Abstract
Together with nitrogen (N), phosphorus (P) has been described as the main plant macronutrient limiting growth. Although P is abundant in many soils, its availability for plants is low. For this reason, P is provided to plants largely through the application of P-enriched fertilizers. However, since rock phosphate reserves (the main source of P) are predicted to be depleted in the near future, it is crucial to understand the processes linked with a better P use efficiency. P is a target structural constituent of energetic compounds (ATP, ADP), nucleic acids, phosphate sugars, etc., that are essential for cell metabolism and plant development. Current knowledge highlights that low P availability negatively affects above- and below-ground organ growth, as a consequence, in part, of poor photosynthetic performance. While essential for all plants, the P requirement of N2-fixing plants has been described as larger than that of non N2-fixing plants, mainly as a consequence of the large P demand for biological N2 fixation (BNF) processes. Moreover, three main factors have been suggested to affect BNF under low P conditions: carbon supply, N-feedback and O2 diffusion have been identified as the main factors conditioning N2 fixation under low P availability conditions. In this chapter, we summarize current knowledge regarding P content in plant performance, with special emphasis on N2-fixing plants and the symbiotic relationship.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
The world’s population is expected to increase from 7.5 billion in 2016 to 9.3 billion in 2050, and to 11.2 billion by 2100 (UN 2015). Along with the increase in population, dietary changes towards a preference for meat products due to the emergence of a middle class in developing regions such as Asia and South America will increase the demand for seed crops. To meet this demand, present crop production should be doubled by the end of the century. In the past, during the 1960s, similar challenges were faced, and the population doubled at the same pace as crop production due to unforeseen advances in plant germplasm, the unprecedented use of fertilizers such us the NPK, and the use of new irrigation techniques (Dyson 1999). The use of NPK fertilizers is paramount for crop production. Although nitrogen (N) is among the most abundant elements, it is critical for the growth of most plants due to its unavailability for absorption by plants (Vance et al. 2000). Plants assimilate N from two main sources: through absorption of the N available in soil, and through the N2 fixation that occurs in the nodules of leguminous plants thanks to symbiosis with bacteria from the Rhizobiaceae family. The discovery of industrial N2 fixation during the 1930s makes the use of N almost ubiquitous in agriculture, although this technique is energetically and environmentally costly (Vance 2001). Potassium (K) is a limited macronutrient on our planet, but, with current reserves, the K supply is thought to be enough to last for centuries (USGS 2014). Phosphorus (P) is the second most limiting element for plant growth after N (Vance et al. 2000), and, unlike N, global supplies of P are not infinite. The amount of phosphate (Pi) reserves readily available for its use in agriculture has been a matter of discussion in the last 10 years (Vaccari 2009; Cooper et al. 2011; Edixhoven et al. 2014; Scholz and Wellmer 2016), with some authors claiming that Pi reserves will last until the end of the century, and others that these reserves will last for at least 350 years. Therefore, the study of reserves available for future use in agriculture and other commodities, and understand the keys to P availability in soil and the effects of its deficiency in crop plants is paramount when facing the challenge of feeding an increasing population in an ever-changing world.
2.1.1 P Availability for Plants
P is an abundant element in natural and agricultural soils, and is found in three main “pools”. (1) The soluble P pool is a very small fraction of the total P available in the soil. This fraction is very important because it is the only fraction with mobility in the soil, and is the pool from which plants absorb P in the form of orthophosphate. (2) The active P pool, which is found in the solid phase, contains inorganic Pi that is absorbed by soil particles, Pi that reacts with cations such as Ca2+, Mg2+, Fe2+, and Al3+, and organic P, which is mineralized by the action of soil microorganisms (Vance 2001). The active pool is the fraction that replenishes the soluble pool when plants take up the available Pi from it. The P fertility of a soil will depend on how easily Pi is released into the soluble form. At the same time, Pi derived from a fertilizer can be immobilized through adsorption reactions with several metals and pass to the third-fixed pool. This fraction is very insoluble, and Pi is sequestered from the active and soluble pools, making Pi less available for plant acquisition.
Therefore, although P is an abundant mineral in soils, its availability is limited for plant absorption depending on how it moves through the different pools in the soil. A factor that affects Pi availability and mobility in soils is the pH . Reactions that reduce Pi availability can occur at any pH, but they are more accentuated in acidic (pH ≤ 5.5) or in alkaline (pH ≥ 7.3) soils (Schachtman et al. 1998). Approximately 30% of total land area is acidic soils; of these lands, it is estimated that 50% of the total potential arable land is acidic (soils with a pH < 5.5) (von Uexküll and Mutert 1995). In these conditions, the Pi added with fertilizers reacts with oxidized Al and Fe, forming very stable and insoluble aggregates that pass directly to the fixed pool, making this mineral unavailable for plant absorption (Zheng 2010). At the same time, acidic soils allow the dilution of heavy metals such as Al, which, at high concentrations, are toxic for plants (Horst 1995; Horst et al. 2010). On the other hand, in alkaline soils, Pi reacts mainly with calcium cations (Ca2+) in different reactions, forming less soluble products such as dibasic calcium phosphate dihydrate, octocalcium phosphate, and hydroxyapatite (Busman et al. 2002). The precipitation of Pi from fertilizer in alkaline and acidic soils is one of the main causes of the low efficiency of Pi fertilizers. Due to problems of Pi availability in agricultural soils, crop yield is limited on around 40% of world’s arable land (Vance 2001). To produce 7 tons ha–1 of a field crop in intensive agriculture, it is necessary to add between 90 and 120 kg P ha−1 (Bumb and Baanante 1996). However, only 20% of the applied Pi is removed by the crop in the 1st year (Mousavishalmani et al. 2002). This inefficient plant Pi uptake in comparison with the high amounts of Pi added in the fertilizer might end up in contamination of subterranean aquifers, rivers, lakes and seas; contributing to water eutrophication, and the uncontrolled growth of cyanobacteria and other microorganisms that consume all the oxygen dissolved in the water, causing the death of a large part of the ecosystem, as happened in the Mississippi delta in 2008 (Vaccari 2009).
Research on how to improve the P use efficiency of crops, and how to increase availability of the fixed Pi pool should be undertaken. Considering the objective of how to improve crop P use efficiency, several authors have highlighted two main strategies: (1) increase Pi acquisition through the roots, and (2) improve P conservation (Marschner and Dell 1994; Peoples et al. 1995; Harrison 1997; Gilroy and Jones 2000; Vance et al. 2000; Vance 2001). For better Pi acquisition, several strategies that result in expanded root surface have been applied, such as increased root growth in low P situations and increased root hair development (Gilroy and Jones 2000). Another strategy is to choose species and genotypes with organic acid synthesis and exudation that allow better Pi solubility in acidic soils. In this respect, white lupin (Lupinus albus) roots release organic acids such as malate and citrate that are able to solubilize unavailable inorganic Pi (Vance 2001); at the same time, this species also releases acid phosphatase into the rhizosphere, which helps solubilize organically immobilized P (Vance 2001). Although this species does not have a high relevance in the crop market, its use in intercropping has been shown in several countries, such as in India (Ae et al. 1990). Taking a more molecular approach , the enhancement of Pi transporter and aquaporins in the roots has had proven efficacy (Ragothama 1999). Likewise, research into genes implicated in the symbiosis between plant and mycorrhizal and rhizobium organisms, and the overexpression of such genes, can increase P- and N-use efficiency (Marschner and Dell 1994; Peoples et al. 1995; Harrison 1997; Vance et al. 2000; Vance 2001). Mycorrhizal symbiosis increases root area by at least two-fold thanks to the fungal hyphae; at the same time, fungal secretions solubilize easily the unavailable Pi in the soil, increasing P use efficiency for the plant (Marschner and Dell 1994; Harrison 1997).
