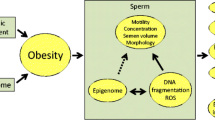
Abstract
Male obesity may have intergenerational and even transgenerational effects in mammals. Studies in rodents have revealed alterations in energy metabolism and disease susceptibility in offspring of obese males, pointing to sperm epigenetic modifications as probable causal factors. To date there is a paucity of studies examining obesity-related changes in the sperm epigenome, and the available epidemiological studies are limited. Upon fertilization, modifications to sperm nuclear and cytoplasmic factors, like RNAs, and to sperm chromatin are likely to influence early embryo gene expression. The available data on the sperm epigenome suggest that sperm DNA methylation status and small noncoding RNA expression patterns are susceptible to obesity-associated modifications. Very little information exists on potential diet-induced modification of sperm histones. Currently, the evidence is most convincing for the involvement of sperm RNA species from obese fathers in the modification of embryo development and offspring energy metabolism.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Obesity
- Sperm
- Epigenome
- High-fat diet
- sncRNA
- miRNA
- tsRNA
- piRNA
- DNA methylation
- Sperm chromatin integrity
- Histone
- Protamine
Introduction
Approximately 19% of the adult population across OECD countries are obese (OECD, Health at a Glance 2015). The high prevalence of human obesity is a major health concern due to its association with increased disease susceptibility.
Several epidemiological studies suggest an association between obesity and reduced male fertility (Nguyen et al. 2007; Ramlau-Hansen et al. 2007). During obesity, adipocyte metabolism and the profile of adipokines secreted by adipose tissue are altered, thus modifying autocrine, paracrine, and endocrine signaling with implications also for spermatogenesis (Galic et al. 2010; Lim et al. 2014). Recently, there has been an increasing awareness that paternal obesity may have inter- and transgenerational effects. Epidemiological studies have shown an association between obesity in fathers and elevated BMI in offspring (Figueroa-Colon et al. 2000; Whitaker et al. 2000). In epidemiological studies, it is difficult to separate effects of genetic factors and shared environment from potential epigenetic hereditary factors. However, experimental studies in rodents suggest that paternal diet-induced obesity has significant negative effects on early embryo development and implantation (Mitchell et al. 2011; Binder et al. 2012a, b) and may lead to increased risk of obesity and altered metabolic programming in offspring (Ng et al. 2010; Fullston et al. 2013).
Through epigenetic mechanisms, mammals are susceptible to changes in nutritional conditions. It is hypothesized that epigenetic modifications in sperm may influence embryo development and thus contribute to the observed paternal inter- and transgenerational effects. In support of the role of epigenetic modifications in paternal effects, two recent experiments using microinjection of obesity-associated sperm-derived RNAs into zygotes have shown changes in embryo gene expression and offspring metabolic phenotype (Grandjean et al. 2015; Chen et al. 2016). Furthermore, a recent cross-fostering experiment following parental pre-conceptional exposure to high-fat diet (HFD) and in vitro fertilization supports the hypothesis that sperm epigenetic changes confer an obesity-prone phenotype to offspring (Huypens et al. 2016).
During spermiogenesis, the chromatin of haploid spermatids is remodeled to allow a high degree of compaction of the spermatozoal DNA. The DNA compaction process involves epigenetic chromatin modifications, guiding a successive replacement of nucleosomal histones with transition proteins and subsequently protamines. Disruption in the histone-to-protamine exchange process may be expressed as alterations in histone retention and chromatin protamine levels and is associated with both reduced fertility and increased sperm DNA fragmentation. Interestingly, both paternal nucleosomes (van der Heijden et al. 2008) and histone modifications can be transmitted to the offspring and possibly influencing gene expression in the early embryo (Carrell and Hammoud 2010).
During spermatogenesis, the DNA methylation pattern changes (Rousseaux et al. 2005; Li et al. 2016). The dynamic changes of the DNA methylation pattern are important for the normal processes of spermatogenesis and fundamental for a successful pregnancy. Furthermore, epigenetic changes such as alterations in DNA methylation are associated with impairments in the histone-to- protamine transition (Schagdarsurengin and Steger 2016), suggesting that these measures are highly interconnected. In addition to DNA compaction, the extrusion of cytoplasm is critical for proper sperm maturation (Cooper 2011). However, some nuclear and cytoplasmic factors, including different RNA species, are retained and transferred to the zygote upon fertilization (Jodar et al. 2013). Lastly, it has been shown that factors in the seminal plasma may contribute to placental growth and affect fetal development (Bromfield 2014). This last aspect is outside the scope of the present review.
