Abstract
Frailty is a result of underlying physiologic processes associated with aging that lead to poor functional reserve. With increasing degrees of frailty, the ability to recover from major stresses on the body such as cancer treatment becomes more difficult. Varying degrees of frailty can be subtle, which explains the difficulty of distinguishing which older adults will have excess toxicity from cancer therapy and ones will tolerate it well. Thus, a biomarker of aging would be a very useful tool to predict toxicity and functional decline with cancer treatment and guide treatment decisions for older patients.
Based on the large body of work in the field of aging research, several processes have emerged as hallmarks of the aging process. There is a decline of the lymphocyte component of the total leukocyte count. Systemic inflammation increases and likely contributes to age-related diseases. Telomere length decreases with cellular replication over time. Finally, repeated exposure to environmental stress results in cellular senescence. Researchers are now exploring biomarkers of these processes and their potential application in geriatric oncology. While they may not be pure aging biomarkers, they may be characterized as biomarkers of frailty that have the potential to identify patients at increased risk for adverse events, functional decline, and poorer survival related to cancer treatment. This chapter will discuss the characteristics of an aging biomarker, the various markers that have been investigated, and the evidence for the use of these biomarkers in older adults with cancer.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
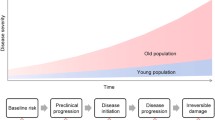
Frailty exists on a continuum from pre-frail to frail, eventually leading to disability. As a person progresses on this continuum, their functional reserve decreases, placing the patient at increased risk for poor outcomes after major physiologic stresses on the body such as injury, surgery, and cancer therapy. While older age is a risk factor for frailty, the development of frailty is not dependent on chronologic age. Frailty is a result of underlying physiologic processes leading to poor functional reserve, and measurement of these processes can give more of a sense of a person’s biologic age.
Understanding a patient’s biologic age is a large need in the field of oncology. Varying degrees of functional reserve among older cancer patients result in great heterogeneity in the ability of older adults to tolerate cancer treatment. Some older patients tolerate treatment well and derive as much benefit from treatment as younger patients, while others have increased rates of toxicity resulting in treatment reductions and/or cessation of treatment. This variation in treatment tolerance is likely a reflection of varying degrees of frailty among older patients, which is a known risk factor for poor tolerance of cancer treatment. A number of studies have shown that pre-frail and frail patients, identified by a geriatric assessment, are at increased risk of toxicity to cancer therapy (Extermann et al. 2012; Hurria et al. 2011; Cohen et al. 2016).
Currently, pre-frail and frail patients are identified by administering a geriatric assessment tool. There is international consensus emphasizing the importance of geriatric assessment in older adults, although there is a lack of consensus on which scale to use (Wildiers et al. 2014). Geriatric assessment tools have a moderate ability to predict toxicity; however, the lack of financial resources and expertise has limited the routine use of these tools in routine clinical practice in the United States. As the cancer incidence rises with the rapidly aging population, this will become an increasing problem.
Although useful for identifying frail patients, geriatric assessments do not give us a measure of the underlying physiologic mechanisms that contribute to frailty. There is a known phenotype associated with both aging and frailty, which includes cellular and systemic inflammation, shortened and/or dysfunctional telomeres, and a decrease in muscle mass. These factors are likely interconnected as part of an “inflammaging” process and may be candidates for easily measuring a patient’s degree of frailty and, therefore, biologic age.
The Ideal Aging Biomarker
The American Federation for Aging provided guidelines on four characteristics of an ideal aging biomarker (Simm et al. 2008; Baker and Sprott 1988; Johnson 2006). The biomarker of aging should be associated with the following abilities:
-
1.
Predict the rate of aging.
-
2.
Monitor the process of aging, not the biology of a disease.
-
3.
Be tested repeatedly without harming the individual.
-
4.
Be able to be tested in both human and animal models.
The difficulty with identifying biomarkers of frailty in the oncology population is that there is a large interplay between the potential biomarkers, disease, and treatment. Patients may be frail related to the disease itself. For instance, patients with widely metastatic disease have a high degree of systemic inflammation leading to anorexia, cachexia, and poor physical performance. In chemosensitive disease, treatment of these patients with systemic therapy can result in improvement of these processes. In addition, the treatment of the disease may contribute to frailty (Demaria et al. 2017; Bailur et al. 2017).
Since many processes related to aging and frailty contribute to diseases associated with aging, it is unlikely that we will find a pure biomarker of aging that is not affected by the underlying disease. However, these markers may be very useful as predictive markers of toxicity and/or functional decline from cancer therapy. These biomarkers could be used to predict adverse outcomes prior to initiating therapy and may be a useful guide during the treatment of the disease.