2.1.2 Phosphate Rock as a Limited Source of P Fertilizers
As stated above, the application of P fertilizers is important to maintain and increase crop production. More than 60% of the P applied in fertilizers comes from phosphate rock (PR), with the remaining 40% coming from recycled P derived from organic residues such us manure, crop scraps and human excreta (Cordell 2010; Liu et al. 2008; Smit et al. 2009). PR is a finite, non-renewable, resource; of the total PR mined, 82% is used for fertilizer production, 5% for livestock feed, and a small percentage in food additives and detergents (Schröder et al. 2010). PR is usually found in sedimentary rock deposits of marine origin, such as former continental shelves—an example of this would be part of the Sahara Desert (Edixhoven et al. 2014). The entire PR is mined and extracted in combination with other materials, which reduces its purity. Around 95% of P in PR is present as calcium-phosphate . According to the United States Geological Survey (USGS), deposits of PR in combination with impurities are commonly known as “PR reserves ”. However, to receive this consideration, the PR found in those deposits has to be extractable economically with current methods of extraction at the time of classification (USGS 2011). Depending on the source of the PR reserves, the purity of Pi that can be extracted can vary between 5% and 50% of the total mined material. If the PR found in a deposit is not economically extractable at the time of classification due to high concentration of impurities and/or difficulties in the mining process, the USGS classify this deposit as “PR reserve base”. Each year, the USGS produces a mineral commodity report that includes annual production, estimation of PR reserves, and estimation of PR reserve base of the top 15 countries with more PR reserves or production. Reserve estimates are not stable, and can change for various reasons (Cooper et al. 2011). For example, they can increase with the discovery of a new deposit; they can be upgraded from reserve base to reserve if new techniques for extraction improve, or if the PR market price increases, encouraging mining companies to open new mines in veins that otherwise would remain unexploded. Conversely, PR reserves can be downgraded to PR reserve base if the market price drops. Although the nature of the estimation of PR reserve is volatile, in the last 10 years the predictions made by the USGS have varied considerably (Cooper et al. 2011; Edixhoven et al. 2014: Scholz and Wellmer 2016). In 2009, the USGS estimated that the PR reserves were around 16,000 million tons (Mt), meanwhile in 2011, the USGS accounted for PR reserves of 65,000 Mt, a 4-fold increase in the prediction (Jasinsky 2011). These differences in the estimation of the PR reserves between different years make it very difficult to predict how long the PR reserves are going to last. The differences in the estimations are due mainly to recalculation of the base reserve estimates of reserves for Morocco, which, according to the USGS , increased from 5500 Mt in 2009, to 50,000 Mt in 2011 (USGS 2011; Edixhoven et al. 2014; Scholz and Wellmer 2016). Some studies based on the USGS estimates before 2009, predicted that PR reserves could be depleted within 60–100 years (De Haes et al. 2009; Smit et al. 2009; Vaccari 2009; Cordell 2010). However, other recent studies based on more optimistic estimations of PR reserves such as USGS (2011), also called PR peak theory , estimate that PR reserves will be available for at least the next 200–300 years (Edixhoven et al. 2014; Scholz and Wellmer 2016). Independently of the estimations of PR used to predict PR reserves, due to the specific conditions needed for PR formation, the occurrence of PR is limited geographically to very specific regions, and therefore a very few countries hold the majority of PR reserves and production. In total, 17 countries account for almost 95% of the total reserves and production (Cooper et al. 2011). Of these, Morocco accounts for 77% of global reserves with 50,000 Mt, followed by China, Algeria, Syria, Jordan, and the United States (Jasinski 2011), with the United States and China being the biggest producers and consumers of PR (Cooper et al. 2011). As the PR demand is linked strongly to crop, food and biofuel production, the Food and Agriculture Organization of the United Nations (Alexandratos and Bruinsma 2012) estimates that PR demand will grow at a pace of 2% until 2050. At that time, several authors predict that PR demand will stabilize, and even drop slightly due to the scarcity of PR reserves in the main PR producers, such as the United States, with a 15% of world’s total production, and China, with 37% (Cordell 2010; Cooper et al. 2011). According to Cooper et al. (2011), if PR production continues increasing at its current pace of 2%, China and United States would deplete their most accessible PR reserves within 55 years. If that happens, Morocco—the country with the biggest PR reserves in the world (77%) but with a moderate share of PR production (15%)—would have to improve its extraction and production industries to fill the gap left by the United States and China (Cooper et al. 2011). In this scenario, it is predicted that the demand for PR would decrease, and that there would be increased use of organic P sources such as manure, due to the higher price of the raw material as well as political uncertainties caused by one country controlling almost 90% of all PR sources (Cooper et al. 2011). Therefore, finding new technologies to make the P immobilized in the soil available to crops, and/or to find new methods to recycle the P used in agriculture or found in organic matter, will be essential to face the future scarcity of P, and the threat of resource monopoly from P producer and reserve holder countries.
2.2 General Importance of P to Plants
Legumes include important agricultural crops, as their high protein content is of primary importance for human food and animal feed. Forage legumes provide a good source of protein, with multiple positive effects on both human and animal nutrition. In addition, the ability of most legumes to establish symbiotic relationships with soil bacteria allows them to obtain their N requirements from N2 fixation in nodules, and therefore avoids the heavy usage of chemical N fertilizers (Peoples et al. 1995; Irigoyen et al. 2014). The symbiotic relationship between rhizobial bacteria and legumes provides access to atmospheric N2. Biological N2 fixation (BNF) provides to the legumes and the surrounding plants an additional N source that is of great value in impoverished soils. As observed by Peoples et al. (1995), this symbiotic relationship is the main source of N2 fixation in terrestrial ecosystems (provides 50% of BNF) and reduces the need to fertilize soils with chemical compounds, which leads to additional economic and environmental benefits. P is one of the essential macronutrients, being a structural component of many metabolites and required for numerous plant biochemical processes. It functions prominently in DNA (genetic information carrier) and RNA (responsible for genetic information translation), and is abundant in bio-membrane phospholipids, playing an important role in the interaction with surrounding ions (Marschner 1995). Most phosphate esters are intermediates in metabolic pathways of biosynthesis and degradation. Moreover, molecules with energy-rich phosphates are required for starch (ATP), sucrose (UTP) and cellulose (GTP) biosynthesis (Marschner 1995).
