Abstract
The management of Potato virus Y (PVY) in potato crops poses a continual challenge due to the non-persistent mode of transmission of the virus and the propagation of seed potato tubers over several generations in the field. While PVY-resistant cultivars remain the most efficient way to protect potato crops against PVY, a vast majority of cultivars grown do not display significant resistance to PVY. Due to the short time period for PVY transmission by non-colonising aphid vectors, efficient control of PVY relies on preventing aphids landing on a crop and on adopting precautionary measures by ensuring that crops are grown in areas of low aphid and low virus pressure and limiting field generation. Prophylactic measures such as roguing and early haulm destruction limit PVY spread but are not efficient alone. Among all existing control methods, spraying potato crops with mineral oils can offer significant protection against PVY spread, but their efficacy do vary in field conditions. The combination of several control methods such as mineral oil treatments, crop borders, intercropping, straw mulching or insecticide treatments can increase protection. These emphasise the importance of controlling virus through appropriate monitoring methods and crop management enforced by seed certification schemes through the use of ‘clean’ input seed and, when possible, the segregation of seed and ware crops to minimise the risk of virus transmission. This chapter presents and discusses the most widely used techniques of control and management of PVY, their effectiveness and their mode of action. This chapter also presents the history, objectives and principles of seed potato certification schemes and their role in minimising the spread of viruses within potato crops worldwide.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The management of Potato virus Y (PVY) in potato crops poses a continual challenge due to the non-persistent mode of transmission of PVY and the propagation of seed potato tubers over several generations in the field, which presents a risk for primary and secondary infections of plants by PVY.
Aphids transmit the virus by flying from plant to plant within or between crops (Sigvald 1984; Boiteau 1997). An aphid will probe an infected plant, acquire the virus and then fly to a healthy plant, probe again and transmit the virus. The length of time required by an aphid for acquisition and subsequent transmission of PVY is very short, with each step generally accomplished in seconds (Fereres and Moreno 2009; Robert et al. 2000; Bragard et al. 2013) (see Chap. 6). The challenge in controlling PVY efficiently lies in either preventing aphids landing on a crop or in promoting a rapid (almost instantaneous) deleterious effect on them after landing to prevent any further transmission. These aspects will be discussed in the following sections.
Potatoes are vegetatively multiplied by seed potato growers in order to maintain the characteristics of a cultivar and to bulk up sufficient quantities of certified seed potatoes to meet the requirements of the market (Frost et al. 2013). This method of propagation enables PVY to be transmitted from one generation of a crop to the next through its translocation from an infected seed tuber into growing plant and daughter tubers (Basky and Almasi 2005). While it is still not possible to prevent translocation of the virus, it is, however, possible to minimise the multiplication of an infected seed lot by a systematic control of its quality. This can be done by the implementation of seed potato certification programmes and appropriate virus testing regimes.
A number of methods have been developed to control the spread of PVY in potato crops, and some of them, either individually or in combination, are now used by seed potato producers. This chapter will present and discuss the most widely used techniques of control and management of PVY, their effectiveness and their mode of action. This chapter will also present the history, objectives and principles of seed potato certification programmes and the role of these programmes in minimising the spread of viruses within potato crops worldwide.
2 Cultural Methods
2.1 Prophylactic Measures
2.1.1 Use of Virus-Free Seed Potatoes
The risk of PVY spread can be reduced by planting seed potatoes which are virus free or have a very low incidence of tuber infection by PVY, thus minimising the number of inoculum sources within a crop (Kerlan et al. 1987; Rolot 2005; Steinger et al. 2014). This will be discussed in the paragraph relating to seed potato certification programmes.
2.1.2 Use of Virus-Resistant Cultivars
The most effective means of controlling virus in potato crops is to grow resistant potato cultivars. This strategy allows potatoes to be produced in areas where aphid pressure is high and hence unsuitable for potato production because of disease. There are several breeding programmes worldwide whose goal is to breed new potato cultivars that are resistant to PVY (see Chap. 8). In summary, there are several different types of resistance (see Chaps. 2 and 8): (i) extreme resistance which does not allow a virus to multiply in a plant, (ii) hypersensitive response is defined as a mechanism of resistance against pathogen invasion by the rapid death of cells at the infection site preventing/reducing significantly the spread of pathogens to other parts of the plant and (iii) tolerance which allows a virus to multiply and spread within a plant without it expressing visible symptoms. However, breeders develop their selection strategy independently depending on market requirements, and the specific disease and pest pressures in the countries intended for marketing. In the USA and Canada, breeders have developed a number of widely grown cultivars which are tolerant to PVY, such as cv. Russet Norkotah, which shows mild or ‘latent’ symptoms of PVY (Whitworth et al. 2010), and cv. Red LaSoda, which is fully susceptible to infection by PVY but does not express symptoms (Draper et al. 2002). This could be one of the reasons for the re-emergence of PVY in these potato production areas (Gray et al. 2010; Schramm et al. 2011). In Germany and France, the selection programmes for new cultivars are different. Breeders mainly aim to develop potato cultivars resistant to the multiplication of PVY and avoid the selection of cultivars susceptible to potato tuber necrotic ringspot disease (PTNRD) (Le Romancer and Kerlan 1991). However, it is widely believed that breeding for cultivars displaying high level of PVY resistance might often not be seen as a major goal due to poor economic return on investments (Ruedi Schwaerzel, personal communication).
2.1.3 Isolation from Virus Sources
Location of crops is important in determining the risk of PVY transmission by aphids (Kerlan et al. 1987). Low-risk areas are usually those with low aphid populations, often as a consequence of low mean temperatures (Robert et al. 2000; Gabriel 1965). Klueken et al. (2009) studied the flight behaviour of alate cereal aphid Sitobion avenae (Fabricius) and demonstrated that, for a 16-h period, no aphid flight occurred at 10°C, while the proportion of aphids flying increased to 70% at 15°C to reach 100% at 20°C. They also showed that the temperature threshold was 3°C higher for Metopolophium dirhodum (Walker) (bird cherry oat aphid), another cereal aphid. This implies that, for cold temperature, these aphid species are likely to stay on their ‘winter host’ instead of colonising crops (‘summer hosts’). Under these conditions, vector pressure and aphid transmission of PVY are minimised. In Switzerland, it was shown that an increase in altitude from 400 m to 800 m resulted in a decrease in infection of potatoes by 57%, mainly due to an average colder temperature at the higher altitude (Steinger et al. 2014). In addition, strong wind speeds (> 0.8 km/h) are not favourable for alate aphid displacement but also for aphid feeding and development, so windy areas tend to be more suited to seed potato production because there is less opportunity for aphids to transmit virus (CIP 1979; Walters and Dixon 1984). The presence of plants infected by PVY in neighbouring crops can also pose a risk with regard to inoculum sources of PVY, especially if the seed potatoes planted are not certified and the crop is being grown for consumption (ware) without management measures to control aphids. Virus is likely to be more prevalent in farm-saved seed potatoes than in certified seed potatoes (Kerlan et al. 1987).
2.1.4 Time of Planting and Haulm Killing
The physiological state of a plant may affect the transmission of PVY by aphids because older plants appear to be less susceptible to PVY infection than younger plants (Gibson 1991; Robert et al. 2000; Sigvald 1985; Dupuis 2016), a phenomenon termed mature plant resistance (Beemster 1972). Sigvald (1985) reported that potato plants are at their most susceptible state up to 25 days post-emergence, becoming more resistant thereafter at the rate of 10% every week. Managing the physiology of plants could, therefore, be integrated into a programme to control PVY in potato crops. Advancing the time of planting and haulm destruction could be important in reducing the risk of PVY spread. Pre-sprouting seed potatoes can enable plants to emerge earlier and daughter tubers to bulk earlier, thus allowing earlier haulm destruction than with unsprouted tubers. However, the effectiveness of pre-sprouting in controlling PVY spread will depend to a great extent on the timing of aphid flights. If aphid flights are late in the growing season, pre-sprouting could be an effective measure. However, if spring aphid flights are earlier than normal, this could pose a greater risk to crops which have been planted with sprouted seed tubers because the plants will have emerged when aphids are flying, whereas those from unsprouted seed potatoes will not (Saucke and Doring 2004). Early haulm destruction will reduce the time of exposure of the crop to aphid flights, thereby reducing the risk of infection (Basky 2003; Kerlan et al. 1987; Steinger et al. 2014). In some conditions, it has been reported that delaying haulm destruction can increase the incidence of PVY infection by 3.5% per day (Steinger et al. 2014). For mature crops, the choice of herbicide or the use of mechanical haulm destruction has a limited impact on reducing virus transmission (Dupuis and Schwaerzel 2011). Nevertheless, rapid and total haulm destruction is required to reduce the risk of late PVY transmission. If new foliage develops on desiccated stems, transmission of PVY by aphids could be possible as the relatively immature leaves are potentially susceptible to PVY transmission (Sigvald 1985). New growth that develops after haulm destruction can be susceptible to aphid-borne infection especially when significant aphid pressure is still observed late in the growing season. However, quantitative data are still lacking to assess accurately the impact of late infection of new growth and PVY incidence at post-harvest.
