, Tobias V, Day AS, Bohane TD. Fat laden macrophages in tracheal aspirates as a marker of reflux aspiration: a negative report. J Pediatr Gastroenterol Nutr. 2002;35(3):309–13.
Mertens V, Blondeau K, Van Oudenhove L, Vanaudenaerde B, Vos R, Farre R, et al. Bile acids aspiration reduces survival in lung transplant recipients with BOS despite azithromycin. Am J Transplant. 2011;11(2):329–35.
De Luca D, Minucci A, Zecca E, Piastra M, Pietrini D, Carnielli VP, et al. Bile acids cause secretory phospholipase A2 activity enhancement, revertible by exogenous surfactant administration. Intensive Care Med. 2009;35(2):321–6.
Krishnan U, Mitchell JD, Messina I, Day AS, Bohane TD. Assay of tracheal pepsin as a marker of reflux aspiration. J Pediatr Gastroenterol Nutr. 2002;35(3):303–8.
Meert KL, Daphtary KM, Metheny NA. Detection of pepsin and glucose in tracheal secretions as indicators of aspiration in mechanically ventilated children. Pediatr Crit Care Med. 2002;3(1):19–22.
Abdallah AF, El-Desoky T, Fathi K, Elkashef WF, Zaki A. Clinical utility of bronchoalveolar lavage pepsin in diagnosis of gastroesophageal reflux among wheezy infants. Can Respir J. 2016;2016:9480843.
Fortunato JE, D’Agostino RB Jr, Lively MO. Pepsin in saliva as a biomarker for oropharyngeal reflux compared with 24-hour esophageal impedance/pH monitoring in pediatric patients. Neurogastroenterol Motil. 2016;29:e12936.
Dy F, Amirault J, Mitchell PD, Rosen R. Salivary pepsin lacks sensitivity as a diagnostic tool to evaluate extraesophageal reflux disease. J Pediatr. 2016;177:53–8.
Rosen R, Johnston N, Hart K, Khatwa U, Nurko S. The presence of pepsin in the lung and its relationship to pathologic gastro-esophageal reflux. Neurogastroenterol Motil. 2012;24(2):129–e85.
Farrell S, McMaster C, Gibson D, Shields MD, McCallion WA. Pepsin in bronchoalveolar lavage fluid: a specific and sensitive method of diagnosing gastro-oesophageal reflux-related pulmonary aspiration. J Pediatr Surg. 2006;41(2):289–93.
Schallom M, Tricomi SM, Chang YH, Metheny NA. A pilot study of pepsin in tracheal and oral secretions. Am J Crit Care. 2013;22(5):408–11.
Emilsson OI, Benediktsdottir B, Olafsson I, Cook E, Juliusson S, Bjornsson ES, et al. Respiratory symptoms, sleep-disordered breathing and biomarkers in nocturnal gastroesophageal reflux. Respir Res. 2016;17(1):115.
Hunt J, Yu Y, Burns J, Gaston B, Ngamtrakulpanit L, Bunyan D, et al. Identification of acid reflux cough using serial assays of exhaled breath condensate pH. Cough. 2006;2:3.
Fitzpatrick AM, Holbrook JT, Wei CY, Brown MS, Wise RA, Teague WG. Exhaled breath condensate pH does not discriminate asymptomatic gastroesophageal reflux or the response to lansoprazole treatment in children with poorly controlled asthma. J Allergy Clin Immunol Pract. 2014;2(5):579–86.e7.
Canani RB, Cirillo P, Roggero P, Romano C, Malamisura B, Terrin G, et al. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117(5):e817–20.
Terrin G, Passariello A, De Curtis M, Manguso F, Salvia G, Lega L, et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics. 2012;129(1):e40–5.
Rosen R. Gastroesophageal reflux in infants: more than just a pHenomenon. JAMA Pediatr. 2014;168(1):83–9.
Garland JS, Alex CP, Johnston N, Yan JC, Werlin SL. Association between tracheal pepsin, a reliable marker of gastric aspiration, and head of bed elevation among ventilated neonates. J Neonatal-Perinatal Med. 2014;7(3):185–92.
Nikaki K, Woodland P, Sifrim D. Adult and paediatric GERD: diagnosis, phenotypes and avoidance of excess treatments. Nat Rev Gastroenterol Hepatol. 2016;13(9):529–42.
Garrigues V, Gisbert L, Bastida G, Ortiz V, Bau I, Nos P, et al. Manifestations of gastroesophageal reflux and response to omeprazole therapy in patients with chronic posterior laryngitis: an evaluation based on clinical practice. Dig Dis Sci. 2003;48(11):2117–23.
