Abstract
The adult myocardium harbours a population of resident (endogenous) multipotent cardiac stem and progenitor cells (eCSCs). Manipulation of these cells in situ and ex vivo has opened new therapeutic avenues for anatomical and functional myocardial regeneration. However, recently the ability of the c-kitpos stem and progenitor cells to transdifferentiate into new cardiomyocytes has been disputed. Within an already highly controversial research field, these publications have caused significant confusion in their interpretation. Importantly, identifying, tracing and characterising stem and progenitor cells according to expression of a single surface receptor such as c-kit do not identify eCSCs. As discussed in this chapter, eCSCs isolated from the adult heart have a specific phenotype, being negative for blood lineage markers such as CD34, CD45 and CD31, and exhibit properties of stem and progenitor cells, being clonogenic, self-renewing and multipotent. Under the appropriate conditions, eCSCs differentiate into fully functional beating cardiomyocytes and regenerate cardiomyocytes lost from damage in vivo. Finally, eCSCs are susceptible to the effects of ageing, making regulation of this parameter highly impactful in the efficacy of myocardial regenerative therapies.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Cardiac Progenitor Cells (CPCs)
- Torella
- Senescence-associated Secretory Phenotype (SASP)
- Adult Mammalian Heart
- Cardiomyogenic Potential
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Despite the adult mammalian heart being composed of terminally differentiated cardiomyocytes that are permanently withdrawn from the cell cycle (Nadal-Ginard 1978; Chien and Olson 2002), it is now apparent that the adult heart has the capacity, albeit low, to self-renew cardiomyocytes over the human lifespan (Bergmann et al. 2009, 2015). This is supported by the detection of small, newly formed, immature cardiomyocytes, which incorporate BrdU/EdU and/or stain positive for Ki67, Aurora B and embryonic/neonatal myosin heavy chain (Fig. 2.1), as well as cardiomyocytes undergoing mitosis, under normal conditions and in response to diverse pathological and physiological stimuli (Urbanek et al. 2003, 2005; Bergmann et al. 2009; Boström et al. 2010; Overy and Priest 1966; Kajstura et al. 1998; Waring et al. 2014). The source of these newly formed cardiomyocytes is still a matter of debate (Laflamme and Murry 2011). Three main sources of origin of the new cardiomyocytes have been claimed: (a) circulating progenitors, which through the bloodstream home to the myocardium and differentiate into cardiomyocytes (Quaini et al. 2002); (b) mitotic division of the pre-existing cardiomyocytes (Boström et al. 2010; Bersell et al. 2009; Kühn et al. 2007; Senyo et al. 2013); and (c) a small population of resident multipotent stem cells able to differentiate into the main cell types of the heart (i.e. cardiomyocytes, smooth and endothelial vascular and connective tissue cells) (Torella et al. 2007; Rasmussen et al. 2011).
Regenerating cardiomyocytes in the adult rat heart. Two small regenerating cardiomyocytes (arrowheads) detected using a mouse monoclonal myosin heavy chain (developmental) primary antibody (Novocastra, Leica Biosystems). This antibody recognises a MHC present during the embryonic and period in the development of skeletal muscle, and the same MHC is re-expressed during regeneration of new skeletal muscle fibres (Ecob-Prince et al. 1989; Williams et al. 2001)
Blood-borne precursors are well documented for having a role in inflammation and healing. When adult mouse bone marrow cells were injected into the chick embryo, they converted to a myocardial phenotype (Eisenberg et al. 2006). Their cardiomyogenic potential in the damaged adult heart is however at best very much limited (Loffredo et al. 2011; Ellison et al. 2013). The evidence so far presented in support of re-entry of terminally differentiated cardiomyocytes into the cell cycle has been limited to show division of cells that express proteins of the contractile apparatus in their cytoplasm (Boström et al. 2010; Bersell et al. 2009; Kühn et al. 2007; Senyo et al. 2013). This evidence is equally compatible with new myocyte formation from the pool of multipotent cardiac stem/progenitor cells, which as precursor cells express contractile proteins, and because newly born myocytes are not yet terminally differentiated, they are capable of a few rounds of division before irreversibly withdrawing from the cell cycle (Nadal-Ginard et al. 2003).
The best documented source of the small, immature, newly formed cardiomyocytes in the adult mammalian heart, including the human, is a small population of endogenous cardiac stem and progenitor cells (eCSCs) distributed throughout the atria and ventricles, which can give rise to functional cardiomyocytes and vasculature in vitro and in vivo (Torella et al. 2007; Ellison et al. 2007a). Importantly, owing to genetic labelling and transitional tracking, it is now documented that the newly formed cardiomyocytes observed in the adult mammalian heart are the product of eCSC differentiation (Hsieh et al. 2007; Ellison et al. 2013; van Berlo et al. 2014).
2.1 Phenotype and Characteristics of eCSCs
The first report of endogenous cardiac stem and progenitor cells in the adult mammalian heart was in 2003 (Beltrami et al. 2003), and since then their existence has been confirmed by a number of independent groups. Although a variety of markers (c-kit, Sca-1, PDGFrα, Wt1) have been proposed to identify eCSCs in different species and throughout development (Oh et al. 2003; Matsuura et al. 2004; Messina et al. 2004; Martin et al. 2004; Laugwitz et al. 2005; Moretti et al. 2006; Kattman et al. 2006; Wu et al. 2006; Smart et al. 2011; Chong et al. 2011; Noseda et al. 2015), it still remains to be determined whether these markers identify different populations of eCSCs or, more likely, different developmental and/or physiological stages of the same cell type (Ellison et al. 2010; Keith and Bolli 2015).
The progeny of a single eCSC is able to differentiate into cardiomyocytes, smooth muscle and endothelial vascular cells and, when transplanted into the border zone of an infarct, regenerates functional contractile muscle and the microvasculature of the tissue (Beltrami et al. 2003; Ellison et al. 2013). In a normal adult myocardium, at any given time, most of the eCSCs are quiescent, and only a small fraction is active to replace the cardiomyocytes and vascular cells lost by wear and tear. In response to stress (hypoxia, exercise, work overload or diffuse damage), however, a proportion of the resident eCSCs are rapidly activated; they multiply and generate new muscle and vascular cells (Urbanek et al. 2003; Ellison et al. 2007a, 2013; Waring et al. 2014), contributing to cardiac remodelling. The activation of the eCSCs is able to regenerate the myocardial cells lost as a consequence of diffuse myocardial damage, which kills up to 10% of the myocardial mass (Ellison et al. 2013), and their transplantation can regenerate the contractile cells lost as a consequence of a major acute myocardial infarction (AMI) affecting up to 25% of the left ventricular mass (Beltrami et al. 2003; Ellison et al. 2013).