2.2 Roguing and Weed Control
For effective control of PVY in crops, it is essential to eliminate any sources of inoculum. These can be plants from infected seed tubers, from weeds or from volunteer plants, i.e. potato plants derived from tubers or parts of a tuber left in the soil after harvesting a previous crop (Jones et al. 1996). The use of certified seed potatoes according to official tolerances can provide assurance regarding the maximum amount of virus disease which could develop in a crop, but cannot rule out the absence of viruses in a crop. When a crop is being grown for marketing as seed potatoes, roguing is a key component in maintaining the health of a crop. Roguing is the removal of potato plants which are atypical of the cultivar in appearance or diseased including virus (Kerlan et al. 1987). For virus diseases, roguing is efficient in reducing PVY spread, but the effect can vary from none to a 20% reduction of PVY spread (Broadbent et al. 1950). To be effective, roguing should be conducted as soon as possible after plant emergence to minimise the opportunity for aphids to acquire virus from infected plants within a crop. However, roguing will be ineffective if a cultivar is tolerant to a virus because infected plants will be symptomless and, therefore, not recognised and removed. There is a risk that excessive amounts of virus can build up in crops of such cultivars cultivated over several generations and pose a serious threat to the health of crops of susceptible cultivars from aphid transmission (Ragsdale et al. 2011). It is also possible that roguing could enhance the transmission of PVY by aphids if too many plants are removed in a small area creating bare patches. Aphids land preferentially in areas where there is a contrast between bare soil and potato plants (reviewed in Döring (2014)). Excessive gaps might promote the landing of aphids and, consequently, increase the risk of infection of plants surrounding a gap. The risk of infection increases with the size of the gap (Davis et al. 2009). The incidence of PVY infection was 13% around gaps of ≤ 0.6 m2 and 29% around gaps of ≥ 0.6 m2.
Volunteer plants are a major concern in potato-growing areas. It has been estimated that in 1 hectare, 20,000–300,000 tubers could remain in a potato field following harvest because a significant proportion of tubers are too small to be collected by harvesters and will remain in the soil over the winter (Yves Le Hingrat, personal communication). Some of these tubers will survive the winter if soil temperatures are not sufficiently cold enough. The extent of tuber overwintering will vary with their depth in soil, the severity and length of cold periods (Lutman 1977; Boydston et al. 2006; Cooke et al. 2011). The growth of plants from surviving tubers (volunteer potatoes) even after several years of crop rotation could be impaired by the foliar growth of the newly planted crop, depending on its capacity to cover and shade volunteer potato plants. In France between 2007 and 2011, the density of volunteer plants in fields in the Brittany region was estimated to range from none to six stems per m2 depending on cultivar, cropping practices and succeeding crop (Rakotonindraina et al. 2011). In addition to volunteer potatoes being a source of varietal mixtures in potato crops and posing weed control issues in other field crops, they can also act as a reservoir for potato diseases and could impact on the phytosanitary status of seed potatoes. A survey was conducted in the UK in 1996 in which volunteer plants were collected from three different sites and the percentage of volunteer plants infected by PVY was found to range from 2 to 54% (Jones et al. 1996). Inoculum from within or near a crop can also originate from weeds. In the late 1990s, 36 weed species belonging to 13 different botanical families were identified as potential host plants for PVY (in natural and/or artificial conditions) (Edwardson and Christie 1997). More recently, seven additional weed species have been identified as potential hosts (Kaliciak and Syller 2009; Kazinczi et al. 2004) (see Chap. 6). It is almost certain that other weed hosts for PVY will be reported in the future. However, the epidemiological role and impact of those weeds in the field are not fully understood, and it is unclear whether the presence of weed species will contribute significantly to the spread and prevalence of PVY in potato crops.
2.3 Crop Borders
Crop borders can be planted in order to limit the amount of virus introduced into a crop (Boiteau et al. 2009; Difonzo et al. 1996). Crop borders display two distinct modes of action, serving as a ‘virus barrier’ and also as a ‘virus sink’ (Boiteau et al. 2009). Viruliferous aphids landing on a border crop will probe the plants and shed any virus particles that they might be carrying into the barrier plant. In this case, the border crop serves the role of ‘virus sink’ by retaining the virus. To be effective, border crops must be planted with a plant species which is not susceptible to PVY. Border plants can also act as a physical barrier interrupting aphid flight (Simons 1957). To provide this type of protection, the border plants have to be taller than the potato plants at all stages of growth. A border must also be wide enough to maximise the probability of aphids landing on it (Boiteau et al. 2009; Difonzo et al. 1996). With a soybean border of 24 m, Difonzo et al. (1996) obtained an efficacy of PVY control of 27 and 60% over the 2 years of an experiment, while Boiteau et al. (2009) obtained 32% efficacy for an experiment with a narrower border of 4 m sown with grass. This technique has the advantage of being effective for the entire cropping season, whatever the environmental conditions. Nevertheless, the use of crop borders requires relatively large areas of a field to be removed from potato production with no commercial return so the method may not be practical, especially if fields are relatively small. To solve this issue, a potato cultivar resistant to PVY can be used as a border. A border of 4 m of cv. Kennebec (known to be relatively resistant to PVY) was used in a 3-year field trial in Canada to protect a central plot of cv. Russet Burbank, susceptible to PVY, and its effectiveness was compared with applying mineral oil (Boiteau et al. 2009). The border crop alone reduced PVY transmission by about 20% in the first 2 years of the trial and 60% in the third year, while mineral oil application alone reduced PVY transmission by 20% in the first 2 years and by 70% in the third year.
2.4 Mulching and Intercropping
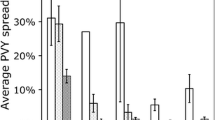
Straw mulching (Fig. 7.1) is effective in controlling PVY spread (Heimbach et al. 2004; Saucke and Doring 2004; Kirchner et al. 2014; Dupuis et al. 2010). The mode of action of straw mulching is not well understood, but the main hypothesis is that straw impacts on the visual perception of a crop by an aphid (Döring 2014). The contrast between potato foliage and the yellow straw is considerably lower than potato foliage and bare soil, so that potato plants in mulched plots are less easily seen by aphids (Döring 2014; Döring and Schmidt 2007). This was confirmed when fewer aphids were captured in mulched plots compared with those in bare soil plots (Heimbach et al. 2004; Saucke and Doring 2004). In the study reported by Saucke and Doring (2004), aphids were counted on the foliage of potato plants in straw-mulched and bare soil plots. An average reduction of 54% in aphid numbers was recorded in the mulched plots. This reduction of aphid populations on foliage was 18% in an independent study (Heimbach et al. 2004), while in the same experiment, 85% fewer winged aphids were captured by sticky net traps within the mulched plots. Thus, the lower number of winged aphids landing on potato plants in mulched plots might be the main factor in controlling PVY spread by mulching. PVY control by mulching is more effective in the early stages of crop development, declining as the crop canopy develops over the mulch (Heimbach et al. 2004). This was shown clearly in a field experiment undertaken over a 3-year period (Saucke and Doring 2004) in which the efficacy of PVY control recorded in the mulched plots was about 48% in the first year, 33% in the second year and 27% in the third year. In the first year of the trial, aphids were most prevalent a few days after potato emergence in mid-May, whereas in the third year of the trial, aphids were most active 2 weeks later. In the second year of the trial, aphid activity remained low during the entire growing period. Intercropping was also tested for its effectiveness in reducing PVY spread in potato crops (Dupuis et al. (2010); Fig. 7.2). Oats sown between rows of potatoes but killed before becoming large enough to provide unwanted competition for the potatoes resulted in a significant reduction in PVY spread (Fig. 7.3). The mode of action of intercropping is comparable to the ‘sink effect’ of a border crop in which inoculum being carried by aphids is lost during probing on the associated crop (Boiteau et al. 2009). Intercropping offers an additional mode of action called the ‘dilution effect’ by significantly decreasing the probability of an aphid landing on potato plants. Given the same plot surface, plots containing an associated crop present many more plants to potential vectors, thereby reducing the risk of potatoes being infected.
Percentage of PVY-infected plants assessed by testing daughter tubers for six treatments and untreated (1 year; four replications of 100 plants per plot). The acronyms for the treatments are SM = straw mulching, MO = mineral oil, OI = oat intercropping. Error bars show the standard error, and stars show the treatments with a percentage of infection significantly lower than untreated (Dunnett’s test; * for p<0.05 and ** for p<0.01) (Dupuis et al. 2010)
3 Chemical Methods
3.1 Oil Treatments
Spraying oil on potato plants has been reported to provide a means of controlling the spread of PVY (Boiteau et al. 2009, Bell 1980, Bell 1989, Boiteau and Wood 1982, Bradley et al. 1962, 1966, Dewijs 1980, Dupuis et al. 2014, Hansen and Nielsen 2012, Kirchner et al. 2014, Martin-Lopez et al. 2006, Milosevic 1996, Steinger et al. 2014, Rolot 2005). Mineral oil was found to be more effective than vegetable oil (Martin-Lopez et al. 2006; Rolot 2005; Wrobel 2012), although among the latter, refined oils were more effective than raw oils (Martin-Lopez et al. 2006). The mechanism of action of the oils is not fully understood. It has been suggested that oil on aphid mouthparts reduces acquisition and retention of PVY by aphids. Oil may impede binding of virus particles on to the stylet (Bradley 1963; Boquel et al. 2013; Loebenstein et al. 1964; Powell 1992) and/or shorten the duration of virus retention in the stylets (Wróbel 2009). Mineral oil has also been shown to reduce virus replication and accumulation in inoculated plants, possibly due to the general activation of defence mechanisms in a plant (Loebenstein et al. 1964; Peters and Lebbink 1973; Martoub 2010; Al-Daoud et al. 2014). Oil also has the ability to kill aphids by interfering physically with their respiration (Martin-Lopez et al. 2006; Hesler and Plapp 1986). Studies have investigated the effects of mineral oil treatment of potato plants on selection of host plant, growth and reproduction of the colonising potato aphid, Macrosiphum euphorbiae (Thomas) (Ameline et al. 2009). Olfactometry experiments showed that mineral oil treatment induced a transient repellent effect shortly after spraying that lasted a day. While probing behaviour was not drastically affected in oil-treated plants, the treatment resulted in antagonistic effects with a significant reduction in nymph survival shortly after treatment and concomitantly a higher fitness and fecundity rate in adult aphids (Ameline et al. 2009). Other studies have reported antagonistic effects of mineral oil on M. euphorbiae that were dose dependent; topical contact at the highest concentration of oil resulted in complete mortality, while lower concentrations and exposure to oil volatiles enhanced aphid fecundity but had no effect on aphid survival (Martoub 2010). Tan et al. (2005) suggested that, after spraying, oil is ‘absorbed’ in various plant tissues and cells and transported throughout the plant, resulting in a decrease in local concentrations and the induction of physiological changes (Tan et al. 2005), altering photosynthesis (Helson and Minshall 1951; Wedding et al. 1952), and triggering the expression of pathogenesis-related proteins (Kachroo et al. 2001; Lin et al. 1996).
