Abstract
Patients with traumatic brain injury present with complex, challenging medical issues. Minimizing neurologic deficit post injury is the ultimate goal. It’s important to understand the intracranial physiology involving increased intracranial pressure (ICP), cerebral perfusion pressure (CPP), and cerebral blood flow (CBF) as it relates to systemic perfusion. Patients with intracranial hypertension must be identified, monitored, and treated in order to optimize outcome.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Intracranial pressure
- Cerebral perfusion pressure
- Cerebral blood flow
- Cushing’s Triad
- Lundberg A waves
- Osmotherapy
- Hypertonic saline
A 27-year-old male involved in a motorcycle accident. He presented to emergency room unresponsive and intubated. Vital signs: blood pressure 180/100, pulse 50, SaO2 = 96%. Physical exam: unresponsive, intubated male. HEENT: facial abrasions, pupils were unequal, and sluggishly reactive; c-collar in place, and aside from a femur fracture, the remaining PE was unremarkable. He was sent for CT and found to have a large subdural hematoma requiring emergent evacuation in the operating room. Once taken to the operating room and the bone flap is removed, the surgeon notes the brain is “bulging and tense.” Postoperatively, an intraventricular catheter was left in place to monitor ICP. In the ICU the ICP monitor showed the following (Fig. 5.1):

Fig. 5.1 ICP waveform
-
1.
What are normal values for intracranial pressure?
-
2.
How does a patient with intracranial hypertension present?
-
3.
What is cerebral perfusion pressure?
-
4.
What are the causes for intracranial hypertension?
-
5.
What is the significance of increased intracranial pressure?
-
6.
Describe the Monro-Kellie hypothesis and Cushing’s Triad.
-
7.
Name some indications and contraindications for invasive ICP monitoring.
-
8.
Name some types of ICP monitors, and what kind of information can an invasive monitor display?
-
9.
Name some therapeutic maneuvers that could be used to reduce intracranial hypertension?
Answers
-
1.
Normal values for intracranial pressure (ICP) are 7–15 mmHg in supine adults. ICP is positional resulting in lower values with head elevation. ICP > 15 mmHg is considered abnormal, and >20 mmHg is considered pathological. ICPs over 20 mmHg, particularly if sustained can lead to worse outcomes.
-
2.
Intracranial hypertension may present with headache, hypertension, bradycardia, and irregular respirations or apnea (Cushing’s Triad). Rarely do these symptoms occur concurrently. A focused neurological exam may reveal papilledema, neurological deficits, and altered consciousness as assessed by the Glasgow Coma Scale.
Uncontrolled intracranial hypertension may lead to brain herniation. Herniation can occur in the supratentorial or infratentorial region of the brain. Common sites for herniation include cingulum (subfalcine), medial temporal lobe (uncal), and inferior cerebellum (tonsillar) [1]. Signs of herniation include dilated and nonreactive pupils, asymmetric pupils, motor exam that demonstrates extensor posturing or no response, and progressive decline in neurologic condition (decrease in GCS > 2 points) that is not associated with non-TBI causes. Signs of uncal herniation specifically include acute loss of consciousness, ipsilateral pupillary dilation (CN III), and contralateral hemiparesis. Transtentorial herniation may cause ipsilateral cerebral infarction because of posterior cerebral artery occlusion.
-
3.
Cerebral perfusion pressure (CPP) is the driving force of blood across the intracranial arterioles and a major determinant of cerebral blood flow (CBF). The relationship between CPP and CBF can be described by the expression CBF = CPP/CVR (cerebral vascular resistance). CPP can be estimated using the formula CPP = MAP–ICP since ICP is generally higher than CVP. Management of patients with intracranial hypertension focuses on optimizing cerebral perfusion by minimizing ICP and maximizing MAP and minimizing increases in CVR. CPP < 60–70 mmHg adversely affect brain tissue oxygenation and metabolism. Attempts to exceed a CPP of 70 mmHg are counterproductive (Level II), and a CPP of <50 mmHg should be avoided. [2]
-
4.
Causes can be grouped into three processes: extra-axial, focal, and diffuse. Extra-axial process would include epidural hemorrhage, subdural hemorrhage, subdural empyema, extra-axial brain tumor, and pneumocephalus. Focal brain process would include brain tumor (primary, metastatic), ischemic stroke, primary intracerebral hemorrhage, brain abscess, traumatic brain injury, and hydrocephalus. Diffuse brain process would include traumatic brain injury, aneurysmal subarachnoid hemorrhage, infectious meningitides and encephalitides, noninfectious neuroinflammatory disorders, hepatic encephalopathy, and toxic-metabolic encephalopathies. Additionally, an increase in venous pressure due to cerebral venous sinus thrombosis, heart failure, superior vena cava, or jugular vein thrombosis/obstruction can cause increased ICP. Metabolic disorders like hypo-osmolality, hyponatremia, or uremic encephalopathy may manifest with increased ICP. Pseudotumor cerebri, idiopathic intracranial hypertension, and choroid plexus tumors (increased CSF production) must also be considered in the differential [1].