2.2.1 P Availability and Plant Growth
Despite relatively high soil total P content, it is limiting and often not easily available to plants due to low P solubility, P fixation to soil particles, and slow replenishment of the extracted P from the soil by the labile pool (Holford 1997; Ribot et al. 2008). Optimal plant P requirement is in the range 0.3–0.5% dry weight (Marschner 1995). P limitation manifests first by heavily affecting growth, and later affects photosynthesis (Halsted and Lynch 1996), typically reducing growth rates (Bottrill et al. 1970; Plesnicar et al. 1994). Moreover, P deficiency has also been described to induce a reduction in the number of leaves (Lynch et al. 1991), premature senescence of the leaves formed (Marschner 1995), and a reduction in leaf surface area or expansion (Terry and Ulrich 1973; Fredeen et al. 1989). The latter is related to the extension of epidermal cells, due to their low P content (Treeby et al. 1987), and a decrease in root hydraulic conductivity (Radin 1990). In contrast to shoot growth, root growth is much less impaired under P deficiency (Marschner 1995; Vysotskaya et al. 2016) and can even be enhanced (Anuradha and Narayanan 1991), coincident with higher root P retention and net P translocation from the shoot (Smith et al. 1990) and correlated with an increase in partitioning of carbohydrates (particularly sucrose) towards the roots (Khamis et al. 1990). Formation of “proteoid roots” is another type of response of certain plant species to P deficiency (Marschner 1995). Also, the ability of other species to associate with mycorrhizal fungi allows them to take up P from the nutrient-poor soils that generally induce P deficiency.
As mentioned above, together with N, P is major limiting mineral governing plant development (Vance et al. 2000). Plant growth inhibition caused by low P availability might be more marked in N2-fixing legumes, because symbiotic plants require more P than non-symbiotic plants. This differing dependence is due to the fact that symbiotic N2 fixation is a high-P-demand process. Nodules have been described to concentrate up to 20% of total plant P (Gunawardena et al. 1992). P concentration in nodules is three-fold higher and is less affected by P deficiency than in other organs (Vadez et al. 1996). Although P is necessary for both growth and N2 fixation, plants involved in BNF are particularly sensitive to P scarcity because P deficiency has greater impact on the N2 fixation process than on shoot growth. Thus, P deficiency prevents nodulation or stops nodule growth when plants have formed nodules in, among others (see Divito and Sadras 2014 for a meta-analysis), white clover, lupins, bean, soybean and Virgilia divaricata (Almeida et al. 2000; Kouas et al. 2005; Miao et al. 2007; Kleinert et al. 2014; Vardien et al. 2014). As a consequence, nitrogenase (Nase) activity decreases (Schulze et al. 2011; Divito and Sadras 2014; Kleinert et al. 2014), and the proportion of whole plant N derived from the symbiotic process decreases (Almeida et al. 2000). However, there is genetic variability within legume species to be exploited, and some mungbean (cultivar T-77; Gunawardena et al. 1992) and cowpea (Ankomah et al. 1996) cultivars are capable of good growth and high N2 fixation, satisfying their total N requirements, in low P soils.
2.2.2 Plant Photosynthesis Is Affected by P Deficiency
P deficiency may, or may not, impact photosynthesis (Bottrill et al. 1970; Brooks 1986; Lauer et al. 1989; Rao and Terry 1989b, 1990). In Eucalyptus globulus, for instance, reductions in photosynthesis can be as large as 55% (Turnbull et al. 2007). P may affect photosynthesis via diffusional and biochemical processes. Some studies have argued that P affects photosynthesis through anatomical effects on stomata (Yang et al. 2016) or stomatal function (Fig. 2.1). In fact, P deficiency decreases stomatal conductance (Brooks 1986; Rao and Terry 1989a; Xu et al. 2007; Yang et al. 2016)—a process in which ATP is involved (Agbariah and Roth-Bejerano 1990). When P deficiency is sensed, the transcription levels of photosynthetic proteins decrease, resulting in reduced photosynthetic rates due to biochemical limitations (Rao and Terry 1989b, 1990; Qiu and Israel 1992; Warren 2011). In the chloroplast stroma, severe inhibition of starch biosynthesis occurs below ca. 5 mM Pi (Marschner 1995). Therefore, a low P supply limits photosynthesis through both stomatal and non-stomatal biochemical processes (Fig. 2.1).
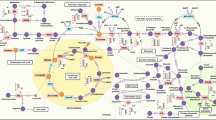
Model representing the most visible changes in carbon and nitrogen primary metabolism of leaves and nodules of N2 fixing plants exposed to P deficiency. This figure is a tentative summary representing the main findings described in the literature. Arrows up (↑) and down (↓) represent compounds/reactions whose content/activity is up- or down-regulated. A n net photosynthesis, Asn asparagine, C c chloroplast CO2 concentration, g s stomatal conductance (Based on Aranjuelo et al. (2014))
The simplest explanation for a positive P relationship with photosynthesis is that Pi is one of the primary substrates of photosynthesis (Walker and Sivak 1986). Hence, there are dramatic and rapid effects on the photosynthetic machinery of sudden changes in internal Pi concentrations (Rao and Terry 1995). For instance, Pi limitation has a greater effect on C reduction than on light reactions (Lauer et al. 1989), lowering ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) content, specific activity and efficiency (Brooks 1986; Lauer et al. 1989). It is known that the presence of Pi accelerates Rubisco activation (Sawada et al. 1992). Both accumulation of leaf starch (and/or sucrose) owing to reduced sink demand (Pieters et al. 2001), or insufficient Pi for operation of triose phosphate transport (Herold 1980), can impair photosynthesis. Furthermore, photosynthetic metabolism is particularly sensitive to Pi insofar as ribulose-1,5-bisphosphate (RuBP) pool size (Brooks 1986; Fredeen et al. 1989) and regeneration efficiency (Brooks 1986; Plesnicar et al. 1994) can be reduced in P-limited plants. Evidence comes from experiments carried out with spinach (Brooks 1986), soybean (Fredeen et al. 1989) and sunflower (Plesnicar et al. 1994). Nevertheless, the amount of P, and the fraction involved in the primary processes of photosynthesis are variable and often small (Bieleski 1973), which makes it less clear why correlations of P with photosynthesis are so strong. In line with this, levels of Pi that reduces photosynthesis in sucrose-accumulating species might barely influence photosynthesis in starch-accumulating species (Walker and Sivak 1985). This is because starch biosynthesis liberates Pi from reduced C, thereby making Pi available for different metabolic reactions.