In this chapter, we describe findings from human and rodent studies obtained through a PubMed database search, of all English language articles published up to August 2016, using the search words obesity or high-fat diet in combination with sperm and epigenetic, as well as selected references referred to in the publications retrieved. The studies identified that included analysis of epigenetic modifications in sperm from either obese humans or rodents, are described in the text and are listed in Table 1.
Sperm DNA Methylation in Obesity
Most DNA methylation marks are erased during gametogenesis and fertilization. However, some marks, e.g., in imprinted genes, are resistant to the global methylation reprogramming in the embryo (Hackett et al. 2013; Wang et al. 2014). Imprinted genes are involved in regulation of early embryonic and fetal growth and are candidates for paternal modulation of the epigenome in offspring.
Human Studies
In spite of a small sample size (10 obese vs 13 lean men), Donkin and co-workers showed a different DNA methylation profile of motile spermatozoa isolated by a swim-up procedure from obese men compared to normal weight men (Donkin et al. 2016). Using reduced representation bisulfite sequencing (RRBS), a total of 9,081unique genes were found to be differentially methylated between the two groups. The number and degree of CpG methylation level differences were higher in protamine-bound regions compared to histone-retained regions. Among the differentially methylated genes, several are involved in central control of appetite including melanocortin-4 receptor (MC4R), brain-derived neurotrophic factor (BDNF), neuropeptide Y (NPY), and cannabinoid receptor type 1 (CR1), cocaine and amphetamine regulated transcript (CART). Further, genes related to obesity and metabolism pathways, like fat mass and obesity-associated (FTO) gene, carbohydrate sulfotransferase 8 (CHST8), and SH2 binding domain-containing protein 1 (SH2B1) were also differentially methylated in obese men. The authors suggest that genes affecting energy metabolism and brain development are susceptible to germ cell epigenetic modulation in response to nutritional status. However, the potential consequences on the offspring epigenome are not known.
Furthermore, in a group of six morbidly obese men, the authors investigated whether gastric bypass-induced weight reduction was associated with changes in sperm DNA methylation status. Semen samples were collected 1 week before, 1 week after, and 1 year after surgery. Already 1 week after surgery, the methylation status of 1509 genes was changed. One year after surgery, when the weight loss had stabilized and the BMI of the group had fallen from 42.6 to 33.9, 3910 genes were differentially methylated. This included several genes involved in obesity (transmembrane protein 18 (TMEM18), CHST8, SH2B1, BDNF, FTO, and MC4R). Since significant changes in DNA methylation were observed already 1 week after surgery, the authors speculate that changes in sperm DNA methylation may occur during the last stages of sperm maturation, which is supported by the observation that DNA methyltransferases are expressed throughout human spermatogenesis (Marques et al. 2011).
As part of the TIEGER (The Influence of the Environment on Gametic Epigenetic Reprogramming) male study, CpG sites in spermatozoa at 12 differentially methylated regions (DMRs) at regulatory regions of imprinted genes were examined by bisulfite pyrosequencing (Soubry et al. 2016). Twenty-three overweight/obese and 44 normal weight Caucasian men were included in the analyses. The semen samples were subjected to gradient centrifugation to select for motile spermatozoa. The study revealed changes in DNA methylation patterns at multiple DMRs in spermatozoa of overweight/obese men compared with men with normal weight. Spermatozoa of overweight or obese men had significantly lower DNA methylation levels at the maternally expressed gene 3 (MEG3), necdin (NDN), small nuclear ribonucleoprotein polypeptide N (SNRPN), and epsilon-sarcoglycan (SGCE)/paternally expressed gene 10 (PEG10). In contrast, the methylation level was higher at the MEG3-IG (intergenic) DMR and the long noncoding RNA gene H19 DMR of spermatozoa of overweight and obese men. Notably, in the group of overweight and obese men, DMRs at several of the studied genes had a tendency to be closer to the theoretically expected levels for methylation than in men of normal weight (closer to 0% methylation for maternally methylated DMRs and closer to 100% methylated for paternally DMRs). The authors speculate that the differences in DNA methylation may reflect normal epigenetic variation at the DMRs at a population level, making the phenotype flexible in response to environmental changes.
