Abstract
Because of their modulatory role, cell-type-specific expression in the CNS, and anti-inflammatory properties, the metabotropic glutamate receptors (mGluRs) have generated significant interest as potential therapeutic targets for various brain disorders. In addition, preclinical studies in animal models of Parkinson’s disease have revealed that specific mGluR subtypes mediate significant neuroprotective effects that reduce midbrain dopaminergic neuronal death. Although the underlying mechanisms of these effects remain to be established, there is evidence that intracellular calcium regulation, anti-inflammatory effects, and glutamatergic network regulation contribute to these properties. These protective effects extend beyond midbrain dopaminergic neurons for some mGluRs. In this review, we discuss recent evidence for mGluR-mediated neuroprotection in PD and highlight the challenges to translate these findings into human trials.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Parkinson’s disease (PD ) is a progressive neurodegenerative disorder clinically characterized by bradykinesia (slowness), rigidity (stiffness), resting tremor, and gait dysfunction with postural instability. The major pathological hallmark of PD is the degeneration of dopaminergic (DA) nigrostriatal neurons in the substantia nigra pars compacta (SNC) and the presence of cytoplasmic α-synuclein-positive inclusions (Lewy bodies) (Fearnley and Lees 1991; Forno 1996; Spillantini et al. 1998; Giasson et al. 2000; Dauer and Przedborski 2003). This progressive degeneration of the nigrostriatal system results in complex pathophysiological changes of neuronal activity and neurochemical imbalance in neurotransmitter release throughout the basal ganglia circuitry and their projection targets (Albin et al. 1989; DeLong 1990; Gerfen et al. 1990; Wichmann and DeLong 2006). The onset of parkinsonian motor symptoms appears only when a critical threshold of 50–60% DA neurons loss in SNC and 70–80% degeneration of striatal DA terminals have been reached (Hornykiewicz 1975, 1998). This lag between the development of motor deficits and the protracted extent of the nigrostriatal degenerative process provides an opportunity for neuroprotective intervention that could slow down the degeneration of the dopaminergic system and delay or prevent the development of parkinsonian motor symptoms .
Significant effort has, indeed, been devoted at identifying disease-modifying therapeutics in different animal models of PD. Unfortunately, despite encouraging preclinical data, none of these compounds were found to be neuroprotective when tested in human trials (Siderowf and Stern 2008; Schapira 2009a, b, c; Smith et al. 2012). Various potential explanations have been raised to explain the failure of these trials, including the lack of PD biomarkers and the late start of the neuroprotective therapy (Mandel et al. 2003, Schapira 2004, Siderowf and Stern 2008, Lang 2009, Olanow 2009, Rascol 2009, Schapira 2009a, c). Thus, the need for effective neuroprotective drugs that could alter the progressive degeneration of the dopaminergic nigrostriatal system and the development of PD biomarkers are key interrelated attributes to the successful development of effective neuroprotective therapy in PD.
Although the mechanisms that underlie nigral DA degeneration in PD remain poorly understood, evidence suggests that glutamate excitotoxicity contributes to the cascade of events that lead to DA cell death in the SNc (Fornai et al. 1997; Sonsalla et al. 1998; Greenamyre et al. 1999; Golembiowska et al. 2002; Blandini et al. 2004; Przedborski 2005). Glutamate elicits its action through the activation of ionotropic (iGlu) and metabotropic glutamate receptors (mGluRs). The use of compounds blocking iGlu, particularly of the N-methyl-D-aspartate (NMDA) subtype, has shown significant neuroprotective effects of midbrain dopaminergic neurons in experimental models of parkinsonism (Turski et al. 1991; Zuddas et al. 1991; Storey et al. 1992; Nash et al. 1999; Konitsiotis et al. 2000). However, the use of these compounds failed human trials because of narrow therapeutic windows and marked CNS side effects (Montastruc et al. 1992; Muir 2006). An alternative approach to the development of glutamate-mediated therapies with a good profile of safety and tolerability for brain disorders is the targeting of glutamate receptors that “modulate” rather than “mediate” fast excitatory synaptic transmission. Because the G-protein-coupled mGluRs meet these criteria, they have generated significant interest as new therapeutic targets for brain diseases, including symptomatic and neuroprotective agents in PD (Lovinger et al. 1993, Conn et al. 2005, Bonsi et al. 2007, Ossowska et al. 2007, Gasparini et al. 2008, Niswender and Conn 2010, Nicoletti et al. 2011, Finlay and Duty 2014, Nickols and Conn 2014, Williams and Dexter 2014, Amalric 2015, Nicoletti et al. 2015).
6.2 MGluR Localization in the Basal Ganglia Circuitry
Eight mGluR subtypes (mGluR1–mGluR8) have been cloned and divided into three groups (groups I–III) on the basis of sequence homologies, signal transduction pathways, and ligand-binding patterns (Pin and Duvoisin 1995; Conn and Pin 1997; Nakanishi et al. 1998; Anwyl 1999; Pin and Acher 2002). Members of these three groups of mGluRs are strongly expressed throughout the basal ganglia circuitry , where they regulate neuronal excitability and synaptic transmission via pre- and postsynaptic mechanisms (Calabresi et al. 1992, Lovinger et al. 1993, Lovinger and McCool 1995, Conn and Pin 1997, Pisani et al. 1997, Rouse et al. 2000, Smith et al. 2000, Pisani et al. 2001, Corti et al. 2002, Marino et al. 2002, 2003a, Gubellini et al. 2004, Conn et al. 2005, Johnson et al. 2009, Lovinger 2010). Furthermore, midbrain dopaminergic neurons in the SNc also express specific mGluRs which represent potential therapeutic targets for neuroprotection in PD (see below). Thus, the foundation of preclinical studies that have been performed so far to assess the potential relevance of mGluR-mediated neuroprotection in PD has been based on the assumption that excitotoxic effects upon midbrain dopaminergic neurons can be mediated either directly through overactivation of calcium-dependent postsynaptic glutamate receptors or via overactivity of glutamatergic inputs onto SNc neurons (Battaglia et al. 2002, 2004; Johnson et al. 2009; Masilamoni et al. 2011). In either case, drugs aimed at postsynaptic (i.e., group I) or presynaptic (i.e., groups II and III) mGluRs may have beneficial effects in reducing toxic insults upon midbrain dopaminergic neurons and possibly other vulnerable populations of neurons in PD (Johnson et al. 2009).
Group I mGluRs (mGluRs 1 and 5) are mainly expressed postsynaptically, couple to Gq proteins, and positively modulate neuronal excitability through activation of phospholipase C leading to an increase in intracellular Ca2+ and protein kinase C (Kim et al. 1994; Pin and Duvoisin 1995; Conn and Pin 1997; Fiorillo and Williams 1998; Nakanishi et al. 1998; Sala et al. 2005; Zhang et al. 2005), while group II (mGluRs 2 and 3) and group III (mGluRs 4, 6, 7, and 8) mGluRs are mainly localized presynaptically, classically couple to Gi/o, and negatively modulate neuronal excitability (Conn and Pin 1997). Group I mGluRs are largely expressed postsynaptically in dendrites and spines, where they enhance excitability through various postsynaptic mechanisms that involve regulation of intracellular calcium, functional interactions with NMDA receptors, and modulation of voltage-gated calcium channels (Conn and Pin 1997; Nakanishi et al. 1998; Alagarsamy et al. 1999; Pisani et al. 2001; Sala et al. 2005; Zhang et al. 2005)
On the other hand, group III mGluRs are predominantly located in the presynaptic active zones of glutamatergic and non-glutamatergic synapses, where they act as autoreceptors or heteroreceptors that reduce neurotransmitter release (Cartmell and Schoepp 2000; Ferraguti and Shigemoto 2006). Although mGluRs 4, 7, and 8 are expressed to a variable degree in different brain regions, mGluR6 is restricted to the retina (Vardi et al. 2000).
The two members of the group II mGluR subtypes , mGluR2 and mGluR3, are differentially expressed in neurons and astrocytes, respectively (Mineff and Valtschanoff 1999; Geurts et al. 2003). At the neuronal level, mGluR2 is located presynaptically in glutamatergic and non-glutamatergic terminals, but its pattern of subcellular expression is different from that of group III mGluRs; instead of being aggregated at the active zones close to the transmitter release sites, as is the case from group III mGluRs, mGluR2 is most commonly expressed away from the synapses, often in preterminal axonal segments, of glutamatergic and GABAergic afferents (Schoepp and Conn 1993; Pin and Duvoisin 1995; Conn and Pin 1997; Cartmell and Schoepp 2000; De Blasi et al. 2001; Galvan et al. 2006; Ferraguti et al. 2008; Niswender and Conn 2010). Thus, upon activation, groups II and III mGluRs can reduce glutamatergic signaling and dampen neuronal excitability giving them potential neuroprotective properties (Nicoletti et al. 1996). On the other hand, group I mGluR antagonists display neuroprotective properties, likely through reduced calcium release from intracellular stores and decreased neuroinflammation (Kim et al. 1994; Pin and Duvoisin 1995; Conn and Pin 1997; Nakanishi et al. 1998; Sala et al. 2005; Zhang et al. 2005).
In this review, we will discuss evidence for neuroprotective properties of different subtypes of mGluRs in PD. We will also summarize findings of mGluR-mediated neuroprotection in other brain diseases (Table 6.1). These will be followed by a brief discussion of the subtype-selective mGlu receptor ligands that still hold the promise to become disease-modifying drugs in neurodegenerative disorders.
