Abstract
Artemisinin is one of the major active ingredients used in artemisinin combination therapies (ACTs) used in malarial treatment. It is produced from Artemisia annua L. Malaria being one of the most severe tropical diseases, dependency on the production of artemisinin has been increasing. Lower yield (0.01–1.1%) further complicates the production process. This has led to the development of alternate strategy to improve plant productivity and enhance the active ingredient. Biostimulants like Piriformospora indica and Azotobacter chroococcum have been well known for their beneficial interaction with plants. Here, we studied the impact of dual inoculation of these stimulants in the growth and productivity of artemisinin in the poly house condition. The plant growth was monitored by measuring parameters like height of plant, total dry weight, and leaf yield with an increase of 63.51, 52.61, and 79.70%, respectively, for treatment with dual biological consortium, as compared to that of control plants. This significant improvement in biomass was associated with higher total chlorophyll content (59.29%) and enhanced nutrition (especially nitrogen and phosphorus, 55.75 and 86.21%, respectively). The concentration of artemisinin along with expression patterns of artemisinin biosynthesis genes was appreciably higher in dual treatment, which showed positive correlation. The study suggested the potential use of the consortium P. indica strain DSM 11827 and A. chroococcum strain W-5 in A. annua L.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Chroococcum
- Piriformospora Indica
- Artemisinin Combination Therapy (ACTs)
- Leaf Yield
- Higher Total Chlorophyll Content
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
15.1 Introduction
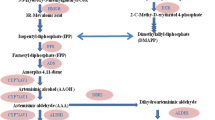
Artemisia annua L. (sweet wormwood) is an important medicinal plant due to presence of artemisinin. It belongs to genus Artemisia, family Asteraceae (Compositae) with an annual growth cycle (Willcox et al. 2004). The phyto-molecule artemisinin, sesquiterpene lactone containing endoperoxide bridge, is obtained from aerial parts of A. annua L. plants (Mandal et al. 2015). Artemisinin is an effective anti-malarial drug discovered by Miller and Su (2011). It has been also reported that artemisinin is not only effective against malaria but also for human cancer (Singh and Lai 2004) and hepatitis B virus (Romero et al. 2005). So far artemisinin-based combination therapies (ACTs) have been the choice for the treatment of people worldwide (Abdin et al. 2003). A. annua L. produces small amount of artemisinin (0.01–1.1%). Such low yields of artemisinin results in relatively high cost for isolation and purification of the useful chemical. Also, the demand of artemisinin production from dried plant material of A. annua L. has been estimated to about 289 tons as against the annual production of about 232–262 tons (Arora et al. 2016).
Rhizosphere microbiota like arbuscular mycorrhizal fungi (AMF) are well-known plant beneficial soilborne microsymbionts. They significantly contribute toward improved agricultural performance by triggering diverse plant physiological responses. Hence, these have been employed for many agricultural production systems as well as for medicinal and endangered plant species (Pozo et al. 2010). The symbiotic association of arbuscular mycorrhizal fungi (AMF) with the plant is in synergistic coordination with the plant growth-promoting rhizobacteria (PGPR) (Bandyopadhyay et al. 2016a; Bandyopadhyay et al. 2016b; Bakker et al. 2013; Berendsen et al. 2012; Bhuyan et al. 2015). The overall plant performance relies on both bacteria and the fungi whereby the nitrogen-fixing ability of bacteria is stimulated by improved phosphate uptake due to AMF association and vice versa (Javot et al. 2007). PGPRs show phosphate-solubilizing mechanisms, enhancement in phytohormone production, increased antifungal activity, etc. (Awasthi et al. 2011; Prasad et al. 2015). The synergistic interaction between plant and microbes in rhizosphere critically improves growth and productivity of plants through an array of processes like increased nutrient uptake, availability, nitrogen fixation, nutrient recycling, photosynthetic rate, and pathogen resistance (Jeffries et al. 2003).
P. indica as well as arbuscular mycorrhiza fungi individually have also been shown to enhance artemisinin content in A. annua L. plants (Kapoor et al. 2007; Rapparini et al. 2008; Chaudhary et al. 2008; Sharma and Agrawal 2013). Kapoor et al. (2007) reported an increase in artemisinin concentration in leaves of A. annua from 0.1% (control) to 0.3% (Glomus fasciculatum treated) while investigating the effect of two AMF Glomus fasciculatum and Glomus macrocarpum singly and along with addition of phosphorous. The increased artemisinin concentration was attributed to high leaf yield and shoot growth which was further validated by high glandular trichome (artemisinin biosynthesis and assembly sites) density in the mycorrhizal-treated plants. Azotobacter is a Gram-negative aerobic soil-dwelling nitrogen-fixing bacteria (Lakshminarayana et al. 1992). It is found in soil and water systems and in association with plants (Martyniuk and Martyniuk 2003). Only, recently studies analyzing synergistic effect of PGPRs and AMF on medicinal and crop plants have been conducted (Awasthi et al. 2011; Walker et al. 2012; Vafadar et al. 2014).