Rodent Studies
De Castro Barbosa and co-workers studied how paternal HFD affected the epigenetic signature of rat spermatozoa isolated by a swim-up procedure. The study reported altered DNA methylation signatures in the spermatozoa of male rats, and their male offspring fed an HFD compared with spermatozoa from rats fed a control diet (CD) (de Castro Barbosa et al. 2016). The authors identified 18 loci that were differentially and commonly methylated in the spermatozoa of fathers and their offspring (Fig. 1) – 11 out of the 18 DMRs were hypermethylated, while seven DMRs were hypomethylated, compared to their respective controls. Several of these DMRs were located near the transcription start sites of their respective genes suggesting gene expression regulatory roles.
Changes in gene-specific DNA methylation in sperm from rats fed a HFD and their offspring. The data used to construct the graph are a reanalysis of supplementary data from de Castro Barbosa and co-workers (de Castro Barbosa et al. 2016) on DNA methylation levels upon HFD, at 18 loci that were differentially methylated in both fathers (F0) and their male offspring (F1), in comparison with the respective controls (CD). Fold changes of the methylation levels were calculated by dividing the level of F0/F1-HFD by the level of F0/F1-CD for each gene. Fold change >1, hypermethylated and fold change <1, hypomethylated
Youngson and co-workers analyzed global DNA methylation status in the spermatozoa from rats by LC-MS assay and targeted locus-specific methylation levels of selected repetitive elements and differentially methylated regions (DMRs) by bisulfite pyrosequencing (Youngson et al. 2016). They observed that the global 5-methyl-C level in spermatozoal DNA from obese rats was slightly higher than in DNA from control rats. Further, they reported significant increases in the methylation levels of satellite repeats (SATI, ISAT, and 91ES8) in the sperm from obese rats compared with controls. However, rat male obesity did seem to be associated with altered methylation status of DMRs of three imprinted genes (H19, Peg3, and Snrpn) (Youngson et al. 2015). Similarly, no difference in DNA methylation level was observed in seven imprinted loci (H19, Zac1, Snrpn, Lit1, Peg1, Peg3, and Igf2r) in spermatozoa from mice on either a HFD or a CD (Terashima et al. 2015).
Fullston and co-workers investigated global DNA methylation level of late-elongated spermatids from male mice fed a HFD or CD, using semiquantitative immunohistochemistry analyses of whole testis sections (Fullston et al. 2013). They observed a HFD-mediated reduction in the global DNA methylation levels of both testes (27%) and late-elongated spermatids (25% reduction) compared to control samples levels. However, in a study by Binder and co-workers, global methylation of DNA extracted from spermatozoa did not differ significantly between normal and obese male mice (Binder et al. 2015).
Interestingly, the two epidemiological studies (Donkin et al. 2016; Soubry et al. 2016) suggest that obesity, as well as weight loss, has an impact on sperm DNA methylation status. However, the sample sizes in these studies are small and subject to confounding factors. From the rodent studies of spermatozoal global DNA methylation, the reported HFD-associated changes were inconsistent, possibly due to methodological differences. Furthermore, no DNA methylation differences in imprinted genes were identified in either rats or mice, in contrast to the findings in the epidemiological study by Soubry and co-workers. The sensitivity of sperm DNA methylation to obesity or overnutrition, and the potential contribution to obesity-related delays in embryo development, needs further substantiation.
Sperm Chromatin Structure in Obesity
Alterations in the composition and structural organization of sperm chromatin may affect early events in embryonic development (D’Occhio et al. 2007). The histone-to-protamine exchange is incomplete in mammals; approximately 15% and 1% residual histones remain in sperm from human and mice, respectively (Hammoud et al. 2009; Erkek et al. 2013). Nucleosomes appears to be moderately retained at unique DNA sequences and regulatory regions; however, whether nucleosome are also retained in repetitive DNA elements is debated (Dansranjavin and Schagdarsurengin 2016; Royo et al. 2016).
The importance of histone methylation for normal offspring development has been demonstrated in a mouse model producing spermatozoa with reduced H3K4me2 levels (Siklenka et al. 2015). More recently, posttranslational modifications of the transition proteins and the protamines have been described as well (reviewed in (Bao and Bedford 2016)), but their functional significance is less well established. They are, however, considered to play a role in the histone-to- protamine transition and hence regulation of the DNA compaction process. Protamine deficiency and altered ratio between protamine 1 and protamine 2 in human and mouse sperm have been associated with reduced fertility and sperm DNA fragmentation (Simon et al. 2011, 2014). Protamines in paternal DNA are replaced by histones upon fertilization.
