Abstract
Microporous and mesoporous silica catalysts, MCM-41, derived from zeolite type catalysts, are easily synthesized in laboratory scale and commercially available SiO2 have applications in reduction reactions. Nickel boride (Ni B) silica catalysts denoted as Cat A were characterized by XRD, IR, SEM, BET surface area and chemisorption studies. Nickel boride generated in situ on silica were found to be super active catalysts for reduction of nitro aromatics, aldehydes, ketones, alkenes, phenols and in reductive amination of aldehydes and ketones at low temperatures, whereas Pd(II)-MCM-41 denoted as Cat B exhibited catalytic activity for reduction of nitro aromatics, aldehydes, and hydrodehalogenation reactions. Efficient catalytic activity for reduction reactions was exhibited by the Ni and Pd catalysts which were found to be reusable, atom economy, reproducible and environmentally friendly. A comparative study of these catalysts is presented.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
21.1 Introduction
Many traditional organic reactions use stoichiometric reagents because they provide excellent activity, selectivity and specificity at low temperatures. However, the necessity to regenerate these materials after reaction leads to the production of large quantities of waste. Due to growing environmental concerns, the laws and regulations governing the disposal of industrial effluents are becoming increasingly tighter. The foremost challenge for the fine chemical manufacturing industries is therefore to seek cleaner processes in order to minimize the production of waste [1].
There are many methods of effecting reduction which may or may not lead to hydrogenation, but in this article only processes leading to the addition of hydrogen or replacement of a functional group by hydrogen will be considered. Reduction of organic functional groups can be categorized into (i) addition of hydrogen to unsaturated groups in the reduction of ketones to alcohols, and (ii) addition of hydrogen across single bonds leading to cleavage of functional groups (hydrogenolysis). The early pioneering work was largely ignored because of poor yields and long reaction times, but the situation has changed considerably following the appearance of stimulating heterogeneous catalysts and the introduction of greater catalyst loadings and different hydrogen donors. A new family of mesoporous molecular sieves was discovered by the researchers at Mobil Oil Corporation, and scientists have shown considerable and continuously growing interest since its discovery at the beginning of the 1990s [2, 3]. This fact led to extend the application of redox molecular sieves for oxidation of organic molecules. One member of mesoporous family MCM-41 with hexagonal arrangement and narrow pore size distribution varying between 3 and 10 nm, according to the surfactant used in the synthesis, high specific surface area (ffi1000 m2/g) and structural thermal stability, but with low hydrothermal stability and adjustable heteroatom compositions enable these materials to be widely used as adsorbents, catalytic supports, and heterogeneous catalysts [4]. The incorporation of active metals into the framework of MCM-41 molecular sieves makes them particularly valuable for catalytic applications, some of which are mentioned in the text. Since the first account of the synthesis of mesoporous molecular sieves in alkaline medium appeared, a large number of publications on the synthesis of mesoporous materials, mainly MCM-41 have been reported. The synthesis of mesoporous materials has also been carried out in acidic and neutral medium. Pinnavaia [5] proposed a neutral template synthesis mechanism based on hydrogen bonding between primary amines and neutral inorganic species. Such mesoporous molecular sieves are called hexagonal mesoporous silica (HMS).
The recently discovered family of mesoporous materials, MCM-41, have potential for industrial application due to their being ‘tunable’, having a large pore size. However, our work is to develop a suitable catalyst to promote the conversion of reactants while maintaining well-dispersed active sites and exhibiting good structural stability, which still remains a challenge.
