Abstract
The aortic regurgitation (AR) is the backflow of blood, from the aorta to the left ventricle, through the aortic valve during diastole. In elderly patients, the most frequent cause, of AR, is the enlargement of the aortic annulus. This regurgitation can be the result of a primary disease, which causes dilation of the ascending aorta or sinus of Valsalva, or can be caused by aortic dilatation, when it occurs with other diseases that affect the valve ring and the aorta, such as hypertension, resulting in an ascending aneurysm. We report the case of a male patient with dyspnea and angina during moderate efforts, whom was diagnosed with hypertension 10 years ago and was being treated. The cardiovascular physical examination identified a systolic murmur (3+/6+), audible in the aortic focus, preserved B1 and hypophonic B2, and a palpable ictus displaced inferiorly and to the left. Exams were performed, and the final diagnosis was an aortic valve insufficiency associated with an ascending aortic aneurysm, with a transverse diameter of 65 mm, and a left ventricular hypertrophy, with a left ventricular ejection fraction of 50%. In patients that have both diseases, the replacement of aorta and aortic valve, by a valvar tube, performed by the Bentall-De Bono technique, and the reimplantation of the coronary ostiums, is the gold standard, having a very high survival rate of 87%, at 10 years. The surgery, in this case, was successful, reducing the aortic diameter to 30 mm and maintaining normal values for LV function. It is recommended for these patients a biannual follow-up, for the first years, and annual after.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Clinical Presentation
J.C.H. a 53-year-old male patient, caucasian race, retired, married and catholic. The patient reported recent dyspnea during moderate effort and had been diagnosed with hypertension 10 years ago, making use of medication. The cardiovascular physical test identified a regurgitation systolic murmur (3+/6+) audible in the aortic focus, preserved B1 and hypophonic B2, and palpable ictus displaced inferiorly to the left.
Diagnosis, Assessment, and Treatment
Additional tests: The electrocardiogram showed sinus rhythm, heart rate of 70 bpm, and overloading of the left atrium and ventricle. The 2-D echocardiogram showed a severe aortic valve insufficiency, due to non-coaptation of the aortic valve leaflets, with a mild thickening, a LV diastolic dysfunction with an altered relaxation pattern, enlarged left chambers, an ascending aortic aneurysm, with an aortic ascending transverse diameter of 65 mm, in its middle third and 61 mm at the level of the sinus of Valsalva and aortic arch diameter of 31 mm, a left ventricular hypertrophy and an ejection fraction of 50%. Cardiac catheterization found an elevation of Pd2 in the left ventricle. The aortography showed an aneurysm of the ascending aorta (Fig. 1b). The aortography showed an aneurysm in the ascending aorta. The left ventriculography shows dilated left ventricle with hypertrophy and end-systolic volume increase, due to diffuse hypokinesia (2+/4+). CT angiography of the thoracic aorta showed an aneurysm affecting the entire length of the ascending thoracic aorta and dilation of the tubular segment and aortic root, with a transverse diameter of 65 mm in the middle third of the tubular portion and 61 mm in the level of sinus Valsalva (Fig. 1a). The aortic diameter before the emergence of the innominate artery was estimated at 38 mm and the diameter in the middle third of the aortic arch 33 mm. The isthmus and the descending thoracic aorta have diameters within the normal range, estimated at 30 and 29 mm, respectively.
Diagnosis: Aneurysm of the ascending aorta and aortic valve insufficiency.
Treatment: Surgical treatment with Bentall-De Bono technique and aortic valve and ascending aorta replacement with the anastomosis of the coronary buttons. On-pump surgery was performed and the cardioplegic solution used to protect the heart with Custodiol®.
The patient recovered well from the surgery, and after 2 months, the tests showed no abnormalities. The echocardiogram shows the maintenance of normal left ventricular ejection fraction values. The preoperative 58.5 mm’s valve area was replaced by the diameter of St. Jude’s valvar tube No. 29, with 30 mm, as seen in CT (Fig. 2). As a follow-up, it recommends the monitoring of this patient by a cardiologist in the first 6 months and annually thereafter. If asymptomatic we perform echocardiography, CT, and prothrombin time tests.
