Abstract
This chapter provides an overview of innovative strategies in the field of oral cavity regeneration. In particular, the benefits of using injectable biomaterials in dentistry, especially for their restorative and regenerative applications are reported. Here also a detailed analysis of several experimental models for teeth regeneration is carried out. Over the past decade, recent findings in stem cell biology and tissue engineering suggest novel approaches for the regeneration of dental tissues or entire new teeth. Stem cells have the potential to self-renew and to give rise to a variety of cell types that ensure tissue regeneration. This chapter provides the various stem cell-based treatment strategies that could be translated in dental practice. The clinical translation of stem-cell-based oral cavity regeneration requires the use of injectable scaffolds that are able to reproduce dental pulp-like microenvironment in order to make odontoblast precursors capable of generating new tubular dentin. In this context, a considerable attention has been given to sol-gel process a new strategy of manufacturing composite biomaterials useful in periodontology and in oral and maxillofacial surgery. For example, nano-hydroxyapatite (the main constituent of the mineral part of teeth) obtained by using sol-gel process showed significant improvements in its mechanical and biological properties. Recent studies demonstrated that the sol-gel process confers distinctive features to composite materials for teeth repair, thus contributing to a more efficient replacement of oral cavity bone tissue.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Root Canal
- Dental Pulp
- Calcium Phosphate Cement
- Dental Pulp Stem Cell
- Stem Cell From Human Exfoliate Deciduous
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
3.1 Introduction
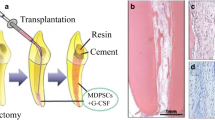
The dentistry health is critical to ensure life quality. Oral cavity defects often raise risk of several disorders including heart diseases [1]. As life expectancy increases the requirement for new bone substitute for tooth is growing very rapidly in the last decade. As a result, there is a great request of biomaterials with detailed properties such as anti-inflammatory, antibacterial and regenerative properties [2]. Currently people with a greater loss of alveolar bone has a risk 6.6 times higher of suffering from heart attack and stroke compared to people who have a healthy mouth. This correlation is more significant in younger people and may be more direct because mouth microorganisms are able to spread easily to the heart. The disorders caused by mouth microorganisms concern especially heart valve defects (such as mitral valve prolapse) because the germs are located directly on the valve, turning a trivial infection of the mouth in a much more serious disease such as endocarditis [3]. Another important direct binding between the heart and the mouth is the pain. The toothache is considered one of the most severe pain. It is well known that all particularly strong pain stimuli can cause a narrowing of the blood vessels. This reduces the normal blood supply to the heart. Vasoconstriction leads to increased blood pressure and may increase the risk of heart damage. It is necessary to prevent infections and dental problems that can cause intense pain, especially in the presence of risk factors for cardiovascular disease. Tooth loss is caused by periodontitis (i.e., a severe inflammation of the periodontium), advanced carious lesions, age-related alternations, or cancer [4]. Hence, the therapy of oral (traumatic and degenerative) diseases which lead to tooth loss including alveolar resorption is crucial. Oral disorders include periodontal disease that is an infectious, complex, multifactorial, chronic inflammatory disease of supporting periodontal tissues. Periodontal chronic inflammation not only damages the bone morphology but also leads to the reduction in bone height [5]. Different issues are associated to chronic periodontal disease: loss of attachment due to destruction of periodontal ligament, loss of adjacent supporting bone, a period of rapid destruction localized. In the case of deep intrabony defects the regeneration is difficult to attain because anatomy impedes the accessibility and obstructs the integration of the grafted material into the physiological architecture [6]. The oral surgery is yet considered the first approach to treat tooth degenerative diseases. In recent years, considerable attention has been given to regenerative medicine and tissue engineering in order to replace oral tissues. In this context, the main challenge in tissue engineering is to introduce biomaterial-based techniques which stimulate stem cell response in terms of oral tissue regeneration. Repair of dental pulp and periodontium is considered an enormous clinical challenge since human teeth have a very limited capacity to regenerate [7]. Teeth regeneration needs a big knowledge of the cellular and molecular events linked to odontogenesis. It is well known that mesenchymal cells give rise to the dental pulp, the dentin-secreting odontoblasts, and the periodontal ligament cells that anchor the tooth to the surrounding alveolar bone. As a result, the dental pulp is capable to generate a connective tissue that conveys vascularization and innervation and hosts stem cells, as well as the dentin [4]. Root growth, cementum matrix deposition, and periodontium formation occur simultaneously to dental pulp innervation [8]. Dental pulp integrity is crucial because it provides trophic support, sensation, and defense against the various pathogens; in fact, devitalized teeth are subject to severe complications that cause tooth fragility and fracture [9]. Hence, the maintenance of dental pulp vitality has a prominent role in endodontic clinics (Fig. 3.1).
Current regenerative therapies in dentistry involve biomaterials and implants with still questionable efficacy and durability [10]. Moreover, these treatments do not preserve the appropriate physiological function of the tooth organ. For this reason, there is an increasing need for new techniques based on biomaterial enabling a balance between new dental tissue formation and unaltered physiological functions of the tooth organ [11]. The endodontic surgery plays a key role in the treatment of traumatic or degenerative diseases that lead to a tissue loss and utilizes techniques that have been improved over time.
Since 1990s, numerous materials for supporting cell attachment, growth, and differentiation, as well as novel stem cell sources and bioactive molecules are identified and tested in order to improve tissue regeneration after lesions due to trauma and/or diseases. In this context, scaffolds in regenerative dentistry can repair dental tissue damaged by inflammation and/or trauma. Inflammation often causes pulp necrosis thus promoting the death of odontoblasts and tooth fracture. The tooth structure is hard to regenerate for the presence of dentin. In fact the dentin is a substance produced only by odontoblasts and consequently dentin-like tissue can be released only by odontoblast-like cells. The researchers developed new experimental models for dentin-like tissue regeneration through the combination of three key elements for tissue regeneration, namely, stem cells, bioactive molecules (e.g., growth factors), and scaffolds [12]. Scaffolds mimicking extracellular-matrix endow mechanical support, promote biological response and regulate bioactive molecule effects [13].
