Abstract
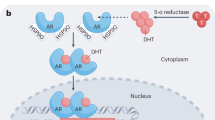
Androgens are endocrine secretions produced mainly by the testes under stimulation of the pituitary gland. They are also synthesized from the adrenal glands in both sexes and from ovaries in females. Luteinizing hormone (LH) produced by the anterior pituitary gland regulates the secretion of androgens from the Leydig cells in the testes. LH secretion is controlled by the hypothalamus via gonadotropin-releasing hormone (GnRH). Androgens play a major role in the development and maintenance of male sex characteristics. The primary and most well-known androgen is testosterone that is rapidly and irreversibly converted to dihydrotestosterone (DHT) in prostate by types 1 and 2 5α-reductase . Androgens stimulate the growth of both normal and cancerous prostate cells by binding to and activating the androgen receptor (AR), a protein that is expressed in prostate cells. Then, AR stimulates the expression of specific genes that cause prostate cells to grow. The role of androgens in prostate cancer was first established in 1941 by Huggins and Hodges. Since then androgen deprivation therapy (ADT) has become the standard of care for patients with advanced prostate cancer. In this chapter ADT and its use in prostate cancer will be discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
20.1 Androgen Deprivation Therapy
ADT aims to reduce the serum testosterone to castrate level . The castrate level was defined as testosterone being less than 50 ng/dL (1.7 nmol/L), many years ago. However contemporary laboratory testing methods showed that the mean value after surgical castration is 15 ng/dL [1]. Thus, recently the level is defined as being less than 20 ng/dL (1 nmol/L). Recent definition is associated with better outcomes compared to the previous one [8,9,4]. However, current guidelines from the National Comprehensive Cancer Network (NCCN) recommend testosterone level of <50 ng/dL (1.7 nmol/L) as the castration level [5].
ADT can be used as adjuvant or neoadjuvant therapy in conjunction with initial treatment of patients with intermediate- or high-risk prostate cancer, patients with rising PSA after curative treatment, or patients with metastatic disease at diagnosis. It can either be used before radiotherapy in patients with large prostate to decrease the tumor volume.
ADT can be accomplished either by surgical or medical orchiectomy. The decision between two treatment options is based upon factors like the preference of the patient, cost, and availability. Combined androgen blockade (CAB) refers to the combination of any ADT with an antiandrogen.
20.2 Surgical Castration
Bilateral orchiectomy is a simple and a cheap procedure which results in rapid decrease in serum testosterone to castration levels and improvement in disease-related symptoms. Although less frequently used in North America and Europe, it is a widely used method in many countries where availability and cost of medical castration are an issue. This type of castration is permanent and irreversible; thus the psychological impact of the treatment should be discussed with the patient. Subcapsular orchiectomy is another method in which the tunica albuginea and epididymis remain intact.
20.3 Medical Castration
There are several methods of medical castration. Measuring serum PSA levels is a way to monitor patient’s response to treatment.
20.3.1 Estrogens
Estrogens inhibit the release of GnRH from the hypothalamus resulting in reduction in testicular production of testosterone via suppressed LHRH secretion from the pituitary. Historically, diethylstilbestrol (DES) was used as an alternative to surgical orchiectomy. However it was shown that DES significantly increased the risk of dying from heart disease and stroke without any survival benefit [6–7]. Due to severe side effects related to DES, estrogens are not considered as a first-line treatment.
20.3.2 Gonadotropin-Releasing Hormone Agonists
Gonadotropin-releasing hormone (GnRH) agonists bind to GnRH receptors on pituitary gland resulting in initial release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). This causes a subsequent increase in testosterone production from testes. However, this is a transient rise that is followed by a downregulation of the GnRH receptors on gonadotropin-producing cells in a week. Decline in serum LH and FSH decreases testosterone levels within 3–4 weeks. Transient rise in LH 2–3 days after first injection leads to a surge in serum testosterone that lasts about a week and results in increase in the tumor growth. This is called “flare-up” phenomenon and is associated with increase in bone pain, acute bladder outlet obstruction, or other disease-related symptoms. Thus, initial treatment with GnRH is contraindicated in patients with severe urinary tract obstruction, painful bone metastases, or spinal cord compression. This can be prevented by antiandrogen treatment at least 1 week before GnRH application.
Approved GnRH analogs are leuprolide , goserelin , triptorelin, and histrelin. GnRH agonists are delivered as depot injections on a one-, two-, three-,or six-monthly periods. A castration level is usually obtained within 2–4 weeks and is reversible upon cessation of GnRH analog [8]. Klotz et al. showed that low nadir serum testosterone (<0.7 mmol/L) within the first year of ADT correlates with improved cause-specific survival (CSS) and duration of response to treatment in men being treated for biochemical failure undergoing continuous ADT [9].
20.3.3 Gonadotropin Hormone-Releasing Hormone Antagonists
GnRH antagonists immediately and reversibly bind to GnRH receptors of the anterior pituitary leading to a rapid decrease in LH, FSH, and testosterone levels without any flare. Currently approved GnRH antagonists are degarelix , abarelix , ganirelix, and cetrorelix . They are administered in parental way. They are used in the treatment where fast control of disease is needed. They do not have a long-acting depot formulation. Early GnRH antagonists leaded to histamine release and resulted in anaphylactic reactions [16,17,12].
