Abstract
Histone post-translational modifications include methylation at N-terminal of histone tails. Such modifications play important roles in many biological processes through divergent transcription activities. Recently, aberrant histone modifications have been shown to contribute significantly towards many diseases, notably cancer. Here, we summarize the known drivers leading to misregulation of DNA and histone methylation in cancer, and current therapeutic options to counter these aberrations.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The advent of next-generation sequencing has substantially accelerated drug development towards targeted therapeutics. Early drug target discovery tends to focus on mutated kinases [1, 2]. For example, vemurafenib, a BRAF kinase inhibitor, is used to treat metastatic melanoma patients harboring BRAF V600E mutation [3]. Despite the profound anticancer effects of targeting activated kinase pathways, such benefit is often temporary in a subset of patients with advanced solid tumors [4]. The lack of durable response motivates the search for enzymes involved in other functional roles such as epigenetics [5] and metabolism [6] as alternative drug targets.
Epigenetic modifications occur both at DNA and histone proteins. The amino acid residues at the N-terminal histone tails are subjected to post-translational modification, such as methylation, acetylation, ubiquitination, phosphorylation and SUMOylation [7, 8]. Recent studies have shown that notably, misregulation at histone methylation leads to diseases including cancer. For example, enhancers showing gain or loss of H3 lysine 4 mono methylation (H3K4me1) can clearly distinguish between normal colon crypts and colorectal cancer [9]. Burgeoning research focusing on epigenetic alterations in cancer have identified a collection of genes involved in epigenetic programming with direct influence on chromatin structure and cell identity [10]. These findings strongly suggest that the transformation of healthy cells to cancer cells may be dependent on the underlying aberrant modifications at histone level. Therefore, identifying drivers to such aberrant transformation may provide new insights for therapeutics to treat cancer. In this chapter, we will summarize the known drivers of aberrant histone methylation in cancer, and current therapeutic strategies to counter aberrant histone methylation.
2 Regulators of Histone Methylation
Unlike the permanent DNA sequences, histone methylation is a highly dynamic process. It is constantly written and erased by histone modifying enzymes (Table 2). This dynamic process forms the basis of lineage specification during development [11], imprinting [12, 13] and disease state. The resulting histone code, containing “on” and “off” signals, is then interpreted by histone readers who can dock on the histone modifications and recruit other co-factors.
Histone can be methylated at lysine, arginine and rarely histidine. Well-characterized sites of lysine methylation include H3K4, H3K9, H3K20, H3K27, H3K36 and H3K79. Each lysine can exist as unmethylated (me0), monomethylated (me1) [14], dimethylated (me2) [15] and trimethylated (me3) [16]. Arginine residues are commonly modified at H3R2, H3R8, H3R17, and H4R3. Arginine residues exist as unmethylated (me0), monomethylated (me1) [17], symmetrically dimethylated (me2s) and asymmetrically dimethylated [18]. Symmetrically dimethylated arginines have a single methyl group on two different nitrogens whereas asymmetrically dimethylated arginines have two methyl group on a single nitrogen. It is estimated that approximately 2% of the arginine residues are methylated in total nuclear proteins of rat liver cells [19].
The different positions of lysine methylation can be associated with vastly divergent transcription activity. In general, H3K4me3 is associated with transcriptional activation. H3K4me3 is thought to be causal for transcriptional activation because TAF3, a subunit of the basal transcription factor TFIID, directly binds to the H3K4me3 [20]. TFIID mediates formation of preinitiation complex assembly for transcription [21]. In contrast, H3K27me3 is associated with transcriptional silencing [22]. The EZH2 subunit of the transcriptional repressor polycomb repressive complex (PRC) [23] catalyzes the methylation of H3K27 which in turn recruits PRC through the EED subunit and stimulates its methyltransferase activity in a positive feedback loop [24]. Similarly, H3K9me3 is also associated with transcriptional repression because it serves as the binding site for heterochromatin protein (HP1) which compacts the chromatin [25]. H3K36me3 is enriched in transcriptionally active regions as its level is sharply elevated after transcription start sites [26, 27]. However, in some other regions, such as the repressed Snurf–Snrpn locus, H3K36me3 is not associated with transcription activity but associated with heterochromatin [28]. H3K79 is associated with both silencing and active transcription. It regulates telomeric silencing and is thus tightly regulated during cell cycle [29]. At the same time, H3K79 methylation is associated with active transcription and correlates with euchromatin [30, 31], likely through inhibiting the non-specific binding of the repressive Sir proteins [32].
In terms of arginine residues, activating marks are H3R8me2a, H3R17me2a and H4R3me2a [33]. H4R3me2a deposited by arginine methyltransferase 1 (PRMT1) promotes transcriptional activation by enhancing lysine acetylation by P300 [33]. Another arginine methyltransferase member, Coactivator-associated arginine methyltransferase 1 (CARM1), enhances transcriptional activation by nuclear receptors [34] and it catalyzes the methylation of H3R2, H3R17 and H3R26 [35]. Repressive marks are H4R3me2s, H3R2me2s and H3R8me2s and H3R2me2a. Lysine methylation and arginine methylation can have antagonistic roles: H3R2me2a deposited by PRMT6 exerts its repressive role by abrogating H3K4 methylation by mixed lineage leukaemia (MLL) complex [36, 37]. Conversely, H3K4me3 also prevents H3R2 methylation by PRMT6 [36, 37].
Different histone modifications mark chromatin states. H3K4me3 marks are generally associated with promoters [38]; H3K4me1 with enhancers [38] and H3K36me3 with transcribed regions [22]. Chromatin states also vary with developmental stage. Embryonic stem (ES) cells have the most permissive chromatin state, but transition to a more restrictive state by gaining H3K27me3 as ES cells differentiate into embryoid bodies, to neural progenitors and finally to differentiated neurons [39]. Large scale chromatin maps showed that chromatin states defined by combination of histone modifications can distinguish between various cell types. In particular, enhancers mark, H3K4me1, is the most tissue-specific [39,40,41]. Hierarchical clustering using H3K4me1 can group cell types of similar origin, for example, haemotopoietic stem cells, B cells, T cells, monocytes fall under the same module [41]. Therefore, histone modifications mark cis-regulatory regions that contain lineage-specific genes. Finally, in cancer, clusters of enhancers called super-enhancers [42] or stretch enhancers [43], are located near key oncogenes, further illustrating the fact that histone modifications play integral roles to the regulation of key master regulators.
2.1 Writers
The majority of lysine methyltransferases contain the highly conserved SET domain. SET domains bind to donor S-Adenosyl-L-Methionine (SAM) and lysine substrate on opposite faces, and catalyze the transfer of methyl group through the methyltransfer pore [44]. Lysine methylation at multiple histone positions is performed by SET domain-containing histone methyltransferases. MLL family of proteins methylate H3K4 [45]. SUV39H1 [46] and G9a [47] can both methylate H3K9. EZH2 catalyzes the formation of H3K27me3 [48,49,50]. KMT3B/NSD1 primarily dimethylates H3K36 [51, 52] and SETD2 is responsible for forming nearly all transcription-dependent H3K36me3 [53]. Besides SET domain, another domain capable of methyltransferase activity is the DOT1 domain. DOT1L specifically mono-, di, and tri-methylates H3K79 [29], and is found at the proximal transcribed region of active genes [54].
For methylation of arginine residues, PRMTs use the same donor SAM as lysine methylation. PRMT1, a transcriptional activator, is the major enzyme catalyzing the active mark H4R3me2a which promotes subsequent histone acetylation by P300 recruitment [33, 55]. PRTM1 is a component of the MLL complex and PRTM1-MLL fusion promotes self-renewal of haematopoietic cells [56]. On the other hand, PRMT5, usually acts as a transcriptional repressor, symmetrically dimethylating H3 and H4 to produce H4R3me2s and H3R8me2s respectively [57]. Because PRMT5 can repress tumor suppressors including the RB family, it is generally considered as an oncogene [57].
2.2 Erasers
The most common domain found in histone demethylases is the JumonjiC (JmjC) domain. In the presence of co-factors Fe (II) and alpha-ketoglutarate (α-KG), JmjC domain undergoes oxidative demethylation reaction to produce hydroxylmethyl-lysine, succinate and CO2 [58]. Because of this dependency on α-KG, mutations affecting TCA cycle enzymes IDH1 and IDH2 can deplete a-KG and subsequently impair histone demethylation [59]. Stable transfection of mutant IDH resulted in progressive accumulation of histone methylation, including H3K9. KDM5A, KDM5B and KDM5C are responsible for erasing tri-, di- and monomethylation mark of H3K4 [60,61,62,63]. UTX and JMJD3 catalyze demethylation of H3K27me3/2 [64]. JHDM1 is the first JmjC-containing enzyme that has been shown to have demethylation activity and its substrate is H3K36 [58]. JHDM2A specifically demethylates mono- and dimethyl-H3K9, and its depletion led to H3K9 demethylation and transcriptional activation [65]. No known enzyme has been found to demethylase H3K79.
