Abstract
Disorders of menstrual period are very frequent in years immediately after menarche. Variation of menstrual rhythm can be related to (Table 7.1) a dysfunction of the hypothalamic–pituitary axis as a consequence of energy deficiency related to eating disorders (Chap. 8) or to elevated energy expenditure, of stress conditions (physical and/or emotional), of metabolic stress secondary to chronic diseases. All the previous mentioned states can result in functional hypothalamic amenorrhea (FHA). Prolactin excess is another possible pathogenesis of menstrual disturbances. Organic CNS pathologies affecting hypothalamic–pituitary region are rare, but they exist.
Menstrual dysfunction can also be related to specific ovarian diseases as polycystic ovary syndrome (Chap. 9), increasingly rarely to functioning ovarian cysts or premature ovarian insufficiency. Other endocrine disorders as thyroid dysfunction or adrenal pathologies can be responsible for alteration of menstrual rhythm as well.
A competent diagnostic workup is required to identify these different clinical conditions and to guide a proper therapeutic management.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
7.1 Pathophysiology of Post-menarche Menstrual Function
Girls, during adolescence, go through the maturation of a complex endocrinological system, which involves the hypothalamus, the pituitary gland, the ovaries and their interactions. All the above should lead to a healthy reproductive function, but it doesn’t occur immediately, so, in postpubertal girls we can find frequent menstrual disorders like polymenorrhea and oligomenorrhea (Tables 7.1 and 7.2) [1,2,3,4].
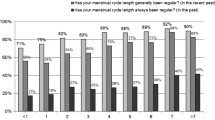
Menstrual irregularity is virtually always the result of anovulatory cycles. However, the opposite is not always true monthly, menstrual regularity does not necessarily indicate underlying regular ovulatory cyclicity. Within 1 year after menarche, menstrual regularity approximates adult standards in most girls, although there is considerable interindividual variation in the time it takes for menstrual cyclicity to mature [1, 4]. Average menstrual cycle length is 21–45 days in 75% of girls 1 year post-menarche, and further 5% falls within these bounds for each of the following 3 years [5, 6]. During the first 2 post-menarcheal years, about half of menstrual cycles are anovulatory, but the duration half of these anovulatory cycles is 21–45 days [2, 3, 6].
Thus, normal menstrual frequency is much greater than ovulatory frequency. Within the first 5 gynecological years, 95% of menstrual cycles lasts 21–40 days, and about 75% of cycles are ovulatory; over the next several years the mature menstrual pattern is established with approximately an 80% ovulatory rate. During the following years the menstrual pattern is mature, and consequently ovulatory, in about 80% of cases [2, 3, 6]. As earlier age of menarche is associated with earlier ovulatory maturation, the opposite applies and late maturation [7]. Normally adolescent anovulation causes only minor menstrual cycle irregularity (Table 7.3).
7.2 Functional Immaturity
Normal, pubertal maturation, menstrual function requires a series of neuroendocrine steps involving numerous districts of the body that can provide a physiological latency. Ovarian follicular structures, i.e., granulosa cells and theca cells, may experience a period of mild dysfunction due to the immaturity of their cross-talk mechanisms and, probably, to the elevated stimulus on the ovary by physiologically increased insulin concentrations. In these cases of functional immaturity, androgen production by thecal cells (mainly androstenedione) is increased but their aromatization in estradiol is reduced, leading to a delay in follicular maturation with oligomenorrhea as clinical manifestation.
7.3 Functional Hypothalamic Amenorrhea (FHA)
FHA includes all clinical conditions characterized by stress, low energetic intake, intense physical activity, and chronic disease affecting metabolic homeostasis.
7.3.1 Mean Pathogenic Mechanisms
In a dangerous, acute situation the production of norepinephrine (also responsible for the feeling of fear) from locus coeruleus starts. In the same time, norepinephrine increases production of CRH with activation of hypothalamus–pituitary–adrenal axis and of sympathetic autonomous nervous system. The answer to stressing stimuli is mediated by β-endorphin, even if modulated by genetic variables. All physiological functions not fundamental for survival are restricted, as the ovarian function. The anatomic and functional proximity between GnRH-gonadotropin axis and CRH-ACTH axis allows rapid short inhibitory mechanisms on reproductive function in response to stress hormone hyperproduction. In a chronic stress state, the answer to chronic stimuli is modulated by feedback of rearrangement, acting through the same pathway of increased limbic–hypothalamic–pituitary–adrenal axis activity [9] and reduced central gonadotropin-releasing hormone (GnRH) drive [10,11,12].
