Abstract
5-methylcysine (5-mC) and N 6-methyladenosine (m6A) are important epigenetic marks occurring in nucleic acids of plants with regulatory roles in a broad range of biological processes. Recently, some novel modifications with potential regulatory roles such as 5-hydroxymethylcytosine (5-hmC), 5-formylcytosine (5-foC), and 5-carboxylcytosine (5-caC) have also been discovered in plants. Systematic investigation of the functions of nucleic acid modifications will promote the understanding of the mechanism underlying association of epigenetic modifications with plant development and response to environmental stresses. In this respect, great advances have been made in the development of methods for investigation of the occurrence and localization of these epigenetic modifications in nucleic acids of plants. Here, we focus on the recent methodological advances for the analysis of the global levels of DNA and RNA methylation. In addition, we will discuss the mostly used methods for mapping the genome-wide distribution of DNA and RNA methylation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- DNA methylation
- RNA methylation
- 5-methylcysine
- 5-hydroxymethylcytosine
- 5-formylcytosine
- 5-carboxylcytosine
- N 6-methyladenosine
- global detection
- mapping
1 General Functions of DNA and RNA Methylation in Plants
Methylation modifications, notably in the forms of 5-methylcytosine (5-mC) and N 6-methyladenosine (m6A) in both DNA and RNA, perform important regulatory functions in various biological processes (Chen et al. 2016; Shen et al. 2014; Wu and Zhang 2014). DNA and RNA methylation occurs in almost all living organisms, from bacteria to fungi, plants, and mammals (Motorin and Helm 2011; Zemach and Zilberman 2010). The fine-tuning of chromatin structure by DNA and RNA methylation is one of the major hallmarks of gene regulation during cellular development (He 2010; Jones 2012).
1.1 DNA Cytosine Methylation in Plants
Although the levels of 5-mC are relatively low in mammalian genomes (3–8% of total cytosine), 5-mC presents a much abundant level in plant genomes (5–25% of total cytosine) (Rangwala and Richards 2004). Plants have more complicated and sophisticated system of the genome methylation compared to animals. In plants, cytosine can be methylated at CG, CHG, and CHH sites (H is A, T, C) (Matzke et al. 2015). DNA methylation in plants is predominantly controlled by domains rearranged as methyltransferases 2 (DRM2) via the RNA-directed DNA methylation pathway and maintained by DNA methyltransferases 1 (MET1), chromomethylase 3 (CMT3), and DRM2 (Chan et al. 2005). CG methylation is mediated by MET1; CHH methylation is controlled by DRM2, while the plant-specific CMT3 regulates CHG methylation (Chan et al. 2005). DNA methylation in plants is involved in the control of genetic functions including transcription, replication, gene transposition, and cell differentiation (Law and Jacobsen 2010).
1.2 RNA Cytosine Methylation in Plants
In addition to occurring in DNA, 5-mC has also been identified in different RNA species from all kingdoms of life (Motorin and Helm 2011; Motorin et al. 2010). 5-mC residues in tRNAs are known to influence their secondary structural, stabilization, and codon recognition (Helm 2006; Squires and Preiss 2010). 5-mC sites are also found in rRNA where they play critical roles in recognition of tRNA and translational fidelity (Chow et al. 2007). And internal 5-mC in mRNA was also identified (Edelheit et al. 2013). In plants, the level of 5-mC in total RNA is about 0.88% in Lepidium sativum. When plant was exposed to abiotic stress, such as Cd(II) or Se(IV), 5-mC in both DNA and RNA changed (Yanez Barrientos et al. 2013). The advances in the field of epigenetics suggest that RNA cytosine methylation might play a similar role in the modulation of genetic information as DNA cytosine methylation in plants (Mattick et al. 2009).
1.3 RNA Adenine Methylation in Plants
Recent discovery of reversible m6A modification on mRNA and mapping of m6A in mammals revealed potential regulatory functions of this RNA adenine modification. In Arabidopsis thaliana, m6A content in mRNA varies across tissues with a high ratio of m6A/A found in flower buds, and defects in m6A methyltransferase cause an embryo-lethal phenotype, suggesting a critical role of m6A in plant development (Zhong et al. 2008). Recently, m6A mapping analysis showed that m6A is a highly conserved modification in mRNA of Arabidopsis thaliana (Luo et al. 2014). m6A in mRNA of Arabidopsis thaliana is enriched around both the stop codon and the start codon. A positive correlation between m6A deposition and mRNA levels indicates a regulatory role of m6A in plant gene expression (Luo et al. 2014).
