Abstract
Several mechanisms can contribute to respiratory manifestations in patients with gastro-oesophageal reflux (GOR), but pathological and causal relationship is uncommon. In most infants apnoea of short duration is a physiologic phenomenon occurring frequently in relation to an episode of GOR and a protective mechanism to prevent aspiration. Diagnostic gold standard, cut-off values and follow-up data are currently lacking making the relation between GOR or GOR disease and respiratory system difficult to clarify. When compared with pH monitoring, oesophageal impedance with simultaneous polysomnography can better demonstrate the temporal association in selected patients but should be reserved to severe or recurrent otherwise unexplained respiratory events. Empirical treatment for GOR is not recommended due to lack of evidence of efficacy and possible pharmacologically related adverse events.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Gastro-oesophageal reflux (GOR) has been associated with all chronic respiratory disorders, but in the vast majority of cases, neither temporal nor causal relation with apnoea or apparent life-threatening event (ALTE) (an episode characterized by some combination of apnoea, colour change, marked change in muscle tone, choking or gagging, i.e. frightening to the observer) is demonstrated [1, 2]. However, coexistence of GOR may occur in many infants, particularly in preterms and in postprandial time [3, 4], mainly because apnoeas and regurgitations are frequent physiologic events in the first months of life. Outcomes of studies are difficult to compare because of heterogeneity in the population recruited and diagnostic criteria of both apnoea and GOR or GOR disease GOR(D) [1, 2]. Intervention trials and follow-up data are needed to clarify the relation between the two phenomena.
Pathophysiology
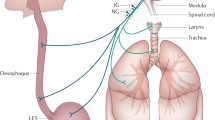
The two major theories that attribute respiratory symptoms to GOR are (micro)aspiration of gastric contents during a reflux episode and vagal reflex arc. The neural theory is based on stimulation of oesophageal afferent receptors by oesophageal distention, inflammation or irritation caused by GOR with subsequent laryngospasm or bronchospasm (triggered by airway efferents) via vagal pathways. However, the association between GOR and respiratory system is more complex and not completely clarified. Negative intrathoracic pressure (i.e. in tracheomalacia), congenital malformation (i.e. oesophageal atresia and/or fistula, laryngomalacia) or other comorbidities (i.e. neurological impairment, cystic fibrosis, achalasia) or swallowing disorders or impaired oesophageal peristalsis or increased abdominal pressure (due to recurrent cough or obesity) all can facilitate reflux. Thus, in each patient with recurrent respiratory events, all the following mechanisms should be considered: presence or coexistence of (micro)aspiration, stimulation of laryngeal (chemo)reflexes, alteration of vagal response, oesophageal and/or laryngeal inflammation, oesophageal or respiratory hyperreactivity or hypersensitivity, increased negative intrathoracic or positive abdominal pressure, impaired airway protection, reduced oesophageal sphincter tone, anatomic abnormalities or congenital malformation and abnormal motility (Fig. 12.1). Because no diagnostic gold standard exists for any of these variables, pathophysiology of respiratory symptoms in GOR(D) is far to be elucidated.
Furthermore, GOR can occur as a consequence of apnoea, facilitated by a negative intrathoracic pressure or as a preceding event that causes apnoea as an exaggerated laryngeal reflex or a natural protective mechanism for the respiratory airways [1, 5]. GOR can even be considered as a protective concurrent disorder for ALTE and SIDS as reflux facilitates arousals [6, 7]. Both acid and nonacid refluxes have been reported to be associated with apnoea. However, the temporal relation and specific association with obstructive apnoea (compared to central or mixed apnoeas) are still controversial.
In healthy individuals, a series of barriers and protective responses prevent refluxed gastric contents from entering the airway. These protective mechanisms include the upper oesophageal sphincter, oesophageal-glottal closure reflex (with consequent apnoea), pharyngeal clearance, cough and airway clearance of aspirated materials [8]. The consequence of oesophageal distension is also complex and difficult to measure. In normal subjects, when GOR is of small volume, the upper oesophageal sphincter contracts, whilst after a large volume reflux, oesophageal distention leads to vagal reflexes that cause vocal cord closure, central apnoea and upper oesophageal sphincter relaxation which allow the entry of gastric contents into the pharynx, followed by a swallow to clear the pharynx and rapid resumption of respiration [8]. If reflux enters the larynx, normally, a cough burst expels the material from the airway and bronchoconstriction prevents aspirated material from reaching the alveolar spaces [8]. If any of this complex sequence occurs out of order or abnormally, there is a high risk of aspiration and respiratory complications [8].
To complicate even more the relationship between respiratory system and GOR, several studies suggested a primary or secondary autonomic or parasympathetic alteration.
A 24 h analysis of heart rate variability showed that in (adult) patients with GOR(D), the function of the autonomic nervous system is altered and vagal tone is (primary?) reduced [8,9,11]. Similarly, lower rate of heart high frequency HF was reported in patients with laryngopharyngeal reflux compared to healthy adults [12].
Data in children are lacking, but in neonates short-term GOR-related changes of vagal activity, with lower parasympathetic tone in the minutes preceding GOR, have been reported [13]. A significant increase in the sympathovagal ratio (+32%, p = 0.013) was observed in the period immediately prior to reflux (due to a 15% reduction in parasympathetic activity (p = 0.017)), relative to the control period. This phenomenon was observed during both wakefulness and active sleep and suggests that a pre-reflux change in autonomic nervous system activity is one of the factors contributing to the mechanism of reflux in neonates [13]. Parasympathetic dysfunction has also long been implicated in GOR(D) through an impaired regulation of the lower oesophageal sphincter (LOS) [14].
However, the potential role of inflammation has not been clarified, and distinguishing between cause and effect is challenging also because (individual) hypersensitivity to oesophageal stimuli produces changes in autonomic nervous system even in healthy subjects [15, 16].
The vigilance state also significantly influenced the distribution of GOR events, with 53% observed during wakefulness, 38% observed during active sleep and only 9% observed during quiet sleep [13]. Poor quality of sleep characterized by irregular breathing patterns is associated with reflux [3, 16,17,18,19,20,21,23]. GOR is a frequent cause of interrupting sleep among infant patients [3], and nonacid GOR proved to be equally important as acid GOR for causing arousals and awakenings in infants [24]. Pain or discomfort also occurs both with weakly acid and acid reflux episodes [25] and may mediate GOR and arousals.