A wide variety of polymer scaffolds—both synthetic (e.g., poly[lactic] acid) and natural (e.g., collagen), ranging from macroporous structures obtained through salt leaching/solvent casting and gas foaming, to nanofibrous scaffolds processed via electrospinning, self-assembly, and phase-separation—have been realized for regeneration of the pulp-dentin complex [14,15,16]. In regenerative medicine, medical devices are usually realized on the basis of a particular approach that utilizes specific bioactive, biodegradable synthetic or natural scaffolds combined with cells and/or biological molecules, to replace damaged tissue site. In medical research over the past 50 years, different biomaterials in order to replace tissue function, have been identified. Starting from 1950s, there was a predominant use of metal implants and associated devices with a good effectiveness on local tissues. Throughout the 1970s and 1980s, there was a wide use of polymers and synthetic materials for enhancing cell biological responses. Recently, there has been an increasing interest in the design of both natural and degradable scaffolds. These scaffolds are gaining more functions over the time. They are becoming: in three dimensions, structurally more acceptable, able to totally regenerate tissue [17].
At first tissue engineering proposed the use of platelet concentrates, which favored and accelerated the post-surgical with a lot of benefits for patients. These platelet concentrates have been enriched with growth factors that promote tissue regeneration. Many authors have emphasized the advantages of the use of growth factors in tissue repair processes. The first studies were published on the use of growth factors (GFs) contained in platelet gel, called Platelet-Rich Plasma (PRP), which required a complex and expensive protocol for its production [18, 19]. The evolution of the PRP was the PRGF (Plasma Rich in Growth Factors) containing a higher concentration of growth factors. Moreover, the PRGF has produced by using a procedure relatively faster. Marrelli et al. have shown that the filling with PRF of a large osteolytic cavity promoted complete bone reformation [20]. Tatullo et al. have demonstrated the osteoinductive potential of PRF related to neoangiogenic ability and concentration of GFs that promoted the totipotent cell migration and activation of pre-osteoblastic cells present in the surgical site [21]. In fact, PRF when used as a membrane or as a grafting material promotes cell events such as osteoblast proliferation leading to mineralized tissue formation [22]. The latest discoveries related to the use of scaffolds and/or stem cells in regenerative endodontics have been focused on injectable materials synthesis because these materials, besides inducing cell response in terms of proliferation, adhesion and differentiation, are capable of controlling growth factor delivery and angiogenesis more effectively than other materials. Gelatin produced by the partial hydrolysis of collagen plays a pivotal role as biomaterial for tissue regeneration due to its useful properties such as biodegradability, biocompatibility and anti-immunogenicity [12]. Recent findings showed that also alginate and/or chitosan (natural polymers) are useful to achieve injectable biomaterial based scaffold for clinical applications aimed to regenerate teeth including dentinal-wall-thickening, root maturation, and, in the same cases, the formation of reparative cementum-like tissue [23, 24].
3.2 Mesenchymal Stem Cells: Tools for Tissue Regeneration in Dentistry
Many research studies have been performed on MSC capability of generating several tissue types including oral tissue. It was widely reported that MSC isolated from bone marrow in combination with scaffolds and growth factors promote bone repair in several in vivo and in vitro experimental models [25]. These studies demonstrated that MSC residing in the oral cavity represent a source for formation of new connective tissues such as dentin, cementum and periodontal ligament [26]. Nowadays the frontier of regenerative medicine is represented by the individuation of the ideal scaffold that enhances MSC residing response in terms of cell growth, spreading, adhesion and differentiation. Phenotypically, MSCs express the CD13, CD29, CD44, CD59, CD73, CD90, CD105, CD146 and STRO-1 surface antigens, and they do not express CD45 (leukocyte marker), CD34 (the primitive hematopoietic progenitor and endothelial cell marker), CD14 and CD11 (the monocyte and macrophage markers), CD79 and CD19 (the B cell markers), or HLA class II. Investigations on MSC from oral origin began in 2000 and oral tissues appear simply available for dentists and a rich source for mesenchymal stem cells [27]. Most recent approaches aimed to tissue regeneration are performed by using MSCs taken from sites that are even more accessible and rich in stem cells: the oral cavity represent an important source of MSCs due to its easily accessibility to the surgeon. In oral cavity tissue regeneration exists naturally thanks to the ability of stem cells to renew themselves indefinitely and differentiate into multiple more specialized cell phenotypes. However, these regenerative mechanisms decrease with age and cells lose the capacity to repair damaged tissues [28].
The regenerative medicine introduced the combination of biomaterials, growth factors and stem cells for avoiding the lack of “self-renewal” in damaged tissue [29]. Recently, different materials with optimal physical and mechanical features have been identified. These biomaterial-based scaffolds used in tissue engineering approaches, have been produced using natural or synthetic polymers that are biocompatible and biodegradable. Scaffold properties are crucial for enhancing MSC biological response (Fig. 3.2). Furthermore, the stem cells for regenerative medicine should comply with the following features: they should be in abundant number, they should be able to differentiate in multiple cell lineages, they can be isolated by minimally invasive procedure, produced according to GMP (Good manufacture Practice) and transplanted safely [30, 31].
In the last decade, three main types of stem cells useful for tissue repair were identified: (1) the embryonic stem cells derived from embryos (ES); (2) the adult stem cells that are derived from adult tissue; and (3) the induced pluripotent stem (iPS) cells that have been produced artificially via genetic manipulation of the somatic cells [32]. ES and iPS cells are pluripotent stem cells because they can differentiate into all types of cells from all three germinal layers. By contrast, adult stem cells are multipotent because they can only differentiate into a restricted number of cell types. It is well known that each tissue consists of a specific area named “stem cell niche” containing adult stem cells. The first time MSCs were isolated from bone marrow by Friedenstein et al. in 1974 [33]. Currently, MSCs can be isolated from different tissues such as peripheral blood, umbilical cord blood, amniotic membrane, adult connective, adipose and dental tissues [34]. Mesenchymal stem cells (MSCs) represent an advantageous therapeutic option for dental defects in presence of specific biomaterials that can manipulate the fate of stem cells leading to high quality tissue regeneration [35]. Nowadays, in bone tissue engineering, encapsulating the cells within hydrogel biomaterials is the major challenge because stem cell encapsulation in hydrogels prevents also the host pro-inflammatory response. Besides controlling the fate of stem cells, the biomaterials play a key role in regulating MSC physiological functions such as survival and host immune system control [36].
It is well known that pro-inflammatory mediators such as TNF-α (tumor necrosis factor alpha) and IFN-γ (Interferon gamma) induced down-regulation of osteogenesis thus inhibiting MSC-mediated bone regeneration [37]. Hence, by using encapsulating hydrogel biomaterials is possible to protect MSCs from the host immune cell/cytokine insult and regulate the crosstalk between immune cells and MSCs. For this purpose several preclinical immunocompromised animal models have been carried out for testing different types of scaffolds and stem cell sources in association with growth factors [12].