2 Global Detection of DNA and RNA Methylation in Plants
Established methods for the determination of global DNA and RNA methylation in plants mainly include liquid chromatography (LC), liquid chromatography-mass spectrometry (LC-MS), capillary electrophoresis (CE), thin layer chromatography (TLC), and immuno-based detection. The global detection of DNA and RNA methylation requires the liberation of DNA/RNA components with enzymatic/chemical treatments followed by determination of the components with various methods.
2.1 Liquid Chromatography
The analysis of global DNA and RNA methylation by LC is based on the chromatographic separation of the components by enzymatic or chemical hydrolysis of DNA/RNA. Therefore, the baseline separation of the DNA/RNA components is necessary to avoid co-elution of analytes.
Johnston et al. (2005) examined the methodological factors in LC analysis of plant DNA methylation using in vitro cultures of Ribes ciliatum. The results demonstrated that complete removal of RNA from plant DNA is difficult using RNase digestions and LiCl precipitation, suggesting that nucleobases analysis should be avoided as nucleobases from residual RNA fragments will interfere DNA-derived nucleobases. Nucleoside or nucleotide analysis is therefore recommended as a more suitable option. Liquid chromatographic techniques generally are quantitative, reproducible, but less sensitive. In this respect, a relatively large amount of genomic DNA (~1–50 μg) is normally needed.
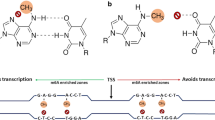
To increase the detection sensitivity of 5-mC by LC-based detection, selective derivatization of cytosine moieties with 2-bromoacetophenone followed by reversed phase LC with spectrofluorimetric detection was developed (Torres et al. 2011; Yanez Barrientos et al. 2013) (Fig. 1). The proposed method was capable for the detection of as low as 0.06% of methylation in 80 ng of DNA and can be used for the evaluation of RNA methylation at the same time. With this method, Barrientos et al. (2013) analyzed the global DNA and total RNA methylation in Lepidium sativum and further assessed the effect of Cd(II) and Se(IV) exposure on DNA and RNA methylation of Lepidium sativum.
(a) Schematic illustration for selective derivatization of cytosinemoieties with 2-bromoacetophenone for the determination of global DNA methylation by reversed phase liquid chromatography with spectrofluorimetric detection (Reprinted with permission from Torres AL, Barrientos EY, Wrobel K. Anal Chem, 2011, 83: 7999–8005). (b) LC-FLD chromatograms of control plant (solid line) and plant exposed to 1.0 mgL−1 Se(IV) (dashed line). Left panel: Y-scale and insert adjusted to visualize 5-mC and C signals (RNA methylation); right panel: Y-scale adjusted to visualize 5-mdC and dC signals (DNA methylation) (Reprinted with permission from Yanez Barrientos E, Wrobel K, Lopez Torres A, Gutierrez Corona F, Wrobel K. Anal Bioanal Chem, 2013, 405: 2397–2404)
2.2 Liquid Chromatography-Mass Spectrometry
Due to the good selectivity and sensitivity, LC-MS has been widely used in the analysis of nucleic acid modifications.
Our group developed various methods for the detection of DNA and RNA methylation by LC-MS (Chen et al. 2013; Huang et al. 2016a, b, 2015; Jiang et al. 2015, 2016; Shen et al. 2015; Tang et al. 2013, 2014, 2015; Wang et al. 2013; Xiong et al. 2015; Yuan 2014; Yuan and Feng 2014; Yuan et al. 2011; Zhang et al. 2016). Specifically, we recently established a chemical derivatization strategy combined with liquid chromatography-electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS) method to determine 5-formyl-2′-deoxycytidine (5-fodC) and 5-carboxyl-2′-deoxycytidine (5-cadC) in plants (Tang et al. 2014) (Fig. 2). Derivatization of 5-fodC and 5-cadC by Girard’s reagents significantly increased the detection sensitivities of 5-fodC and 5-cadC by 52 to 260folds. Using this method, we demonstrated the widespread existence of 5-fodC and 5-cadC in genomic DNA of various plant tissues, with contents of 5-fodC ranging from 2.1 to 4.7 modifications per 106dG and 5-cadC ranging from 0.2 to 3.6 modifications per 106dG. Moreover, we found that environmental stresses of drought and salinity can change the contents of 5-fodC and 5-cadC in plant genomes, suggesting the functional roles of 5-fodC and 5-cadC in response to environmental stresses.
