Abstract
Physical exercise is important for limiting fat mass and preventing obesity. Aerobic exercise after breakfast is effective in helping to maintain lipid consumption. Study subjects were 18 young Japanese women who did not exercise regularly. They then engaged in 30 min of aerobic exercise with loads that were equivalent to 25%, 40%, 55%, 70%, and 25% of the maximum heart rate in sequential 6-minute intervals. Respiratory metabolism and heart rate were measured with a bicycle ergometer. The amount of energy, lipid, and carbohydrate consumed were calculated from respiratory quotients. Energy/carbohydrate consumptions and relative heart rates increased significantly compared with the resting control, which increased linearly as exercise load increased. However, lipid consumption was not different across exercise loads. Our findings show that aerobic exercise at a level to increase relative heart rate by 25% to 70% is effective in reducing body fat in women who do not exercise regularly.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
- Body fat
- Lipid consumption
- Energy consumption
- Carbohydrate consumption
- Respiratory metabolism
- Aerobic exercise
- Japanese young adult female
- Oxygen intake
- Heart rate
1 Introduction
The main cause of illness-related death since World War II in Japan has changed to the geriatric diseases such as cancer, heart disease, and cerebrovascular disease from infectious diseases such as tuberculosis or pneumonia. The onset and progression of geriatric diseases have been considered to be attributed to aging; however, it has become clear that lifestyle is greatly related [1]. In addition, because the symptoms of these geriatric diseases have increased in children because of lifestyle factors [2], the name of geriatric diseases was changed to lifestyle-related disease in 1996 by the Ministry of Health, Labour and Welfare. One of the causes of lifestyle-related disease includes obesity, and it is said that approximately 90% of obesity is simple obesity. Obesity [3, 4] is considered a contributing factor to geriatric diseases such as diabetes [5], high blood pressure [6], dyslipidemia [7], myocardial infarction [8, 9], and cerebral infarction [10]. To prevent obesity and live a healthy life, we should be mindful of our eating habits and participate in regular exercise. In addition, it is necessary to keep body fat (BF) at an appropriate level. Excessive caloric intake [11] and lack of exercise contribute to reduced fitness and possibly long-term illness. Further, vigorous exercise is a considerably demanding task for a person unaccustomed to such activity. According to Mersy et al., aerobic exercise benefits health [12], which affects lipoprotein metabolism in sedentary healthy young women [13]. Thus, we used aerobic exercise in this study measured with an ergometer as a simple way to assess the work performed during exercise. This study aimed to identify easily achievable conditions that enhance BF metabolism during aerobic exercise by assigning moderate exercise in combination with normal eating habits [14,15,16].
2 Method
2.1 Subjects and Experimental Schedule
The study subjects were 18 healthy Japanese females in their twenties who did not normally participate in regular exercise. The physical characteristics and intensity of exercise loads of study subjects are displayed in Table 1. Subjects were non-smokers, with no history of systemic disease, and were not engaged in physical training or dietary programs. Respiratory metabolism was measured in the follicular phases of subjects’ menstrual cycles [17] in winter [18, 19].
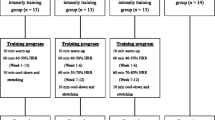
Figure 1 illustrates the experimental schedule. Subjects awoke at 06:00 after 7 h of sleep and consumed a prescribed diet shown in Table 2 (breakfast typical for youth) until 07:00. They then entered a climate-controlled room (environmental temperature, 24.5 ± 0.3 °C; relative humidity, 50.3 ± 2.5%; lumination, 827 ± 27 lx; air current, 8.0 ± 0.1 cm/s) at 08:00. Subjects were seated at rest for 1 h while wearing a short-sleeved 100% cotton t-shirt and 100% polyester running pants. Their respiratory metabolism levels were measured by a Vmax-229 (Sensormedics, CA, USA) [20] using the breath-by-breath method for 40 min starting at 9:00. BF percentage, muscle, and basal metabolic rate (BMR) were measured using an electric bioelectrical impedance analysis [21] scale (BC-520, TANITA, Tokyo, Japan). Body weight was measured with a balance (IPS-150 K, Shimazu, Kyoto, Japan). Metabolism of BF was assessed by measuring respiratory metabolism before, during, and after aerobic exercise; the device used measured the amount of inhaled oxygen (L/min) and exhaled carbon dioxide (L/min) for every lungful of air. The energy consumption per one liter of oxygen (EC, kcal/O2 l) was calculated from obtaining the respiratory quotient (RQ) [22, 23] using a Zunts-Schumberg-Lusk table [24], which shows analysis of the oxidation of mixtures of carbohydrate and fat. EC was calculated using the following equation:
This was used to determine the EC ratio supplied from carbohydrate versus lipid metabolism. For example, the carbohydrate ratio Z for EC is determined from this equation:
while the lipid ratio X is calculated as:
Therefore, the amount of energy consumed (kcal/O2 l) during respiration was divided into the relative ECs (kcal/min) supplied by carbohydrates (CC) or lipids (LC) using oxygen intake (L/min). Afterward, the carbohydrates and lipids metabolized during respiration were calculated using the relationship of 4.1 kcal per gram of carbohydrate and 9.3 kcal per gram of lipid.