In practice, the protection achieved with mineral oil spraying varies greatly depending on the time of application (Figs. 7.3, 7.5 and 7.6). Mineral oils are more effective on older plants in reducing the speed with which PVY moves in the vascular system (Al-Daoud et al. 2014). The reduction in the transmission of PVY by oil treatments is likely to vary among different potato cultivars and with different aphid and virus pressures. Steinger et al. (2014) reported that oil treatments reduced the average incidence of PVY by 39% (p<0.001) over a 4-year period. The protective effect of mineral oil was slightly greater in the year in which the incidence of PVY was greatest (50% decrease in infection Ntreated = 432, Nnon-treated = 86) than in other years and with the susceptible cvs Bintje and Charlotte (54% decrease in infection, Ntreated = 819, Nnon-treated = 79) compared with more resistant cultivars. In a separate study over 3 years of field trials in the UK, mineral oil reduced PVY infection two- to threefold in cvs King Edward and Maris Piper (N = 160 plants per cultivar per treatment per year) during a year of relatively high virus pressure (overall 30% incidence of plants infected by PVY) (Dawson et al. 2015). However, there was no significant effect of mineral oil treatments on the incidence of PVY in years of low virus pressure (3–13% PVY incidence). In this study, significant variation in the effectiveness of the oil treatment in controlling PVY suggests that local differences in aphid phenology and aphid vector pressure strongly influenced the effectiveness of the treatment. These results are consistent with the expected reduction in the incidence of PVY for oil-based treatments compared with untreated plants, usually between 30 and 60% (reviewed by Al-Mrabeh et al. (2010)).
Mineral oils are usually applied weekly to a seed potato crop. The protection provided by oil is generally limited to the leaf surfaces exposed to the spray being applied (Simons et al. 1977). Effective coverage of foliage is easier to achieve on older plants than on younger plants on which new leaves are constantly developing. The rate of foliar development should be considered when determining the optimum interval between sprays for effective application of oil. An increase in the frequency of foliar applications on young plants should be considered for maximum effectiveness in order to protect recently opened leaves (Fageria et al. 2014a, b; Demeulemeester 2013). The usual practice in France to protect high-grade seed is to spray mineral oil three times a week starting at 30% plant emergence and continuing until complete emergence followed by weekly applications thereafter. In Belgium, the practice is very similar except that only two applications are recommended during the early period of crop growth followed by one each week until haulm destruction is complete (Yves Le Hingrat and Pierre Lebrun, personal communication).
Mineral and vegetable oils must be used with caution to avoid plant phytotoxicity (Kirchner et al. 2014). It is essential to use a labelled product (Dewijs 1980). Paraffinic oils are preferably used for the treatment of plants instead of naphthenic oils (phytotoxic) and aromatic oils (phytotoxic and unstable) (Rolot 2005). The linear structure of saturated paraffinic oils is relatively stable and not phytotoxic. Dewijs et al. (1979) demonstrated that the viscosity of the oil, which is related to the number of carbon atoms in the molecular chain, is an important character determining the efficacy of the oil. Nevertheless, when the chains have more than 25 carbon atoms, phytotoxicity can be observed (Walsh 2000). The phytotoxicity of the paraffinic oil also depends on its degree of refining. Less than 8% of sulphonated residues (residues reacting with sulphuric acid) are required for plant treatments (Walsh 2000). For treatments with paraffinic oils, it is also required to not exceed the maximum rate and to avoid application during hot weather because oil can become so hot that it can burn potato foliage on application after being heated in the sprayer pipes during prolonged sun exposure. Mineral oil treatment can alter plant physiology and, in some cases, be phytotoxic. This can have an adverse effect on the appearance of plants and potentially might affect crop inspection by reducing a seed potato inspector’s ability to identify cultivars and virus symptoms visually in a growing crop. A recent study in the UK of the effect of oil treatments on a range of virus-infected and healthy plants of various potato cultivars concluded that the ability of inspectors to identify both cultivars and virus symptoms was not diminished by applications of mineral oil to foliage (Dawson et al. 2015), even though phytotoxic symptoms (localised necrotic spots) were occasionally observed (Fig.7.4). However, some loss of tuber yield was reported for some cultivars after mineral oil treatment, emphasising the necessity for a cautious use of mineral oil to control PVY (Kirchner et al. 2014). Delaying haulm destruction by several days could compensate for this reduction in yield (Dawson et al. 2015).
Phytotoxic symptoms (localised necrotic spots and leaf midrib necrosis) developing on leaves of potato cv. Desiree following mineral oil spraying (Dawson et al. 2015). Note the beading of rainwater on the foliage of sprayed plants (Photo: Courtesy of SASA Crown copyright)
3.2 Insecticide Treatments
The acquisition and inoculation periods of non-persistent viruses like PVY by their aphid vectors are extremely short (seconds to minutes) (Bragard et al. 2013). To be effective, an insecticide has to kill or incapacitate an aphid very quickly to limit the infection of a plant. However, the effect of an insecticide may impair the capacity of a viruliferous aphid to fly from a treated plant to another plant. Various insecticides and their formulations have been tested for their effectiveness in controlling PVY transmission in the field (Table 7.1). Pyrethroids have a near-instantaneous ‘knock-down’ effect and can provide a reduction in PVY transmission in controlled conditions (Perring et al. 1999; Gibson et al. 1982; Collar et al. 1997). Unfortunately, results obtained in field-grown potato crops with the same products have proven to be variable (Table 7.1). Other group of insecticides with different modes of action can potentially interfere with the transmission of viruses either by repelling aphids (deltamethrin, Rice et al. (1983)), altering feeding behaviour (flonicamid, imidacloprid, pymetrozine and thiamethoxam, Morita et al. (2007), Cho et al. (2011), Boquel et al. (2014)) and reducing aphid’s movement (aldicarb, Boiteau et al. (1985)). However, no significant reduction of PVY transmission was reported in field trials for imidacloprid, pymetrozine (Table 7.1) and flonicamid (Fig. 7.5). Pyrethroids such as deltamethrin (Gibson et al. 1982) and cypermethrin (Collar et al. 1997) can provide a significant degree of PVY protection in controlled conditions, but it is not known whether virus acquisition or transmission is affected. Lambda-cyhalothrin, dimethoate and pymetrozine reduce PVY acquisition by aphids (Boquel et al. 2014; Margaritopoulos et al. 2010), but the effect of these insecticides is too slow to prevent PVY spread in the field (Table 7.1). In summary, for most of the field trial studies reported, the efficacy of an insecticide in controlling PVY spread was either not significant, of a limited impact or highly variable between independent experiments. One of the reasons that insecticides have been demonstrated to be either ineffective or only of limited effectiveness is because many aphids which transmit PVY do not colonise plants in a crop and thus only come in contact with the insecticides for a very limited period (reviewed in Perring et al. (1999)).