Of note, stress and its resulting hormonal changes could trigger either undernutrition or overnutrition, depending on fuel availability, attitudes about food, and dietary behaviors such as bingeing, purging, overeating, or restricting. The answer to hormonal stress depends on age and weight, for this reason in adolescence it is stronger than in adults.
The energetic homeostasis is another crucial point to allow physiological reproductive function. Vagal nerve is the principal nervous input to central nervous system (CNS); this nerve is connected to gastric distension and cholecystokinin (CCK) and glucose production. Peripheral endocrine signals are various: leptin and adiponectin are signals produced by adipose tissue acting as feedback on hypothalamic nuclei regulating feeding and reproductive control. On the contrary, ghrelin, produced by cells of stomach fundus, is a short-term signal of energetic request. Both leptin and ghrelin play their role, respectively inhibitory and stimulating on appetite center, through arcuate nuclei, probably through pro-opiomelanocortin and the peptides derived from its cleavage. The feedbacks from other peripheral hormones produced by gastrointestinal tract are integrated at hypothalamic level: Peptide YY, GLP-1, Insulin, and Pancreatic polypeptide. These peripheral endocrine messages mix their information to hypothalamus with central afferences (as endocannabinoids and oxytocin).
The inhibitory effect of energetic deficit on hypothalamic function is a key point in menstrual dysfunctions of adolescent athletes, due to strenuous exercise sometimes associated to inadequate energy intake. This can be expressed by inappropriate luteal phase, anovulatory cycles, oligomenorrhea, or secondary amenorrhea. It is important to keep in mind that the same endocrine system that binds energetic restriction and menstrual alteration produces effects on bone turnover, increasing reabsorption and reducing neoformation in the adolescent age, causing a problem in reaching or maintaining peak bone mass.
Amenorrhea, low bone density, and eating disorders, the so-called “Athletic Triade,” expose these girls to high risk of stress fractures. Stress fractures are normally not due a single traumatic event, but to multiple bone stresses. The bones most frequently interested are tibia, metatarsi, and navicular, due to their particular exposure to micro traumatic events.
7.3.2 Diagnosis
Functional hypothalamic amenorrhea is a diagnosis established after exclusion of other conditions having similar manifestation. The diagnostic workup should be based on the history of menstrual disorders. In the majority of cases normal menses with ovulatory cycles are followed by gradual loss of ovulation, then the menses become rare till they completely disappear. A lack of menarche can also be the main manifestation of FHA in early pubertal girls (Chap. 2). A careful clinical history is essential for identifying these patients; familiar osteoporosis, low birth weight, bowel malabsorption, previous fractures, late menarche, and low sun exposition are also important factors for bone mineral density deficiency; the number of hours of physical activity per day or week, eating diary, and menstrual diaries are also useful. Family conflicts, problems with the peers, school difficulties, and stressful events should also be investigated. The measure of height and weight and body mass index (BMI) is fundamental. DXA (Dual energy X-ray Absorption) and BIA (Bioelectrical Impedance Assessment) are useful tools to assess body composition. The impedance assessment is based on low frequency electric energy modifications passing through the body. The attenuation (resistance) is mainly due to the presence of water, which is mainly related to muscle mass. This exam primarily evaluates hydration and nutritional state. Generally, a good hydration should be around 60% and lean mass 78–80%. The measure of fat mass is indirect and for this reason is not totally reliable. Athletic girls often present reduced levels of intracellular water due to thermoregulation induced by physical effort. The body cellular mass (BCM) expresses the metabolically active part of the body: a cutoff level of 7 is considered expression of undernutrition. The DXA total body is a more precise mean of studying body composition, even if it is expensive and minimally radiant. It consents the evaluation of Bone Mineral Density (BMD) using references for age. Ultrasound pelvic is a useful complementary examination because endometrial thickness is an indirect measure of estradiol levels. Ovary echo-structures can be extremely various: multi-follicular or micro-follicular with low vascularization to color Doppler (Table 7.4).
We can use progesterone challenge test to check the endometrial estrogenization [13]; performing the test blood sampling for hormonal profile can take place during bleeding. In the past medroxyprogesterone acetate 10 mg for 10 days has been extensively used: menstrual bleeding could be expected with an endometrial thickness >6 mm. Nowadays micronized progesterone 100 mg/day twice/day for 10 days is preferred, but it doesn’t exist yet a definition of endometrial thickness related to the bleeding answer.
7.3.3 Management
Treatment of menstrual disorders, and secondary amenorrhea resulting from hypothalamic disorders should be aimed at the elimination of the primary cause, i.e., a decrease in psycho-emotional strain, avoidance of chronic stressors, reduction of physical exercise level, or optimization of BMI in patients who lose weight [14].