The Ideal Frailty Biomarker for Oncology
Given the interplay of biomarkers of frailty between the patient, the disease, and the treatment, it will be important to assess these markers over time to determine their ability to predict which patients are at risk. Biomarkers of frailty need to be tested in prospective clinical trials to determine the following:
-
1.
Feasibility of rapid, repeated measurement before, during, and after treatment.
-
2.
Correlation with toxicity (development of adverse events).
-
3.
Correlation with adjustments in doses, withholding doses, or discontinuation of treatment.
-
4.
Correlation with physical performance and functional decline.
Identifying frailty among cancer patients is extremely important for multiple reasons. First, it is important to recognize upfront which patients are at increased risk for toxicity and decreased functional decline with cancer treatment. This information helps guide the clinician and patient to make informed choices regarding the type and aggressiveness of cancer treatment that patient should pursue. Second, while on cancer treatment, it is important to recognize if frailty is developing and/or worsening, so that adjustments in doses and interventions such as physical therapy and nutritional support can be offered to prevent the transition into disability. Third, investigating the underlying biology is the identification of targets for the development of therapeutic and/or pharmacologic interventions.
Classes of Biomarkers of Aging
Cellular Markers of Inflammation
Increased age is associated with decreased cell-mediated immunity termed immunosenescence. In the peripheral blood, this manifests as an increase in myeloid cells and a decrease in lymphoid cells (Sieburg et al. 2006). In the general geriatric population, lymphopenia is considered to be a marker of frailty. In the general geriatric population, higher neutrophil and lower lymphocyte counts are associated with lower physical activity and poor muscular strength (Fernandez-Garrido et al. 2014).
Numerous studies have evaluated lymphopenia as a marker for outcomes in oncology patients. Lymphopenia is independently associated with both progression-free and overall survival in a variety of cancers (Ray-Coquard et al. 2009). Low lymphocyte counts may also be a predictor for hematologic toxicity. When measured prior to treatment, lymphopenia has been shown to be associated with higher levels of chemotherapy-induced neutropenia and febrile neutropenia (Blay et al. 1996; Ray-Coquard et al. 2003).
The exact mechanism is unknown, but there is a suggestion that early lymphoid precursors are the most sensitive to induction of differentiation in response to DNA damage (Wang et al. 2012). Regardless of the cause, lymphopenia is considered a marker of immunosenescence (Falandry et al. 2013; Pawelec and Solana 1997). Multiple studies have evaluated the lymphocyte count in relation to other leukocytes and platelets as a marker of aging and/or frailty as well as a prognostic factor. The neutrophil-lymphocyte ratio (NLR), the lymphocyte-monocyte ratio (LMR), and the platelet-lymphocyte ratio (PLR) all have robust data showing an association with oncologic outcomes. Because these ratios can be derived from a complete blood count test routinely measured prior to treatment, they may be a readily accessible, easily measured biomarker.
Neutrophil-Lymphocyte Ratio (NLR)
A recent meta-analysis of 100 studies including 40,559 patients with solid tumors reported by Templeton et al. (2014a) evaluated the effect of the neutrophil-lymphocyte ratio (NLR) on cancer-specific and overall survival outcomes. The hazard ratio for overall survival among all tumor types and stages showed poorer outcomes for patients with a high NLR (cutoff 4.0) in terms of cancer-specific survival (HR 1.61, 95% CI = 1.36–1.91; P <0.001), progression-free survival (HR 1.63, 95% CI = 1.39–1.91; P <0.001), and overall survival (HR 1.81, 95% CI = 1.67–1.97; P <0.001). These effects were consistent regardless of the disease subtype, site of disease, and stage of disease.
More recently, the relationship between frailty and ratios of leukocyte counts was evaluated in older adults with cancer (Nishijima et al. 2017). Using the Carolina Frailty Index (CFI), 133 patients who had a geriatric assessment were characterized as robust (54%), pre-frail (22%), and frail (24%). The CFI was positively correlated with the NLR (r = 0.22, p = 0.025). On multivariate analysis, those with an elevated NLR were more likely to be frail or pre-frail (OR 3.8, 95% CI = 1.1–12.8). Higher NLR was also significantly associated with instrumental activity of daily living (IADL) scores (p = 0.040) and a prolonged timed up and go (TUG) test (p = 0.016).
The relationship between the NLR both frailty and survival outcomes in these studies warrants further investigation to determine how best to utilize this information to guide cancer treatment for older adults.