2.1.1 c-kitpos Stem and Progenitor Cells
c-kit, also known as CD117, is a tyrosine kinase type III receptor, which is expressed in several cell types and plays a significant role in a variety of cell functions, including identifying haematopoietic stem cells while regulating their cell fate (Roskoski 2005). c-kit positive (c-kitpos) stem, and progenitor cells have been identified in the myocardium that are also positive for Sca-1 and MDR-1 (ABCG2) yet are negative for markers of the blood cell lineage, CD31, CD34 and CD45 (described as lineage-negative). They are self-renewing, clonogenic and multipotent and exhibit significant regenerative potential when injected into the adult rat heart following a myocardial infarction (MI), forming new cardiomyocytes and vasculature and restoring cardiac function (Beltrami et al. 2003; Ellison et al. 2013). c-kitpos eCSCs with similar properties to those originally identified in the rat have been identified and characterised in the mouse (Messina et al. 2004; Fransioli et al. 2008), dog (Linke et al. 2005), pig (Ellison et al. 2011) and human (Messina et al. 2004; Torella et al. 2006; Bearzi et al. 2007; Arsalan et al. 2012). These cells are present at a similar density in all species (~1 eCSC per 1000 cardiomyocytes or 45,000 human eCSCs per gram of tissue) (Torella et al. 2007). Similar to the rodent heart, the distribution of c-kitpos eCSCs in the pig and human heart varies with cardiac chamber, and this will differ in the human depending on disease status (our unpublished findings). The adult-derived c-kitpos eCSCs are very similar in their characteristics and potential to a population of cardiac-specific (c-kitpos/Nkx2.5pos) cells identified in the mouse embryo that differentiate into cardiomyocytes and also smooth muscle cells (Wu et al. 2006). Indeed, similar to adult-derived eCSCs, embryonic cardiac c-kitpos/Nkx2.5pos cells possessed the capacity for long-term expansion in vitro, clonogenicity and differentiation into both cardiomyocytes and smooth muscle cells from a single-cell-derived colony (Wu et al. 2006).
Recently considerable confusion has mounted because of the development of genetic lineage tracing mouse models according to the expression of c-kit. Stem cells, as defined by Potten and Loeffler, are “undifferentiated cells capable of (1) proliferation, (2) self-maintenance, (3) production of large number of differentiated progeny, (4) regeneration of the tissue after injury, and (5) flexibility in the use of these options” (Potten and Loeffler 1990). It is important to iterate that a cell must possess these characteristics to be defined a stem cell; identifying, tracing and characterising stem cells according to expression of a single surface receptor (Tallini et al. 2009; Jesty et al. 2012; van Berlo et al. 2014; Sultana et al. 2015), such as c-kit, do not identify eCSCs. Thus, relying on genetic labelling of c-kitpos cells or quantifying c-kitpos cells within any tissue, including the heart, to extrapolate their plasticity or regenerative potential is in our view a major biological and practical pitfall that brings data which require a careful interpretation.
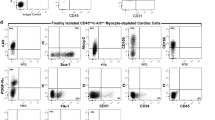
In the adult heart, the total population of c-kitpos cells (including the CD45pos fraction representing cardiac mast cells and CD34/CD31pos cells representing vascular progenitors and cells; Fig. 2.2) have little cardiomyogenic potential (van Berlo et al. 2014; Sultana et al. 2015) and following cryogenic injury (induced by touching a 1 mm diameter copper probe that is equilibrated in liquid nitrogen to the apex of the left ventricle) or myocardial infarction (induced by ligation of left anterior descending coronary artery) contribute predominantly through revascularisation of the damaged tissue (Tallini et al. 2009; Jesty et al. 2012; van Berlo et al. 2014; Sultana et al. 2015). These cells express Flk-1 and/or Pecam-1 (CD31) suggesting they are primarily vascular progenitors and bear more resemblance to the bone marrow-derived c-kitpos/Sca-1pos/Flk-1pos cells identified by Fazel and colleagues, which following a myocardial infarction home to the heart and contribute to the revascularisation of the infarcted/damaged area by establishing a pro-angiogenic milieu (Fazel et al. 2006). Importantly, the c-kitpos eCSCs are CD34 and CD31 negative (Smith et al. 2014) making them distinguishable from these vascular progenitor c-kitpos cells (Fang et al. 2012) (Fig. 2.2).
c-kitpos cells in the adult heart are not all stem/progenitor cells. c-kit-positive cells in the adult represent cardiac mast cells (MAST), endothelial cells (EC), endothelial progenitor cells (EPC) and cardiac stem cells (CSC). CSCs express c-kit at a lower level compared to mast cells, endothelial cells and endothelial progenitor cells. CSCs have a phenotype of c-kitpos/low, CD45neg, tryptaseneg, CD31neg and CD34neg and are clonogenic, self-renewing and multipotent, differentiating into the three cardiac lineages: cardiomyocyte, endothelial and smooth muscle cells
Despite the extensive characterisation of c-kitpos eCSCs, where they meet all five properties of the ‘stem cell’ definition given above, their role and significance in the adult mammalian heart have been continually questioned (Passier et al. 2008; Pouly et al. 2008; Zaruba et al. 2010; van Berlo and Molkentin 2014). Pouly et al. investigated c-kitpos cells in endomyocardial, right ventricular (RV) biopsies and right atrial appendages of heart transplant recipients 73.5 months post-transplantation. Using immunohistochemistry they found that c-kitpos cells were rare (1/mm2 atrial tissue and 2.7/mm2 RV tissue). None of the c-kitpos cells identified expressed Nxk2.5 or CD105; however, all of these cells expressed CD45 and tryptase, identifying them as cardiac mast cells. It is not surprising that the authors only identified mast cells, as cardiac mast cells account for ~80% of the total number of c-kitpos cells in the atria (Ellison et al. 2011).
An important consideration when isolating c-kitpos eCSCs using the enzymatic tissue digestion method (Smith et al. 2014) is to allow liberation of all eCSCs from deep within the myocardium, but also being aware that the c-kit receptor can be affected by over-enzymatic digestion becoming internalised (Lévesque et al. 2003).
2.1.2 Sca-1pos and Side Population Progenitor Cells
Sca-1pos, lineage-negative cardiac progenitor cells (CPCs) were first described in 2003 and are resident non-myocyte cells from the adult murine heart expressing stem cell antigen 1 (Sca-1). While the total Sca-1 CPCs express early cardiac-specific factors such as Gata-4 and MEF2C (Oh et al. 2003; Matsuura et al. 2004), only a fraction of them exhibit stem cell properties of self-renewal and clonogenicity (Ye et al. 2012; Chong et al. 2011; Matsuura et al. 2004; Noseda et al. 2015). Sca-1pos CPCs are capable of cardiomyogenic differentiation in vitro (Oh et al. 2003; Ye et al. 2012; Matsuura et al. 2004; Takamiya et al. 2011; Chong et al. 2011; Wang et al. 2006) and exhibit in vivo cardiomyogenic regenerative potential (Oh et al. 2003; Noseda et al. 2015; Wang et al. 2006; Takamiya et al. 2011). Sca1pos CPCs also show differentiation into both endothelial and smooth muscle lineages (Ye et al. 2012; Wang et al. 2006; Takamiya et al. 2011; Iwakura et al. 2011; Noseda et al. 2015). It is worth noting that there is also a population of Sca1pos vascular progenitor cells which resides within the arterial adventitia (AdvSca1 cells) that have been shown to be regulated by sonic hedgehog signalling (Shh) (Passman et al. 2008).