In many cases, positive relationships between P supply and photosynthesis are best explained by leaf P content, rather than by active (cytoplasmic located) P pool (Turnbull et al. 2007). In a plant with an adequate P supply, 85–95% of total Pi is located in vacuoles, from which release is usually slow (Marschner 1995). Under optimal P nutrition , Pi concentration in the cytosol is ca. 6 mM (Marschner 1995). Better relationships with leaf P content can be explained, at least in some cases, because P affects photosynthesis indirectly through effects on N allocation to the photosynthetic machinery. In Pinus pinaster, for example, there is a strong positive correlation between P supply, P storage as orthophosphate, and N allocated to Rubisco (Warren and Adams 2002). This may come about because, at longer time scales, P deficiency causes accumulation of carbohydrates that downregulate expression of genes coding for the photosynthetic machinery (Krapp and Stitt 1995).
2.3 Nodule Performance Under Low P Conditions
It has been postulated that legumes, since they are capable of fixing atmospheric N2, will have an advantage in plant growth over non-N2-fixing plants (Rogers et al. 2006). Within the symbiotic relationship established between plants and rhizobia, the plant provides photoassimilates to nodules while the bacteria return ammonium to the plant for the further synthesis of amino acids (Fig. 2.2). High metabolic activity of nodules demands a large supply of energy and C skeletons (in the form of sucrose) to the nodules (Aranjuelo et al. 2014). In order to fuel Nase activity, photosynthetically fixed C is supplied to symbiosomes mainly in the form of dicarboxylic acids (such as malate), to produce energy and reducing power in the tricarboxylic acid (TCA) cycle . Organic C supply to nodules is also employed as C skeletons to synthesize N-transport compounds. In exchange for C supply, fixed N is transferred from the bacteroids to the plant cytosol either as ammonia and/or alanine and aspartic acid, where they are assimilated into ureides or amides. Typically, in tropical legumes (such as soybean and cowpea), ureides are the major form of N transported. In contrast, in temperate legumes (such as Medicago and Pisum), amides are reported as the major organic N compounds transported. The exchange of C and N between plant and nodules is well known, and it is clear that amino acids play a pivotal role in the interaction between both metabolisms (Lea and Forde 1994). However, the role of amino acids in nodule metabolism has led to controversy (Lodwig and Poole 2003), and the lack of knowledge about the metabolic fluxes of amino acids between plants and nodules is remarkable. Due to the dependency of C and N metabolism between bacteroids and plant, it is necessary to identify the amino acid exchange in order to understand the importance of amino acids in this symbiotic relationship (Lodwig and Poole 2003).
Model representing the most visible changes in carbon and nitrogen primary metabolism of N2 fixing nodules exposed to P deficiency. This figure is a tentative summary representing the main findings described in the literature. Arrows up (↑) and down (↓) represent compounds/reactions whose content/activity is up- or down-regulated. Asn asparagine, ETC electron transport chain, Lb leghemoglobin, MDH malate dehydrogenase, N ase nitrogenase, OAA oxaloacetate, PEP phosphoenolpyruvate, PEPc phosphoenolpyruvate carboxylase, ROS reactive oxygen species, TCA tricarboxylic acid pathway (Based on Sulieman et al. (2013) and Aranjuelo et al. (2014))
The large P demand in nodules is linked with the large nodule energy requirements (Schulze et al. 1999). As described by Plaxton (2004), nodule and energy generating metabolism depends strongly upon the availability of P. P starvation has proved to negatively affect nodule P and adenylate levels (Le Roux et al. 2006; Vardien et al. 2016). According to published reports, the reductions in Pi adenylate pools, which accompany P, have important implications for nodule functioning. Reduced levels of ADP and P, and increased ADP/ATP ratios, have been reported to result in restricted electron flow in the cytochrome pathway (Vardien et al. 2016). In order to minimize the effects of P deficiency on BNF during low P supply, the nodules require very flexible mechanisms to maintain functional P homeostasis (Vardien et al. 2016). As observed by Vardien et al. (2016), in low P conditions, nodules develop very flexible P recycling and internal P conservation mechanisms, rather than enhanced mechanisms aimed at acquiring external P. Within this context, according to Nasr Esfahani et al. (2016), when exposed to low Pi conditions , chickpea plants tend to reallocate Pi from both leaves and roots to their nodules (Fig. 2.1). A sharp reduction (78%), observed particularly in the Pi level in roots, would help prevent the complete depletion of nodular P level (Nasr Esfahani et al. 2016). Moreover, under P deficiency conditions, plants have been characterized to be able to remobilize P from internal resources, such as nucleic acids and phospholipids (Hernández et al. 2009). In this regard, the induction of genes involved in membrane-phospholipid degradation has been reported in different plant species (Hernández et al. 2009).
Previous studies conducted in N2-fixing plants exposed to P deficiency and other stressful growth conditions (Almeida et al. 2000; Aranjuelo et al. 2014; Sulieman et al. 2013, 2014; Nasr Esfahani et al. 2016) remark that the main processes limiting nodule functioning (summarized in Fig. 2.2) are: (1) carbohydrate availability, (2) accumulation of nitrogenous compounds, (3) oxygen (O2) permeability, and (4) accumulation of reactive oxygen species (ROS):
2.3.1 Carbohydrate Availability
Jakobsen (1985) observed that the impaired nodule
performance under P deficient
conditions is associated with the above-mentioned inhibition of photosynthetic apparatus, and the consequent depletion in nodule carbohydrate availability. As previously described, nodule N2 fixation requires C provided by the host, mostly in the form of malate, for bacteroid respiration. The fact that low P availability negatively affects photosynthetic apparatus implies that less C is delivered to nodules, with consequent impact on nodule performance (Jakobsen 1985). The high level of metabolic activity in nodules requires a large amount of C supplied by the host plant to the nodule. It has been estimated that, during the photoperiod, up to 45% of photoassimilates
may be exported towards the nodules (Gordon et al. 1987). So, within this context, a decline in nodule performance under low P conditions could be associated with the inhibition of the photosynthetic machinery. However, according to Almeida et al. (2000), while leaf photosynthesis can be negatively affected by P, poor growth or poor performance of the nodules cannot be associated with limitations in C availability. According to the same study, starch accumulated in the leaves, and the concentration of water soluble carbohydrates in the nodules of Medicago truncatula was highest under P deficiency. In a recent study with N2-fixing M. truncatula plants exposed to sub-optimal P conditions, the authors observed that nodule sucrose content was relatively stable, and not markedly affected by low P supply (Sulieman et al. 2014). Such results suggest that, under P-limiting conditions, there appeared to be no shortage of carbohydrate provision from the host plant to the nodules. Moreover, in agreement with this conclusion, it was observed that previous studies (Le Roux et al. 2008; Sulieman et al. 2013), also detected large increases in organic acids, such as malate, in nodules subjected to P deprivation. Malate
is believed to be one of the major substrates entering the N2-fixing bacteroids (Schulze et al. 2002). This dicarboxylic acid
is the major energy source for bacteroids and plant mitochondria in different species, and is used for NH
4
+ assimilation as the C skeleton in the glutamine synthetase/glutamate synthase (GS/GOGAT) pathway. It has been shown (Aranjuelo et al. 2014) that an accumulation of organic acids, in nodules under environmental constraints, reflects an impairment of BNF prior to a decline in the glycolytic pathway or sucrose supply from the shoots. On the other hand, a decrease in organic acids is due either to an impaired sucrose synthase activity or decreased glycolytic flux in nodules leading to a diminished symbiosome performance (González et al. 2001; Gálvez et al. 2005). Within this context, increases in nodule
organic acid content detected in N2-fixing plants grown with low P have been associated with the stimulation of nodule respiration derived from the large energy requirements (Schulze and Drevon 2005; Le Roux et al. 2008). Moreover, the large nodule carbohydrate demand is sustained by the increase in nodule dark CO2 fixation (Le Roux et al. 2008; Cabeza et al. 2014). In nodules subjected to low P, an increase in alternative glycolytic pathway (phosphoenolpyruvate, PEP, metabolism via phosphoenolpyruvate carboxylase, PEPc) has been associated with an increase in malate levels (Le Roux et al. 2006, 2008; Cabeza et al. 2014). In a previous study where transcript and metabolite analyses were characterized in nodules of N2-fixing bean plants exposed to P stress
, it was revealed that the glycolysis/C-fixation pathway is induced in P-stressed nodules (Hernández et al. 2009).