Another domain capable of histone demethylation is amine oxidase, present in KDM1A/LSD1. The LSD1 can specifically demethylate H3K4me1 and H3K4me2 in a FAD (flavin adenine dinucleotide)-dependent oxidative reaction [66]. LSD1 is unable to demethylate H3K4me3 because it requires a protonated nitrogen in the substrates [66]. Because LSD1 removes methylation from H3K4, it acts as a transcriptional repressor [66]. No demethylases have been found for arginine methylation.
2.3 Readers
The most common domain found in readers of methylated lysine is the plant homeodomain (PHD domain). First discovered in ING2 tumor suppressor, the PHD domain binds with increasingly affinity to methylated H3K4, with strongest association to H3K4me3, and no association with H3K4me0/1 [67]. It is thought that PHD domain contributes to the tumor suppressive role of ING2 by stabilizing histone deacetylase, mSin3a-HDAC1, at promoters of proliferation genes [67]. At the same time, it is also found that PHD domain is present in bromodomain and PHD domain transcription factor (BPTF), the largest subunit of nucleosome remodeling factor NURF, suggesting that NURF-mediated chromatin remodeling is directly coupled to H3K4me3 [68, 69].
However, not all PHD domains have similar affinity of histone methyl lysines. Unlike the PHD domain in ING2 and BPTF, PHD domain found in BHC80 binds to unmethylated H3K4. Interestingly, BHC80 influences LSD1 binding, and its depletion leads to de-repression of LSD1 target genes [70].
PHD domains are also present in many histone writers, so there are many histone writers that read. The MLL family of histone methyltransferases all contain multiple PHD domains whose functions are not identical. For instance, the second PHD domain of KMT2A and KMT2B shows E3 ubiquitin ligase activity whereas the third PHD domain binds with the highest affinity to H3K4me3 and less to H3K4me1/2 [71]. The recognition of its own mark suggests a positive feedback system of histone methylation.
In terms of histone methylarginine, the transcriptional activator, TDRD3, reads the active marks H3R17me2a and H4R3me2a using its Tudor domain [72]. Specifically, TDRD3 can distinguish between the asymmetrical, active mark H4R3me2a from the symmetrical, repressive mark H4R3me2s [72]. TDRD3 is a transcriptional co-activator that binds to H4R3me2a and H3R17me2a located upstream of transcriptional start sites.
3 Mechanism of Misregulation
Given the multi-faceted roles of histone modifiers in gene regulation, it is no surprise that cancer hijacks them via diverse mechanisms: mutations, gene rearrangements and misregulation of gene expression. The resulting change is often genome-wide, affecting multiple gene targets and pathways.
3.1 Mutations in the Catalytic Domains
Mutations in the catalytic domains of histone methyltransferases affect methylation differently. The most common mutation residing within the SETD domain of EZH2, Y646 (previously Y641), is a gain of the function mutation. EZH2 Y646 increases global H3K27me3 levels because it displays enhanced catalytic activity towards H3K27me2/3 whereas the wildtype EZH2 has the greatest affinity towards H3K27me0/1 substrate [73]. Although EZH2 Y646F causes global increases of H3K27me3, the gain of H3K27me3 is not monotonic across the genome, as some loci exhibit a loss of H3K27me3 and increased transcription [74]. As a result, EZH2 Y646F induces both repression and activation of polycomb target genes.
KMT2C/MLL3 is frequently inactivated in a number of different cancers by inactivating, truncating or even activating mutations [75,76,77,78,79,80]. The N4848S mutation leads to a loss of the catalytic activity of MLL3, similar to frame shifts or other inactivating mutations. In contrast, the Y4884C mutation of MLL3 is a gain of function mutation as it adopts a higher catalytic activity towards H3K4me1 than the wildtype MLL3, in a manner highly analogous to EZH2 Y646. Knockout of MLL2/3 globally decreases H3K4me1 and H3K4me2 levels in macrophages and HCT116 colon cancer cells [81]. Since MLL3 and MLL2 co-localize with markers of enhancers including H3K4me1, P300 binding and H3K27ac [81,82,83], perturbation of enhancer landscape by inactivating mutations of MLL2/3 could contribute to tumorigenesis.
Mutations can also target the catalytic domains of histone demethylases. The Jumonji-C domain-containing KDM6A/UTX (demethylase of H3K27me3) [11, 64] is a tumor suppressor frequently associated with inactivating mutations [84]. Ectopic expression of UTX leads to a strong decrease of H3K27me3 levels and delocalization of polycomb proteins [64, 84]. Conversely, knockdown of UTX by siRNA decreases its occupancy at promoters of polycomb target genes, HOXA13 and HOXC4, and brings about a concomitant increase in the levels of H3K27me2/3 at these promoters [85]. It remains to be elucidated what is the genome-wide effect of UTX depletion on H3K27me2/3 levels. Current evidence suggests that the loss of UTX may be a reciprocal mechanism to EZH2 gain, and both may lead to increasing and redistributing H3K27me3 mark, and deregulating the transcription of polycomb target genes.
Finally, chromatin readers can also be targeted by inactivating mutations that abrogate their binding to histone marks. Four mutations targeting ING1 (C215, N216S, V218I and G221V) are either located within or near the H3K4me3 binding site of the PHD finger [86]. The hotspot C215 mutation disrupts the three-dimensional structure of the PHD finger and abolishes interaction with H3K4me3 [86]. Similarly, the other three mutations all decrease the affinity of the PHD finger with H3K4me3.
3.2 Gene Arrangement
Gene arrangement involving MLL1 gene on chromosome band 11q23 occurs frequently in leukemia. The first form of gene rearrangement involves MLL gene fusions [87,88,89], which occur in the 8.3 kb breakpoint cluster region (BCR) of the MLL gene, between exons 8 and 12. The fusion forms include MLL-AF4, MLL-AF9, MLL-AF10, MLL-AF17, MLL-AF5q31, MLL-ENL. What is common amongst these fusion partners is that they all form stable complex with KMT4/DOT1L, a histone methyltransferase of H3K79 [90, 91]. MLL-AF9 binds to the promoters of target genes and induces H3K79me2 at the binding region. The increase in H3K79me2 induced by MLL-AF9 causes increased expression of direct targets including HoxA [92] which are important for hematopoiesis. In addition to H3K79me2, other active marks including H3K4me3, H3K36me3 are also concomitantly elevated and the repressive mark H3K27me3 is depleted [92]. Another important histone methyltransferase targeted by MLL-AF9 is LSD1 [93]. LSD1 sustains the leukemia stem cell potential of MLL-AF9 cells [93]. Knockdown of LSD1 increases the level of H3K4me2 at MLL-AF9 bound genes, suggesting that expression of MLL-AF9 target genes is dependent upon H3K4me2 [93].
The second form of gene rearrangement involves partial tandem duplication of MLL from exon 5 to 11/12 (MLL PTD) in the absence of a partner gene [94], found in 5–10% of patients with acute myeloid leukemia (AML). In AML patients, MLL PTD also co-occurs with the loss of MLL in the second allele [95]. MLL PTD displays activation of similar target genes as MLL fusions such as HoxA, but through increased methylation of H3K4 [96].
Another gene fusion involves the chromatin reader, plant homeodomain (PHD) finger 23 (PHF23) with nucleoporin 98-KDa (NUP98) [97]. NUP98 fuses with either HOX or non-HOX partners. Interestingly, NUP98-PHF23 can also achieve activation of HOX, but through binding to H3K4me3 regions spanning HOX genes. The binding of NUP98-PHF23 to H3K4me3 is highly specific, occupying only 1.6% of total H3K4me3 regions, but it remains unknown how such specificity is achieved [98]. In addition to fusing with the chromatin reader PHF23, NUP98 can also fuse with NSD1, the methyltransferase of H3K36 in AML [99]. In a manner similar to the MLL fusion and other NUP98 fusions, NUP98–NSD1 activates HoxA and Meis proto-oncogenes by recruiting p300 (histone acetyl transferase) and suppression of EZH2 [100].