6.3 Neuroprotective Effects of Group I mGluRs
There is evidence that mice treated with the mGluR5 antagonist 2-methyl-6-(phenylethynyl) pyridine (MPEP) or mice that lack mGluR5 receptors show increased survival of nigrostriatal DA neurons after administration of the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Battaglia et al. 2004; Aguirre et al. 2005; Armentero et al. 2006; Vernon et al. 2007). mGluR5 receptor antagonists are also protective against nigrostriatal dopamine denervation in the methamphetamine model of parkinsonism (Battaglia et al. 2002). The mGluR5 antagonist, MTEP, was also found to be neuroprotective against the loss of midbrain DA neurons and norepinephrine neurons in the locus coeruleus (LC) in a chronic MPTP -treated monkey model of PD (Masilamoni et al. 2011) (Fig. 6.1). In this study, we showed that neurons in the SNc and LC of monkeys chronically treated with low doses of MPTP over a period of over 20 weeks were significantly spared in animals that received daily administration of MTEP (Masilamoni et al. 2011). Altogether, these rodent and monkey data suggest that the use of mGluR5 receptor antagonists may be a useful strategy to reduce degeneration of catecholaminergic neurons in PD. Taking into consideration evidence that mGluR5 antagonists also have significant anti-dyskinetic effects in rodent and nonhuman primate models of PD (Dekundy et al. 2006; Mela et al. 2007; Gravius et al. 2008; Levandis et al. 2008; Rylander et al. 2009; Johnston et al. 2010; Morin et al. 2010; Rylander et al. 2010; Gregoire et al. 2011) and some antiparkinsonian effects in 6-OHDA-treated rats (Breysse et al. 2002), these findings provide additional support for the potential use of mGluR5 antagonist as PD-relevant therapeutic.
Neuroprotective effects of MTEP towards MPTP-induced neurotoxicity of the midbrain dopaminergic nigrostriatal system in rhesus monkeys. (a–c) Coronal sections at the level of the pre-commissural striatum showing binding of the 18F-FECNT dopamine transporter ligand at baseline (a) and after chronic treatment with MPTP/vehicle (b) or MPTP/MTEP (C). (d–f) show corresponding levels of the striatum in the same groups of animals immunostained with a DAT antibody. (g–l) Coronal sections showing 18F-FECNT binding (g–i) or TH immunostaining (j–l) at the level of the Fig 6.1 (continued) ventral midbrain of the three animal groups used in this study. Abbreviations: PA associative putamen, CA associative caudate nucleus, AL limbic nucleus accumbens, SNC substantia nigra, PM motor putamen, CA associative caudate nucleus. Scale bar: 10 mm (applies to a–c and g–i) and 5 mm (applies to d–f and j–l). M: Densitometry analysis N. Stereological estimate of the total number of TH-positive neurons (means ± SD) in SNC-v, SNC-d and VTA regions of control, MPTP/vehicle and MPTP/MTEP treated monkeys. **p < 0.001 and *p< 0.05 for differences from control and MPTP/vehicle. #, p < 0.05 for differences between the vehicle and MTEP-treated animals. There were no significant difference found in the SNCd and VTA between control and MPTP/MTEP treated monkeys (see Masilamoni et al. 2011 for details)
The evidence that various mGluR5 antagonists (AFQ056-mavoglurant; ADX-48621-dipraglurant) currently being tested in human trials as anti-dyskinetic drugs are well tolerated and do not worsen PD motor symptoms (Berg et al. 2011; Stocchi et al. 2013) is encouraging and provides further supports toward the chronic use of mGluR5-related compounds as potential neuroprotective drug in PD.
Although the exact mechanisms by which mGluR5 antagonists mediate their neuroprotective effects upon catecholaminergic neurons in toxin-based models of PD remain to be established, some interesting possibilities have been raised. In light of evidence that calcium dysregulation, mitochondrial respiration impairment, and excitotoxic insults contribute to the degeneration of nigral dopaminergic neurons in PD (Sherer et al. 2002; Maesawa et al. 2004; Wallace et al. 2007; Caudle and Zhang 2009; Chan et al. 2009; Cannon and Greenamyre 2010; Winklhofer and Haass 2010; Van Laar et al. 2011), the blockade of mGluR5 may provide its protective effects through reduction of intracellular calcium levels in dopaminergic neurons via decreased mGluR5-mediated activation of intracellular IP3 receptors (Kim et al. 1994; Pin and Duvoisin 1995; Conn and Pin 1997; Nakanishi et al. 1998; Sala et al. 2005; Zhang et al. 2005) and/or reduction of the potentiating effects of mGluR5 upon NMDA receptor function (Calabresi et al. 1992; Alagarsamy et al. 1999; Tu et al. 1999; Awad et al. 2000). An alternative mechanism could involve the reduction of overactive glutamatergic inputs from the subthalamic nucleus (STN) to SNC neurons in the PD state (Bezard et al. 1998; Rodriguez et al. 1998; Awad et al. 2000; Breysse et al. 2003; Fazal et al. 2003; Shimo and Wichmann 2009; Piallat et al. 2011). Finally, because glial mGluR5 expression is upregulated in some inflammatory processes (Byrnes et al. 2009a; Loane et al. 2009, 2012; Drouin-Ouellet et al. 2011), the blockade of these receptors may reduce these harmful effects and attenuate MPTP-induced toxicity toward midbrain dopaminergic neurons (see further discussion below).
6.4 Neuroprotective Effects of Group II mGluRs in PD
Results from several groups reveal a moderate neuroprotective effect of Group II mGluR agonists in rodent models of PD, the magnitude of which was dependent on lesion severity (Battaglia et al. 2002, 2003, Murray et al. 2002; Vernon et al. 2005; Battaglia et al. 2009). Both mGluR2 and mGluR3 are expressed in the BG circuitry including in STN neurons (Testa et al. 1994). Activation of presynaptic group II mGluRs at the STN-SNc synapse reduces excitatory postsynaptic current amplitudes in rat SNc neurons (Bradley et al. 2000; Wang et al. 2005). In line with these observations, systemic treatment or intranigral administration of the group II mGluR agonists LY379268 and (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (2R,4R-APDC) reduces the extent of 6-OHDA-induced toxicity of dopaminergic neurons in the rat SNc (Murray et al. 2002; Vernon et al. 2005; Chan et al. 2010). In addition, activation of group II mGluRs by dual mGlu2/3 receptor agonist LY379268 or DCG-IV reduces SNc degeneration in mice after intrastriatal MPP+ or systemic MPTP administration, further supporting a role for group II mGluRs as disease-modifying agents in PD (Battaglia et al. 2003). Activation of mGluR2/3 could also contribute to neurorestoration via the production and release of neurotrophic factors from glial cells in vitro (Bruno et al. 1998a; D’Onofrio et al. 2001) and in vivo (Matarredona et al. 2001; Corti et al. 2007; Battaglia et al. 2009). Through the use of the mGluR2/3 receptor agonist LY379268 in mGluR2 and mGluR3 receptor knockout mice, Corti et al. (2007) suggested that the neuroprotective properties of the mGluR2/3 receptor agonist LY379268 in MPTP-treated mice were entirely mediated by activation of the astrocytic mGluR3 receptor and that these effects were amplified in the absence of neuronal mGluR2 receptors, suggesting that mGluR2 activation might be harmful to toxin exposure (Corti et al. 2007). The studies of the neuroprotective properties of the selective mGluR3 receptor agonist, N-acetylaspartylglutamate (NAAG) (Orlando et al. 1997; Wroblewska et al. 1997; Bruno et al. 1998a; Bergeron et al. 2005), have been limited by the poor blood-brain barrier permeability for this compound (Westbrook et al. 1986; Sekiguchi et al. 1992).
6.5 Neuroprotective Effects of Group III mGluRs in PD
Several lines of evidence based on cellular and physiological data predict that activation of group III mGluRs in the BG could have neuroprotective effects in PD (Wigmore and Lacey 1998; Valenti et al. 2002, 2003, 2005). These Gi/Go-coupled auto- or heteroreceptors regulate GABAergic and glutamatergic synaptic transmission, most likely through inhibition of voltage-gated calcium entry required for triggering transmitter release (Trombley and Westbrook 1992; Conn and Pin 1997). In regard to neuroprotection in PD, activation of mGluR4 at the striatopallidal synapse reduces the activity of the indirect pathway, which attenuates hyperactivity of the STN, and thereby reduces its excitotoxic effects toward SNc dopaminergic neurons (Valenti et al. 2005). Group III mGluRs activation protects against NMDA-induced toxicity in cultured neurons and in vivo, neuroprotection against excitotoxic insult (Gasparini et al. 1999; Bruno et al. 2000; Flor et al. 2002).
Consistent with the prediction that group III mGluRs activation might have neuroprotective effects in PD, both acute and subchronic intranigral infusion of the group III mGluRs agonist L-AP4 reduce the extent of 6-OHDA toxicity in the rat SNc (Vernon et al. 2005, 2007, 2008; Jiang et al. 2006; Betts et al. 2012). Furthermore, systemic or intrapallidal administration of the mGluR4 allosteric potentiator, PHCCC (Phenyl-7-(hydroxyimino) cyclopropa [b] chromen-1a-carboxamide), reduces the extent of nigrostriatal MPTP toxicity in wild-type mice, but not in mice lacking mGluR4, further supporting the selective activation of mGluR4 as a neuroprotective approach in PD (Battaglia et al. 2006). The study of mGluR7 and mGluR8 receptor subtypes in neuroprotection awaits the development of specific compounds that selectively regulate these receptors.
6.6 Neuroprotective Effects of mGluR-Related Drugs in Other Brain Diseases
Chronic treatment with the prototypic mGluR5 receptor antagonist, MPEP, attenuates cell death, delays the onset of motor symptoms, and prolongs survival in mutant mice carrying a mutation of SOD1 associated with familiar ALS (Rossi et al. 2008). There is evidence that modulation of mGluR1 and mGluR5 on oligodendrocytes is protective in a rodent model of periventricular leukomalacia during the developmental peak of vulnerability to hypoxia/ischemia, and modulation of these receptors is protective in a rodent model of periventricular leukomalacia (Jantzie et al. 2010). Recent evidence from Huntington’s disease (HD) mouse model indicates that the mGluR5 allosteric potentiator, CDPPB, protects striatal neurons against excitotoxic neuronal death, maybe through activation of Akt pathway, without triggering increased intracellular Ca2+ concentration (Ribeiro et al. 2010; Doria et al. 2013, 2015). There is also evidence that the mGluR5 antagonist MPEP might reduce disease progression in HD mouse models (Schiefer et al. 2004; Ribeiro et al. 2011).