Most studies [38, 39] are focus on modification of the scaffold to enhance odontogenic differentiation and biomineralization. At present the effect of matrix stiffness on MSC fate in terms of odontogenic differentiation is still largely unclear. However, a study of Engler et al. showed that the elasticity of the matrix influences the differentiation of MSCs into osteoblast-like-phenotype in an ascending manner, with the stiffest matrices supporting MSC differentiation to osteoblasts [40]. Recently, MSC-like cells exhibited a tumorigenic potential but they might lose carcinogenic activities, implanting them safer into humans [41]. For this purpose, in a recent research the generation of iPSCs by combining primary human gingival fibroblasts and episomal plasmid vectors has been assessed. Such iPSCs could represent a promising source of stem cells in order to evaluate SC potential for future clinical applications.
Numerous investigations for evaluating the in vivo application of MSCs isolated from the oral cavity were carried out on animal models. MSCs isolated from the gingiva showed self-renewal and multipotent differentiation capacity similar to that of MSCs [42]. Moreover, MSCs isolated from the salivary glands could generate the salivary gland duct cells as well as mucin and amylase producing acinar cells in vitro [43]. In addition, MSCs isolated from peri-osteum are able to differentiate into bone tissue cells [44]. Unlike bone marrow that is a not easily accessible tissue, the orofacial tissues are the most accessible stem cell sources. MSCs can be isolated also from periapical cysts (hPCy-MSCs) thus overcoming surgical methods or tooth or pulp extraction [45]. MSCs obtained from the periapical cysts can be simply expanded and represent a promising source of adult stem cells in dentistry for oral tissue regeneration.
Hence, stem cell-based therapies are very promising long-term alterative in dentistry since they could restore dental tissues keeping structural integrity and physiological functions of teeth. In vivo studies confirmed the successful of stem cell-based therapies in dentistry not only in animal models but also in humans. Stem cells could be used for several applications in dentistry such as reestablishment of dental pulp vitality and new dentin formation. The use of stem cell-based strategies has started to be applied in endodontic clinics. The main goal after tooth loss would be the regeneration of an entire tooth by using stem cell-based approaches. The distinction of various dental stem cell populations as well as their behavior after transplantation in ectopic sites keys a pivotal role in applying these novel approaches. Moreover, the innervation and vascularization control stem cell niche homeostasis, thus influencing stem cell fate and behavior [46]. Despite the limitations related to the translation of stem cell-based approaches into the clinics, these emerging strategies represent the future of dentistry that will benefit millions of patients worldwide. Due to the limitations of cell injection therapy, the investigation of biological mechanisms underlying tissue regeneration is of primary importance. In oral regenerative medicine the most likely candidate for such therapies remains the human oral mucosa-/gingiva-derived MSCs due to their immunomodulatory and anti-inflammatory properties. In fact, MSCs can modulate the intensity of immune response by inducing T-cell apoptosis, which have a great therapeutic potential in terms of antinflammatory effect when utilizing biomaterials for tissue engineering applications [28]. In order to generate a new oral tissue MSCs will be isolated, expanded in culture and finally seeded within or onto a natural or synthetic scaffold that can reproduces the shape of the newly forming tissue and then the newly formed “organoid” can be transplanted into the patient. Another opportunity is to directly implant acellular scaffolds into the oral defect thus the body cells can populate the scaffold to form the new tissue in situ. In this context, many authors have highlighted a relevant synergistic role of biological molecules for cell-based therapies in order to achieve properly functioning dental tissue regeneration.
3.3 Injectable Scaffolds in Dentistry: State of Art
3.3.1 Injectable Polymeric Scaffolds
No single implantable scaffold involved in the functional regeneration of the pulp-dentin complex exists. Tissue-engineering-based strategies for regenerative endodontics include very promising injectable-based scaffold. Injectable biomaterials allow the incorporation and the release of therapeutic agents, such as antimicrobial and anti-inflammatory drugs thus promotingoral cavity disinfection, as well as bioactive molecules that can trigger stem cell differentiation to aid in regeneration of the pulp-dentin complex. More recently, injectable electrospun-based scaffolds [47] have also shown an excellent structural stability over time, with better chances for overcoming the adaptation issue associated with initial testing of macroporous scaffolds [14, 15]. Notably, the use of injectable hydrogel polymers shows advantages compared to the use of non-injectable scaffolds because of their capability of intracanal delivery, which allows stem cell niche formation [16, 48]. Moreover, drugs such as antibiotics may also be incorporated into injectable hydrogel polymers, thus treating oral cavity infections. In addition, growth factors may be encapsulated into hydrogels laded to the neovascularization and regeneration of tissues relevant to the dentin-pulp complex [49, 50]. Recently several evidences on potential clinical impact of a very promising hydrogel-based nanofibrous scaffold named Puramatrix™ have been reported. Puramatrix™, is a hydrogel bioactivated through a peptide that, upon interaction with physiological conditions, polymerizes and forms a biodegradable nanofiber hydrogel scaffold [16]. This mechanism favors clinical application that requires not only a biocompatible matrix, but also that can be rapidly formed. It was shown that Puramatrix™ supports dental pulp stem cell survival and proliferation in vitro [48]. The commercially available peptide hydrogel scaffold PuraMatrix™, a synthetic matrix comprising a repeated polymer of four amino acids (R-A-D-A) and water, supported the development of a capillary network when the HUVEC are co-cultured with DPSCs. Furthermore, several reports have demonstrated that the HUVECs had an inducing effect on mineralization by the DPSCs due to a direct cell–cell contact of HUVECs with osteoblasts. In vivo studies confirmed that the transplantation of PuraMatrix™ allows the partial regeneration of pulp-like tissue within the root canals. PuraMatrix™ hydrogel, through a pre-vascularization process, can enhance vascularization within a cell construct, because the regeneration of full-length pulps is inhibited when only the apical region is available for vascular connection. Hence, injectable systems like PuraMatrix™ is particularly attractive for clinical translation of dental pulp regeneration, because it can be easily realized with growth factors or drugs and cells by simple mixing. Moreover, PuraMatrix™ can conform to the variable shape of the pulp chamber, following injection [51]. In the design of the scaffold for dental pulp tissue engineering, to overcome the disadvantages associated to the use of natural biopolymer gels (collagen, Matrigel, PuraMatrix, and hyaluronic acid), which do not tune the mechanical properties independently from matrix composition and architecture, semisynthetic hydrogels have been realized. For example, PEG-fibrinogen (PF) based scaffold is able to retain mechanical properties by the addition of cross-linker that controls the hydrogel cross-linking degree, while maintaining a constant fibrinogen backbone.