For C metabolism, a major point of divergence in glycolysis is at the PEP branchpoint. Three enzymes at this branchpoint implicated in primary PEP metabolism are pyruvate kinase (PK) , PEPc and phosphoenolpyruvate phosphatase (PEP phosphatase) (Le Roux et al. 2008). Previous transcriptomic studies conducted in P-deficient plants showed that the over-expression of PEPc and malate dehydrogenase (MDH) in P-deficient plants was linked to an increase in nodule malate (and other organic acid) content (Hernández et al. 2009; Cabeza et al. 2014). Apart from the generation of pyruvate via conventional glycolysis, pyruvate can alternatively be synthesized from malate via the combined action of cytosolic PEPc , MDH and mitochondrial malic enzyme (ME) (Le Roux et al. 2008). From this perspective, apart from supplying anaplerotic C (to replenish TCA-cycle intermediates), the increase in PEPc would be a response to increased demands for pyruvate and/or Pi recycling (Juszczuk and Rychter 2002). These findings support the fact that stimulation of the PEPc pathway in P-deficient nodules ensures nodule respiration.
Although PEPc has a target role in alternative routes of C metabolism under P stress, the enzyme has also a target role in N2 fixation. In amide-exporting legumes, this route via PEPc is vital to provide C skeletons for the assimilation of NH4 + (Le Roux et al. 2006). Furthermore, PEPc provides a portion of the C for both malate and aspartate synthesis. The double implication of PEPc on C and N metabolism implies that there is a competition of organic acids for the TCA cycle and for amino acid synthesis (Le Roux et al. 2008; Hernández et al. 2009). Moreover, it was further reported that excessive malate accumulation in P-deficient nodules inhibits N2 fixation and subsequent N assimilation.
2.3.2 Accumulation of Nitrogenous Compounds
A deleterious impact of low P availability in nodule performance has also been associated with processes of plant N adjustment. According to Almeida et al. (2000), the slower growth of plants exposed to low P implies that plant N requirements are also diminished. Consequently, these authors observed that depleted N2 fixation is a consequence of an adjustment between plant N sink/source. Accordingly, the host plant would decrease the N2 fixation rate in order to adjust to real N requirements . In accordance with what has been described in nodules subjected to other stresses such as drought and salinity (Aranjuelo et al. 2014), the decrease in Nase activity under low P availability conditions has been associated with the accumulation of nitrogenous compounds in nodules (Almeida et al. 2000; Sulieman et al. 2013, 2014; Nasr Esfahani et al. 2016). The accumulation of these compounds can originate from decreases in carbohydrate fluxes to the nodules and the consequent decrease in the transport of nitrogenous compounds to the plant (Sulieman et al. 2013, 2014). According to Sulieman et al. (2013), the reduction in host plant growth would result in a reduction in N demand for the newly fixed N2—a component known to be highly expensive in terms of C energy. As a result, a shoot-derived N-signal would probably be sent out to the nodule through the phloem to induce a down-regulation of Nase activity (Sulieman et al. 2013, 2014). The fact that analyses of total amino acids in the phloem sap of M. truncatula P-stressed plants strongly increased (as compared with a control treatment) would support the hypothesis that amino acid build up is involved in the poorer nodule performance (Sulieman et al. 2013). The authors also observe that asparagine, or a precursor that interacts with nodule machinery and down-regulates the Nase activity, might be the shoot signal (Sulieman et al. 2013). In addition to asparagine, other compounds, such as glutamate, glutamine, ureides, γ-aminobutyric acid (GABA), proline, polyamines, or a combination of these compounds, have been proposed as signaling candidates involved in nodule N-feedback signaling (Sulieman et al. 2014 and references therein). However, according to what observed by Nasr Esfahani et al. (2016) increases in nodule amino acid content could be also associated to other processes. When exposed to low P conditions, activities of the main enzymes associated with N metabolism increased, with the consequent accumulation of identified amino acids. Alternatively, the increase in the availability of amino acids could also have been attributed to protein degradation in P-deficient nodules (Nasr Esfahani et al. 2016).
2.3.3 O2 Permeability
O2 is required by the nodule for respiration processes; however, O2 regulation is critical for BNF since most Nase are sensitive to its presence (Aranjuelo et al. 2014). Nodule permeability to O2 via the regulation of the O2 diffusion barrier has been suggested as a key factor conditioning Nase performance (Hunt and Layzell 1993). Previous studies have shown that water stress causes a diminishment in the permeability to O2 diffusion, which leads to a reduction in nodule respiration, and, therefore, a lower production of energy via ATP synthase (Serraj and Sinclair 1996). Nodule cortex permeability to O2, and consequently nodule O2 content, has also been described to be affected by P availability (Jebara et al. 2005). Previous studies conducted with soybean, common bean and alfalfa exposed to low P reported an increase in cortex permeability (Ribet and Drevon 1995; Jebara et al. 2005; Schulze and Drevon 2005). According to those studies, such an increase might be a response to enhanced O2-limitation due to wasteful O2 alternative respiration. However, Jebara et al. (2005) showed that a decline in nodule permeability to O2 of P deficiency in common bean plants, and that high P supply increases nodule conductance to O2 diffusion. Another, not mutually exclusive, hypothesis is that the increase in nodule permeability might be the result of a higher shoot N demand per nodule mass under P deficiency (Ribet and Drevon 1995).