Degarelix is the most extensively studied and widely available new GnRH antagonist with a monthly subcutaneous formulation. The standard dosage is 240 mg in the first month, followed by monthly injections of 80 mg. Most patients achieve a castrate level at the third day. An extended follow-up has been published, suggesting a better PFS compared to monthly leuprorelin [13]. Compared with GnRH agonists, degarelix is associated with faster decline in serum testosterone and PSA levels [8]. Its definitive superiority over the LHRH analogues remains to be proven.
20.3.4 Antiandrogens
Antiandrogens (AAs) are oral compounds that competitively inhibit the binding of androgens to the androgen receptor . They can be either steroidal (e.g., cyproterone acetate (CPA), megestrol acetate, and medroxyprogesterone acetate) or nonsteroidal (e.g., bicalutamide, flutamide, and nilutamide). Nonsteroidal AAs competitively inhibit the binding of androgens to the androgen receptor. Thus serum testosterone levels are not suppressed and may even be elevated by nonsteroidal AAs. However steroidal AAs have additional progestational and antigonadotropic properties. Its application via a feedback suppression of pituitary LHRH release thus leads to a reduction of serum testosterone levels.
20.3.4.1 Steroidal AAs
They are synthetic derivatives of hydroxyprogesterone . Their main side effects are suppression of libido and erectile dysfunction. Cardiovascular toxicity and hepatotoxicity may also be seen. Cyproterone acetate (CPA), megestrol acetate, and medroxyprogesterone acetate are examples of steroidal AAs. CPA has no overall survival (OS) advantage compared to LHRH analogues [14]. Another study comparing CPA with flutamide in M1b disease did not show any difference in disease specific- and OS at a median follow-up of 8.6 years [15].
20.3.4.2 Nonsteroidal AAs
Bicalutamide is the most widely used form of nonsteroidal AAs. The licensed dosages are 50 mg or 150 mg. Bicalutamide monotherapy seems to be a tolerable regimen for patients with biochemical failure following 3D-CRT and TAD and may be effective in patients with low PSA levels at biochemical failure [16]. Its main side effects are gynecomastia and breast pain that occur in 70–80% of patients [23,24,19]. However it was shown that bicalutamide monotherapy increases bone mineral density , lessens fat accumulation, and has fewer bothersome side effects than treatment with a gonadotropin-releasing hormone agonist [20].
Nilutamide and flutamide are the other forms of nonsteroidal AAs. All of them have potential liver hepatotoxicity; thus liver enzymes should be regularly monitored during treatment. Nilutamide is not licensed for monotherapy. Its side effects are visual disturbances, alcohol intolerance, nausea, and specifically severe interstitial pneumonitis. Flutamide has been studied as monotherapy. The half-life of the active metabolite of flutamide is 5–6 h; thus it should be used three times daily. Its frequent side effect is diarrhea.
Castration resistance may occur during the course of the disease. Castration-resistant prostate cancer (CRPC) is considered to be mediated through two mechanisms: either androgen-receptor (AR)- dependent or AR-independent ways. Abiraterone acetate is a CYP17 inhibitor . It significantly decreases the intracellular testosterone level by suppressing its synthesis at the adrenal level and inside the tumor cells. It must be used together with prednisone/prednisolone (2 × 5 mg) to prevent drug-induced hyperaldosteronism.
Enzalutamide is another AA with a higher affinity for the androgen receptor . Nonsteroidal AAs allow transfer of ARs to the nucleus, but enzalutamide additionally blocks AR transfer leading to suppression of any possible agonist-like activity.
20.4 Combined Androgen Blockade with Antiandrogens
CAB is defined as the combination of an AA with orchiectomy or medical castration. It blocks the effect of both testicular and adrenal androgens. AAs are not indicated as monotherapy for treatment naïve patients. However it is used in conjunction with medical castration to block the side effects associated with the flare phenomenon at the initiation of ADT. Given 7–10 days before the initiation of GnRH analogue, GnRH receptors are downregulated at the hypophysis. This results in decline of LH and FSH secretion leading to decrease in testosterone to castrate level within 3–4 weeks after the start of treatment.
The decrease in testosterone production is generally reversible. However depending on the duration of ADT and patient-related other factors, it may not return to baseline levels after treatment cessation. Murthy et al. showed that after LHRHa treatment and radiotherapy, the testosterone levels of most men had recovered to normal by 18–24 weeks after the last injection [21]. D’Amico et al. showed that time to testosterone recovery was associated with a lower risk of death in men with no or minimal comorbidity [22].
Studies of short-term and long-term neoadjuvant ADT all have used CAB. To date, there are no trials comparing the use of initial CAB with AA monotherapy in nonmetastatic prostate cancer patients. Safety and Efficacy Study of Enzalutamide Plus Leuprolide in Patients with Nonmetastatic Prostate Cancer (EMBARK) trial is an ongoing trial that randomizes patients to enzalutamide plus leuprolide , enzalutamide monotherapy, or leuprolide monotherapy after radical prostatectomy (RP) or radiotherapy [23].