Side population (SP) cells were first characterised as a primitive population of haematopoietic stem cells characterised by their unique ability to efflux the DNA-binding dye, Hoechst 33342 (Goodell et al. 1996). SP cells have since been isolated from extra-haematopoietic tissues, including bone marrow, skeletal muscle, liver, brain, heart and lung (Asakura and Rudnicki 2002), and the ATP-binding cassette transporter (ABCG2) has been identified as a molecular determinant of the SP phenotype (Zhou et al. 2001; Martin et al. 2004). Hierlihy et al. first reported that the adult myocardium contained an endogenous cardiac SP with stem cell-like activity and identified that this Hoechst dye-excluding population constituted ~1% of total cardiac cells in the mouse postnatal heart (Hierlihy et al. 2002). Transcriptional profiling revealed that the cardiac SP exhibits a Sca-1pos, c-kitlow, CD34neg and CD45neg phenotype (Martin et al. 2004), and further interrogation of these cells revealed that 75% express the endothelial marker, CD31. However, the Sca-1pos CD31neg population was subsequently identified as having the greatest cardiomyogenic potential and was found to represent ~10% of the total cardiac SP (Martin et al. 2004; Wang et al. 2006; Pfister et al. 2005; Oyama et al. 2007).
Although Sca-1 appears to be an ideal marker for isolating and identifying CPCs, its homology hasn’t been confirmed in any species, other than mouse. This poses a significant problem when translating research to develop human regenerative therapies. As c-kitpos eCSCs express Sca-1 (Smith et al. 2014) and the c-kitposCD45negCD31neg and Sca-1posCD31neg cell populations exhibit similar number, self-renewing, clonogenicity and differentiation potential in vitro and in vivo, it can be concluded that they are probably the same cell population and will only differ in their level of expression of c-kit and/or Sca-1 depending on their physiological/differentiation state.
2.2 Cardiac Differentiation Potential of eCSCs
Despite the extensive published data from different groups in support of the regenerative cardiomyogenic potential of the eCSCs in vivo (Ellison et al. 2013; Beltrami et al. 2003; Li et al. 2011; Noseda et al. 2015; Hsieh et al. 2007; Mohsin et al. 2012; Fischer et al. 2009; Angert et al. 2011), scepticism exists over an eCSC’s potential to differentiate into a fully functional synchronised beating cardiomyocyte. The first demonstration that a cardiosphere-forming progenitor cell type isolated from the mouse heart could form spontaneous beating myocyte colonies in vitro was from Messina et al. (2004). Then it was shown that Sca-1pos/CD31neg/CD34neg/CD45neg eCSCs isolated from adult mice hearts differentiated into active contracting cardiomyocytes in vitro (Pfister et al. 2005). We have also shown that clonal c-kitpos eCSCs differentiate into functionally competent beating cardiomyocytes following supplementation with a stage-specific growth factor cocktail targeting TGFβ and Wnt signalling pathways, recapitulating the morphogens present during embryonic development (Smith et al. 2014). This stage-specific regime is not dissimilar to that used to induce differentiation of ESCs and iPSCs into the functional cardiomyogenic embryoid bodies in vitro (Yang et al. 2007). Therefore, like other stem cells, under the appropriate conditions eCSCs do have cardiomyogenic capability, differentiating into functionally competent, beating cardiomyocytes in vitro.
When c-kitpos cells are transplanted intramyocardially in the border/infarct zone of myocardial infarcted hearts, reports have also shown lack of their ability to differentiate into cardiomyocytes. This lack of differentiation capability is most likely due to lack of characterisation of the transplanted cell type, poor cell survival and retention, hostile host environment and subsequent restriction of cell proliferation and integration and differentiation in this damage-regeneration infarct model. Similar findings have been shown for Sca-1pos CPCs (Noseda et al. 2015) and other stem/progenitor cells, including ESCs (Don and Murry 2013). Furthermore, whether the cells are injected as freshly isolated or pre-cultured and expanded in vitro or clonogenic cells will influence their survival and subsequent proliferation, integration and differentiation post-transplantation. Stem cells are maintained in a quiescent state until activated by injury in vivo (Ellison et al. 2007b) or another stimulus ex vivo (i.e. cell culture). Therefore, a freshly isolated stem cell, as well as being highly stressed following isolation from its niche, is quiescent and, unless activated, will not exit from G0 and, upon transplantation, coupled with the hostile host environment, will be more prone to death and/or not likely to proliferate. A cycling-competent stem cell that has been propagated in vitro is more robust and shows increased survival and proliferation post-transplantation (our unpublished findings). Additionally, a clonogenic population, derived from a single cell, is multipotent and able to give rise to cells of all three cardiac lineages. We have shown that clonogenic eCSCs injected intramyocardially following myocardial infarction can replenish up to 20% of cardiomyocytes in the infarct zone, resulting in improved LV function (Ellison et al. 2013).
As stated above certain criteria need to be met to ensure that a cell can be defined as a ‘stem/progenitor’ cell. These include being self-renewing, clonogenic and multipotent. A cell that is injected in vivo to test its regenerative potential should at least show these characteristics in vitro and prior to transplantation. Unfortunately, only a few publications show that the cells they inject have the properties of stem and progenitor cells. Instead because they express stem cell markers such as c-kit or Sca-1 and have been isolated from myocardial tissue, they assume that they are eCSCs, when in fact they are very likely not, but rather CD34pos/CD31pos vascular progenitors and will give rise to new vasculature once transplanted.
It is currently disputed if adult tissue-specific stem cells possess true pluripotency. Indeed, Sca1pos CPCs and c-kitpos eCSCs have shown capability of differentiation into noncardiac lineages in vitro and in vivo (Takamiya et al. 2011; Chong et al. 2011; Miyamoto et al. 2010). Interestingly it has been reported that the level of Sca-1 expression may actually play a role in their differentiation potential with Sca-1 high CPCs having a broader differentiation potential, showing osteogenic, chondrogenic, smooth muscle, endothelial and cardiac differentiation in vitro than Sca-1 low CPCs (Takamiya et al. 2011). In vivo teratoma formation assays have also shown that while Sca-1pos CPCs alone do not form tumours, when injected alongside ESCs, they differentiate into cells of the three germ layers (Chong et al. 2011), although this broad developmental plasticity is yet to be shown in tissue regeneration and repair in vivo.
2.3 The Controversy
As outlined above eCSCs are small primitive cells, positive for stem cell surface receptor markers (i.e. c-kit, Sca-1) and negative for markers of the haematopoietic and endothelial lineage (i.e. CD45 and CD31) and mast cells (i.e. tryptase). They exhibit properties of stem cells, being clonogenic and self-renewing, and differentiate into cardiomyocytes, smooth muscle and endothelial cells, both in vitro and in vivo. Despite these reputable published data, recently, by targeting the c-kit locus with multiple reporter genes in mice, the significance of c-kitpos eCSCs to give rise to cardiomyocytes in vivo has been challenged (van Berlo et al. 2014; Sultana et al. 2015). Instead, these papers suggest a largely vasculogenic and advential lineage predisposition of c-kitpos cells, which isn’t surprising considering 90% of c-kitpos cells from the adult heart are CD31-positive (our unpublished data).