-
5.
The significance of increased ICP depends on its effect on cerebral perfusion pressure. A retrospective look at 427 patients in the NMDA antagonist Selfotel trial found that the most powerful predictor of neurological worsening was ICP > 20 mmHg either initially or during neurologic deterioration. There was no correlation with cerebral perfusion pressure (CPP) as long as it was greater than 60 mmHg. CBF is kept constant by autoregulation (Fig. 5.2). Autoregulation is a process of adjustment by the brain’s arterioles to keep cerebral blood flow constant over a wide range of MAP or CPP. When MAP is high, those arterioles constrict increasing cerebral vascular resistance (CVR) and reducing the pressure. When MAP is low, CVR decreases and maintains CPP/CBF. When MAP is less than 65 mmHg or greater than 150 mmHg, the arterioles are unable to autoregulate, and CBF becomes dependent on the MAP, described as “pressure-passive flow” which also occurs in abnormal brain. MAP < 65 mmHg place the brain at risk for ischemia and MAP > 150 mmHg may cause excess CBF and may result in increased ICP and edema.
Optimally the goal is to maintain CPP greater than 60 mmHg by either decreasing ICP or increasing MAP using vasopressors (only vasopressors that do not increase ICP). As long as CPP > 60 mmHg, ICP control is more important than further increases in CPP in terms of neurologic outcome [2].

Fig. 5.2 Cerebral autoregulation

Fig. 5.3 Compliance curve
-
6.
Normal or abnormal ICP is a function of the volume and compliance of each component of the intracranial compartment. Known as the Monro-Kellie hypothesis, this doctrine states that the cranial compartment is enclosed in a nonexpandable case of bone, and thus the volume inside the cranium is fixed. In an incompressible cranium, the blood, CSF, and brain exist in a state of volume equilibrium. A volume increase in one component is compensated by a reciprocal decrease in each of the other components. Once the compensation limits are exceeded, intracranial pressure rises, and CBF can fall (Fig. 5.3).
In 1903, Cushing described as a clinical tool what is now widely known as the “Cushing’s reflex.” It consists of a widening pulse pressure (rising systolic, declining diastolic) and bradycardia [2]. When the arterial pressure is less than the intracranial pressure, a reflex called the “CNS ischemic response” or “Cushing’s reflex” is initiated by the hypothalamus in the brain. The hypothalamus activates the sympathetic nervous system, causing peripheral vasoconstriction and an increase in cardiac output. These two effects serve to increase arterial blood pressure. When arterial blood pressure exceeds the intracranial pressure, blood flow to the brain is restored. The increased arterial blood pressure caused by the CNS ischemic response stimulates the baroreceptors in the carotid bodies, thus slowing the heart rate drastically often to the point of a bradycardia. The Cushing reflex helps save brain tissues during periods of poor perfusion. It’s a late sign of increasing intracranial pressure and indicates that brainstem herniation is imminent. A related term is “Cushing’s triad,” which is the presence of hypertension, bradycardia, and irregular respirations in a patient with increased intracranial pressure. These findings are another manifestation of the Cushing reflex. The irregular respirations are due to reduced perfusion of the brainstem from swelling or possible brainstem herniation.
-
7.
Indications: [6]
-
An ICP monitor should be placed in patients with a Glasgow Coma Score less than 8 T after resuscitation and after reversal of paralytics or sedatives that may have been used during intubation.
-
GCS 3–8 and abnormal CT scan.
-
GCS 3–8 with normal CT scan and 2 or more of the following:
Age > 40 years
Motor posturing
SBP < 90 mmHg
-
GCS 9–15 and CT scan:
Mass lesion (extra-axial >1 cm thick, temporal contusion, ICH > 3 cm)
Effaced cisterns
Shift >5 mm
-
Following craniotomy.
-
A patient at risk for increased ICP undergoing a necessary non-neurosurgical procedure under general anesthesia rendering clinical observation impossible.
-
Patients who have nonsurgical intracranial hemorrhage but are intubated for non-neurosurgical reasons preventing clinical examination.
-
Patients with moderate head injury due to brain parenchymal contusions that are at risk of developing cerebral edema or continued hemorrhage. Extreme vigilance and clinical judgment must be used for lesions in the temporal fossa, since their proximity to the brainstem can lead to herniation and brainstem compression with little change in global ICP.