21.2 Valuable Catalytic Applications
Supported nickel catalysts find widespread applications in many important industrial hydrogenation and hydrogenolysis processes such as steam reforming of methane and higher paraffins, or methanation of synthetic gas. In view of good results obtained, the authors explored the use of Pd(II)-MCM41 (Cat B) prepared by functionalizing MCM-41 with 3-triethoxysilylpropylamine and subsequent complexation with dichlorobis(benzylcyano) palladium(II) for reduction of nitro-aromatics and hydrodehalgenation reactions [5]. On the other hand the supported Ni silica catalysts were tested for catalytic reactions such as reduction of functional groups such as nitro, aldehydes, ketones, phenols, alkenes, and bromo benzene. Hydrogenation of aqueous glucose has also been carried out using supported nickel and ruthenium catalysts. Catalysts were prepared by precipitation, impregnation, sol-gel and template synthesis using SiO2, TiO2, Al2O3 and carbon as support materials. Industrially nickel catalysts were used for the polymerization of cyclo-olefins, and conversion of ethene to propene which is used as an intermediate for the production of polypropylene. Conversion of CO2 to CO with CH4 over Ni–SiO2 catalyst [1, 4,5,6,7] has also been investigated.
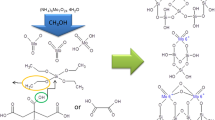
The manuscript details a comparison of Cat A (Ni–SiO2) and Cat B (Pd(II)-MCM-41) for reduction reactions at moderate conditions (Scheme 21.1), avoiding high temperature and high hydrogen pressure to minimize the thermal risks during industrial production [8,9,10]. It addresses also the achievement of high conversion and selectivity using mild reducing agents, to produce the desired chemical compound in the required quality. Additional features looked into are the operational simplicity, safety, energy efficient procedure and scope to recover the investment and developmental costs in a reasonable time.

Scheme 21.1
Thus, the objective of this work is to study the capability of Ni loaded support catalysts on silica for selective reduction reactions of substituted aromatic compounds to their corresponding products. Table 21.1 illustrates the effect of the Ni loading on the process and the catalytic aptitude of the system for a potential use in industrial processes. Aromatic amines and aromatic alcohols are generally prepared by the reduction of nitro aromatics and aromatic aldehydes usually by the use of Pd, Ni, Rh, Pt, Ru or calcined Ni-hydrotalcite [11]. The main limitations of the earlier reports are the high temperature, pressure, and sophisticated autoclaves required for the catalytic activity of metals such as Ni, Pt and Pd [4].
The authors studied the oxidation of p-nitro benzyl alcohol using nickel supported silica catalytic system at 65 °C into p-nitrobenzaldehyde, and the reduction of nitro aromatics, aldehydes, ketones, alkenes, phenols and reductive amination of aldehydes and ketones at low temperatures. Also reduction of nitroaromatics and hydrodehalogenation reactions were also performed using Pd(II)-MCM-41 (Cat B). Both catalysts showed successful completion of these reactions with excellent conversion and selectivity (Table 21.1).
21.3 Experimental Section
21.3.1 Materials and Methods
All chemicals, dichlorobis(benzylcyano)palladium(II), tetraethyl orthosilicate (TEOS), hexadodecyl amine, 2-propanol, nickel nitrate and silica gel particle size 63–200 μm, 70–230 mesh, pore size 100 Å, were obtained from Fluka.
21.3.2 Preparation of Ni–SiO2 (Cat A)
The catalyst was prepared by impregnation method by dissolving nickel nitrate nonahydrate (2.5 g) in distilled water (20.0 ml) and adding to it silica gel (5.0 g), stirring for 2 h using a magnetic stirrer at room temperature (20–22 °C) and ageing at room temperature overnight. The excess water is removed by heating the mixture on a water bath and using a rotavapor under mild vacuum to evaporate the water. The catalyst material is dried in an oven at 100 °C for 12 h [11].