In the following sections we like to point out specific limitations of previous studies questioning the existence of eCSCs:
-
1.
c-kit pos cells vs. c-kit pos eCSCs: c-kit is expressed in numerous cell types in the bone marrow (haematopoietic stem and progenitor cells and mast cells), endothelial (and circulating progenitor) cells, prostate stem cells and interstitial cells of Cajal. Elimination of these cells, and in particular of CD45pos/c-kitpos/tryptasepos mast cells and CD34pos/CD31pos/c-kitpos endothelial progenitors which are several-fold higher in number in the heart than the eCSCs, from analysis is essential (Ellison et al. 2011, 2013; Smith et al. 2014). The CD45neg/CD31neg/CD34neg/tryptaseneg/c-kitpos eCSCs make up a small population (~2–8%) of the total c-kitpos cells (Smith et al. 2014). Therefore, when using genetic lineage tracing to target the c-kit locus at large, definitive conclusions cannot be drawn on the cardiomyogenic potential of the eCSCs per se. Finally, the presented data is in agreement with our observation that a very small percentage of the tagged c-kitpos cells can generate cardiomyocytes and can therefore be considered.
-
2.
Level of c-kit expression: Our preliminary, unpublished data show that c-kit in eCSCs is expressed at a significantly lower level than in the mast cells and endothelial progenitor cells. Whether c-kit/cre lineage tracing models are able to tag and enable effective cre recombination to occur over the time periods tested in the lower c-kit-expressing eCSC cohort has not yet been determined (Nadal-Ginard et al. 2014).
-
3.
Injury model: The adult heart has a low cardiomyocyte renewal rate, and although this rate may increase somewhat after injury, the heart itself is unable to effect large-scale cardiac regeneration, as would be expected from it following a myocardial infarction. Since the discovery of eCSCs, investigators have used the small animal myocardial infarction model to claim the lack of significance and cardiomyogenic regenerative potential of eCSCs. We question whether this specific model in light of the naturally low abundance of eCSCs is suited to support this claim. No solid organ, even with a large stem cell reserve and renewal capability, can regenerate itself from ligation of its main artery resulting in large segmental loss of tissue (Ellison et al. 2012). Therefore, when using the myocardial infarction model there will be very little spontaneous regeneration of cardiomyocytes (<0.01%), whether coming from resident, endogenous stem cells (Smart et al. 2011; van Berlo et al. 2014; Sultana et al. 2015; Hsieh et al. 2007) or proliferation of the survived cardiomyocytes (Senyo et al. 2013).
We have developed in our view a more physiologically relevant cardiac damage model that is in the presence of a patent coronary circulation and more recapitulates muscle wear and tear. When a single excessive (200 mg/kg for mouse; 5 mg/kg for rat) dose injection (s.c.) of the synthetic catecholamine, isoproterenol (ISO), is administered, there is significant diffuse sub-endocardial and apical cardiomyocyte necrotic death, resulting in a dropout of ~10% cardiomyocytes at 24 h post ISO (Goldspink et al. 2004; Ellison et al. 2007b, 2013). This leads to the development of acute cardiac failure; however, the myocardial damage and heart failure spontaneously reverse anatomically and functionally by 28 days (Ellison et al. 2013). Using the acute ISO model, we showed that the adult heart has intrinsic regenerative capacity, where the eCSCs restore cardiac function by regenerating the lost cardiomyocytes. When ISO injury was followed by a 4-week regime of the anti-proliferative agent 5-FU for ablation of eCSC expansion and consequent differentiation, no cardiac regeneration and functional recovery was apparent with animals ending in overt heart failure. However, the regenerative process is completely restored by replacing the ablated eCSCs with the progeny of one eCSC. After regeneration, selective suicide of these exogenous CSCs and their progeny abolishes regeneration, severely impairing ventricular performance. Thus, eCSCs are necessary and sufficient for the regeneration and repair of myocardial damage (Ellison et al. 2013). Incidentally, the acute ISO model should not be confused with chronic administration of ISO over a minimum of 7 days leading to heart failure and cardiac remodelling with significant fibrotic scar formation and as used by van Berlo et al. (2014).
2.4 Origin of eCSCs
An intriguing question concerning eCSCs resident in the heart is whether they are directly descended from lineages which have been present since early development or have possibly ‘migrated’ to the heart later in life. c-kitpos/Nkx2.5pos eCSCs have been identified in early cardiogenic mesoderm (Wu et al. 2006) and in murine embryonic hearts at E6.5 (Ferreira-Martins et al. 2012), a period of development currently thought to be confined solely to first heart field progenitors during primitive heart tube formation.
A study of Nkx2.5-positive, multipotent cardiac stem/progenitor cells early in development found expression of c-kit in ~28% of these cells, which were also negative for CD45, demonstrating that c-kit expression marks a major subset of cardiac progenitors during development (Wu et al. 2006). Furthermore, Nkx2.5pos, c-kitpos cells were more proliferative and less differentiated than Nkx2.5pos, c-kitneg cells; this correlation was not found with Sca-1 expression levels in Nkx2.5pos cells (Wu et al. 2006). However, it has not been determined if the adult c-kitpos eCSCs are directly descended from these cells. Analysis of GFP-positive cells in the embryo of a c-kit-GFP transgenic mouse during cardiac development showed a c-kit-expressing population of progenitor cells that was resident in the heart and did not migrate from extra-cardiac tissue (although a contribution to the c-kit-positive population from extra-cardiac sources could not be excluded) and were present in the postnatal period (Ferreira-Martins et al. 2012). These cells were also shown to have comparable properties to c-kitpos eCSCs in adult life in terms of proliferation, multipotency and myocardial regenerative capacity (Ferreira-Martins et al. 2012).
Recently, using high-resolution genetic fate-mapping approaches with c-kitCreERT2/+ and Wnt1::Flpe mouse lines, Hare and colleagues have shown that c-kit identifies a population of multipotent progenitors of cardiac neural crest origin (Hatzistergos et al. 2015). Recent evidence reviewed by Keith and Bolli (Keith and Bolli 2015) support the concept that c-kit-expressing cells in the heart are not limited to originating from one progenitor cell; rather c-kit expression is a property of cells that originate from multiple pools of progenitors in the developing and postnatal heart (e.g. FHF, proepicardium). Moreover, c-kit expression by itself does not define the embryonic origin, lineage commitment capabilities or differentiation potential of the various groups of progenitors (Keith and Bolli 2015).
2.5 Impact of Ageing and Senescence on eCSCs
Ageing poses the largest risk factor for cardiovascular disease (North and Sinclair 2012). Although long-term exposure to known cardiovascular risk factors strongly drives the development of cardiovascular pathologies, intrinsic cardiac ageing is considered to highly influence the pathogenesis of heart disease (Dutta et al. 2012). However, the fields of the biology of ageing and cardiovascular disease have been studied separately, and only recently their intersection has begun to receive the appropriate attention.