In addition, Liu et al. (2013) reported the use of a reversed-phase LC coupled with tandem mass spectrometry method and stable isotope-labeled standards for assessing the levels of the oxidized 5-mC nucleosides in DNA of Arabidopsis thaliana. The quantitative results showed that the occurrence of 5-hmC, 5-foC, and 5-caC in DNA of Arabidopsis thaliana were 0.79, 0.79, and 0.78 modifications per 106nucleosides, respectively. This method is involved in the pre-enrichment of these modified nucleosides by LC, which can minimize the interference from other abundant nucleosides during subsequent mass spectrometry analysis but will increase the analytical time.
Recently, Magana et al (2016) investigated the effect of CuO nanoparticles on global DNA and RNA methylation in Lepidium sativum by liquid chromatography/ion trap mass spectrometry. Enhanced selectivity toward cytosine-containing nucleosides was achieved using proton-bound dimers formed in positive electrospray ionization as precursor ions for multiple reaction monitoring (MRM) quantification. The quantitative results showed that 13.03% and 0.92% methylated cytosines were found in DNA and RNA, respectively. Upon CuO nanoparticles treatment, DNA hypomethylation was observed, but RNA methylation did not present significant changes.
2.3 Capillary Electrophoresis
Several studies have developed capillary electrophoresis methods for the detection of genomic DNA methylation content in plant tissues. Fraga et al. (2000) used open-tube capillary electrophoresis system to separate acid hydrolyzed genomic DNA (nucleobases) from Pinus radiate trees for the evaluation of genomic DNA methylation. The problem is that the contamination of RNA may also contribute to the acid hydrolyzed products of nucleobases. In this respect, later they developed micellar capillary electrophoresis with UV-Vis detection to analyze the nucleosides of enzymatic products of DNA from Pinus radiate trees (Fraga et al. 2002). The detection and quantification of nucleosides through enzymatic hydrolyses notably increases the specificity and allows its exploitation in the analysis of poorly purified and/or concentrated DNA samples.
The capillary electrophoresis method offers the advantages of high resolution and cost-effective separations, providing an efficient approach to quantify nucleic acids methylation. The drawback of capillary electrophoresis method is that sample loading volume is limited and separation reproducibility can be affected by slight variations, which requires further improvements.
2.4 Thin Layer Chromatography
TLC-based method normally requires ribo- and deoxyribonucleotides that are generally distinguishable. After isolation of nucleic acids, the digested nucleosides were labeled with radioactive phosphate which enables sensitive determination of the contents of global DNA and RNA methylation. With the TLC method, global DNA methylation was successfully detected and quantified in various plant samples, including Arabidopsis and Cardaminopsis arenosa (Madlung et al. 2002), Artemisia annua (Pandey and Pandey-Rai 2015), Pyruscommunis (Michalak et al. 2013), and Quercus robur (Michalak et al. 2015).
TLC method is cost-effective, and there is no need for sophisticated instrumentation. So TLC method is frequently used in biological lab for discovery and quantification of modified DNA and RNA. However, TLC-based method involves radioactive isotope labeling, and the analytical procedure is relatively tedious.
2.5 Immuno-Based Detection
Immunostaining is a technique widely used to evaluate the presence of DNA and RNA methylation. This technique relies antibodies that can selectively recognize the corresponding modified DNA and RNA inside cells for cell-based visualization.
Zluvova et al. (2001) employed immunostaining technique to examine the global changes of DNA methylation during seed germination and shoot apical meristem development in Silene latifolia. The data showed that a rapid decrease in global DNA methylation during seed germination occurred first in endosperm tissue and subsequently in the hypocotyl. To reveal the dynamics of the methylation pattern, correct epitope retrieval sometimes is essential and a deep denaturation step is needed.