2.3.4 Oxidative Stress
Oxidative stress has been identified as another mechanism responsible for N2 fixation inhibition under stressful growth conditions (Aranjuelo et al. 2014). Some environmental conditions (such as drought or salinity) have been described (Aranjuelo et al. 2014) to cause an O2 content imbalance (necessary to ensure successful nodule performance) responsible for nodule senescence. The imbalance in O2 control is associated with the formation of ROS, which could produce cellular damage. ROS production and removal is a complex process that requires a tight biochemical control involving enzymatic and non-enzymatic detoxification mechanisms that have been developed by plants (Aranjuelo et al. 2014). Despite its proven relevance in N2 fixation, information on the impact of P deficiency in nodule oxidative status is scarce. A recent study (Nasr Esfahani et al. 2016) conducted in chickpea plants revealed that, in P-deficient conditions, the plants develop different strategies to overcome oxidative stress. As revealed by the authors, the increase in nodule pyruvate and GABA availability of P-deficient plants could be associated with their ROS scavenging properties and adjustment of cellular redox status. Moreover, Nasr Esfahani et al. (2016) also showed that increased activity of the main enzymes involved with the NH4 + assimilation (such as NADH-dependent glutamate dehydrogenase, NADH-GDH) was induced in nodules in response to low Pi stress. Such an increase, together with the above-mentioned increase in glutamate content, would help prevent potential ROS development derived from toxic accumulation of NH4 +.
2.4 Conclusions
This chapter has reviewed the importance of P availability to plants, taking the perspective of a future P fertilizer shortage. As shown, P deficiency has strong impact in plants, functioning at both aboveground and belowground levels. P deficiency has been shown to affect negatively N2-fixation and photosynthesis (as Pi is a direct subtract of the photosynthesis) and, consequently, plant growth. It should also be noted that effects derived from P-deficiency are more severe in non-fixative plants than in N2-fixing plants. Although the effect of P-deficiency has been studied in the past, such studies were focused mainly on growth characterizations, especially of aboveground organs. However, in the current chapter, it is remarked that, as highlighted by studies carried out in recent decades, plant growth under P-deficiency conditions will be also conditioned by the inhibitory effect of P-deficiency in nodule performance. While, classically, carbohydrate availability, accumulation of nitrogenous compounds, together with O2 diffusion and oxidative stress, have been identified as target process that mediate nodule functioning, as noted in this chapter, still little is known about the impact of P-deficiency in such processes. Overall, these advances emphasize the need to increase our knowledge on the P availability effect in N2 fixation.
References
Ae N, Arihara J, Okada K, Yoshihara T, Johansen C (1990) Phosphorus uptake by pigeon pea and its role in cropping systems of the Indian subcontinent. Science 248:477–480
Agbariah K-T, Roth-Bejerano N (1990) The effect of blue light on energy levels in epidermal strips. Physiol Planta 78:100–104
Alexandratos N, Bruinsma J (2012) World agriculture towards 2030/2050: the 2012 revision. ESA Working paper No. 12–03. FAO, Rome
Almeida JPF, Hartwig UA, Frehner M, Nosberger J, Luscher A (2000) Evidence that P deficiency induces N feedback regulation of symbiotic N2 fixation in white clover (Trifolium repens L.) J Exp Bot 51:1289–1297
Ankomah AB, Zapata F, Hardarson G, Danso SKA (1996) Yield, nodulation, and N2 fixation by cowpea cultivars at different phosphorus levels. Biol Fert Soils 22:10–15
Anuradha M, Narayanan A (1991) Promotion of root elongation by phosphorus deficiency. Plant Soil 136:273–275
Aranjuelo I, Arrese-Igor C, Molero G (2014) Nodule performance within a changing environmental context. J Plant Physiol 98:32–39
Bieleski RL (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol 24:225–252
Bottrill DE, Possingham JV, Kriedemann PE (1970) The effect of nutrient deficiencies on photosynthesis and respiration in spinach. Plant Soil 32:424–438
Brooks A (1986) Effects of phosphorus nutrition on ribulose-1,5-bisphosphate carboxylase activation, photosynthetic quantum yield and amounts of some Calvin cycle metabolites in spinach leaves. Aust J Plant Physiol 13:221–237
Bumb BL, Baanante CA (1996) The role of fertilizer in sustaining food security and protecting the environment. Food, Agriculture, and the Environment, Discussion Paper 17. International Food Policy Research Institute, Washington DC
Busman L, Lamb J, Randall G, Rehm G, Schmitt M (2002) http://www.extension.umn.edu/agriculture/nutrient-management/phosphorus/the-nature-of-phosphorus/#quality
Cabeza RA et al (2014) RNA-seq transcriptome profiling reveals that Medicago truncatula nodules acclimate N2 fixation before emerging P deficiency reaches the nodules. J Exp Bot 65(20):6035–6048
Cooper J, Lombardi R, Boardman D, Carliell-Marquet C (2011) The future distribution and production of global phosphate rock reserves. Resour Conserv Recycl 57:78–86
Cordell D (2010) The story of phosphorus. Sustainability implications of global phosphorus scarcity for food security. Linköping University, Linköping
De Haes HAU, Jansen J, Van Der Weijden LA, Smit WJAL (2009) Phosphate – from surplus to shortage. In: Policy memorandum of the Steering Committee for Technology Assessment. Ministry of Agriculture, Nature and Food Quality, Utrecht
Divito GA, Sadras VO (2014) How do phosphorus, potassium and sulphur affect plant growth and biological nitrogen fixation in crop and pasture legumes? A meta-analysis. Field Crop Res 156:161–171
Dyson T (1999) World food trends and prospects to 2025. Proc Natl Acad Sci USA 96:5929–5936
Edixhoven JD, Gupta J, Savenije HHG (2014) Recent revisions of phosphate rock reserves and resources: a critique. Earth Syst Dynam 5:491–507. doi:10.5194/esd-5-491-2014
Fredeen AL, Rao IM, Terry N (1989) Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol 89:225–230
Gálvez L, González EM, Arrese-Igor C (2005) Evidence for carbon flux shortage and strong carbon/nitrogen interactions in pea nodules at early stages of water stress. J Exp Bot 56: 2551–2561
Gilroy S, Jones DL (2000) Through form to function Broot hair development and nutrient uptake. Trends Plant Sci 3:56–60
González EM, Gálvez L, Royuela M, Aparicio-Tejo PM, Arrese-Igor C (2001) Insights into the regulation of nitrogen fixation in pea nodules: lessons from drought, abscisic acid and increased photoassimilate availability. Agronomie 21:607–613