Percentage of PVY-infected daughter tubers produced by plants treated with a range of insecticides and mineral oil. The insecticides used belong to different chemical groups such as pyrethroids (esfenvalerate, etofenprox, zeta-cypermethrin), neonicotinoids (thiacloprid, thiamethoxam) and pyridine (flonicamid, pymetrozine). The ‘standard’ programme refers to a weekly application of mineral oil, together with a pyrethroid in the first week after emergence (esfenvalerate) and a systemic insecticide every 2 weeks (one treatment with flonicamid followed by pymetrozine and thiacloprid). The ‘intense’ programme is similar to the ‘standard’ programme with, in addition, two applications of mineral oil in the first week after emergence. The letter (w) means weekly application, (2w) means one application every 2 weeks and (fract) means two applications per week with half dose for the first 3 weeks after emergence and thereafter one application per week (Demeulemeester 2013)
3.3 Limitation of Chemical Control: Insecticide Resistance in Aphids
The (intensive) use of insecticides has led to the development of different mechanisms of resistance in some aphid populations. The main aphid vectors displaying various levels of resistances to insecticides worldwide are Myzus persicae (Sulzer), Aphis nasturtii (Kaltenbach), Macrosiphum euphorbiae (Thomas) and Sitobion avenae (Fabricius) (Foster et al. 2014, 2002). Different types of biochemical and molecular mechanisms of insecticide resistance can be found with Myzus persicae (Bass et al. 2014), which includes (i) the overproduction of carboxylesterases (conferring broad-spectrum resistance to members of the organophosphate (OP), (mono-methyl) carbamate and, to a much lesser extent, pyrethroid classes) that hydrolyse the active compound before it affects the nervous system of the insect; (ii) mutation of the acetylcholinesterase enzyme (MACE – modified acetylcholinesterase) resulting in the insensitivity of target site of the insecticides, the acetylcholinesterase enzyme; (iii) mutation of the voltage-gated sodium channel, also named ‘knock-down resistance’ (kdr or super-kdr for the enhanced allelic form), which is conferred by a mutation of a transmembrane ion channel that plays an essential role in the initiation and propagation of action potentials in neurons; and (iv) the mutation of the nicotinic acetylcholine receptor (nAChR), a neurotransmitter-gated ion channel that plays an important role in nerve signalling (Bass et al. 2014). In addition, there are new types of resistance that are currently being identified (such as resistance to pyridine compounds) (Table 7.2; Bass et al. (2014)). Table 7.2 presents the chemical groups of insecticides frequently used in potato production and the corresponding resistance mechanism displayed by Myzus persicae. The incidence of Myzus persicae susceptible to the insecticides listed in Table 7.2 is highly variable from one year to another. For example, in aphid samples collected in the UK in 2004, about 60% of the sampled aphids were genotypically associated with carboxylesterases-mediated resistance, 70% with MACE and 80% with super-kdr, while 5 years before, this proportion was about 20%–5%–80% respectively (IRAG 2008). Myzus persicae individuals resistant to neonicotinoids (with an alteration of the nAChR) have not yet been found in the UK; however, a survey performed in Southern France and Northern Spain in 2010 revealed that resistance to neonicotinoids is relatively widespread in these regions, with an average of 76% of resistance to thiamethoxam (Slater et al. 2012). Combinations of the three main resistance mechanisms (MACE, carboxylesterase and kdr) can be found in individuals of Myzus persicae, but the most common association of resistances found in the field in the UK are MACE with super-kdr (IRAG 2008). Consequently, growers are recommended to minimise whenever possible the use of insecticide mixtures in order to prevent the outbreak of Myzus persicae populations displaying cross-resistances. Populations of Aphis nasturtii (Buckthorn-potato aphid) sampled in Belgium and northern France in the 1990s were found to be resistant to insecticide; however, resistance in Aphis nasturtii seems to be a relatively rare event (Duvauchelle et al. 1997). Macrosiphum euphorbiae has developed a resistance mechanism based on overproduction of an esterase after treatment with pirimicarb and λ-cyhalothrin. This mechanism of resistance is analogous to the one found in M. persicae. However, it is not known if this mode of resistance is effective when treatments are applied at the recommended rate (Foster et al. 2002). Kdr resistance to pyrethroids (λ-cyhalothrin) was found in Sitobion avenae in the UK (Foster et al. 2014), and the prevalence of resistant individuals in 2015 varied from 45 to 75% depending on the location of sampling (Malloch et al. 2016).
3.4 Synthetic Pheromones
Numerous aphid species secrete pheromones as a behavioural mechanism when attacked by natural enemies (Kislow and Edwards 1972; Nault et al. 1973). (E)-β-farnesene (EβF) is the main alarm pheromone (Pickett et al. 1992; Zhang et al. 1997). EβF has a repellent effect on aphid colonies and affects the development and fecundity of the aphid populations (Su et al. 2006; Gibson et al. 1984). Additionally, it was shown in the laboratory that EβF inhibits the acquisition and subsequent inoculation of PVY by Myzus persicae (Sulzer) (Gibson et al. 1984). Another laboratory experiment performed with tobacco plantlets using apterous aphids (Myzus persicae (Sulzer) and Macrosiphum euphorbiae (Thomas)) revealed a higher spread of PVY when the aphids are in contact with EβF (Lin et al. 2016). This result suggests an increase in the movement of apterous aphids from plant to plant in the presence of EβF. Apterous aphids are known to be able to move from plant to plant even without the overlapping of crop canopy, by walking on the soil surface (Alyokhin and Sewell 2003; Narayandas and Alyokhin 2006). Nevertheless, winged aphids are more motile in the crop by flying from plant to plant and, hence, have a higher impact on PVY spread in the field (Boiteau 1997). A 1-year experiment undertaken in field conditions showed that PVY spread was identical with or without the diffusion of EβF (Crutzen et al. 2014). While pheromone treatment might have a limited impact on the aphid transmission of PVY, the efficacy of insecticide in controlling aphid populations can be improved by using EβF treatments, as reported in Chinese cabbage fields treated simultaneously with imidacloprid and EβF (Cui et al. 2012). The strategy of combining EβF diffusion with insecticide treatments to control PVY spread has yet to be tested and might represent an interesting approach to control aphid populations and consequently virus transmission.
3.5 Elicitors
Several chemicals are able to induce systemic resistance of plants to pathogens (Kessmann et al. 1994). Among those chemicals, salicylic acid (SA) is known to induce resistance to a wide range of pathogens including viruses (Nakayama et al. 1996; Vasyukova and Ozeretskovskaya 2007). It was shown that Potato virus X (PVX) accumulation in tobacco leaves is reduced after SA treatment (Naylor et al. 1998). This effect was also observed in tomato leaves inoculated with PVY and treated with acibenzolar-S-methyl (Bion®), a functional analogue of SA (Petrov and Andonova 2012). Bion® was also tested in a field trial to control PVY spread in potatoes, which resulted in a relatively low (14%) but nevertheless significant reduction of PVY transmission (Dupuis et al. 2014).
4 Combined Methods
Many of the techniques cited above have often been tested in combination. Crop borders and mineral oil treatments have been tested together, and the combination of those two techniques was more effective than when they were applied individually. In the field trials of Boiteau et al. (2009), the combination of applying oil sprays to potato crops and a border crop improved the efficacy of PVY control compared with oil treatment and borders used alone: 47–59% reduction in the incidence of PVY in the first year, 57–63% in the second year and 79–97% in the third year of field trials. Insecticide treatment of a border crop did not produce a reduction in the incidence of tuber infection by PVY (Difonzo et al. 1996). A synergistic effect of applying straw mulching and mineral oil together was recorded in a 1-year trial in Switzerland (Fig. 7.3), but no synergy was found in a similar trial in Finland (Kirchner et al. 2014). In the Swiss trial, the efficacy of PVY control with straw mulching was about 47%, while spraying mineral oil produced a similar efficacy of a 55% reduction in PVY. However, an efficacy of 70% was achieved when both techniques were used together. In the Finnish trial, straw mulching produced a reduction of 26% in PVY infection, but a combined mineral oil spraying and straw mulching treatment gave only a 19% reduction. It is likely that the extent of any synergistic effect of mineral oil with straw mulch may depend upon the experimental conditions of a trial. As explained above, the efficacy of mineral oil treatment increases the older the plants are when they are sprayed, while the efficacy of straw mulching is greatest early in the season before the soil surface becomes covered by the crop canopy. Thus, the timing and location of the peak of aphid activity (data not shown) could explain the differences between those two trials. Oat intercropping was also tested with mineral oil applications, and some synergy was recorded (Fig. 7.3). Oat intercropping resulted in 63% reduction in PVY infection in the potato crop, and the efficacy of PVY reduction reached 89% when oat intercropping was used together with mineral oil spraying. The combination of insecticides and mineral oil treatments can also provide some synergy, increasing the protection of treated crops against PVY. A Canadian survey of 56 crops of seed potatoes being multiplied using distinct cropping techniques revealed that mineral oil was more effective when combined with insecticide applications, particularly when used early in the season (Mackenzie et al. 2014; 2016). This synergy was not found in all circumstances. A weekly treatment with esfenvalerate together with mineral oil gave better protection (42%) of a crop against PVY spread than a weekly spray of mineral oil alone (9%; p<0.05) (Fig 7.5). Contrastingly, the combination of a weekly treatment of thiomethon + fluvalinate and a twice-weekly treatment with mineral oil did not improve the protection of the crop against PVY infection (p > 0.05 for all years of trial) than when treatments were applied individually (Fig. 7.6). Additional studies conducted in a greenhouse have shown a significant reduction in the transmission of PVY in plants treated with mineral oil and insecticides (Martin-Lopez et al. 2006; Gibson and Rice 1986). This synergistic effect of insecticides and oil may be influenced by the particular chemistry of an insecticide, e.g. synthetic pyrethroids (Fig. 7.5), and by the time of application because mineral oil alone is less efficient when applied early in the season (Mackenzie et al. 2014; Gibson and Cayley 1984; Demeulemeester 2013).
