Abstract
Acute respiratory distress syndrome (ARDS) continues to be a devastating acute lung process with high short-term mortality and potential for significant long-term consequences. Ventilator strategies are keystone in the treatment of this disease, along with other supportive therapies as adjuncts. The ability to quickly diagnose this syndrome with clinical data, including chest X-ray and computed tomography, is crucial for prompt initiation of appropriate treatments that could positively affect outcome. In this chapter, we review the current evidence base for such therapies, both pharmacological and non-pharmacological. We emphasize lung protective ventilation, the concept of low plateau pressures and low tidal volumes, and the benefit of positive end expiratory pressure (PEEP).
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
-
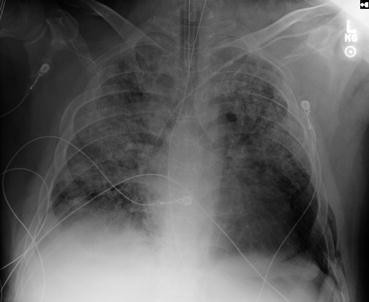
Fig. 47.1 Chest X-ray
-
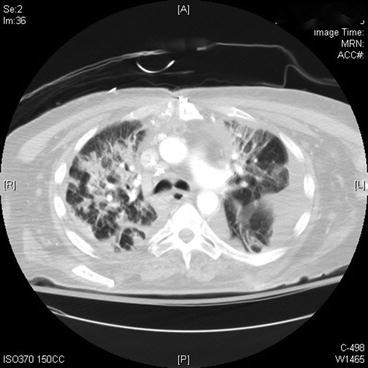
Fig. 47.2 Chest computed tomography
-
1.
What do the images above show and what is the differential diagnosis based on the appearance seen in the images above?
-
2.
What is the current definition of acute respiratory distress syndrome?
-
3.
Name some common triggers for the development of ARDS.
-
4.
What is the approach for mechanical ventilation on patients with the above diagnosis?
-
5.
Is there an indication for steroids, statins, or neuromuscular blockade (NMB) in ARDS?
-
6.
Which nonconventional therapies can be used to enhance oxygenation in severe ARDS?
-
7.
What is the role of nitric oxide and prostaglandins in ARDS?
Answers
-
1.
The chest X-ray (Fig. 47.1) shows diffuse bilateral coalescent opacities, whereas the CT chest (Fig. 47.2) shows ground-glass opacification, reflecting an overall reduction in the air content of the affected lung. It is also possible to visualize bronchial dilatation within areas of ground-glass opacification. Differential diagnosis include (a) ARDS, (b) congestive heart failure, (c) pulmonary hemorrhage, (d) pneumonia, (e) transfusion-related acute lung injury, and (f) non-cardiogenic pulmonary edema.
-
2.
The Berlin definition, dated 2012, states that acute respiratory distress syndrome is an entity characterized by hypoxemia and stiff lungs that occurs within a week of a known clinical insult or new/worsening respiratory symptoms. It presents with bilateral opacities on the chest X-ray involving at least three quadrants that are not fully explained by effusions, atelectasis, or nodules. Chest computed tomography (CT) findings are opacification that is denser in the most dependent regions as compared to more normal and hyper-expanded lung in the nondependent ones. In addition, CT chest shows widespread ground-glass attenuation, which is a nonspecific sign that reflects an overall reduction in the air content of the affected lung. Respiratory failure in ARDS must not be fully explained by cardiac failure, and an objective assessment for exclusion of such cause may be necessary by echocardiography. Finally, ARDS is classified as mild, moderate, or severe based on PaO2/FiO2 ratio and PEEP. If PaO2/FiO2 ratio is between 200 and 300 mmHg with PEEP ≥5, it is classified as mild. If PaO2/FiO2 ratio between 100 and 200 mmHg with PEEP ≥5, it is moderate. PaO2/FiO2 ratio less than 100 mmHg with PEEP ≥5 is classified as severe. Note that the term acute lung injury has been removed, as well as the requirement of pulmonary capillary wedge pressure ≤18 mmHg [1].