Human Studies
Using micrococcal nuclease sequencing (Mnase-seq), Donkin and co-workers showed that the positioning of histones was similar in spermatozoa of obese men compared to normal weight men (Donkin et al. 2016). In both groups, histones were retained in approximately 2% of the genome, and the histone retention occurred mainly at CpG islands and at transcription start sites. The genes with retained histones were enriched in biological terms associated with metabolic processes and developmental processes. This study suggests that nutritional status may not influence spermatozoal nucleosome positioning.
Rodent Studies
Terashima and co-workers (Terashima et al. 2015) examined the effects of HFD on sperm histone distribution. In this study, male C57Bl/6 J mice fed a HFD or a CD for 10–12 weeks from the age of 6 weeks. The males were mated to females on CD, and a differential expression of several selected hepatic genes was reported in the offspring of the two dietary groups. Paternal sperm histones were analyzed by ChIP-seq using either a pan histone H3 antibody or an antibody directed to methylated histone H3 (H3K4me1). The authors reported an increased number of H3 peaks in sperm DNA from HFD mice suggesting an H3 enrichment compared to control mice. H3 occupancy appeared more enriched at GC-rich promotor in sperm from mice on HFD than CD. Gene ontology analysis suggested enrichment at regulatory regions for genes involved in embryonic morphogenesis and in transcription. The authors also reported a differential enrichment of H3K4me1 at promoter regions of genes encoding transcription regulators but with a limited overlap with transcription regulatory factors showing differential histone H3 retention. Of the top 5% of genes with significant differential H3K4me1 enrichment, the enrichment was found in sperm from CD mice compared to HFD mice.
In a study by Palmer and co-workers (Palmer et al. 2011), the level of sirtuin 6 was examined in male mice fed a CD or a HFD for 16 weeks. Sirtuins comprise a family of histone deacetylases whose activity is sensitive to caloric intake. Hyperacetylation of histones H3 and H4 has been shown to be required for proper histone replacement by protamines (Kurtz et al. 2009). Sirtuin 6 is involved in DNA damage repair and displays ADP-ribosyltransferase and histone 3 deacetylase activity. The study by Palmer et al. (2011) showed that the level of sirtuin 6 in transitional spermatids was decreased in HFD-fed mice, and this decrease was associated with increased levels of acetylated H3K9 in the spermatid nucleus (i.e., open chromatin structure) and increased DNA fragmentation in both spermatids and sperm.
Impairment of the histone replacement process during spermatid elongation may lead to a less compact chromatin structure in mature spermatozoa. The published literature reports different findings concerning the potential effect of obesity on the degree of compaction of sperm head DNA. However, in a meta-analysis by Campbell and co-workers, they concluded that a less compact sperm chromatin structure did not appear to be a common finding in epidemiological studies (Campbell et al. 2015). In our recent studies in mice, impaired chromatin compaction was not observed in response to a moderate HFD (Duale et al. 2014; Gutzkow et al. 2016).
The potential effects of obesity and overnutrition on the composition and modifications of sperm nuclear proteins clearly deserve further investigation.
Sperm Noncoding RNAs in Obesity
Spermatozoa contain a variety of noncoding RNAs, but so far, the focus of obesity studies has been on potential changes in small noncoding RNAs (sncRNAs), including microRNAs (miRNA), PIWI-interacting RNAs (piRNA), and transfer RNA-derived small RNAs (tsRNA). SncRNAs are implicated in epigenetic processes and may modulate DNA methylation, histone codes, and gene expression. Interestingly, sncRNAs in sperm are transferred to the zygote during fertilization, and injection of sncRNAs from obese males into zygotes has been reported to influence the embryo and offspring metabolism (Grandjean et al. 2015; Chen et al. 2016).
Human Studies
Donkin and co-workers showed that expression of 37 piRNAs was significantly altered in the motile spermatozoal fractions from obese men compared to normal weight men (Donkin et al. 2016). piRNA are predominantly found in germinal cells and involved in retrotransposon silencing, but reports on other effects of piRNAs on cellular processes are emerging (Sarkar et al. 2016). Several of the differentially expressed piRNAs were predicted to target genes belonging to the gene ontology terms “chromosome,” “chromatin,” and gene annotation term “chemdependency.” Four of the differentially expressed piRNAs in obese men (piR-31,445, piR-30,924, piR-34,810, piR-34,604) were predicted to target the CART gene, a regulator of food intake involved in obesity (Asnicar et al. 2001; Vicentic and Jones 2007). The authors speculated that the altered piRNA expression could modulate expression of genes involved in behavior and food intake and thereby predispose offspring to obesity.