Lymphocyte-Monocyte Ratio (LMR)
The aforementioned study reported by Nishijima et al. (2017) also evaluated the relationship between the LMR and frailty. The LMR was positively correlated with the IADL score (r = 0.197, p = 0.046). Patients with prolonged TUG tests also had a lower LMR (p = 0.013).
The LMR has also been shown to correlate with oncologic survival outcomes in multiple different malignancies. A meta-analysis involving 4260 patients with head and neck cancer demonstrated a significant associated with elevated LMR and improved DFS (HR 0.70; 95% CI 0.62–0.80) as well as improved OS (HR 0.5; 95% CI 0.44–0.57) (Tham et al. 2018). Another meta-analysis of 1795 patients with various stages of pancreatic cancer also demonstrated improved disease control DFS/RFS/TTP (HR =0.38, 95% CI: 0.15–0.95, P = 0.04) and OS (HR = 0.56, 95% CI: 0.38–0.83, P = 0.004) for those with an elevated LMR. These results were independent of ethnicity, surgical treatment, and cancer stage (Li et al. 2017).
The largest meta-analysis evaluating the relationship between LMR and non-hematologic solid tumors was reported by Nishijima et al. (2015). This analysis included 11,197 patients from 29 studies. Patients that had a LMR <3.0 had poorer cancer-specific survival (HR, 1.73; 95% CI: 1.55–1.93; P <0.001), disease-free survival (HR, 1.56; 95% CI: 1.31–1.86; P <0.001), and overall survival (HR, 1.56; 95% CI: 1.27–1.91; P <0.001). The LMR was prognostic for survival for multiple tumor types and stages of disease.
Platelet-Lymphocyte Ratio (PLR)
Templeton et al. (2014b) also performed a large meta-analysis investigating the prognostic role of the PLR in 12,574 patients with solid tumor malignancies. Among the 12 studies with a dichotomous definition for elevated PLR (median cutoff 185), the hazard ratio for PLR on overall survival was stronger for patients with metastatic disease (HR 2.0; 95% CI: 1.6–2.7) than patients with nonmetastatic disease (HR 1.5; 95% CI: 1.0–2.2). Within the eight studies that categorized PLR into three groups (<150/150–300/>300), the association with poorer overall survival was significant for metastatic disease (HR 1.6; 95% CI: 1.1–2.4) but nonsignificant for early-stage disease (HR 1.0; 95% CI: 0.8–1.3). The negative prognostic association for PLR was significant for colorectal, hepatocellular, gastroesophageal, ovarian, and pancreatic carcinomas in the dichotomous group and for colorectal cancers among the studies that categorized PLR into three groups. Based on these results, PLR may also provide prognostic information for older adults with cancer, especially for those with metastatic disease.
Circulating Markers of Systemic Inflammation
Levels of circulating pro-inflammatory mediators such as IL-6 and TNF-alpha D-dimer, and plasminogen activator inhibitor (PAI)-1, increase with age (Ershler et al. 1993; Fagiolo et al. 1993; Sindermann et al. 1993). These markers are thought to accelerate the aging process and exacerbate multiple age-related diseases (Bruunsgaard et al. 2001; Franceschi et al. 2007; Vasto et al. 2007). There is a co-stimulatory affect between markers of inflammation and pro-thrombotic factors. Cytokines such as TNF-α and IL-6 stimulate production of pro-thrombotic factors such as PAI-1 and fibrinogen (Kanapuru and Ershler 2009). In turn, D-dimer, a marker of the clotting process, has been shown to induce synthesis and release cytokines IL-1B, IL-6, and PAI-1 (Robson et al. 1994). Likewise, when vascular cell adhesion molecule (VCAM) is exposed to inflammatory markers TNF-α and IL-1B, it is cleaved to soluble (s)-VCAM, which is elevated in patients with age-related diseases (Carter and Wicks 2001).
Several studies have shown that inflammatory mediators correlate with measures of physical function and are elevated to a greater degree in frail patients than in age-matched, non-frail controls (Cesari et al. 2004; Ferrucci et al. 2002a; Hubbard et al. 2009; Leng et al. 2007; Pieper et al. 2000; Walston et al. 2002; Yao et al. 2011; Collerton et al. 2012). A study of 110 patients >75 years demonstrated that a combination of inflammatory markers (TNF-α, IL-6, CRP) and low albumin correlated with lower physical function scores, independent of age, sex, body mass index (BMI), smoking status, number of comorbidities, and number of medications (Hubbard et al. 2009).