Over the course of ageing, the heart undergoes a number of anatomical, functional and cellular alterations. Early diastolic left ventricular (LV) filling, LV contractility and ejection fraction all decrease during ageing leading to a reduced cardiac output (Schulman et al. 1992; Fleg et al. 1995; Lakatta and Levy 2003a). In an attempt to compensate for the reduction in cardiac output, the myocardium is triggered to increase its muscle mass by undergoing hypertrophy, which in the long-term results in weakened cardiac function. Ageing of the arterial system is exemplified by increased arterial thickening and stiffness, luminal enlargement and dysfunctional endothelium with decreased responsiveness to stress and injury (Lakatta and Levy 2003b). Arterial stiffness contributes to LV pathological hypertrophy and stimulates fibroblast proliferation causing myocardial and arterial fibrosis. Impaired heart rate is another characteristic of the ageing heart. Loss of sinoatrial node cells together with fibrosis and hypertrophy, slow electric impulse propagation throughout the heart causes decreased maximum heart rate and arrhythmias (Antelmi et al. 2004). Thus, age-imposed anomalies of the cardiovasculature led to the onset of a variety of age-related pathologies, including ischemia, hypertension, atherosclerosis, age-related macular degeneration and stroke (North and Sinclair 2012).
Mammalian ageing has been defined as a gradual loss of the capacity to maintain tissue homeostasis or to repair tissues after injury or stress (Jeyapalan and Sedivy 2008). It is now well known that tissue regeneration and homeostasis are controlled by the tissue-specific stem-progenitor cell compartment present in every tissue (Weissman 2000; Li and Clevers 2010). Therefore, it is logical to postulate that pathological and pathophysiological conditions associated with distorted homeostasis and regenerative capacity, such as ageing, correlate with impairments in the corresponding stem cell pool (Sharpless and DePinho 2007; Rossi et al. 2008; Beltrami et al. 2011). Indeed, there is an already well-established overlap between ageing and stem cell impairment, observed in a number of organs and tissues (Martin et al. 1998; Flores et al. 2005; Liang et al. 2005; Nishimura et al. 2005; Janzen et al. 2006; Krishnamurthy et al. 2004; Molofsky et al. 2006; Beerman et al. 2010). Tissue-specific stem cells decline with age due to several factors including telomere shortening, DNA damage and external influences affecting stem cell niche homeostasis (Sharpless and DePinho 2007). In recent years, accumulated evidence signified that cardiac ageing and pathology affects eCSC activity and potency, and therefore this diminishes the capacity of the myocardium to maintain homeostasis (Chimenti et al. 2003; Torella et al. 2004; Urbanek et al. 2005; Sharpless and DePinho 2007; Rossi et al. 2008; Thijssen et al. 2009; Kajstura et al. 2010; Cesselli et al. 2011). As the majority of cardiovascular disease patients are of advanced age, we should focus on the biology of aged CSCs to reflect the aetiology of cardiovascular disease observed in the clinic.
In the heart, ageing and disease are shown to be associated with a significant accumulation of senescent and dysfunctional cardiomyocytes and eCSCs displaying attenuated telomerase activity, telomeric erosion, high incidence of telomere-induced dysfunction foci and elevated expression of the cyclin-dependent kinase inhibitors (CDKIs) p16INK4a and p21Cip1 (Chimenti et al. 2003; Torella et al. 2004; Gonzalez et al. 2008; Kajstura et al. 2010; Cesselli et al. 2011; Rota et al. 2006; Urbanek et al. 2005) (Fig. 2.3). Nevertheless, a population of functional eCSCs, which express telomerase, lack expression of senescent markers and express the cycling protein, Ki67, have been shown to persist in aged hearts (Urbanek et al. 2003; Dawn et al. 2005). Indeed, in the setting of pathophysiological ageing, telomerase-competent eCSCs with normal telomerases can still be found in various cardiac regions, which have the capacity to migrate to injured zones and generate a healthy progeny partly reversing the senescent phenotype and improving cardiac performance (Gonzalez et al. 2008). Unpublished data from our lab has found that the number of eCSCs that can be isolated from human myocardial samples is similar regardless of age, gender and pathology (~45,000/g of tissue). While eCSCs isolated from human hearts showed age-correlated increased expression of ageing/senescence markers and decreased expression of stemness/multipotency and proliferation markers. Moreover, ‘aged-senescent’ eCSCs show limited cloning and growth capacity and impaired cardiac differentiation capacity. Importantly, although the cloning efficiency was inversely age-related, single-cell-derived eCSC clones obtained from younger and older human hearts are indistinguishable by their gene expression and differentiation potential. These data suggest that while the loss of functionally competent eCSCs may underlie the progressive functional deterioration documented with age, eCSC ageing itself may be a stochastic process that does not affect all eCSCs in a cell autonomous manner.
Pathways contributing to eCSC dysfunction in the ageing process. eCSC ageing is regulated by a combination of intrinsic and extrinsic factors. Intrinsic changes include increased senescent marker expression, e.g. p16INK4a, DNA damage, telomere attrition, increased intracellular ROS, mitochondrial dysfunction and ageing-associated epigenetic changes, all of which are challenging to reverse in a clinically translatable manner. Extracellular changes include systemic circulating factors, local factors secreted by the niche and the SASP, which can negatively modulate cell function. These extrinsic pathways are potentially reversible and provide potential therapeutic targets to rejuvenate eCSCs and reverse the senescent, dysfunctional phenotype
Senescent cells are characterised by impaired proliferation, an altered gene expression profile, resistance to apoptosis and epigenetic modifications, as well as producing an altered secretome, which acts on adjacent as well as distant cells, causing fibrosis, inflammation and a possible carcinogenic response (Kuilman and Peeper 2009; Kuilman et al. 2010; Rodier and Campisi 2011; Baker et al. 2011; Tchkonia et al. 2013). Although a universal marker exclusively expressed in senescent cells has not been identified, most senescent cells express p16INK4a, which is not commonly expressed by quiescent or terminally differentiated cells (Baker et al. 2011; Rodier and Campisi 2011). p16INK4a, which becomes progressively expressed with age, enforces cell-cycle arrest by activating retinoblastoma (RB) tumour-suppressor protein (Krishnamurthy et al. 2004; Kim and Sharpless 2006).
Interestingly, a recent study demonstrated silencing of p16INK4a in geriatric satellite cells restored their quiescence and regenerative potential (Sousa-Victor et al. 2014). Similarly, induction of p16INK4a has been shown to induce features of ageing and inhibit proliferation of intestinal stem cells; however, subsequent withdrawal of p16INK4a even after several weeks of induction is sufficient to allow rapid recovery of the affected cells (Boquoi et al. 2015). A recent study demonstrated that genetic reduction of p16INK4a reverses the pathology observed in dilated cardiomyopathy (Gonzalez-Valdes et al. 2015). Together these findings suggest that p16INK4a-expressing cells may exist in a pre-senescent state, which is potentially reversible.
Accumulation of p16INK4a-positive senescent cells within a tissue has been reported to exacerbate dysfunction as these impaired cells have an altered secretome consisting of matrix metalloproteinases, growth factors and inflammatory cytokines, known collectively as the senescent-associated secretory phenotype (SASP) (Coppé et al. 2010) (Fig. 2.3). The SASP can promote senescence of neighbouring cells, and this bystander effect has been shown to negatively affect the host tissue composition in a paracrine fashion (Acosta et al. 2008; Campisi 2005). Researchers at the Mayo clinic have shown that in the BubR1 progeroid mouse, removal of p16Ink4a senescent cells delayed the acquisition of age-related pathologies in adipose, skeletal muscle and eye, while late-life clearance attenuated progression of already established age-related disorders (Baker et al. 2011). Moreover, recently Kirkland and colleagues have also shown that the SASP can be supressed by targeting the JAK pathway and activin A, contributing to alleviating frailty (Xu et al. 2015a, b). Once the process of senescence is initiated in an organ of limited regenerative potential, such as the heart, this can lead to widespread cellular deterioration with the remaining unaffected cells unable to compensate for this cellular loss, ultimately leading to impaired cardiac function (Siddiqi and Sussman 2013). Therefore, therapeutic approaches inhibiting the SASP-mediated decline may improve eCSC, cardiomyocyte and vascular function and alleviate global cardiac deterioration.