Physiologic data suggest that when there is a temporal relationship, apnoea is more likely to predispose to GOR via oesophageal sphincter relaxation than vice versa [26].
When compared with pH monitoring [3], combined pH-oesophageal impedance (pH-MII), detecting both acid and nonacid GOR, could better demonstrate that apnoea of short duration following GOR is a physiologic protective phenomenon to prevent aspiration.
In children and adults, GOR has been linked to obstructive sleep apnoea (OSA) syndrome (OSAS). OSA is characterized by repetitive narrowing or collapse of the upper airway during sleep, with the development of large negative intrathoracic pressures during inspiratory efforts against the occluded airway, until restoration of airway patency with arousal from sleep [27]. In adults, OSA has been associated with increased occurrence of nocturnal symptoms of GOR [28] as well as increased number and length of overnight GOR episodes (2000). In children the relation has not been investigated so far.
Continuous positive airway pressure (CPAP), the mainstay therapy for OSA (in adults), may reduce reflux events and improve symptoms of nocturnal GOR [28] through a beneficial effect (increase pressure and/or reduced transient relaxations) on LOS [29].
However, adult studies reported an association between nocturnal GOR episodes and apnoea or hypopnea in a range of 54–70% [27, 29,30,32], suggesting a (mild) causal relationship between obstructive respiratory events and nocturnal GOR events, but also, reflecting the large number of apnoeas and hypopneas that occur during the night in patients with OSA, the high probability, by chance, of a nocturnal GOR event occurring in proximity to any given respiratory event [27].
The arousal accompanying the re-establishment of upper airway patency after occlusion and the associated stimulation of sympathetic nervous activity as well as the apnoea-associated increased parasympathetic (vagal) nervous activity [33] do not appear to influence significantly transient LOS relaxation in adults [27].
Conversely, obesity predisposes to OSA and GOR(D) both in adults [27] and in children [1].
The clinical relevance of the proximal extension of a reflux in generating respiratory events or other symptoms is still unclear. A stronger association between symptoms and proximal reflux than with non-proximal reflux was sustained by some authors [34, 35] but could not be confirmed by others [35,36,38]. The majority of reflux events in asymptomatic preterms reached the proximal oesophagus or pharynx, and there were no differences between acid and nonacid reflux [39]. The lack of differences between asymptomatic and diseased infants contravenes the hypothesis for macro- or microaspiration but does not exclude hypersensitivity to reflux as a cause for respiratory symptoms [39]. In our population, symptoms were associated with proximal reflux in 70% of all the reflux-related episodes without influence of age [38]. However, proximal extension of reflux was not a necessary condition to cause symptoms. As expected, the proportion of proximal reflux was higher for “vomiting” as a symptom than for all the other occurring symptoms [38].
A recent report investigated 20 preterm infants (10 with ALTE and 10 controls) with simultaneous pharyngo-oesophageal manometry, respiratory plethysmography and nasal thermistors and suggested a possible role of oesophageal motility. The analysis showed more frequent and prolonged spontaneous respiratory events (defined as apnoea >2″ with ≥2 “missing” breathing), less amplitude of protective contraction of upper oesophageal sphincter, more frequent disturbed oesophageal propagation, mixed apnoea and gasping in patients with ALTE compared to controls [40].
Apnoea, ALTE and GOR(D)
Current knowledge on the relationship between apnoea or ALTE and GOR(D) in infants is limited because of the small number of patients investigated, differences in methodology and controversial results. Furthermore, patient selection and grouping are made difficult by the absence of “gold standard” diagnostic criteria both for apnoea and GOR(D) in infant population.
First, relation between apnoea or ALTE and GOR was based on concurrent clinical symptoms of regurgitation and/or results of pH monitoring. Pathological pH monitoring has been overall reported in a range of 20% [41] to 77% [19] of infants with ALTE and of 32% [20, 21, 42] to 100% [43] of infants with apnoeas.
ALTE and GOR(D)
GOR(D) is the most commonly attributed cause of ALTE in a range of 31–55% of ALTE cases [44, 45]. However, in most studies proper investigations for GOR(D) were not performed, and diagnosis of GOR(D) was based simply on reported regurgitations concomitant to the episode or even just in previous weeks.
In old studies, patients with ALTEs had a 60–70% prevalence of recurrent regurgitation or emesis [46, 47], and case reports and series described ALTEs triggered by overt regurgitation into the oropharynx, by aspiration of refluxed gastric contents and by reflux induced by positional change after feedings [43, 45,46,50]. In selected patients with ALTE, acid perfusion of the oesophagus induced obstructive apnoea [49] or oxygen desaturation [51], suggesting that one mechanism for ALTE is acid stimulation of laryngeal, pharyngeal or oesophageal chemoreceptors with subsequent laryngospasm. Three small studies showed no significant difference in terms of acid reflux percentage or duration between infants who had experienced an ALTE and controls [18, 52, 53]. However, in a previous report, abnormal pH-metry results were found in 42% of 62 infants with episodes of paleness possibly suggestive of an ALTE, compared with 8.5% of the 378 control infants [3]. In 67 infants with ALTE investigated with pH monitoring for ≥10 ore, Arad-Cohen reported pathological GOR in 53% of infants, but 81% of apnoeic events were not associated with GOR. In the minority of cases with demonstrated association, apnoeas preceded GOR in nearly all (94%) of the episodes [54]. A large study reported the prevalence of ALTE to be less (20%) in a sample of 173 infants with GOR(D) (defined as a reflux index greater than 5% on pH monitoring) than in 169 healthy controls (31%, p < 0.12) [41].
In 2008 retrospectively reviewed records from a large group of 313 infants hospitalized for ALTE showed a discharge diagnosis of GOR(D) as the most common (49%) diagnosis, but that again was not based on pH monitoring except that in one patient. Interestingly, within 6 months, 14 patients (9%) of this subgroup had recurrent ALTE [55]. A large revision of 12,067 infants discharged with a diagnosis of ALTE in the USA confirmed that the most common associated diagnosis was GOR (37%) but with a considerable hospital-based variation particularly in the evaluation and diagnosis of GOR. An increased likelihood of readmission for patients discharged with a diagnosis of cardiovascular disorders (odds ratio [OR] = 1.68; 95% confidence interval [CI] = 1.30–2.16) and GOR (OR = 1.32; 95% CI = 1.03–1.69) compared with other discharge diagnoses was also reported [44].