6.7 Anti-inflammatory Properties of mGluRs: Implications for Neuroprotection
In the normal brain, microglia , the resident CNS macrophages, are found in a resting state with ramified morphology, but when chronically activated in response to insults, they display an amoeboid morphology (Kreutzberg 1996; Nimmerjahn et al. 2005) and exacerbate neurodegeneration by releasing glutamate and neurotoxic pro-inflammatory cytokines and cytotoxic factors (Barger and Basile 2001; Parker et al. 2002; Cunningham et al. 2005; Barger et al. 2007; McCoy and Tansey 2008; Lee et al. 2009; Tansey and Goldberg 2010). In addition, prolonged activation of microglia prevents them from carrying out their neuro-supportive functions such as the release of key growth factors (Benoit et al. 2008). Microglial toxicity toward midbrain dopaminergic neurons is well documented in a number of cell culture studies and animal models of PD (Gao et al. 2002a, b; Liu and Hong 2003; McCoy and Tansey 2008; Lee et al. 2009; Tansey and Goldberg 2010; Barnum et al. 2014). It has been postulated that both the initiation and progression of PD are triggered by neuroinflammation (Qin et al. 2007a, b; McCoy and Tansey 2008; Lee et al. 2009; Tansey and Goldberg 2010) and there is a growing body of evidence that in some cases, PD is linked to head trauma, viruses, and infections which subsequently trigger microglial activation (Liu and Hong 2003; Herrera et al. 2005). Inflammation is also heavily implicated in the pathogenesis of other neurodegenerative diseases including AD, ALS, MS, and HD (McGeer and McGeer 2002; Kutzelnigg et al. 2005; Solomon et al. 2006; Gao and Hong 2008; Hickman et al. 2008; Jimenez et al. 2008; Moller 2010).
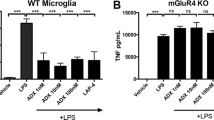
As discussed above, glia (astrocytes, microglia, and oligodendrocytes) express mGluRs to variable degrees. Although mGluR3 is the most abundant subtype of glial mGluRs, there is evidence that groups I and III mGluRs also display light glial expression. These glial receptors are activated under normal and pathophysiological conditions (Loane et al. 2012). The selective mGluR5 orthosteric agonist, (RS)-2-chloro-5-hydroxyphenylglycine (CHPG), reduces microglial activation and the associated release of pro-inflammatory mediators following stimulation with either lipopolysaccharide (LPS) or interferon-γ (IFNγ) (Byrnes et al. 2009a, b; Loane et al. 2009). CHPG treatment also attenuates NADPH oxidase (NOX2) activity levels and abolishes the neurotoxic potential of activated microglia in microglia/neuron co-culture models. The anti-inflammatory effects of CHPG are abolished in mGluR5 knockout mice or by addition of the mGluR5 antagonist, MTEP, demonstrating selective mGluR5-mediated neuroprotective effects through microglia (Byrnes et al. 2009a; Loane et al. 2009). Recently, mGluR5 positive allosteric modulators were found to significantly attenuate both LPS- and IFNγ-induced nitric oxide (NO) and tumor necrosis factor-α (TNFα) release in cultured microglia with higher potency compared to the orthosteric agonist, CHPG (Xue et al. 2014). These data support the use of mGluR5 PAMs as anti-inflammatory neuroprotective agents. MGluR5 PAMs are currently being investigated as potential treatment for various CNS conditions ranging from schizophrenia and anxiety disorders (Kinney et al. 2003, 2005; Homayoun et al. 2004; Lecourtier et al. 2007; Gravius et al. 2008) to learning and cognition-related problems (Gravius et al. 2008; Ayala et al. 2009; Gass and Olive 2009; Cleva et al. 2010; Reichel et al. 2011). Whether their neuroprotective/anti-inflammatory properties contribute to the potential benefit of these compounds in these disorders remain to be established.
Activation of group III mGluRs also mediates anti-inflammatory effects. For example, LPS-induced microglial activation in vitro is attenuated by the group III mGluR agonists L-AP4 and RS-PPG (Taylor et al. 2003). Activation of glial mGluR4 reduces the production of RANTES, a chemokine involved in neuroinflammation that circulates at higher levels in humans with PD compared with age- and sex-matched controls (Besong et al. 2002; Rentzos et al. 2007). L-AP4 is similarly protective against myelin-induced microglial neurotoxicity in cell culture, a finding attributable to the mGluR-mediated inhibition of soluble toxin production by activated microglia (Pinteaux-Jones et al. 2008). Similarly, oligodendrocyte precursor cells found in lesion sites are particularly vulnerable to cytotoxic and pro-inflammatory factors released by activated microglia, and stimulation of group III mGluRs by L-AP4 prevents microglial-induced inhibition of oligodendrocyte precursor cell proliferation (Taylor et al. 2010). These studies suggest that targeting microglia by group III mGlu receptors has the potential to encourage neuroprotection and regeneration of lost myelin in MS.
Activation of mGluR2/3 stimulates the production and release of neurotrophic factors from glial cells in vitro (Bruno et al. 1998b; D’Onofrio et al. 2001) and in vivo (Matarredona et al. 2001; Corti et al. 2007; Battaglia et al. 2009). Results from several groups reveal a moderate neuroprotective effect of group II mGluR agonists in rodent models of PD, the magnitude of which was dependent on lesion severity (Battaglia et al. 2002, 2003, 2009, Murray et al. 2002; Vernon et al. 2005; ).
6.8 mGluR-Mediated Neuroprotection vs Disease Biomarkers
The mGluRs are therapeutic targets of great interest for various brain diseases (Conn et al. 2005; Marino and Conn 2006; Johnson et al. 2009; Niswender and Conn 2010). Their beneficial symptomatic effects are further amplified by their potential neuroprotective properties in PD and other brain diseases . However, caution must be exercised in translating the promising preclinical neuroprotective data discussed in this review to human trials (Olanow 2009; Smith et al. 2012; Schapira et al. 2014). The constant failure of previous neuroprotective human trials in PD patients (Olanow 2009) indicates that the effective assessment of mGluR neuroprotective properties in PD must await the development of biomarkers that will allow to identify at-risk candidates for the disease prior to the appearance of motor symptoms. We hope that the current effort of the Parkinson Progression Marker Initiative (PPMI) multicenter study (Parkinson Progression Marker 2011) will help identify reliable progression biomarkers that could be used toward the future assessment of the disease-modifying properties of mGluRs in PD.
References
Aguirre JA, Kehr J, Yoshitake T, Liu FL, Rivera A, Fernandez-Espinola S, Andbjer B, Leo G, Medhurst AD, Agnati LF, Fuxe K (2005) Protection but maintained dysfunction of nigral dopaminergic nerve cell bodies and striatal dopaminergic terminals in MPTP-lesioned mice after acute treatment with the mGluR5 antagonist MPEP. Brain Res 1033:216–220
Alagarsamy S, Marino MJ, Rouse ST, Gereau RW, Heinemann SF, Conn PJ (1999) Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat Neurosci 2:234–240
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375
Allen JW, Ivanova SA, Fan L, Espey MG, Basile AS, Faden AI (1999) Group II metabotropic glutamate receptor activation attenuates traumatic neuronal injury and improves neurological recovery after traumatic brain injury. J Pharmacol Exp Ther 290:112–120
Amalric M (2015) Targeting metabotropic glutamate receptors (mGluRs) in Parkinson’s disease. Curr Opin Pharmacol 20:29–34
Anwyl R (1999) Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev 29:83–120
Armentero MT, Fancellu R, Nappi G, Bramanti P, Blandini F (2006) Prolonged blockade of NMDA or mGluR5 glutamate receptors reduces nigrostriatal degeneration while inducing selective metabolic changes in the basal ganglia circuitry in a rodent model of Parkinson’s disease. Neurobiol Dis 22:1–9
Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ (2000) Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci 20:7871–7879
Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ (2009) mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology 34:2057–2071
Banasik T, Jasinski A, Pilc A, Majcher K, Brzegowy P (2005) Application of magnetic resonance diffusion anisotropy imaging for the assessment neuroprotecting effects of MPEP, a selective mGluR5 antagonist, on the rat spinal cord injury in vivo. Pharmacol Rep 57:861–866
Bao WL, Williams AJ, Faden AI, Tortella FC (2001) Selective mGluR5 receptor antagonist or agonist provides neuroprotection in a rat model of focal cerebral ischemia. Brain Res 922:173–179
Barger SW, Basile AS (2001) Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem 76:846–854
Barger SW, Goodwin ME, Porter MM, Beggs ML (2007) Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem 101:1205–1213
Barnum CJ, Chen X, Chung J, Chang J, Williams M, Grigoryan N, Tesi RJ, Tansey MG (2014) Peripheral administration of the selective inhibitor of soluble tumor necrosis factor (TNF) XPro(R)1595 attenuates nigral cell loss and glial activation in 6-OHDA hemiparkinsonian rats. J Parkinsons Dis 4:349–360
Battaglia G, Fornai F, Busceti CL, Aloisi G, Cerrito F, De Blasi A, Melchiorri D, Nicoletti F (2002) Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity. J Neurosci 22:2135–2141
Battaglia G, Busceti CL, Pontarelli F, Biagioni F, Fornai F, Paparelli A, Bruno V, Ruggieri S, Nicoletti F (2003) Protective role of group-II metabotropic glutamate receptors against nigro-striatal degeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Neuropharmacology 45:155–166
Battaglia G, Busceti CL, Molinaro G, Biagioni F, Storto M, Fornai F, Nicoletti F, Bruno V (2004) Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigro-striatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J Neurosci 24:828–835
Battaglia G, Busceti CL, Molinaro G, Biagioni F, Traficante A, Nicoletti F, Bruno V (2006) Pharmacological activation of mGlu4 metabotropic glutamate receptors reduces nigrostriatal degeneration in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurosci 26:7222–7229
Battaglia G, Molinaro G, Riozzi B, Storto M, Busceti CL, Spinsanti P, Bucci D, Di Liberto V, Mudo G, Corti C, Corsi M, Nicoletti F, Belluardo N, Bruno V (2009) Activation of mGlu3 receptors stimulates the production of GDNF in striatal neurons. PLoS One 4:e6591