In the general geriatric population, elevated chronic inflammatory and pro-coagulant markers predict functional decline (Cohen et al. 2003; De Martinis et al. 2006; de Saint-Hubert et al. 2011; Ferrucci et al. 1999, 2002b; Huffman et al. 2011; Puts et al. 2005; Reuben et al. 2002). An analysis of disabled women ≥65 years showed higher IL-6 levels at baseline were associated with higher levels of functional decline including decreased mobility, activities of daily living deficits, increased walking limitations, and decreased walking speed, compared to women with low IL-6 levels (Ferrucci et al. 2002a). These markers also correlate with functional decline after hospitalization and postoperative complications from oncologic surgery (de Saint-Hubert et al. 2011; Ronning et al. 2010).
Markers of chronic inflammation and coagulation are also associated with all-cause mortality risk in the elderly. In a population of community-dwelling adults (mean age 78), soluble (s)-VCAM was independently correlated with poorer functional status at baseline (HR 1.2, p = 0.002) (Huffman et al. 2011). After adjusting for functional status, demographic factors, and comorbidities, higher plasma s-VCAM, D-dimer, and IL-6 concentrations were independently related to mortality within 4 years. Inflammatory mediators can have greater predictive ability among patients without baseline functional impairments, suggesting they may identify pre-frail patients that may not otherwise have been identified without extensive geriatric assessment testing (Pieper et al. 2000; Cohen et al. 2003; Reuben et al. 2002).
Circulating Inflammatory Markers in Oncologic Studies
Markers of systemic inflammation correlate with physical function, functional decline, and mortality in the general geriatric population. These circulating inflammatory markers are felt to reflect underlying biologic aging and frailty. Multiple studies have evaluated these biomarkers’ association with frailty, toxicity, and survival outcomes for cancer patients.
Systemic inflammatory markers have been shown to correlate with frailty in cancer patients. Browers et al. (2015) measured systemic markers of inflammation felt to be related to aging and frailty including telomere length, interleukin-6 (IL-6), regulated upon activation normal T cell expressed and secreted (RANTES), monocyte chemotactic protein 1 (MCP-1), and insulin-like growth factor 1 (IGF-1) among young (n = 42) and older (n = 162) patients with nonmetastatic breast cancer. The biomarker levels were then correlated with patients’ Leuven Oncogeriatric Frailty Score (LOFS). The investigators found that IL-6 levels correlated with frailty among older patients. IL-6, telomere length, IGF-1, and MCP-1 correlated with age.
Multiple studies have shown elevated inflammatory markers are associated with a poorer prognosis among oncology patients. For example, higher circulating IL-6 levels have been shown to correlate with poorer survival in patients with hormone-refractory metastatic breast cancer (Bachelot et al. 2003). Likewise, elevated C-reactive protein (CRP) is associated with worse survival in multiple urologic cancers, including renal, bladder, and prostate cancers as well as colorectal and gastroesophageal cancers (Saito and Kihara 2011; Roxburgh and McMillan 2010).
Investigators have shown that systemic inflammation plays a major role in the decline of cancer patients, especially in terms of nutrition and physical function. The systemic inflammation-based Glasgow Prognostic Score (GPS), based on levels of CRP and hypoalbuminemia, was derived as a surrogate of systemic inflammatory status. Data from 8333 patients from 28 studies in patients with operable cancer demonstrate the GPS was a predictor of survival independent of stage, pathological features, and comorbidity (HR range 1.5–5.1) (McMillan 2013). Similar prognostic ability of the GPS was seen in 11 studies involving 1504 patients with metastatic cancer. In a study of 56 patients with advanced-stage colorectal cancer, the GPS not only predicted survival, but it also predicted toxicity to chemotherapy (Sharma et al. 2008). A higher GPS score correlated with higher grade 2/3 diarrhea and higher incidence of grade 2/3 toxicity compared to those with lower scores (p = 0.023 and 0.015, respectively).
One argument against using inflammatory mediators has been that they may reflect other underlying processes such as the response to surgical intervention and the underlying cancer itself. If used in the adjuvant setting, circulating acute phase reactants from the surgery itself should be resolved 6–8 weeks postoperatively (Baigrie et al. 1992; Wirtz et al. 2000). In addition, multiple studies have associated inflammatory markers with poorer prognosis and toxicity, independent of tumor stage (Bachelot et al. 2003; Laird et al. 2013).