15.2 Effect of P. indica and A. chroococcum on Plant Growth Parameters
Inoculation of A. annua L. plants with Piriformospora indica and A. chroococcum either singly or in combination under poly house conditions improved the growth of plants in terms of plant height, biomass, and total leaf yield per plant as compared with control plants (Table 15.1). A. annua L. plants treated with either P. indica or A. chroococcum enhanced the growth compared with control. When combined, inoculation of plants with both P. indica and A. chroococcum was highly effective in improving the plant height, biomass, and leaf yield with an observed increase of 63.51, 52.61 and 79.70% respectively, compared with control (Table 15.1).
Rhizospheric soil from A. annua L. plants treated with A. chroococcum alone or in combination with P. indica was used for determination of the viable count of A. chroococcum by using standard serial dilution pour plate method. A. annua L. plants treated only with A. chroococcum showed 18.33 × 105 CFU/g soil, whereas dual treated plants exhibited high population of A. chroococcum (21.12 × 105 CFU/g soil) in the rhizospheric soil. P. indica colonization was evaluated by randomly selected fine roots from 2-month-old A. annua L. as method followed by Phillips and Hayman (1970), and percentage colonization of P. indica was calculated using the formula as described by McGonigle et al. (1990). A. annua L. plants cocultivated with P. indica resulted in 50.23% colonization, while dual treated plants have better root colonization of 78.99% by P. indica (Arora et al. 2016).
15.3 Effect of P. indica and A. chroococcum on Nitrogen and Phosphorus
Phosphorus and nitrogen are the important macromolecules that are responsible for increased growth, yield, and quality of plant. Concentrations of phosphorus and nitrogen were significantly higher in those plants cocultivated with dual treatment (Fig. 15.1). On individual basis, plants treated with P. indica significantly increased P content by 65.95% and with A. chroococcum resulted in 31.90% higher P content in A. annua L. plants compared to the control plants, respectively. Likewise, plants treated with P. indica significantly increased N content by 13.27% and with A. chroococcum resulted in 29.20% higher N content in A. annua L. plants compared to the control plants, respectively. The colonization of A. annua L. with dual treatment resulted in 86% increase in P content and 55.75% increase in N content (Fig. 15.1). P. indica is known to enhance phosphorous uptake in plants, which in turn might enable more energy available for nitrogen fixation by A. chroococcum; this could be the reason for higher P and N content in dual treated plants (Arora et al. 2016).
Phosphorus (a) and nitrogen (b) concentration (%) in leaves of A. annua L. plants, grown for 2 months after transplanting, under poly house conditions. Columns with different letters are indicating significant differences between each treatment at 5% probability level according to Tukey’s post hoc test, and the error bars represent the standard error
15.4 Effect of P. indica and A. chroococcum on Chlorophyll Content
Chl a, chl b, and total chlorophyll content was quantified in leaves of A. annua L. and found significantly increased in plants treated with P. indica, A. chroococcum alone, or in combination as compared to the control plants. Chl a showed values of 4.5 and 4.7 mg/g, respectively, for plant treated with A. chroococcum and P. indica, separately, and 5.6 mg/g fresh weight for plant dual treated with P. indica and A. chroococcum together. Similarly, the content of chl b exhibited values of 0.7 and 0.8 mg/g, respectively, for plant treated with A. chroococcum and P. indica, separately, and 1.0 mg/g fresh weight for plant dual treated with P. indica and A. chroococcum together. The plants dual treated with P. indica and A. chroococcum together also enhanced total chlorophyll content by 57.91% than control plants (Fig. 15.2). However, the chlorophyll content of A. annua L. plants treated with P. indica and A. chroococcum, separately, was not significantly different. More chlorophyll content in the plants is attributed to the fact that an increase in plant nutrition by an increase in P and N uptake will optimize the rate of photosynthesis by increasing the amount of plant chlorophyll, which will lead to an increase in biomass production by C fixation from CO2. Nitrogen is part of the chlorophyll molecule, which gives green color to plants and is involved in creating food for the plant through photosynthesis.