A cognitive-behavioral therapy can be proposed to help coping with stress response or modifying habits related to diet and physical exercise, working on body image difficulties or problem-solving skills. A reduction of stress response and the restoration of metabolic equilibrium is the main street to resume normal menses and ovulation. Usually, menstrual function resumes spontaneously as a result of lifestyle modification or of environmental changes (e.g., changing school).
If menses do not resume after a period of 6 months or primary causative treatment is not possible, e.g., in competitive athletes or ballet dancers, neutralization of hypoestrogenism consequences especially unfavorable effects on bone metabolism becomes the main issue. Hormonal preparations should be introduced into therapeutic protocol on an individualized basis; the patient’s expectations with regard to treatment outcomes should also be considered. In situations with long-lasting low energy intake, the bone sparing effect of estroprogestins is probably ineffective.
7.4 Chronic Diseases
Systemic diseases affecting metabolic homeostasis can induce menstrual dysfunction and bone impairment.
Congenital bile atresia: Rare, inflammatory damage to intra-extra hepatic bile ducts with bile tree sclerosis and narrowing up to obliteration.
Celiac disease: The disease is an immune-mediated inflammatory enteropathy triggered by gluten exposure in genetically susceptible individuals. It has a high prevalence approaching 1% but it is very poorly diagnosed. The enzyme transglutaminase, through deamidation, modifies gluten, so the protein is presented like an antigen and triggers a systemic inflammatory reaction. Several studies have shown that celiac disease, mostly if not recognized, can impair women’ reproductive life eliciting delayed puberty, infertility, amenorrhea, and early menopause [15]. Therapy is a gluten-free diet.
Systemic lupus erythematosus (SLE) is an autoimmune disorder; during its active phases, it may affect the hypothalamic–pituitary functioning and reproductive health status. Additionally, cyclophosphamide treatment can affect gonadal function [16].
Inflammatory bowel disease (IBD) is an autoimmune disease related to individual genetic susceptibility, modifications of gut microbiota, and trigger events that modify the physiological immune barrier inducing an inflammatory chronic condition. Menstrual disorders occur commonly in women with Crohn’s diseases, linked both to malabsorption and to the elevated inflammatory reaction, present even in the years preceding the diagnosis. In these patients, we prefer the use of progesterone with natural estrogen rather than hormonal contraceptive due to theirs higher level of thromboembolic risk, related to pathology. The treatment of the underlying condition is the main therapeutic aid.
Chronic kidney disease: Girls with kidney dysfunction often experience menstrual disorders especially patients in dialysis. Malnutrition and modification in body composition are probably the main pathogenetic factors. As a treatment it is possible to use progesterone or progestins.
7.5 Hyperprolactinemia
An increase in circulating prolactin (PRL) levels may reveal itself with menstrual disturbances. 5.5% of menstrual dysfunction in adolescents are due to hyperprolactinemia. Hyperprolactinemia is not a unique disease per se; rather, it has multiple etiologies [17,18,19] (Table 7.5).
PRL size is heterogeneous in terms of circulating molecular forms. The predominant form in healthy subjects and in patients with prolactinomas is monomeric PRL. Dimeric or big PRL (45–60 kDa), and big-big PRL or macroprolactin (150–170 kDa) correspond to less than 20% of the total PRL Though still controversial, studies indicate that macroprolactin has both low bioactivity and bioavailability [20,22,23], thus explaining why most patients with increases in macroprolactinemia lack typical symptoms related to hyperprolactinemia [22,23,24].
Considering prevalence, prolactinoma is the most common cause of chronic hyperprolactinemia, followed by drugs stimulating PRL production, pseudoprolactinoma, pregnancy, and primary hypothyroidism.
Prolactin secreting pituitary adenomas or prolactinomas represent the most common type of pituitary adenoma (about 40%) being the main cause of pathological hyperprolactinemia [17,18,19]. Pituitary adenomas secreting PRL can be distinguished in micro if they are <10 mm and macro if they are bigger than 10 mm.
The term pseudoprolactinoma is comprehensive of all compressive situations that disrupt or reduce inhibitory connections (Tubero Infundibular Dopaminergic neurons or TIDA) between hypothalamus and pituitary. They may be not functioning adenomas, tumors as craniopharyngiomas, traumatic lesions, infective, infiltrative or vascular pathologies that reduce the hematic flow or directly damage the neurovascular bundle.