21.3.3 Typical Reduction Procedure Using Cat A
To a solution of nitrobenzene (2.0 mmol) in 10 ml methanol, catalyst (200 mg) was added followed by slow addition of sodium borohydride (200 mg) at 0 °C for 5 min. The reaction mixture was magnetically stirred continuously for 10 min during which all starting material was consumed. The reaction progress was monitored by TLC. The reaction mixture was quenched with deionized water and extracted with ethyl acetate. The organic layer was dried with sodium sulfate and the solvent was evaporated on a rotavapor to give crude aniline which was then subjected to column chromatography to afford pure aniline product. The final product was characterized by 1H and 13C NMR spectroscopy using a 400 MHz Bruker instrument. GC–MS samples were run on an Agilent 6890 GC/5973, MS column: J & W HP5-MS. GC results were obtained on a GC System 6820, Agilent Technologies equipped with flame ionization detection (FID) and a carbowax OVI capillary column and compared with standard sample.
21.3.4 Preparation of Pd(II)-MCM-41 (Cat B)
The mesoporous material MCM-41 (pure silica) was synthesized as described below using a reported procedure [17]. First a solution was prepared by mixing 1 mmol of TEOS with 6 mol of ethanol and 1 mol of isopropanol. At the same time a second solution was obtained by mixing hexadecylamine (0.3 mol) in water (36 mol). The two solutions were then mixed under stirring at room temperature for about 1 h. The product obtained was aged at 25 °C for 12 h under static conditions. The resulting solid was recovered by centrifugation, washed with distilled water 8–10 times and filtered. Finally, the synthesized sample was air dried at room temperature for 24 h. One gram of MCM-41 was calcined at 550 °C overnight and refluxed with 0.686 mmol of 3-triethoxypropylamine in dry toluene in inert atmosphere for 48 h. This was then complexed with dichlorobis(benzylcyano) palladium(II) in dry benzene under stirring at room temperature. The bright yellow coloured complex obtained was then filtered, soxhlet extracted with benzene for 8 h, filtered, washed with benzene and dried under vacuum to afford the final required catalyst.
21.3.5 Typical Reduction Procedure Using Cat B
Pd(II)-MCM-41 catalyst (25 mg) was added to a solution of nitrobenzene (2.0 mmol) in 10 ml THF. The resulting solution was magnetically stirred in the presence of a hydrogen balloon attached to the round bottom flask at room temperature and monitored by TLC, till the starting material was consumed. Then the reaction mixture was filtered and the solvent was evaporated on a rotavapor to give crude product aniline which was then subjected to column chromatography to afford pure aniline product. The final product was characterized by 1H and 13C NMR spectroscopy using a JEOL Eclipse 270 NMR spectrometer (δC = 146.6, 129.3, 118.4 and 115.1 ppm and δH = 7.1, 6.7, 6.6 and 3.6 ppm). GC–MS samples were run on an Agilent 6890 GC/5973, MS column: J&W HP5-MS and compared with standard sample.
21.3.6 Characterization of Catalysts
Ni silica catalysts were characterized by the following techniques: XRD, BET, IR spectroscopy, SEM, UV DRS, and chemisorption.
21.4 Results and Discussion
Aromatic compounds with functional groups are key intermediates for the manufacture of dye stuff, pharmaceutical, agricultural, photographic chemicals, additives, surfactants, textiles auxiliaries, chelating agents and polymers [1,2,3] in chemical industries. The benefits of simple preparation, low cost, ease of handling and the efficiency of anchored palladium complexes in selective organic transformations, prompted us to explore the possibility of their use in the reduction of nitro aromatics, azides and in hydrodehalogenation reactions. Pd(II)-MCM-41 (Cat B) exhibited encouraging results for the reduction of nitro aromatics, aldehydes, ketones, azides and for hydrodehalogenation reactions of chlorobenzene, bromobenzene, 3-chlorotoluene at room temperature with excellent conversions and selectivity. The catalyst showed 3 cycles of reusability and no leaching of catalysts was observed. Nickel silicates with layered structure are well known as phyllosilicates, hydro silicates, or surface silicates. The authors prepared the catalysts Ni–SiO2 (Cat A) by simple impregnation method. Fine amorphous powders of Ni–SiO2 (Cat A) were obtained showing that Ni particles are well distributed over the silica surface which is confirmed by XRD characterization technique. Selective oxidation of alcohols is an important transformation from industry point of view [1, 2]. The authors reported previously the use of 10% Ni–SiO2 (Cat A) for the oxidation of p-nitrobenzyl alcohol to p-nitrobenzaldehyde in the presence of hydrogen peroxide and the controlled oxidation of benzhydrol to benzophenone with excellent conversion and selectivity [9, 12, 13]. When NaBH4 is added to nickel silica catalysts at 0–5 °C, a black precipitate is formed with the spontaneous evolution of hydrogen which indicates that NiB species are generated in situ and behave as superactive catalysts for reduction reaction of bromobenzene, benzaldehyde, phenol, styrene, toluene, and for reductive amination of aldehydes and ketones as shown in Table 21.1 for some of the reactions.