-
3.
Common risk factors for ARDS are divided into two categories: direct and indirect.
-
(a)
Direct causes are pneumonia, aspiration of gastric contents, inhalational injury, pulmonary contusion, pulmonary vasculitis, and drowning.
-
(b)
Indirect causes are non-pulmonary sepsis, major trauma, pancreatitis, severe burns, non-cardiogenic shock, drug overdose, and multiple transfusions or transfusion-associated acute lung injury (TRALI).
-
(a)
-
4.
Protective lung strategy (also known as open lung approach or lung protective ventilation) is the standard of care for the management of patients with ARDS. The ARDS Network was a randomized controlled trial designed based on the concept that the limitation of end inspiratory lung stretch may reduce mortality in this patient population. Patients that received lower tidal volume (Vt 4–6 ml/kg ideal body weight) and maintenance of plateau pressure between 25 and 30 mmHg had a survival benefit, with a decrease in mortality from 40% to 31% [2]. Drawbacks from this mode of ventilation were hypoventilation leading to permissive hypercapnia and shear injury due to repetitive opening and closing of alveoli with each cycle. For that reason, PEEP should be set at above lower inflection point to prevent cyclic atelectasis. It is difficult to describe an efficient method of applying optimal PEEP in any given patient. Applying the highest PEEP that allows for maintenance of goal plateau pressure could be a reasonable approach. In that study, the survival benefit was also associated with a reduction of plasma IL-6, supporting the hypothesis that a lung protective strategy limits the spill of inflammatory mediators into the systemic circulation, which may induce multiple system organ failure. In refractory hypoxemia, prolonging the inspiratory time by increasing the I:E ratio may improve oxygenation; however, close attention must be directed to avoid air trapping, auto-PEEP, barotrauma, and hemodynamic compromise.
-
5.
The use of glucocorticoid treatment for ARDS remains contradictory. The ARDS Network LaSRS study showed no benefit in mortality from the routine use of steroids in patients with ARDS. In addition, it was associated with increased risk of neuromuscular complications, as well as risk of death if started 2 weeks after onset of ARDS. The potential adverse effects of steroids also include immunosuppression, superadded infection, and higher blood glucose levels. The mineralocorticoid component contributes to fluid/sodium retention; both of which could result in positive fluid balance, a known factor associated with poor outcomes in lung injury. At the moment, there is insufficient evidence to justify the routine use of steroids in patients with ARDS [3]. The SAILS trial published in 2014 compared statin with placebo in patients with ARDS in the setting of sepsis. Statin therapy did not reduce mortality or increase ventilator-free days; therefore there is no evidence to support its use in ARDS [4]. Neuromuscular blockade therapy for hypoxia has a few potential benefits. Avoidance of large tidal volumes that predispose to volutrauma decreased oxygen consumption from lack of muscle activity and improved patient–ventilator synchrony. Literature shows that the use of NMB in early (first 48 h) ARDS is associated with improved mortality rate. Having said that, judicious use is warranted since paralysis interferes with neurological exam and has been linked to ICU-acquired weakness and posttraumatic stress disorder.
-
6.
Airway pressure release ventilation (APRV) is a combination of pressure-controlled ventilation and inverted ratio ventilation on a time-triggered, pressure-targeted, and time-cycled mode (Fig. 47.3). A higher and a lower PEEP are set, and 80–95% of the respiratory cycle is spent during inspiration at the higher PEEP. The patient is allowed to breathe spontaneously during both high and low PEEP. The mean airway pressure increases without much increase in the peak pressure, favoring lung protection. This mode has been found to be associated with shorter ICU stay and duration of ventilation in patients with ARDS, but contradictory literature still exists, mostly in regard to the lack of evidence of mortality benefit.