Efficacy in the reduction of the incidence of PVY in progeny tubers in response to foliar treatments with either mineral oil (3 l twice a week) sprayed alone or in combination with a pyrethroid insecticide (0.6 l of thiomethon (200g/l) +fluvalinate (72g/l) once a week) over a 3-year period. Each year, the trials were conducted in three different locations with three replications of each treatment (Rolot 2005)
5 Seed Certification Schemes
5.1 History and Evolution
Potato is a vegetatively propagated crop, and consequently tubers can potentially be contaminated with a large range of organisms (Franc 2001). These pathogens can then be transmitted from the seed tuber to the new plant in the next generation of multiplication and also to healthy plants in the crop. The pathogens can range from fungi to viroids and can be carried within and/or on the surface of a tuber. The diseases caused by the pathogens can have a serious effect on the yield and the quality of a crop. Seed potatoes are potatoes intended for planting, unlike those intended for end uses such as consumption and processing. As commercial trade in seed potatoes developed, a perceived need arose for some form of independent verification of the quality of seed potatoes being marketed. This resulted in the establishment of seed potato certification schemes under official control with tolerances for quality aspects of seed potatoes being applied at each year of propagation (Rousselle et al. 1996; EPPO 1999). The first potato seed certification scheme was established in Europe (Germany) in the first years of the nineteenth century and about 10 years later in the USA (Shepard and Claflin 1975). Similar schemes were introduced in New Zealand in the late 1920s and in Australia in the 1930s (Maunder 2005; Crump 2008). Until early 1970s, production of the initial planting material was largely by ‘clonal selection’ (or ‘positive selection’), which involved selecting disease-free tubers from apparently healthy mother plants (Gildemacher et al. 2011). As far as possible, testing was conducted on mother plants and tubers to ensure freedom from viruses known to be present in a country. However, appropriate testing was not available to confirm freedom from bacterial and fungal tuber pathogens. Subsequently, tissue culture techniques were developed for producing initial planting material in which greater security of plant health could be achieved through an extensive testing programme (Espinoza et al. 1984; Dodds 1988). In Scotland, the first technique used was ‘stem cuttings’ starting in the late 1960s with the aim of eliminating latent tuber-borne pathogens, especially those causing blackleg, from the initial planting material (Jeffries 1986; Hall 1993), but this was superseded by micropropagation to produce ‘nuclear stock’. Nowadays, this method is used as the starting point in almost all certification schemes worldwide because it allows material to be tested comprehensively for quality and notifiable pathogens and pests (EPPO 1999; Donnelly et al. 2003; Frost et al. 2013). One exception is the Netherlands, where clonal selection is also used, together with micropropagation, to produce the initial planting material (NIVAP 2016). Tubers produced from the initial planting material are then multiplied for a number of generations in the field as seed potatoes under certification control until finally marketed for end use (Frost et al. 2013). In Asia, Africa and South America, ‘informal’ seed potato production systems account for 94% of the market (Thomas-Sharma et al. 2016). In informal seed potato production systems, seed tubers are sourced on farm, neighbouring farms, local markets and unofficial specialist producers (Hirpa et al. 2010). The health status of this type of seed potatoes can be very variable, and consequently crop yields fall far short of their potential. This is largely due to the inherently high amounts of virus in the potatoes and greater aphid pressure in more favourable environments for spread. Gildemacher et al. (2011) assessed the economic return of three different seed potato schemes: (i) the use of the farmer seed stock, (ii) the use of the ‘positive selection’ technique and (iii) the use of certified seed from the market. This showed that buying high-quality seed is generally more economically effective than the other schemes because the yield obtained with certified seed is usually greater. This does assume that farmers can afford buying high-quality seed potatoes and that the required cultivar is available for planting.
5.2 Objectives of Seed Potato Certification Schemes
The main goal of a potato certification scheme is to assure the quality of seed potatoes being marketed through an independent process of verification and testing conducted by a designated certifying authority. The aspects covered by certification are identity of cultivar, purity of crop, diseases and pests affecting quality or yield, external quality, physiology, size and labelling (UNECE 2015). Seed potatoes are propagated over a number of years so genealogy and traceability are key elements of a well-developed certification scheme. In order to maintain the health of seed potatoes during propagation, tolerances for disease and quality characteristics are set for each generation, being strictest for first generation and becoming slightly more relaxed for subsequent generations in the field (EPPO 1999). Verification of tolerances is largely conducted by visual inspection of plants or tubers supported by testing as appropriate (Franc 2001; Frost et al. 2013).
5.3 Principles of Certification
Although varying degrees of complexity exist, the basic principles of seed certification remain the same (EPPO 1999). Currently, the first step is the multiplication of an in vitro pathogen-free plant (nuclear stock) which can then be multiplied in an approved facility to produce large numbers of in vitro plantlets or microtubers (i.e. tubers produced in vitro by a micropropagated plantlet). Nuclear stock is normally subject to an extensive testing programme to ensure its freedom from a range of specified pathogens before being used to produce minitubers in insect-free greenhouses or screenhouses (Frost et al. 2013; EPPO 1999). These tubers are then planted in the field the following year to produce the first generation of seed potatoes (Franc 2001). The import of healthy plant material or clonal selection can be used as an alternative to tissue culture (Franc 2001; Schulte-Geldermann et al. 2012). Several years of field multiplication will ensue before being used by ware potato producers. In European countries, most certification schemes classify seed potatoes into three categories: pre-basic, basic and certified in accordance with United Nations Economic Commission for Europe (UNECE) Standard for Seed Potatoes (UNECE 2015). At least two classes within each category are normally set nationally although the possibility of agreed common classes is being explored by EU (2002). The number of years that seed potatoes can be multiplied is also limited in a scheme, normally less than nine (Frost et al. 2013), and with a maximum of nine generations allowed in the EU since 2016 (directives 2014/20/CE and 2014/21/CE). Some schemes, especially in North America, are based solely on generation number although the number does not always correspond to the number of generations in the field from initiation (Willem Schrage, personal communication). Each year, crops are visually examined by seed potato inspectors for varietal purity and a range of faults including virus diseases around the time when plants are flowering. Inspected crops have to comply with specific tolerances for faults for the class for which they have been entered; otherwise the crop is downgraded to the appropriate class or, in extreme circumstances, not accepted as being suitable for marketing as seed potatoes (Frost et al. 2013). The UNECE has developed an international standard for certification and marketing of seed potato that sets out a common terminology and minimum commercial quality requirements for the certification of high-quality seed intended for marketing internationally (UNECE 2015). This standard is a useful blueprint to facilitate seed potato trade between countries that already have their own certification schemes in accordance with the UNECE standard. It is also a good model for countries aiming to develop their own seed potato certification scheme.
5.4 Virus Diagnostic Methods Used in Certification Schemes
Historically, the incidence of viruses, including PVY, in seed potato crops was assessed visually at two or more inspections according to disease symptoms reported as severe mosaic or mild mosaic (see Chap. 5). Knowledge of the virus causing symptoms was not essential for certification purposes but was nevertheless collected for future analysis. If any tolerance is exceeded at inspection, a crop is downgraded as appropriate or rejected (Shepard and Claflin 1975). This method is still used in seed potato production areas where PVY pressure is low (e.g. Scotland). Diseased plants seen at inspection are generally the result of secondary transmission from infected tubers although symptoms of primary infection can sometimes be observed, but this can underestimate the true extent of such infection. The incidence of symptomatic plants derived from primary infection varies with environment and time of infection. Late-season infection of a susceptible potato plant will probably result in no development of foliar symptoms although virus may be translocated to daughter tubers, highlighting the necessity for post-harvest testing (Franc 2001; Frost et al. 2013). Therefore, in some environments, relying on visual inspection may be insufficient to provide assurance that seed potatoes will meet the expectations of customers (Lindner et al. 2015). Additional measures such as a targeted post-harvest assessment were introduced in some certification schemes to check for late-season virus infection or, in some cases, symptomless infection of tolerant cultivars.
There are numerous post-harvest diagnostic methods for viruses. The first method to be developed was termed ‘post-harvest grow-out assay’ or ‘growing-on assay’. A sample of a prescribed number of tubers is collected at random from a crop before harvest or shortly after harvest. Seed pieces consisting of a single eye are removed from each tuber and planted (with or without a treatment to break dormancy) in a greenhouse or outdoors if the climate of a country is suitable. When these tests were first conducted, the plants grown from each seed piece were then examined visually for symptoms of virus infection (Franc 2001). Microscopy (Igel-Lange test) or serological (radial-immunodiffusion) techniques were used to support these assessments (Shepard and Claflin 1975; Gugerli and Fries 1983). Visual observation was later replaced by an enzyme-linked immunosorbent assay (ELISA) for the detection of virus (see Chap. 5). France and Belgium still use ELISA for this type of testing.
A variation of this method is the so-called winter test. After breaking tuber dormancy, a standard sample from a crop is shipped to a ‘warmer’ location for planting. After emergence, each plant is visually inspected, and/or a leaf sample is taken for further testing by serological or molecular methods. The northern states of the USA use this approach and ship seed samples to southern states for a ‘winter test’. These programmes are expensive and are gradually being replaced by laboratory-based tuber tests (Shepard and Claflin 1975; Franc 2001). Laboratory-based tests of tubers were first developed in Switzerland in the early 1980s. After dormancy of the tubers is broken by fumigation with Rindite (a mixture of 2-chloroethanol, 1,2-dichloroethane and carbon tetrachloride 7:3:1), sap from the basis of the sprouts of each tested tuber is tested by ELISA (Gugerli and Gehriger 1980). In Europe, this product is banned due to its toxicity and is replaced by a solution of gibberellic acid. In this case, ELISA test is performed on recently emerged potato leaflets. ELISA is gradually being replaced by molecular techniques such as real-time RT-PCR, which has been employed in seed potato certification schemes in the Netherlands and Scotland since 2013 and in Switzerland and France since 2015 (this list of countries is not exhaustive).
5.5 The Role of Seed Certification Schemes for PVY Control and Future Challenges
Seed certification schemes are essential in managing and controlling non-persistent viruses such as PVY in seed potato production through inspection of seed potato crops and statutory virus testing. They provide a purchaser with assurance that seed potatoes have been produced according to scheme requirements and standards, particularly with regard to health and cultivar purity. A post-harvest test should provide a reasonable estimate of the incidence of PVY in the daughter crop, provided the sample collected is representative of the crop (Frost et al. 2013). Certification, therefore, offers the opportunity to discard or ‘flush through’ crops, which pose a risk to the health of subsequent crops. The population dynamics of PVY are changing (see references in Chap. 3). Ordinary and veinal necrotic PVY strains (i.e. PVYO and PVYN) are gradually becoming less prevalent in Europe and are being replaced by recombinant strains PVYNTN and PVYN-Wi. PVYO and PVYN are generally believed to be more aggressive than the now-prevalent above-mentioned recombinant strains and elicit severe foliar symptoms on potato plants, although this does vary between cultivars. On certain cultivars, the recombinant PVYNTN and PVYN-Wi strains tend to produce milder symptoms, and in some occasions, higher copy numbers of viral RNA were found in leaves displaying milder symptoms (Lindner et al. 2015; Karasev and Gray 2013). This suggests an apparent lack of correlation between symptom severity, virus concentration and virus strains; therefore, adopting different tolerances with respect to the severity of virus symptoms might no longer be appropriate in certification schemes when it comes to control PVY (Lindner et al. 2015). Consequently, the Specialised Section on Standardisation of Seed Potatoes at UNECE has revised the UNECE Standard (S-1) Seed Potatoes and adopted new virus tolerances irrespective of the severity of viral symptoms observed in growing crops (UNECE 2014). As mild mosaic/viral symptoms may be missed during crop inspection, particularly if conditions are less favourable for visual inspection of plants (e.g. bright sunlight or water on foliage) or, as stated above, when plants are infected by less aggressive virus strains or if a cultivar is tolerant to some virus species, visual inspections should be complemented by post-harvest virus testing, as undertaken by most developed certification schemes worldwide.