-
Patients who have undergone tumor or arteriovenous malformation resection and are at risk for cerebral edema who cannot be assessed clinically.
-
Placement of an ICP monitor has no absolute contraindications because of its relatively low risk. The following conditions increase the risk for hemorrhage and merit careful clinical judgment.
-
(a)
Patients with a known bleeding disorder
-
(b)
Patients with thrombocytopenia (platelets count < 10,000/μL)
-
(c)
Known platelets dysfunction (aspirin/clopidogrel or uremic encephalopathy)
-
(d)
Prothrombin time > 13 s
-
(e)
International normalized ratio greater than 1.3
Potential complications include intraparenchymal, interventricular, or subdural hemorrhage. Catheter-related hemorrhages occur in 1–33% of patients. Infection occurs in 1–12% of patients. Higher rates of ventriculitis/meningitis occur with longer duration of EVD placement [3]. Infection rates increase exponentially after 5 days [4].
-
8.
Types of intracranial pressure monitors: invasive vs noninvasive
-
Epidural
-
Subarachnoid
-
Intraparenchymal fiber-optic
-
Intraventricular
-
Transcranial Doppler
-
Optic nerve sheath diameter
-
ICP monitoring allows measurement of ICP at a given point but also provides information about intracranial dynamics and brain compliance from waveform analysis. Prognosis of survival following head injury and optimization of CPP-guided therapy can be based on parameter analysis.
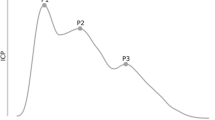
A normal ICP trace is pulsatile and reflects cardiac and respiratory cycles. Amplitude reflects changes in intrathoracic pressure and varies between 2 and 10 mmHg (Fig. 5.4). Respiratory variation diminishes with rising ICP and eventually disappears entirely.
The pulse component of a normal ICP waveform consists of 3 peaks generally 1–4 mmHg in amplitude and correlate with the arterial waveform that occurs with each cardiac cycle.

Fig. 5.4 Normal ICP waveform
The P1 wave or percussion wave reflects the arterial pulse transmitted through the choroid plexus into the CSF. The P2 or the tidal wave reflects cerebral compliance as the arterial pulse wave bounces off the springy brain parenchyma. The dicrotic wave or P3 correlates with aortic valve closure making the trough prior to P3 equivalent to the dicrotic notch.
Based on the morphology of the CSF pulsations, the state of brain compliance can be estimated. As ICP increases above resting level, the cardiac pulse component amplitude increases while the variability of the respiratory component decreases (Fig. 5.5). The waveform can provide information about altered intracranial dynamics and compliance such as increased waveform amplitude, elevated P2, waveform rounding, and plateau wave presence, all suggesting significant increase in ICP that would warrant intervention.

Fig. 5.5 ICP waveform reflecting decreased brain compliance
As intracranial compliance decreases, the greater the effect a 1 cc withdrawal of CSF has on ICP (>5 mmHg). Additionally, pathological waves or Lundberg A, B, and C waves appear (Fig. 5.6). Lundberg A waves or plateau waves are characteristic of conditions that lead to reduced intracranial compliance. With amplitude of 50–100 mmHg occurring for 5–10 min duration, they indicate a situation of low CPP and ischemia. If ICP is left untreated, they are an ominous sign for the development of brain herniation.
Lundberg B waves are rhythmic oscillations, sharply peaked occurring every 1–2 min. In a crescendo manner, ICP increases to 20–30 mmHg from a variable baseline and are not sustained.
Lundberg C waves have no clinical significance being documented in healthy patients. These waves correspond to fluctuations in arterial pressure brought about by oscillations in baroreceptor and chemoreceptor reflex control systems [5].

Fig. 5.6 ICP waveform with illustrated Lundberg waves
-
9.
Management of increased intracranial pressure can fall along three tiers of therapy.
-
(a)
Tier 0: Standard measures which include assessment of adequate circulation, airway patency, and ventilation. The head of the bed should be elevated to 30° or higher to facilitate cerebral venous drainage. Any stimuli like tracheal suctioning should be minimized as it can raise ICP. Lower body and brain temperature if hyperthermia is present. Only iso or hyperosmotic fluids should be administered as intravenous solutions. Correct hyponatremia slowly. Vasogenic edema from brain tumors, abscesses, or noninfectious neuroinflammatory conditions should be treated with high-dose corticosteroid therapy. A non-contrast head CT should be obtained when patient can be safely transported.