In addition to 5-mC in DNA, Yao et al. (2012) found that DNA in leaves and flowers of Arabidopsis thaliana contains low level of 5-hmC by immuno-based dot-blot technique (Fig. 3). Using in vitro binding assays, the authors observed that full-length VIM1 protein binds preferentially to hemi-methylated DNA. However, when 5-hmC replaces one or both cytosine residues at CpG site, VIM1 binds with tenfold lower affinity. These results suggest that 5-hmC may contribute to VIM-mediated passive loss of cytosine methylation in vivo during DNA replication in Arabidopsis thaliana.
Dot-blot detection of 5-hmC in DNA of Arabidopsis thaliana. (a) Dot-blot assay of synthetic DNA and Arabidopsis thaliana genomic DNA containing biotin-N3-5-gmC. The amount of loaded DNA increased from left to right. Detection top row: leaf DNA (25, 50, 100, 200 ng); middle row: 5-hmCcontaining DNA standard (boxed) (1–8 ng); bottom row: flower DNA (25, 50, 100, 200 ng). (b) Dot-blot assay using antibody to 5-hmC to detect 5-hmC in synthetic DNA or Arabidopsis thaliana genomic DNA. Spotted samples in top row (boxed): N Control: H2O; C: Synthetic DNA with C; 5-mC: Synthetic DNA with 5mC; 5-hmC: Synthetic DNA with 5-hmC; 0.5 ng of each synthetic DNA were loaded; middle row and bottom rows (leaf and flower DNA, respectively): The amount of Arabidopsis thaliana DNA (0.5, 1, 2, 4 ng) increased from left to right (Reprinted with permission from Yao Q, Song CX, He C, Kumaran D, Dunn JJ. Protein Expr Purif, 2012, 83:104–111)
In the past several years, many commercially available kits, such as enzyme linked immunosorbent assay (ELISA) with chemiluminescence detection-based analysis, were also produced and employed for the detection of global 5-mC in Phelipanche ramosa (Lechat et al. 2015) and rice (Ferreira et al. 2015) and global 5-mC and 5-hmC in B. oleracea and C. sativus (Moricova et al. 2013).
3 Location Analysis of DNA and RNA Methylation in Plants
The quantitative distribution information of DNA and RNA methylation is crucial to understand their biological functions. The advance in sequencing technologies accelerates and revolutionizes the genome-wide distribution studies of DNA and RNA methylation (Mardis 2013). Two major strategies, including affinity enrichment and bisulfite conversion followed by next-generation sequencing, have been widely used to profile the location of 5-mC in DNA and m6A in RNA of plants.
3.1 Affinity Enrichment-Sequencing Analysis
Affinity enrichment of modified DNA and RNA using antibodies or affinity binding proteins has been proved to be a powerful tool for comprehensive profiling of 5-mC and its derivatives (Thu et al. 2010). The enriched DNA-antibody/protein complex can be analyzed using sequencing-based technologies (Down et al. 2008). However, affinity enrichment-sequencing methods do not provide location information at single-base resolution.
Methylated DNA immunoprecipitation sequencing (MeDIP-seq) relies on the use of antibody to precipitate fragments of DNA containing methylated cytosine (Down et al. 2008). Related methods include MBD-seq (Serre et al. 2010) and MethylCap-seq (Brinkman et al. 2010), which employ methyl CpG binding domain protein to precipitate DNA fragments containing methylated CpG sites. These approaches have the advantage that a greatly reduced portion of the genome needs to be sequenced. However, the major drawback of these approaches is the low resolution for identifying methylation sites.
Genome-wide profiles of DNA methylation for maize (Zea mays) inbred lines by MeDIP-seq demonstrated that DNA methylation variation is influenced by genetic and epigenetic changes that are often stably inherited and can affect the expression of nearby genes (Eichten et al. 2013). Hu et al. (2015) used MeDIP-seq to profile DNA methylation in the rice PTGMS line PA64S under two different phenotypes (sterility and fertility). The results revealed that hypermethylation was observed in PA64S (sterility), and 1258 differentially methylated regions were found between PA64S (sterility) and PA64S (fertility).