6 Conclusion
Controlling the spread of PVY remains a challenge to the potato industry worldwide because of its non-persistent mode of transmission and the evolution of new strains and variants. Prophylactic measures such as roguing and early haulm destruction are required to limit PVY spread but are not efficient alone; the implementation of additional control strategies is needed to protect the susceptible potato cultivars. The control strategies presented in this chapter can help to reduce PVY transmission by aphids; however, each individual control strategy has its own limitations. Border crops can reduce the introduction of virus from outside the field but will not reduce the risk of transmission within a crop. While displaying variable levels of protection, mineral oil is generally used by seed potato growers to reduce aphid transmission of non-persistent viruses. Nevertheless, the effectiveness of mineral oil is dependent on the timing of aphid flights. For ‘early’ aphid flights that occur during the first weeks after plant emergence when plants are at their most susceptible stage, frequent oil application (two to three times per week to maximise coverage of new leaves) might provide some level of protection by reducing PVY transmission if inoculum is present. For ‘late’ aphid flights (3–4 weeks after emergence), most leaves on a potato plant should be more effectively covered with a film of oil, which reduces the risk of infection. The efficacy of insecticides in controlling PVY spread is highly variable and at best of a limited impact. The effectiveness of control of PVY transmission through combined insecticide and mineral oil treatment may be more effective than individual insecticide or mineral oil treatment. Due to the rapid development of leaves and the difficulty of forecasting aphid flights during early crop development, it is advisable to combine mineral oil treatments with other control techniques such as intercropping, straw mulching and insecticide treatments. These emphasise the importance of controlling virus through appropriate monitoring methods and crop management enforced by seed certification schemes through the use of ‘clean’ input seed (ideally virus free or seed potatoes with a low incidence of virus) and, when possible, the segregation of seed and ware crops to minimise the risk of virus transmission between them.
References
Al-Daoud F, Fageria MS, Zhang JH, Boquel S, Pelletier Y (2014) Mineral oil inhibits movement of potato virus Y in potato plants in an age-dependent manner. Am J Potato Res 91:337–345
Al-Mrabeh A, Anderson E, Torrance L, Evans A, Fenton B (2010) A literature review of insecticide and mineral oil use in preventing the spread of non-persistent viruses in potato crops In: Council P (ed) Agriculture and horticulture development board 2010. Warwickshire, UK, 65. (Council P, ed.)
Alyokhin A, Sewell G (2003) On-soil movement and plant colonization by walking wingless morphs of three aphid species (Homoptera : Aphididae) in greenhouse arenas. Environ Entomol 32:1393–1398
Alyokhin A, Sewell G, Groden E (2002) Aphid abundance and potato virus Y transmission in imidacloprid-treated potatoes. Am J Potato Res 79:255–262
Ameline A, Couty A, Martoub M, Giordanengo P (2009) Effects of mineral oil application on the orientation and feeding behaviour of Macrosiphum euphorbiae (Homoptera: Aphidae). Acta Entomol Sin 52:617–623
Basky Z (2003) Virus vector aphid activity and seed potato tuber virus infection in Hungary. Anzeiger Fur Schadlingskunde-Journal of Pest Science 76:83–88
Basky Z, Almasi A (2005) Differences in aphid transmissibility and translocation between PVYN and PVY0 isolates. J Pest Sci 78:67–75
Bass C, Puinean AM, Zimmer CT et al (2014) The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem Mol Biol 51:41–51
Beemster ABR (1972) Virus translocation in potato plants and mature plant resistance. In: De Bokx JA (ed) Viruses of potatoes and seed potato production. Pudoc, Wageningen, pp 144–151
Bell AC (1980) The use of mineral oil to inhibit aphid transmission of potato veinal necrosis virus: a laboratory and field experiment. Record of agricultural Research, Northern Ireland Dept. Agric. Res. 28, 13–7
Bell AC (1989) Use of oil and pyrethroid sprays to inhibit the spread of potato virus-Yn in the field. Crop Prot 8:37–39
Boiteau G (1997) Comparative propensity for dispersal of apterous and alate morphs of three potato-colonizing aphid species. Can J Zool 75:1396–1403
Boiteau G, Singh RP (1999) Field assessment of imidacloprid to reduce the spread of PVYO and PLRV in potato. Am J Potato Res 76:31–36
Boiteau G, Wood FA (1982) Persistence of mineral-oil spray deposits on potato leaves. Am Potato J 59:55–63
Boiteau G, King RR, Levesque D (1985) Lethal and sublethal effects of aldicarb on two potato aphids (Homoptera: Aphididae): Myzus persicae (Sulzer) and Macrosiphum euphorbiae (Thomas). J Econ Entomol 78:41–44
Boiteau G, Singh M, Lavoie J (2009) Crop border and mineral oil sprays used in combination as physical control methods of the aphid-transmitted potato virus Y in potato. Pest Manag Sci 65:255–259
Boquel S, Giguere MA, Clark C, Nanayakkara U, Zhang JH, Pelletier Y (2013) Effect of mineral oil on potato virus Y acquisition by Rhopalosiphum padi. Entomol Exp Appl 148:48–55
Boquel S, Zhang J, Goyer C, Giguère M-A, Clark C, Pelletier Y (2014) Effect of insecticide-treated potato plants on aphid behavior and potato virus Y acquisition. Pest Manag Sci 71:1106–1112
Boydston RA, D-Seymour M, Brown CR, Alva AK (2006) Freezing behavior of potato (Solanum tuberosum) tubers in soil. Am J Potato Res 83:305–315
Bradley RHE (1963) Some ways in which a paraffin oil impedes aphid transmission of potato virus Y. Can J Microbiol 9:369–380
Bradley RHE, Wood FA, Wade CV (1962) Aphid transmission of potato virus Y inhibitted by oils. Virology 18:327–329
Bradley RHE, Moore CA, Pond DD (1966) Spread of potato virus Y curtailed by oil. Nature 209:1370–1371
Bragard C, Caciagli P, Lemaire O, et al. (2013) Status and prospects of plant virus control through interference with vector transmission. In: Vanalfen NK (ed) Annual review of phytopathology, Vol 51. Annual Reviews, 4139 El Camino Way, Po Box 10139, Palo Alto, Ca 94303-0897 USA, 177-201. (Annual Review of Phytopathology; vol. 51.)