Chlorophyll content (mg/g fresh weight) in leaves of A. annua L. plants, grown for 2 months after transplanting, under poly house conditions. Columns with different letters are indicating significant differences between each treatment at 5% probability level according to Tukey’s post hoc test, and the error bars represent the standard error
15.5 Effect of P. indica and A. croococcum on Artemisinin Content
One gram of dry leaf material was used for the estimation of artemisinin using the method as described by Zhao and Zeng (1986). Derivatized artemisinin was analyzed and quantified through reverse phase column (C18, 5 μm, 4.6 × 250 mm) using premix methanol: 100 mM K-phosphate buffer, pH, 6.5 (60:40), as mobile phase at constant flow rate of 1 ml min−1 with the detector set at 260 nm. Artemisinin was quantified with the help of standard curve prepared by HPLC (Fig. 15.3). An overlay of the results obtained with preparative HPLC of a standard solution of artemisinin prior to analysis of samples is shown in Fig. 15.3. Artemisinin content was expressed as % as well as mg g−1 dw of leaves.
The symbiotic effectiveness was much evident when artemisinin content was recorded 70% higher in A. annua L. plants subjected to dual inoculation (Fig. 15.4). P. indica colonization or A. croococcum inoculation independently enhanced artemisinin content to approximately similar levels. The enhanced concentration of artemisinin by dual treatment may be due to improved growth and nutrient status of the plants (Arora et al. 2016; Davies et al. 2009).
Artemisinin content (%) in leaves of A. annua L. plants, grown for 2 months after transplanting, under poly house conditions. Columns with different letters are indicating significant differences between each treatment at 5% probability level according to Tukey’s post hoc test, and the error bars represent the standard error
15.6 Conclusion
Interaction of A. annua L. with both P. indica and A. chroococcum in cocultivation resulted in improved plant biomass and concentration of artemisinin in the plant as compared to control and singly treated plants. The combinatorial application of P. indica with A. chroococcum induces reprogramming of many cellular activities like phytohormone biosynthesis, nutrient acquisition, and secondary metabolite synthesis in A. annua L. leading to higher biomass and enhanced artemisinin content and yield. The use of this microbial consortium as bio-fertilizer in place of chemical fertilizers, hence, presents a viable option for increased artemisinin availability.
15.7 Future Prospects
The current study provides a perspective into study of combined inoculation of symbiotic fungus and nitrogen-fixing bacteria and their interaction with plants. Different beneficial and symbiotic bacterial fungal associations can also be studied with plants to check their effect on plant yield, disease resistance, abiotic and biotic stress response, production of important molecules, and plant products. It will also help to understand the molecular mechanism between the microorganisms and determine the active compounds released that help in plant trait enhancement. Proteomic studies can also be carried out to check the effect of consortium on plants. Hence, this consortium can also be used to check their effect on other plant species. Further study is also required to check the effectiveness of microbial consortia in making the plant resistant to pathogens through systemic induced resistance.
References
Abdin MZ, Israr M, Rehman RU, Jain SK (2003) Artemisinin, a novel antimalarial drug: biochemical and molecular approaches for enhanced production. Planta Med 69:289–299
Arora M, Saxena P, Choudhary DK, Abdin MZ, Varma A (2016) Dual symbiosis between Piriformospora indica and Azotobacter chroococcum enhances the artemisinin content in Artemisia annua L. World J Microbiol Biotechnol 32:19. doi:10.1007/s11274-015-1972-5
Awasthi A, Bharti N, Nair P et al (2011) Synergistic effect of Glomus mosseae and nitrogen fixing Bacilluc subtilis strain Daz26 on artemisinin content in Artemisia annua L. Appl Soil Ecol 49:125–130
Bakker PAHM, Berendsen RL, Doornbos RF, Wintermans PCA, Pieterse CMJ (2013) The rhizosphere revisited: root microbiomics. Front Plant Sci 4:165. doi:10.3389/fpls.2013.00165
Bandyopadhyay P, Bhuyan S, Yadava PK, Varma A (2016a) Soluble factors from Azotobacter chroococcum modulate growth of Piriformospora indica in co-cultures. Endocytobiosis Cell Res 27:9–13
Bandyopadhyay P, Bhuyan SK, Yadava PK, Varma A, Tuteja N (2016b) Emergence of plant and rhizospheric microbiota as stable interactomes. Protoplasma 254:617–626. doi:10.1007/s00709-016-1003-x