The authors have reported the use of catalyst Ni–SiO2 (Cat A) in a number of reactions including Knoevenagel condensation in liquid phase. All the Knoevenagel reactions resulted in 100% selectivity but the conversions differed with varying amounts of catalysts, with 10% Ni–SiO2 (Cat A) proving to be the superior catalyst. The catalytic activity of 10% Ni–SiO2 (Cat A) towards Knoevenagel reaction of 4-hydroxy benzaldehyde and malononitrile and ethyl cyanoacetate with substituted benzaldehydes were investigated and were found to be effective. All the Ni-supported catalysts were characterized by XRD, IR, BET, SEM instruments, and chemisorption studies. Ni silica catalysts of all samples showed particle size in the range of 20–250 Å and the moisture content observed was 7% [12].
The characterized catalysts showed better results compared with the literature reported catalysts [8, 9]. Extension of this work is being carried out with different borohydrides in order to evaluate the best catalytic system. A model reaction was tested for leaching of Ni metal from Ni–SiO2 (Cat A). The filtrate was subjected to reduction reactions with model substrate under the same conditions and it was observed that the reaction did not occur. This indicates that the Ni metal was intact on the surface of the silica support which is confirmed by IR studies showing that nickel silica are heterogeneous catalysts [14].
21.4.1 X-Ray Diffraction
The XRD studies on the Ni silica (Cat A) were carried out on a Phillips-PW 1830 powder X-ray diffraction instrument. The XRD spectra (Fig. 21.1) showed the characteristic bands of the nickel phase and support, but no mixed nickel oxide support phases were identified. The pattern for the supports was that of crystallized materials with well-defined bands except for the amorphous structure, as only one broad peak around 2θ = 25° appeared, which is characteristic of silica [8, 9, 11].
21.4.2 UV Visible Spectroscopy
Spectra of the supported materials, after initial drying, but before calcination and reduction, were measured by diffuse reflectance uv spectroscopy and their absorption maxima and assignments are recorded. Ni–SiO2 of all percentages showed a band in the range of 740 nm−1 while nickel nitrate aqueous solution and nickel nitrate showed respectively UV bands in the range of 728 and 707 nm−1.
21.4.3 BET Surface Area
BET surface area analysis of 10% Ni-silica (Cat A) was carried out using the Brunauer–Emmett–Teller equation. As a higher catalytic activity was observed with 10% Ni–SiO2 (Cat A) the surface area of only 10% Ni–SiO2 was estimated and was found to be 180 m2g−1.