Rodent Studies
In their study of rats fed an HFD, de Castro Barbosa and co-workers have identified diet-induced changes in sncRNA subtypes in spermatozoa (de Castro Barbosa et al. 2016). They observed 15 miRNAs, 41 tsRNAs, and 1092 piRNAs that were differentially expressed in spermatozoa from rats on HFD compared with rats on CD. Some significantly differentially expressed miRNAs, tsRNAs, or piRNAs in the founder mice (F0) are illustrated in Fig. 2. Some sncRNAs had similar expression profile in both spermatozoa from HFD F0 mice and their F1 offspring suggesting that these epigenetic modifications may persist across generations.
Differentially expressed sncRNAs in sperm from rats on HFD compared with rats on CD. The supplementary data from de Castro Barbosa and co-workers (de Castro Barbosa et al. 2016) were reanalyzed and used in these graphs. (a) The expression levels of significantly differentially expressed tsRNAs and miRNAs in sperm from rats on HFD in comparison to the respective controls (CD). (b) The expression levels of the top ten significantly upregulated and downregulated piRNAs in sperm from rats on HFD in comparison to the respective controls (CD)
Chen and co-workers investigated the expression profiles of sperm sncRNAs from male mice on HFD by small RNA sequencing (Chen et al. 2016). The expression levels of tsRNAs and miRNAs in the sperm samples from mice on HFD were significantly altered compared with the sperm samples from mice on CD. A large proportion of tsRNAs (~12%) was significantly affected by the HFD suggesting that sperm tsRNAs may be sensitive to HFD exposure. Furthermore, they identified several RNA modifications, particularly 5-methylcytidine (m5C) and N2-methylguanosine (m2G), in sperm RNAs (predominantly tsRNAs), and these RNA modifications were significantly changed in sperm samples from HFD mice.
Fullston and co-workers investigated the effects of feeding mice an HFD on expression profile of miRNAs in testicular tissue and spermatozoa isolated from the vas deferens (Fullston et al. 2013). The miRNAs that were found to be differentially expressed in the testis in response to a HFD were examined in sperm. The authors found that four of the eleven miRNAs examined (mmu-miR-133b-3p, mmu-miR-196a-5p, mmu-miR-205-5p, and mmu-miR-340-5p) were differentially expressed also in sperm. The functional pathways affected by these miRNAs were investigated by in silico analysis. Genes targeted by these miRNAs were enriched in metabolic disease, cell death, production of ROS, DNA replication, NF-κB signaling, p53 signaling, recombination and repair, lipid metabolism, spermatogenesis, and embryonic development.
Further, Grandjean and co-workers identified 13 diet-induced differentially expressed miRNAs in the testis by RNA sequencing (Grandjean et al. 2015). Of these 13 miRNAs, five (miR-182, miR-19a, miR-19b, miR-29a, and miR-340) were found to be differentially expressed following qPCR analysis of both testis and sperm samples from mice on Western diet compared with mice on standard diet. miR-19b and miR-29a were identified as the two most abundant deregulated microRNAs in mice on a Western diet. Intriguingly, microinjection of miR-19b, but not of miR-29a, into fertilized one-cell embryos resulted in offspring with higher body weights than controls.
Obesity and HFD have been shown to be associated with differential expression of sperm sncRNA subtypes, and some of these appear to play a role in intergeneration epigenetic inheritance. Studies of sperm sncRNA in obesity are thus an important focus of further investigations.
Concluding Remarks
There are strong indications that paternal HFD may lead to changes in energy metabolism in offspring, and data suggest that such effects persist also in F2 generations. Recently, studies are emerging that examine the epigenome of sperm from obese men or in sperm from HFD-induced obesity rodent models (Table 1). Spermatozoa have been considered resistant with respect to the introduction of epigenetic changes due to the compact chromatin structure and lack of transcriptional activity. However, it was recently revealed that macromolecules including tsRNA and miRNA are delivered to maturing sperm by the fusion with epididymosomes during epididymal transit (Sharma et al. 2016). Furthermore, mature sperm contains DNA methyltransferases, and it has been suggested that DNA methylation changes occur also in mature sperm (Donkin et al. 2016).
To date there is inconsistent information on the association between obesity and alterations in sperm DNA methylation and potential consequences for embryo development. However, Donkin and co-workers recently demonstrated differences in gene-specific DNA methylation between sperm from lean and obese men and a marked change in sperm DNA methylation following gastric bypass-induced weight loss in morbidly obese men (Donkin et al. 2016). Less is currently known about the potential role of sperm histones in intergenerational transmission of paternal effects, and the role of histone modifications clearly deserves further examination.