3.3 Gene Deregulation
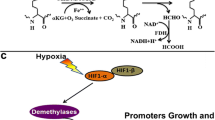
Overexpression of EZH2 is an alternative but analogous mechanism to inactivating mutations. While mutations of EZH2 mainly occurs in hematopoietic cancers including diffuse large B-cell lymphoma and follicular lymphoma, EZH2 overexpression can occur in a variety of solid cancers including prostate, breast, gastric, bladder, kidney, liver and ovarian [101,102,103,104,105]. Multiple transcription factors can stimulate EZH2 overexpression. MYC binds to EZH2 promoter and directly activates its transcription [106]. Other transcription factors that cause EZH2 overexpression include E2F [107], EWS-FLI1 fusion [108], SOX4 [109], and HIF1α [110].
Transcriptional upregulation of MLL1 and MLL2 can be induced by gain of function (GOF) p53 mutants. R273H p53 mutant, but not wildtype p53, binds at the promoter of MLL1 and MLL2 [111]. GOF p53 results in slight elevation in the global levels of H3K4me3, including regions around the hoxa gene cluster [111]. The oncogenic role of GOF p53 mutant is dependent on MLL1 expression [111].
Another example of overexpressed histone reader is PRMT5, found in leukemia, lymphoma [57], lung, gastric cancer [112] and glioblastoma [113]. PRMT5 can be directly upregulated by MYC [114] which physically associates with PRMT5 and stimulate H4R4me2s [115]. This implies that gene misregulation of chromatin modifiers often stem from mutations in classic oncogenes or tumor suppressors.
4 How do Changes in Histone Methylation Lead to Oncogenesis?
4.1 Activation of Developmental Master Regulators
Various mutational changes and gene rearrangements converge to activate developmentally important master regulators. Often expressed in progenitor cells, these factors are essential for maintaining stemness during development but are turned off in differentiated cells. However, deregulated histone modifiers often re-activate these developmental regulators, thus contributing to tumorigenesis.
In vivo mouse model shows that Hoxa9 can collaborate with meis1a to induce AML in less than 3 months [116]. DOT1L induced by MLL fusion is targeted to hoxa9 [91], and the presence of DOT1L resulted in enhanced H3K79me2 at HoxA clusters and Meis1 [92]. Another translocation mentioned earlier, NUP98-PHF23 fusion, also caused overexpression of Hoxa and Hoxb cluster. AML-derived myeloblastic cells with NUP98-PHF23 demonstrate both enrichment of H3K4me3 and depletion of H3K27me3 across the Hoxa, Hoxb and Meis loci [98]. Similarly, EZH2 Y641F causes a re-distribution of H3K27me3. Even though the global level of H3K27me3 is elevated, this repressive mark is depleted from Hoxc cluster and Meis1 which are densely covered with H3K27me3 in normal B cells [74]. The liberation of the repressive H3K27me3 from developmental regulators causes their overexpression and contributes to tumorigenesis.
4.2 Suppression of Tumor Suppressors
The redistribution of histone mark can simultaneously activate oncogenes and repress tumor suppressors. The tumor suppressor Ink4a/Arf locus is epigenetically silenced in leukemia-initiating cells by strong enrichment of H3K27me3 [117]. Ezh2 knockout decreases H3K27me3 at Ink4a-Arf locus, implying that Ezh2 is required to maintain H3K27me3 and repression of the Ink4a/Arf locus [118].
Another important group of tumor suppressors inactivated by histone methylation is the retinoblastoma protein (RB family). PRMT5 recruitment to the promoters of RB, RBL1 and RBL2 is increased 3–4.7-fold, 4–9.5-fold and 3–5.2-fold respectively in transformed lymphoid cell lines compared to that of normal B cells [57]. The increase in PRMT5 recruitment results in corresponding enrichment of the repressive marks, H3R8me2s and H4R3me2s, that suppress the mRNA levels of the RB tumor suppressor genes [57].
An example of a histone demethylase that contributes to suppression of tumor suppressor is the H3K4 demethylase KDM5B/PLU-1. Its recruitment and resulting depletion of H3K4me3 mark represses tumor suppressors including BRCA1, therefore its overexpression can contribute to breast cancer cell proliferation [63].
4.3 Splicing Defects
Besides marking transcriptionally active regions, H3K36me3 also plays an important role in safeguarding splicing fidelity. H3K36me3 recruits polypyrimidine tract–binding protein (PTB) which results in exon silencing [119]. Truncating mutations of SETD2 in ccRCC result in a global loss of H3K36me3 [120]. H3K36me3-deficient ccRCCs display a drastic increase in intron retention, affecting 95% of the transcripts [120]. Other defects in exon utilization, start and termination site usage were also observed [120]. The most affected genes include tumor suppressors, genes in the DNA repair pathway and cell cycle regulators [120].
4.4 Genomic Instability
Histone modifications may also influence genomic instability even though the exact mechanism is not completely well understood. SETD2 loss has also been associated with genomic instability. Even though the main cause of genomic instability due to SETD2 depletion may be a result of decreased methylation in microtubules, a non-histone substrate [121], it is also observed that chromosomal breakpoints are located away from H3K36me3 [122]. SETD2 loss decreases nucleosomal occupancy and increases sensitivity to micrococcal nuclease, suggesting that SETD2 plays a role in maintaining nucleosome stabilization and coordination of DNA repair [122].
Another histone modifying enzyme that safeguards genomic stability is MLL2. Tumor cells with MLL2 knockout had higher levels of sister chromatid exchange compared with the two control cell lines [123]. Deletion of SET domain alone mimics the genomic instability seen in MLL2 knockouts, indicating that the catalytic domain is necessary for maintaining genomic stability [123]. However intriguingly, MLL2 deficient cells do not display differential H3K4 levels compared to MLL2 wildtype at mutated genes since MLL2 mutation predominantly affects H3K4 methylation at enhancers [123]. Therefore, the connection between histone methylation and genomic instability remains to be elucidated.
5 Histone Methyltransferase/Demethylase Inhibitors as Treatment in Cancer
Unlike genetic abnormalities, epigenetic alteration are reversible, enabling restoration of original function in cells showing disease phenotypes without altering the DNA sequences [124, 125]. Taken together, such findings has fueled immense interest in using chromatin-associated proteins as anticancer drug targets.
Indeed, several epigenetic inhibitors have been approved by the Food and Drug Administration (FDA). These approved drugs include azacitidine (5-azacytidine) and decitabine (5-aza-2′-deoxycytidine) as DNA methyltransferase (DNMT) inhibitors; suberoylanilide hydroxamic acid (SAHA) romidepsin (depsipeptide or FK228) as histone deacetylase (HDAC) inhibitors. On the other hand, since inhibitors of lysine methyltransferases (KMT) and demethylases (KDM) have been recently discovered, many of them are still in (pre-) clinical development (see Table 1 for a list of inhibitors and their drug development stage). Interestingly, the utility of such inhibitors in academic research demonstrated promising results in treating cancer with KMT/KDM inhibitors. Given the increasing importance of these compounds in cancer, pharmaceutical companies strive to develop more epigenetic drugs through collaborative discovery and development. Recently, Merck Sharp & Dohme (MSD) initiated collaboration with Cancer Research Technology to develop a portfolio of inhibitors of protein arginine methyltransferase 5 (PRMT5) for treatment of blood cancers. Other pharmaceutical companies developing PRMT inhibitors include Epizyme and GlaxoSmithKline [157]. Business acquisition of EpiTherapeutics by Gilead Sciences, and Quanticel Pharmaceuticals by Celgene Corporations further suggest the immense interest of pharmaceutical giants in epigenetic drugs. In essence, inhibitors targeting histone methylases/demethylases may render a previously-untapped reservoir of cancer therapeutic interventions.
Here, we highlight the clinical development of selected pharmacological inhibitors targeting histone methyltransferases and demethylases. Anti-tumour effects followed by treatment with inhibitors are also briefly discussed.
5.1 EZH2 Inhibitors
As mentioned in the previous section, EZH2 is a histone-lysine N-methyltransferase enzyme that methylates H3K27, and thus has repressive effect on gene expression. Studies have shown that EZH2 overexpression associates with cancer development and poor prognosis in human cancer, including lymphoma, breast, prostate, kidney and lung [77, 158,159,160,161]. Therefore, inhibiting EZH2 could be an important therapeutic intervention.
Recent studies have revealed an array of small molecule inhibitors targeting EZH2 (Table 2). Amongst these inhibitors, 3-Deazaneplanocin A (DZNep) was previously reported as a SAH-hydrolase inhibitor, acting as an indirect EZH2 inhibitor [188, 189]. It is also a derivative of the antibiotic neplanocin-A. Despite indirect inhibition, DZNep was shown to induce apoptosis in cancer by reactivating PRC2 target genes [188].