Battaglia G, Riozzi B, Bucci D, Di Menna L, Molinaro G, Pallottino S, Nicoletti F, Bruno V (2015) Activation of mGlu3 metabotropic glutamate receptors enhances GDNF and GLT-1 formation in the spinal cord and rescues motor neurons in the SOD-1 mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 74:126–136
Benoit M, Desnues B, Mege JL (2008) Macrophage polarization in bacterial infections. J Immunol 181:3733–3739
Berg D, Godau J, Trenkwalder C, Eggert K, Csoti I, Storch A, Huber H, Morelli-Canelo M, Stamelou M, Ries V, Wolz M, Schneider C, Di Paolo T, Gasparini F, Hariry S, Vandemeulebroecke M, Abi-Saab W, Cooke K, Johns D, Gomez-Mancilla B (2011) AFQ056 treatment of levodopa-induced dyskinesias: results of 2 randomized controlled trials. Mov Disord 26:1243–1250
Bergeron R, Coyle JT, Tsai G, Greene RW (2005) NAAG reduces NMDA receptor current in CA1 hippocampal pyramidal neurons of acute slices and dissociated neurons. Neuropsychopharmacology 30:7–16
Besong G, Battaglia G, D’Onofrio M, Di Marco R, Ngomba RT, Storto M, Castiglione M, Mangano K, Busceti CL, Nicoletti FR, Bacon K, Tusche M, Valenti O, Conn PJ, Bruno V, Nicoletti F (2002) Activation of group III metabotropic glutamate receptors inhibits the production of RANTES in glial cell cultures. J Neurosci 22:5403–5411
Betts MJ, O’Neill MJ, Duty S (2012) Allosteric modulation of the group III mGlu4 receptor provides functional neuroprotection in the 6-hydroxydopamine rat model of Parkinson’s disease. Br J Pharmacol 166:2317–2330
Bezard E, Bioulac B, Gross CE (1998) Glutamatergic compensatory mechanisms in experimental parkinsonism. Prog Neuro-Psychopharmacol Biol Psychiatry 22:609–623
Biber K, Laurie DJ, Berthele A, Sommer B, Tolle TR, Gebicke-Harter PJ, van Calker D, Boddeke HW (1999) Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J Neurochem 72:1671–1680
Blandini F, Armentero MT, Fancellu R, Blaugrund E, Nappi G (2004) Neuroprotective effect of rasagiline in a rodent model of Parkinson’s disease. Exp Neurol 187:455–459
Bond A, O’Neill MJ, Hicks CA, Monn JA, Lodge D (1998) Neuroprotective effects of a systemically active group II metabotropic glutamate receptor agonist LY354740 in a gerbil model of global ischaemia. Neuroreport 9:1191–1193
Bond A, Ragumoorthy N, Monn JA, Hicks CA, Ward MA, Lodge D, O’Neill MJ (1999) LY379268, a potent and selective group II metabotropic glutamate receptor agonist, is neuroprotective in gerbil global, but not focal, cerebral ischaemia. Neurosci Lett 273:191–194
Bonsi P, Cuomo D, Picconi B, Sciamanna G, Tscherter A, Tolu M, Bernardi G, Calabresi P, Pisani A (2007) Striatal metabotropic glutamate receptors as a target for pharmacotherapy in Parkinson’s disease. Amino Acids 32:189–195
Bradley SR, Marino MJ, Wittmann M, Rouse ST, Awad H, Levey AI, Conn PJ (2000) Activation of group II metabotropic glutamate receptors inhibits synaptic excitation of the substantia Nigra pars reticulata. J Neurosci 20:3085–3094
Breysse N, Baunez C, Spooren W, Gasparini F, Amalric M (2002) Chronic but not acute treatment with a metabotropic glutamate 5 receptor antagonist reverses the akinetic deficits in a rat model of parkinsonism. J Neurosci 22:5669–5678
Breysse N, Amalric M, Salin P (2003) Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basal-ganglia structures in parkinsonian rats. J Neurosci 23:8302–8309
Bruno V, Sureda FX, Storto M, Casabona G, Caruso A, Knopfel T, Kuhn R, Nicoletti F (1997) The neuroprotective activity of group-II metabotropic glutamate receptors requires new protein synthesis and involves a glial-neuronal signaling. J Neurosci 17:1891–1897
Bruno V, Battaglia G, Casabona G, Copani A, Caciagli F, Nicoletti F (1998a) Neuroprotection by glial metabotropic glutamate receptors is mediated by transforming growth factor-beta. J Neurosci 18:9594–9600
Bruno V, Wroblewska B, Wroblewski JT, Fiore L, Nicoletti F (1998b) Neuroprotective activity of N-acetylaspartylglutamate in cultured cortical cells. Neuroscience 85:751–757
Bruno V, Battaglia G, Ksiazek I, van der Putten H, Catania MV, Giuffrida R, Lukic S, Leonhardt T, Inderbitzin W, Gasparini F, Kuhn R, Hampson DR, Nicoletti F, Flor PJ (2000) Selective activation of mGlu4 metabotropic glutamate receptors is protective against excitotoxic neuronal death. J Neurosci 20:6413–6420
Byrnes KR, Stoica B, Loane DJ, Riccio A, Davis MI, Faden AI (2009a) Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia 57:550–560
Byrnes KR, Stoica B, Riccio A, Pajoohesh-Ganji A, Loane DJ, Faden AI (2009b) Activation of metabotropic glutamate receptor 5 improves recovery after spinal cord injury in rodents. Ann Neurol 66:63–74
Cai Z, Lin S, Rhodes PG (2002) Neuroprotective effects of N-acetylaspartylglutamate in a neonatal rat model of hypoxia-ischemia. Eur J Pharmacol 437:139–145
Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G (1992) Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci 12:4224–4233
Cannon JR, Greenamyre JT (2010) Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog Brain Res 184:17–33
Caraci F, Molinaro G, Battaglia G, Giuffrida ML, Riozzi B, Traficante A, Bruno V, Cannella M, Merlo S, Wang X, Heinz BA, Nisenbaum ES, Britton TC, Drago F, Sortino MA, Copani A, Nicoletti F (2011) Targeting group II metabotropic glutamate (mGlu) receptors for the treatment of psychosis associated with Alzheimer’s disease: selective activation of mGlu2 receptors amplifies beta-amyloid toxicity in cultured neurons, whereas dual activation of mGlu2 and mGlu3 receptors is neuroprotective. Mol Pharmacol 79:618-626.
Cartmell J, Schoepp DD (2000) Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75:889–907
Caudle WM, Zhang J (2009) Glutamate, excitotoxicity, and programmed cell death in Parkinson disease. Exp Neurol 220:230–233
Chan CS, Gertler TS, Surmeier DJ (2009) Calcium homeostasis, selective vulnerability and Parkinson’s disease. Trends Neurosci 32:249–256
Chan CS, Gertler TS, Surmeier DJ (2010) A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson’s disease. Mov Disord 25(Suppl 1):S63–S70
Chen L, Liu J, Ali U, Gui ZH, Hou C, Fan LL, Wang Y, Wang T (2011) Chronic, systemic treatment with a metabotropic glutamate receptor 5 antagonist produces anxiolytic-like effects and reverses abnormal firing activity of projection neurons in the basolateral nucleus of the amygdala in rats with bilateral 6-OHDA lesions. Brain Res Bull 84:215–223
Chen T, Cao L, Dong W, Luo P, Liu W, Qu Y, Fei Z (2012a) Protective effects of mGluR5 positive modulators against traumatic neuronal injury through PKC-dependent activation of MEK/ERK pathway. Neurochem Res 37:983–990
Chen T, Zhang L, Qu Y, Huo K, Jiang X, Fei Z (2012b) The selective mGluR5 agonist CHPG protects against traumatic brain injury in vitro and in vivo via ERK and Akt pathway. Int J Mol Med 29:630–636
Chiamulera C, Albertini P, Valerio E, Reggiani A (1992) Activation of metabotropic receptors has a neuroprotective effect in a rodent model of focal ischaemia. Eur J Pharmacol 216:335–336
Cleva RM, Gass JT, Widholm JJ, Olive MF (2010) Glutamatergic targets for enhancing extinction learning in drug addiction. Curr Neuropharmacol 8:394–408
Colwell CS, Levine MS (1999) Metabotropic glutamate receptor modulation of excitotoxicity in the neostriatum: role of calcium channels. Brain Res 833:234–241
Conn PJ, Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237
Conn PJ, Battaglia G, Marino MJ, Nicoletti F (2005) Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci 6:787–798
Corti C, Aldegheri L, Somogyi P, Ferraguti F (2002) Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience 110:403–420
Corti C, Battaglia G, Molinaro G, Riozzi B, Pittaluga A, Corsi M, Mugnaini M, Nicoletti F, Bruno V (2007) The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J Neurosci 27:8297–8308
Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH (2005) Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci 25:9275–9284
D’Antoni S, Berretta A, Seminara G, Longone P, Giuffrida-Stella AM, Battaglia G, Sortino MA, Nicoletti F, Catania MV (2011) A prolonged pharmacological blockade of type-5 metabotropic glutamate receptors protects cultured spinal cord motor neurons against excitotoxic death. Neurobiol Dis 42:252–264
D’Onofrio M, Cuomo L, Battaglia G, Ngomba RT, Storto M, Kingston AE, Orzi F, De Blasi A, Di Iorio P, Nicoletti F, Bruno V (2001) Neuroprotection mediated by glial group-II metabotropic glutamate receptors requires the activation of the MAP kinase and the phosphatidylinositol-3-kinase pathways. J Neurochem 78:435–445
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
De Blasi A, Conn PJ, Pin J, Nicoletti F (2001) Molecular determinants of metabotropic glutamate receptor signaling. Trends Pharmacol Sci 22:114–120
Degos V, Peineau S, Nijboer C, Kaindl AM, Sigaut S, Favrais G, Plaisant F, Teissier N, Gouadon E, Lombet A, Saliba E, Collingridge GL, Maze M, Nicoletti F, Heijnen C, Mantz J, Kavelaars A, Gressens P (2013) G protein-coupled receptor kinase 2 and group I metabotropic glutamate receptors mediate inflammation-induced sensitization to excitotoxic neurodegeneration. Ann Neurol 73:667–678
Dekundy A, Pietraszek M, Schaefer D, Cenci MA, Danysz W (2006) Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res Bull 69:318–326
DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13:281–285
Di Liberto V, Bonomo A, Frinchi M, Belluardo N, Mudo G (2010) Group II metabotropic glutamate receptor activation by agonist LY379268 treatment increases the expression of brain derived neurotrophic factor in the mouse brain. Neuroscience 165:863–873
Domenici MR, Potenza RL, Martire A, Coccurello R, Pezzola A, Reggio R, Tebano MT, Popoli P (2005) Chronic treatment with the mGlu5R antagonist MPEP reduces the functional effects of the mGlu5R agonist CHPG in the striatum of 6-hydroxydopamine-lesioned rats: possible relevance to the effects of mGlu5R blockade in Parkinson’s disease. J Neurosci Res 80:646–654
Domin H, Jantas D, Smialowska M (2015) Neuroprotective effects of the allosteric agonist of metabotropic glutamate receptor 7 AMN082 on oxygen-glucose deprivation- and kainate-induced neuronal cell death. Neurochem Int.