Obesity and HFD induce changes in germ cell transcriptome in the testis and epididymis that may result in alterations also in sperm RNA composition. Intriguingly, recent studies with injection of sncRNA into zygote suggest that sncRNA including miRNA and tsRNA may transmit a paternal metabolic phenotype to offspring (Grandjean et al. 2015; Chen et al. 2016; Sharma et al. 2016). Several piRNAs also seem to be sensitive to nutritional status (Donkin et al. 2016); however, the functional role of the changes in piRNA levels for embryo development and offspring phenotype remains to be shown. Chen and co-workers have recently shown that sperm tsRNA modification is subject to dietary modulation (Chen et al. 2016) implying that modifications of sncRNAs may play a role in the control of paternal epigenetic intergenerational heritance. As somatic cells contain much more RNA than spermatozoa, the absence of contaminating somatic cell RNA should be confirmed in studies of spermatozoal RNA expression.
In the years to come, more and exciting information is likely to increase our understanding of the adaptive nature of the sperm epigenome to nutrition and obesity, along with an increasing awareness of paternal influence on the epigenetic programming of their offspring.
Definitions of Words and Terms
- Overweight and obesity:
-
According to the WHO (World Health Organization), overweight and obesity are defined as abnormal or excessive fat accumulation that may impair health. For adults, overweight is defined as a BMI ≥ 25 kg/m2; obesity denotes BMI ≥ 30 kg/m2.
- Body mass index (BMI):
-
A simple index of weight for height that is commonly used to classify overweight and obesity in adults. It is defined as a person’s weight in kilograms divided by the square of his height in meters (kg/m2).
- Diet-induced obesity:
-
Defined diets high in fat are commonly used in experimental rodent studies of obesity and associated diseases. The calorie content (% kcals) from fat in most studies varies between approximately 10% in control diets and 30–60% in high-fat diets. In some studies, both the fat and the sucrose levels are high to simulate the human Western diet.
- Sperm chromatin:
-
During spermiogenesis, the chromatin of haploid spermatids is remodeled to allow a high degree of DNA compaction. The DNA compaction process involves a successive replacement of nucleosomal histones with transition proteins and subsequently protamines.
- Sperm protamines:
-
Protamines are small, arginine-rich, nuclear proteins that bind to and package all but a very small subset of the sperm genome. Protamine binding allows the DNA to be condensed into a volume approximately 1/20th that of a somatic nucleus. Humans and mice express two main types of protamines (P1 and P2), whereas rats have one main type (P1). Protamines in paternal DNA are replaced by histones after fertilization.
- Sperm histones:
-
In humans and rodents, between 15 and 1% residual histones remain in sperm. Most of the genes that retain their histone packaging in human sperm have not yet been identified, but they seem to represent a unique subset of the sperm genome.
Key Facts of Paternal Obesity
-
Across OECD countries, about one in five adults is obese, with similar rates in men and women in most countries.
-
Paternal obesity is associated with alterations in energy metabolism and disease susceptibility in offspring.
-
Data suggest that paternal obesity may have effects that can be sustained across generations.
-
In rodent studies, genetic and environmental factors can be carefully controlled allowing for mechanistic examination of causal relationships between paternal obesity and effects in offspring.
-
In rodents, paternal diet-induced obesity has negative effects on early embryo development. It is hypothesized that epigenetic modifications in sperm from obese males may influence embryo development and contribute to the observed paternal inter- and transgenerational effects.
Summary Points
-
The current obesity epidemic is a huge societal concern due to associated diseases.
-
Together, human and rodent studies indicate that paternal obesity may affect embryo development and modify offspring energy metabolism.
-
Evidence is accumulating showing that the sperm epigenome is modifiable in response to nutritional status and diet.
-
This review has identified two human studies and seven rodent studies focusing on analysis of obesity-associated epigenetic modifications in mature sperm.
-
Modification of the sperm epigenome may occur throughout spermatogenesis including during epididymal transit.
-
Arguably, the strongest evidence for a paternal obesity-associated epigenetic inheritance currently relates to the role of small noncoding RNAs.
-
Differential expression of several small noncoding RNAs (e.g., miRNAs, piRNAs, and tsRNAs) between obese and nonobese subjects has been identified.
-
Modification of tsRNA species in obese mice has been reported.
-
Injection of obesity-associated sperm sncRNAs into a fertilized embryo may affect offspring metabolism.