There are two studies comparing CAB with AA monotherapy in metastatic prostatic cancer. Intergroup trial INT 0036 is a randomized, double-blind trial that compared leuprolide in combination with either placebo or flutamide in 603 patients with disseminated, previously untreated prostate cancer. Patients who received leuprolide and flutamide had a longer progression-free survival (16.5 vs. 13.9 months; p = 0.039) and an increase in the median length of survival (35.6 vs. 28.3 months; p = 0.035) with symptom control [24]. Intergroup trial INT 0105 enrolled 1387 patients with metastatic prostate cancer to orchiectomy and either flutamide or placebo. The addition of flutamide to orchiectomy does not result in a clinically significant improvement in survival. Patients who received flutamide had more toxicity compared to placebo group [25].
20.5 Intermittent Androgen Deprivation
ADT has several side effects including loss of libido, hot flashes , night sweats, psychological stress, osteoporosis, anemia, fatigue, loss of muscle mass, glucose intolerance, and changes in lipid profile. Prolonged ADT may also lead to progression of androgen independence. The aim of intermittent androgen deprivation (IAD) is to minimize the adverse effects of continuous ADT and to delay progression of castration resistance. IAD is delivered for a period of time or until a maximal response is achieved based on PSA levels. Then ADT is withdrawn and patient is followed with PSA. ADT is initiated in case of recurrence or disease progression based on PSA levels.
20.5.1 Metastatic Disease
The intergroup trial INT 0162 was designed to assess whether intermittent therapy was noninferior to continuous therapy with respect to survival in patients with metastatic, hormone-sensitive prostate cancer. Patients with PSA level of ≥5 ng/mL received aN LHRH analogue and an AA agent for 7 months. Patients in whom the PSA level fell to ≤4 ng/mL were randomly assigned to continuous or IAD [26]. ADT was reinitiated in the IAD group when the PSA level rose to 20 ng per milliliter (or returned to baseline in the case of patients who had PSA levels of <20 ng/mL before enrollment). A total of 3040 patients were enrolled, of whom 1535 were included in the analysis. The median follow-up period was 9.8 years. Median survival was 5.8 years in the continuous-therapy group and 5.1 years in the IAD group (hazard ratio for death with intermittent therapy, 1.10; 90% confidence interval, 0.99–1.23). Intermittent therapy was associated with better erectile function and mental health (p < 0.001 and p = 0.003, respectively) at month 3 but not thereafter. There were no significant differences between the groups in the number of treatment-related high-grade adverse events. Based on these results, continuous ADT remains the standard of care in patients with metastatic prostate cancer.
20.5.2 Rising PSA
Crook et al. evaluated the noninferiority of IAD compared to continuous ADT in terms of overall survival. Patients with PSA level greater than 3 ng/mL more than 1 year after primary or salvage radiotherapy for localized prostate cancer were included. All of the 1386 enrolled patients did not have detectable metastases. Intermittent treatment was provided in 8 month cycles, with nontreatment periods determined according to the PSA level. Median follow-up was 6.9 years. Median OS was 8.8 years in the IAD group versus 9.1 years in the continuous-therapy group (hazard ratio for death, 1.02; 95% confidence interval, 0.86–1.21). There were no significant differences in adverse events. IAD provided potential benefits with respect to physical function, fatigue, and hormonal, urinary, and erectile function. This study showed that IAD was noninferior to continuous therapy with respect to OS and some quality-of-life factors improved with intermittent therapy [27].
20.6 Timing of Hormonal Therapy
ADT was shown to improve OS and PFS in patients with locally advanced disease [34,35,30]. Table 20.1 represents the results of ADT combined with RT.
ADT was shown to be the most cost-effective therapy if started at the time that the patient developed symptomatic metastases [35] Thus ADT should be started immediately in case of symptomatic metastases in order to palliate symptoms and prevent complications; however, controversy still exists regarding asymptomatic metastatic patients because of the lack of high-quality studies.
20.7 Hormonal Treatment Combined with Chemotherapy
Three large RCTs compared ADT alone as the standard of care with ADT combined with immediate docetaxel (75 mg/m2 every 3 weeks; within 3 months of ADT initiation) in terms of OS [42,43,38].
In the GETUG-15 trial [36], all patients had newly diagnosed M1 prostate cancer, either primary or after a primary treatment. After a median follow-up of 83.9 months, updated results of GETUG-15 trial were published [37]. Median OS was 62.1 months and 48.6 months for ADT plus docetaxel and ADT arms, respectively (hazard ratio [HR]: 0.88 [95% CI, 0.68–1.14]; p = 0.3). Median OS in ADT plus docetaxel and ADT arms, respectively, was for high-volume disease (HVD) patients 39.8 months versus 35.1 months (HR: 0.78 [95% CI, 0.56–1.09]; p = 0.14) for low-volume disease (LVD) patients; median was not reached and 83.4 months (HR: 1.02 [95% CI, 0.67–1.55]; p = 0.9). For up-front metastatic patients, OS was 52.6 months and 41.5 months, respectively (HR: 0.93 [95% CI, 0.69–1.25]; p = 0.6). The bPFS (HR: 0.73 [95% CI, 0.56–0.94]; p = 0.014) and rPFS (HR: 0.75 [95% CI, 0.58–0.97]; p = 0.030) were significantly longer in the ADT plus D arm. Docetaxel should not be used as part of first-line treatment for patients with non-castrate metastatic prostate cancer.