These mechanical properties of PEG-fibrinogen confer to the structure biofunctional features that influence adhesion, proliferation and differentiation of dental stem cells and progenitors. Collectively, the injectable PF hydrogels are cytocompatible and determine an increase of odontogenic differentiation but lesser extent of proliferation. Notably, the injectable PF hydrogels are able to upregulate Col I gene expression, one of the most important components of extracellular matrix (ECM) of the demineralized dentin. These PF properties suggest that hydrogels as scaffolds can support the formation of new tubular dentin and pulp tissue complex for dental pulp regeneration [52].
Subperiosteal tunnelling injection is a method that allows bone regeneration in a minimally invasive manner. However, because of the poor plasticity of most of the injectable bone substitute materials used for this protocol the technique has not been used widely. To overcome this problem in a recent study authors have been developed an injectable, sol-gel reversible thermosensitive alginate hydrogel. The flowable material obtained by using sol-gel transformation was injected in vivo through a syringe needle into tissue and at body temperature, in situ the biomaterial turned into a gel form and was stable on the bone surface. Alginate based hydrogel showed a degradation time of 28 days matching osteogenesis and retains RhBMP-2 through an electrostatic interaction thus providing sustained rhBMP-2 release. BMP-2 in presence of this alginate based-hydrogel stored its bioactivity, increased the ALP activity of hBMSCs until day 15 and promoted mineralization processes. Also marker of mature osteoblasts such as osteopontin and osteocalcin were induced in presence of alginate hydrogel and BMP-2 [53].
In recent studies, it is reported that also scaffolds made of chitosan form a dentine-pulp complex in vivo [24] in presence of stem cells and hydroxyapatite (HA).
In a specific study, porous chitosan/collagen scaffolds were manufactured by using a freeze-drying process, and then were loaded with the plasmid vector encoding human bone morphogenetic protein-7 (BMP-7) gene. These scaffolds in vitro and in vivo enhanced dental stem cell response in terms of oral tissue regeneration. In particular, chitosan/collagen-based scaffolds enhanced DPSCs differentiation toward an odontoblast-like phenotype in vitro and in vivo. Moreover chitosan/collagen-loaded with the plasmid vector encoding human bone morphogenetic protein-7 (BMP-7) gene showed good properties as substrate for gene delivery [54].
3.3.2 Injectable Calcium Phosphate Scaffolds
Since 1982 calcium phosphate cements (CSCs) have been investigated extensively as injectable bone replacement biomaterials due to their successful properties. In fact, CSCs possess a chemical composition similar to the mineral component of bone, a proven biocompatibility, osteoconductive capabilities and fast setting times (<5 min). Moreover, CPCs showed higher solubility than apatite and resorb more rapidly. Thus, CPCs have attracted considerable attention in recent years for orthopedic and cranio-maxillofacial applications [55].
In this context, some authors have proposed the regeneration of the periodontium using the enamel matrix (EMD) derivative in combination with injectable bone cements. By combining EMD and CaP is possible to obtain a synergistic effect, stimulating both soft periodontal tissue healing and bone regeneration. This model is cost-effective and especially easy to apply in patients [56]. In order to obtain fast resorption of the grafts, the CaP cement was tuned with a low molecular PLGA. In this device, CaP appeared to act much like a “membrane” in supplying wound stabilization. Besides as wound stabilizer, CaP is the major determining factor of cementum formation and bone regeneration due to its osteoconductive properties. Because the use of an injectable calcium phosphate cement accelerates bone formation, the combination with EMD is a promising curative strategy for bone tissue regeneration in the periodontium [56].
Another experimental study in dogs demonstrated for the first time that the use of an injectable bone substitute, composed of a calcium phosphate ceramic and a polymeric carrier, favors bone regeneration around dental implants immediately placed into fresh extractions sockets [57].
After calcium phosphate-based ceramics such as hydroxyapatite (HA), beta-tricalcium phosphate (β-TCP) and the HA/β-TCP association that replaced bone autografts thanks their chemical composition closely related to that of bone mineral, a ready-to-use injectable bone substitute (IBS) based on an association of BCP granules with a cellulosic hydrogel has been developed [58]. This IBS has been ranked among innovative biomaterials with osteoconductive properties in tooth bone regeneration. The effectiveness of IBS is comparable to that of conventional implants placed after a 3-month healing period thus encouraging its use in clinics. Furthermore, IBS confirmed its osteoconductive potential because the newly formed bone contains the same Ca and P values as in basal bone. Thus, IBS may satisfy immediate implantation requirements. Hence, the advantages of an injectable bone substitute (IBS) appear to be clear because these composite biomaterials are able to promote bone regeneration immediately placed after tooth extraction [59]. For this reason, injectable composite biomaterials are becoming of primary importance for clinical applications such as socket filling and pre-implant reconstruction. Novel cell aggregate-loaded macroporous scaffolds combining the osteoinductive properties of titanium dioxide (TiO2) with hydroxyapatite-gelatin nanocomposites (HA-GEL) for regeneration of craniofacial defects were also approached. An in vivo study showed the applicability of these macroporous (TiO2)-enriched HA-GEL scaffolds because they were able to promote osteointegration and newly formed bone tissue production in a craniofacial defect model [60].