Another retrospective cohort study of 469 infants admitted for ALTE found that adverse outcomes associated with GOR(D) (including aspiration pneumonia, failure to thrive, or anti-reflux surgery), second ALTE or death were rare (3.8%) and significantly related to neurological impairment or long hospital staying, in a follow-up period of approximately 8 years [2].
In the last two decades, many studies analysed the temporal association between reflux episodes and ALTE or apnoea in infants using 24 h pH-impedance monitoring (pH-MII) which offers a higher diagnostic sensitivity for GOR compared to pH monitoring, particularly in the first months of life and in postprandial period when nonacid (pH >4) reflux is more common.
Mousa et al. [56] analyzed the temporal relationship between apnoea and GOR by pH-MII in a group of 25 infants who presented with an ALTE event or pathologic apnoea. In this report a time interval of as long as 5 min between apnoea and reflux during pH-MII investigation was considered acceptable to demonstrate a “temporal link” between the two phenomena. In total, 527 episodes of apnoea were recorded, but only 80 (15.2%) were temporally linked to a reflux episode (despite the large criterion of 5 min). Of these 80 episodes, 37 (7% of the total number of apnoea events) were related to an acid reflux episode and 43 (8%) were related to a nonacid reflux episode. Thus, even considering both acid and nonacid GOR and a time interval of as long as 5 min, the relation between reflux and apnoea appears rare [56]. Recently, the analysis of 39 infants with ALTE reported abnormal GOR parameters in 33 (85%) with combined pH impedance reduced to 14 (36%) when only pH monitoring was considered, confirming an increased frequency of nonacid reflux events and usefulness of combined investigation to detect underlying GOR(D) [57].
As all studies were retrospective regarding the episode of ALTE, they could not document the rate of ALTEs that occurred during reflux or vice versa but only the underlying condition of GOR and possible temporal association between GOR and apnoeas occurring during GOR investigation.
Medical therapy of ALTEs suspected of being GOR related has not been adequately studied. Avoidance of overfeeding and approaches that decrease the frequency of regurgitation and the volume of reflux such as thickened feeding is suggested in infants with frequent regurgitation [1]. Pharmacotherapy has not been shown to be effective and the use of acid inhibitors has been related to an increased risk of infections in infants. Furthermore, the incidence of ALTEs diminishes significantly with age and without therapy in most cases, suggesting that anti-reflux therapy should be reserved in the rare infant in whom ALTEs are truly life threatening and are shown to be clearly related to GOR [1].
Similarly, even if supine position is associated with increased rate of reflux events, prone sleeping should be avoided in infants because of related increased risk of SIDS.
Although rare, SIDS has been reported to occur in patients with a previous ALTE and documented GOR [7, 22, 58]. However, in none of these patients, a correlation between oesophageal acidification and a cardiopulmonary event was ever recorded. At present there is no evidence that the characteristics of the ALTE or the polysomnographic record can predict which infants with ALTE are at risk for future life-threatening episodes or sudden death or GOR(D).
In a recent review on ALTE [59] Tieder concluded that routine investigation for GOR is not necessary, but patients with recurrent ALTEs or symptoms of GOR not responsive to behaviour and diet treatment can benefit from pH (or, better, impedance pH) monitoring combined with symptom (and polysomnography) registration to establish a cause-effect relation or another aetiology [59].
In the new classification of ALTE, in case of brief resolved unexplained events (BRUE) and infant at low risk, GOR can be associated with or without overt regurgitation and should be considered as a (co)factor for respiratory abnormalities and recurrent BRUE events [60].
It is clear that ALTE is the preceding referring manifestation and investigation for GOR(D) can only reveal an underlying excessive oesophageal acid exposure or GOR-associated apnoea/desaturation that occurred during the (impedance)-pH monitoring. As ALTE rarely recurs during the diagnostic test, the causal relation with the episode of ALTE is impossible to prove as well as the related benefit of GOR treatment unless follow-up data are available.
Apnoea and GOR(D): Studies in Infants
In highly selected cases, reflux is temporally associated with pathological, central and obstructive apnoea [7] but no study has conclusively shown a cause and effect relation between reflux and pathologic apnoea.
In one old report, short apnoea or bradycardia was tightly tied to vomit or regurgitation, whereas the majority of prolonged apnoea spells (>20 s) were not [61]. Using pH monitoring to assess GOR, several studies reported an occasional correlation of GOR with obstructive or short-mixed central apnoeas (5–15 s) [3, 20, 21, 23, 62] but also showed that all of the patients presented episodes of apnoea unrelated to episodes of GOR, suggesting a primary impairment in the regulation of respiration. Large case series did not find a significant relation between GOR and pathologic apnoea or ALTEs [17, 18].
To examine the temporal relationship between apnoea and GOR and its effect on apnoea duration, 119 preterm infants underwent 12 h cardiorespiratory monitoring studies using respiratory inductance plethysmography, heart rate, oxygen saturation and oesophageal pH monitoring [63]. Among 6255 episodes of acid GOR detected by pH monitoring, only 1% were associated with apnoea >15 s. There was also no difference in rate of apnoea >10 s before versus during GOR, but a decrease in apnoea rate was found immediately after GOR. The presence of reflux during apnoea did not prolong apnoea duration, and GOR had no effect on the lowest oxygen saturation or heart rate during apnoea. Hence, there was no evidence of a temporal relationship between acid-based GOR and apnoea in these preterm infant cohorts [63].
One retrospective study showed that GOR-related apnoea improved rapidly following commencement of gastrojejunal feeding, suggesting that in some cases reflux may cause apnoea [64].
A strong temporal association between acid and nonacid GOR and respiratory abnormalities was reported in 1 study using 6 h combined pH-MII that recorded 364 episodes of reflux, of which only 11% were acid, in a group of 22 infants who presented with repetitive regurgitation and chronic respiratory symptoms [65]. Of these reflux episodes, 312 (85%, 12% of which were acid) could be associated with irregular breathing. In a minority of these episodes (n:19), oxygen desaturations of 10% occurred (19% [3 of 19] of these episodes were acid). Analysis of the polysomnographic recording revealed 165 episodes of apnoea (>5 s), of which 49 (30%) were associated with a reflux episode. Again, the majority (78%) of reflux episodes were detected with impedance only [65].