In the CHAARTED trial [38], the same inclusion criteria applied. High-volume disease was defined as either presence of visceral metastases or four or more bone metastases, with at least one outside the spine and pelvis. After a median follow-up of 28.9 months, the median overall survival was 13.6 months longer with ADT plus docetaxel than with ADT alone (57.6 months vs. 44.0 months; p < 0.001). The median time to progression was 20.2 months in the combination group, as compared with 11.7 months in the ADT-alone group (p < 0.001). The rate of a prostate-specific antigen level of less than 0.2 ng/mL at 12 months was 27.7% in the combination group versus 16.8% in the ADT-alone group (p < 0.001). In the combination group, the rate of grade 3 or 4 febrile neutropenia was 6.2%, the rate of grade 3 or 4 infection with neutropenia was 2.3%, and the rate of grade 3 sensory neuropathy and of grade 3 motor neuropathy was 0.5%. It was concluded that ADT plus docetaxel for metastatic prostate cancer resulted in significantly longer overall survival than that with ADT alone.
STAMPEDE [39] was a multiarm, multistage trial including high-risk, locally advanced, metastatic, or recurrent prostate cancer who is starting first-line long-term hormone therapy. The standard of care (SOC) arm was ADT (n = 1184). The experimental arms were ADT combined with docetaxel (n = 593) and ADT combined with zoledronic acid (n = 593), and another was ADT combined with docetaxel and zoledronic acid (n = 593). Median follow-up was 43 months. Median overall survival was 71 months for SOC only, not reached for SOC + ZA (p = 0.450), 81 months for SOC + docetaxel (p = 0.006), and 76 months for SOC + ZA + docetaxel (p = 0.022). Grade 3–5 adverse events were reported for 399 (32%) patients receiving SOC, 197 (32%) receiving SOC + ZA, 288 (52%) receiving SOC + docetaxel, and 269 (52%) receiving SOC + ZA + docetaxel. Zoledronic acid showed no evidence of survival improvement, but docetaxel chemotherapy, given at the time of long-term hormone therapy initiation, showed evidence of improved survival accompanied by an increase in adverse events. Thus it was concluded that docetaxel treatment should become part of standard of care for adequately fit men commencing long-term hormone therapy. Table 20.2 summarizes the results of those four important trials.
20.8 Side Effects of Hormonal Treatment
ADT has been shown to improve survival when used with radiation for patients with intermediate- and high-risk disease and locally advanced and node-positive disease. However it may cause side effects on bone, metabolic, cardiovascular, sexual, and cognitive health as well as body composition that negatively affect quality of life of patients.
20.8.1 Osteoporosis and Bone Fractures
ADT decreases bone mineral density (BMD). It was shown that ADT significantly increases risk for any clinical fracture, hip fractures, and vertebral fractures in men with prostate cancer, and the duration of treatment affects the onset of complications [40, 41]. Calcium (1000–1200 mg daily from diet and supplements) and vitamin D (800–1000 IU daily) are recommended to reduce the ADT side effects [42]) Osteoclast inhibition with either bisphosphonates or denosumab is recommended for men with bone metastases. Osteoclast inhibition can decrease bone turnover and increase bone mineral density in men receiving ADT [43].
20.8.2 Cardiovascular Events
The first report identifying a possible CV risk with LHRH agonists was by Keating et al., who analyzed the Surveillance, Epidemiology, and End Results (SEER) Medicare data of 73,196 men with locoregional prostate cancer [44]. A significantly increased risk of coronary heart disease, myocardial infarction, and sudden cardiac death was reported for men receiving an LHRH agonist compared with those not undergoing ADT. Prospective clinical trials have demonstrated that ADT may increase cardiovascular disease risk by increasing body weight, reducing insulin sensitivity, and/or resulting in dyslipidemia. In a prospective 12-month study of 40 men with prostate cancer, ADT increased serum total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides by 9%, 7%, 11%, and 27%, respectively [45].
It is advisable that patients in whom ADT is initiated be on periodic follow-up that includes assessment of blood pressure, lipid profile, and glucose level. Some of the effects of ADT occur within the first 3 months of treatment; thus it may be reasonable for an initial follow-up evaluation to occur within 3–6 months after initiation of therapy. There are no data to guide at what intervals periodic further follow-up should occur, and this is left to the discretion of the physician initiating ADT and to the patient’s primary care physician. Prudence and good medical care dictate that patients with cardiac disease receive appropriate secondary preventive measures as recommended by the American Heart Association and other expert organizations, including, when appropriate, lipid-lowering therapy, antihypertensive therapy, glucose-lowering therapy, and antiplatelet therapy [46].
20.8.3 Sexual Dysfunction
Since the incidence of prostate cancer is higher among older patients, at least one-third of men have sexual problems at diagnosis. However, most of the patients receiving continuous ADT who are potent prior to therapy develop sexual dysfunction. Loss of libido in patients receiving LHRH agonists usually develops within the first months followed by erectile dysfunction [47]. Erections do not recover in about one-half of men, even if ADT is discontinued. Although intermittent ADT allows some recovery of sexual function, serum testosterone requires 9–12 months off ADT to recover [48].