21.4.4 Dispersion of Ni on Silica Surface
The gels with macropores have micro- and mesopores and show a high specific surface area. Thus, Ni–SiO2 with a distinct aggregated structure was prepared in this work. Incidentally, the wet gel contains nickel as a cation dissolved in the solvent phase. The solvent phase can move relatively fast in the wet silica gel, and aggregation of the nickel salt easily occurs during drying even in the gel without macropores [8]. In the gel with hierarchical pores, therefore, inhomogeneous aggregation probably occurs in the macropores during drying. In order to prepare a catalyst with high activity, it is necessary to disperse the nickel species only in the silica gel skeleton. For the sample prepared by impregnation on silica gel, diffraction peaks of NiO were observed. The SEM-EDX result clearly shows that the aggregation of large NiO particles occurs in the macropores. Because of sintering, the NiO aggregates grow in the large spaces during calcination at 500 °C especially for the sample with high NiO loading. The NiO aggregates in the macropores are easily reduced to large Ni particles. Despite the inhomogeneity of the Ni dispersion in the samples, they show relatively high Ni surface areas. As suggested from the XRD results, a part of the Ni in the samples is trapped in the mesopore of the gel skeleton, which cannot grow during heating and reduction due to the pore walls of the silica. The small Ni particles probably contribute to the high Ni surface area. Dispersion of the Ni species in both CG and SE samples without macropores can be regarded as standard in the nickel/silica prepared by the usual sol-gel process. In the samples, aggregation of Ni occurs during drying to become a crystal of nickel nitrate salt [15]. A wet silica gel prepared under acidic conditions consists of a polymeric network rather than particle aggregates. Therefore, the aggregation of the nickel nitrate salt can proceed even in the mesoscale space during drying. The NiO particle size agrees well with the correlation length of the wet silica gel, typically of the order of 10 nm [11, 12, 16].
21.4.5 SEM Characterization
The morphology and location of metallic species on the surface of the catalyst were examined by scanning electron microscopy (SEM) using a JEOL JSM-6100 microscope equipped with an energy-dispersive X-ray analyzer (EDX). Images were taken with an emission current of 100 μA by a tungsten filament and an accelerator voltage of 12 kV. The SEM figures of 5 and 10% Ni loaded silica and morphology (Fig. 21.2) depicted that crystalline Ni of 2–4 μm are well distributed over the silica surface and Fig. 21.2d showed the scans of 15% Ni loaded silica, which indicates that the distribution of Ni on support is either in conglomerates or in layers, thus hindering the participation of both Ni and silica at active sites in the reaction. SiO2 or Ni2+ individually had no catalytic effect on the reaction. The observed reactivity of Ni supported on silica material can be possibly attributed to the metal and support interactions, and the resultant changes in surface properties of the reactive sites. Earlier studies by Urbano et al. [15] have revealed that supported Ni catalysts prepared with Ni(NO3)2 by impregnation exhibit wide size distribution in contrast to those prepared by more controlled deposition and precipitation method route which generate low metallic Ni particles. The low metallic surface of nickel on the silica support encourages the formation of nickel oxide crystals. The presence of weak interaction between the metal and support, due to little distribution of Ni entailing the formation of the layer of impregnated Ni, probably contributed to the increase of catalytic activity [16,17,18]. The observed non-reactivity with 15% Ni loaded material, could be possibly due to the multilayer of Ni loading on the support resulting in loss of activity as suggested by Blanco et al. [14]. This is also supported by the SEM analysis of the different amounts of Ni loaded silica in the current study. An examination of SEM results (Fig. 21.2) suggest that the Ni particles are well distributed in 5 and 10% Ni loaded material with fine particles but the 15% loaded material shows Ni multilayers and conglomeration of Ni particles on the silica surface.
21.4.6 FT-IR Spectroscopy of Ni–SiO2 (Cat A)
As the results of both 10 and 5% Ni–SiO2 were similar, only the 10% Ni–SiO2 (Cat A) results are discussed. The examination of IR spectra of 5 and 10% Ni loaded SiO2 showed band at 1100 cm−1 (asymmetrical Si–O–Si) due to formation of silicates [12]. The vibrational stretching frequency of the hydrogen atom in the hydroxide catalysts, 5 and 10% Ni–SiO2 (Cat A) appears at 3422 cm−1 indicating it has ordered cation distribution [14,15,16]. In the IR results of 10% Ni–SiO2, the strong and intense absorption band between 1078 and 1050 cm−1 showed the presence of Si–O–Ni bonds. This Ni active species in association with silica is responsible for the activation of sodium borohydride with the evolution of hydrogen with immediate formation of nickel boride species. These results conclusively demonstrate that 10% Ni-silica catalyst is ideally suited for selective nickel boride silica initiated reduction of nitro aromatics to aromatic amines with good conversion and high selectivity [11, 12].