The use of the Bentall-De Bono technique has had lower mortality rates. Studies show a percentage of 87.17% survival in patients followed up for 10 years [6].
Questions
1. What are the epidemiological and pathophysiological features of aortic aneurysms and aortic insufficiency?
It is understood as pathological, the diameter of the ascending aorta where the aortic dilatation is greater than 50% predicted for age and body surface of the patient. This results from the progressive weakening or defect in the layers of the aortic wall [10]. An aortic regurgitation (AR), in turn, is characterized by the backflow of blood from the aorta to the left ventricle (LV) through the aortic valve during diastole. The presence of a congenital bicuspid aortic valve is considered the more likely cause of this condition; however, in elderly patients, the most frequent cause is calcification of the leaflets and in developing countries, there is a relationship with rheumatic heart disease. In addition, aortic regurgitation results from primary diseases, causing dilation of the ascending aorta or sinus of Valsalva [5].
The regurgitated blood increases the final diastolic volume and wall tension in the left ventricle, causing a progressive compensatory myocardial hypertrophy. The natural evolution of this heart condition is characterized, in most cases, by a lack of symptoms and a normal LV ejection fraction for years. Diseases that affect the valve ring or ascending aorta and cause simultaneous AR, such as Marfan and Ehlers-Danlos syndromes, ankylosing spondylitis, syphilitic aortitis, and hypertension, should be also considered. In these situations, the AR is a consequence of the ostium dilatation, followed by coaptation failure of the leaflets [7].
2. From the symptomatology of the disease, which additional tests should be performed?
Some additional tests should jointly assist in clarifying the diagnostic hypotheses and possibly confirm the diagnosis. Among them, there is a chest X-ray and the echocardiography for AR. The gold standard for the investigation of the ascending aortic aneurysm is computed tomography (CT) with iodinated contrast; it is used for diagnosis, monitoring, and surgical planning. Magnetic resonance imaging (MRI) is a high-accuracy test, whose advantage is the nonuse of contrast and radiation and, however, presents long-time acquisition of images, which impairs their use in emergencies.
When we suspect an aortic aneurysm , the chest X-ray is important at the beginning of the evaluation process, because it can identify abnormalities such as calcification and aortic dilation. To measure the increased diameter, a 2-D echocardiography is used. The transesophageal echocardiogram can be used as a supplement due to the high-accuracy rate for the ascending aorta, sometimes confirming the initial diagnostic. The CT is useful to visually reconstruct different dimensional planes, providing a better visualization of an important structure. This exam also complements the understanding of anatomical structures with a sensitivity of 98–100%. MRI is highly sensitive as CT and allows to assess the left ventricular function and the involvement of aortic branches. The aortic angiography, in turn, fell into disuse because it is more invasive and less specific than CT, but it is important for the evaluation of the coronary arteries.
The AR investigation by chest radiograph shows anatomical changes such as calcification and cardiomegaly with left ventricular dilatation expense. Echocardiography can give information about morphological features of the valve, assessing the number of brochures, the fusion of the cusps, the calcification, and the failure of coaptation. It is considered especially useful in measuring the diastolic and systolic diameter, ejection fraction, and degree of reflux in conjunction with aortography and MR [4].
3. What is the classification of the patient as the aortic regurgitation?
Aortic regurgitation can be divided into four categories measured by anatomy and valve hemodynamics and the presence of symptoms. Internships vary among patients at risk for aortic insufficiency (stage A), with progressive AR medium-moderate asymptomatic (stage B), with asymptomatic severe AR (stage C), and with symptomatic AR (D stage) [4, 5].
The patient showed a small calcification in a leaflet of the aortic valve, ascending aortic aneurysm, left ventricular ejection fraction of 50% with the presence of dilation, and symptoms of dyspnea and angina. So, the patient was classified as severe AR, stage D.