In the last years, although some authors suggested a relation between (long, >30 s) apnoea or bradycardias of prematurity and reflux [66, 67], most studies did not support reflux as a cause of pathologic apnoea in (premature) infants [39, 63, 68, 69]. Nineteen preterm infants (gestational age, 30 weeks) who presented with apnoea were studied at a mean age of 26 days, and 2039 episodes of apnoea (median, 67; range, 10–346), 188 oxygen desaturations (median, 6; range, 0–25), 44 bradycardias (median, 0; range, 0–24) and 524 episodes of GOR (median, 25; range 8–62) were detected by pH-MII lasting 6 h [68]. The frequency of apnoea (≥4 s) in a 20 s period before and after an episode of GOR was not different from the frequency of apnoea not related to a reflux episode (0.19/min (range, 0.00–0.85/min) vs. 0.25/min (range, 0.00–1.15/min)) [68]. The analysis and conclusions were identical for oxygen desaturations and bradycardias [68].
In a small group of 6 premature infants with apnoea (defined as abnormal respiratory pause ≥20 s or of shorter duration if associated with cyanosis or marked pallor or hypotonia or bradycardia <80 beats/min) or hypoxaemia (defined as pulse oximeter saturation ≤80%) not responsive to caffeine treatment, a total of 405 reflux events (306 (76%) weakly acid and 99 acid reflux) and 142 apnoeas were detected. The sub-analysis based on chemical composition and duration of refluxate showed that the frequency of apnoeas associated with nonacid reflux events was significantly greater than the one calculated for reflux-free period (0.416/min (0.00–1.30) vs. 0.016/min (0.003–0.028), respectively; p < 0.05) and that the frequency of apnoeas occurring during reflux events longer than 30 s was significantly higher than those occurring during shorter reflux events (22% vs. 11%; p < 0.004) [67].
Corvaglia et al. investigated 52 preterm infants with simultaneous polysomnography and combined 24 h pH-MII and showed that 154 (14%) apnoeas out of 1136 were related in time to GOR. The frequency of apnoea during the 1 min time (30 s before and after) within the onset of GOR was significantly higher than the apnoea in GOR-free periods (p = 0.03). Furthermore, the frequency of apnoea in the 30 s after GOR (GOR-triggered apnoeas) was greater than that detected in the 30 s before (p = 0.01) suggesting that a number of apnoeas were induced by GOR [66].
In a subsequent report, the same authors confirmed in 58 preterm infants with recurrent apnoeas an increased frequency of apnoea after (both acid and nonacid) GOR compared to periods before or without GOR [70]. No difference was found regarding proximal extension or duration of GOR between reflux events associated or not associated with apnoea [70].
The influence of body position on GOR has also been assessed in a number of studies. In ten healthy preterm infants, a “crossover position study” and postprandial evaluation showed more liquid GOR in the right than in the left lateral position (median 9.5 (range 6.0–22.0) vs. 2.0 (range, 0.0–5.0) episodes/h; p = 0.002). Conversely, gastric emptying was faster in the right than in the left lateral position (37.0 + 21.1 vs. 61.2 + 24.8 min; p = 0.006) [71]. Similar findings were reported by another group in 22 preterm babies presenting with regurgitation and postprandial desaturations: the number of acid and nonacid reflux episodes was significantly smaller when the subjects were in the prone and left-side sleeping position in comparison with the supine and right-side positions [72]. The left-side position showed the lowest oesophageal acid exposure in the early postprandial period, whereas in the prone position acid reflux was smallest in the late postprandial period [73].
History and physical examination are still important, in infants with ALTE, to exclude warning signs (for GOR and other extra-oesophageal diseases), but the presence or absence of regurgitation is not sufficient to discriminate physiological reflux from GOR(D). Regurgitation is neither specific (even if associated to crying or back arching or feeding problem) nor sufficient to make a diagnosis of GOR(D). Indeed regurgitation is extremely common in the first months of life and represents a physiological manifestation in most infants who do not need any investigation or pharmacological treatment. Similarly, desaturation or apnoea or laryngeal inflammation does not imply GOR(D) [5]. Conversely, malformation (such as laryngomalacia) or respiratory disease can facilitate (secondary) GOR by negative intrathoracic pressure or increased abdominal pressure caused by cough. No specific symptom or cluster of symptoms for GOR(D) and response to acid inhibitors have been identified in infants and young children so far. Therefore, empirical pharmacological treatment is not recommended in infants because of lack of symptomatic efficacy and possible adverse events (i.e. increased incidence of infections with acid inhibitors and cardiac problems with prokinetics) [1]. Domperidone is also not beneficial for GOR in newborns because it increases GER episodes per hour compared to the baseline despite reducing the duration without modifying the pH value or the proximal extent reached by the refluxes [74].
In accordance with the ESPGHAN-NASPGHAN guidelines [1], the NICE guidelines, after reviewing 13 studies, confirmed that GOR only rarely causes episodes of apnoea or ALTEs and recommended specialist investigations if GOR(D) is suspected as a possible factor following a general paediatric assessment or in cases of unexplained apnoeas [75]. MII/pH oesophageal monitoring in combination with polysomnographic recording and precise, synchronous symptom recording may aid in establishing the relationship between apnoea and GOR [1].
A recent systematic review [76] has highlighted the limited data available on the association between GOR and apnoea with small patients recruited, heterogeneous inclusion and diagnostic criteria and therapeutic outcomes. Only one study was considered eligible using pH-MII recording [77] which found, in 71 preterms, no association between GOR and apnoeas with only 3% of apnoea (>10 s) following GOR and only 9% respiratory events preceding reflux considering a time window of 30 s [77]. Based on the current literature, the authors concluded that there is insufficient evidence to prove an association between the two disorders.
However, the association between pathologic central, obstructive and mixed apnoea has never been demonstrated (but has also not been well studied yet), and clear cut-off values discriminating normal from pathologic children still need to be determined.
In conclusion, the available evidence suggests that in the vast majority of infants, GOR is not related to pathologic apnoea or to ALTE [1, 2, 26], and thus there is no evidence to support an empirical treatment of GOR in infants presenting with apnoea or ALTE. However, a clear temporal association based on history, observation or testing occurs in individual infants. pH-MII in combination with polysomnographic recording is recommended to demonstrate the relation in these infants [1].