3.3.3 Injectable Polymeric Scaffolds for Dentin Reconstitution
The most difficult challenge in tooth regeneration is to reconstitute dentin tissue. Dentin problems involve the entire adult population and about 60–70% of the pediatric population because of the prevalence of dental caries [61]. In the tooth, the role of dentin is crucial because dentin provides strong mechanical support and protection to delicate dental pulp tissue. When dentin is damaged loses its structural integrity, the pulp is exposed and may be affected by periodontitis, and other infections [62]. Current dental treatments to cure dentin disorders include pulp capping and root canal therapy [63]. However, these treatments cause several side effects such as tooth discoloration, increased brittleness, and tooth loss [64]. Therefore, novel alternative dentin repair therapies are highly required. Dentin is hard to regenerate because dentin matrix is only secreted by odontoblasts, a terminal differentiated cell type. This cell population is present in a limited number and is complicated to isolate. Tissue engineering suggests for dentin regeneration the use of stem cells that can differentiate under odontogenic stimuli. For this purpose, porous scaffolds have been explored as a biomimetic odontogenic microenvironment to guide stem cell differentiation in odontoblastic-like phenotype cell lines. New approaches to replace damaged dentin include dental pulp stem cells (DPSCs), stem cells from the apical part of the papilla (SCAPs), and stem cells from human exfoliated deciduous teeth (SHED) in presence of a favorable microenvironment consists of a beneficial scaffolding for the cell attachment, proliferation and differentiation. To facilitate biological response in terms of cell seeding, adhesion and differentiation, scaffolds have to possess specific features such as high porosity and a high interconnection of pores thus scaffold can better mimic ECM [65]. Natural biomaterials such as gelatin, collagen, chitosan, and hyaluronic acid have been investigated for oral tissue regeneration but they present disadvantages due to their physical properties such as a poor mechanical behavior and uncontrolled degradation kinetics. To overcome the drawbacks of natural biomaterials, synthetic polymers with tailored degradation rates and high processability are increasingly introduced in tissue engineering. Hence, three-dimensional (3D) macroporous and nanofibrous PLLA scaffolds with a high porosity and well-interconnected pores have been realized for enhancing hDPSCs odontogenic differentiation [66]. Injectable formulations are preferable for dentin defects due to the small defect size and irregular defect shape. To this end, the clinical translation of stem-cells in presence of injectable scaffolds for dental pulp regeneration has been approached. The authors demonstrated that stem cells from exfoliated deciduous teeth (SHED) mixed with Puramatrix™ (peptide hydrogel) after 7 days, or when mixed with recombinant human Collagen (rhCollagen) type I after 14 days and injected into the root canals of human premolars can generate a functional dental pulp. After subcutaneous implantation in immuno-deficient mice self-assembling peptide hydrogel (Puramatrix™) and rhCollagen type I induced pulp-like tissues formation that consist of odontoblasts capable of generating new tubular dentin throughout the root canals. Surprisingly, newly formed tissue showed similar cellularity and vascularization of control human dental pulps. Moreover, the new-engineered pulp was capable of generating new dentin. The self-assembling peptide hydrogel (Puramatrix™) and rhCollagen type-I scaffold without surrounding tooth structure was not able to promote odontoblastic differentiation because is necessary dentin-derived signaling molecules [67, 68]. Interestingly, scaffolds increased expression of dentin sialophosphoprotein (DSPP) that is the first marker of odontoblastic differentiation. DSPP overexpression predicted mineralization processes [69]. Furthermore, the physical properties of the scaffold directly contributed to dental pulp tissue regeneration. Dentin stimulation plays a key role in dental pulp regeneration because dentin contains functional pro-angiogenic factors and chemotactic factors that induce blood vessels generation [70].
For dental tissue engineering, an injectable scaffold is more effective than an implantable 3D bulk scaffold because dental defects are often small and have irregular shapes. Porous microspheres are proposed as injectable cell carriers for tissue repair [71]. In fact, in a study novel injectable microspheres (NF-SMS) made of biodegradable and biocompatible poly (l-lactic acid)-block-poly (l-lysine) copolymers were tested as a cell carrier to regenerate dentin [65]. The biomimetic nanofibrous feature and the porous structure of the NF-SMS significantly improved hDPSC biological response in terms of cell attachment, proliferation and odontogenic differentiation. The diameter of NF-SMS pores is around 10–20 μm in order to facilitate the cell infiltration into the internal space. The high interconnection of pores enhanced cell-cell interaction thus promoting the activation of several differentiation pathways. Consequently cell-cell interactions, DSPP expression and odontoblast maturation were observed. Notably, NF-SMS increased not only DSPP expression but also the levels of other important osteogenic markers such as ALP, an early marker of osteogenic differentiation, that regulates organic and inorganic phosphate metabolism [71]. The expression of OCN an important late marker of mineralization during odontogenic differentiation was induced by NF-SMS. Several research studies reported the effect of various scaffolds, such as gelatin, collagen sponge, porous ceramics or fibrous titanium meshes, on hDPSCs in order to form a connective tissue than a dentin-like tissue [65], but in presence of NF-SMS the largest newly formed tissue volume was obtained. In conclusion, the injectable NF-SMS seems to create a microenvironment useful for hDPSC proliferation, odontogenic differentiation, and dentin tissue regeneration. Hence, NF-SMS showed features useful for clinical applications as an injectable cell carrier with high potential for dentin repair [71].
3.4 The Sol-Gel Approach to Prepare Calcium Phosphate Injectable Biomaterials
Sol-gel method has recently attracted much attention because is capable of improving chemical homogeneity of the resulting HA compared to conventional methods such as solid state reactions, wet precipitation, and hydrothermal synthesis. In fact, the sol-gel approach improves the conditions for the synthesis of HA thus providing a much better structural integrity compared to the defects related to plasma spraying method [72]. Moreover, the lower temperature, used during the process, allows the inclusion of thermolabile drugs and bioactive molecules (i.e. growth factors, peptides, dendrimer, antibiotics) in the variously shaped materials [72]. Furthermore, hybrid organic-inorganic materials may be formed through sol-gel method by using three different approaches. The first one is based on the dissolution of organic molecules in a liquid sol-gel [72]. The second one consists of the impregnation of a porous gel in the organic solution. In the third approach, the inorganic precursor either already has an organic group or reactions occur in a liquid solution to form chemical bonds in the hybrid gel. The sol-gel process consists of four steps: (1) the evolution of inorganic networks, (2) formation of colloidal suspension (sol), (3) the gelation of the sol to form a network in a continuous liquid phase (gel) and (4) the “aging” step (the sol-gel derived material expulses the liquid phase). Variously porous materials may be formed by sol-gel technique and the pore size depends on such factors as time and temperature of the hydrolysis and the kind of catalyst used. The sol-gel method is useful for the synthesis of hydroxyapatite (HA)-based injectable materials due to the possibility to obtain nanoparticles that are able to rapidly improve