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Bhuyan SK, Bandyopadhyay P, Kumar P, Mishra DK, Prasad R et al (2015) Interaction of Piriformospora indica with Azotobacter chroococcum. Sci Rep 5:13911. doi:10.1038/srep13911
Chaudhary V, Kapoor R, Bhatnagar AK (2008) Effectiveness of two arbuscular mycorrhizal fungi on concentrations of essential oil and artemisinin in three accessions of Artemisia annua L. Appl Soil Ecol 40:174–181. doi:10.1016/j.apsoil.2008.04.003
Davies MJ, Atkinson CJ, Burns C et al (2009) Enhancement of artemisinin concentration and yield in response to optimization of nitrogen and potassium supply to Artemisia annua. Ann Bot 104:315–323
Javot H, Pumplin N, Harrison MJ (2007) Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant Cell Environ 30:310–322
Jeffries P, Gianinazzi S, Perotto S, Turnau K, Barea JM (2003) The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fertil Soils 37:1–16
Kapoor R, Chaudhary V, Bhatnagar AK (2007) Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza 17:581–587
Lakshminarayana K, Narula N, Hooda IS, Faroda AS (1992) Nitrogen economy in wheat (Triticum aestivum) through use of Azotobacter chroococcum. Ind J Agric Sci 62:75–76
Mandal S, Upadhyay S, Wajid S et al (2015) Arbuscular mycorrhiza increase artemisinin accumulation in Artemisia annua by higher expression of key biosynthesis genes via enhanced jasmonic acid levels. Mycorrhiza 25(5):345–357. doi:10.1007/s00572-014-0614-3
Martyniuk S, Martyniuk M (2003) Occurrence of Azotobacter spp. in some polish soils. Pol J Environ Stud 12:371–374
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Miller LH, Su X (2011) Artemisinin: discovery from the Chinese herbal garden. Cell 146:855–858
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 5:158–161
Pozo MJ, Jung SC, Lopez-Raez JA, Azcon-Aguilar C (2010) Impact of Arbuscular Mycorrhizal Symbiosis on plant response to biotic stress: the role of plant defence mechanisms. In: Koltai H, Kapulnik Y (eds) Arbuscular mycorrhizas: physiology and function, vol 193. Springer Science+Business Media B.V., Dordrecht, pp 193–207. doi:10.1007/978-90-481-9489-6_9
Prasad R, Kumar M and Varma A (2015) Role of PGPR in soil fertility and plant health. In: Egamberdieva D, Shrivastava S, Varma A (eds) Plant growth-promoting rhizobacteria and medicinal plants. Springer International Publishing, pp 247–260
Rapparini F, Llusia J, Penuelas J (2008) Effect of arbuscular mycorrhizal (AM) colonization on terpene emission and content of Artemisia annua L. Plant Biol 10:108–122
Romero MR, Efferth T, Serrano MA et al (2005) Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an ‘in vitro’ replicative system. Antiviral Res 68(2):75–83
Sharma G, Agrawal V (2013) Marked enhancement in the artemisinin content and biomass productivity in Artemisia annua L. shoots co-cultivated with Piriformospora indica. World J Microbiol Biotechnol 29:1133–1138
Singh NP, Lai HC (2004) Artemisinin induces apoptosis in human cancer cells. Anticancer Res 24:2277–2280
Vafadar F, Amooaghaie R, Otroshy M (2014) Effects of plant growth promoting rhizobacteria and arbuscular mycorrhizal fungi on plant growth, stevioside, NPK, and chlorophyll content of Stevia rebaudiana. J Plant Interact 9:128–136. doi:10.1080/17429145.2013.779035
Walker V, Couillerot O, Felten AV, Bellvert F et al (2012) Variation of secondary metabolite levels in maize seedling roots induced by inoculation with Azospirillum, Pseudomonas and Glomus consortium under field conditions. Plant Soil 356:151–163
Willcox M, Bodeker G, Bourdy G et al (2004) Artemisia annua as a traditional herbal antimalarial. In: Wilcox ML, Bodeker G, Rasoanaivo P, Addae-Kyereme J (eds) Traditional medicinal plants and malaria. CRC Press, Boca Raton, IL
Zhao SS, Zeng MY (1986) Determination of Qinghaosuin in Artemisia annua L. by high performance liquid chromatography. Chinese J Pharma Anal 6:3–5
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Bandyopadhyay, P., Arora, M., Abdin, M.Z., Varma, A. (2017). Cocultivation of Piriformospora indica and Azotobacter chroococcum for Production of Artemisinin. In: Varma, A., Prasad, R., Tuteja, N. (eds) Mycorrhiza - Eco-Physiology, Secondary Metabolites, Nanomaterials. Springer, Cham. https://doi.org/10.1007/978-3-319-57849-1_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-57849-1_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57848-4
Online ISBN: 978-3-319-57849-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)