There are also inhibitors imposing direct inhibition on EZH2, such as GSK126 and EPZ005687 leading to global decrease of H3K27me3 and also reactivation of silenced PRC2 target genes in haematological cancers, including diffuse large B-cell lymphoma (DLBCL) [134] and non-Hodgkin’s lymphoma [190]. Since activating mutations in EZH2 were reported in DLBCL and follicular lymphoma [75, 77, 191,192,193], inhibitors were designed to be specific to EZH2 mutants. Specifically, GSK126 (GlaxoSmithKline) effectively inhibited the proliferation of EZH2 mutant DLBCL cell lines as well as xenografts in mice [134]. EPZ005687 (Epizyme) enables apoptotic cell killing in heterozygous Y641 or A677 mutant cells with non-Hodgkin’s lymphoma [136]. Treatment with UNC1999 also selectively killed DLBCL cell lines harboring the EZH2Y641N mutant [139]. EI1 (Novartis) showed reduced proliferation, cell cycle arrest and apoptosis in DLBCL cells carrying the Y641 mutations.
Unlike DLBCL and NHL, EZH2 is often overexpressed but not mutated in solid tumors. Inhibition of EZH2 in solid tumors has not been studied as extensively as in haematological cancers. This raises an important problem whether existing EZH2 inhibitors are able to inhibit the expression carrying wild type EZH2. To address this concern, a previous study conducted on three-dimensional culture of epithelial ovarian cancer shows that the tumor culture is sensitive to EZH2 methyltransferase inhibition by GSK343 [135]. The inhibition results in suppression of cell growth and invasion, and induction of apoptosis. Additionally, treatment of non-small cell lung carcinoma with genetically engineered mouse models using JQEZ5 promotes regression of these tumors [140], and EPZ-6438 treatment in malignant rhabdoid tumors with mutated SMARCB1 caused apoptosis and differentiation [137]. In general, most of the EZH2 inhibitors show effect in cancer cells with mutant and wild type EZH2, but with a few exceptions. For example, EPZ005687 is effective in targeting cells carrying EZH2 mutant, but its effect on the proliferation of NHL cells with wild type EZH2 is minimal [136]. It is also worth noting that the kinetics of H3K27me3 turnover is slow, therefore prolonged EZH2 inhibition for several days is required to reduce tri-methylation of H3 lysine 27 and alter the transcriptional program in cancer cells [194].
5.2 DOT1L Inhibitors
As discussed in the previous section, misregulation of DOT1L, a H3K79 methyltransferase, may serve as a potential oncogenic driver in leukaemia with MLL-fusion proteins [91]. Therefore, pharmacological inhibition of DOT1L may treat patients suffering from leukaemia.
Most DOTL1 inhibitors are SAM competitive inhibitors. Analysis of structure-activity relationships and co-crystal structures design principles using S-Adenosyl-L-Homocysteine (SAH), the demethylated form of SAM, [195] is used to identify small molecules targeting DOT1L catalytic activity. The first compound, EPZ004777, is a potent and highly selective DOT1L aminonucleoside inhibitor [146]. It competes with the universal methyl donor for binding to the DOT1L’s active site. Previous studies showed that the compound exhibited cell-killing effect in murine myeloid progenitors and human AML cells harbouring MLL rearrangement [145,146,147, 196, 197]. The treatment with EPZ004777 led to dosage-dependent global depletion of H3K79 methylation [146]. However, such response to the inhibitor was only observed in primary AML cells with both wild type MLL and IDH1/IDH2 mutations [198]. Primary AML cells with wild type MLL alone demonstrated limited antitumor effect [145,146,147, 196, 197].
EPZ-5676, an alternative DOT1L inhibitor with improved pharmacokinetics was demonstrated to activate apoptosis in MLL-translocated leukemia cell lines in a time- and dosage-dependent manner. Continuous infusion is necessary to achieve maximal efficacy. For example, complete regression of MV4-11 subcutaneous xenograft tumors in rats was observed after 21 days of continuous infusion of EPZ-5676. Similar to EPZ004777, treatment with the compound is also associated with depletion of H3K79me2 in the tumor [145]. Currently, EPZ-5676 is at phase I clinical trial targeting AML patients with MLL-rearrangement (http://clinicaltrials.gov).
Taken together, the results showed that the aminonucleoside DOT1L inhibitors show favorable pre-clinical outcome, including improved survival in rats (MV4-11 subcutaneous xenograft model) and treatment response. Such achievement should motivate more drug development and optimization to address some of the limitations including insignificant oral bioavailability [199].
5.3 PRMT Inhibitors
Protein arginine methyltransferases (PRMT) have been identified as coactivators for nuclear hormone receptors [34, 200]. Misregulation of PRMT is associated with development in multiple diseases, notably cancer. Specifically, PRMT was found to be overexpressed in a wide range of cancers, including breast, prostate, lung, bladder and leukaemia [201]. Furthermore, aberrant activation of different PRMT isoforms, which have distinct functional role were also implicated in cancer [202,203,204]. Taken together, these findings motivated drug discovery effort in identifying lead compounds targeting specifically for one particular isoform enzyme.
A series of compounds were identified as PRMT1-specific inhibitors using virtual and structure-based screening. AMI-1 and AMI-8 are among the earliest small molecule inhibitors [148], followed by allantodapsone [149]. A newer inhibitor of PRMT1, compound 6 [150], showed improvement in the selectivity profile since it is inactive to CARM1 and SET7/9. Compound 6 showed growth inhibition of breast cancer cell line MCF-7a and prostate cancer cell line LNCaP [205]. It also showed significant reduction in androgen-dependent transcription [205]. Treatment with other compounds, such as DCLX069 and DCLX078, demonstrated reduced proliferation rate by 40% in HepG2, MCF7 and leukemic monocyte cell line THP1 [151]. MHI-21 (compound 11) treatment on cervical cancer cell line HeLa caused cell arrest in the S-phase and led to cell growth inhibition [152]. Another compound E84 was tested for cellular activity in three different hemotological cancer cell lines: Meg01 (chronic myelogenous leukaemia), MOLM13 (AML) and HEL (erythroleukemia) [153]. Notably, the compound repressed cell proliferation and associated with depletion in global cellular methylation after 24 h treatment. The methylation-depleting effect is significant at 100 nM treatment for Meg01 and MOLM13 cells. Stilbamidine (compound 13) shows better activity than AMI-1 on reducing the transcription activation of an estrogen-dependent gene in MCF-7-2a cells [149]. In short, these drug treatments in vitro delivered promising results, but more studies are needed to bring these compounds further to clinical testing.
Overexpression of PRMT4/CARM1 was reported in hormone-dependent cancer [206, 207]. For example, CARM1 expression associates with androgen-dependent transcription in prostate carcinoma. It also promotes tumour progression in androgen-independent prostate cancer [208]. Besides prostate cancer, elevated CARM1 expression is also linked to high-grade breast cancer tumours. Interestingly, inhibition of estrogen-dependent transcription, cell cycle progression and cancer cell growth were observed in breast cancer cells with CARM1 mutant [200, 209]. Taken these findings together suggest CARM1 as a novel anti-cancer drug target. In this regard, several pharmacological inhibitors, such as compounds by Methylgene [156], 17b by Bristol-Myers Squibb [155, 210] and 7g [211] were synthesized. The compound, 7g was tested in the prostate cancer cell line LNCaP, and showed a significant reduction of the prostate-specific antigen promoter activity in a dose-dependent manner. However, such treatment did not affect cancer cell viability [211].
5.4 LSD1/2 Inhibitors
Overexpression of LSD1, histone demethylase of H3K4me1/2 and H3K9me1/2 is oncogenic in several cancers including leukaemia [93], colon [212], breast [213], prostate [214] and liver [215]. For example, high expression of LSD1 was linked to activation of epithelial-mesenchymal transition (EMT) and cancer progression in estrogen receptor-negative tumors [213]. On the contrary, depleting LSD1 expression using small interfering (si) RNAs led to the suppression of proliferation in various cancer cell lines [172]. Taken together, the LSD1 expression is important to tumorigenesis, thus making it as an attractive target for therapeutic intervention.