4. About the therapeutic approach, what types of treatments this patient could be submitted?
The recommended treatment of AR is the surgery, for valve replacement in cases of symptomatic patients at rest or after exercise testing. The valve replacement is also indicated for patients with lower left ventricular ejection fraction or equal to 50%, although asymptomatic, or those who have other comorbidities that require surgical intervention.Until surgery can be performed, the use of vasodilators is recommended, such as nifedipine, captopril, and enalapril, which act reducing the diastolic blood pressure and, consequently, the aortic regurgitation. The patient had an aortic aneurysm, with a diameter greater than 60 mm, which implies a surgical recommendation grade A, mainly due to the risk of rupture and/or dissection that increases 2–30% in similar cases [4, 5].
Clinical treatment is controversial, mainly by the use of beta-blockers, even reducing the impact of blood on the walls of the aneurysmal aorta; it can cause side effects by decreasing the elasticity of the same. Thus, the clinical management involves the regulation of risk factors for rupture of an aneurysm, such as hypertension, dyslipidemia, and evaluation of correlated genetic diseases [10].
5. How is the surgical procedure done?
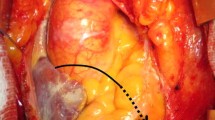
The Bentall-De Bono technique - a median transsternal thoracotomy is performed, followed by the opening of the pericardial sac; the ascending aortic aneurysm and dilated left ventricle, was viewed (Fig. 3a). The patient is placed in extracorporeal circulation and hypothermia at 32 °C is achieved. The aorta is clamped, opened and its structures visualized; the aneurysmal portion of the ascending aorta and the aortic cusps are excised, after separating the coronary ostiums, and replaced by a valvar tube with a mechanical valve; both coronary buttons are anastomosed to the tube(Fig. 3b).
All anastomosis are performed by means of continuous suture; hemostatic sealant is used. Deairing manouvers are performed, during reheating and the aortic clamp is opened, and gradually the patirent is withdraw from the extracorporeal circulation. A drain is placed in the anterior mediastinum as weel as of two temporary epicardial pacemaker wires, in the right ventricle and right atrial appendage. A complete review of hemostasis is performed. Partial closure of the pericardial sac, sternum and underlying plans, complets the surgery [1].
6. How is the coronary arteries’ anastomosis technique done? What is its relevance?
With the patient in cardiopulmonary bypass and hypothermia, and after removing the calcified aortic valve, the coronary arteries are removed while maintaining a generous portion of the aortic wall, called button. This structure is useful for the coronary implant since it avoids bending and unwanted tissue tension. After the valved tube placement, the coronary’s ostium is implanted directly on the prosthesis. When the diameter of an aneurysm is very significant, laterally away from the ostium, it is advisable to use the second graft between this and the prosthesis. Such behavior prevents tension on the anastomosis, which is usually the most frequent point of bleeding after repair. After that, holes are made in the graft on the opposite sides of the arteries’ ostium, and an adequate amount of the graft must be left out between the prosthesis’ suture ring and the new way to the coronary artery. After that, the anastomosis of the left coronary artery is initially made, all the suture loops around the bottom edge of the coronary ostium are placed before pulling, so the area comes close to graft accurately. Cartwheel stitches are made around the coronary artery ostium. The right coronary anastomosis is made after complete anastomosis of the left coronary artery [2, 3].
The procedures that reconstruct the coronary ostium using homologous or autologous tissues are susceptible to intimal hyperplasia, thrombosis, or calcification. Furthermore, the new ostium location after implantation allows easy catheterization in future interventional procedures [2, 3].
7. What are the most common types of complication of the Bentall-De Bono procedure?
In the short term, the most common complications of the Bentall-De Bono technique include bleeding due to the positioning and fixation of the prosthesis or valved tube, which can result in cardiac tamponade. In addition, a complete heart block and arrhythmias may occur by an injury to the cardiac conduction system. The thrombus formation can also happen with the withdrawal of the stenotic valve structures, mainly causing neurological injuries by embolism and stroke by occlusion.
The prolonged use of cardiopulmonary bypass should be considered as a factor that increases the incidence of inflammatory reactions and is directly related to the prevalence of these [3, 8].