2.2 Exercise Program
The exercise load of the exercise bike (Aerobic Exercise Ergometer, STB-1400, NIHONKHODEN, Tokyo, Japan) is shown in Fig. 2. Subjects engaged in 30 min of aerobic exercise with loads that were equivalent to 25%, 40%, 55%, 70%, and 25% of the maximum heart rate using the Karvonen equation [16, 25] in sequential 6-minute intervals (the second 25% interval served as a cool-down period). Respiratory metabolism and heart rate were measured during exercise and 5 min before and after as a control. EC, LC, and CC were calculated from RQs.
2.3 Lipid and Carbohydrate Metabolism
A typical example of the relationship between oxygen intake (OI) and carbon dioxide output using the breath-by-breath method is shown in Fig. 3, with dots representing the number of breaths. No points were detected that exceeded the anaerobic threshold (AT), confirming that these exercises were aerobic.
According to Rennie et al., heavy exercise and long-term exercise resulted in the consumption of body protein in men [26]. The RQs in the present study were regarded as “non-protein RQs,” as the exercise was carried out at light loads and the 30-minute exercise duration was relatively short. Further, the subjects did not eat a lot of protein [27] (see Table 2). CCs and LCs were then calculated according to the fix rule [28, 29] and compared with each exercise load.
2.4 Perspiration and Insensitive Perspiration Amount
Body weights while wearing clothes were measured by a balance before and after exercise, and the increased weight of the clothes post-exercise was attributed to perspiration. The weight of the sweat was deducted from the loss of body weight over the exercise period to determine the amount of insensitive perspiration.
2.5 Statistics
ECs, CCs, LCs, and relative heart rates (RHR) measured before exercise at rest were compared with those from each exercise stage using a paired t-test in consideration of individual differences. Significant findings between each exercise stage were confirmed in EC, CC, and RHR from stages 1–4 after analysis by one-way ANOVA and examining significant differences using Tukey’s method. The correlation coefficients between EC and LC in each stage and the following relative factors were calculated: weight, sweat, insensitive water loss, BF, muscle, BMR, CC, LC, OI, and RHR were calculated. From the respiration metabolism determined by the breath-by-breath method, the averages of EC, CC, LC, and RHR were calculated every 10 s. The data collected in this way were analyzed by path analysis using LC as a dependent variable and OI, RHR, and CC as independent variables (IBM SPSS Ver. 22, Armonk, NY, USA).
3 Results
3.1 Effects on Energy Metabolism
A typical example of EC, CC, LC and RHR every 10 s is shown in Fig. 4. The variation of the CC and RHR resembled the variation of the EC (which changed by exercise stage), but was different from the variation of the LC. Therefore, these data were distributed and compared across each exercise stage.
EC, CC, LC, and RHR values for each stage compared with resting measurements are shown in Fig. 5. EC, CC, and RHR for each exercise stage and after exercise were significantly increased compared with each measurement before exercise (paired t-test). EC, CC, and RHR significantly increased during aerobic exercise in comparison with resting state. The significant findings between each exercise stage are shown in Table 3. EC, CC, and RHR increased significantly and linearly with exercise intensity. Conversely, for LC, no significant differences were found.
Table 4 displays the correlation coefficients between EC and LC in each stage and selected relative factors: weight, sweat, insensitive water loss, BF, muscle, BMR, CC, LC, OI, and RHR. There were significant positive correlations between EC and weight, sweat (except E1: EC in stage 1), insensitive water loss, BF, muscle, BM, CC, LC, and OI. Conversely, there was no significant correlation between LC and other factors except BF and OI in stage 1. This result showed that increased LC was not obtained even if the intensity of exercise was increased. That is, it was not necessary to increase the exercise load to burn BF with aerobic exercise.