-
Differences in gene-specific DNA methylation between sperms from lean and obese subjects have been demonstrated.
-
Data on differential sperm DNA methylation of imprinted genes in obese subjects are not consistent for human and rodent studies.
-
The data on potential modification of sperm histones in obesity is particularly limited.
Abbreviations
- CD:
-
Control diet
- DFI:
-
DNA fragmentation index
- DMRs:
-
Differentially methylated regions
- HFD:
-
High-fat diet
- m2G:
-
N2-methylguanosine
- m5C:
-
5-Methylcytidine
- miRNA:
-
MicroRNA
- piRNA:
-
PIWI-interacting RNA
- RRBS:
-
Reduced-representation bisulfite sequencing
- SCSA:
-
Sperm chromatin structure assay
- sncRNA:
-
Small non-coding RNA
- tsRNA:
-
tRNA-derived small RNA
References
Asnicar MA, Smith DP, Yang DD et al (2001) Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology 142:4394–4400
Bao J, Bedford MT (2016) Epigenetic regulation of the histone-to-protamine transition during spermiogenesis. Reproduction 151:R55–R70
Binder NK, Hannan NJ, Gardner DK (2012a) Paternal diet-induced obesity retards early mouse embryo development, mitochondrial activity and pregnancy health. PLoS One 7:e52304
Binder NK, Mitchell M, Gardner DK (2012b) Parental diet-induced obesity leads to retarded early mouse embryo development and altered carbohydrate utilisation by the blastocyst. Reprod Fertil Dev 24:804–812
Binder NK, Sheedy JR, Hannan NJ et al (2015) Male obesity is associated with changed spermatozoa Cox4i1 mRNA level and altered seminal vesicle fluid composition in a mouse model. Mol Hum Reprod 21:424–434
Bromfield JJ (2014) Seminal fluid and reproduction: much more than previously thought. J Assist Reprod Genet 31:627–636
Campbell JM, Lane M, Owens JA et al (2015) Paternal obesity negatively affects male fertility and assisted reproduction outcomes: a systematic review and meta-analysis. Reprod Biomed Online 31:593–604
Carrell DT, Hammoud SS (2010) The human sperm epigenome and its potential role in embryonic development. Mol Hum Reprod 16:37–47
Chen Q, Yan M, Cao Z et al (2016) Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351:397–400
Cooper TG (2011) The epididymis, cytoplasmic droplets and male fertility. Asian J Androl 13:130–138
Dansranjavin T, Schagdarsurengin U (2016) The rationale of the inevitable, or why is the consideration of repetitive DNA elements indispensable in studies of sperm nucleosomes. Dev Cell 37:13–14
De Castro Barbosa T, Ingerslev LR, Alm PS et al (2016) High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol Metab 5:184–197
D'occhio MJ, Hengstberger KJ, Johnston SD (2007) Biology of sperm chromatin structure and relationship to male fertility and embryonic survival. Anim Reprod Sci 101:1–17
Donkin I, Versteyhe S, Ingerslev LR et al (2016) Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab 23:369–378
Duale N, Steffensen IL, Andersen J et al (2014) Impaired sperm chromatin integrity in obese mice. Andrology 2:234–243
Erkek S, Hisano M, Liang C-Y et al (2013) Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa. Nat Struct Mol Biol 20:868–875
Figueroa-Colon R, Arani RB, Goran MI et al (2000) Paternal body fat is a longitudinal predictor of changes in body fat in premenarcheal girls. Am J Clin Nutr 71:829–834
Fullston T, Ohlsson Teague EM, Palmer NO et al (2013) Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J 27:4226–4243
Galic S, Oakhill JS, Steinberg GR (2010) Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316:129–139
Grandjean V, Fourre S, De Abreu DA et al (2015) RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci Rep 5:18193
Gutzkow KB, Duale N, Danielsen T et al (2016) Enhanced susceptibility of obese mice to glycidamide-induced sperm chromatin damage without increased oxidative stress. Andrology 4:1102–1114
Hackett JA, Sengupta R, Zylicz JJ et al (2013) Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science 339:448–452
Hammoud SS, Nix DA, Zhang H et al (2009) Distinctive chromatin in human sperm packages genes for embryo development. Nature 460:473–478
Huypens P, Sass S, Wu M et al (2016) Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genet 48:497–499