Studies in Children
In one study that analysed 28 children (mean age, 6.5 ± 5.6 years) with chronic respiratory symptoms (on treatment with antacid medications), multivariate analysis confirmed a stronger association between respiratory symptoms with nonacid reflux episodes than with acid reflux episodes and pointed out the importance of the height of the refluxate: the higher the reflux, the stronger the association [78].
The complexity in understanding the role of acid and nonacid GOR in respiratory symptoms still exists and involves the possible presence of primary or secondary reflux in different subjects, the low chance of occurrence of respiratory symptoms (especially if they have no daily frequency) during the investigation (making the association impossible to be determined) and the difficulty in identifying the correct temporal sequence of “respiratory reflux” or “reflux respiratory” or “respiratory reflux-reflux respiratory” without combined sensitive investigation tool.
In a selected group of 22 adults, a relation between chronic coughing and GER has been, for the first time, accurately studied by combined manometry and pH-MII in 2005 [79]. Using a time frame of 2 min and symptom association probability, 69% of the coughing episodes were considered “independent” of a reflux episode. When a “reflux-cough” sequence occurred, the reflux in 65% of the cases was acid, weakly acid in 29% and weakly alkaline in 6% [79].
The feasibility and accuracy of these combined investigations for cough were then confirmed in children [80].
No similar method has been reported for the temporal detection of apnoea and GOR. Polysomnography has demonstrated a better accuracy compared to transcutaneous oxygen saturimeter to detect and define apnoeas, but it has not been a widespread use because of the cost and complexity of the analysis [81]. Furthermore most studies used synchronization of the internal clock of the two instruments (polysomnography and impedance), but the related tracings do not appear on the same screen of the computer limiting the accuracy of the temporal association and sequence between apnoea and GOR.
OSAS and GOR
It is estimated that 9–10% of children are habitual snorers or have sleep-disordered breathing-related illnesses [82]. Conventionally, an apnoea is considered as a cessation of airflow for 10 s and is often associated with oxygen desaturation, whereas a lesser reduction in airflow is termed a hypopnoea [81]. Snoring and occasional apnoeic breath holding in sleep are common, but only when witnessed repetitive apnoeas and symptoms of sleep fragmentation, such as excessive daytime sleepiness, occur, a diagnosis of obstructive sleep apnoea syndrome (OSAS) can be made [81]. In adult, sleep studies measure the apnoea/hypopnoea index (AHI), which is the number of respiratory events an hour. Excessive sleepiness becomes more prevalent once the AHI exceeds five events an hour, and this value has become a lower cut-off for the diagnosis of OSAS [81].
The ideal method for diagnosis of sleep apnoeas is full polysomnography, which involves overnight admission for supervised multichannel recording, including electroencephalography. Restricted availability of polysomnography and the cost mean that oximetry and limited respiratory monitoring are more widely used [81]. Overnight oximetry is widely available, but oxygen desaturation is an inexact surrogate for apnoeic events, and the ideal frequency and depth of desaturation events are still debated although an oxygen desaturation of 4% is conventionally used to indicate apnoea [81]. Obstructive sleep apnoea occurs in approximately 3% of children, most frequently aged from 2 to 6 years [83]. OSAS diagnosis is clinically relevant because recurrent episodes of air flow cessation, oxygen desaturation and sleep disruption are associated with behaviour disorders, neurocognitive deficits, disturbances of somatic development as well as cardiovascular and metabolic sequelae [78, 84].
The aetiology of OSAS is multifactorial consisting of a complex interplay between airway anatomical characteristics and dynamic control of upper airway muscular tone [85]. Obstructive sleep apnoea is hypothesized to be influenced by genes involved with obesity, craniofacial development, inflammation and ventilator control [86]. Adenotonsillar hypertrophy is recognized as the most frequent cause of OSAS in childhood [87]. The association between GOR and OSAS in children has been less explored compared to apnoea in infants and, as well as in adults, remains controversial.
In several studies acidification of the distal oesophagus was suggested in the mechanism of OSA in children and adults and in persisting OSAS after adenoidectomy [85,86,87,88,92]. The role of GOR in OSAS in infants has been less investigated and in residual OSA among young children is unclear.
A report in 18 children with adenotonsillar hypertrophy and OSAS evaluated the OSA-18 questionnaire, nasofibrolaringoscopy and full overnight polysomnography performed simultaneously with oesophageal pH monitoring. Seven children (41%) presented episodes of acid reflux during the registered sleep time. The authors concluded that GOR is frequent and should be assessed in children from 6 to 12 years with OSAS [91]. However, reflux parameters did not correlate to OSAS severity and a temporal relationship between GOR and apnoea-hypopnea events was not observed [91].
The main treatment options of OSAS are essentially physical solutions to narrowing of the upper airway, namely, continuous positive airway pressure (CPAP), oral appliances and upper airway surgery. Weight loss and bariatric surgery may also be appropriate interventions in adults [81].
Treatment of GOR has been shown to improve OSAS [90, 93], and OSAS therapy with CPAP has been demonstrated to reduce GOR [94] confirming a bidirectional association between these two conditions. The favourable effect of CPAP on nocturnal GOR is possibly due to an increase in nadir LOS pressure and decrease in the duration of LOS relaxation [27].
In eight newborn lambs, continuous oesophageal pH-impedance monitoring and polysomnography were performed for 6 h during both spontaneous breathing and nCPAP application at 6 cm H2O (nCPAP6, of common usage in newborns), in a randomized order. CPAP6 virtually abolished GER (mean ± SD reflux number for 6 h = 9.1 ± 8.6 without nCPAP vs. 0.6 ± 1 with nCPAP, p < 0.05) and decreased the depth and duration of LOS relaxation suggesting that nCPAP may enhance the barrier function of the LOS against GOR [95]. Hence, CPAP may reduce in patients with OSAS both acid and nonacid GOR and, eventually, proximal refluxes, which are especially prominent in infants and can be responsible for cardiorespiratory inhibition via the laryngeal chemoreflexes [96].
However, temporal relationship between GOR and apnoea-hypopnea events was not clearly demonstrated, and heterogeneity for both apnoea and GOR definition and detection does not allow a general conclusion. Even in studies when a simultaneous recording of pH monitoring and polysomnography was applied, the registration from the two investigations was not integrated. Additionally, since a pH probe and not a pH-MII was used to assess GOR in many studies, it is possible that GOR has been underestimated in these patients. Prospective studies assessing natural evolution of patients with concomitant GOR and apnoeas and benefit of GOR treatment are lacking.