Doria JG, Silva FR, de Souza JM, Vieira LB, Carvalho TG, Reis HJ, Pereira GS, Dobransky T, Ribeiro FM (2013) Metabotropic glutamate receptor 5 positive allosteric modulators are neuroprotective in a mouse model of Huntington’s disease. Br J Pharmacol 169:909–921
Doria JG, de Souza JM, Andrade JN, Rodrigues HA, Guimaraes IM, Carvalho TG, Guatimosim C, Dobransky T, Ribeiro FM (2015) The mGluR5 positive allosteric modulator, CDPPB, ameliorates pathology and phenotypic signs of a mouse model of Huntington’s disease. Neurobiol Dis 73:163–173
Drouin-Ouellet J, Brownell AL, Saint-Pierre M, Fasano C, Emond V, Trudeau LE, Levesque D, Cicchetti F (2011) Neuroinflammation is associated with changes in glial mGluR5 expression and the development of neonatal excitotoxic lesions. Glia 59:188–199
Durand D, Carniglia L, Caruso C, Lasaga M (2013) mGlu3 receptor and astrocytes: partners in neuroprotection. Neuropharmacology 66:1–11
Faden AI, Ivanova SA, Yakovlev AG, Mukhin AG (1997) Neuroprotective effects of group III mGluR in traumatic neuronal injury. J Neurotrauma 14:885–895
Faden AI, O’Leary DM, Fan L, Bao W, Mullins PG, Movsesyan VA (2001) Selective blockade of the mGluR1 receptor reduces traumatic neuronal injury in vitro and improvesoOutcome after brain trauma. Exp Neurol 167:435–444
Fazal A, Parker F, Palmer AM, Croucher MJ (2003) Characterisation of the actions of group I metabotropic glutamate receptor subtype selective ligands on excitatory amino acid release and sodium-dependent re-uptake in rat cerebrocortical minislices. J Neurochem 86:1346–1358
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt 5):2283–2301
Fei Z, Zhang X, Bai HM, Jiang XF, Wang XL (2006) Metabotropic glutamate receptor antagonists and agonists: potential neuroprotectors in diffuse brain injury. J Clin Neurosci 13:1023–1027
Feng JF, Zhao X, Gurkoff GG, Van KC, Shahlaie K, Lyeth BG (2012) Post-traumatic hypoxia exacerbates neuronal cell death in the hippocampus. J Neurotrauma 29:1167–1179
Ferraguti F, Shigemoto R (2006) Metabotropic glutamate receptors. Cell Tissue Res 326:483–504
Ferraguti F, Crepaldi L, Nicoletti F (2008) Metabotropic glutamate 1 receptor: current concepts and perspectives. Pharmacol Rev 60:536–581
Ferrigno A, Vairetti M, Ambrosi G, Rizzo V, Richelmi P, Blandini F, Fuzzati-Armentero MT (2015) Selective blockade of mGlu5 metabotropic glutamate receptors is protective against hepatic mitochondrial dysfunction in 6-OHDA lesioned Parkinsonian rats. Clin Exp Pharmacol Physiol 42:695–703
Finlay C, Duty S (2014) Therapeutic potential of targeting glutamate receptors in Parkinson’s disease. J Neural Transm 121:861–880
Fiorillo CD, Williams JT (1998) Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature 394:78–82
Flor PJ, Battaglia G, Nicoletti F, Gasparini F, Bruno V (2002) Neuroprotective activity of metabotropic glutamate receptor ligands. Adv Exp Med Biol 513:197–223
Fornai F, Vaglini F, Maggio R, Bonuccelli U, Corsini GU (1997) Species differences in the role of excitatory amino acids in experimental parkinsonism. Neurosci Biobehav Rev 21:401–415
Forno LS (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55:259–272
Fuzzati-Armentero MT, Cerri S, Levandis G, Ambrosi G, Montepeloso E, Antoninetti G, Blandini F, Baqi Y, Muller CE, Volpini R, Costa G, Simola N, Pinna A (2015) Dual target strategy: combining distinct non-dopaminergic treatments reduces neuronal cell loss and synergistically modulates l-DOPA-induced rotational behavior in a rodent model of Parkinson’s disease. J Neurochem 134:740–747
Galvan A, Kuwajima M, Smith Y (2006) Glutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function? Neuroscience 143:351–375
Gao HM, Hong JS (2008) Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol 29:357–365
Gao HM, Hong JS, Zhang W, Liu B (2002a) Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22:782–790
Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B (2002b) Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem 81:1285–1297
Gasparini F, Bruno V, Battaglia G, Lukic S, Leonhardt T, Inderbitzin W, Laurie D, Sommer B, Varney MA, Hess SD, Johnson EC, Kuhn R, Urwyler S, Sauer D, Portet C, Schmutz M, Nicoletti F, Flor PJ (1999) (R,S)-4-phosphonophenylglycine, a potent and selective group III metabotropic glutamate receptor agonist, is anticonvulsive and neuroprotective in vivo. J Pharmacol Exp Ther 289:1678–1687
Gasparini F, Bilbe G, Gomez-Mancilla B, Spooren W (2008) mGluR5 antagonists: discovery, characterization and drug development. Curr Opin Drug Discov Devel 11:655–665
Gass JT, Olive MF (2009) Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry 65:717–720
Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, Sibley DR (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432
Geurts JJ, Wolswijk G, Bo L, van der Valk P, Polman CH, Troost D, Aronica E (2003) Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain 126:1755–1766
Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290:985–989
Golembiowska K, Konieczny J, Ossowska K, Wolfarth S (2002) The role of striatal metabotropic glutamate receptors in degeneration of dopamine neurons: review article. Amino Acids 23:199–205
Golembiowska K, Konieczny J, Wolfarth S, Ossowska K (2003) Neuroprotective action of MPEP, a selective mGluR5 antagonist, in methamphetamine-induced dopaminergic neurotoxicity is associated with a decrease in dopamine outflow and inhibition of hyperthermia in rats. Neuropharmacology 45:484–492
Gravius A, Dekundy A, Nagel J, More L, Pietraszek M, Danysz W (2008) Investigation on tolerance development to subchronic blockade of mGluR5 in models of learning, anxiety, and levodopa-induced dyskinesia in rats. J Neural Transm 115:1609–1619
Greenamyre JT, MacKenzie G, Peng TI, Stephans SE (1999) Mitochondrial dysfunction in Parkinson’s disease. Biochem Soc Symp 66:85–97
Gregoire L, Morin N, Ouattara B, Gasparini F, Bilbe G, Johns D, Vranesic I, Sahasranaman S, Gomez-Mancilla B, Di Paolo T (2011) The acute antiparkinsonian and antidyskinetic effect of AFQ056, a novel metabotropic glutamate receptor type 5 antagonist, in L-Dopa-treated parkinsonian monkeys. Parkinsonism Relat Disord 17:270–276
Gubellini P, Pisani A, Centonze D, Bernardi G, Calabresi P (2004) Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Prog Neurobiol 74:271–300
Hamilton A, Esseltine JL, DeVries RA, Cregan SP, Ferguson SS (2014) Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer’s disease. Mol Brain 7:40
He X, Lakkaraju SK, Hanscom M, Zhao Z, Wu J, Stoica B, MacKerell AD Jr, Faden AI, Xue F (2015) Acyl-2-aminobenzimidazoles: a novel class of neuroprotective agents targeting mGluR5. Bioorg Med Chem 23:2211–2220
Henrich-Noack P, Hatton CD, Reymann KG (1998) The mGlu receptor ligand (S)-4C3HPG protects neurons after global ischaemia in gerbils. Neuroreport 9:985–988
Henrich-Noack P, Flor PJ, Sabelhaus CF, Prass K, Dirnagl U, Gasparini F, Sauter A, Rudin M, Reymann KG (2000) Distinct influence of the group III metabotropic glutamate receptor agonist (R,S)-4-phosphonophenylglycine [(R,S)-PPG] on different forms of neuronal damage. Neuropharmacol 39:911–917
Herrera AJ, Tomas-Camardiel M, Venero JL, Cano J, Machado A (2005) Inflammatory process as a determinant factor for the degeneration of substantia nigra dopaminergic neurons. J Neural Transm 112:111–119
Hickman SE, Allison EK, El Khoury J (2008) Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci 28:8354–8360
Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B (2004) Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology 29:1259–1269
Hornykiewicz O (1975) Brain monoamines and Parkinsonism. Psychopharmacol Bull 11:34–35
Hornykiewicz O (1998) Biochemical aspects of Parkinson’s disease. Neurology 51:S2–S9
Jantas D, Greda A, Golda S, Korostynski M, Grygier B, Roman A, Pilc A, Lason W (2014) Neuroprotective effects of metabotropic glutamate receptor group II and III activators against MPP(+)-induced cell death in human neuroblastoma SH-SY5Y cells: the impact of cell differentiation state. Neuropharmacology 83:36–53
Jantzie LL, Talos DM, Selip DB, An L, Jackson MC, Folkerth RD, Deng W, Jensen FE (2010) Developmental regulation of group I metabotropic glutamate receptors in the premature brain and their protective role in a rodent model of periventricular leukomalacia. Neuron Glia Biol 6:277–288
Jiang Q, Yan Z, Feng J (2006) Activation of group III metabotropic glutamate receptors attenuates rotenone toxicity on dopaminergic neurons through a microtubule-dependent mechanism. J Neurosci 26:4318–4328
Jimenez S, Baglietto-Vargas D, Caballero C, Moreno-Gonzalez I, Torres M, Sanchez-Varo R, Ruano D, Vizuete M, Gutierrez A, Vitorica J (2008) Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer’s disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci 28:11650–11661