the stability at the artificial/natural bone interface [72]. Hydroxyapatite has long been among the most studied biomaterials for medical applications due to both its high biocompatibility and for being the main constituent of the mineral part of bone and teeth [73]. To overcome the limitations related to the preparation of HA by using sol-gel process such as the possible hydrolysis of phosphates, the high cost of the raw materials, a strict pH control, the vigorous agitation and a long time for, is possible to use a non-alkoxide based sol-gel approach where the calcium and phosphate precursors are calcium nitrate tetrahydrate and phosphorous pentoxide, respectively [72]. Organic-inorganic composite materials such as PCL/HA can be synthetized by sol-gel method. Sol-gel process allows mixing at molecular-level calcium and phosphorous precursors with the polymer chains in order to obtain composites having enhanced dispersion and exhibiting good interaction between the inorganic phase and the polymer matrix. A homogeneous distribution of nanoscale hydroxyapatite particles in the polymeric matrix by using sol-gel technique was observed (Fig. 3.3). This homogeneous distribution of nanoscale hydroxyapatite particles enhanced the bioactivity and the ability in bone repair of composites. In fact, these materials were able to increase osteoblast adhesion, proliferation and to inhibit osteoclast functions [72]. In addition, metals coated with nanoscale hydroxyapatite particles induced new bone formation compared to conventional apatite. Innovative injectable composite materials based on hydroxyapatite containing strontium (Sr-HA) and carbon nanotubes (CNTs) as a reinforcing component for the treatment remodeling compromised bone have been developed. Besides the conventional processes to produce HA-CNT composite materials, innovative techniques such as sol-gel have been approached to obtain an increasing of the bone mineral density and a decreasing of bone resorption by strontium intake [2]. It is well known that Strontium (Sr) plays a key role both in the stimulation of bone formation and in the reduction in bone resorption. Moreover, Sr is able to enhance the bioactivity and biocompatibility of biomaterials. Conventional processes to produce HA-CNT composite materials are based on physicochemical blending methods including ball milling [74] and mixing in solvent [75]. Initially, the sol-gel method was used in the preparation of silicate from tetraethylorthosilicate (TEOS, Si(OC2H5)4), which is mixed with water and a mutual solvent, to form a homogeneous solution. Recently, new reagents are appeared, so novel inorganic oxides and hybrid organic-inorganic materials can be synthesized using this methodology. Furthermore, the sol-gel technology provides the opportunity of working at lower temperature during the synthesis thus preventing mechanical degradation of substrates and/or of thermolable drugs and growth factors. Therefore, the literature reported that the sol-gel process leads to a high-quality HA coating after heat treatment at lower temperatures. The synthesis of HA requires a correct molar ratio of 1.67 between Ca and P in the final product. A number of combinations between calcium and phosphorus precursors were employed for sol-gel HA synthesis. However, calcium phosphate (CaP) materials show limited compressive strength and their uses are limited to non-stress-bearing applications exactly as maxillofacial surgery, or the repair of craniofacial defects and dental fillings [2]. On this basis, recent research studies are aimed to investigate the synthesis of an injectable composite material based on hydroxyapatite containing strontium (Sr-HA) and CNTs as a reinforcing component (Fig. 3.4). CNTs as a reinforcing component showed no acute toxicity and a good effect on the attachment and spreading of osteoblast cells [76]. Nayak et al. [77] have also shown that surface roughness of CNT thin films may show effects on proteins adsorption on the material surface thus improving biological response in terms of proliferation and differentiation of hMSCs into bone lineage. Moreover, a recent study [78] reported that MWCNT (multiwalled carbon nanotubes) has beneficial effects on inhibition of osteoclastic bone resorption in vivo and through the suppression of essential transcription factors involved in osteoclastogenesis in vitro. The injectable strontium-modified CaP gels reinforced with CNT material are able to induce osteogenic marker expression such as the phosphatase activity (ALP) that is one of the most widely used markers for osteogenic differentiation and is considered a necessary prerequisite for the onset on mineralization [2]. Furthermore, the expression of some bone-related molecules such as OPN and OCN was promoted in presence of the injectable strontium-modified CaP gels reinforced with CNTs thus confirming the ability of these biomaterials to support MSC differentiation toward the osteoblast-like phenotype [79]. These results suggest potential applications in regenerative endodontics of injectable hydrogels that can be dispersed inside a closed, small space, such as the root canal system. Injectable biomaterials can be involved also in angiogenic processes because they promote cell-cell and cell-extracellular matrix cross-talk. This function plays a key role in pulp regeneration because these injectable scaffolds may create an interaction between DPSCs (Dental Pulp Stem Cells) and HUVECs (Human Umbilical Vein Endothelial Cells) thus remodeling of capillary-like structures. However, it is difficult to fabricate a stable vascular network in vitro because ECs (Endothelial Cells) require specific environment elements, such as a specific pH range, signaling molecules, and growth factors, for their survival, proliferation, migration, and vascular morphogenesis.
3.5 Conclusions
The main challenge of the biomedical sciences is to regenerate all tissue types starting from an initial stem cell line by using innovative scaffolds. This goal is opening the door to new stem cells based therapies for tissue regeneration. New therapies based on combination of scaffold and stem cells could ameliorate the expectation of quality of life in more than two billion of patients undergone to a regenerative surgery. In dentistry, the aim is to simply replace damaged or degenerated tissues with MSCs from dental and oral sources. Hence, the use of injectable biomaterials is particularly attractive for dental pulp and bone tissue engineering, as they can be easily formulated with growth factors, drugs and cells by simple mixing. In conclusion, the tunable of injectable biomaterials makes them appropriate for induction of odontogenic differentiation and mineralization of human dental MSCs.
References
Drangsholt MT. A new causal model of dental diseases associated with endocarditis. Ann Periodontol. 1998;3(1):184–96.
Raucci MG, Alvarez-Perez M, Giugliano D, Zeppetelli S, Ambrosio L. Properties of carbon nanotube-dispersed Sr-hydroxyapatite injectable material for bone defects. Regen Biomater. 2016;3(1):13–23. doi:10.1093/rb/rbv026.
Wennström A, Boman UW, Ahlqwist M, Björkelund C, Hakeberg M. Perceived mental stress in relation to oral health over time in middle-aged Swedish women. Community Dent Health. 2015;32(4):241–6.
Miran S, Mitsiadis TA, Pagella P. Innovative dental stem cell-based research approaches: the future of dentistry. Stem Cells Int. 2016;2016:7231038.
Salaria SK, Ghuman SK, Kumar S, Sharma G. Management of localized advance loss of periodontal support associated grade II furcation and intrabony defect in chronic periodontitis patient through amalgamation of platelet-rich fibrin and hydroxyapatite bioactive glass composite granules. Contemp Clin Dent. 2016;7(3):405–8.
Gupta G. Clinical and radiographic evaluation of intra-bony defects in localized aggressive periodontitis patients with platelet rich plasma/hydroxyapatite graft: a comparative controlled clinical trial. Contemp Clin Dent. 2014;5(4):445–51.
Mitsiadis TA, Orsini G, Jimenez-Rojo L. Stemcell-based approaches in dentistry. Eur Cells Mater. 2015;30:248–57.