Although chronic inflammatory mediators have not been established as true aging biomarkers, the fact that they correlate with measures of physical status, functional decline, and mortality in the older adult population suggests they may reflect underlying biologic processes that could predict a patient’s risk of toxicity from cancer treatment and, therefore, their ability to tolerate it. The levels of inflammatory mediators can be measured with ELISA assays on plasma samples collected during blood draws routinely done for cancer management. Therefore, the measurement of these levels could become an efficient way of providing the oncologist insight into potential tolerance of cancer therapy as well as prognosis and provide guidance as to the most appropriate regimens and doses for the older cancer patient.
Telomere Shortening
Telomeres are DNA protein complexes capping the end of chromosomes that provide chromosomal stability and prevent DNA degradation and recombination. Telomeres shorten with each cell division, eventually leading to cellular senescence and apoptosis (de Lange 2002). Cawthon et al. (2003) reported a pivotal study of 143 patients >60 years of age which showed that shorter telomere length correlated with both age and higher mortality. Subsequent studies have not shown a consistent correlation with telomere length and mortality, but several have shown positive association with telomere length and better health in older age (Atzmon et al. 2010; Bekaert et al. 2005; Njajou et al. 2009; Pallis 2013). Various lifestyle factors, including obesity, smoking, and marital status, also affect telomere length (Mishra et al. 2012).
Because there appears to be associations with telomere length and health status, investigators have studied telomere length as a potential biomarker among cancer patients. Willeit et al. (2010) evaluated telomere length of 787 individuals. Shorter telomere length was associated with developing cancer over the course of 10 years (HR 1.60; 95% CI: 1.30–1.98; P <0.001). The shortest telomere length also correlated with higher cancer mortality (HR 2.13; 95% CI: 1.58–2.86; P <0.001). There have been reports of shortened telomere length and poorer prognosis in a number of different cancers including colorectal, breast, lung, and sarcoma (Pallis et al. 2014). However, not all studies consistently show a correlation between telomere length and cancer incidence and/or mortality (Pooley et al. 2010; Prescott et al. 2012).
It does not appear that telomere length is associated with frailty as measured by geriatric assessment. A study reported by Falci et al. (2013) evaluated telomerase activity and telomere length of cancer patients ≥70 years of age (n = 52) compared with 39 age-matched controls that underwent a geriatric assessment. Telomere length was significantly shorter in cancer patients, but only correlated with age in non-cancer patients. They did not find that telomere length was associated with geriatric assessment scores. Several other studies have not found a correlation between measures of frailty and telomere length in the general population (Lorenzi et al. 2018; Saum et al. 2014).
Telomere length does not appear to be associated with frailty, which would potentially predict an increased risk for toxicity. There are a few studies on telomere length and toxicity. One study in colorectal cancer did find an association of telomere length with hematologic toxicity and mucositis in patients receiving 5-fluorouracil (Garg et al. 2012). Among breast cancer patients receiving paclitaxel, those with a higher percentage of critically shortened telomeres had higher toxicity, but there was no correlation with toxicity and average telomere length (Quintela-Fandino et al. 2017).
While telomere length plays a role in the aging process, the conflicting results in studies related to cancer incidence and mortality make the role of this biomarker in geriatric oncology unclear. In addition, there is not universal agreement on the method of measuring of telomere length, which can be complex (shortest telomere length by fluorescent in situ hybridization, quantitative real-time polymerase chain reaction [qPCR], measuring cleaved fragments). Further studies are needed to determine if telomere length can consistently predict outcomes for cancer patients.
Cellular Senescence Markers
Senescent cells accumulate with age as a reaction to lifelong cellular stress (Ressler et al. 2006; Liu et al. 2009). Although senescent cells are not mitotically active, they acquire a senescence-associated secretory phenotype (SASP), with increased production of proteins involved in chronic inflammation and coagulation, very similar to the markers associated with poorer physical function and mortality in the elderly (Campisi 2005; Coppe et al. 2008). p16INK4a is considered a marker of cellular senescence and is felt to be a potential biomarker for aging.
The p16INK4a gene is a cell cycle regulator that inhibits downstream activation of cyclin-dependent kinases 4/6 leading to permanent cell cycle arrest, otherwise known as cellular senescence (Romagosa et al. 2011). p16INK4a is activated during cellular stress responses and considered a tumor suppressor gene. Circulating levels of p16INK4a can be measured in T lymphocytes by qPCR.
p16INK4a age levels increase with age and age-related diseases (Lawrence et al. 2018; Tsygankov et al. 2009). Higher levels of p16INK4a expression in T lymphocytes are seen with lifestyle factors such as inactivity and tobacco use (Song et al. 2010). p16INK4a levels appear to be associated with frailty in the general geriatric population. In a small study of older women (n = 11) in a resistance training program, higher levels of p16INK4a-positive cells in adipose tissue were associated with poorer grip strength, 400-meter walk time, gait speed, and their perception of mobility (Justice et al. 2017). Waaijer et al. (2017) found a stronger association of p16INK4a levels with functional measures than with age.