Low basal pressure of LOS detected in some OSA patients raises the possibility of weakening of the gastro-oesophageal junction from repetitive strain associated with obstructed breathing events.
Conclusions
Several authors have suggested an association between GOR and apnoea in infants. However, current studies present low number of patients, high heterogeneity in terms of population recruited, diagnostic tools, definition of apnoeas, GOR and GOR(D), means of association and time intervals, hampering a direct comparison among results. Most studies fail to show a temporal link between apnoea or ALTE and GOR, and when an association is found, apnoea episodes more frequently precede GOR, than follow GOR. Empirical treatment for GOR is not recommended due to lack of evidence of efficacy and possible pharmacologically related adverse events. In selected patients with recurrent idiopathic respiratory events, pH-MII with simultaneous polysomnography recording should be performed to detect underlying GOR(D) and to prove the relationship with apnoeas.
References
Vandenplas Y, Rudolph CD, Di Lorenzo C, North American Society for Pediatric Gastroenterology Hepatology and Nutrition, European Society for Pediatric Gastroenterology Hepatology and Nutrition, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498–597.
Zimbric G, Bonkowsky JL, Maloney CG, Srivastava R. Adverse outcomes associated with gastroesophageal reflux disease are rare following an apparent life-threatening event. J Hosp Med. 2012;7:476–81.
Sacre L, Vandenplas Y. Gastroesophageal reflux associated with respiratory abnormalities during sleep. J Pediatr Gastroenterol Nutr. 1989;9:28–33.
Wenzl TG, Schneider S, Scheele F, et al. Effects of thickened feeding on GER in infants: a placebo-controlled crossover study using intraluminal impedance. Pediatrics. 2003;114:e355–9.
Tolia V, Vandenplas Y. Systematic review: the extra-oesophageal symptoms of gastro-oesophageal reflux disease in children. Aliment Pharmacol Ther. 2009;29(3):258–72.
Kahn A, Rebuffat E, Sottiaux M, et al. Prevention of airway obstructions during sleep in infants with breath-holding spells by means of oral belladonna: a prospective double-blind crossover evaluation. Sleep. 1991;14(5):432–8.
Vandenplas Y, Hauser B. Gastro-oesophageal reflux, sleep pattern, apparent life threatening event and sudden infant death. The point of view of a gastroenterologist. Eur J Pediatr. 2000;159:726–9.
Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distension. Am J Phys. 2001;281:G1246–63.
Cuomo R, De Giorgi F, Adinolfi L, et al. Oesophageal acid exposure and altered neurocardiac function in patients with GERD and idiopathic cardiac dysrhythmias. Aliment Pharmacol Ther. 2006;24:361–70.
Dobrek L, Nowakowski M, Mazur M, et al. Disturbances of the parasympathetic branch of the autonomic nervous system in patients with gastroesophageal reflux disease (GERD) estimated by short-term heart rate variability recordings. J Physiol Pharmacol. 2004;55(S2):77–90.
Milovanovic B, Filipovic B, Mutavdzin S, et al. Cardiac autonomic dysfunction in patients with gastroesophageal reflux disease. World J Gastroenterol. 2015;21:6982–9.
Huang W-J, Shu C-H, Chou K-T, et al. Evaluating the autonomic nervous system in patients with laryngopharyngeal reflux. Otolaryngol—Head Neck Surg. 2013;148:997–1002.
Djeddi D-D, Kongolo G, Stéphan-Blanchard E, et al. Involvement of autonomic nervous activity changes in gastroesophageal reflux in neonates during sleep and wakefulness. PLoS One. 2013;8:e83464.
Heatley RV, Collins RJ, James PD, Atkinson M. Vagal function in relation to gastro oesophageal reflux and associated motility changes. Br Med J. 1980;280:755–7.
Sharma A, Paine P, Rhodes S, et al. The autonomic response to human esophageal acidification and the development of hyperalgesia. Neurogastroenterol Motil. 2012;24:e285–93.
Tougas G, Spaziani R, Hollerbach S, et al. Cardiac autonomic function and oesophageal acid sensitivity in patients with non-cardiac chest pain. Gut. 2001;49:706–12.
Ariagno RL. Evaluation and management of infantile apnea. Pediatr Ann. 1984;13:210–3. 216–217
Kahn A, Rebuffat E, Sottiaux M, et al. Lack of temporal relation between acid reflux in the proximal oesophagus and cardiorespiratory events in sleeping infants. Eur J Pediatr. 1992;151(3):208–12.
Newman LJ, Russe J, Glassman MS, et al. Patterns of gastroesophageal reflux (GER) in patients with apparent life-threatening events. J Pediatr Gastroenterol Nutr. 1989;8:157–60.
Paton JY, Nanayakkara CS, Simpson H. Observations on gastroesophageal reflux, central apnoea and heart rate in infants. Eur J Pediatr. 1990;149:608–12.
Paton JY, Macfadyen U, Williams A, et al. Gastro-oesophageal reflux and apnoeic pauses during sleep in infancy – no direct relation. Eur J Pediatr. 1990;149:680–6.
Veereman-Wauters G, Bochner A, Van Caillie-Bertrand M. Gastroesophageal reflux in infants with a history of near-miss sudden infant death. J Pediatr Gastroenterol Nutr. 1991;12:319–23.
Walsh JK, Farrell MK, Keenan WJ, et al. Gastroesophageal reflux in infants: relation to apnea. J Pediatr. 1981;99:197–201.
Machado R, Woodley FW, Skaggs B, et al. Gastroesophageal reflux causing sleep interruptions in infants. J Pediatr Gastroenterol Nutr. 2013;56:431–5.
Cresi F, Castagno E, Storm H, et al. Combined esophageal intraluminal impedance, pH and skin conductance monitoring to detect discomfort in GERD infants. PLoS One. 2012;7:e43476.
Abu Jawdeh EG, Martin RJ. Neonatal apnea and gastroesophageal reflux (GER): is there a problem? Early Hum Dev. 2013;89(Suppl 1):S14–6.
Shepherd K, Hillman D, Holloway R, Eastwood P. Mechanisms of nocturnal gastroesophageal reflux events in obstructive sleep apnea. Sleep Breath. 2011;15(3):561–70.