4 Discussion
In this study, we investigated a condition for metabolizing BF while identifying an exercise intensity that is not burdensome to maintain. As shown in Fig. 4 and Table 4, a larger exercise load raised EC and this increase derived from carbohydrate sources rather than lipid sources. EC, CC, and RHR increased as the exercise loads increased. However, LCs for each exercise stage were 311.4 ± 105.6, 289.6 ± 102.8, 298.6 ± 117.2, and 316.0 ± 123.3 mg/min in stages 1–4, respectively. Metabolism was not different between exercise loads. That is, even if exercise intensity increases, the LC does not necessarily increase under aerobic exercise.
Hansen et al. [29] reported oxygen uptake as related to work rate increment during cycle ergometer exercise. They employed a heavy exercise load and found that OI increased with each increment of exercise load. We therefore adopted OI as a related factor to LC. We found a significant positive correlation between OI and RHR (0.88, P < 0.001) as shown in Fig. 6, which illustrates the path analysis result. The significant multiple correlation coefficient revealed a positive correlation between LC and OI (0.64, P < 0.001) and negative correlations between RHR (−0.14, P < 0.001) and CC (−0.87, P < 0.001). For increasing LC, it is necessary to increase OI, which increased by increasing the exercise load. However, the resulting decrease in LC may be attributed to corresponding increases in the negatively correlated factors RHR and CC. Therefore, for increasing LC, it is necessary to prevent RHR and CC increases with increasing OI.
OI and RHR showed a positive correlation (Fig. 6). The regression line between OI and HR with exercise was calculated in each subject, and VdotO2 (VO2) max for HR of “220-subject’s age” (Karvonen equation) was calculated [22]. The ratios of OI in each four stage for VO2 max were calculated for each subject. Figure 7 shows the relationship between LC and relative OI for VO2 max, which was the mean of 18 subjects (Mean ± SD: 1st stage, 23.6 ± 0.2%; 2nd stage, 31.0 ± 0.1%; 3rd stage, 38.4 ± 0.1%; 4th stage, 45.2 ± 0.2%; incidentally 9.1 ± 0.0% in the rest). In this way, the relationship showed a U-shaped curve, which revealed that BF was not burning under 30–39% OI compared with under 24% or 45% OI.
Why would the lipid consumption of 31–39% VO2 max be low? Romijn et al. report energy consumption when five male athletes in their twenties were made to exercise for 2 h with 25%, 65%, and 85% VO2 max load as measured by an isotope infusion. During exercise at 25% VO2 max, 90% of metabolized energy came from lipids, the majority of which came from the oxidation of plasma free fatty acids (FFA). While exercising at 65% VO2 max, half of the metabolized energy comes from lipid, half from TR in muscle and half from plasma FFA. Furthermore, during exercise at 85% VO2 max, more than 70% of metabolized energy comes from sugar (glucose and glycogen) and fat becomes hard to metabolize [30].
During 24% OI exercise (the first stage) in the present study, plasma FFA were metabolized and TR in muscle began to be metabolized at 45% OI (the fourth stage). Meanwhile, 30–39% OI shifts the energy source of supply from plasma FFA alone to a combination of TR in muscle. Because the total energies increase, it is thought the decreased lipid metabolic was supplied with by sugar metabolism, thus reducing the efficiency of lipid metabolism. It was found that exercise at 30% VO2, which a Ministry of Health, Labour and Welfare and exercise enforcement teachers’ textbooks recommended to people without regular exercise habits, was inefficient because of the fat burning potential. An exercise intensity of 25% VO2 max (HR = 93.9 ± 9.09 beats, RHR = 123.4 ± 6.9%) or exercise with an increased intensity of 45% VO2 max (HR = 128.7 ± 10.5 beats, RHR = 169.4 ± 11.3%) is appropriate. The rate of total lipid metabolism during aerobic exercise increased to 97.1 ± 33.1 times faster than the control. Because RHR at this stage was equivalent to an increase from 20% to 30% at rest, such exercise was effective and could be maintained in young adult women who do not normally participate in regular exercise.