8. Did we find any abnormalities in the ascending aortic wall?
During surgery, there was a tear in the tunica intima, of the ascending aortic wall , with approximately 4 cm in length and 10 cm distally from the aortic valve. The lesion showed signs of fibrosis, which probably prevented the development of a dissection (Fig. 4). It is understood that it began as a spontaneous aortic dissection due to the rupture of the tunica intima and the commitment of the tunica media, without tearing it. There is almost no connection between patients with aortic regurgitation, ascending aortic aneurysm, and a loss of elastic fibers. Generally, in cases of Marfan syndrome, it is expected that a preexisting injury is responsible for starting the laceration and degeneration of the tunica media. However, this patient did not have specific comorbidities that could cause histological alterations. Although the patient was hypertensive, the presence of hypertension is not a confirmed cause for the appearance of spontaneous dissection, but it may promote the progression of the medial hematoma. Thus, antihypertensive medications can effectively limit a developing dissection, justifying its use for tissue healing [6, 7].
Review About the Addressed Disease or Treatment
Suffering from valvular and vascular comorbidities, the treatment recommended by the AHA/ACC’s guidelines is the surgery for valve replacement and aortic tube placement. These guidelines indicate the surgical option to correct the aortic insufficiency due to the symptoms of the patient during the moderate effort, ejection fraction lower or equal to 50%, and vascular comorbidities. Having an aortic diameter greater than 60 mm, the same procedure is indicated to treat an aneurysm. Thus, the option of performing the Bentall-De Bono surgery and valve tube implantation was in consonance with international recommendations.
The high survival rate of the technique, approximately 87% in 10 years, and the long life span of the mechanical aortic prosthesis were convincing factors to the patient.
The surgery was successful, reducing the aortic diameter to 30 mm and obtaining normal values for left ventricular ejection fraction. During surgery, a laceration was found in the tunica intima of the ascending aorta with approximately 4 cm in length and with signs of fibrosis, indicating a possible early dissection.
It is recommended for this patient initially semiannual follow-up and annual after the symptoms cease.
Bibliography
Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax. 1968;23:338–9.
Bongiovani HL, Haddad JL. Re-implant of the right coronary artery: a surgical technique for treatment of ostial lesion. Rev Bras Cir Cardiovasc. 2002;17(4):352–4.
Kouchoukos NT, et al. (2013). Cardiac surgery. vol. 1(4). Philadelphia: Elsevier Saunders.
Liguori GR, et al. Manual acadêmico de cirurgia cardiovascular SBCCV/DBLACCV. São Paulo: Atheneu; 2014.
Nishimura RA, et al. Guideline for the management of patients with valvular heart disease: executive summary. J Am Coll Cardiol. 2014;63(22):2438–88.
Roberts, W. C. et al. (2010). Comparison of the structure of the aortic valve and ascending aorta in adults having aortic valve replacement for aortic stenosis versus for pure aortic regurgitation and resection of the ascending aorta for aneurysm. Circulation [online]. 123(8). Available at: http://circ.ahajournals.org/content/123/8/896 [Accessed 12 Aug 2016].
Paola AAV, et al. Cardiologia: Livro Texto da Sociedade Brasileira de Cardiologia. Manole: Burueri; 2012.
Paparellaa D, Yaua TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. Eur J Cardiothorac Surg. 2002;21:232–44.
Silva VF, et al. Bentall and Bono surgery for correction of valve ascending aortic disease: long-term results. Rev Bras Cir Cardiovasc. 2008;23:256–61.
Townswend CM. Tratado de Cirurgia: as bases biológicas da prática cirúrgica moderna. 6th ed. Rio de Janeiro: Guanabara Kooogan; 2003.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Almeida, F.T.K.S., Vieira, L.L., Silva, A.F., Flores, E.L.Q., Almeida, R.M.S. (2019). Bentall-De Bono Technique in the Ascending Aorta Aneurysm, Aortic Regurgitation, and Coronary Reimplantation. In: Almeida, R., Jatene, F. (eds) Cardiovascular Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-57084-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-57084-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57083-9
Online ISBN: 978-3-319-57084-6
eBook Packages: MedicineMedicine (R0)