Wo JM, Grist WJ, Gussack G, Delgaudio JM, Waring JP. Empiric trial of high-dose omeprazole in patients with posterior laryngitis: a prospective study. Am J Gastroenterol. 1997;92(12):2160–5.
Fass R, Noelck N, Willis MR, Navarro-Rodriguez T, Wilson K, Powers J, et al. The effect of esomeprazole 20 mg twice daily on acoustic and perception parameters of the voice in laryngopharyngeal reflux. Neurogastroenterol Motil. 2010;22(2):134–41. e44-5
Vaezi MF, Richter JE, Stasney CR, Spiegel JR, Iannuzzi RA, Crawley JA, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope. 2006;116(2):254–60.
Faruqi S, Molyneux ID, Fathi H, Wright C, Thompson R, Morice AH. Chronic cough and esomeprazole: a double-blind placebo-controlled parallel study. Respirology. 2011;16(7):1150–6.
Shaheen NJ, Crockett SD, Bright SD, Madanick RD, Buckmire R, Couch M, et al. Randomised clinical trial: high-dose acid suppression for chronic cough - a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33(2):225–34.
Stordal K, Johannesdottir GB, Bentsen BS, Knudsen PK, Carlsen KC, Closs O, et al. Acid suppression does not change respiratory symptoms in children with asthma and gastro-oesophageal reflux disease. Arch Dis Child. 2005;90(9):956–60.
Orenstein SR, Hassall E, Furmaga-Jablonska W, Atkinson S, Raanan M. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J Pediatr. 2009;154(4):514–20. e4
Vandenplas Y. Management of paediatric GERD. Nat Rev Gastroenterol Hepatol. 2014;11(3):147–57.
Kastelik JA, Redington AE, Aziz I, Buckton GK, Smith CM, Dakkak M, et al. Abnormal oesophageal motility in patients with chronic cough. Thorax. 2003;58(8):699–702.
Ou XM, Feng YL, Wen FQ, Wang K, Yang J, Deng ZP, et al. Macrolides attenuate mucus hypersecretion in rat airways through inactivation of NF-kappaB. Respirology. 2008;13(1):63–72.
Mertens V, Blondeau K, Pauwels A, Farre R, Vanaudenaerde B, Vos R, et al. Azithromycin reduces gastroesophageal reflux and aspiration in lung transplant recipients. Dig Dis Sci. 2009;54(5):972–9.
Solidoro P, Braido F, Boffini M, Corsico AG. New life for macrolides. Minerva Med. 2013;104(6 Suppl 1):7–14.
Rosen R, Amirault J, Liu H, Mitchell P, Hu L, Khatwa U, et al. Changes in gastric and lung microflora with Acid suppression: Acid suppression and bacterial growth. JAMA Pediatr. 2014;168(10):932–7.
Goldin AB, Garrison M, Christakis D. Variations between hospitals in antireflux procedures in children. Arch Pediatr Adolesc Med. 2009;163(7):658–63.
Tannuri AC, Tannuri U, Mathias AL, Velhote MC, Romao RL, Goncalves ME, et al. Gastroesophageal reflux disease in children: efficacy of Nissen fundoplication in treating digestive and respiratory symptoms. Experience of a single center. Dis Esophagus. 2008;21(8):746–50.
Frongia G, Ahrens P, Capobianco I, Kossler-Ebs J, Stroh T, Fritsche R, et al. Long-term effects of fundoplication in children with chronic airway diseases. J Pediatr Surg. 2015;50(1):206–10.
Lee SL. Short- and long-term antireflux and asthma medication use in children after nissen fundoplication. Perm J. 2009;13(2):4–11.
Goldin AB, Sawin R, Seidel KD, Flum DR. Do antireflux operations decrease the rate of reflux-related hospitalizations in children? Pediatrics. 2006;118(6):2326–33.
Richards CA, Milla PJ, Andrews PL, Spitz L. Retching and vomiting in neurologically impaired children after fundoplication: predictive preoperative factors. J Pediatr Surg. 2001;36(9):1401–4.
Spiegel B. Diagnostic testing in extraesophageal GERD: another case of "furor medicus"? Am J Gastroenterol. 2013;108(6):912–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Duncan, D.R., Rosen, R.L. (2017). Gastroesophageal Reflux and Respiratory Tract Symptoms. In: Vandenplas, Y. (eds) Gastroesophageal Reflux in Children. Springer, Cham. https://doi.org/10.1007/978-3-319-60678-1_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-60678-1_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-60677-4
Online ISBN: 978-3-319-60678-1
eBook Packages: MedicineMedicine (R0)