21.4.7 FT-IR Spectroscopy of Pd(II)-MCM-41 (Cat B)
The IR spectra of the ligand and the complex showed silylpropyl amine bands. The complex showed an additional band at 356 cm−1 indicating the presence of terminal Pd-Cl [12]. Pd content of the catalysts was determined by plasma analysis (1.99%).
21.4.8 Chemisorption of Ni Silica Catalysts
The absence of carbon monoxide adsorption when helium was used as the carrier was amazing in the light of the TPR experiments and a range of other physical techniques which indicated the presence of nickel metal. Catalysts were reduced in dihydrogen/dinitrogen conditions. The samples were heated to 723 K well above the maximum rate of hydrogen abstraction observed and the catalysts were held at this temperature until no further hydrogen uptake was observed. The reason for the absence of carbon monoxide adsorptive capacity was confirmed as the carbon dioxide chemisorption was due to the presence of surface oxygen [11, 12]. These results suggest that the removal of monolayer and sub-monolayer levels of oxygen from nickel catalysts is kinetically slow and that in the absence of a reducing atmosphere will block the surface to carbon monoxide adsorption. Interestingly the use of di-hydrogen as a carrier gas was sufficient to allow adsorption of carbon monoxide. No evidence was obtained to suggest that the di-hydrogen removed the surface oxygen at 298 K. Two explanations can be advanced although neither is entirely acceptable. If the oxygen was randomly distributed over the surface under helium and the action of di-hydrogen was to cause agglomeration into islands then a significant proportion of the adsorptive capacity could be reclaimed. Alternatively if the surface oxygen was to diffuse into the bulk or be re-dispersed throughout the crystallite, then adsorptive capacity would also be regained.
21.5 Conclusions
Ni–B–SiO2 (Cat A) are found to be super active catalysts compared to Pd(II)-MCM-41 (Cat B) for reduction reactions. Various functional groups attached to aromatic substrates were reduced with Ni B silica (Cat A) where nickel boride species are formed in situ when Ni is reacted with NaBH4 for reduction of aromatic substrates at low temperature with high conversion, and 100% selectivity. Characterization of Ni–SiO2 catalyst by XRD, FT-IR, UV diffusion, SEM, chemisorption studies, particle size and moisture content, confirms the crystallinity of nickel incorporated on silica. 15% nickel on silica is found to be inactive for reduction reactions from XRD and SEM studies which confirm that excess nickel reduces the activity of the catalysts. A variety of functional groups attached to aromatics such as p-nitrophenol, nitrobenzene, benzaldehyde, benzene, bromobenzene, phenol, 4-bromobenzaldehyde are reduced at 0–5 °C by 5 and 10% Ni–SiO2 to their respective products with maximum conversion and 100% selectivity while Pd(II)-MCM-41 (Cat B) reduces nitro aromatics at room temperature and aromatic alkyl halides, 4-bromobenzaldehyde, aromatic substituted azides such as benzyl azide to corresponding amines. The characterization of 5 and 10% Ni–SiO2 (Cat A) and the results demonstrate that they are super active catalysts compared to Pd(II)-MCM-41 (Cat B) for reduction reactions, in terms of reusability, atom economy, environmentally friendly and applicability for large scale reactions which excludes harsh reaction conditions and use of additives.