20.8.4 Vasomotor Symptoms
The most common symptom associated with ADT is hot flashes . Hot flashes are usually described as an intense sensation of warmth in the face and upper part of the body which seems similar to postmenopausal symptoms in women. The treatment should be decided depending on the degree of symptoms and potential side effects of the treatment. Megestrol and estrogen appear substantially more effective than venlafaxine. However, estrogen is associated with breast symptoms, megestrol is associated with increased appetite and weight, and venlafaxine is associated with dry mouth. It is recommended to start with a selective serotonin reuptake inhibitor and reserve hormonal treatment (estrogen, megestrol) for refractory cases.
20.8.5 Body Composition and Metabolism
A prospective study of 32 men receiving 12 months of GnRH agonists found a 2.4% weight gain, 9.4% increase in body fat percentage, and 2.7% decrease in lean body mass at 12 months [49]. Another prospective study of 25 patients without diabetes found that 12 weeks of combined androgen blockade resulted in a 12.8% decrease in insulin sensitivity and a 25.9% increase in fasting plasma insulin [50]. Basaria et al. conducted a cross-sectional study among 53 men, including 18 men with PCa who received ADT for at least 12 months prior to the onset of the study (the ADT group), 17 age-matched men with nonmetastatic PCa who had undergone prostatectomy and/or received radiotherapy and who were not receiving ADT (the non-ADT group), and 18 age-matched controls (the control group). It was shown that men on long-term ADT had significantly higher fasting glucose (131 vs. 103; p < 0.01) and greater insulin resistance (17 vs. 6; p < 0.01) and that 44% of the men on ADT had fasting glucose in the diabetic range (>126 mg/dL) compared with 11–12% for the controls [51].
Per the Adult Treatment Panel III (ATP III) guidelines, the diagnosis of metabolic syndrome requires the presence of three of the following five criteria: (1) serum triglycerides ≥150 mg/dL, (2) high-density lipoprotein (HDL) < 40 mg/dL, (3) fasting serum glucose >110 mg/dL, (4) waist circumference ≥ 40 inches, and (5) blood pressure ≥ 130/85 [52]. As noted earlier, patients receiving ADT are at risk for higher fasting serum glucose and increased waist size due to central weight gain. Triglycerides have also been reported to rise by 26.5% (±10%; p = 0.01) with 1 year of ADT [49]. There are currently no specific recommendations regarding the management of insulin resistance and lipid increases for men on ADT.
20.8.6 Gynecomastia
Gynecomastia and breast pain may be seen in patients on ADT. The incidence of gynecomastia was reported to be as high as 85% in patients receiving high-dose 150 mg daily bicalutamide antiandrogen monotherapy [53]. However, the incidence is lower (13–22%) in men receiving combined androgen blockade [54]. Di Lorenzo et al. investigated the role of tamoxifen and radiotherapy for the prevention and treatment of gynecomastia and breast pain during adjuvant bicalutamide monotherapy after RP in patients with prostate cancer [55]. It was shown that gynecomastia and breast pain induced by bicalutamide monotherapy after RP can be prevented and treated. Tamoxifen has been shown to be more effective and safe than RT (12 Gy) in this setting, and QOL and sexual function are not negatively influenced by these two treatment options. Ozen et al. investigated the efficacy of prophylactic radiotherapy for gynecomastia/breast pain induced by 150 mg bicalutamide in a prospective, randomized, multi-institutional trial [56]. After definitive treatment for localized prostate cancer, 125 patients were randomized to 12 Gy radiotherapy before bicalutamide as prophylactic radiotherapy or bicalutamide only for nonprophylactic radiotherapy. With a follow-up of 12 months, the gynecomastia rate was 15.8% in the prophylactic group and 50.8% in the nonprophylactic group (p < 0.001). Although prophylactic breast irradiation seemed to decrease the gynecomastia rate in patients on 150 mg bicalutamide, not all patients need prophylaxis since only 52% were significantly bothered by gynecomastia. Thus, it was recommended to select patients who need prophylactic radiation based on individual assessment.
20.8.7 Other
Fatigue is another side effect of ADT. The main strategy to reduce fatigue is exercise. Anemia and reduction in penile and testis size may be seen. Hypogonadism has been linked to cognitive declines in patients on ADT [57].
20.9 Follow-Up During Hormonal Treatment
EAU-ESTRO recommends follow-up of 3–6 month intervals. As a minimum, tests should include serum PSA measurement, physical examination, serum testosterone, and careful evaluation of symptoms to assess the treatment response and side effects. Patients should be warned about the signs of metastatic situations like occult cord compression, urinary tract complications/obstruction signs, and bone pain. Routine imaging is not indicated asymptomatic in patients. However, new-onset bone pain requires a bone scan, as does PSA progression suggesting CRPC status, if a treatment modification is considered [43].
The measurement of serum testosterone levels should be a part of follow-up of patients on LHRH therapy. Although timing of measurements is not clearly defined, a 3–6 month assessment of the testosterone level might be performed to evaluate the effectiveness of treatment and to ensure that the castration level is being maintained. If it is not achieved, switching to another type of treatment modality can be attempted. In patients with rising PSA and/or clinical progression, serum testosterone must be evaluated in all cases to confirm a castrate-resistant state.