Tranylcypromine (TCP) is one of the firstly discovered KDM inhibitors. Interestingly, it is initially used clinically to treat depression. Mechanistically, it is an unselective compound that acts as an inhibitor of monoaminooxidase, and bonds to the cofactor of FAD at the C-terminal end of LSD1 [216]. Although the treatment with TCP showed anticancer effect in a mouse model [93], it also caused side-effects, such as dizziness, drowsiness [217, 218] and drug-induced anaemia in mice [93]. In light of these limitations, a more potent drug is desired. Treatment with ORY-1001, a potent and selective LSD1 inhibitor designed by Oryzon, demonstrated the accumulation of H3K4me2 at LSD1 target genes in a time- and dosage-dependent manner. It also activates gene expression involving in differentiation in THP-1 cells with MLL translocation (MLL-AF9). ORY-1001 treatment also shows reduced tumor growth in rodent MV (4;11) xenograft [219]. Its rival, GlaxoSmithKline, also reported a selective irreversible LSD1 inhibitor, GSK2879552, which is in a phase I study in AML and in small cell lung cancer. The compound promotes differentiation in AML cells. Treatment in SCLC and AML cells demonstrated a potent anti-proliferative growth effect and favourable clinical outcome in mouse models [220]. Besides the irreversible inhibitor GSK2879552, GlaxoSmithKline also developed a reversible LSD1 inhibitor, GSK690. Favorable clinical outcome after treatment could be attributed to the underlying changes in epigenomic landscape in tumors, both locally or globally. For example, a previous study showed that an elevated enrichment of H3K4me2 at gene promoters is associated with myeloid differentiation after inhibiting LSD1 in AML [132]. Another study also demonstrated a global increase of H3K4me2 and growth inhibition in breast cancer cells overexpressing LSD1 after treating with pharmacologic inhibitors targeting amine oxidases [213]. Besides targeting the enzyme directly to repress demethylation activity at H3K4, similar effect can be achieved through downregulating LSD1 expression by inhibiting Sp1 with pan-HDAC inhibitor (HDI) treatment [221].
Despite promising therapeutics effect, these preclinical studies largely focused on the treatment in haematological cancer. More studies using solid tumor samples should be conducted in future to attest the benefit of LSD1 inhibitors in a wider spectrum of cancer. Given the dual capability of LSD1 in activating and repressing different sets of gene through modification of H3K4 and H3K9, the design of therapeutic strategies for targeting LSD1 should account for its multifaceted actions.
JMJC demethylases are another class of lysine demethylase. However, unlike LSD1 inhibitors, clinical candidates targeting JMJC domain-containing demethylases are still lacking. The drug development process is hindered by two factors: (1) high structural similarity of JMJC members, thus causing poor selectivity, and (2) poor cellular permeability of the inhibitors. To address this concern, selective pharmacological intervention across the JMJ family has been achieved by designing small-molecule inhibitors [141]. For example, EPT-103182 (EpiTherapeutics) targeting KDM5B/JARID1B showed anti-proliferative growth effect in cancer lines as well as in xenograft model [131]. GlaxoSmithKline also reported another compound, GSKJ1/4 targeting KDM6 [141]. It induced cell death and caused loss of self-renewal and tumor-initiating capacity in ovarian cancer cell lines [142]. Studies to date have covered only a subset of lysine demethylases. Other lysine demethylases such H3K79 demethylase remain unknown, which might be distinct from existing classes of histone demethylase [222].
6 Application of Combined Epigenetic Therapies with Other Cancer Treatments
In the previous section, we have discussed the application of single agent alone leading to antitumor effect. However, combining epigenetic therapies with other cancer treatments has become an emerging trend. Recent reports have demonstrated that combining epigenetic therapies with other treatment exerts synergistic activity and yields significantly improved clinical outcome in AML. For example, the engraftment of primary AML cells in vivo in the NOD/SCID-IL-2receptor-γ-deficient (NSG) mice diminished after co-treatment with the LSD1 inhibitor tranylcypromine (TCP) and all-trans-retinoic acid [132]. Interestingly, a recent study using the combined therapy using a very potent LSD1 inhibitor (SP2509) and a pan-HDAC inhibitor (panobinostat) yielded synergistic lethal effect against cultured and primary AML [223]. Such co-treatment also demonstrated more superior survival outcome in mice engrafted with the human AML cells. There is also evidence that EPZ-5676, a DOT1L inhibitor shows synergistic anti-proliferative activity with standard agents (cytarabine and daunorubicin) in the treatment of patients with AML [224]. Combined therapies with all-trans retinoic acid (ATRA) differentiation therapy with KDM1A inhibition also show potent anti-leukemic effect [132]. Such combination could sensitize otherwise ATRA-insensitive cells towards differentiation. In summary, these findings highlight the importance and potential application of combined therapies with standard cancer treatments.
References
Vogelstein B et al (2013) Cancer genome landscapes. Science 339(6127):1546–1558
Torkamani A, Verkhivker G, Schork NJ (2009) Cancer driver mutations in protein kinase genes. Cancer Lett 281(2):117–127
Chapman PB et al (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364(26):2507–2516
Misale S et al (2012) Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486(7404):532–536
Arrowsmith CH et al (2012) Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov 11(5):384–400
Furuhashi M, Hotamisligil GS (2008) Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7(6):489–503
Berger SL (2007) The complex language of chromatin regulation during transcription. Nature 447(7143):407–412
Kouzarides T (2007) Chromatin modifications and their function. Cell 128(4):693–705
Akhtar-Zaidi B et al (2012) Epigenomic enhancer profiling defines a signature of colon cancer. Science 336(6082):736–739
Wutz A (2013) Epigenetic regulation of stem cells: the role of chromatin in cell differentiation. Adv Exp Med Biol 786:307–328
Lan F et al (2007) A histone H3 lysine 27 demethylase regulates animal posterior development. Nature 449(7163):689–694
Lewis A et al (2004) Imprinting on distal chromosome 7 in the placenta involves repressive histone methylation independent of DNA methylation. Nat Genet 36(12):1291–1295
Umlauf D et al (2004) Imprinting along the Kcnq1 domain on mouse chromosome 7 involves repressive histone methylation and recruitment of Polycomb group complexes. Nat Genet 36(12):1296–1300
Murray K (1964) The occurrence of epsilon-N-methyl lysine in histones. Biochemistry 3:10–15
Woon Ki Paik SK (1967) Epsilon-N-dimethyllysine in histones. Biochem Biophys Res Commun 27(4):479–483
Hempel K, Lange HW, Birkofer L (1968) Epsilon-N-trimethyllysine, a new amino acid in histones. Naturwissenschaften 55(1):37
Byvoet P et al (1972) The distribution and turnover of labeled methyl groups in histone fractions of cultured mammalian cells. Arch Biochem Biophys 148(2):558–567
Borun TW, Pearson D, Paik WK (1972) Studies of histone methylation during the HeLa S-3 cell cycle. J Biol Chem 247(13):4288–4298
Boffa LC et al (1977) Distribution of NG, NG,-dimethylarginine in nuclear protein fractions. Biochem Biophys Res Commun 74(3):969–976
Lauberth SM et al (2013) H3K4me3 interactions with TAF3 regulate preinitiation complex assembly and selective gene activation. Cell 152(5):1021–1036
Buratowski S et al (1989) Five intermediate complexes in transcription initiation by RNA polymerase II. Cell 56(4):549–561
Mikkelsen TS et al (2007) Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448(7153):553–560
Boyer LA et al (2006) Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441(7091):349–353
Margueron R et al (2009) Role of the polycomb protein EED in the propagation of repressive histone marks. Nature 461(7265):762–767
Bannister AJ et al (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410(6824):120–124
Bannister AJ et al (2005) Spatial distribution of di- and tri-methyl lysine 36 of histone H3 at active genes. J Biol Chem 280(18):17732–17736
Barski A et al (2007) High-resolution profiling of histone methylations in the human genome. Cell 129(4):823–837
Chantalat S et al (2011) Histone H3 trimethylation at lysine 36 is associated with constitutive and facultative heterochromatin. Genome Res 21(9):1426–1437
Feng Q et al (2002) Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr Biol 12(12):1052–1058
Ng HH et al (2003) Lysine-79 of histone H3 is hypomethylated at silenced loci in yeast and mammalian cells: a potential mechanism for position-effect variegation. Proc Natl Acad Sci U S A 100(4):1820–1825