Gordon AJ, Mitchell DF, Ryle GJA, Powell CE (1987) Diurnal production and utilization of photosynthates in nodulated white clover. J Exp Bot 38:84–98
Gunawardena SFBN, Danso SKA, Zapata F (1992) Phosphorus requirements and nitrogen accumulation by 3 mungbean (Vigna radiata (L) Welzek) cultivars. Plant Soil 147:267–274
Halsted M, Lynch J (1996) Phosphorus responses of C3 and C4 species. J Exp Bot 47:497–505
Harrison MJ (1997) The arbuscular mycorrhizal symbiosis: an underground association. Trends Plant Sci 2:54–60
Hernández G, Valdés-López O, Ramírez M, Goffard N, Weiller G, Aparicio-Fabre R, Fuentes SI, Erban A, Kopka J, Udvardi MK, Vance CP (2009) Global changes in the transcript and metabolic profiles during symbiotic nitrogen fixation in phosphorus-stressed common bean plants. Plant Physiol 151:1221–1238
Herold A (1980) Regulation of photosynthesis by sink activity—the missing link. New Phytol 86:131–144
Holford ICR (1997) Soil phosphorus: its measurement, and its uptake by plants. Aust J Soil Res 35:227–239
Horst WJ (1995) The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Z Pflanzenernähr Bodenkd 158:419–428
Horst WJ, Wang Y, Eticha D (2010) The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann Bot 106:185–197
Hunt S, Layzell DB (1993) Gas exchange of legume nodules and the regulation of nitrogenase activity. Annu Rev Plant Physiol Plant Mol Biol 44:483–511
Irigoyen JJ, Goicoechea N, Antolín MC, Pascual I, Sánchez-Díaz M, Aguirreolea J, Morales F (2014) Growth, photosynthetic acclimation and yield quality in legumes grown under climate change simulations: an updated survey. Plant Sci 226:22–29
Jakobsen I (1985) The role of phosphorus in nitrogen-fixation by young pea-plants (Pisum-Sativum). Physiol Plant 64:190–196
Jasinski SM (2011) Phosphate rock, mineral commodity summaries. U.S. Geological Survey, Reston
Jebara M, Aouani ME, Payre H, Drevon JJ (2005) Nodule conductance varied among common bean (Phaseolus vulgaris) genotypes under phosphorus deficiency. J Plant Physiol 162:309–315
Juszczuk IM, Rychter AM (2002) Pyruvate accumulation during phosphate deficiency stress of bean roots. Plant Physiol Biochem 40(9):783–788
Khamis S, Chaillou S, Lamaze T (1990) CO2 assimilation and partitioning of carbon in maize plants deprived of orthophosphate. J Exp Bot 41:1619–1625
Kleinert A, Venter M, Kossmann J, Valentine A (2014) The reallocation of carbon in P deficient lupins affects biological nitrogen fixation. J Plant Physiol 171:1619–1624
Kouas S, Labidi N, Debez A, Abdelly C (2005) Effect of P on nodule formation and N fixation in bean. Agron Sustain Dev 25:389–393
Krapp A, Stitt M (1995) An evaluation of direct and indirect mechanisms for the sink regulation of photosynthesis in spinach—changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195:313–323
Lauer MJ, Pallardy SG, Blevins DG, Randall DD (1989) Whole leaf carbon exchange characteristics of phosphate deficient soybeans (Glycine max L.) Plant Physiol 91:848–854
Le Roux MR, Ward CL, Botha FC, Valentine AJ (2006) Routes of pyruvate synthesis in phosphorus-deficient lupin roots and nodules. New Phytol 169(2):399–408
Le Roux MR, Khan S, Valentine AJ (2008) Organic acid accumulation may inhibit N2 fixation in phosphorus-stressed lupin nodules. New Phytol 177(4):956–964
Lea PJ, Forde BG (1994) The use of mutants and transgenic plants to study amino acid metabolism plant. Cell Environ 17:541–556
Liu Y, Villalba G, Ayres RU, Schroder H (2008) Global phosphorus flows and environmental impacts from a consumption perspective. J Ind Ecol 12:229–247
Lodwig E, Poole P (2003) Metabolism of rhizobium bacteroids. Crit Rev Plant Sci 22:37–78
Lynch J, Lauch HA, Epstein E (1991) Vegetative growth of the common bean in response to phosphorus nutrition. Crop Sci 31:380–387
Marschner H (1995) Mineral nutrition of higher plants. Academic, London
Marschner H, Dell B (1994) Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159:89–102
Miao SJ, Qiao YF, Han XZ, An M (2007) Nodule formation and development in soybeans (Glycine max L.) in response to phosphorus supply in solution culture. Pedosphere 17:36–43
Mousavishalmani MA, Sagheb N, Hobbi MS, Rafh H, Khorasani A (2002) Fertilizer P distribution into different parts of plant and soil under trickle fertigation on tomato by 32P. In: 17th Word Congress Soil Science, Paper 2286, Thailand
Nasr Esfahani MN, Kusanob M, Nguyend KH, Watanabee Y, Ha CV, Saitoc K, Sulieman S, Herrera-Estrella L, Tran LSP (2016) Adaptation of the symbiotic Mesorhizobium–chickpea relationship to phosphate deficiency relies on reprogramming of whole-plant metabolism. Proc Natl Acad Sci USA 113(32):E4610–E4619
Peoples MB, Herridge DF, Ladha JK (1995) Biological nitrogen fixation: an efficient source of nitrogen for sustainable agricultural production. Plant Soil 174:3–28
Pieters AJ, Paul MJ, Lawlor DW (2001) Low sink demand limits photosynthesis under Pi deficiency. J Exp Bot 52:1083–1091
Plaxton WC (2004) Plant response to stress: biochemical adaptations to phosphate deficiency. In: Goodman R (ed) Encyclopedia of plant and crop science. Marcel Dekker, New York, pp 976–980
Plesnicar M, Kastori R, Petrovic N, Pankovic D (1994) Photosynthesis and chlorophyll fluorescence in sunflower (Helianthus annuus L.) leaves as affected by phosphorus nutrition. J Exp Bot 45:919–924
Qiu J, Israel DW (1992) Diurnal starch accumulation and utilization in phosphorus-deficient soybean plants. Plant Physiol 98:316–323
Radin JW (1990) Responses of transpiration and hydraulic conductance to root temperature in nitrogen- and phosphorus-deficient cotton seedlings. Plant Physiol 92:855–857
Ragothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50:665–693
Rao IM, Terry N (1989a) Leaf phosphate status, photosynthesis and carbon partitioning in sugar beet. I. Changes in growth, gas exchange and Calvin cycle enzymes. Plant Physiol 90:814–819
Rao IM, Terry N (1989b) Leaf phosphate status, photosynthesis and carbon partitioning in sugar beet. II. Diurnal changes in sugar phosphates, adenylates and nicotinamide nucleotides. Plant Physiol 90:820–826
Rao IM, Terry N (1990) Leaf phosphate status, photosynthesis and carbon partitioning in sugar beet. III. Diurnal changes in carbon partitioning and carbon export. Plant Physiol 92:29–36
Rao IM, Terry N (1995) Leaf phosphate status, photosynthesis, and carbon partitioning in sugar beet. IV: changes with time following increased supply of phosphate to low phosphate plants. Plant Physiol 107:1313–1321
Ribet J, Drevon JJ (1995) Increase in conductance to oxygen and in oxygen uptake of soybean nodules under limiting phosphorus nutrition. Physiol Plant 94:298–304