Autoimmune lymphocytic hypophysitis is generally the consequence of an inflammatory process affecting the whole gland or only the infundibular-posterior region, with autoimmune partial parenchyma destruction and consequent hypofunction. It can alter menstrual cycle both through with hyperprolactinemia and low level of FSH and LH.
A primary empty sella syndrome is characterized by the increase of cerebrospinal fluid through a hole in the sella diaphragm, with compression of pituitary parenchyma. It is often asymptomatic, but it can sometimes appear with hyperprolactinemia and intracranial hypertension. Secondary empty sella is mainly related to hypophysitis.
In case of untreated primary hypothyroidism, we can register an increase of prolactin due to drag effect of TRH.
We define an idiopathic hyperprolactinemia if specific causes are not evident and the imaging is negative; in about 30% of cases, the levels of the hormone will reestablish spontaneously.
A high level of prolactin due to drugs is very common in the adolescent girls. Drugs act through different mechanisms: increased transcription of PRL gene (estrogens), antagonism of dopamine receptor (risperidone, haloperidol, metoclopramide, domperidone, sulpiride, etc.), dopamine depletion (reserpine, methyldopa), inhibition of hypothalamic dopamine production (verapamil, heroin, morphine, enkephalin analogs, etc.), inhibition of dopamine reuptake (tricyclic antidepressants, cocaine, amphetamine, monoamine oxidase inhibitors), inhibition of serotonin reuptake (opiates, fenfluramine, fluoxetine, sibutramine), etc. [19, 26,27,28,29,30,31].
Among antipsychotics, the most frequently involved are haloperidol, phenothiazine, and risperidone, while tricyclic drugs are the main observed among the antidepressants. Other studies [26] found the following rates of hyperprolactinemia associated with each therapeutic drug class: 31% neuroleptics, 28% neuroleptic-like drugs, 26% antidepressants, 5% H2-receptor antagonists, and 10% other drugs. The newer atypical antipsychotics (AAP) are characterized by increased antipsychotic efficacy, and fewer neurological and endocrine related side effects as compared to classical antipsychotic drugs. With the exception of risperidone, amilsulpride, and molindone that are often associated with high PRL levels [32], most of AAP elicit poor hyperprolactinemic response or no hyperprolactinemia at all [28]. Furthermore, addition of drugs like quetiapine and aripiprazole has been shown to reverse the hyperprolactinemia induced by other AAPs [29].
The evaluation of drug-induced hyperprolactinemia can be challenging; it is noteworthy to consider the concomitance of a pathologic cause. In an ideal situation, if the severity of the disease consents and is corroborated by a psychiatrist, it is recommended to perform repeated PRL measurements after discontinuing the medication for at least 3–4 days. When drug withdrawal is unsafe, an MRI should be performed to rule out a sella mass. If drug-induced hyperprolactinemia is confirmed, the switch to an alternative medication is the safer option [25, 30].
7.5.1 Diagnosis
The diagnosis of hyperprolactinemia is based on repeated findings (two at least) of an increase of PRL serum concentration (above 25 ng/ml or 600 UI/l in women). Blood samples should be collected in the morning in the patient in fasting state, who should be in a comfortable setting after a good night’s sleep at least 2–3 h after waking up (samples drawn earlier may show sleep-induced peak levels). The TSH and IGF-1 dosage is a useful tool to confirm the diagnosis. Magnetic resonance imaging (MRI) with gadolinium as a contrast mean virtually visualizes all macroprolactinomas (diameter ≥10 mm) and pseudoprolactinomas, as well as most microprolactinomas (diameter <10 mm) [32,33,34]. In case of macroprolactinomas is worth a check of visual field.
7.5.2 Therapy
D2 receptor agonists induce the inhibition of stored PRL and the reduction of its synthesis and secretion through suppression of gene. A daily dopamine agonist administration can produce side effects such as nausea and vomiting; for these reasons it is worth starting with the lowest dose, monitoring the hormone plasma levels. Bromocriptine is less effective in adolescent microadenomas. Cabergoline is the drug of choice because it is characterized by a long half-life, low clearance and enterohepatic circulation.
The treatments with dopaminoagonist could, even if rarely, cause cardiac valves’ lesions, so an echocardiogram in prolonged treatments is recommended. In patients with hyperprolactinemia, we can use also oral estroprogestin. In case of no symptoms, with negative imaging, the necessity of therapy is under debate.
7.6 Secondary Menstrual Disorders
In case of Congenital Adrenal Hyperplasia, menstrual cycles are irregular for higher levels of progesterone in follicular phase.