Mitsiadis TA, Harada H. Regenerated teeth:the future of tooth replacement. An update Regen Med. 2015;10(1):5–8.
Ricketts D. Management of the deep carious lesion and the vital pulp dentine complex. Br Dent J. 2001;191(11):606–10.
Jiménez-Rojo L, Granchi Z, Woloszyk A, Filatova A, Pagella P, Mitsiadis TA. Regenerative dentistry: stem cells meet nanotechnology (chapter 1). Boca Raton: Pan Stanford; 2016.
Pagella P, Neto E, Lamghari M, Mitsiadis TA. Investigation of orofacial stem cell niches and their innervation through microfluidic devices. Eur Cells Mater. 2015;29:213–23.
Albuquerque MT, Valera MC, Nakashima M, Nör JE, Bottino MC. Tissue-engineering-based strategies for regenerative endodontics. J Dent Res. 2014;93(12):1222–31.
Bottino MC, Thomas V, Schmidt G, Vohra YK, Chu TM, Kowolik MJ, et al. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—a materials perspective. Dent Mater. 2012;28:703–21.
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–9.
Huang GT. Pulp and dentin tissue engineering and regeneration: current progress. Regen Med. 2009;4:697–707.
Rosa V, Zhang Z, Grande RH, Nör JE. Dental pulp tissue engineering in full-length human root canals. J Dent Res. 2013;92:970–5.
Abou Neel EA, Chrzanowski W, Salih VM, Kim HW, Knowles JC. Tissue engineering in dentistry. J Dent. 2014;42(8):915–28.
Whitman DH, Berry R, Green D. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294–9.
Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46.
Marrelli M, Pacifici A, Di Giorgio G, Cassetta M, Stefanelli LV, Gargari M, Promenzio L, Annibali S, Cristalli MP, Chiaravalloti E, Pacifici L, Tatullo M. Diagnosis and treatment of a rare case of adenomatoid odontogenic tumor in a young patient affected by attenuated familial adenomatosis polyposis (aFAP): case report and 5 year follow-up. Eur Rev Med Pharmacol Sci. 2014;18(2):265–9.
Tatullo M, Marrelli M, Cassetta M, Pacifici A, Stefanelli LV, Scacco S, Dipalma G, Pacifici L, Inchingolo F. Platelet rich fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci. 2012;9(10):872–80.
TatulloM, MarrelliM, PaduanoF. The regenerative medicine in oral and maxillofacial surgery: the most important innovations in the clinical application of mesenchymal stem cells. Int J Med Sci2015;12(1):72–77. doi:10.7150/ijms.10706. eCollection 2015.
Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate hydrogels and TGF-β1 on human dental pulp repair in vitro. Connect Tiss Res. 2002;43:387–90.
Yang X, Han G, Pang X, Fan M. Chitosan/collagen scaffold containing bone morphogenetic protein-7 DNA supports dental pulp stem cell differentiation in vitro and in vivo. J Biomed Mater Res A. 2012; doi:10.1002/jbm.a.34064.
van den Dolder J, Farber E, Spauwen PH, Jansen JA. Bone tissue reconstruction using titanium fiber mesh combined with rat bone marrow stromal cells. Biomaterials. 2003;24:1745–50.
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55.
Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54.
Moshaverinia A, Chen C, Xu X, Ansari S, Zadeh HH, Schricker SR, Paine ML, Moradian-Oldak J, Khademhosseini A, Snead ML, Shi S. Regulation of the stem cell-host immune system interplay using hydrogel coencapsulation system with an anti-inflammatory drug. Adv Funct Mater. 2015;25(15):2296–307.
Sundelacruz S, Kaplan DL. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol. 2009;20:646–55.
Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60.
Gimble JM. Adipose tissue-derived therapeutics. Expert Opin Biol Ther. 2003;3:705–13.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20.
Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006 Dec 1;99(5):1285–97.
Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195–202.
Xiao L, Nasu M. From regenerative dentistry to regenerative medicine: progress, challenges, and potential applications of oral stem cells. Stem Cells Cloning. 2014;7:89–99.
AndersonHJ, SahooJK, UlijnRV, DalbyMJ. Mesenchymal stem cell fate: applying biomaterials for control of stem cell behavior. Front Bioeng Biotechnol2016 13;4:38. doi: 10.3389/fbioe.2016.00038. eCollection 2016.
Kovach TK, Dighe AS, Lobo PI, Cui Q. Interactions between MSCs and immune cells: implications for bone healing. J Immunol Res. 2015;2015:752510. doi:10.1155/2015/752510. Epub 2015 Apr 27.
Qu T, Jing J, Jiang Y, Taylor RJ, Feng JQ, Geiger B, Liu X. Magnesium-containing nanostructured hybrid scaffolds for enhanced dent in regeneration. Tissue Eng Part A. 2014;20(17–18):2422–33.
Qu T, Liu X. Nano-structured gelatin/bioactive glass hybrid scaffolds for the enhancement of odontogenic differentiation of human dental pulp stem cells. J Mater Chem B Mater Biol Med. 2013;1(37):4764–72.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89.
Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–23.
Zhang QZ, Nguyen AL, Yu WH, Le AD. Human oral mucosa and gingiva: a unique reservoir for mesenchymal stem cells. J Dent Res. 2012;91:1011–8.
Kishi T, Takao T, Fujita K, Taniguchi H. Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem Biophys Res Commun. 2006;340:544–52.
De Bari C, Dell’Accio F, Vanlauwe J, Eyckmans J, Khan IM, Archer CW, Jones EA, McGonagle D, Mitsiadis TA, Pitzalis C, Luyten FP. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209–21.
Marrelli M, Paduano F, Tatullo M. Cells isolated from human periapical cysts express mesenchymal stem cell-like properties. Int J Biol Sci. 2013;9:1070–8.
Kumarand A, Brockes JP. Nerve dependence in tissue,organ, and appendage regeneration. Trends Neurosci. 2012;35(11):691–9.
Baylan N, Bhat S, Ditto M, Lawrence JG, Lecka-Czernik B, Yildirim-Ayan E. Polycaprolactone nanofiber interspersed collagen type-I scaffold for bone regeneration: a unique injectable osteogenic scaffold. Biomed Mater. 2013;8:045011.
Cavalcanti BN, Zeitlin BD, Nör JE. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent Mater. 2013;29(1): 97–102.