-
(b)
Tier 1: If acute obstructive hydrocephalus is present on CT, an external ventricular drainage (EVD) system should be placed emergently. If an EVD is in place, 5–10 mL of CSF should be drained. Mannitol should be administered as a 0.5–0.1 g/kg bolus and repeated every 4–6 h if monitoring serum osmolality. Stop osmotic therapy if serum osmolality is >320 mOsm/kg. A brief course of hyperventilation (<2 h) to a PaCO2 of 30–35 mmHg may be considered. Hyperventilation is known to lower ICP but also decreases CBF 3–4% for every mmHg decrease in PaCO2. This can lead to dangerous drops in CBF in TBI scenarios. Decompressive surgical options may be considered if Tier 1 interventions do not resolve clinical signs of herniation or increased ICP. If surgery is not appropriate, move to Tier 2 therapies.
-
(c)
Tier 2: Hypertonic saline in concentrations ranging from 2–23.4% can effectively treat brain edema and ICP. Concentrations > 3% are preferably administered via a central venous catheter. However, with careful monitoring of the peripheral IV site, 3% and 7.5% saline solutions should not be withheld when treatment indicated. Bolus 23.4% saline has been associated with ICP reduction and transtentorial herniation reversal. A target serum sodium level should be identified and checked every 4–6 h when using hypertonic saline therapies.
Propofol reduces cerebral metabolic oxygen consumption (CMRO2) and cerebral blood volume (CBV) consequently reducing ICP. Administer as a bolus of 1–3 mg/kg and continue as an infusion at 25–200 μg/kg/min titrated as vital signs allow. CPP may be supported with intravenous fluids and/or vasopressors/inotropes. If these fail reconsider decompressive surgery.
-
(d)
Decompressive surgical interventions include:
-
Placement of a ventricular drain
-
Evacuation of extra-axial lesion (epidural hematoma)
-
Resection of intracerebral lesion (lobar hemorrhage)
-
Removal of brain parenchyma (cerebellar mass)
-
Uni- or bilateral craniectomies
-
-
(e)
Tier 3: This is the most aggressive level of management. These guidelines are consensus driven.
-
Barbiturate coma induction can be administered while following EEG continuous monitoring. Using pentobarbital, induction is started with a 10 mg/kg bolus over 30 min, and then 5 mg/kg/h × 3 h, followed by a maintenance infusion of 1–4 mg/kg/h titrated to ICP goal. The infusion rate is adjusted according to either burst suppression of 5–20 s or ICP. This infusion can be continued for 24–96 h while the underlying processes causing the ICP issues are managed. This therapy is not without complications. Pentobarbital is associated with respiratory depression, circulatory instability, immune suppression, and paralytic ileus. Pupillary reactivity is the only neurologic assessment finding, and drug clearance may take days after infusion has stopped.
-
Moderate hypothermia to a target core temperature of 32–34°C can be induced with external cooling or cold intravenous fluids to decrease CMO2 and theoretically achieve some brain protection. Treatment may be associated with shivering, cardiac dysrhythmias, sepsis, coagulopathy, and electrolyte disturbances.
-
Moderate hypocapnia to a PaCO2 of 25–35 mmHg thru hyperventilation can be used if patients have not responded to other therapeutic maneuvers to decrease ICP. Cerebral ischemia can be avoided if some cerebral oxygenation monitor like jugular venous oximetry or a brain tissue oxygen probe is used along with hyperventilation. Prolonged hyperventilation for >6 h is not beneficial and may cause ischemic brain injury [1].
-
-
(a)
References
Stevens RD, Huff JS, Duckworth J, Papangelou A, Weingart SD, Smith WS. Emergency neurological life support: intracranial hypertension and herniation. Neurocrit Care. 2012; doi:10.1007/s12028-012-9754-5.
Gupta G. Intracranial pressure monitoring: background, indications, and contraindications. Emedicine. Medscape.com/article/1829950-overview.
Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurosurg. 2001;95:560–8.
Kirmani AR, Sarmast AH, Bhat AR. Role of external ventricular drainage in the management of intraventricular hemorrhage; its complications and management. Surg Neurol Int. 2015;6:188.
Abraham M, Singhal V. Intracranial pressure monitoring. J Neuroanaesthesiol Crit Care. 2015;2:193–203.
Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, et al. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma. 2007;24(Suppl 1):s55–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Smith, J.J. (2017). Intracranial Pressure. In: Raj, T. (eds) Data Interpretation in Anesthesia. Springer, Cham. https://doi.org/10.1007/978-3-319-55862-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-55862-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55861-5
Online ISBN: 978-3-319-55862-2
eBook Packages: MedicineMedicine (R0)