Transgenic mouse models allow researchers to track and eliminate senescent cells. After exposure to a cellular senescence-inducing agent such as chemotherapy, Demaria et al. (2017) showed senescent cells in mice contribute to local and systemic inflammation leading to worsening side effects, and clearing these cells reduces those effects. They also found that humans with increased senescent marker expression in T cells prior to receiving chemotherapy had a greater degree of chemotherapy-induced fatigue. These results suggest that markers of senescence may be able to predict greater toxicity to cancer treatment.
Importantly, chemotherapy may also affect p16INK4a expression in patients exposed to chemotherapy. In a study of breast cancer patients undergoing adjuvant anthracycline-based chemotherapy, chemotherapy exposures were associated with an increase in p16INK4a expression that would be found with 10.4 years of aging (Sanoff et al. 2014). Therefore, this biomarker may also predict accelerated aging in cancer survivors.
Discussion
It is likely that all of the aforementioned potential markers of aging and/or frailty are interrelated. For instance, telomere shortening and the resultant dysfunction lead to cellular senescence (Wang et al. 2012). Cellular senescence is felt to be an underlying process contributing to the increase in inflammation associated with functional decline and mortality in the elderly (Franceschi et al. 2007; De Martinis et al. 2006; Freund et al. 2010). Inflammation can lead to impaired lymphopoiesis (Wang et al. 2012). Systemic inflammation is also associated with sarcopenia, which can result in a more sedentary lifestyle that is also associated with shortened telomeres (Mishra et al. 2012).
Regardless of the underlying process that set off the interrelated processes associated with biologic aging, further study is needed to determine which markers are the most easily measured, low burden to patient and the financial system, and the most reliable at identifying frail patients vulnerable to the toxicities from cancer treatment. This research will also provide valuable information on the underlying biologic mechanisms contributing to increased toxicity and poorer survival outcomes. Ultimately, identifying the underlying mechanisms of frailty leaves the potential for pharmacologic interventions that may improve the tolerability of cancer treatment for frail patients.
References
Atzmon G, Cho M, Cawthon RM, Budagov T, Katz M, Yang X, et al. Evolution in health and medicine Sackler colloquium: genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc Natl Acad Sci U S A. 2010;107(Suppl 1): 1710–7.
Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003;88(11):1721–6.
Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. Systemic cytokine response after major surgery. Br J Surg. 1992;79(8):757–60.
Bailur JK, Pawelec G, Hatse S, Brouwers B, Smeets A, Neven P, et al. Immune profiles of elderly breast cancer patients are altered by chemotherapy and relate to clinical frailty. Breast Cancer Res. 2017;19(1):20.
Baker GT 3rd, Sprott RL. Biomarkers of aging. Exp Gerontol. 1988;23(4–5):223–39.
Bekaert S, De Meyer T, Van Oostveldt P. Telomere attrition as ageing biomarker. Anticancer Res. 2005;25(4): 3011–21.
Blay JY, Chauvin F, Le Cesne A, Anglaret B, Bouhour D, Lasset C, et al. Early lymphopenia after cytotoxic chemotherapy as a risk factor for febrile neutropenia. J Clin Oncol. 1996;14(2):636–43.
Brouwers B, Dalmasso B, Hatse S, Laenen A, Kenis C, Swerts E, et al. Biological ageing and frailty markers in breast cancer patients. Aging. 2015;7(5):319–33.
Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8(3):131–6.
Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–22.
Carter RA, Wicks IP. Vascular cell adhesion molecule 1 (CD106): a multifaceted regulator of joint inflammation. Arthritis Rheum. 2001;44(5):985–94.
Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–5.
Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3): 242–8.
Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114(3):180–7.
Cohen HJ, Smith D, Sun CL, Tew W, Mohile SG, Owusu C, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865–72.
Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133(6): 456–66.
Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–68.
de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21(4):532–40.
De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80(3):219–27.
de Saint-Hubert M, Jamart J, Morrhaye G, Martens HJ, Geenen V, Duy Vo TK, et al. Serum IL-6 and IGF-1 improve clinical prediction of functional decline after hospitalization in older patients. Aging Clin Exp Res. 2011;23(2):106–11.
Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7(2):165–76.