van Welsem T et al (2008) Synthetic lethal screens identify gene silencing processes in yeast and implicate the acetylated amino terminus of Sir3 in recognition of the nucleosome core. Mol Cell Biol 28(11):3861–3872
van Leeuwen F, Gafken PR, Gottschling DE (2002) Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell 109(6):745–756
Wang H et al (2001) Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science 293(5531):853–857
Chen D et al (1999) Regulation of transcription by a protein methyltransferase. Science 284(5423):2174–2177
Schurter BT et al (2001) Methylation of histone H3 by coactivator-associated arginine methyltransferase 1. Biochemistry 40(19):5747–5756
Guccione E et al (2007) Methylation of histone H3R2 by PRMT6 and H3K4 by an MLL complex are mutually exclusive. Nature 449(7164):933–937
Kirmizis A et al (2007) Arginine methylation at histone H3R2 controls deposition of H3K4 trimethylation. Nature 449(7164):928–932
Heintzman ND et al (2007) Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39(3):311–318
Zhu J et al (2013) Genome-wide chromatin state transitions associated with developmental and environmental cues. Cell 152(3):642–654
Ernst J et al (2011) Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473(7345):43–49
Roadmap Epigenomics Consortium et al (2015) Integrative analysis of 111 reference human epigenomes. Nature 518(7539):317–330
Whyte WA et al (2013) Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153(2):307–319
Parker SC et al (2013) Chromatin stretch enhancer states drive cell-specific gene regulation and harbor human disease risk variants. Proc Natl Acad Sci U S A 110(44):17921–17926
Trievel RC et al (2002) Structure and catalytic mechanism of a SET domain protein methyltransferase. Cell 111(1):91–103
Milne TA et al (2002) MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell 10(5):1107–1117
Rea S et al (2000) Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406(6796):593–599
Tachibana M et al (2002) G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev 16(14):1779–1791
Kuzmichev A et al (2002) Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev 16(22):2893–2905
Czermin B et al (2002) Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111(2):185–196
Muller J et al (2002) Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111(2):197–208
Rayasam GV et al (2003) NSD1 is essential for early post-implantation development and has a catalytically active SET domain. EMBO J 22(12):3153–3163
Qiao Q et al (2011) The structure of NSD1 reveals an autoregulatory mechanism underlying histone H3K36 methylation. J Biol Chem 286(10):8361–8368
Edmunds JW, Mahadevan LC, Clayton AL (2008) Dynamic histone H3 methylation during gene induction: HYPB/Setd2 mediates all H3K36 trimethylation. EMBO J 27(2):406–420
Steger DJ et al (2008) DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. Mol Cell Biol 28(8):2825–2839
Huang S, Litt M, Felsenfeld G (2005) Methylation of histone H4 by arginine methyltransferase PRMT1 is essential in vivo for many subsequent histone modifications. Genes Dev 19(16):1885–1893
Cheung N et al (2007) Protein arginine-methyltransferase-dependent oncogenesis. Nat Cell Biol 9(10):1208–1215
Wang L, Pal S, Sif S (2008) Protein arginine methyltransferase 5 suppresses the transcription of the RB family of tumor suppressors in leukemia and lymphoma cells. Mol Cell Biol 28(20):6262–6277
Tsukada Y et al (2006) Histone demethylation by a family of JmjC domain-containing proteins. Nature 439(7078):811–816
Lu C et al (2012) IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483(7390):474–478
Christensen J et al (2007) RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell 128(6):1063–1076
Iwase S et al (2007) The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell 128(6):1077–1088
Klose RJ et al (2007) The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell 128(5):889–900
Yamane K et al (2007) PLU-1 is an H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol Cell 25(6):801–812
Agger K et al (2007) UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 449(7163):731–734
Yamane K et al (2006) JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell 125(3):483–495
Shi Y et al (2004) Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119(7):941–953
Shi X et al (2006) ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature 442(7098):96–99
Wysocka J et al (2006) A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature 442(7098):86–90
Li H et al (2006) Molecular basis for site-specific read-out of histone H3K4me3 by the BPTF PHD finger of NURF. Nature 442(7098):91–95
Lan F et al (2007) Recognition of unmethylated histone H3 lysine 4 links BHC80 to LSD1-mediated gene repression. Nature 448(7154):718–722
Wang Z et al (2010) Pro isomerization in MLL1 PHD3-bromo cassette connects H3K4me readout to CyP33 and HDAC-mediated repression. Cell 141(7):1183–1194
Yang Y et al (2010) TDRD3 is an effector molecule for arginine-methylated histone marks. Mol Cell 40(6):1016–1023
Sneeringer CJ et al (2010) Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas. Proc Natl Acad Sci U S A 107(49):20980–20985
Souroullas GP et al (2016) An oncogenic Ezh2 mutation induces tumors through global redistribution of histone 3 lysine 27 trimethylation. Nat Med 22(6):632–640
Pasqualucci L et al (2011) Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet 43(9):830–837
Parsons DW et al (2011) The genetic landscape of the childhood cancer medulloblastoma. Science 331(6016):435–439
Morin RD et al (2011) Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 476(7360):298–303
Zang ZJ et al (2012) Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet 44(5):570–574
Fujimoto A et al (2012) Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet 44(7):760–764
Gui Y et al (2011) Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet 43(9):875–878
Hu D et al (2013) The MLL3/MLL4 branches of the COMPASS family function as major histone H3K4 monomethylases at enhancers. Mol Cell Biol 33(23):4745–4754
Lee JE et al (2013) H3K4 mono- and di-methyltransferase MLL4 is required for enhancer activation during cell differentiation. eLife 2:e01503
Kaikkonen MU et al (2013) Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell 51(3):310–325
van Haaften G et al (2009) Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet 41(5):521–523
Lee MG et al (2007) Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science 318(5849):447–450
Pena PV et al (2008) Histone H3K4me3 binding is required for the DNA repair and apoptotic activities of ING1 tumor suppressor. J Mol Biol 380(2):303–312
Djabali M et al (1992) A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nat Genet 2(2):113–118
Gu Y et al (1992) The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell 71(4):701–708
Tkachuk DC, Kohler S, Cleary ML (1992) Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell 71(4):691–700
Mohan M et al (2010) Linking H3K79 trimethylation to Wnt signaling through a novel Dot1-containing complex (DotCom). Genes Dev 24(6):574–589
Okada Y et al (2005) hDOT1L links histone methylation to leukemogenesis. Cell 121(2):167–178
Bernt KM et al (2011) MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell 20(1):66–78
Harris WJ et al (2012) The histone demethylase KDM1A sustains the oncogenic potential of MLL-AF9 leukemia stem cells. Cancer Cell 21(4):473–487
Caligiuri MA et al (1994) Molecular rearrangement of the ALL-1 gene in acute myeloid leukemia without cytogenetic evidence of 11q23 chromosomal translocations. Cancer Res 54(2):370–373
Whitman SP et al (2005) The MLL partial tandem duplication: evidence for recessive gain-of-function in acute myeloid leukemia identifies a novel patient subgroup for molecular-targeted therapy. Blood 106(1):345–352
Dorrance AM et al (2008) The Mll partial tandem duplication: differential, tissue-specific activity in the presence or absence of the wild-type allele. Blood 112(6):2508–2511
Reader JC et al (2007) A novel NUP98-PHF23 fusion resulting from a cryptic translocation t(11;17)(p15;p13) in acute myeloid leukemia. Leukemia 21(4):842–844
Gough SM et al (2014) NUP98-PHF23 is a chromatin-modifying oncoprotein that causes a wide array of leukemias sensitive to inhibition of PHD histone reader function. Cancer Discov 4(5):564–577
Cerveira N et al (2003) Frequency of NUP98-NSD1 fusion transcript in childhood acute myeloid leukaemia. Leukemia 17(11):2244–2247
Wang GG et al (2007) NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat Cell Biol 9(7):804–812
Raman JD et al (2005) Increased expression of the polycomb group gene, EZH2, in transitional cell carcinoma of the bladder. Clin Cancer Res 11(24 Pt 1):8570–8576
Matsukawa Y et al (2006) Expression of the enhancer of zeste homolog 2 is correlated with poor prognosis in human gastric cancer. Cancer Sci 97(6):484–491