References
Sheldon RA (1996) Selective catalytic synthesis of fine chemicals: opportunities and trends. J Mol Catal A 107:75–83
Kresge CT, Leonowicz ME, Roth JW, Vartuli JC, Beck JS (1992) Ordered mesoporous molecular sieves synthesized by a liquid crystal template mechanism. Nature 359:710–712
Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CT-W, Olson DH, Sheppard EW, McCullen SB, Higgins JB, Schlenker JL (1992) A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc 114:10834–10843
Vartuli JC, Degnan TF Jr (2007) Applications of mesoporous molecular sieves in catalysis and separations. Stud Surf Sci Catal 168:837–854
Pinnavaia TJ (1983) Intercalated clay catalysts. Science 220:365–371
Arnold H, Dobert F, Gaube J, Ertl G, Knozinger H, Weitkempl J (eds) (1997) Handbook of heterogeneous catalysis, vol 5. Wiley, New York, pp 2165–2184
Blaser HU, Siegrist U, Steiner H, Studer M, Sheldon RA, van Bekkum H (eds) (2001) Fine chemicals through heterogeneous catalysis. Wiley, New York, pp 389–394
Saadi A, Merabti R, Rassoul Z, Bettahar MM (2006) Benzaldehyde hydrogenation over supported nickel catalysts. J Mol Catal A 253:79–85
Kockritz A, Sebek M, Dittmar A, Radnik J, Bruckner A, Bentrup U, Pohl M, Hugl H, Magerlein W (2006) Ru catalyzed oxidation of primary alcohols. J Mol Cat A 246:85–99
Bonelli B, Cozzolino M, Tesser R, Serio Di M, Piumrtti M, Garrone E, Santacesaria E (2007) Study of the surface acidity of TiO2/SiO2 catalysts by means of FTIR measurements of CO and NH3 adsorption. J Catal 246:293–300
Ferreira-Aparicio P, Rodriguez-Ramos I, Anderson JA, Guerrero-Ruiz A (2000) Mechanistic aspects of the dry reforming of methane over ruthenium catalysts. Appl Catal 202:183–196
Khodadadi B (2016) Nickel doping effect on the photo catalytic activity of TiO2/SiO2 nano-composite. Bulg Chem Commun 48:238–243
Mobley JK, Crocker M (2015) Catalytic oxidation of alcohols to carbonyl compounds over hydrotalcite and hydrotalcite-supported catalysts. RSC Adv 5:65780–65797
Blanco PH, Wu C, Williams PT (2014) Influence of Ni/SiO2 catalyst preparation methods on hydrogen production from the pyrolysis/reforming of refuse derived fuel. Int J Hydrogen Energy 39:5723–5732
Urbano FJ, Marinas JM (2001) Hydrogenolysis of organohalogen compounds over palladium supported catalysts. J Mol Catal A 173:329–345
Ermakova MA, Ermakov DY (2002) Ni/SiO2 and Fe/SiO2 catalysts for production of hydrogen and filamentous carbon via methane decomposition. Catal Today 77:225–235
Komiya S, Sone T, Usui Y, Hirano M, Fukuoka A (1996) Condensation reactions of benzaldehyde catalysed by gold alkoxides. Gold Bull 29:131–136
Solymosi F (1991) The bonding, structure and reactions of CO2 adsorbed on clean and promoted metal surfaces. J Mol Catal 65:337–358
Acknowledgement
This study was supported by the Environmental Investment Fund (EIF) of Namibia: Matching fund subsidy from National Commission on Research Science and Technology (NCRST) for strengthening capacity at universities and research institution in Namibia.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Rahman, A., Likius, D., Uahengo, V. (2018). Pd-MCM-41 and Ni–Boride–Silica Catalyst Synthesis, Characterization and Its Application for Reduction of Substituted Aromatics: An Environmentally Benevolent Approach. In: Ramasami, P., Gupta Bhowon, M., Jhaumeer Laulloo, S., Li Kam Wah, H. (eds) Emerging Trends in Chemical Sciences. ICPAC 2016. Springer, Cham. https://doi.org/10.1007/978-3-319-60408-4_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-60408-4_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-60407-7
Online ISBN: 978-3-319-60408-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)