Ershler WB, Sun WH, Binkley N, Gravenstein S, Volk MJ, Kamoske G, et al. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12(4): 225–30.
Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377–86.
Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23(9):2375–8.
Falandry C, Gilson E, Rudolph KL. Are aging biomarkers clinically relevant in oncogeriatrics? Crit Rev Oncol Hematol. 2013;85(3):257–65.
Falci C, Gianesin K, Sergi G, Giunco S, De Ronch I, Valpione S, et al. Immune senescence and cancer in elderly patients: results from an exploratory study. Exp Gerontol. 2013;48(12):1436–42.
Fernandez-Garrido J, Navarro-Martinez R, Buigues-Gonzalez C, Martinez-Martinez M, Ruiz-Ros V, Cauli O. The value of neutrophil and lymphocyte count in frail older women. Exp Gerontol. 2014;54: 35–41.
Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–46.
Ferrucci L, Cavazzini C, Corsi A, Bartali B, Russo CR, Lauretani F, et al. Biomarkers of frailty in older persons. J Endocrinol Investig. 2002a;25(10 Suppl):10–5.
Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002b;50(12):1947–54.
Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105.
Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16(5):238–46.
Garg MB, Lincz LF, Adler K, Scorgie FE, Ackland SP, Sakoff JA. Predicting 5-fluorouracil toxicity in colorectal cancer patients from peripheral blood cell telomere length: a multivariate analysis. Br J Cancer. 2012;107(9):1525–33.
Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103–9.
Huffman KM, Pieper CF, Kraus VB, Kraus WE, Fillenbaum GG, Cohen HJ. Relations of a marker of endothelial activation (s-VCAM) to function and mortality in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2011;66(12):1369–75.
Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457.
Johnson TE. Recent results: biomarkers of aging. Exp Gerontol. 2006;41(12):1243–6.
Justice JN, Gregory H, Tchkonia T, LeBrasseur NK, Kirkland JL, Kritchevsky SB, et al. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J Gerontol A Biol Sci Med Sci. 2017;73:939.
Kanapuru B, Ershler WB. Inflammation, coagulation, and the pathway to frailty. Am J Med. 2009;122(7):605–13.
Laird BJ, Kaasa S, McMillan DC, Fallon MT, Hjermstad MJ, Fayers P, et al. Prognostic factors in patients with advanced cancer: a comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin Cancer Res. 2013;19(19): 5456–64.
Lawrence I, Bene M, Nacarelli T, Azar A, Cohen JZ, Torres C, et al. Correlations between age, functional status, and the senescence-associated proteins HMGB2 and p16(INK4a). Geroscience. 2018;40:193–9.
Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–71.
Li W, Tao L, Zhang L, Xiu D. Prognostic role of lymphocyte to monocyte ratio for patients with pancreatic cancer: a systematic review and meta-analysis. Onco Targets Ther. 2017;10:3391–7.
Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8(4):439–48.
Lorenzi M, Bonassi S, Lorenzi T, Giovannini S, Bernabei R, Onder G. A review of telomere length in sarcopenia and frailty. Biogerontology. 2018;19: 209–21.
McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–40.
Mishra MV, Showalter TN, Dicker AP. Biomarkers of aging and radiation therapy tailored to the elderly: future of the field. Semin Radiat Oncol. 2012;22(4): 334–8.
Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):971–8.
Nishijima TF, Deal AM, Williams GR, Guerard EJ, Nyrop KA, Muss HB. Frailty and inflammatory markers in older adults with cancer. Aging. 2017;9(3): 650–64.
Njajou OT, Hsueh WC, Blackburn EH, Newman AB, Wu SH, Li R, et al. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64(8):860–4.
Pallis AG, et al. Evaluating the physiological reserves of older patients with cancer: the value of potential biomarkers of aging? J Geriatr Oncol. 2013;5:204.
Pallis AG, Hatse S, Brouwers B, Pawelec G, Falandry C, Wedding U, et al. Evaluating the physiological reserves of older patients with cancer: the value of potential biomarkers of aging? J Geriatr Oncol. 2014;5(2): 204–18.
Pawelec G, Solana R. Immunosenescence. Immunol Today. 1997;18(11):514–6.
Pieper CF, Rao KM, Currie MS, Harris TB, Cohen HJ. Age, functional status, and racial differences in plasma D-dimer levels in community-dwelling elderly persons. J Gerontol A Biol Sci Med Sci. 2000;55(11):M649–57.
Pooley KA, Sandhu MS, Tyrer J, Shah M, Driver KE, Luben RN, et al. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res. 2010;70(8):3170–6.
