Abstract
A membrane bioreactor (MBR) and a hybrid moving bed biofilm reactor-membrane bioreactor (hybrid MBBR-MBR) for municipal wastewater treatment were studied to determine the effect of salinity on the nitrogen removal and autotrophic kinetics. The biological systems were analyzed during the start-up phase under regular and variable salinities, with a hydraulic retention time (HRT) of 6 h. The variable salinity affected the autotrophic biomass, which caused a reduction of the nitrogen degradation rate and an increase of time to remove ammonium from municipal wastewater.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The growing awareness of environmental protection has led to more stringent legislation regarding the treatment of saline wastewater before discharge. This aspect is especially important for activities such as the fish canning, petroleum, petrochemical and tannery industries, as well as the wastewater produced during shipboard activities that is characterized by high saline concentrations (Sun et al. 2010; Abdollahzadeh Sharghi et al. 2014).
In recent years, membrane bioreactor (MBR) systems have been applied to treat saline wastewater (Di Bella et al. 2013). To improve the performance of MBR regarding the pollutant removal and membrane filtration, the hybrid moving bed biofilm reactor-membrane bioreactor (hybrid MBBR-MBR) systems have been developed during the last years (Leyva-Díaz et al. 2014).
The analysis of available scientific literature shows a lack of knowledge in terms of autotrophic biomass respiratory activity in MBR and hybrid MBBR-MBR used to treat municipal wastewater under salinity variation. In light of this, kinetic modeling can be an important tool for design and operation of MBR and hybrid MBBR-MBR plants (Leyva-Díaz et al. 2014).
The aim of this study was to assess the effect of salinity on the autotrophic bacteria kinetics during the start-up of MBR and hybrid MBBR-MBR working at low hydraulic retention time (HRT) (6 h).
2 Materials and Methods
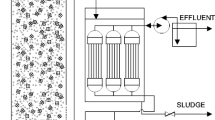
An MBR (Fig. 1a) and a hybrid MBBR-MBR (Fig. 1b), working in parallel, were fed with municipal wastewater containing regular (1.05 mS cm−1) and variable salinity (1.2–6.5 mS cm−1). These systems included a bioreactor divided into four zones: one anoxic zone and three aerobic ones, as well as a membrane tank. There was a biomass recycling from the membrane tank to the bioreactor to get the working mixed liquor suspended solids (MLSS) concentration and the nitrogen removal. The total biomass concentration for the steady state was 2,500 mg L−1. Wastewater treatment plants (WWTPs) operated at HRT of 6 h. The working volumes of the bioreactor and the membrane tank were 24 L and 4.32 L, respectively, and the anoxic zone of the bioreactor had a volume of 6 L. Regarding the hybrid MBBR-MBR, the K1 media filling-fraction had a value of 35% in the aerobic zone and the anoxic zone did not contain carriers. Furthermore, the dissolved oxygen (DO) in the aerobic zone varied between 2.0 and 2.5 mg O2 L−1 and DO in the anoxic zone fluctuated between 0.2 and 0.4 mg O2 L−1.
Respirometric tests were carried out on suspended biomass for the MBR. In order to evaluate the effect of biomass adhered to carriers, respirometric assays were performed on total biomass, suspended and attached, for the hybrid MBBR-MBR. Kinetic parameters for autotrophic bacteria and the total nitrogen (TN) degradation rate (rsu,A) were evaluated through respirometric techniques according to Leyva-Díaz et al. (2013). The evolution of the dynamic oxygen uptake rate (Rs) was registered from the respirograms obtained (Leyva-Díaz et al. 2013).
The concentrations of MLSS and mixed liquor volatile suspended solids (MLVSS) were calculated from standard methods (APHA 2012). The concentrations of autotrophic biomass (XA) were determined by supposing the percentages of autotrophic bacteria that were obtained by Leyva-Díaz et al. (2015) for the same MBR and hybrid MBBR-MBR under similar operation conditions.