Green BT, Broughton WA, O’Connor B. Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea treated with continuous positive airway pressure. Arch Intern Med. 2003;163:41–5.
Shepherd KL, Holloway RH, Hillman DR, Eastwood PR. The impact of continuous positive airway pressure on the lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol. 2007;292(5):G1200–5.
Ing AJ, Ngu MC, Breslin AB. Obstructive sleep apnea and gastroesophageal reflux. Am J Med. 2000;108(Suppl. 4A):120S–5S.
Ozturk O, Ozturk L, Ozdogan A, et al. Variables affecting the occurrence of gastroesophageal reflux in obstructive sleep apnea patients. Eur Arch Otorhinolaryngol. 2004;261(4):229–32.
Penzel T, Becker HF, Brandenburg U, et al. Arousal in patients with gastro-oesophageal reflux and sleep apnoea. Eur Respir J. 1999;14:1266–70.
Urata M, Fukuno H, Nomura M, et al. Gastric motility and autonomic activity during obstructive sleep apnea. Aliment Pharmacol Ther. 2006;24(S 4):132–40.
Jadcherla SR, Gupta A, Fernandez S, et al. Spatiotemporal characteristics of acid refluxate and relationship to symptoms in premature and term infants with chronic lung disease. Am J Gastroenterol. 2008;103:720–8.
Rosen R, Nurko S. The importance of multichannel intraluminal impedance in the evaluation of children with persistent respiratory symptoms. Am J Gastroenterol. 2004;99:2452–8.
Condino AA, Sondheimer JM, Pan Z, et al. Evaluation in infantile acid and non-acid gastroesophageal reflux utilizing combined pH monitoring and impedance measurement. J Pediatr Gastroenterol Nutr. 2006;42:16–21.
Condino AA, Sondheimer J, Pan Z, et al. Evaluation of GER in pediatric patients with asthma using impedance-pH monitoring. J Pediatr. 2006;149:216–9.
Salvatore S, Arrigo S, Luini C, Vandenplas Y. Esophageal impedance in children: symptom-based results. J Pediatr. 2010;157:949–54.e1-2.
Lopez-Alonso M, Moya MJ, Cabo JA, et al. 24-hour esophageal impedance-pH monitoring in healthy preterm neonates: rate and characteristics of acid, weakly acidic, and weakly alkaline gastro-esophageal reflux. Pediatrics. 2006;118:e299–308.
Hasenstab KA, Jadcherla SR. Respiratory events in infants presenting with apparent life threatening events: is there an explanation from esophageal motility? J Pediatr. 2014;165:250–5.
Tolia V, Wuerth A, Thomas R. Gastroesophageal reflux disease: review of presenting symptoms, evaluation, management, and outcome in infants. Dig Dis Sci. 2003;48(9):1723–9.
Barrington KJ, Tan K, Rich W. Apnea at discharge and gastro-esophageal reflux in the preterm infant. J Perinatol. 2002;22(1):8–11.
Spitzer AR, Boyle JT, Tuchman DN, et al. Awake apnea associated with gastroesophageal reflux: a specific clinical syndrome. J Pediatr. 1984;104:200–5.
Tieder JS, Cowan CA, Garrison MM, Christakis DA. Variation in inpatient resource utilization and management of apparent life-threatening events. J Pediatr. 2008;152(5):629–35.
Okada K, Miyako M, Honma S, et al. Discharge diagnoses in infants with apparent life-threatening event. Pediatr Int. 2003;45(5):560–3.
Rosen CL, Frost JD Jr, Harrison GM. Infant apnea: polygraphic studies and follow-up monitoring. Pediatrics. 1983;71:731–6.
Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47–52.
Herbst JJ, Book LS, Bray PF. Gastroesophageal reflux in the “near miss” sudden infant death syndrome. J Pediatr. 1978;92:73–5.
Herbst JJ, Minton SD, Book LS. Gastroesophageal reflux causing respiratory distress and apnea in newborn infants. J Pediatr. 1979;95:763–8.
Leape LL, Holder TM, Franklin JD, et al. Respiratory arrest in infants secondary to gastroesophageal reflux. Pediatrics. 1977;60:924–8.
Friesen CA, Streed CJ, Carney LA, et al. Esophagitis and modified Bernstein tests in infants with apparent life-threatening events. Pediatrics. 1994;94:541–4.
Gorrotxategi P, Eizaguirre I, Saenz de Ugarte A, et al. Characteristics of continuous esophageal pH-metering in infants with gastroesophageal reflux and apparent life-threatening events. Eur J Pediatr Surg. 1995;5(3):136–8.
Kahn A, Rebuffat E, Sottiaux M, et al. Sleep apneas and acid esophageal reflux in control infants and in infants with an apparent life-threatening event. Biol Neonate. 1990;57(3–4):144–9.
Arad-Cohen N, Cohen A, Tirosh E. The relationship between gastroesophageal reflux and apnea in infants. J Pediatr. 2000;137:321–6.
Doshi A, Bernard-Stover L, Kuelbs C, et al. Apparent life-threatening event admissions and gastroesophageal reflux disease: the value of hospitalization. Pediatr Emerg Care. 2012;28(1):17–21.
Mousa H, Woodley FW, Metheney M, Hayes J. Testing the association between gastroesophageal reflux and apnea in infants. J Pediatr Gastroenterol Nutr. 2005;41:169–77.
Blasco-Alonso J, Yun-Castilla C, Girón-Fernández-Crehuet F, et al. Esophageal multichannel intraluminal impedance and pH testing in the study of apparent life threatening episode incidents in infants. Rev Esp Enferm Dig. 2014;106:159–64.
Jolley SG, Halpern LM, Tunell WP, et al. The risk of sudden infant death from gastroesophageal reflux. J Pediatr Surg. 1991;26(6):691.
Tieder JS, Altman RL, Bonkowsky JL, et al. Management of apparent life-threatening events in infants: a systematic review. J Pediatr. 2013;163(1):94–9.
Tieder JS, Bonkowsky JL, Etzel RA, Subcommittee on apparent life threatening events, et al. Brief resolved unexplained events (formerly apparent life-threatening events) and evaluation of lower-risk infants. Pediatrics. 2016;137(5):e20160590.
Menon AP, Schefft GL, Thach BT. Apnea associated with regurgitation in infants. J Pediatr. 1985;106:625–9.
de Ajuriaguerra M, Radvanyi-Bouvet MF, Huon C, Moriette G. Gastroesophageal reflux and apnea in prematurely born infants during wakefulness and sleep. Am J Dis Child. 1991;145:1132–6.