Cushing Syndrome is not so common in adolescent period and it is characterized by high level of cortisol. An increase in free urinary cortisol is required for the diagnosis.
7.6.1 Dysfunctional Thyroid
Hashimoto’s thyroiditis (HT), the most common autoimmune thyroid disease at any age, is often associated with other autoimmune diseases.
Girls with hypothyroidism suffer from menstrual irregularity three times more than general population. In hypothyroid patients, TRH increases stimulation of both TSH and PRL and a deficit in LH production with inadequate luteal phase has also been observed; reduction of SHBG, estradiol and testosterone circulating levels reduce endometrial growth.
In case of hyperthyroidism, we can find higher level of estrogen due to increase of SHBG and heavy menstrual bleeding.
7.7 Premature Ovarian Insufficiency (POI)
Numerous studies are available on the pathophysiology of premature ovarian insufficiency (POI). Spontaneous POI involves the precocious cessation of normal ovarian function, causing infertility, menopausal symptoms, and general health concerns. It affects approximately 0.1% of women under 30 years [35, 36].
There are multiple etiologies for primary POI, including genetic, autoimmune, and idiopathic presentations, but also iatrogenic related to chemotherapy or radiation, or pelvic surgery.
In adolescent girls, gonadal dysgenesis revealing after menarche is a frequent cause of gonadal defect (see Chap. 4). The most common genetic cause of POI is Turner syndrome, affecting about 1: 2500 girls [37] characterized by aneuploidy of X chromosome. But spontaneous 46, XX POI with ovarian insufficiency in girls with normal karyotype is frequent and related to various gene mutations related to X chromosomes or to autosomes.
A premutation in the Fragile X Mental Retardation 1 (FMR1) gene is responsible for an estimated 2–5% of cases of isolated sPOI and 14% of familial sPOI cases [37, 38]. The FMR1 gene contains a polymorphic trinucleotide (CGG): more than 200 CGG repeats cause fragile X syndrome, the most common heritable form of mental retardation. The FMR1 gene premutation, which may expand to the full mutation across generations, contains 55–199 CGG repeats, and entails about 24% risk of developing POI in carriers [37].
Approximately 4% of sPOI cases are due to lymphocytic autoimmune oophoritis caused by autoimmunity against steroidogenic cells, a process that may affect function of both the ovary and the adrenal glands [39, 40].
Classic galactosemia (ORPHA-79239) is caused by deficient activity of galactose-1-phosphate uridyl transferase (GALT), as a result of mutations in the GALT gene located on chromosome 9p13. GALT is the second of the three enzymes in the Leloir pathway, the main pathway of galactose metabolism. The incidence of classic galactosemia varies between 1:16,000 [41] and 1:60,000 [42] in Western countries. Galactose is needed for energy metabolism and glycosylation of complex molecules. It may be derived from exogenous (dietary) sources, most importantly lactose from dairy products, or endogenous production. Deficiency of the GALT enzyme leads to accumulation of galactose and its metabolites and results in secondary glycosylation abnormalities. Patients usually present in the first weeks of life with signs of liver and renal disease, cataracts, and an Escherichia coli sepsis. Diagnostic tests include elevated galactose and galactitol in body fluids, elevated Gal-1-P in erythrocytes, severely diminished enzyme activity in erythrocytes, and mutations in the GALT gene. A galactose-restricted diet quickly resolves the early signs, but cannot prevent the development of later-onset complications, such as cognitive impairment, neurological sequelae, bone health abnormalities, and, in female patients, POI with subsequent infertility. Although POI in classic galactosemia represents a major concern for these patients and/or their parents [43], there are no published recommendations concerning fertility preservation in this group [44].
Symptoms of POI differ between affected women, varying from subfertility, to early development of irregular menstrual cycles and infertility, to primary amenorrhea and absence of spontaneous puberty [45]. The cause of POI in classic galactosemia is not yet understood. Several mechanisms have been postulated, including direct toxicity of metabolites (i.e., galactose-1-phosphate), altered gene expression, or aberrant function of hormones and or receptors due to glycosylation abnormalities [46,47,48,49]. It is also possible that not one, but several mechanisms act in unison to cause POI in classic galactosemia.
In general, POI can be caused by either the formation of a smaller primordial follicle pool or more rapid loss of primordial follicles [45] and there is evidence for both mechanisms in classic galactosemia. In classic galactosemia, there is some evidence that the follicle pool at birth is as large as in girls without this disease [44].