Broadbent L, Gregory PH, Tinsley TW (1950) Roguing potato crops for virus diseases. Ann Appl Biol 37:640–650
Cho S-R, Koo H-N, Yoon C, Kim G-H (2011) Sublethal effects of flonicamid and thiamethoxam on green peach aphid, Myzus persicae and feeding behavior analysis. J Korean Soc Appl Biol Chem 54:889–898
Cip IPC (1979) Insect vector transmission of potato viruses. CIP, International Potato Center
Collar JL, Avilla C, Duque M, Fereres A (1997) Behavioral response and virus vector ability of Myzus persicae (Homoptera : aphididae) probing on pepper plants treated with aphicides. J Econ Entomol 90:1628–1634
Cooke LR, HTaM S, Hermansen A et al (2011) Epidemiology and integrated control of potato late blight in Europe. Potato Res 54:183–222
Crump NS, (2008) Seed potato certification and its role in soilborne disease management. In: Vispa VCSPA (ed) ViSPA, Voctoria, 1
Crutzen F, Moreau V, Francis F, Bragard C (2014) The alarm pheromone E-β-Farnesene as biocontrol semiochemical to reduce the propagation of potato virus Y in potato fields. In: Research EaFP, ed. 19th Triennial Conference of the European Association for Potato Research. Brussels, European Association for Potato Research, 65. 1
Cui LL, Dong J, Francis F et al (2012) E-beta-farnesene synergizes the influence of an insecticide to improve control of cabbage aphids in China. Crop Prot 35:91–96
Davis JA, Radcliffe EB, Ragsdale DW (2009) Planter skips and impaired stand favors potato virus Y spread in potato. Am J Potato Res 86:203–208
Dawson G, Anderson F, Bain R, et al. (2015) Effectiveness of mineral and vegetable oils in minimising the spread of non-persistent viruses in potato seed crops in Great Britain. In Potato Council project R449 final report. Potato Council
Demeulemeester K (2013) Control of potato virus Y spread in seed potatoes. In: Virology E (ed) Proceedings of the EAPR Section Virology Meeting, Antalya, 57
Dewijs JJ (1980) The caracteristics of mineral-oils in relation to their inhibitory activity on the aphid transmission of potato virus Y. In. Netherlands. J Plant Pathol 86:291–300
Dewijs JJ, Sturm E, Schwinn FJ (1979) Viscosity of mineral-oils in relation to their ability to inhibit the transmission of stylet-borne viruses. In. Netherlands. J Plant Pathol 85:19–22
Difonzo CD, Ragsdale DW, Radcliffe EB, Gudmestad NC, Secor GA (1996) Crop borders reduce potato virus Y incidence in seed potato. Ann Appl Biol 129:289–302
Dodds JH (1988) Tissue culture technology: practical application of sophisticated methods. Am J Potato Res 65:167–180
Donnelly DJ, Coleman WK, Coleman SE (2003) Potato microtuber production and performance: a review. Am J Potato Res 80:103–115
Döring TF (2014) How aphids find their host plants, and how they don't. Ann Appl Biol 165:3–26
Döring TF, Schmidt T (2007) Response of apterous potato aphids to visual contrasts (Hemiptera : Aphididae). Entomologia Generalis 30:190–191
Draper MD, Pasche JS, Gudmestad NC (2002) Factors influencing PVY development and disease expression in three potato cultivars. Am J Potato Res 79:155–165
Dupuis B (2016) The movement of potato virus Y (PVY) in tehe vascular system of potato plants. Eur J Plant Pathol:1–9
Dupuis B, Schwaerzel R (2011) Impact of Haulm killing on potato virus Y (PVY) spread. Proceedings of the The 18th Triennial Conference of the European Association for Potato Research, . Oulu, 167
Dupuis B, Schwaerzel R, Goy G, Tallant M, Derron J (2010) Stepwise development of an efficient method to control potato virus Y spread in seed potato fields. In: Focus B (ed) Proceedings of the EAPR virology. Bioforsk Focus, Hamar, 22
Dupuis B, Schwaerzel R, Derron J (2014) Efficacy of three strategies based on insecticide, oil and elicitor treatments in controlling aphid populations and potato virus Y epidemics in potato fields. J Phytopathol 162:14–18
Duvauchelle S, Dubois L, Nguyen N (1997) Aphids and viruses on ware potatoes in northern France particularly in 1995 and 1996. Mededelingen Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen Universiteit Gent 62:545–546
Edwardson JR, Christie RG (1997) Potyviruses. University of Florida, Gainesville
Eppo (1999) EPPO standard PM 4/28, certification schemes – seed potatoes. Bull OEPP/EPPO 29:253–267
Espinoza N, Estrada R, Tovar P, Bryan J, Dodds JH, (1984) Tissue culture micropropagation, conservation, and export of potato germplasm. Lima, Peru: International Potato Center (CIP)
Eu (2002) Council Directive 2002/56/EC of 13 June 2002 on the marketing of seed potatoes. In: Eu (ed) Union TCOTE
Fageria M, Boquel S, Leclair G, Pelletier Y (2014a) Quantification of mineral oil accumulation and movement in potato plants and its significance in potato virus Y management. Pest Manag Sci 70:1243–1248
Fageria MS, Boquel S, Leclair G, Pelletier Y (2014b) The use of mineral oil in potato protection: dynamics in the plant and effect on potato virus Y spread. Am J Potato Res 91:476–484
Fereres A, Moreno A (2009) Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res 141:158–168
Foster SP, Hackett B, Mason N, et al (2002) Resistance to carbamate, organophosphate and pyrethroid insecticides in the potato aphid (Macrosiphum euphorbiae). Proceedings of the Brighton crop conference: Pests and diseases Brighton, 811–816
Foster SP, Paul VL, Slater R et al (2014) A mutation (L1014F) in the voltage-gated sodium channel of the grain aphid, Sitobion avenae, is associated with resistance to pyrethroid insecticides. Pest Manag Sci 70:1249–1253
Franc GD (2001) Seed certification as a virus management tool. Virus and virus-like diseases of potatoes and production of seed-potatoes. Kluwer, Dordrecht, pp 407–420
Frost KE, Groves RL, Charkowski AO (2013) Integrated control of potato pathogens through seed potato certification and provision of clean seed potatoes. Plant Dis 97:1268–1280
Gabriel W (1965) Influence of temperature on spread of aphid-borne potato virus diseases. Ann Appl Biol 56:461
Gibson RW (1991) The development of mature plant-resistance in 4 potato cultivars against aphid-inoculated potato-virus Yo and Yn in 4 potato cultivars. Potato Res 34:205–210
Gibson RW, Cayley GR (1984) Improved control of potato virus-Y by mineral-oil plus the pyrethroid cypermethrin applied electrostatically. Crop Prot 3:469–478
Gibson RW, Rice AD (1986) The combined use of mineral-oils and Pyrethroids to control plant-viruses transmitted nonpersistently and semi-persistently by Myzus persicae. Ann Appl Biol 109:465–472
Gibson RW, Rice AD, Sawicki RM (1982) Effect of the pyrethroid deltamethrin on the acquisition and inoculation of viruses by Myzus persicae. Ann Appl Biol 100:49–54
Gibson RW, Pickett JA, Dawson GW, Rice AD, Stribley MF (1984) Effects of aphid alarm pheromone derivatives and related compounds on non-persistent and semi-persistent plant virus transmission by Mysus persicae. Ann Appl Biol 104:203–209
Gildemacher PR, Schulte-Geldermann E, Borus D et al (2011) Seed potato quality improvement through positive selection by smallholder farmers in Kenya. Potato Res 54:253–266
Gray S, De Boer S, Lorenzen J et al (2010) Potato virus Y: an evolving concern for potato crops in the United States and Canada. Plant Dis 94:1384–1397
Gugerli P, Fries P (1983) Characterization of monoconal antibodies to potato virus Y and their use for virus detection. J Gen Virol 64:2471–2477
Gugerli P, Gehriger W (1980) Enzyme-linked immunosorbent assay (ELISA) for the detection of potato leafroll virus and potato virus Y in potato tubers after artificial break of dormancy. Potato Res 23:353–359
Hall TD (1993) Seed potato certification in the UK. In: Council BCP (ed) Proceedings of the plant health and the Single European Market: British Crop Protection Council, pp 77–82
Hansen LM, Nielsen SL (2012) Efficacy of mineral oil combined with insecticides for the control of aphid virus vectors to reduce potato virus Y infections in seed potatoes (Solanum tuberosum). Acta Agric Scand B Soil Plant Sci 62:132–137
Heimbach U, Eggers C, Thieme T (2004) Effect of mulch on aphid populations and virus transmissions in some arable crops. In: Simon JC, Dedryver CA, Rispe C, Hulle M (eds) Proceedings of the Aphids in a new millennium. 2001 Rennes, France, pp 307–312
Helson VA, Minshall W (1951) Effects of petroleum oils on the carbon dioxide output in respiration of parsnip and mustard. Plant Physiol 31:5–11
Hesler LS, Plapp FW (1986) Uses of oils in insect control. Southwest Entomol 11:1–8
Hirpa A, Meuwissen MPM, Tesfaye A et al (2010) Analysis of seed potato systems in Ethiopia. Am J Potato Res 87:537–552
Irag (2008) Guidelines for preventing and managing insecticide resistance in aphids on potatoes. In: Council P (ed) Oxford, 4
Irag (2014) Guidelines for preventing and managing insecticide resistance in aphids on potatoes. In: Board AaHD (ed) 8
Jeffries CJ (1986) The Scottish seed potato classification scheme and the production of nucleus stocks using micropropagation. In: Council BCP (ed) Proceedings of the symposium on healthy planting material, British Crop Protection Council, 239–247
Jones DC, Woodford JT, Main SC, Pallett D, Barker H (1996) The role of volunteer potatoes in the spread of potato virus Y-N in ware crops of cv. record. Ann Appl Biol 129:471–478
Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF (2001) A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci U S A 98:9448–9453
Kaliciak A, Syller J (2009) New hosts of potato virus Y (PVY) among common wild plants in Europe. Eur J Plant Pathol 124:707–713
Karasev AV, Gray SM (2013) Continuous and emerging challenges of potato virus Y in potato. Annu Rev Phytopathol 51(51):571–586
Kazinczi G, Horvath J, Takacs AP, Gaborjanyi R, Beres I (2004) Experimental and natural weed host-virus relations. Commun Agric Appl Biol Sci 69:53–60
Kerlan C, Robert Y, Perennec P, Guillery E (1987) Survey of the level of infection by PVYo and control methods developed in France for potato seed production. Potato Res 30:651–667
Kessmann H, Staub T, Hofmann C, et al (1994) Induction of systemic acquired disease resistance in plants by chemicals. In: Cook RJ, (ed) Annual Review of Phytopathology. Annual Reviews Inc. {a}, P.O. Box 10139, 4139 El Camino Way, Palo Alto, California 94306, USA, 439–59. (Annual Review of Phytopathology; vol. 32.)