Jodar M, Selvaraju S, Sendler E et al (2013) The presence, role and clinical use of spermatozoal RNAs. Hum Reprod Update 19:604–624
Kurtz K, Saperas N, Ausio J et al (2009) Spermiogenic nuclear protein transitions and chromatin condensation. Proposal for an ancestral model of nuclear spermiogenesis. J Exp Zool B Mol Dev Evol 312b:149–163
Li N, Shen Q, Hua J (2016) Epigenetic remodeling in male germline development. Stem Cells Int 2016:3152173
Lim JM, Wollaston-Hayden EE, Teo CF et al (2014) Quantitative secretome and glycome of primary human adipocytes during insulin resistance. Clin Proteomics 11:20
Marques CJ, Joao Pinho M, Carvalho F et al (2011) DNA methylation imprinting marks and DNA methyltransferase expression in human spermatogenic cell stages. Epigenetics 6:1354–1361
Mitchell M, Bakos HW, Lane M (2011) Paternal diet-induced obesity impairs embryo development and implantation in the mouse. Fertil Steril 95:1349–1353
Ng SF, Lin RCY, Laybutt DR et al (2010) Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 467:963–U103
Nguyen RHN, Wilcox AJ, Skjaerven R et al (2007) Men's body mass index and infertility. Hum Reprod 22:2488–2493
OECD (2015), Health at a Glance 2015: OECD Indicators, OECD Publishing, Paris. https://doi.org/10.1787/health_glance-2015-en
Palmer NO, Fullston T, Mitchell M et al (2011) SIRT6 in mouse spermatogenesis is modulated by diet-induced obesity. Reprod Fertil Dev 23:929–939
Ramlau-Hansen CH, Thulstrup AM, Nohr EA et al (2007) Subfecundity in overweight and obese couples. Hum Reprod 22:1634–1637
Rousseaux S, Caron C, Govin J et al (2005) Establishment of male-specific epigenetic information. Gene 345:139–153
Royo H, Stadler MB, Peters AH (2016) Alternative computational analysis shows no evidence for nucleosome enrichment at repetitive sequences in mammalian spermatozoa. Dev Cell 37:98–104
Sarkar A, Volff JN, Vaury C (2016) piRNAs and their diverse roles: a transposable element-driven tactic for gene regulation? FASEB J 31(2):436-446
Schagdarsurengin U, Steger K (2016) Epigenetics in male reproduction: effect of paternal diet on sperm quality and offspring health. Nat Rev Urol 13:584–595
Sharma U, Conine CC, Shea JM et al (2016) Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351:391–396
Siklenka K, Erkek S, Godmann M et al (2015) Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science 350:aab2006
Simon L, Castillo J, Oliva R et al (2011) Relationships between human sperm protamines, DNA damage and assisted reproduction outcomes. Reprod Biomed Online 23:724–734
Simon L, Liu L, Murphy K et al (2014) Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Hum Reprod 29:904–917
Soubry A, Guo L, Huang Z et al (2016) Obesity-related DNA methylation at imprinted genes in human sperm: results from the TIEGER study. Clin Epigenetics 8:51
Terashima M, Barbour S, Ren J et al (2015) Effect of high fat diet on paternal sperm histone distribution and male offspring liver gene expression. Epigenetics 10:861–871
van der Heijden GW, Ramos L, Baart EB, et al. (2008) Sperm-derived histones contribute to zygotic chromatin in humans. BMC Dev Bio 8:34
Vicentic A, Jones DC (2007) The CART (cocaine- and amphetamine-regulated transcript) system in appetite and drug addiction. J Pharmacol Exp Ther 320:499–506
Wang L, Zhang J, Duan J et al (2014) Programming and inheritance of parental DNA methylomes in mammals. Cell 157:979–991
Whitaker RC, Deeks CM, Baughcum AE et al (2000) The relationship of childhood adiposity to parent body mass index and eating behavior. Obes Res 8:234–240
Youngson NA, Lecomte V, Maloney CA et al (2016) Obesity-induced sperm DNA methylation changes at satellite repeats are reprogrammed in rat offspring. Asian J Androl 18:930-936
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Duale, N., Witczak, O., Brunborg, G., Haugen, T.B., Lindeman, B. (2019). Sperm Epigenome in Obesity. In: Patel, V., Preedy, V. (eds) Handbook of Nutrition, Diet, and Epigenetics. Springer, Cham. https://doi.org/10.1007/978-3-319-55530-0_53
Download citation
DOI: https://doi.org/10.1007/978-3-319-55530-0_53
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55529-4
Online ISBN: 978-3-319-55530-0
eBook Packages: MedicineReference Module Medicine