2.6 Therapeutic Targets to Activate eCSCs and Reverse the Senescent, Dysfunctional eCSC Phenotype
To reverse the senescent eCSC phenotype, targeting extracellular signals appears to be a promising therapeutic avenue with early work showing that exposure of old skeletal muscle satellite cells to a youthful environment promotes restoration of their function (Conboy et al. 2005). Thus, manipulation of the cardiac microenvironment could alleviate eCSC dysfunction (Fig. 2.3). The IGF-1 signalling pathway has been implicated as a mediator of eCSC senescence, with increased IGF-1 signalling shown to attenuate ageing-associated markers (Torella et al. 2004). In a 22-month-old mice, c-kitpos eCSCs show senescence, evidenced through impaired proliferation and differentiation potential, p16INK4a expression, reduced telomerase activity, telomere shortening, senescence and increased apoptosis (Torella et al. 2004). Senescent eCSCs become largely unable to generate new functionally competent myocytes, compromising cardiomyocyte turnover and favouring the accumulation of old poorly contracting cardiomyocytes (Torella et al. 2004). These findings show that cardiovascular ageing impairs eCSCs, leading to their decline and dysfunction, which leads to the development of cardiac dysfunction and failure. Interestingly, this progression is altered favourably in IGF-1 transgenic mice (Torella et al. 2004). Moreover, reduced phos-Akt expression associated with ageing is now thought to act as a main modulator of telomerase activity; thus, therapies aimed at counteracting this through stimulation of Akt have been shown to circumvent some of the effects of ageing (Torella et al. 2004; D'Amario et al. 2011). Another promising study focused on ex vivo modification with Pim-1, a serine/threonine kinase to alleviate senescent characteristics. Mohsin et al. (2013) showed that Pim-1 rejuvenated the phenotypic and functional properties of eCSCs with restoration of youthful telomeric length, enhanced replicative capacity and decreased levels of p16Ink4a and p53 (Mohsin et al. 2013). More recently the cardioprotective effects of Pim-1 have been shown to be most effective when targeted to nuclear or mitochondrial compartments of eCSCs (Samse et al. 2015).
A number of molecular pathways involved in the reversal of eCSC senescence still remain unexplored; however, given the evidence available for other self-renewing tissues, potential future directions can be identified. One potential target is the Wnt signalling pathway, with a shift from canonical to non-canonical Wnt signalling reported in aged haematopoietic stem cells (HSCs) due to elevated expression of Wnt5a. Conversely, stem cell-intrinsic reduction of Wnt5a expression resulted in functionally rejuvenated aged HSCs (Florian et al. 2013). Mice which overexpressed the Wnt receptor, Frizzled, had reduced infarct size and improved cardiac function (Barandon et al. 2003), suggesting that this pathway may have a role to play in maintaining eCSC regenerative capacity. Bmi-1, necessary for self-renewal and regulator of p16Ink4a and p19, has also been shown to limit dilated cardiomyopathy by limiting heart senescence (Gonzalez-Valdes et al. 2015). The precise role played by reactive oxygen species, mitochondrial dysfunction and epigenetic changes associated with aged eCSCs also remains to be determined. Moreover, to date many of these pathways have only been studied either in cardiomyocytes in vitro or in rodent models; therefore, it is vital that we begin to uncover the mechanisms regulating senescence in human eCSCs and cardiomyocytes in order to move towards translation into clinical therapies.
In summary, the adult heart harbours a small population of cells, which exhibit all the necessary properties to be defined as bona fide stem and progenitor cells, being clonogenic, self-renewing and multipotent, in vitro and in vivo. In order to assess the role of the eCSCs in the adult myocardium, it is indispensable to first be able to identify and track the fate of these cells in contradistinction from other myocardial cells with which they share some specific marker(s), particularly expression of c-kit. Therefore, alternative markers should be sought and an in-depth sequencing analysis carried out. Finally, eCSCs are affected by ageing rendering a proportion of them senescent and dysfunctional. Regulation of eCSC ageing and senescence will impact the efficacy of regenerative therapies, considering the majority of patients in need of treatment are of advanced age. This should not be overlooked and should be considered at the forefront when designing and optimising protocols to repair and regenerate the injured and old myocardium.
Compliance with Ethical Standards
References
Acosta JC, O'Loghlen A, Banito A et al (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133(6):1006–1018
Angert D, Berretta RM, Kubo H et al (2011) Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells. Circ Res 108(10):1226–1237
Antelmi I, de Paula RS, Shinzato AR et al (2004) Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol 93(3):381–385
Arsalan M, Woitek F, Adams V et al (2012) Distribution of cardiac stem cells in the human heart. ISRN Cardiol 2012:483407
Asakura A, Rudnicki MA (2002) Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol 30(11):1339–1345
Baker DJ, Wijshake T, Tchkonia T et al (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479:232–236
Barandon L, Couffinhal T, Ezan J et al (2003) Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation 108(18):2282–2289
Bearzi C, Rota M, Hosoda T et al (2007) Human cardiac stem cells. PNAS 104(35):14068–14073
Beerman I, Maloney WJ, Weissmann IL et al (2010) Stem cells and the aging hematopoietic system. Curr Opin Immunol 22(4):500–506
Beltrami AP, Barlucchi L, Torella D et al (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114:763–776
Beltrami AP, Cesselli D, Beltrami CA (2011) At the stem of youth and health. Pharmacol Ther 129(1):3–20
Bergmann O, Bhardwaj RD, Bernard S et al (2009) Evidence for cardiomyocyte renewal in humans. Science 324(5923):98–102
Bergmann O, Zdunek S, Felker A et al (2015) Dynamics of cell generation and turnover in the human heart. Cell 161(7):1566–1575
Bersell K, Arab S, Haring B et al (2009) Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138:257–270
Boquoi A, Arora S, Chen T et al (2015) Reversible cell cycle inhibition and premature aging features imposed by conditional expression of p16Ink4a. Aging Cell 14(1):139–147
Boström P, Mann N, Wu J et al (2010) C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143:1072–1083
Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120:513–522
Cesselli D, Beltrami AP, D'Aurizio F et al (2011) Effects of age and heart failure on human cardiac stem cell function. Am J Pathol 179(1):349–366
Chien KR, Olson EN (2002) Converging pathways and principles in heart development and disease: CV@CSH. Cell 110:153–162
Chimenti C, Kajstura J, Torella D et al (2003) Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res 93(7):604–613
Chong JJ, Chandrakanthan V, Xaymardan M et al (2011) Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell 9:527–540
Conboy IM, Conboy MJ, Wagers AJ et al (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764
Coppé J, Desprez P, Krtolica A et al (2010) The Senescence-Associated Secretory Phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118