Kirchner SM, Hiltunen LH, Santala J et al (2014) Comparison of straw mulch, insecticides, mineral oil, and birch extract for control of transmission of potato virus Y in seed potato crops. Potato Res 57:59–75
Kislow CJ, Edwards LJ (1972) Repellent odours in aphids. Nature 235:108–109
Klueken AM, Hau B, Ulber B, Poehling HM (2009) Forecasting migration of cereal aphids (Hemiptera: Aphididae) in autumn and spring. J Appl Entomol 133:328–344
Le Romancer M, Kerlan C (1991) Superficial ringspot necrosis of potato tubers, a recent disease caused by potato virus Y. Agronomie 11:889–900
Lin K, Bushnell W, Szabo L, Smith A (1996) Isolation and expression of a host response gene family encoding thaumatinlike proteins in incompatible oat-stem rust fungus interactions. Mol Plant-Microbe Interact 9:511–522
Lin FJ, Bosquee E, Liu YJ, Chen JL, Liu Y, Francis F (2016) Impact of aphid alarm pheromone release on virus transmission efficiency: when pest control strategy could induce higher virus dispersion. J Virol Methods 235:34–40
Lindner K, Trautwein F, Kellermann A, Bauch G (2015) Potato virus Y (PVY) in seed potato certification. J Plant Dis Protect 122:109–119
Loebenstein G, Deutsch M, Alper M (1964) Preventing aphid-spread cucumber mosaic virus with oils. Phytopathology 54:960
Lutman PJ (1977) Investigations into some aspects of the biology of potatoes as weeds. Weed Res 17:123–132
Mackenzie TDB, Fageria MS, Nie XZ, Singh M (2014) Effects of crop management practices on current-season spread of potato virus Y. Plant Dis 98:213–222
Mackenzie TDB, Nie X, Singh M (2016) Crop management practices and reduction of on-farm spread of potato virus Y: a 5-Year study in commercial potato fields in New Brunswick., Canada. Am J Potato Res 93:552–563
Malloch G, Foster S, Williamson M (2016) Monitoring pyrethroid resistance (kdr) and genetic diversity in UK populations of the grain aphid, Sitobion avenae during 2015. In: Council P (ed) Kenilworth, 26
Margaritopoulos JT, Tsamandani K, Kanavaki OM, Katis NI, Tsitsipis JA (2010) Efficacy of pymetrozine against Myzus persicae and in reducing potato virus Y transmission on tobacco plants. J Appl Entomol 134:323–332
Martin-Lopez B, Varela I, Marnotes S, Cabaleiro C (2006) Use of oils combined with low doses of insecticide for the control of Myzus persicae and PVY epidemics. Pest Manag Sci 62:372–378
Martoub M (2010) Impact global de l’huile minerale blanche sur le pathosysteme plante - puceron-virus: Universite de Picardie Jule Verne PhD thesis
Maunder HB, (2005) The history of seed potato certification in New Zealand 1927–2000. Wellington, New-Zealand: Vegfed, the New Zealand Vegetable and Potato Growers’ Federation
Milosevic D (1996) Efficacy of oils and insecticides in potato plant protection against infection by potato virus Y and leaf roll virus (PV and PLRV). Zaštita Bilja 47:333–342
Morita M, Ueda T, Yoneda T, Koyanagi T, Haga T (2007) Flonicamid, a novel insecticide with a rapid inhibitory effect on aphid feeding. Pest Manag Sci 63:969–973
Nakayama M, Matsuura K, Okuno T (1996) Production of salicylic acid in tobacco and cowpea plants by a systemic fungicide ferimzone and induction of resistance to virus infection. J Pestic Sci 21:69–72
Narayandas GK, Alyokhin AV (2006) Interplant movement of potato aphid (Homoptera : aphididae) in response to environmental stimuli. Environ Entomol 35:733–739
Nault LR, Edwards JL, Styer WE (1973) Aphid alarm pheromones: secretion and reception. Environ Entomol 2:101–105
Naylor M, Murphy AM, Berry JO, Carr JP (1998) Salicylic acid can induce resistance to plant virus movement. Mol Plant-Microbe Interact 11:860–868
Nivap (2016) Clonal selection – Netherlands. In: Nivap, ed. (2016.)
Perring TM, Gruenhagen NM, Farrar CA (1999) Management of plant viral diseases through chemical control of insect vectors. Annu Rev Entomol 44:457–481
Peters D, Lebbink G (1973) Effect of oil on transmission of pea enation mosaic-virus during short inoculation probes. Entomol Exp Appl 16:185–190
Petrov N, Andonova R (2012) Bion and exin as sar elicitors against potato virus Y infection in tomato. Plant Stud 2:46–49
Pickett JA, Wadhams LJ, Woodcock CM (1992) The chemical ecology of aphids. Annu Rev Entomol 37:67–90
Powell G (1992) The effect of mineral-oil on stylet activities and potato virus-Y tramsmission by aphids. Entomol Exp Appl 63:237–242
Ragsdale DW, Landis DA, Brodeur J, Heimpel GE, Desneux N, (2011) Ecology and management of the soybean aphid in North America. In: Berenbaum MR, Carde RT, Robinson GE, (eds) Annual Review of Entomology, Vol 56. Annual Reviews, 4139 El Camino Way, Po Box 10139, Palo Alto, Ca 94303-0897 USA, 375–399. (Annual Review of Entomology; vol. 56.)
Rakotonindraina T, Corbière R, Chatot C, et al. (2011) Analysis of volunteer density under the influence of cropping practices: a contribution to the modelling of primary inoculum of Phytophthora infestans in potato crops. Proceedings of the Thirteenth EuroBlight workshop. St. Petersburg: PPO-Special Report, 67–74
Rice AD, Gibson RW, Stirbley MF (1983) Effects of deltamethin on walking flight and potato virus Y-transmission by pyrethroid-resistant Myzus persicae. Ann Appl Biol 102:224–236
Robert Y, Woodford JT, Ducray-Bourdin DG (2000) Some epidemiological approaches to the control of aphid-borne virus diseases in seed potato crops in northern Europe. Virus Res 71:33–47
Rolot J-L (2005) Analyse des facteurs régulant la dissémination du virus Y de la pomme de terre (PVY) en vue de stratégies de lutte raisonnées. Gembloux: Faculté des Sciences agronomiques de Gembloux, PhD thesis
Rolot J-L, Seutin H, Georges G, Deveux L (2006) Evaluation de l’efficacité du nouvel insecticide Plenum pour le contrôle de la dissémination des infections à virus Y dans une parcelle de plants
Rousselle P, Robert Y, Crosnier JC (1996) La pomme de terre. INRA, Paris
Saucke H, Doring TF (2004) Potato virus Y reduction by straw mulch in organic potatoes. Ann Appl Biol 144:347–355
Schramm S, Frost K, Charkowski A, Gray S, Crockford A, Groves RL (2011) Management of potato virus Y (PVY) in Wisconsin seed potato production. University of Wisconsin. Extension (A3951),Madison 8
Schulte-Geldermann E, Gildemacher PR, Struik PC (2012) Improving seed health and seed performance by positive selection in three kenyan potato varieties. Am J Potato Res 89:429–437
Shepard JF, Claflin LE (1975) Critical analyses of the principles of seed potato certification. Annu Rev Phytopathol 13:271–293
Sigvald R (1984) The relative efficiency of some aphid species as vectors of potato virus Yo (PVYo). Potato Res 27:285–290
Sigvald R (1985) Mature-plant resistance of potato plants against potato-virus Yo (PVYo). Potato Res 28:135–143
Simons JN (1957) Effect of insecticides and physical barriers on field spread of pepper veinbanding mosaic virus. Phytopathology 47:139–145
Simons J, Mclean D, Kinsey M (1977) Effects of mineral oil on probing behavior and transmission of stylet-borne viruses by Myzus persicae. J Econ Entomol 70:6
Slater R, Paul VL, Andrews M, Garbay M, Camblin P (2012) Identifying the presence of neonicotinoidresistant peach-potato aphid (Myzus persicae) in the peach-growing regions of southern France and northern Spain. Pest Manag Sci 68:634–638
Steinger T, Gilliand H, Hebeisen T (2014) Epidemiological analysis of risk factors for the spread of potato viruses in Switzerland. Ann Appl Biol 164:200–207
Su JW, Zhu SR, Zhang ZN, Ge F (2006) Effect of synthetic aphid alarm pheromone (E)-beta-farnesene on development and reproduction of Aphis gossypii (Homoptera : Aphididae). J Econ Entomol 99:1636–1640
Tan BL, Sarafis V, Beattie GC, White R, Darley EM, Spooner-Hart R (2005) Localization and movement of mineral oil in plants by fluorescence and confocal microscopy. J Exp Bot 56:2755–2763
Thomas-Sharma S, Abdurahman A, Ali S et al (2016) Seed degeneration in potato: the need for an integrated seed health strategy to mitigate the problem in developing countries. Plant Pathol 65:3–16
Unece (2014) Virus tolerances, proposals by the United States. In: Unece (ed) Proceedings of the meeting of the extended bureau of the specialized section on standardization of seed potatoes, Melbourne, UNECE, 5
Unece (2015) United Nations international standard for certification and marketing of seed potatoes. In: Unece (ed) Geneva, Switzerland, 2
Van Toor RF, Drayton GM, Lister RA, Teulon DJ (2009) Targeted insecticide regimes perform as well as a calendar regime for control of aphids that vector viruses in seed potatoes in New Zealand. Crop Prot 28:599–607
Vasyukova NI, Ozeretskovskaya OL (2007) Induced plant resistance and salicylic acid: a review. Appl Biochem Microbiol 43:367–373
Walsh D (2000) Horticultural spray oils: useful year-round tactic for IPM systems. Agrichem Environ News 165:5–7
Walters KFA, Dixon AFG (1984) The effect of temperature and wind on the flight activity of cereal aphids. Ann Appl Biol 104:17–26
Wedding RT, Riehl LA, Rhaods WA (1952) Effect of petroleum oil spray on photosynthesis and respiration in citrus leaves. Plant Physiol 60:3–12
Whitworth JL, Hamm PB, Mcintosh CS (2010) Effect of potato virus Y on yield of a clonal selection of russet norkotah. Am J Potato Res 87:310–314
Wróbel S (2009) The retention of PVY in the stylet of Myzus persicae Sulz. After the application of mineral oil on potato plants. Plant Breed Seed Sci 60:3–12
Wrobel S (2012) Comparison of mineral oil and rapeseed oil used for the protection of seed potatoes against pvy and pvm infections. Potato Res 55:83–96
Zhang Z, Tu M, Du Y et al (1997) Behavioral and electrophysiological response of Myzus persicae to stimulus of (E)- beta -farnesene. Acta Entomol Sin 40:40–44
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Crown Copyright
About this chapter
Cite this chapter
Dupuis, B. et al. (2017). Potato virus Y: Control, Management and Seed Certification Programmes. In: Lacomme, C., Glais, L., Bellstedt, D., Dupuis, B., Karasev, A., Jacquot, E. (eds) Potato virus Y: biodiversity, pathogenicity, epidemiology and management. Springer, Cham. https://doi.org/10.1007/978-3-319-58860-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-58860-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58858-2
Online ISBN: 978-3-319-58860-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)