Di Fiore JM, Arko M, Hitehouse M, et al. Apnea is not prolonged by acid gastroesophageal reflux in preterm infants. Pediatrics. 2005;116:1059–63.
Misra S, Macwan K, Albert V. Transpyloric feeding in gastroesophageal-reflux-associated apnea in premature infants. Acta Paediatr. 2007;96:1426–9.
Wenzl TG, Silny J, Schenke S, et al. Gastro-esophageal reflux and respiratory phenomena in children: status of the intraluminal impedance technique. J Pediatr Gastroenterol Nutr. 1999;28:423–8.
Corvaglia L, Zama D, Gualdi S, et al. Gastro-oesophageal reflux increases the number of apnoeas in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 2009;94:F188–92.
Magistà A, Indio F, Baldassarre M, et al. Multichannel intraluminal impedance to detect relationship between gastroesophageal reflux and apnoea of prematurity. Dig Liver Dis. 2007;39:216–21.
Peter CS, Sprodowski N, Bohnhorst B, et al. Gastroesophageal reflux and apnea of prematurity: no temporal relationship. Pediatrics. 2002;109:8–11.
Poets CF. Gastroesophageal reflux: a critical review of its role in preterm infants. Pediatrics. 2004;113:e128–32.
Corvaglia L, Zama D, Spizzichino M, et al. The frequency of apneas in very preterm infants is increased after non-acid gastro-esophageal reflux. Neurogastroenterol Motil. 2011;23:303–7.
van Wijk MP, Benninga MA, Dent J, et al. Effect of body position changes on postprandial gastroesophageal reflux and gastric emptying in the healthy premature neonate. J Pediatr. 2007;151:585–90.
Corvaglia L, Rotatori R, Ferlini M, et al. The effect of body positioning on gastroesophageal reflux in premature infants: evaluation by combined impedance and pH monitoring. J Pediatr. 2007;151:591–6.
van Wijk MP, Benninga MA, Davidson GP, et al. Small volumes of feed can trigger transient lower esophageal sphincter relaxation and gastroesophageal reflux in the right lateral position in infants. J Pediatr. 2010;156:744–8.
Cresi F, Marinaccio C, Russo MC, et al. Short-term effect of domperidone on gastroesophageal reflux in newborns assessed by combined intraluminal impedance and pH monitoring. J Perinatol. 2008;28:766–70.
National Institute of Health and Care Excellence (NICE) Clinical Knowledge Summaries on Gastroesophageal reflux disease in children available on www.nice.org.uk/guidance/NG1.
Smits MJ, Van Wijk MP, Langendam MW, et al. Association between gastroesophageal reflux and pathologic apneas in infants: a systematic review. Neurogastroenterol Motil. 2014;26:1527–38.
Di Fiore J, Arko M, Herynk B, et al. Characterization of cardiorespiratory events following gastroesophageal reflux in preterm infants. J Perinatol. 2010;30:683–7.
Rosen CL, Storfer-Isser A, Taylor HG, et al. Increased behavioral morbidity in school-aged children with sleep-disordered breathing. Pediatrics. 2004;114:1640–8.
Sifrim D, Dupont I, Blondeau K, et al. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut. 2005;54:449–54.
Blondeau K, Mertens V, Dupont L, et al. The relationship between gastroesophageal reflux and cough in children with chronic unexplained cough using combined impedance-pH-manometry recordings. Pediatr Pulmonol. 2011;46(3):286–94.
Greenstone M, Hack M. Ostructive sleep apnoea. BMJ. 2014;348:g3745.
Ali NJ, Pitson D, Stradling JR. Natural history of snoring and related behaviour problems between the ages of 4 and 7 years. Arch Dis Child. 1994;71(1):74–6.
Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–12.
Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182:676–83.
Katz ES, D’Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:253–62.
Larkin EK, Patel SR, Goodloe RJ, et al. A candidate gene study of obstructive sleep apnea in European Americans and African Americans. Am J Respir Crit Care Med. 2010;182:947–53.
Marcus CL. Pathophysiology of childhood obstructive sleep apnea: current concepts. Respir Physiol. 2000;119:2–3.
Berg S, Hoffstein V, Gislason T. Acidification of distal esophagus and sleep related breathing disturbances. Chest. 2004;125:2101–6.
Carr MM, Poje CP, Ehrig D, Brodsky LS. Incidence of reflux in young children undergoing adenoidectomy. Laryngoscope. 2001;111:2170–2.
Friedman M, Gurpinar B, Lin HC, et al. Impact of treatment of gastroesophageal reflux on obstructive sleep apnea–hypopnea syndrome. Ann Otol Rhinol Laryngol. 2007;116:805–11.
Noronha AC, de Bruin AC, Nobre e Souza VM, et al. Gastroesophageal reflux and obstructive sleep apnea in childhood. Int J Pediatr Otorhinolaryngol. 2009;73(3):383–9.
Stapleton A, Brodsky L. Extra-esophageal acid reflux induced adenotonsillar hyperplasia: case report and literature review. Int J Pediatr Otorhinolaryngol. 2008;72:409–13.
Bortolotti M, Gentilini L, Morselli C, Giovannini M. Obstructive sleep apnoea is improved by a prolonged treatment of gastroesophageal reflux with omeprazole. Dig Liver Dis. 2006;38(2):78–81.
Tawk M, Goodrich S, Kinasewitz G, Orr W. The effect of 1 week of continuous positive airway pressure treatment in obstructive sleep apnea patients with concomitant gastroesophageal reflux. Chest. 2006;130(4):1003–8.
Djeddi D, Cantin D, Samson N, Praud JP. Nasal continuous positive airway pressure inhibits gastroesophageal reflux in newborn lambs. PLoS One. 2014;9(9):e107736.
Praud JP. Upper airway reflexes in response to gastric reflux. Paediatr Respir Rev. 2010;11:208–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Salvatore, S., Vandenplas, Y. (2017). GOR(D) and Apnoea. In: Vandenplas, Y. (eds) Gastroesophageal Reflux in Children. Springer, Cham. https://doi.org/10.1007/978-3-319-60678-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-60678-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-60677-4
Online ISBN: 978-3-319-60678-1
eBook Packages: MedicineMedicine (R0)