Thus, respirometric tests allowed for assessing the maximum specific growth rate (µm,A), substrate half-saturation coefficient (KM,A) and yield coefficient (YA) for autotrophic biomass. The assessment of these parameters was carried out in six steps:
-
(1)
Determination of the oxygen consumption (OC) through the integration of Rs, as shown in Eq. (1):
$$ {\text{OC}} = \mathop \smallint \limits_{{{\text{t}}_{0} }}^{\text{t}} {\text{R}}_{\text{s}} {\text{dt}}\quad ({\text{mgO}}_{2} \,\,{\text{L}}^{ - 1} ) $$(1) -
(2)
Estimation of YA according to Eq. (2) described by Helle (1999):
$$ {\text{Y}}_{\text{A}} = \frac{{{\text{S}} - {\text{OC}}}}{{{\text{S}}_{\text{NH}} \cdot {\text{f}}_{\text{cv}} }}\quad ({\text{mgVSS}}\,\,{\text{mgTN}}^{ - 1} ) $$(2)where S is the ammonium concentration expressed as oxygen (mgO2 L−1), SNH is the ammonium concentration expressed as total nitrogen (mgTN L−1) and fcv is a conversion factor (1.42 mgO2 mgVSS−1).
-
(3)
Evaluation of the substrate degradation rate (rsu) from Rs:
$$ {\text{r}}_{\text{su}} = \frac{{{\text{R}}_{\text{s}} }}{{1 - \frac{{{\text{Y}}_{\text{A}} \cdot{\text{S}}_{\text{NH}} \cdot{\text{f}}_{\text{cv}} }}{\text{S}}}}\quad ({\text{mgO}}_{2} {\text{L}}^{ - 1} {\text{h}}^{ - 1} ) $$(3) -
(4)
Assessment of the empirical specific growth rate (µemp) from the relation between the cell growth rate and rsu:
$$ \upmu_{\text{emp}} = \frac{{\frac{1}{{{\text{f}}_{\text{cv}} }}\cdot{\text{R}}_{\text{s}} }}{{\left( {1 - \frac{{{\text{Y}}_{\text{A}} \cdot{\text{S}}_{\text{NH}} \cdot{\text{f}}_{\text{cv}} }}{\text{S}}} \right)\cdot{\text{X}}_{\text{A}} }}\quad ({\text{h}}^{ - 1} ) $$(4)where XA is the concentration of autotrophic biomass (mgVSS L−1)
-
(5)
Estimation of µm,A and KM,A through the linearization of the Monod model (Monod 1949):
$$ \frac{1}{{\upmu_{\text{emp}} }} = \frac{1}{{\upmu_{{{\text{m}},{\text{A}}}} }} + \frac{{{\text{K}}_{{{\text{M}},{\text{A}}}} }}{{\upmu_{{{\text{m}},{\text{A}}}} }}\cdot\frac{1}{{{\text{S}}_{\text{NH}} }}\quad ({\text{h}}) $$(5)
Therefore, the rsu,A can be expressed as a function of the autotrophic kinetic parameters, as well as the substrate and biomass concentrations, as indicated in Eq. (6):
3 Results and Discussion
Table 1 shows the autotrophic kinetic parameters for MBR and hybrid MBBR-MBR working at regular and variable salinities. It should be noted that the values of µm,A were higher for the systems operating under regular salinity than those obtained for the technologies working under variable salinity, which favored the substrate degradation rate. Moreover, the values of KM,A were higher for the systems working under variable salinity, which could be due to the inability of autotrophic biomass to use the whole substrate.
The multivariate analysis linking the operational conditions of the MBR and hybrid MBBR-MBR systems working with municipal wastewater under regular and variable salinity with the Monod growth model parameters clearly showed the influence of salinity and dissolved oxygen concentration in the autotrophic kinetics of the systems (Fig. 2). The yield coefficient for autotrophic biomass (YA) (purple triangle) and the half-saturation coefficient for ammonium nitrogen (KM,A) (red triangle) had a positive correlation with conductivity and dissolved oxygen concentration in the aerobic zone. On the other hand, the maximum specific growth rate for autotrophic biomass (µm,A) (yellow triangle) was negatively correlated with electric conductivity and dissolved oxygen concentration in the aerobic zone.
Multivariate redundancy analysis triplot linking the autotrophic kinetics of MBR and hybrid MBBR-MBR systems working under regular and variable salinity wastewater with the conductivity and dissolved oxygen in the anoxic and aerobic chambers, DOanox and DOaer, respectively, of the bioreactors. (Color figure online)
In light of this, it was clearly shown that high variations of electric conductivity led to the lowest rsu,A values concerning total nitrogen removal during the start-up of MBR and hybrid MBBR-MBR under variable salinity (Fig. 3). Therefore, the autotrophic kinetics was slower for the systems working under variable salinity. Johir et al. (2013) demonstrated an inhibitory effect of salinity on ammonium removal efficiency for an MBR system.