An immune-mediated premature ovarian insufficiency could be associated in some case of thyroidits immune-mediated, Addison and dyabets and many others immune-mediated disease. Many targets have been identified in the ovary: steroid secretion, gonadotropin, and oocyte. Many of the health complications associated with POI are directly related to ovarian hormone deficiency, primarily estrogen deficiency. They include menopausal symptoms (hot flashes, night sweats, insomnia, dyspareunia, decreased sexual desire, and vaginal dryness), decreased bone mineral density (BMD) and increased risk of fracture, infertility, increased risk of mood disorders, namely, depression and anxiety, cognitive decline, sexual dysfunction, increased rates of autoimmune disease, increased risk of cardiovascular disease, increased risk of type 2 diabetes mellitus (T2DM) or pre-DM, and dry eye syndrome [39].
7.7.1 Diagnosis
The most commonly applied definition of POI is 4 months of amenorrhea, with serum levels of FSH greater than 40 IU/L on two occasions. Dosage of FSH >30 UI/l twice, even if an FSH level >15 UI/l in adolescent, is already significant for an ovarian damage. The level of anti-mullerian hormone (AMH) produced by granulosa cells follicle can give us an estimate of ovarian reserve.
Pelvic ultrasound is useful to measure ovarian volume and to perform the antral follicle count. Ovarian volume in these patients is reduced, and cutoff is <6 cm3; antral follicles are those with diameter between 2 and 10 mm; in a normal condition they should be between 3 and 8 per ovary. The minimum amount in both ovaries is 10 antral follicles.
In case of autoimmune etiology, we must check autoantibodies. Generally, we check autoantibodies directed to ovarian cell, against thyroglobulin, TRH, adrenal cells, and gastric mucosa.
In these patients it is also important to measure basal bone mineral density (especially in patients treated with chemotherapy during infancy), and to propose specific counseling for bone loss prevention: physical activity, diet, and vitamin D supplementation if required.
Genetic counselling, extended to other familiar, is particularly useful in case of FMR1 premutation; but it could be of interest also in X aneuploidies or other known mutations.
In subjects with previous antineoplastic treatment a thorough clinical and laboratoristic evaluation, considering also thromboembolic risk, is mandatory before choosing the hormonal therapy. A cardiological evaluation after chemotherapy in case of use of cardiotoxic drugs is also advised. In subjects recovered from Hodgkin disease extended to mediastinal area, we recommend strict mammary control for the well-known higher risk of breast cancer after radiotherapy.
If it is possible for a very precocious diagnosis we can suggest the patient an oocyte cryopreservation.
In case of POI, the communication of the diagnosis with the patient is very heavy, because they often do not accept their condition and can remove the information we give them. In our experience the possibility of a psychological counselling is precious.
7.7.2 Therapy
It is widely accepted that the mainstay of treatment of POI is hormone replacement therapy (HRT). The choice of HRT should closely mimic normal ovarian steroid hormone production and provide sufficient levels of E2 to reduce menopausal symptoms, maintain bone density, minimize psychologic impacts of estrogen deficiency, and protect against early progression of CVD and dementia [37]. In these patients it is very important for a very precocious start of hormonal therapy for ensuring a correct genital tropism and minimizing endothelial dysfunction due to hypoestrogenism. For these patients, especially those with presumptive autoimmune disorder, the possibility of pregnancy is very low but it exists and we have to inform them about it.
We usually prescribe oral or transdermal estradiol; the transdermal route is more advisable if a minimal venous risk is suspected. Risk of venous thromboembolism is increased by oral estrogen compared with transdermal estrogen use [35, 50,51,52,53].
An adequate dosage for estradiol in this age range is about 75–100 mcg transdermal and 1.5–2 mg per os daily. The individual variability of absorption, especially for oral route, is very wide. So we suggest to check the clinical answer through ultrasound examination evaluating uterus dimension and endometrial thickness and, in some cases, the dosage of 17 β estradiol in precocious follicular phase.
The progestin component of HRT for women with POI should be cyclical and will protect the endometrium by inducing regular withdrawal bleeds. Natural progesterone or dydrogesterone is preferable because of the low metabolic impact. HRT should be continued until the age of natural menopause, at which time the dose may be tapered to postmenopausal levels or stopped, depending on a woman’s specific risks and needs.