Kondo Y et al (2007) Alterations of DNA methylation and histone modifications contribute to gene silencing in hepatocellular carcinomas. Hepatol Res 37(11):974–983
Lee HW, Choe M (2012) Expression of EZH2 in renal cell carcinoma as a novel prognostic marker. Pathol Int 62(11):735–741
Rao ZY et al (2010) EZH2 supports ovarian carcinoma cell invasion and/or metastasis via regulation of TGF-beta1 and is a predictor of outcome in ovarian carcinoma patients. Carcinogenesis 31(9):1576–1583
Koh CM et al (2011) Myc enforces overexpression of EZH2 in early prostatic neoplasia via transcriptional and post-transcriptional mechanisms. Oncotarget 2(9):669–683
Bracken AP et al (2003) EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J 22(20):5323–5335
Richter GH et al (2009) EZH2 is a mediator of EWS/FLI1 driven tumor growth and metastasis blocking endothelial and neuro-ectodermal differentiation. Proc Natl Acad Sci U S A 106(13):5324–5329
Tiwari N et al (2013) Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell 23(6):768–783
Mahara S et al (2016) HIFI-alpha activation underlies a functional switch in the paradoxical role of Ezh2/PRC2 in breast cancer. Proc Natl Acad Sci U S A 113(26):E3735–E3744
Zhu J et al (2015) Gain-of-function p53 mutants co-opt chromatin pathways to drive cancer growth. Nature 525(7568):206–211
Kim JM et al (2005) Identification of gastric cancer-related genes using a cDNA microarray containing novel expressed sequence tags expressed in gastric cancer cells. Clin Cancer Res 11(2 Pt 1):473–482
Yan F et al (2014) Genetic validation of the protein arginine methyltransferase PRMT5 as a candidate therapeutic target in glioblastoma. Cancer Res 74(6):1752–1765
Koh CM et al (2015) MYC regulates the core pre-mRNA splicing machinery as an essential step in lymphomagenesis. Nature 523(7558):96–100
Mongiardi MP et al (2015) Myc and Omomyc functionally associate with the Protein Arginine Methyltransferase 5 (PRMT5) in glioblastoma cells. Sci Rep 5:15494
Kroon E et al (1998) Hoxa9 transforms primary bone marrow cells through specific collaboration with Meis1a but not Pbx1b. EMBO J 17(13):3714–3725
Volanakis EJ, Boothby MR, Sherr CJ (2013) Epigenetic regulation of the Ink4a-Arf (Cdkn2a) tumor suppressor locus in the initiation and progression of Notch1-driven T cell acute lymphoblastic leukemia. Exp Hematol 41(4):377–386
Chen H et al (2009) Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev 23(8):975–985
Zhou HL et al (2014) Regulation of alternative splicing by local histone modifications: potential roles for RNA-guided mechanisms. Nucleic Acids Res 42(2):701–713
Simon JM et al (2014) Variation in chromatin accessibility in human kidney cancer links H3K36 methyltransferase loss with widespread RNA processing defects. Genome Res 24(2):241–250
Park IY et al (2016) Dual chromatin and cytoskeletal remodeling by SETD2. Cell 166(4):950–962
Kanu N et al (2015) SETD2 loss-of-function promotes renal cancer branched evolution through replication stress and impaired DNA repair. Oncogene 34(46):5699–5708
Kantidakis T et al (2016) Mutation of cancer driver MLL2 results in transcription stress and genome instability. Genes Dev 30(4):408–420
Baylin SB, Jones PA (2011) A decade of exploring the cancer epigenome – biological and translational implications. Nat Rev Cancer 11(10):726–734
Ahuja N, Easwaran H, Baylin SB (2014) Harnessing the potential of epigenetic therapy to target solid tumors. J Clin Invest 124(1):56–63
Kubicek S et al (2007) Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol Cell 25(3):473–481
Liu F et al (2013) Discovery of an in vivo chemical probe of the lysine methyltransferases G9a and GLP. J Med Chem 56(21):8931–8942
Sweis RF et al (2014) Discovery and development of potent and selective inhibitors of histone methyltransferase g9a. ACS Med Chem Lett 5(2):205–209
Yuan Y et al (2012) A small-molecule probe of the histone methyltransferase G9a induces cellular senescence in pancreatic adenocarcinoma. ACS Chem Biol 7(7):1152–1157
Liu F et al (2009) Discovery of a 2,4-diamino-7-aminoalkoxyquinazoline as a potent and selective inhibitor of histone lysine methyltransferase G9a. J Med Chem 52(24):7950–7953
Maes T et al (2015) Advances in the development of histone lysine demethylase inhibitors. Curr Opin Pharmacol 23:52–60
Schenk T et al (2012) Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat Med 18(4):605–611
Mohammad HP, Kruger RG (2016) Antitumor activity of LSD1 inhibitors in lung cancer. Mol Cell Oncol 3(2):e1117700
McCabe MT et al (2012) EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492(7427):108–112
Amatangelo MD et al (2013) Three-dimensional culture sensitizes epithelial ovarian cancer cells to EZH2 methyltransferase inhibition. Cell Cycle 12(13):2113–2119
Knutson SK et al (2012) A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol 8(11):890–896
Knutson SK et al (2013) Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci U S A 110(19):7922–7927
Qi W et al (2012) Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci U S A 109(52):21360–21365
Konze KD et al (2013) An orally bioavailable chemical probe of the Lysine Methyltransferases EZH2 and EZH1. ACS Chem Biol 8(6):1324–1334
Zhang H et al (2016) Oncogenic deregulation of EZH2 as an opportunity for targeted therapy in lung cancer. Cancer Discov 6(9):1006–1021
Kruidenier L et al (2012) A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature 488(7411):404–408
Sakaki H et al (2015) GSKJ4, a selective Jumonji H3K27 demethylase inhibitor, effectively targets ovarian cancer stem cells. Anticancer Res 35(12):6607–6614
Ferguson AD et al (2011) Structural basis of substrate methylation and inhibition of SMYD2. Structure 19(9):1262–1273
Wang J et al (2016) Silencing the epigenetic silencer KDM4A for TRAIL and DR5 simultaneous induction and antitumor therapy. Cell Death Differ 23(11):1886–1896
Daigle SR et al (2013) Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood 122(6):1017–1025
Daigle SR et al (2011) Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell 20(1):53–65
Yu W et al (2012) Catalytic site remodelling of the DOT1L methyltransferase by selective inhibitors. Nat Commun 3:1288
Cheng D et al (2004) Small molecule regulators of protein arginine methyltransferases. J Biol Chem 279(23):23892–23899
Spannhoff A et al (2007) Target-based approach to inhibitors of histone arginine methyltransferases. J Med Chem 50(10):2319–2325
Hu H et al (2016) Small molecule inhibitors of protein arginine methyltransferases. Expert Opin Investig Drugs 25(3):335–358
Xie Y et al (2014) Virtual screening and biological evaluation of novel small molecular inhibitors against protein arginine methyltransferase 1 (PRMT1). Org Biomol Chem 12(47):9665–9673
Sinha SH et al (2012) Synthesis and evaluation of carbocyanine dyes as PRMT inhibitors and imaging agents. Eur J Med Chem 54:647–659
Hu H et al (2015) Exploration of cyanine compounds as selective inhibitors of protein arginine methyltransferases: synthesis and biological evaluation. J Med Chem 58(3):1228–1243
Liu F et al (2013) Exploiting an allosteric binding site of PRMT3 yields potent and selective inhibitors. J Med Chem 56(5):2110–2124
Wan H et al (2009) Benzo[d]imidazole inhibitors of Coactivator Associated Arginine Methyltransferase 1 (CARM1)--Hit to Lead studies. Bioorg Med Chem Lett 19(17):5063–5066
Allan M et al (2009) N-Benzyl-1-heteroaryl-3-(trifluoromethyl)-1H-pyrazole-5-carboxamides as inhibitors of co-activator associated arginine methyltransferase 1 (CARM1). Bioorg Med Chem Lett 19(4):1218–1223
Chan-Penebre E et al (2015) A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. Nat Chem Biol 11(6):432–437
Varambally S et al (2002) The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419(6907):624–629
Kleer CG et al (2003) EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A 100(20):11606–11611
Wagener N et al (2010) Enhancer of zeste homolog 2 (EZH2) expression is an independent prognostic factor in renal cell carcinoma. BMC Cancer 10:524
Takawa M et al (2011) Validation of the histone methyltransferase EZH2 as a therapeutic target for various types of human cancer and as a prognostic marker. Cancer Sci 102(7):1298–1305
Peifer M et al (2012) Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 44(10):1104–1110
Cancer Genome Atlas Research Network et al (2013) Integrated genomic characterization of endometrial carcinoma. Nature 497(7447):67–73
Sjoblom T et al (2006) The consensus coding sequences of human breast and colorectal cancers. Science 314(5797):268–274
Okosun J et al (2014) Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet 46(2):176–181
Tan J et al (2015) Genomic landscapes of breast fibroepithelial tumors. Nat Genet 47(11):1341–1345
Xiang Y et al (2007) JARID1B is a histone H3 lysine 4 demethylase up-regulated in prostate cancer. Proc Natl Acad Sci U S A 104(49):19226–19231