Long-term ADT reduces bone mineral density (BMD) and increases the risk of fractures [58]. In the absence of associated risk factors, it is recommended that BMD and serum vitamin D and calcium levels should be measured every 2 years [59]. Patients should be screened for the development of alterations in lipid profiles and decreased insulin sensitivity [60]. ADT may increase the risk of diabetes and cardiovascular disease [61]. Patients should be given advice on modifying their lifestyle (e.g., diet, exercise, smoking cessation) and should be treated for any existing conditions, such as diabetes, hyperlipidemia, and/or hypertension. Furthermore, the risk–benefit ratio of ADT must be considered for patients with a higher risk of cardiovascular complications, especially if it is possible to delay starting ADT.
20.10 Castration-Resistant Prostate Cancer
CRPC is defined as castrate serum testosterone <50 ng/dl plus either biochemical or radiological progression. Biochemical progression is defined as three consecutive rises in PSA 1 week apart, resulting in two 50% increases over the nadir, and PSA >2 ng/mL. Radiological progression is defined as the appearance of new lesions, either two or more new bone lesions on bone scan or a soft tissue lesion using the Response Evaluation Criteria in Solid Tumors.
20.10.1 First-Line Treatment in Metastatic Castration-Resistant Prostate Cance r
Abiraterone was evaluated in 1088 chemonaïve metastatic CRPC patients in the phase 3 trial. Patients were randomized to abiraterone acetate or placebo, both combined with prednisone [62]. After a median follow-up of 22.2 months, there was significant improvement of radiographic PFS (median: 16.5 vs. 8.2 months; HR: 0.52; p < 0.001). At the final analysis, with a median follow-up of 49.2 months, the OS end point was significantly improved (34.7 vs. 30.3 months; HR: 0.81; 95% CI, 0.70–0.93; p = 0.0033) [63].
PREVAIL is a randomized phase 3 trial that included a similar patient population and compared enzalutamide with placebo. It was conducted in 1717 chemonaïve mCRPC patients and showed significant improvement in both radiographic PFS (HR: 0.186; 95% CI, 0.15–0.23; p < 0.0001) and OS (HR: 0.706; 95% CI, 0.6–0.84; p < 0.001). The most common clinically relevant AEs were fatigue and hypertension. The results showed that enzalutamide significantly decreased the risk of radiographic progression and death and delayed the initiation of chemotherapy in men with metastatic prostate cancer [64].
In a phase 2 randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic CRPC, 125 patients were randomly assigned in a multicenter trial of vaccination series. After a median follow-up of 34 months, median survival was 25.8 months in the sipuleucel-T group compared with 21.7 months in the placebo group (p = 0.03). PFS was similar in both groups and treatment tolerance was very good [65].
Tannock et al. compared docetaxel plus prednisone with mitoxantrone plus prednisone in 1006 men with metastatic hormone-refractory prostate cancer. The median survival was 16.5 months in the mitoxantrone group, 18.9 months in the group given docetaxel every 3 weeks, and 17.4 months in the group given weekly docetaxel. Among these three groups, 32%, 45%, and 48% of men, respectively, had at least a 50% decrease in the serum PSA level (p < 0.001 for both comparisons with mitoxantrone); 22%, 35% (p = 0.01), and 31% (p = 0.08) had predefined reductions in pain; and 13%, 22% (p = 0.009), and 23% (p = 0.005) had improvements in the quality of life. Adverse events were also more common in the groups that received docetaxel [66].
ALSYMPCA is a phase 3 trial that enrolled 921 patients with symptomatic mCRPC who failed or were unfit for docetaxel. Patients were randomized to six injections of 50 kBq/kg Ra 223, every 4 weeks. or placebo, plus standard of care. Ra 223, an alpha emitter, significantly improved median OS by 3.6 months (HR: 0.70; p < 0.001) [67]. Ra-223 treatment was associated with prolonged time to first skeletal event and improvement in pain scores and QoL. Radium-223 was associated with low myelosuppression rates and fewer adverse events. The updated analysis of ALSYMPCA trial showed that Ra-223 was effective and safe regardless of previous docetaxel use [68].
20.10.2 Second-Line Treatment Options and Beyond in Metastatic Castration-Resistant Prostate Cancer
Cabazitaxel is a form of taxane that has an activity in docetaxel-resistant cancers. TROPIC trial is a large prospective randomized phase 3 trial comparing cabazitaxel plus prednisone versus mitoxantrone plus prednisone in 755 patients with mCRPC who had progressed after or during docetaxel-based chemotherapy [69]. Patients received a maximum of ten cycles of cabazitaxel (25 mg/m2) or mitoxantrone (12 mg/m2) plus prednisone (10 mg/dL), respectively. OS was significantly longer with cabazitaxel (median: 15.1 vs. 12.7 months; p < 0.0001). Treatment-associated World Health Organization grade 3–4 AEs developed significantly more often in the cabazitaxel arm, particularly hematological toxicity (68.2% vs. 47.3%; p < 0.0002) but also nonhematological toxicity (57.4% vs. 39.8%; p < 0.0002). This drug should be administered preferably with prophylactic granulocyte colony-stimulating factor and by physicians with expertise in handling neutropenia and sepsis [70].