5 Conclusions
Obesity is considered a contributing factor to geriatric diseases, and it is necessary to keep BF at an appropriate level. Excessive caloric intake and lack of exercise contribute to reduced fitness and possibly long-term illness. Therefore, the present study aimed to identify easily achievable conditions that enhance BF metabolism during aerobic exercise by assigning moderate exercise in combination with normal eating habits. Eighteen young Japanese women participated in an experiment that assessed their respiratory metabolism while performing aerobic exercise at low and moderate intensities. EC, CC, and RHR increased linearly with exercise intensity and were significantly increased compared with each measurement taken before exercise as a control. Conversely, no significant differences were found in LC across exercise stages.
There were significant positive correlations between ECs and weight, sweat, insensitive water loss, BF, muscle, BMR, CCs, LCs, and OI. In contrast, there were no significant correlations found between these variables and LCs. These results showed that the effect of increasing LC was not obtained, even if the intensity of exercise was increased. Thus, it was not necessary to increase the exercise load to burn BF with aerobic exercise. Using path analysis, we found that it is necessary to prevent RHR and CC increases to create an increase in LC with adequate OI during exercise.
Our findings show that aerobic exercise at a level to increase RHR 20%–30% was effective in reducing body fat in women who do not exercise regularly.
5.1 List of Abbreviations Used
-
EC: energy consumption
-
CC: carbohydrate consumption
-
LC: lipid consumption
-
BF: body fat
-
BMR: basal metabolic rate
-
OI: oxygen intake
-
RHR: relative heart rate
-
VO2 max: VdotO2 max
References
Japan Ministry of Health Labour and Welfare: Basic directionality of the illness measures that focused its attention on a lifestyle (Verdict of the concrete opinion). http://www.mhlw.go.jp/index.shtml, 1996.12.17 (in Japanese)
Department of Epidemiology and Health Policy: Infant lifestyle-related disease (geriatric-type disease of children). http://www.med.u-toyama.c.jp/healpro/meeting.html. (in Japanese)
Bouchard, C.: Physical Activity and Obesity, pp. 1–389. Human Kinetic Publishers Inc., USA, Canada, Europe, Australia, New Zealand (2000)
Galgani, J., Ravussin, E.: REVIEW; Energy metabolism, fuel selection and body weight regulation. Int. J. Obes. 2, S109–S119 (2008)
Fujioka, K., Seaton, T.B., Rowe, E., Jelinek, C.A., Raskin, P., Lebovitz, H.E., Weinstein, S.P., The Sibutramin/Diabetes Clinical Study Group: Weight loss with sibutramine improves control and other metabolic parameters in obese patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2, 175–187 (2000)
Whyte, H.M., Phil, D.: Blood pressure and obesity. Circulation XIX, 511–516 (1959)
Carr, M.C., Brunzell, J.D.: Abdominal obesity and dyslipidemia in the metabolic syndrome: importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery disease risk. J. Clin. Endocrinol. Metab. 89, 2601–2607 (2004)
Yusuf, S., Hawken, S., Ounpuu, S., Bautista, L., Frnzosi, M.G., Commerford, P., Lang, C.C., Rumboldt, Z., Ones, C.L., Lisheng, L., Tanomsup, S., Wangai, P., Razak, F., Sharma, A.M., Anand, S.S.: Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case-control study. Lancet 366, 1640–1649 (2005)
Aoyagi, K., Kusano, Y., Takamura, N., Abe, Y., Osaki, M., Une, H.: Obesity and cardiovascular risk factors among men and women aged 40 years and older in a rural area of Japan. J Physiol. Anthoropol. 25, 371–375 (2006). http://www.jstage.jst.go.jp/browse/jpa2. doi:10.2114/jpa2.25.371
Xiaodong, Y., Shujuan, W., Yaru, X., Jing, L.: A clinical follow-up study on the risk of cerebral infarction in Chinese aging overweight and obese population. Obes. Res. Clin. Pract. 5, e1–e78 (2011). doi:10.1016/j.orcp.2010.09.251
Rogge, M.M.: The role of impaired mitochondrial lipid oxidation in obesity. Biol. Res. Nurs. 10, 356–373 (2009)