D'Amario D, Cabral-Da-Silva MC, Zheng H et al (2011) Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circ Res 108(12):1467–1481
Dawn B, Stein AB, Urbanek K et al (2005) Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. PNAS 102:3766–3771
Don CW, Murry CE (2013) Improving survival and efficacy of pluripotent stem cell-derived cardiac grafts. J Cell Mol Med 17(11):1355–1362
Dutta D, Calvani R, Bernabei R et al (2012) Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ Res 110(8):1125–1138
Ecob-Prince M, Hill M, Brown W (1989) Immunocytochemical demonstration of myosin heavy chain expression in human muscle. J Neurol Sci 91:71–78
Eisenberg CA, Burch JB, Eisenberg LM (2006) Bone marrow cells transdifferentiate to cardiomyocytes when introduced into the embryonic heart. Stem Cells 24:1236–1245
Ellison GM, Torella D, Karakikes I et al (2007a) Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J Biol Chem 282:11397–11409
Ellison GM, Torella D, Karakikes I et al (2007b) Myocyte death and renewal: modern concepts of cardiac cellular homeostasis. Nat Clin Pract Cardiovasc Med 4(Suppl 1):S52–S59
Ellison GM, Galuppo V, Vicinanza C et al (2010) Cardiac stem and progenitor cell identification: different markers for the same cell? Front Biosci 2:641–652
Ellison GM, Torella D, Dellegrottaglie S et al (2011) Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol 58(9):977–986
Ellison GM, Nadal-Ginard B, Torella D (2012) Optimizing cardiac repair and regeneration through activation of the endogenous cardiac stem cell compartment. J Cardiovasc Transl Res 5(5):667–677
Ellison GM, Vicinanza C, Smith AJ et al (2013) Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 154(4):827–842
Fang S, Wei J, Pentinmikko N et al (2012) Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol 10(10):e1001407
Fazel S, Cimini M, Chen L et al (2006) Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest 116(7):1865–1877
Ferreira-Martins J, Ogórek B, Cappetta D et al (2012) Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ Res 110(5):701–715
Fischer KM, Cottage CT, Wu W et al (2009) Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation 120(21):2077–2087
Fleg JL, O'Connor F, Gerstenblith G et al (1995) Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol 78(3):890–900
Flores I, Cayuela ML, Blasco MA (2005) Effects of telomerase and telomere length on epidermal stem cell behavior. Science 309(5738):1253–1256
Florian MC, Nattamai KJ, Dörr K (2013) A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature 503:392–396
Fransioli J, Bailey B, Gude NA et al (2008) Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells 26(5):1315–1324
Goldspink DF, Burniston JG, Ellison GM et al (2004) Catecholamine-induced apoptosis and necrosis in cardiac and skeletal myocytes of the rat in vivo: the same or separate death pathways? Exp Physiol 89(4):407–416
Gonzalez A, Rota M, Nurzynska D et al (2008) Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res 102(5):597–606
Gonzalez-Valdes I, Hidalgo I, Bujarrabal A et al (2015) Bmi1 limits dilated cardiomyopathy and heart failure by inhibiting cardiac senescence. Nat Commun 6:6473
Goodell MA, Brose K, Paradis G et al (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183(4):1797–1806
Hatzistergos KE, Takeuchi LM, Saur D et al (2015) cKit+ cardiac progenitors of neural crest origin. PNAS 112(42):13051–13056
Hierlihy AM, Seale P, Lobe CG et al (2002) The post-natal heart contains a myocardial stem cell population. FEBS Lett 530(1–3):239–243
Hsieh PC, Segers VF, Davis ME et al (2007) Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med 13(8):970–974
Iwakura T, Mohri T, Hamatani T et al (2011) STAT3/Pim-1 signaling pathway plays a crucial role in endothelial differentiation of cardiac resident Sca-1+ cells both in vitro and in vivo. J Mol Cell Cardiol 51(2):207–214
Janzen V, Forkert R, Fleming HE et al (2006) Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443(7110):421–426
Jesty SA, Steffey MA, Lee FK et al (2012) c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. PNAS 109(33):13380–13385
Jeyapalan JC, Sedivy JM (2008) Cellular senescence and organismal aging. Mech Ageing Dev 129(7–8):467–474
Kajstura J, Leri A, Finato N et al (1998) Myocyte proliferation in end-stage cardiac failure in humans. PNAS 95(15):8801–8805
Kajstura J, Gurusamy N, Ogórek B et al (2010) Myocyte turnover in the aging human heart. Circ Res 107:1374–1386
Kattman SJ, Huber TL, Keller GM (2006) Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell 11:723–732
Keith MC, Bolli R (2015) "String theory" of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results. Circ Res 116(7):1216–1230
Kim WY, Sharpless NE (2006) The regulation of INK4/ARF in cancer and aging. Cell 127(2):265–275
Krishnamurthy J, Torrice C, Ramsey MR et al (2004) Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114:1299–1307
Kühn B, del Monte F, Hajjar RJ et al (2007) Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 13:962–969
Kuilman T, Peeper DS (2009) Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9(2):81–94
Kuilman T, Michaloglou C, Mooi WJ et al (2010) The essence of senescence. Genes Dev 24(22):2463–2479
Laflamme MA, Murry CE (2011) Heart regeneration. Nature 473:326–335
Lakatta EG, Levy D (2003a) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation 107(1):139–146
Lakatta EG, Levy D (2003b) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises, part II: the aging heart in health: links to heart disease. Circulation 107:346–354
Laugwitz KL, Moretti A, Lam J et al (2005) Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 433:647–653
Lévesque JP, Hendy J, Winkler IG et al (2003) Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol 31(2):109–117
Li L, Clevers H (2010) Coexistence of quiescent and active adult stem cells in mammals. Science 327(5965):542–545
Li TS, Cheng K, Malliaras K et al (2011) Expansion of human cardiac stem cells in physiological oxygen improves cell production efficiency and potency for myocardial repair. Cardiovasc Res 89(1):157–165
Liang Y, Van Zant G, Szilvassy SJ (2005) Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 106(4):1479–1487
Linke A, Müller P, Nurzynska D et al (2005) Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. PNAS 102(25):8966–8971
Loffredo FS, Steinhauser ML, Gannon J et al (2011) Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell 8:389–398
Martin K, Kirkwood TB, Potten CS (1998) Age changes in stem cells of murine small intestinal crypts. Exp Cell Res 241(2):316–323
Martin CM, Meeson AP, Robertson SM et al (2004) Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol 265:262–275
Matsuura K, Nagai T, Nishigaki N et al (2004) Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem 279:11384–11391
Messina E, De Angelis L, Frati G et al (2004) Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 95:911–921
Miyamoto S, Kawaguchi N, Ellison GM et al (2010) Characterization of long-term cultured c-kit+ cardiac stem cells derived from adult rat hearts. Stem Cells Dev 19(1):105–116
Mohsin S, Khan M, Toko H et al (2012) Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J Am Coll Cardiol 60(14):1278–1287
Mohsin S, Khan M, Nguyen J et al (2013) Rejuvenation of human cardiac progenitor cells with pim-1 kinase. Circ Res 113(10):1169–1179
Molofsky AV, Slutsky SG, Joseph NM et al (2006) Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443:448–452