AFFIRM is a trial including 1199 mCRPC patients with randomization in a 2:1 fashion to enzalutamide or placebo [71]. The patients had progressed after docetaxel treatment, according to the PCWG2 criteria. After a median follow-up of 14.4 months, median survival in the enzalutamide group was 18.4 months compared with 13.6 months in the placebo arm (HR: 0.63; p < 0.001). The benefit was observed regardless of age, baseline pain intensity, and type of progression. All secondary objectives including soft tissue response, QoL response rate, and time to PSA progression or objective progression were in favor of enzalutamide. Rates of fatigue, diarrhea, and hot flashes were higher in the enzalutamide group. Seizures were reported in five patients (0.6%) receiving enzalutamide compared to none in the placebo group.
Conclusion
ADT has become the standard of care for patients with advanced prostate cancer. ADT aims to reduce the serum testosterone to castrate level . The contemporary laboratory testing methods showed that the mean value after surgical castration is 15 ng/dL. Thus, recently the level is defined as being less than 20 ng/dL (1 nmol/L). Recent definition is associated with better outcomes compared to the previous one. ADT can be used as adjuvant or neoadjuvant therapy in conjunction with initial treatment of patients with intermediate or high-risk prostate cancer, patients with rising PSA after curative treatment, or patients with metastatic disease at diagnosis. It can either be used before radiotherapy in patients with large prostate to decrease the tumor volume.
References
Oefelein MG, Feng A, Scolieri MJ, et al. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56:1021–4.
Morote J, Planas J, Salvador C, et al. Individual variations of serum testosterone in patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 2009;103:332–5.
Pickles T, Hamm J, Morris WJ, et al. Incomplete testosterone suppression with luteinizing hormone-releasing hormone agonists: does it happen and does it matter? BJU Int. 2012;110:E500–7.
Klotz L, O’Callaghan C, Higano T, et al. MP74-01 nadir testosterone on ADT predicts for time to castrate resistant progression: a secondary analysis of the PR-7 intermittent vs continuous ADT trial. J Urol. 2014;191(4):e855–6.
https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed Jan 2017.
Klotz L, McNeill I, Fleshner N. A phase 1-2 trial of diethylstilbestrol plus low dose warfarin in advanced prostate carcinoma. J Urol. 1999;161:169–72.
Farrugia D, Ansell W, Singh M, Philp T, et al. Stilboestrol plus adrenal suppression as salvage treatment for patients failing treatment with luteinizing hormone-releasing hormone analogues and orchiectomy. BJU Int. 2000;85:1069–173.
Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–8.
Klotz L, O’Callaghan C, Ding K, et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J Clin Oncol. 2015;33(10):1151–6.
Schmidt F, Sundaram K, Thau RB, Bardin CW. [Ac-D-NAL(2)1,4FD-Phe2,D-Trp3,D-Arg6]-LHRH, a potent antagonist of LHRH, produces transient edema and behavioral changes in rats. Contraception. 1984;29(3):283–9.
Hook WA, Karten M, Siraganian RP. Histamine release by structural analogs of LHRH. Fed Am Soc Exp Biol. 1985;44:1323.
Broqua P, Riviere P, Conn P, Rivier J, Aubert M, Junien J. Pharmacological profile of a new, potent, and longacting gonadotropin-releasing hormone antagonist: degarelix. J Pharmacol Exp Ther. 2002;301:95–102.
Crawford ED, Tombal B, Miller K, et al. A phase III extension trial with a 1-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. J Urol. 2011;186:889–97.
Moffat LE. Comparison of Zoladex, diethylstilbestrol and cyproterone acetate treatment in advanced prostate cancer. Eur Urol. 1990;18(Suppl 3):26–7.
Schroder FH, Whelan P, de Reijke TM, et al. Metastatic prostate cancer treated by flutamide versus cyproterone acetate. Final analysis of the “European Organization for Research and Treatment of Cancer” (EORTC) Protocol 30892. Eur Urol. 2004;45:457–64.
Akyol F, Selek U, Ozyigit G, Onal C, Akdogan B, Karabulut E, Ozen H. Preliminary results of bicalutamide monotherapy on biochemical failure of localized prostate cancer. J Natl Med Assoc. 2006;98(7):1058–61.
Wirth M, Tyrrell C, Delaere K, et al. Bicalutamide (‘Casodex’) 150 mg in addition to standard care in patients with nonmetastatic prostate cancer: updated results from a randomised double-blind phase III study (median followup 5.1 y) in the early prostate cancer programme. Prostate Cancer Prostatic Dis. 2005;8:194–200.
Abrahamsson PA, Anderson J, Boccon-Gibod L, et al. Risks and benefits of hormonal manipulation as monotherapy or adjuvant treatment in localised prostate cancer. Eur Urol. 2005;48:900–5.
Tyrrell CJ, Payne H, See WA, et al. Bicalutamide (‘Casodex’) 150 mg as adjuvant to radiotherapy in patients with localised or locally advanced prostate cancer: results from the randomised early prostate cancer programme. Radiother Oncol. 2005;76:4–10.
Smith MR, Goode M, Zietman AL, et al. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol. 2004;22:2546–53.
Murthy V, Norman AR, Shahidi M, et al. Recovery of serum testosterone after neoadjuvant androgen deprivation therapy and radical radiotherapy in localized prostate cancer. BJU Int. 2006;97(3):476–9.