Mersy, D.J.: Health benefits of aerobic exercise. Postgrad. Med. 90, 103–110 (1991)
Ootani, K., Hashimoto, S., Hayashi, S., Kimura, T., Naito, M.: Effects of aerobic exercise training on lipoprotein metabolism and antioxidant status in sedentary but otherwise healthy young women. J. Integr. Study Diet. Habits 22, 114–120 (2011)
Carstens, M.T., Goedecke, J.H., Dugas, L., Evans, J., Kroff, J., Levitt, N.S., Lambert, E.V.: Fasting substrate oxidation in relation to habitual dietary fat intake and insulin resistance in nondiabetic women: a case for metabolic flexibility? Nutr. Metab. 10, 8 (2013). http://www.nutritionandmetabolism.com/content/10/1/8
Mitsuno, T., Hibino, A.: Which support pants are the most effective for burning body fat? JFBI 9, 1–13 (2016)
ACSM Certified Personal Trainer SM: The Heart Rate Debate. http://www.acsm.org/public-information/articles/2016/10/07/the-heart-rate-debate
Lamina, S., Hanif, S., Muhammed, H.: Influence of menstrual cycle on maximal aerobic power of young female adults. AJPARS 3, 36–41 (2011)
Tsumura, Y., Makonakwkeyoon, L., Limtrakul, P., Hirota, N., Sone, Y.: Seasonal variation in amount of unabsorbed dietary carbohydrate from the intestine after breakfast in young female Thai subjects: comparison with that of Japanese subjects. J. Physiol. Anthropol. 29, 141–147 (2010)
Morinaka, T., Wozniewicz, M., Jeszjka, J., Bajerska, J., limtrakul, P., Makonkawkeyoon, L., Hirota, N., Kumagai, S., Sone, Y.: Comparison of seasonal variation in the fasting respiratory quotient of young Japanese, Polish and Thai women in relation to seasonal change in their percent body fat. J. Physiol. Anthropol. 31, 1–12 (2012). http://www.jphysiolanthropol.com/content/31/1/10
Okura, M., Nakaya, H., Yamamoto, S., Ojima, Y., Sakai, S., Sakanoue, N.: The validity of the estimated maximal oxygen up take by different indirect methods. Bull. Kochi Rehabil. Inst. 1, 5–24 (1999)
TANITA HP: Electric bioelectrical impedance analysis. http://www.tanita.co.jp/support/faq.htr (In Japanese)
Rennie, M.J., Edwrds, R.H.T., Krywawch, S., Davies, C.T.M., Hallday, D., Waterlow, J.C., Millward, D.J.: Effect of exercise on protein turnover in man. Clin. Sci. 61, 627–639 (1981)
Du Bois, E.F.: Clinical calorimetry: thirty-fifth paper. A graphic representation of the respiratory quotient and the percentage of calories from protein fat and carbohydrate. J. Biol. Chem. 59, 43–49 (1924)
Lusk, G.: Animal calorimetry: twenty-fourth paper. Analysis of the oxidation of mixtures of carbohydrate and fat. J. Biol. Chem. 59, 41–42 (1924)
Robert, A.: Surprising history of “HRmax = 220-age” equation. J. Exerc. Physiol. 5, 1–10 (2002)
Bender, D.A.: Introduction to Nutrition and Metabolism, 5th edn. CRC Press, New York (2014)
Ferrannin, E.: The theoretical bases of indirect calorimetry: a review. Metabolism 37, 287–301 (1988)
Sugimoto, A.: The measurement of physical activity—a review of recent progress. Rehabil. Med. 37, 53–61 (2007) (English abstract and Japanese text)
Hansen, J.E., Casaburi, R., Cooper, D.M., Wasserman, K.: Oxygen uptake as related to wark rate increment during cycle ergometer exercise. Eur. J. Appl. Physiol. 57, 140–145 (1988)
Romijin, J.A., Coyle, E.F., Sidossis, L.S., Gastaldelli, A., Horowitz, J.F., Endert, E., Wlofe, R.R.: Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 265, E380–E391 (1993)
Acknowledgements
This work was supported by JSPS Kakenhi (grant number 25242011).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Mitsuno, T., Nagayasu, M., Shinohara, Y., Ando, Y. (2018). Most Effective Exercise Load for Burning Body Fat with Aerobic Exercise in Young Japanese Women. In: Duffy, V., Lightner, N. (eds) Advances in Human Factors and Ergonomics in Healthcare and Medical Devices. AHFE 2017. Advances in Intelligent Systems and Computing, vol 590. Springer, Cham. https://doi.org/10.1007/978-3-319-60483-1_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-60483-1_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-60482-4
Online ISBN: 978-3-319-60483-1
eBook Packages: EngineeringEngineering (R0)