Johnson KA, Conn PJ, Niswender CM (2009) Glutamate receptors as therapeutic targets for Parkinson’s disease. CNS Neurol Disord Drug Targets 8:475–491
Johnston TH, Fox SH, McIldowie MJ, Piggott MJ, Brotchie JM (2010) Reduction of L-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor 5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Pharmacol Exp Ther 333:865–873
Kim WT, Rioult MG, Cornell-Bell AH (1994) Glutamate-induced calcium signaling in astrocytes. Glia 11:173–184
Kingston AE, O’Neill MJ, Bond A, Bruno V, Battaglia G, Nicoletti F, Harris JR, Clark BP, Monn JA, Lodge D, Schoepp DD (1999) Neuroprotective actions of novel and potent ligands of group I and group II metabotropic glutamate receptors. Ann N Y Acad Sci 890:438–449
Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ, Conn PJ (2003) Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther 306:116–123
Kinney GG, O’Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL Jr (2005) A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther 313:199–206
Kohara A, Takahashi M, Yatsugi S, Tamura S, Shitaka Y, Hayashibe S, Kawabata S, Okada M (2008) Neuroprotective effects of the selective type 1 metabotropic glutamate receptor antagonist YM-202074 in rat stroke models. Brain Res 1191:168–179
Konitsiotis S, Blanchet PJ, Verhagen L, Lamers E, Chase TN (2000) AMPA receptor blockade improves levodopa-induced dyskinesia in MPTP monkeys. Neurology 54:1589–1595
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318
Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712
Lafon-Cazal M, Fagni L, Guiraud MJ, Mary S, Lerner-Natoli M, Pin JP, Shigemoto R, Bockaert J (1999) mGluR7-like metabotropic glutamate receptors inhibit NMDA-mediated excitotoxicity in cultured mouse cerebellar granule neurons. Eur J Neurosci 11:663–672
Lam AG, Soriano MA, Monn JA, Schoepp DD, Lodge D, McCulloch J (1998) Effects of the selective metabotropic glutamate agonist LY354740 in a rat model of permanent ischaemia. Neurosci Lett 254:121–123
Landucci E, Boscia F, Gerace E, Scartabelli T, Cozzi A, Moroni F, Mannaioni G, Pellegrini-Giampietro DE (2009) Involvement of endocannabinoid signaling in the neuroprotective effects of subtype 1 metabotropic glutamate receptor antagonists in models of cerebral ischemia. Int Rev Neurobiol 85:337–350
Lang AE (2009) When and how should treatment be started in Parkinson disease? Neurology 72:S39–S43
Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B (2007) Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-Methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biol Psychiatry 62:739–746
Lee JK, Tran T, Tansey MG (2009) Neuroinflammation in Parkinson’s disease. J NeuroImmune Pharmacol 4:419–429
Levandis G, Bazzini E, Armentero MT, Nappi G, Blandini F (2008) Systemic administration of an mGluR5 antagonist, but not unilateral subthalamic lesion, counteracts l-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neurobiol Dis 29:161–168
Liu B, Hong JS (2003) Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther 304:1–7
Loane DJ, Stoica BA, Pajoohesh-Ganji A, Byrnes KR, Faden AI (2009) Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J Biol Chem 284:15629–15639
Loane DJ, Stoica BA, Faden AI (2012) Metabotropic glutamate receptor-mediated signaling in neuroglia. Wiley Interdiscip Rev Membr Transp Signal 1:136–150
Loane DJ, Stoica BA, Byrnes KR, Jeong W, Faden AI (2013) Activation of mGluR5 and inhibition of NADPH oxidase improves functional recovery after traumatic brain injury. J Neurotrauma 30:403–412
Loane DJ, Stoica BA, Tchantchou F, Kumar A, Barrett JP, Akintola T, Xue F, Conn PJ, Faden AI (2014) Novel mGluR5 positive allosteric modulator improves functional recovery, attenuates neurodegeneration, and alters microglial polarization after experimental traumatic brain injury. Neurotherapeutics 11:857–869
Lovinger DM (2010) Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacol 58:951–961
Lovinger DM, McCool BA (1995) Metabotropic glutamate receptor-mediated presynaptic depression at corticostriatal synapses involves mGLuR2 or 3. J Neurophysiol 73:1076–1083
Lovinger DM, Tyler E, Fidler S, Merritt A (1993) Properties of a presynaptic metabotropic glutamate receptor in rat neostriatal slices. J Neurophysiol 69:1236–1244
Luo P, Chen T, Zhao Y, Zhang L, Yang Y, Liu W, Li S, Rao W, Dai S, Yang J, Fei Z (2014) Postsynaptic scaffold protein Homer 1a protects against traumatic brain injury via regulating group I metabotropic glutamate receptors. Cell Death Dis 5:e1174
Maesawa S, Kaneoke Y, Kajita Y, Usui N, Misawa N, Nakayama A, Yoshida J (2004) Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: neuroprotection of dopaminergic neurons. J Neurosurg 100:679–687
Maj M, Bruno V, Dragic Z, Yamamoto R, Battaglia G, Inderbitzin W, Stoehr N, Stein T, Gasparini F, Vranesic I, Kuhn R, Nicoletti F, Flor PJ (2003) (-)-PHCCC, a positive allosteric modulator of mGluR4: characterization, mechanism of action, and neuroprotection. Neuropharmacology 45:895–906
Makarewicz D, Duszczyk M, Gadamski R, Danysz W, Lazarewicz JW (2006) Neuroprotective potential of group I metabotropic glutamate receptor antagonists in two ischemic models. Neurochem Int 48:485–490
Mandel S, Weinreb O, Youdim MB (2003) Using cDNA microarray to assess Parkinson’s disease models and the effects of neuroprotective drugs. Trends Pharmacol Sci 24:184–191
Marino MJ, Conn PJ (2006) Glutamate-based therapeutic approaches: allosteric modulators of metabotropic glutamate receptors. Curr Opin Pharmacol 6:98–102
Marino MJ, Awad H, Poisik O, Wittmann M, Conn PJ (2002) Localization and physiological roles of metabotropic glutamate receptors in the direct and indirect pathways of the basal ganglia. Amino Acids 23:185–191
Marino MJ, Valenti O, Conn PJ (2003a) Glutamate receptors and Parkinson’s disease: opportunities for intervention. Drugs Aging 20:377–397
Marino MJ, Williams DL Jr, O’Brien JA, Valenti O, McDonald TP, Clements MK, Wang R, DiLella AG, Hess JF, Kinney GG, Conn PJ (2003b) Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson’s disease treatment. Proc Natl Acad Sci U S A 100:13668–13673
Masilamoni GJ, Bogenpohl JW, Alagille D, Delevich K, Tamagnan G, Votaw JR, Wichmann T, Smith Y (2011) Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys. Brain 134:2057–2073
Matarredona ER, Santiago M, Venero JL, Cano J, Machado A (2001) Group II metabotropic glutamate receptor activation protects striatal dopaminergic nerve terminals against MPP+-induced neurotoxicity along with brain-derived neurotrophic factor induction. J Neurochem 76:351–360
McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation 5:45
McGeer PL, McGeer EG (2002) Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve 26:459–470
Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA (2007) Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J Neurochem 101:483–497
Meltzer LT, Serpa KA, Christoffersen CL (1997) Metabotropic glutamate receptor-mediated inhibition and excitation of substantia nigra dopamine neurons. Synapse 26:184–193
Milanese M, Giribaldi F, Melone M, Bonifacino T, Musante I, Carminati E, Rossi PI, Vergani L, Voci A, Conti F, Puliti A, Bonanno G (2014) Knocking down metabotropic glutamate receptor 1 improves survival and disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 64:48–59
Mineff E, Valtschanoff J (1999) Metabotropic glutamate receptors 2 and 3 expressed by astrocytes in rat ventrobasal thalamus. Neurosci Lett 270:95–98
Moller T (2010) Neuroinflammation in Huntington’s disease. J Neural Transm 117:1001–1008
Montastruc JL, Rascol O, Senard JM, Rascol A (1992) A pilot study of N-methyl-D-aspartate (NMDA) antagonist in Parkinson’s disease. J Neurol Neurosurg Psychiatry 55:630–631
Morin N, Gregoire L, Gomez-Mancilla B, Gasparini F, Di Paolo T (2010) Effect of the metabotropic glutamate receptor type 5 antagonists MPEP and MTEP in parkinsonian monkeys. Neuropharmacol 58:981–986
Movsesyan VA, O’Leary DM, Fan L, Bao W, Mullins PG, Knoblach SM, Faden AI (2001) mGluR5 antagonists 2-methyl-6-(phenylethynyl)-pyridine and (E)-2-methyl-6-(2-phenylethenyl)-pyridine reduce traumatic neuronal injury in vitro and in vivo by antagonizing N-methyl-D-aspartate receptors. J Pharmacol Exp Ther 296:41–47
Muir KW (2006) Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol 6:53–60
Murray TK, Messenger MJ, Ward MA, Woodhouse S, Osborne DJ, Duty S, O’Neill MJ (2002) Evaluation of the mGluR2/3 agonist LY379268 in rodent models of Parkinson’s disease. Pharmacol Biochem Behav 73:455–466