Ribot C, Wang Y, Poirier Y (2008) Expression analysis of three members of the AtPHO1 family reveal differential interactions between signaling pathways involved in phosphate deficiency and the responses to auxin, cytokinin, and abscisic acid. Planta 227:1025–1036
Rogers A, Gibon Y, Stitt M, Morgan PB, Bernacchi CJ, Ort DR, Long SP (2006) Increased C availability at elevated carbon dioxide concentration improves N assimilation in a legume. Plant Cell Environ 29:1651–1658
Sawada S, Usuda H, Tsukui T (1992) Participation of inorganic orthophosphate in regulation of the ribulose-1,5-bisphosphate carboxylase activity in response to changes in the photosynthetic source-sink balance. Plant Cell Physiol 33:943–949
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453
Scholz RW, Wellmer FW (2016) Comment on: “recent revisions of phosphate rock reserves and resources: a critique” by Edixhoven et al. (2014)—clarifying comments and thoughts on key conceptions, conclusions and interpretation to allow for sustainable action. Earth Syst Dynam 7:103–117
Schröder JJ, Cordell D, Smit AL, Rosemarin A (2010) Sustainable use of phosphorus, EU tender EN V. B.1/ETU/2009/0025. Plant Research International, Business Unit Agrosystems, Wageningen UR, Wageningen
Schulze J, Drevon JJ (2005) P-deficiency increases the O2 uptake per N2 reduced in alfalfa. J Exp Bot 56:1779–1784
Schulze J, Adgo E, Merbach W (1999) Carbon costs associated with N2 fixation in Vicia faba L. and Pisum sativum L. over a 14-day period. Plant Biol 1:625–631
Schulze J, Tesfaye M, Litjens R, Bucciarelli B, Trepp G, Miller S et al (2002) Malate plays a central role in plant nutrition. Plant Soil 247:133–139
Schulze J, Mohamed MAN, Carlsson G, Drevon JJ (2011) Phosphorus deficiency decreases nitrogenase activity but increases proton efflux in N2-fixing Medicago truncatula. Plant Physiol Biochem 49:458–460
Serraj R, Sinclair TR (1996) Inhibition of nitrogenase activity and nodule oxygen permeability by water deficit. J Exp Bot 47:1067–1073
Smit AL, Bindraban PS, Schröder JJ, Conijn JG, Van Der Meer HG (2009) Phosphorus in agriculture: global resources trends and developments. Plant Research International B.V, Wageningen
Smith FW, Jackson WA, van den Berg PJ (1990) Internal phosphorus flows during development of phosphorus stress in Stylosanthes hamata. Aust J Plant Physiol 17:451–464
Sulieman S, Ha CV, Schulze J, Tran L-SP (2013) Growth and nodulation of symbiotic Medicago truncatula at different levels of phosphorus availability. J Exp Bot 64(10):2701–2712
Sulieman S, Schulze J, Tran LSP (2014) N-feedback regulation is synchronized with nodule carbon alteration in Medicago truncatula under excessive nitrate or low phosphorus conditions. J Plant Physiol 171:407–410
Terry N, Ulrich A (1973) Effects of phosphorus deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol 51:43–47
Treeby MT, Van Steveninck RFM, de Vries HM (1987) Quantitative estimates of phosphorus concentrations within Lupinus luteus leaflets by means of electron probe X-ray microanalysis. Plant Physiol 85:331–334
Turnbull TL, Warren CR, Adams MA (2007) Novel mannose-sequestration technique reveals variation in subcellular orthophosphate pools do not explain the effects of phosphorus nutrition on photosynthesis in Eucalyptus globulus seedlings. New Phytol 176:849–861
United Nations, Department of Economic and Social Affairs, Population Division (2015). World population prospects: The 2015 revision, key findings and advance tables, Working paper No. ESA/P/WP.241
USGS (2011) Mineral commodity summaries, Phosphate Rock. US Geological Survey, Washington, DC
USGS (2014) Mineral commodity survey: mineral commodity summaries. US Geological Survey, Washington, DC
Vaccari DA (2009) Phosphorus, a looming crisis. Sci Am 300:42–47
Vadez V, Rodier F, Payre H, Drevon JJ (1996) Nodule permeability to O2 and nitrogenase-linked respiration in bean genotypes varying in the tolerance of N2 fixation to P deficiency. Plant Physiol Biochem 34(6):871–878
Vance CP (2001) Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol 127:390–397
Vance CP, Graham PH, Allan DL (2000) Biological nitrogen fixation: phosphorus Ba critical future need? In: Pederosa FO, Hungria M, Yates MG, Newton WE (eds) Nitrogen fixation: from molecules to crop productivity. Kluwer, Dordrecht, pp 506–514
Vardien W, Mesjasz-Przybylowicz J, Przybylowicz WJ, Wang YD, Steenkamp ET, Valentine AJ (2014) Nodules from Fynbos legume Virgilia divaricata have high functional plasticity under variable P supply levels. J Plant Physiol 171:1732–1739
Vardien W, Steenkampb ET, Valentine AJ (2016) Legume nodules from nutrient-poor soils exhibit high plasticity of cellular phosphorus recycling and conservation during variable phosphorus supply. J Plant Physiol 191:73–81
von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant Soil 171:1–15
Vysotskaya LB, Trekozova AW, Kudoyarova GR (2016) Effect of phosphorus starvation on hormone content and growth of barley plants. Acta Physiol Plant 38:108
Walker DA, Sivak MN (1985) Can phosphate limit photosynthetic carbon assimilation in vivo? Physiol Veg 23:829–841
Walker DA, Sivak MN (1986) Photosynthesis and phosphate: a cellular affair? Trends Biochem Sci 11:176–179
Warren CR (2011) How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree Physiol 31:727–739
Warren CR, Adams MA (2002) Phosphorus affects growth and partitioning of nitrogen to Rubisco in Pinus pinaster. Tree Physiol 22:11–19
Xu HX, Weng XY, Yang Y (2007) Effect of phosphorus deficiency on the photosynthetic characteristics of rice plants. Russ J Plant Physiol 54:741–748
Yang N, Zavisic A, Pena R, Polle A (2016) Phenology, photosynthesis, and phosphorus in European beech (Fagus sylvatica L.) in two forest soils with contrasting P contents. J Plant Nutr Soil Sci 179:151–158
Zheng SJ (2010) Crop production on acidic soils: overcoming aluminium toxicity and phosphorus deficiency. Ann Bot 106:183–184
Acknowledgments
This work was funded by the Spanish National Research and Development Programme (AGL2014-56561-P), the “I-COOP Suelos y Legumbres” Programme (2016SU0016) and their corresponding FEDER funding, and Aragón Government (A03 research group).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Sanz-Saez, A., Morales, F., Arrese-Igor, C., Aranjuelo, I. (2017). P Deficiency: A Major Limiting Factor for Rhizobial Symbiosis. In: Sulieman, S., Tran, LS. (eds) Legume Nitrogen Fixation in Soils with Low Phosphorus Availability. Springer, Cham. https://doi.org/10.1007/978-3-319-55729-8_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-55729-8_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55728-1
Online ISBN: 978-3-319-55729-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)