Ishimatsu H, Kitamura C, Morotomi T, Tabata Y, Nishihara T, Chen KK, et al. Formation of dentinal bridge on surface of regenerated dental pulp in dentin defects by controlled release of fibroblast growth factor-2 from gelatin hydrogels. J Endod. 2009;35:858–65.
Nagy MM, Tawfik HE, Hashem AA, Abu-Seida AM. Regenerative potential of immature permanent teeth with necrotic pulps after different regenerative protocols. J Endod. 2014;40:192–8.
Dissanayaka WL, Hargreaves KM, Jin L, Samaranayake LP, Zhang C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng Part A. 2015;21(3–4):550–63.
Lu Q, Pandya M, Rufaihah AJ, Rosa V, Tong HJ, Seliktar D, Toh WS. Modulation of dental pulp stem cell odontogenesis in a tunable PEG-fibrinogen hydrogel system. Stem Cells Int. 2015;2015:525367.
Li Y, Fang X, Jiang T. Minimally traumatic alveolar ridge augmentation with a tunnel injectable thermo-sensitive alginate scaffold. J Appl Oral Sci. 2015;23(2):215–23.
Koop R, Merheb J, Quirynen M. Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: a systematic review. J Periodontol. 2012;83:707–20.
Prieto EM, Page JM, Harmata AJ, Guelcher SA. Injectable foams for regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6:136–54.
Oortgiesen DA, Meijer GJ, Bronckers AL, Walboomers XF, Jansen JA. Regeneration of the periodontium using enamel matrix derivative in combination with an injectable bone cement. Clin Oral Investig. 2013;17(2):411–21.
Boix D, Gauthier O, Guicheux J, Pilet P, Weiss P, Grimandi G, Daculsi G. Alveolar bone regeneration for immediate implant placement using an injectable bone substitute: an experimental study in dogs. J Periodontol. 2004;75(5):663–71.
Liu Q, Cen L, Yin S, Chen L, Liu G, Chang J, Cui L. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and beta-TCP ceramics. Biomaterials. 2008;29(36):4792–9.
Daculsi G, Uzel AP, Weiss P, Goyenvalle E, Aguado E. Developments ininjectable multiphasic biomaterials. The performance of microporous biphasic calcium phosphate granules and hydrogels. J Mater Sci Mater Med. 2010;21(3):855–61.
Ferreira JR, Padilla R, Urkasemsin G, Yoon K, Goeckner K, Hu WS, Ko CC. Titanium-enriched hydroxyapatite-gelatin scaffolds with osteogenically differentiated progenitor cell aggregates for calvaria bone regeneration. Tissue Eng Part A. 2013;19:1803–16.
Joshi N, Sujan S, Joshi K, Parekh H, Dave B. Prevalence, severity and related factors ofdentalcaries in school going children of Vadodara city—an epidemiological study. J Int Oral Health. 2013;5:35.
Srivastava R, Gupta SK, Mathur VP, Goswami A, Nongkynrih B. Prevalence of dental caries and periodontal diseases, and their association with socio-demographic risk factors among older persons in Delhi, India: a community-based study. Southeast Asian J Trop Med Public Health. 2013;44:523.
Hodgdon A. Dental and related infections. Emerg Med Clin North Am. 2013;31(2):465–80.
Huang GT. Dental pulp and dentin tissue engineering and regeneration: advancement and challenge. Front Biosci (Elite Ed). 2011;3:788–800.
Fonzar F, Fonzar A, Buttolo P, Worthington HV, Esposito M. The prognosis ofroot canal therapy: a 10-year retrospective cohort study on 411 patients with1175 endodontically treated teeth. Eur J Oral Implantol. 2009;2(3):201–8.
Kuang R, Zhang Z, Jin X, Hu J, Gupte MJ, Ni L, Ma PX. Nanofibrous spongy microspheres enhance odontogenic differentiation of human dental pulp stem cells. Adv Healthc Mater. 2015;4(13):1993–2000.
Wang J, Liu X, Jin X, Ma H, Hu J, Ni L, Ma PX. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010;6(10):3856–63.
Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nör JE. Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res. 2010;89:603–8.
Suzuki S, Sreenath T, Haruyama N, Honeycutt C, Terse A, Cho A, et al. Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biol. 2009;28:221–9.
Zhang R, Cooper PR, Smith G, Nör JE, Smith AJ. Angiogenic activity of dentin matrix components. J Endod. 2011;37:26–30.
Fang J, Zhang Y, Yan S, Liu Z, He S, Cui L, Yin J. Poly(L-glutamicacid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014;10(1):276–88.
D’Antò V, Raucci MG, Guarino V, Martina S, Valletta R, Ambrosio L. Behaviour of human mesenchymal stem cells on chemically synthesized HA-PCL scaffolds for hard tissue regeneration. J Tissue Eng Regen Med. 2016;10(2):E147–54.
Pepla E, Besharat LK, Palaia G, Tenore G, Migliau G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Ann Stomatol. 2014;5(3):108–14.
Balazsi C, Konya Z, Weber F, et al. Preparation and characterization of carbon nanotube reinforced silicon nitride composites. Mater Sci Eng C. 2003;23:1133–7.
Xia Z, Riester L, Curtin WA, et al. Direct observation of toughening mechanisms in carbon nanotube ceramic matrix composites. Acta Mater. 2004;52:931–44.
Nassar EJ, Ciuffi KJ, Calefi PS, Rocha LA, De Faria EH, eSilva MLA, Luz PP, Bandeira LC, Cestari A; Fernandes CN. Biomaterials and sol–gel process: a methodology for the preparation of functional materials (Chapter 1). Biomater Sci Eng. 2016. doi:10.5772/23202.
Aoki N, Yokoyama A, Nodasaka Y, et al. Cell culture on a carbon nanotube scaffold. Biomed Nanotech. 2005;1:402–5.
Nayak TR, Jian L, Phua LC, et al. Thin films of functionalized multiwalled carbon nanotubes as suitable scaffold materials for stem cells proliferation and bone formation. ACS Nano. 2010;4:7717–24.
Narita N, Kobayashi Y, Nakamura H, et al. Multiwalled carbon nanotubes specifically inhibit osteoclast differentiation and function. Nano Lett. 2009;9:1406–13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Fasolino, I., Raucci, M.G., Ambrosio, L. (2017). MSCs and Innovative Injectable Biomaterials in Dentistry. In: Tatullo, M. (eds) MSCs and Innovative Biomaterials in Dentistry. Stem Cell Biology and Regenerative Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-55645-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-55645-1_3
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-55644-4
Online ISBN: 978-3-319-55645-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)