Yamamoto S et al (2014) JARID1B is a luminal lineage-driving oncogene in breast cancer. Cancer Cell 25(6):762–777
Dalgliesh GL et al (2010) Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 463(7279):360–363
Varela I et al (2011) Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469(7331):539–542
Komura K et al (2016) Resistance to docetaxel in prostate cancer is associated with androgen receptor activation and loss of KDM5D expression. Proc Natl Acad Sci U S A 113(22):6259–6264
Hayami S et al (2011) Overexpression of LSD1 contributes to human carcinogenesis through chromatin regulation in various cancers. Int J Cancer 128(3):574–586
Gunduz M et al (2000) Genomic structure of the human ING1 gene and tumor-specific mutations detected in head and neck squamous cell carcinomas. Cancer Res 60(12):3143–3146
Bjorkman M et al (2012) Systematic knockdown of epigenetic enzymes identifies a novel histone demethylase PHF8 overexpressed in prostate cancer with an impact on cell proliferation, migration and invasion. Oncogene 31(29):3444–3456
Peters AH et al (2001) Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107(3):323–337
Black JC et al (2013) KDM4A lysine demethylase induces site-specific copy gain and rereplication of regions amplified in tumors. Cell 154(3):541–555
Stephens PJ et al (2012) The landscape of cancer genes and mutational processes in breast cancer. Nature 486(7403):400–404
Le Gallo M et al (2012) Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet 44(12):1310–1315
Xi Q et al (2011) A poised chromatin platform for TGF-beta access to master regulators. Cell 147(7):1511–1524
Schotta G et al (2004) A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev 18(11):1251–1262
Vougiouklakis T et al (2015) SUV420H1 enhances the phosphorylation and transcription of ERK1 in cancer cells. Oncotarget 6(41):43162–43171
Kim JH et al (2014) UTX and MLL4 coordinately regulate transcriptional programs for cell proliferation and invasiveness in breast cancer cells. Cancer Res 74(6):1705–1717
Liu YL et al (2015) Expression and clinicopathological significance of EED, SUZ12 and EZH2 mRNA in colorectal cancer. J Cancer Res Clin Oncol 141(4):661–669
Lee W et al (2014) PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat Genet 46(11):1227–1232
Brien GL et al (2012) Polycomb PHF19 binds H3K36me3 and recruits PRC2 and demethylase NO66 to embryonic stem cell genes during differentiation. Nat Struct Mol Biol 19(12):1273–1281
Zhao Q et al (2009) PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol 16(3):304–311
Nagahata T et al (2004) Expression profiling to predict postoperative prognosis for estrogen receptor-negative breast cancers by analysis of 25,344 genes on a cDNA microarray. Cancer Sci 95(3):218–225
Tan J et al (2007) Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev 21(9):1050–1063
Miranda TB et al (2009) DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol Cancer Ther 8(6):1579–1588
Knutson SK et al (2014) Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol Cancer Ther 13(4):842–854
Morin RD et al (2010) Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 42(2):181–185
McCabe MT et al (2012) Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27). Proc Natl Acad Sci U S A 109(8):2989–2994
Ryan RJ et al (2011) EZH2 codon 641 mutations are common in BCL2-rearranged germinal center B cell lymphomas. PLoS One 6(12):e28585
Bradley WD et al (2014) EZH2 inhibitor efficacy in non-Hodgkin’s lymphoma does not require suppression of H3K27 monomethylation. Chem Biol 21(11):1463–1475
Basavapathruni A et al (2012) Conformational adaptation drives potent, selective and durable inhibition of the human protein methyltransferase DOT1L. Chem Biol Drug Des 80(6):971–980
Chen L et al (2013) Abrogation of MLL-AF10 and CALM-AF10-mediated transformation through genetic inactivation or pharmacological inhibition of the H3K79 methyltransferase Dot1l. Leukemia 27(4):813–822
Deshpande AJ et al (2013) Leukemic transformation by the MLL-AF6 fusion oncogene requires the H3K79 methyltransferase Dot1l. Blood 121(13):2533–2541
Sarkaria SM et al (2014) Primary acute myeloid leukemia cells with IDH1 or IDH2 mutations respond to a DOT1L inhibitor in vitro. Leukemia 28(12):2403–2406
Basavapathruni A et al (2014) Nonclinical pharmacokinetics and metabolism of EPZ-5676, a novel DOT1L histone methyltransferase inhibitor. Biopharm Drug Dispos 35(4):237–252
Yadav N et al (2003) Specific protein methylation defects and gene expression perturbations in coactivator-associated arginine methyltransferase 1-deficient mice. Proc Natl Acad Sci U S A 100(11):6464–6468
Yang Y, Bedford MT (2013) Protein arginine methyltransferases and cancer. Nat Rev Cancer 13(1):37–50
Goulet I et al (2007) Alternative splicing yields protein arginine methyltransferase 1 isoforms with distinct activity, substrate specificity, and subcellular localization. J Biol Chem 282(45):33009–33021
Mathioudaki K et al (2008) The PRMT1 gene expression pattern in colon cancer. Br J Cancer 99(12):2094–2099
Baldwin RM et al (2012) Alternatively spliced protein arginine methyltransferase 1 isoform PRMT1v2 promotes the survival and invasiveness of breast cancer cells. Cell Cycle 11(24):4597–4612
Bissinger EM et al (2011) Acyl derivatives of p-aminosulfonamides and dapsone as new inhibitors of the arginine methyltransferase hPRMT1. Bioorg Med Chem 19(12):3717–3731
El Messaoudi S et al (2006) Coactivator-associated arginine methyltransferase 1 (CARM1) is a positive regulator of the Cyclin E1 gene. Proc Natl Acad Sci U S A 103(36):13351–13356
Majumder S et al (2006) Involvement of arginine methyltransferase CARM1 in androgen receptor function and prostate cancer cell viability. Prostate 66(12):1292–1301
Hong H et al (2004) Aberrant expression of CARM1, a transcriptional coactivator of androgen receptor, in the development of prostate carcinoma and androgen-independent status. Cancer 101(1):83–89
Frietze S et al (2008) CARM1 regulates estrogen-stimulated breast cancer growth through up-regulation of E2F1. Cancer Res 68(1):301–306
Sack JS et al (2011) Structural basis for CARM1 inhibition by indole and pyrazole inhibitors. Biochem J 436(2):331–339
Cheng D et al (2011) Novel 3,5-bis(bromohydroxybenzylidene)piperidin-4-ones as coactivator-associated arginine methyltransferase 1 inhibitors: enzyme selectivity and cellular activity. J Med Chem 54(13):4928–4932
Ding J et al (2013) LSD1-mediated epigenetic modification contributes to proliferation and metastasis of colon cancer. Br J Cancer 109(4):994–1003
Lim S et al (2010) Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis 31(3):512–520
Kahl P et al (2006) Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer Res 66(23):11341–11347
Zhao ZK et al (2012) Overexpression of lysine specific demethylase 1 predicts worse prognosis in primary hepatocellular carcinoma patients. World J Gastroenterol 18(45):6651–6656
Schmidt DM, McCafferty DG (2007) trans-2-Phenylcyclopropylamine is a mechanism-based inactivator of the histone demethylase LSD1. Biochemistry 46(14):4408–4416
Fiedorowicz JG, Swartz KL (2004) The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract 10(4):239–248
Shulman KI et al (2009) Current prescription patterns and safety profile of irreversible monoamine oxidase inhibitors: a population-based cohort study of older adults. J Clin Psychiatry 70(12):1681–1686
Morera L, Lubbert M, Jung M (2016) Targeting histone methyltransferases and demethylases in clinical trials for cancer therapy. Clin Epigenetics 8:57
Mohammad H et al (2014) Novel anti-tumor activity of targeted LSD1 inhibition by GSK2879552. Eur J Cancer 50:72
Huang PH et al (2011) Histone deacetylase inhibitors stimulate histone H3 lysine 4 methylation in part via transcriptional repression of histone H3 lysine 4 demethylases. Mol Pharmacol 79(1):197–206
Shi YG, Tsukada Y (2013) The discovery of histone demethylases. Cold Spring Harb Perspect Biol 5(9):a017947
Fiskus W et al (2014) Highly effective combination of LSD1 (KDM1A) antagonist and pan-histone deacetylase inhibitor against human AML cells. Leukemia 28(11):2155–2164
Klaus CR et al (2014) DOT1L inhibitor EPZ-5676 displays synergistic antiproliferative activity in combination with standard of care drugs and hypomethylating agents in MLL-rearranged leukemia cells. J Pharmacol Exp Ther 350(3):646–656
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Ooi, W.F., Yao, X., Tan, P., Teh, B.T. (2017). Misregulation of Histone Methylation Regulators in Cancer. In: Kaneda, A., Tsukada, Yi. (eds) DNA and Histone Methylation as Cancer Targets. Cancer Drug Discovery and Development. Humana Press, Cham. https://doi.org/10.1007/978-3-319-59786-7_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-59786-7_8
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-59784-3
Online ISBN: 978-3-319-59786-7
eBook Packages: MedicineMedicine (R0)