Nakanishi S, Nakajima Y, Masu M, Ueda Y, Nakahara K, Watanabe D, Yamaguchi S, Kawabata S, Okada M (1998) Glutamate receptors: brain function and signal transduction. Brain Res Brain Res Rev 26:230–235
Nash JE, Hill MP, Brotchie JM (1999) Antiparkinsonian actions of blockade of NR2B-containing NMDA receptors in the reserpine-treated rat. Exp Neurol 155:42–48
Nickols HH, Conn PJ (2014) Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol Dis 61:55–71
Nicoletti F, Bruno V, Copani A, Casabona G, Knopfel T (1996) Metabotropic glutamate receptors: a new target for the therapy of neurodegenerative disorders? Trends Neurosci 19:267–271
Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP (2011) Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacol 60:1017–1041
Nicoletti F, Bruno V, Ngomba RT, Gradini R, Battaglia G (2015) Metabotropic glutamate receptors as drug targets: what’s new? Curr Opin Pharmacol 20:89–94
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318
Niswender CM, Conn PJ (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50:295–322
Olanow CW (2009) Can we achieve neuroprotection with currently available anti-parkinsonian interventions? Neurology 72:S59–S64
Orlando LR, Luthi-Carter R, Standaert DG, Coyle JT, Penney JB Jr, Young AB (1997) N-acetylaspartylglutamate (NAAG) protects against rat striatal quinolinic acid lesions in vivo. Neurosci Lett 236:91–94
Ossowska K, Konieczny J, Wardas J, Pietraszek M, Kuter K, Wolfarth S, Pilc A (2007) An influence of ligands of metabotropic glutamate receptor subtypes on parkinsonian-like symptoms and the striatopallidal pathway in rats. Amino Acids 32:179–188
Parker MA, Bazan HE, Marcheselli V, Rodriguez de Turco EB, Bazan NG (2002) Platelet-activating factor induces permeability transition and cytochrome c release in isolated brain mitochondria. J Neurosci Res 69:39–50
Parkinson Progression Marker I (2011) The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 95:629–635
Pellegrini-Giampietro DE (2003) The distinct role of mGlu1 receptors in post-ischemic neuronal death. Trends Pharmacol Sci 24:461–470
Pellegrini-Giampietro DE, Peruginelli F, Meli E, Cozzi A, Albani-Torregrossa S, Pellicciari R, Moroni F (1999) Protection with metabotropic glutamate 1 receptor antagonists in models of ischemic neuronal death: time-course and mechanisms. Neuropharmacol 38:1607–1619
Piallat B, Polosan M, Fraix V, Goetz L, David O, Fenoy A, Torres N, Quesada JL, Seigneuret E, Pollak P, Krack P, Bougerol T, Benabid AL, Chabardes S (2011) Subthalamic neuronal firing in obsessive-compulsive disorder and Parkinson disease. Ann Neurol 69:793–802
Piers TM, Heales SJ, Pocock JM (2011) Positive allosteric modulation of metabotropic glutamate receptor 5 down-regulates fibrinogen-activated microglia providing neuronal protection. Neurosci Lett 505:140–145
Pin JP, Acher F (2002) The metabotropic glutamate receptors: structure, activation mechanism and pharmacology. Curr Drug Targets CNS Neurol Disord 1:297–317
Pin JP, Duvoisin R (1995) The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34:1–26
Pinteaux-Jones F, Sevastou IG, Fry VA, Heales S, Baker D, Pocock JM (2008) Myelin-induced microglial neurotoxicity can be controlled by microglial metabotropic glutamate receptors. J Neurochem 106:442–454
Pisani A, Calabresi P, Centonze D, Bernardi G (1997) Enhancement of NMDA responses by group I metabotropic glutamate receptor activation in striatal neurones. Br J Pharmacol 120:1007–1014
Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P (2001) Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience 106:579–587
Pizzi M, Consolandi O, Memo M, Spano PF (1996) Activation of multiple metabotropic glutamate receptor subtypes prevents NMDA-induced excitotoxicity in rat hippocampal slices. Eur J Neurosci 8:1516–1521
Popoli P, Pintor A, Tebano MT, Frank C, Pepponi R, Nazzicone V, Grieco R, Pezzola A, Reggio R, Minghetti L, De Berardinis MA, Martire A, Potenza RL, Domenici MR, Massotti M (2004) Neuroprotective effects of the mGlu5R antagonist MPEP towards quinolinic acid-induced striatal toxicity: involvement of pre- and post-synaptic mechanisms and lack of direct NMDA blocking activity. J Neurochem 89:1479–1489
Przedborski S (2005) Pathogenesis of nigral cell death in Parkinson’s disease. Parkinsonism Relat Disord 11(Suppl 1):S3–S7
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007a) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55:453–462
Qin Z, Hu D, Han S, Reaney SH, Di Monte DA, Fink AL (2007b) Effect of 4-hydroxy-2-nonenal modification on alpha-synuclein aggregation. J Biol Chem 282:5862–5870
Rascol O (2009) “Disease-modification” trials in Parkinson disease: target populations, endpoints and study design. Neurology 72:S51–S58
Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE (2011) Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacol 36:782–792
Reiner A, Wang HB, Del Mar N, Sakata K, Yoo W, Deng YP (2012) BDNF may play a differential role in the protective effect of the mGluR2/3 agonist LY379268 on striatal projection neurons in R6/2 Huntington’s disease mice. Brain Res 1473:161–172
Rentzos M, Nikolaou C, Andreadou E, Paraskevas GP, Rombos A, Zoga M, Tsoutsou A, Boufidou F, Kapaki E, Vassilopoulos D (2007) Circulating interleukin-15 and RANTES chemokine in Parkinson’s disease. Acta Neurol Scand 116:374–379
Ribeiro FM, Paquet M, Ferreira LT, Cregan T, Swan P, Cregan SP, Ferguson SS (2010) Metabotropic glutamate receptor-mediated cell signaling pathways are altered in a mouse model of Huntington’s disease. J Neurosci 30:316–324
Ribeiro FM, Pires RG, Ferguson SS (2011) Huntington’s disease and group I metabotropic glutamate receptors. Mol Neurobiol 43:1–11
Ribeiro FM, Hamilton A, Doria JG, Guimaraes IM, Cregan SP, Ferguson SS (2014) Metabotropic glutamate receptor 5 as a potential therapeutic target in Huntington’s disease. Expert Opin Ther Targets 18:1293–1304
Rodriguez MC, Obeso JA, Olanow CW (1998) Subthalamic nucleus-mediated excitotoxicity in Parkinson’s disease: a target for neuroprotection. Ann Neurol 44:S175–S188
Rossi D, Brambilla L, Valori CF, Roncoroni C, Crugnola A, Yokota T, Bredesen DE, Volterra A (2008) Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ 15:1691–1700
Rouse ST, Marino MJ, Bradley SR, Awad H, Wittmann M, Conn PJ (2000) Distribution and roles of metabotropic glutamate receptors in the basal ganglia motor circuit: implications for treatment of Parkinson’s disease and related disorders. Pharmacol Ther 88:427–435
Rylander D, Recchia A, Mela F, Dekundy A, Danysz W, Cenci MA (2009) Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signaling. J Pharmacol Exp Ther 330:227–235
Rylander D, Iderberg H, Li Q, Dekundy A, Zhang J, Li H, Baishen R, Danysz W, Bezard E, Cenci MA (2010) A mGluR5 antagonist under clinical development improves L-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol Dis 39:352–361
Sabelhaus CF, Schroder UH, Breder J, Henrich-Noack P, Reymann KG (2000) Neuroprotection against hypoxic/hypoglycaemic injury after the insult by the group III metabotropic glutamate receptor agonist (R, S)-4-phosphonophenylglycine. Br J Pharmacol 131:655–658
Sala C, Roussignol G, Meldolesi J, Fagni L (2005) Key role of the postsynaptic density scaffold proteins Shank and Homer in the functional architecture of Ca2+ homeostasis at dendritic spines in hippocampal neurons. J Neurosci 25:4587–4592
Sarnico I, Boroni F, Benarese M, Sigala S, Lanzillotta A, Battistin L, Spano P, Pizzi M (2008) Activation of NF-kappaB p65/c-Rel dimer is associated with neuroprotection elicited by mGlu5 receptor agonists against MPP(+) toxicity in SK-N-SH cells. J Neural Transm 115:669–676
Schapira AH (2004) Disease modification in Parkinson’s disease. Lancet Neurol 3:362–368
Schapira AH (2009a) Molecular and clinical pathways to neuroprotection of dopaminergic drugs in Parkinson disease. Neurology 72:S44–S50
Schapira AH (2009b) Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol Sci 30:41–47
Schapira AH (2009c) Neuroprotection in Parkinson’s disease. Parkinsonism Relat Disord 15(Suppl 4):S41–S43
Schapira AH, Olanow CW, Greenamyre JT, Bezard E (2014) Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: future therapeutic perspectives. Lancet 384:545–555
Schiefer J, Sprunken A, Puls C, Luesse HG, Milkereit A, Milkereit E, Johann V, Kosinski CM (2004) The metabotropic glutamate receptor 5 antagonist MPEP and the mGluR2 agonist LY379268 modify disease progression in a transgenic mouse model of Huntington’s disease. Brain Res 1019:246–254
Schoepp DD, Conn PJ (1993) Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol Sci 14:13–20
Sekiguchi M, Wada K, Wenthold RJ (1992) N-acetylaspartylglutamate acts as an agonist upon homomeric NMDA receptor (NMDAR1) expressed in Xenopus oocytes. FEBS Lett 311:285–289
Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT (2002) An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci 22:7006–7015
Shimo Y, Wichmann T (2009) Neuronal activity in the subthalamic nucleus modulates the release of dopamine in the monkey striatum. Eur J Neurosci 29:104–113
Siderowf A, Stern MB (2008) Premotor Parkinson’s disease: clinical features, detection, and prospects for treatment. Ann Neurol 64(Suppl 2):S139–S147
Smith Y, Charara A, Hanson JE, Paquet M, Levey AI (2000) GABA(B) and group I metabotropic glutamate receptors in the striatopallidal complex in primates. J Anat 196(Pt 4):555–576
Smith Y, Wichmann T, Factor SA, DeLong MR (2012) Parkinson’s disease therapeutics: new developments and challenges since the introduction of levodopa. Neuropsychopharmacol 37:213–246
Solomon JN, Lewis CA, Ajami B, Corbel SY, Rossi FM, Krieger C (2006) Origin and distribution of bone marrow-derived cells in the central nervous system in a mouse model of amyotrophic lateral sclerosis. Glia 53:744–753
Sonsalla PK, Albers DS, Zeevalk GD (1998) Role of glutamate in neurodegeneration of dopamine neurons in several animal models of parkinsonism. Amino Acids 14:69–74
Souza LC, Wilhelm EA, Bortolatto CF, Nogueira CW, Boeira SP, Jesse CR (2014) Involvement of mGlu5 receptor in 3-nitropropionic acid-induced oxidative stress in rat striatum. Neurol Res 36:833–840
Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M (1998) Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett 251:205–208
Stocchi F, Rascol O, Destee A, Hattori N, Hauser RA, Lang AE, Poewe W, Stacy M, Tolosa E, Gao H, Nagel J, Merschhemke M, Graf A, Kenney C, Trenkwalder C (2013) AFQ056 in Parkinson patients with levodopa-induced dyskinesia: 13-week, randomized, dose-finding study. Mov Disord 28:1838–1846
Storey E, Hyman BT, Jenkins B, Brouillet E, Miller JM, Rosen BR, Beal MF (1992) 1-Methyl-4-phenylpyridinium produces excitotoxic lesions in rat striatum as a result of impairment of oxidative metabolism. J Neurochem 58:1975–1978
Stover JF, Sakowitz OW, Beyer TF, Dohse NK, Kroppenstedt SN, Thomale UW, Schaser KD, Unterberg AW (2003) Effects of LY379268, a selective group II metabotropic glutamate receptor agonist on EEG activity, cortical perfusion, tissue damage, and cortical glutamate, glucose, and lactate levels in brain-injured rats. J Neurotrauma 20:315–326
Szydlowska K, Kaminska B, Baude A, Parsons CG, Danysz W (2007) Neuroprotective activity of selective mGlu1 and mGlu5 antagonists in vitro and in vivo. Eur J Pharmacol 554:18–29
Tansey MG, Goldberg MS (2010) Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 37:510–518
Taylor DL, Diemel LT, Cuzner ML, Pocock JM (2002) Activation of group II metabotropic glutamate receptors underlies microglial reactivity and neurotoxicity following stimulation with chromogranin A, a peptide up-regulated in Alzheimer’s disease. J Neurochem 82:1179–1191
Taylor DL, Diemel LT, Pocock JM (2003) Activation of microglial group III metabotropic glutamate receptors protects neurons against microglial neurotoxicity. J Neurosci 23:2150–2160
Taylor DL, Pirianov G, Holland S, McGinnity CJ, Norman AL, Reali C, Diemel LT, Gveric D, Yeung D, Mehmet H (2010) Attenuation of proliferation in oligodendrocyte precursor cells by activated microglia. J Neurosci Res 88:1632–1644
Testa CM, Standaert DG, Young AB, Penney JB Jr (1994) Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci 14:3005–3018
Trombley PQ, Westbrook GL (1992) L-AP4 inhibits calcium currents and synaptic transmission via a G-protein-coupled glutamate receptor. J Neurosci 12:2043–2050
Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF (1999) Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23:583–592
Turski L, Bressler K, Rettig KJ, Loschmann PA, Wachtel H (1991) Protection of substantia nigra from MPP+ neurotoxicity by N-methyl-D-aspartate antagonists. Nature 349:414–418
Valenti O, Conn PJ, Marino MJ (2002) Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors Co-expressed in the same neuronal populations. J Cell Physiol 191:125–137
Valenti O, Marino MJ, Conn PJ (2003) Modulation of excitatory transmission onto midbrain dopaminergic neurons of the rat by activation of group III metabotropic glutamate receptors. Ann N Y Acad Sci 1003:479–480
Valenti O, Mannaioni G, Seabrook GR, Conn PJ, Marino MJ (2005) Group III metabotropic glutamate-receptor-mediated modulation of excitatory transmission in rodent substantia nigra pars compacta dopamine neurons. J Pharmacol Exp Ther 313:1296–1304
Van Laar VS, Arnold B, Cassady SJ, Chu CT, Burton EA, Berman SB (2011) Bioenergetics of neurons inhibit the translocation response of Parkin following rapid mitochondrial depolarization. Hum Mol Genet 20:927–940
Vardi N, Duvoisin R, Wu G, Sterling P (2000) Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol 423:402–412
Venero JL, Santiago M, Tomas-Camardiel M, Matarredona ER, Cano J, Machado A (2002) DCG-IV but not other group-II metabotropic receptor agonists induces microglial BDNF mRNA expression in the rat striatum Correlation with neuronal injury. Neuroscience 113:857–869
Vernon AC, Palmer S, Datla KP, Zbarsky V, Croucher MJ, Dexter DT (2005) Neuroprotective effects of metabotropic glutamate receptor ligands in a 6-hydroxydopamine rodent model of Parkinson’s disease. Eur J Neurosci 22:1799–1806
Vernon AC, Zbarsky V, Datla KP, Croucher MJ, Dexter DT (2007) Subtype selective antagonism of substantia nigra pars compacta group I metabotropic glutamate receptors protects the nigrostriatal system against 6-hydroxydopamine toxicity in vivo. J Neurochem 103:1075–1091
Vernon AC, Croucher MJ, Dexter DT (2008) Additive neuroprotection by metabotropic glutamate receptor subtype-selective ligands in a rat Parkinson’s model. Neuroreport 19:475–478
Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J, Benabid AL (2007) Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain 130:2129–2145
Wang L, Kitai ST, Xiang Z (2005) Modulation of excitatory synaptic transmission by endogenous glutamate acting on presynaptic group II mGluRs in rat substantia nigra compacta. J Neurosci Res 82:778–787
Westbrook GL, Mayer ML, Namboodiri MA, Neale JH (1986) High concentrations of N-acetylaspartylglutamate (NAAG) selectively activate NMDA receptors on mouse spinal cord neurons in cell culture. J Neurosci 6:3385–3392
Wichmann T, DeLong MR (2006) Basal ganglia discharge abnormalities in Parkinson’s disease. J Neural Transm Suppl:21–25
Wigmore MA, Lacey MG (1998) Metabotropic glutamate receptors depress glutamate-mediated synaptic input to rat midbrain dopamine neurones in vitro. Br J Pharmacol 123:667–674
Williams CJ, Dexter DT (2014) Neuroprotective and symptomatic effects of targeting group III mGlu receptors in neurodegenerative disease. J Neurochem 129:4–20
Winklhofer KF, Haass C (2010) Mitochondrial dysfunction in Parkinson’s disease. Biochim Biophys Acta 1802:29–44
Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH (1997) N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem 69:174–181
Xue F, Stoica BA, Hanscom M, Kabadi SV, Faden AI (2014) Positive allosteric modulators (PAMs) of metabotropic glutamate receptor 5 (mGluR5) attenuate microglial activation. CNS Neurol Disord Drug Targets 13:558–566
Zhang Y, Rodriguez AL, Conn PJ (2005) Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther 315:1212–1219
Zhou M, Xu W, Liao G, Bi X, Baudry M (2009) Neuroprotection against neonatal hypoxia/ischemia-induced cerebral cell death by prevention of calpain-mediated mGluR1alpha truncation. Exp Neurol 218:75–82
Zuddas A, Corsini GU, Barker JL, Kopin IJ, Di Porzio U (1991) Specific Reinnervation of Lesioned Mouse Striatum by Grafted Mesencephalic Dopaminergic Neurons. Eur J Neurosci 3:72–85
Zwienenberg M, Gong QZ, Berman RF, Muizelaar JP, Lyeth BG (2001) The effect of groups II and III metabotropic glutamate receptor activation on neuronal injury in a rodent model of traumatic brain injury. Neurosurgery 48:1119–1126. discussion 1126-1117
Acknowledgments
This research was supported by NIH grant P50NS071669 and a grant from the National Parkinson Disease Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Masilamoni, G.J., Smith, Y. (2017). Neuroprotective Properties of Glutamate Metabotropic Glutamate Receptors in Parkinson’s Disease and Other Brain Disorders. In: Ngomba, R., Di Giovanni, G., Battaglia, G., Nicoletti, F. (eds) mGLU Receptors. The Receptors, vol 31. Humana Press, Cham. https://doi.org/10.1007/978-3-319-56170-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-56170-7_6
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-56168-4
Online ISBN: 978-3-319-56170-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)