Abbreviations
- AAP:
-
Atypical AntiPsychotics
- ACTH:
-
Adrenocorticotropic hormone
- AMH:
-
Anti-Mullerian hormone
- BCM:
-
Body cellular mass
- BIA :
-
Bioelectrical impedance assessment
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- CCK:
-
CholeCystoKinin
- CNS:
-
Central nervous system
- CRH:
-
Corticotropin-releasing hormone
- CVD:
-
Cardiovascular disease
- DM:
-
Diabetes mellitus
- DXA:
-
Dual energy X-ray absorptiometry
- D2:
-
Dopamine receptors 2
- E2:
-
Estradiol
- FHA:
-
Functional hypothalamic amenorrhea
- FMR1:
-
Fragile X mental retardation protein
- FSH:
-
Follicle stimulating hormone
- fT3:
-
Free thyroxine 3
- fT4:
-
Free thyroxine 4
- GALT:
-
Galactose-1-phosphate-uridyl-transferase
- GLP-1:
-
Glucagon like peptide-1
- GnRH:
-
Gonadotropin-releasing hormone
- HRT:
-
Hormone replacement therapy
- IBD:
-
Inflammatory bowel disease
- IGF-1:
-
Insulinlike growth factors-1
- LH:
-
Luteinizing hormone
- MRI:
-
Magnetic resonance imaging
- PCOS:
-
Polycystic ovary syndrome
- POI:
-
Premature ovarian insufficiency
- PRL:
-
Prolactin
- SLE:
-
Systemic lupus erythematosus
- TIDA:
-
Tubero infundibular dopaminergic neurons
- TRH:
-
Thyrotropin-releasing hormone
- TSH:
-
Thyrotropin stimulating hormone
- T2DM:
-
Type 2 diabetes mellitus
References
Legro RS, et al. Rapid maturation of the reproductive axis during perimenarche independent of body com- position. J Clin Endocrinol Metab. 2000;85:1021–5.
Apter D, Vihko R. Serumpregnenolone, progesterone,17-hydroxy- progesterone, testosterone, and 5-dihydrotestosterone during female puberty. J Clin Endocrinol Metab. 1977;45:1039–48.
Metcalf MG, et al. Incidence of ovulation in the years after the menarche. J Endocrinol. 1983;97:213–9.
Rosenfield R. Adolescent anovulation: maturational mechanisms and implications. J Clin Endocrinol Metab. 2013;98:3572–83.
Treloar AE, et al. Variation of human menstrual cycle through reproductive life. Int J Fertil. 1967;12:77–126.
Vollman RF. The menstrual cycle. Major Probl Obstet Gynecol. 1977;7:1–193.
Apter D, Vihko R. Hormonal patterns of the first menstrual cycles. In: Venturoli S, Flamigni C, Givens JR, editors. Adolescence in females. Chicago, IL: Year Book Medical Publishers; 1985. p. 215–38.
Hillard PJA. Menstruation in adolescents: what do we know? and what do we do with the information? J Pediatr Adolesc Gynecol. 2014;27:309–19.
Berga SL. Stress and reprodution: a tale of false dichotomy? Endocrinology. 2008;149:867–8.
Reame NE, et al. Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: Evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab. 1985;61:851–8.
Crowley WF Jr, et al., editors. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res. 1985;41:473–526.
Prokai D, Berga SL. Neuroprotection via reduction in stress: altered menstrual patterns as a marker for stress and implications for long-term neurologic health in women. IntJ Mol Sci. 2016;17(12):2147.
Dei M, Bruni V. In Guida alla Ginecologia dell’Infanzia e adolescenza Cap 13 Ed. Officine Editoriali Oltrarno; 2016.
Sowińska-Przepiera E, et al. Functional hypothalamic amenorrhoea – diagnostic challenges, monitoring, and treatment. Endokrynologia Polska. 2015;66(3):252–68.
Martinelli D, et al. Reproductive life disorders in Italian celiac women. A case-control study. BMC Gastroenterol. 2010;10:89.
Nonato DR, et al. Menstrual disturbances in systemic lupus erythematosus patients using immunossuppressants. Rev Bras Reumatol. 2010;50(5):501–15.
Bronstein MD. Disorders of prolactin secretion and prolactinomas. In: DeGroot LJ, Jameson JL, editors. Endocrinology. 6th ed. Philadelphia: Saunders/Elsevier; 2010. p. 333–57.
Vilar L, Naves LA. Avaliação diagnóstica da hiperprolactinemia. In: Vilar L, et al., editors. Endocrinologia Clínica. 5a ed. Rio de Janeiro: Guanabara Koogan; 2013. p. 39–49.
Vilar L, et al. Pitfalls in the diagnosis of hyperprolactinemia. Arq Bras Endocrinol Metabol. 2003;47(4):347–57.
Vilar L, et al. Challenges and pitfalls in the diagnosis of hyperprolactinemia. Arq Bras Endocrinol Metabol. 2014;58(1):9–22.