Prescott J, Wentzensen IM, Savage SA, De Vivo I. Epidemiologic evidence for a role of telomere dysfunction in cancer etiology. Mutat Res. 2012;730(1–2): 75–84.
Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol. 2005;63(4):403–11.
Quintela-Fandino M, Soberon N, Lluch A, Manso L, Calvo I, Cortes J, et al. Critically short telomeres and toxicity of chemotherapy in early breast cancer. Oncotarget. 2017;8(13):21472–82.
Ray-Coquard I, Borg C, Bachelot T, Sebban C, Philip I, Clapisson G, et al. Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy. Br J Cancer. 2003;88(2):181–6.
Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13): 5383–91.
Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Durr P, et al. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5(5):379–89.
Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50(4):638–44.
Robson SC, Shephard EG, Kirsch RE. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1 beta, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br J Haematol. 1994;86(2):322–6.
Romagosa C, Simonetti S, Lopez-Vicente L, Mazo A, Lleonart ME, Castellvi J, et al. p16(Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene. 2011;30(18):2087–97.
Ronning B, Wyller TB, Seljeflot I, Jordhoy MS, Skovlund E, Nesbakken A, et al. Frailty measures, inflammatory biomarkers and post-operative complications in older surgical patients. Age Ageing. 2010;39(6):758–61.
Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1): 149–63.
Saito K, Kihara K. C-reactive protein as a biomarker for urological cancers. Nat Rev Urol. 2011;8(12):659–66.
Sanoff HK, Deal AM, Krishnamurthy J, Torrice C, Dillon P, Sorrentino J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106(4):dju057.
Saum KU, Dieffenbach AK, Muezzinler A, Muller H, Holleczek B, Stegmaier C, et al. Frailty and telomere length: cross-sectional analysis in 3537 older adults from the ESTHER cohort. Exp Gerontol. 2014;58: 250–5.
Sharma R, Zucknick M, London R, Kacevska M, Liddle C, Clarke SJ. Systemic inflammatory response predicts prognosis in patients with advanced-stage colorectal cancer. Clin Colorectal Cancer. 2008;7(5):331–7.
Sieburg HB, Cho RH, Dykstra B, Uchida N, Eaves CJ, Muller-Sieburg CE. The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood. 2006;107(6):2311–6.
Simm A, Nass N, Bartling B, Hofmann B, Silber RE, Navarrete Santos A. Potential biomarkers of ageing. Biol Chem. 2008;389(3):257–65.
Sindermann J, Kruse A, Frercks HJ, Schutz RM, Kirchner H. Investigations of the lymphokine system in elderly individuals. Mech Ageing Dev. 1993;70(1–2):149–59.
Song Z, von Figura G, Liu Y, Kraus JM, Torrice C, Dillon P, et al. Lifestyle impacts on the aging-associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell. 2010;9(4):607–15.
Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014a;106(6):dju124.
Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2014b;23(7):1204–12.
Tham T, Olson C, Khaymovich J, Herman SW, Costantino PD. The lymphocyte-to-monocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2018;275:1663.
Tsygankov D, Liu Y, Sanoff HK, Sharpless NE, Elston TC. A quantitative model for age-dependent expression of the p16INK4a tumor suppressor. Proc Natl Acad Sci U S A. 2009;106(39):16562–7.
Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128(1):83–91.
Waaijer ME, Westendorp RG, Goldeck D, Gunn DA, Pawelec G, Stijntjes M, et al. Assessment of health status by molecular measures in adults ranging from middle-aged to old: ready for clinical use? Exp Gerontol. 2017;87(Pt B):175–81.
Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20): 2333–41.
Wang J, Sun Q, Morita Y, Jiang H, Gross A, Lechel A, et al. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell. 2012;148(5):1001–14.
Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–603.
Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1): 69–75.
Wirtz DC, Heller KD, Miltner O, Zilkens KW, Wolff JM. Interleukin-6: a potential inflammatory marker after total joint replacement. Int Orthop. 2000;24(4):194–6.
Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clin Geriatr Med. 2011;27(1): 79–87.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry
Hubbard, J.M. (2020). Biomarkers of Aging (With a Clinical Potential in Oncology). In: Extermann, M. (eds) Geriatric Oncology . Springer, Cham. https://doi.org/10.1007/978-3-319-57415-8_62
Download citation
DOI: https://doi.org/10.1007/978-3-319-57415-8_62
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57414-1
Online ISBN: 978-3-319-57415-8
eBook Packages: MedicineReference Module Medicine