In this regard, it should be highlighted that autotrophic biomass required less time for ammonium oxidation during the start-up of MBR and hybrid MBBR-MBR working under regular salinity due to their higher rsu,A (Fig. 3). Thus, a longer time would be required to accomplish the steady state for the systems working under variable salinity (Fig. 3).
4 Conclusions
Variable salinity slowed down the total nitrogen degradation rate as a consequence of the reduction of the maximum specific growth rate for autotrophic biomass and the increase of the half-saturation coefficient for ammonium nitrogen. Thus, the autotrophic biomass could be inhibited by salinity, implying the inability of this kind of biomass to degrade ammonium substrate.
The MBR and hybrid MBBR-MBR systems that operated at regular salinity showed the highest values for total nitrogen degradation rate, which involved less time to oxidize ammonium during the start-up phase.
References
Abdollahzadeh Sharghi E, Bonakdarpour B, Pakzadeh M (2014) Treatment of hypersaline produced water employing a moderately halophilic bacterial consortium in a membrane bioreactor: effect of salt concentration on organic removal performance, mixed liquor characteristics and membrane fouling. Bioresour Technol 164:203–213
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, Washington DC
Di Bella G, Di Trapani D, Torregrossa M, Viviani G (2013) Performance of a MBR pilot plant treating high strength wastewater subject to salinity increase: analysis of biomass activity and fouling behaviour. Bioresour Technol 147:614–618
Helle S (1999) A respirometric investigation of the activated sludge treatment of BKME during steady state and transient operating conditions. Thesis, University of British Columbia
Johir MAH, Vigneswaran S, Kandasamy J, BenAim R, Grasmick A (2013) Effect of salt concentration on membrane bioreactor (MBR) performances: detailed organic characterization. Desalination 322:13–20
Leyva-Díaz JC, Calderón K, Rodríguez FA, González-López J, Hontoria E, Poyatos JM (2013) Comparative kinetic study between moving bed biofilm reactor-membrane bioreactor and membrane bioreactor systems and their influence on organic matter and nutrients removal. Biochem Eng J 77:28–40
Leyva-Díaz JC, Martín-Pascual J, Muñío MM, González-López J, Hontoria E, Poyatos JM (2014) Comparative kinetics of hybrid and pure moving bed reactor-membrane bioreactors. Ecol Eng 70:227–234
Leyva-Díaz JC, González-Martínez A, González-López J, Muñío MM, Poyatos JM (2015) Kinetic modeling and microbiological study of two-step nitrification in a membrane bioreactor and hybrid moving bed biofilm reactor-membrane bioreactor for wastewater treatment. Chem Eng J 259:692–702
Monod J (1949) The growth of bacterial cultures. Annu Rev Microbiol 3:371–394
Sun C, Leiknes T, Weitzenbock J, Thorstensen B (2010) Development of a biofilm-MBR for shipboard wastewater treatment: the effect of process configuration. Desalination 250:745–750
Acknowledgements
This research was supported by EMASAGRA and the Spanish Ministry of Economy and Competitiveness in the framework of the program “Convocatoria de ayudas a Proyectos de I+D Excelencia 2013” (CTM2013-48154-P).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Leyva-Díaz, J.C., Rodríguez-Sánchez, A., González-López, J., Poyatos, J.M. (2017). Effect of Salinity Variation on the Autotrophic Kinetics of the Start-up of Membrane Bioreactor and Hybrid Moving Bed Biofilm Reactor-Membrane Bioreactor at Low Hydraulic Retention Time. In: Mannina, G. (eds) Frontiers in Wastewater Treatment and Modelling. FICWTM 2017. Lecture Notes in Civil Engineering , vol 4. Springer, Cham. https://doi.org/10.1007/978-3-319-58421-8_82
Download citation
DOI: https://doi.org/10.1007/978-3-319-58421-8_82
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58420-1
Online ISBN: 978-3-319-58421-8
eBook Packages: EngineeringEngineering (R0)