Moretti A, Caron L, Nakano A et al (2006) Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127:1151–1165
Nadal-Ginard B (1978) Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell 15:855–864
Nadal-Ginard B, Kajstura J, Leri A et al (2003) Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res 92:139–150
Nadal-Ginard B, Ellison GM, Torella D (2014) Absence of evidence is not evidence of absence: pitfalls of cre knock-ins in the c-Kit locus. Circ Res 115(4):415–418
Nishimura EK, Granter SR, Fisher DE (2005) Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 307(5710):720–724
North BJ, Sinclair DA (2012) The intersection between aging and cardiovascular disease. Circ Res 110(8):1097–1108
Noseda M, Harada M, McSweeney S et al (2015) PDGFRα demarcates the cardiogenic clonogenic Sca1+ stem/progenitor cell in adult murine myocardium. Nat Commun 6:6930
Oh H, Bradfute SB, Gallardo TD et al (2003) Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. PNAS 100:12313–12318
Overy HR, Priest RE (1966) Mitotic cell division in postnatal cardiac growth. Lab Investig 15(6):1100–1103
Oyama T, Nagai T, Wada H et al (2007) Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol 176(3):329–341
Passier R, van Laake LW, Mummery CL (2008) Stem-cell-based therapy and lessons from the heart. Nature 453(7193):322–329
Passman JN, Dong XR, Wu SP et al (2008) A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. PNAS 105(27):9349–9354
Pfister O, Mouquet F, Jain M et al (2005) CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 97(1):52–61
Potten CS, Loeffler M (1990) Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110(4):1001–1020
Pouly J, Bruneval P, Mandet C et al (2008) Cardiac stem cells in the real world. J Thorac Cardiovasc Surg 135(3):673–678
Quaini F, Urbanek K, Beltrami AP et al (2002) Chimerism of the transplanted heart. N Engl J Med 346:5–15
Rasmussen TL, Raveendran G, Zhang J et al (2011) Getting to the heart of myocardial stem cells and cell therapy. Circulation 123:1771–1779
Rodier F, Campisi J (2011) Four faces of cellular senescence. J Cell Biol 192(4):547–556
Roskoski R Jr (2005) Signaling by Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem Biophys Res Commun 337(1):1–13
Rossi DJ, Jamieson CH, Weissman IL (2008) Stems cells and the pathways to aging and cancer. Cell 132(4):681–696
Rota M, LeCapitaine N, Hosoda T et al (2006) Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res 99(1):42–52
Samse K, Emathinger J, Hariharan N (2015) Functional effect of Pim1 depends upon intracellular localization in human cardiac progenitor cells. J Biol Chem 290(22):13935–13947
Schulman SP, Lakatta EG, Fleg JL (1992) Age-related decline in left ventricular filling at rest and exercise. Am J Phys 263(6 Pt 2):H1932–H1938
Senyo SE, Steinhauser ML, Pizzimenti CL et al (2013) Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493(7432):433–436
Sharpless NE, DePinho RA (2007) How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 8(9):703–713
Siddiqi S, Sussman MA (2013) Cardiac Hegemony of Senescence. Curr Transl Geriatr Exp Gerontol Rep 2(4):247–254
Smart N, Bollini S, Dubé KN et al (2011) De novo cardiomyocytes from within the activated adult heart after injury. Nature 474:640–644
Smith AJ, Lewis FC, Aquila I (2014) Isolation and characterization of resident endogenous c-Kit+ cardiac stem cells from the adult mouse and rat heart. Nat Protoc 9(7):1662–1681
Sousa-Victor P, Gutarra S, García-Prat L et al (2014) Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506:316–321
Sultana N, Zhang L, Yan J et al (2015) Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun 6:8701
Takamiya M, Haider KH, Ashraf M (2011) Identification and characterization of a novel multipotent sub-population of Sca-1+ cardiac progenitor cells for myocardial regeneration. PLoS One 6(9):e25265
Tallini YN, Greene KS, Craven M et al (2009) c-kit expression identifies cardiovascular precursors in the neonatal heart. PNAS 106(6):1808–1813
Tchkonia T, Zhu Y, van Deursen J et al (2013) Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123(3):966–972
Thijssen DH, Bullens LM, van Bemmel MM et al (2009) Does arterial shear explain the magnitude of flow-mediated dilation?: a comparison between young and older humans. Am J Physiol Heart Circ Physiol 296(1):H57–H64
Torella D, Rota M, Nurzynska D et al (2004) Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res 94:514–524
Torella D, Ellison GM, Méndez-Ferrer S et al (2006) Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med 3(Suppl 1):S8–13
Torella D, Ellison GM, Karakikes I et al (2007) Resident cardiac stem cells. Cell Mol Life Sci 64:661–673
Urbanek K, Quaini F, Tasca G et al (2003) Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. PNAS 100:10440–10445
Urbanek K, Torella D, Sheikh F et al (2005) Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. PNAS 102(24):8692–8697
van Berlo JH, Molkentin JD (2014) An emerging consensus on cardiac regeneration. Nat Med 20(12):1386–1393
van Berlo JH, Kanisicak O, Maillet M et al (2014) c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509(7500):337–341
Wang X, Hu Q, Nakamura Y et al (2006) The role of the sca-1+/CD31–cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells 24(7):1779–1788
Waring CD, Vicinanza C, Papalamprou A et al (2014) The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J 35:2722–2731
Weissman IL (2000) Stem cells: units of development, units of regeneration, and units in evolution. Cell 100(1):157–168
Williams P, Simpson H, Kenwright J, Goldspink G (2001) Muscle fibre damage and regeneration resulting from surgical limb distraction. Cells Tissues Organs 169:395–400
Wu SM, Fujiwara Y, Cibulsky SM et al (2006) Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell 127:1137–1150
Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL (2015a) JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A 112:E6301–E6310
Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL (2015b) Targeting senescent cells enhances adipogenesis and metabolic function in old age. elife 4:pii: e12997
Yang MJ, Chen CH, Lin PJ et al (2007) Novel method of forming human embryoid bodies in a polystyrene dish surface-coated with a temperature-responsive methylcellulose hydrogel. Biomacromolecules 8(9):2746–2752
Ye J, Boyle A, Shih H et al (2012) Sca-1+ cardiosphere-derived cells are enriched for Isl1-expressing cardiac precursors and improve cardiac function after myocardial injury. PLoS One 7(1):e30329
Zaruba MM, Soonpaa M, Reuter S et al (2010) Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation 121(18):1992–2000
Zhou S, Schuetz JD, Bunting KD et al (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7(9):1028–1034
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All experimental procedures were performed in accordance with the British Home Office Animals (Scientific Procedures) Act 1986 by appropriately qualified staff and approved by the institutional animal welfare and ethical review board.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Ellison-Hughes, G.M., Lewis, F.C. (2017). Progenitor Cells from the Adult Heart. In: Ieda, M., Zimmermann, WH. (eds) Cardiac Regeneration. Cardiac and Vascular Biology, vol 4. Springer, Cham. https://doi.org/10.1007/978-3-319-56106-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-56106-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-56104-2
Online ISBN: 978-3-319-56106-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)