D’Amico AV, Chen MH, Renshaw AA, et al. Interval to testosterone recovery after hormonal therapy for prostate cancer and risk of death. Int J Radiat Oncol Biol Phys. 2009;75(1):10–5.
https://clinicaltrials.gov/ct2/show/NCT02319837. Accessed Jan 2017.
Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321(7):419–24.
Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339(15):1036–42.
Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368(14):1314–25.
Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367(10):895–903.
Ozyigit G. The role of radiotherapy in the management of prostate cancer. Turkiye Klinikleri J Med Oncol-Special Topics. 2015;8(3):23–30.
Ozdemir Y, Akyol F, Ozyigit G, Hurmuz P, Onal C, Selek U, Karabulut E. Three dimensional conformal radiotherapy and androgen deprivation therapy in patients with clinically localized prostate cancer; Hacettepe University experience. UHOD 2015;25(2):107–17.
Ozyigit G, Akyol F, Onal C, Sari S, Gurdalli S, Yapici B. Combined hormonotherapy and definitive radiation therapy in localized prostate adenocarcinoma. UHOD 2003;13(4):177–89.
Lawton CA, Winter K, Grignon D, Pilepich MV. Androgen suppression plus radiation versus radiation alone for patients with stage D1/pathologic node-positive adenocarcinoma of the prostate: updated results based on national prospective randomized trial radiation therapy oncology group 85-31. J Clin Oncol. 2005;23(4):800–7.
Roach M III, Bae K, Speight J, Wolkov HB, Rubin P, Lee RJ, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26(4):585–91.
Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26(15):2497–504.
Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11(11):1066–73.
Bayoumi AM, Brown AD, Garber AM. Cost-effectiveness of androgen suppression therapies in advanced prostate cancer. J Natl Cancer Inst. 2000;92:1731–9.
Gravis G, Fizazi K, Joly F, Oudard S, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–58.
Gravis G, Boher JM, Joly F, et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70:256–62.
Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46.
James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77.
Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23(31):7897–903.
Krupski TL, Smith MR, Lee WC, et al. Natural history of bone complications in men with prostate carcinoma initiating androgen deprivation therapy. Cancer. 2004;101(3):541–9.
http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf. Accessed Jan 2017.
Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2016;71(4):630–42.
Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–56.
Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87(2):599–603.
Levine GN, D'Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin. 2010;60(3):194–201.
Wilke DR, Parker C, Andonowski A, et al. Testosterone and erectile function recovery after radiotherapy and long-term androgen deprivation with luteinizing hormone-releasing hormone agonists. BJU Int. 2006;97(5):963–8.
Schover LR. Sexual healing in patients with prostate cancer on hormone therapy. Am Soc Clin Oncol Educ Book. 2015:e562–6.
Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87(2):599–603.
Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91(4):1305–8.
Basaria S, Muller DC, Carducci MA, et al. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106(3):581–8.
Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97.
Tyrrell CJ, Payne H, Tammela TL, et al. Prophylactic breast irradiation with a single dose of electron beam radiotherapy (10 Gy) significantly reduces the incidence of bicalutamide-induced gynecomastia. Int J Radiat Oncol Biol Phys. 2004;60(2):476–83.
Di Lorenzo G, Autorino R, Perdonà S, De Placido S. Management of gynaecomastia in patients with prostate cancer: a systematic review. Lancet Oncol. 2005;6(12):972–9.
Di Lorenzo G, Perdonà S, De Placido S, et al. Gynecomastia and breast pain induced by adjuvant therapy with bicalutamide after radical prostatectomy in patients with prostate cancer: the role of tamoxifen and radiotherapy. J Urol. 2005;174(6):2197–203.
Ozen H, Akyol F, Toktas G, Eskicorapci S, Unluer E, Kuyumcuoglu U, Abay E, Cureklibatur I, Sengoz M, Yalcin V, Akpinar H, Zorlu F, Sengor F, Karaman I. Is prophylactic breast radiotherapy necessary in all patients with prostate cancer and gynecomastia and/or breast pain? J Urol. 2010;184(2):519–24.
Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–36.
Smith MR, Boyce SP, Moyneur E, et al. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175(1):136–9.
Conde FA, Aronson WJ. Risk factors for male osteoporosis. Urol Oncol. 2003;21(5):380–3.
Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2008;181(5):1998–2006.
Faris JE, Smith MR. Metabolic sequelae associated with androgen deprivation therapy for prostate cancer. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):240–6.
Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48.
Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–60.
Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33.
Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–105.
Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–12.
Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–23.
Hoskin P, Sartor O, O’Sullivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014;15(12):1397–406.
de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54.
Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of clinical oncology clinical practice guideline endorsement. J Clin Oncol. 2015;33(9):1078–85.
Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Hurmuz, P., Akyol, F., Gultekin, M., Yazici, G., Sari, S.Y., Ozyigit, G. (2017). The Role of Hormonal Treatment in Prostate Cancer. In: Ozyigit, G., Selek, U. (eds) Principles and Practice of Urooncology. Springer, Cham. https://doi.org/10.1007/978-3-319-56114-1_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-56114-1_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-56113-4
Online ISBN: 978-3-319-56114-1
eBook Packages: MedicineMedicine (R0)