-
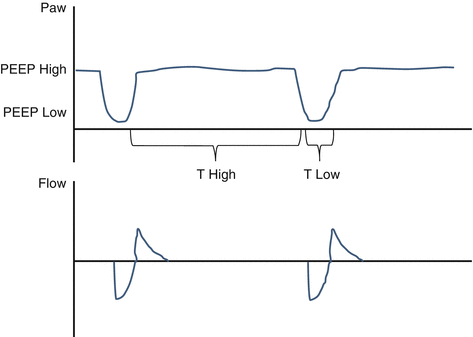
Fig. 47.3 Airway pressure release ventilation (APRV)
-
High-frequency oscillatory ventilation (HFOV) has been evaluated recently by two randomized controlled trials (OSCAR, and OSCILLATE) as well as by a meta-analysis. HFOV has failed to show any mortality benefit. The HFOV group in the OSCILLATE trial had higher mortality, higher requirement for sedatives, paralytics, and vasopressors, and therefore no evidence to support its use.
-
Prone positioning takes advantage of gravity and repositioning of the heart in the thorax to recruit lung regions and improve ventilation–perfusion matching. The mechanisms for the proposed benefit are change in diaphragm movements, increased functional and residual capacity, better secretion clearance, and reduced ventilator-induced lung injury. The PROSEVA trial, published in 2013, brought attention back to this rescue mode after showing association with major decrease in 28-day and 90-day mortality, increase in ventilation-free days, and reduced time to extubation. An increase in PaO2 by 10 mmHg over the first 30 min of prone ventilation usually predicts a sustained increase in PaO2 and deems the patient as a “responder.”
-
Finally, extracorporeal membrane oxygenation (ECMO) remains an important tool for managing refractory hypoxemia that is life-threatening but often considered as a last resort. Literature on its benefit is scarce and controversial. Guidelines suggest it should be used in scenarios that have a potential reversible cause, less than 7 days on mechanical ventilation, age <65 years, no significant comorbidities, no contraindication to anticoagulation, and no significant neurological dysfunction. In case of isolated respiratory failure, a veno-venous approach is advised, whereas in case of hemodynamic instability, a venoarterial approach should be used. More evidence is needed to support its use as standard of care [5].
-
7.
Inhaled vasodilators reduce pulmonary arterial pressure and redistribute blood flow to well-ventilated lung regions with little to no systemic side effects, improving the ventilation–perfusion matching. Inhaled nitric oxide has been shown to improve oxygenation as measured by PaO2/FiO2 ratio and oxygenation index. It is expensive, gets rapidly inactivated by hemoglobin, can result in methemoglobinemia, and carries an increased risk of renal failure. No beneficial effect on mortality or ventilator-free days has been shown with the use of nitric oxide. Inhaled prostaglandins demonstrate similar vasodilator effects when compared to nitric oxide, including improved oxygenation and reduction in pulmonary hypertension; however evidence with large randomized clinical trials is lacking. Patients on these vasodilators are considered “responders” if an improvement on oxygenation is observed within the first 1 h of administration. Based on current evidence, inhaled vasodilators must be considered only as a rescue and temporary therapy for patients with refractory hypoxemia (with or without pulmonary hypertension) when other methods have failed.
References
Fanelli V, et al. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis. 2013;5(3):326–34.
Donahoe M. Acute respiratory distress syndrome: a clinical review. Pulm Circ. 2011;1(2):192–211.
Bihari S, et al. Steroids in ARDS: to be or not to be. Intensive Care Med. 2016;42(5):931–3.
Truwit J, et al. SAILS: statins for acutely injured lungs (ARDS) from sepsis. Am J Respir Crit Care Med. 2014;189:A5088.
Mehta C, et al. Management of refractory hypoxemia. Ann Card Anesth. 2016;19(1):89–96.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Gomes, M.E. (2017). CXR/CT IV. In: Raj, T. (eds) Data Interpretation in Anesthesia. Springer, Cham. https://doi.org/10.1007/978-3-319-55862-2_47
Download citation
DOI: https://doi.org/10.1007/978-3-319-55862-2_47
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55861-5
Online ISBN: 978-3-319-55862-2
eBook Packages: MedicineMedicine (R0)