Suh HK, Frantz AG. Size heterogeneity of human prolactin in plasma and pituitary extracts. J Clin Endocrinol Metab. 1974;39(5):928–35.
Sinha YN. Structural variants of prolactin: occurrence and physio- logical significance. Endocr Rev. 1995;16(3):354–69.
Jackson RD, et al. Characterization of a large molecular weight prolactin in women with idiopathic hyper- prolactinemia and normal menses. J Clin Endocrinol Metab. 1985;61(2):258–64.
Shimatsu A, Hattori N. Macroprolactinemia: diagnostic, clinical, and pathogenic significance. Clin Dev Immunol. 2012;2012:167132.
Glezer A, Bronstein MD. Approach to the patient with persistent hyperprolactinemia and negative sellar imaging. J Clin Endocrinol Metab. 2012;97(7):2211–6.
Petit A, et al. Drug-induced hyperprolactinemia: a case-non-case study from the national pharmacovigilance database. Therapie. 2003;58(2):159–63.
Inder WJ, Castle D. Antipsychotic-induced hyperprolactinaemia. Aust N Z J Psychiatry. 2011;45(10):830–7.
LaTorre D, Falorni A. Pharmacological causes of hyperprolactinemia. Ther Clin Risk Manag. 2007;3(5):929–51.
Kunwar AR, Megna JL. Resolution of risperidone-induced hyper- prolactinemia with substitution of quetiapine. Ann Pharmacother. 2003;37(2):206–8.
Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolac- tinemia: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273–88.
Molitch ME. Drugs and prolactin. Pituitary. 2008;11(2):209–18.
Freda PU, Post KD. Differential diagnosis of sellar masses. Endocrinol Metab Clin North Am. 1999;28:81–117.
Glezer A, all e. Rare sellar lesions. Endocrinol Metab Clin North Am. 2008;37:195–211.
Naidich MJ, Russell EJ. Current approaches to imaging of the sellar region and pituitary. Endocrinol Metab Clin North Am. 1999;28:45–79.
Sullivan SD, et al. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. 2016;106(7):1588–99.
Sassarini J, et al. Sex hormone replacement in ovarian failure - new treatment concepts. Best Pract Res Clin Endocrinol Metab. 2015;29(1):105–14.
Bondy CA. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92:10–25.
Murray A, et al. Population-based estimates of the prevalence of FMR1 expansion mutations in women with early menopause and primary ovarian insufficiency. Genet Med. 2014;16:19–24.
Hoek A, et al. Premature ovarian failure and ovarian autoimmunity. Endocr Rev. 1997;18:107–34.
Rafique S, et al. A new approach to primary ovarian insufficiency. Obstet Gynecol Clin North Am. 2012;39:567–86.
Coss KP, et al. Classical Galactosaemia in Ireland: incidence, complications and outcomes of treatment. J Inherit Metab Dis. 2012;36:21–7.
Berry GT, Elsas LJ. Introduction to the Maastricht workshop: lessons from the past and new directions in galactosemia. J Inherit Metab Dis. 2011;34:249–55.
Bosch AM, et al. Living with classical galactosemia: health-related quality of life consequences. Pediatrics. 2004;113:e423–8.
van Erven B, et al. Fertility preservation in female classic galactosemia patients. Orphanet J Rare Dis. 2013;8:107.
Forges T, et al. Pathophysiology of impaired ovarian function in galactosaemia. Hum Reprod Update. 2006;12:573–84.
Fridovich-Keil JL, et al. Ovarian function in girls and women with GALT-deficiency galactosemia. J Inherit Metab Dis. 2011;34:357–66.
Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2006;2009(360):606–14.
Liu G, et al. Galactose metabolism and ovarian toxicity. Reprod Toxicol. 2000;2000(14):377–84.
Forges T, Monnier-Barbarino P. Premature ovarian failure in galactosaemia: pathophysiology and clinical management. Pathol Biol (Paris). 2003;51:47–56.
Scarabin PY, et al. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428–32.
Canonico M, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–5.
Canonico M, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336:1227–31.
Mohammed K, et al. Oral vs. transdermal estrogen therapy and vascular events: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:4012–20.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Pampaloni, F., Mertino, P. (2018). Menstrual Disorders in Post-menarcheal Girls. In: Fulghesu, A. (eds) Good Practice in Pediatric and Adolescent Gynecology. Springer, Cham. https://doi.org/10.1007/978-3-319-57162-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-57162-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57161-4
Online ISBN: 978-3-319-57162-1
eBook Packages: MedicineMedicine (R0)