In addition to 5-mC in DNA, Luo et al. (2014) established m6A-targeted antibody enrichment coupled with next-generation sequencing to map transcriptome-wide m6A in Arabidopsis thaliana. The results showed that m6A is a highly conserved modification of mRNA in plants. Distinct from mammals, m6A in Arabidopsis thaliana is enriched around both the stop codon and the start codon. The distribution pattern of m6A in Arabidopsis thaliana is associated with plant-specific pathways involving the chloroplast. A positive correlation between m6A deposition and mRNA abundance was observed, suggesting a regulatory role of m6A in plant gene expression. The m6A transcriptome-wide study of Arabidopsis thaliana provides a starting roadmap for uncovering m6A functions that may regulate plant metabolism.
Recently, Shen et al. (2016) employed anti-m6A polyclonal antibody enrichment coupled with next-generation sequencing that further map the m6A sites in mRNA of Arabidopsis thaliana. The results suggested an indispensable role of FIP37 (a core component of the m6A methyltransferase complex) in mediating m6A mRNA modification, which is required for maintaining the shoot meristem as a renewable source for continuously producing aerial organs in plants.
3.2 Bisulfite Conversion-Sequencing Analysis
The discovery that treatment of DNA with sodium bisulfite revolutionized DNA methylation analysis since 1990s (Clark et al. 1994; Frommer et al. 1992). And various methodologies have been developed based on bisulfite treatment that leads to the conversion of unmethylated cytosine to uracil, while methylated cytosine remains unchanged in DNA and RNA (Plongthongkum et al. 2014; Schaefer 2015). Amplification by polymerase chain reaction of converted DNA followed by sequencing can reveal positions of 5-mC in DNA and RNA. Since bisulfite conversion-sequencing strategy can provide single-base resolution for DNA and RNA cytosine methylation, the technique has been widely utilized in various plant samples, including Arabidopsis thaliana (Cokus et al. 2008; Feng et al. 2010; Ibarra et al. 2012; Lister et al. 2008; Shen et al. 2012; Stroud et al. 2013; Yu et al. 2013), rice (Li et al. 2012), soybean (Song et al. 2013), and Marchantia polymorpha (Takuno et al. 2016).
With applications of bisulfite conversion-sequencing to wild-type Arabidopsis thaliana and mutants defective in DNA methyltransferase or demethylase activity, Lister et al. (2008) observed local sequence effects upon methylation state and revealed a direct relationship between the location of smRNAs and DNA methylation. Cokus et al. (2008) identified sequence motifs that associate with high and low methylation for each different context of methylation in Arabidopsis thaliana.
Using the bisulfite conversion-sequencing, Stroud et al. (2013) substantially extended and refined the characterization of regulatory factors of the methylome by examining 86 Arabidopsis thaliana mutants, suggesting that individual sites of methylation may be regulated by novel RNA-directed pathways. Feng et al. (2010) carried out bisulfite conversion-sequencing in the flowering plants rice and Arabidopsis thaliana and found that the patterns of methylation were similar in flowering plants with methylated cytosines detected in all sequence contexts. Shen et al. (2012) found that genome-wide remodeling of DNA methylation mediated by the RNA-directed DNA methylation pathway in Arabidopsis thaliana may play a role in heterosis. Yu et al. (2013) found some transposable elements are demethylated and transcriptionally reactivated during antibacterial defense in Arabidopsis thaliana, which provides evidence that DNA demethylation is part of a plant-induced immune response and can potentially act to transcriptional activation of some defense genes linked to transposable elements and repeats. Ibarra et al. (2012) demonstrated that demethylation in companion cells reinforces transposon methylation in Arabidopsis thaliana gametes and contributes to stable silencing of transposable elements across generations.
In addition to the most studied model plant of Arabidopsis thaliana, Li et al. (2012) generated single-base resolution DNA methylome maps by bisulfite conversion-sequencing for Asian cultivated rice Oryza sativa ssp. japonica, indica, and their wild relatives, Oryza rufipogon and Oryza nivara. The overall methylation level of rice genomes is four times higher than that of Arabidopsis thaliana. Interestingly, the authors discovered that methylation in gene transcriptional termination regions can significantly repress gene expression, and the effect is stronger than that of promoter methylation.
Song et al. (2013) also analyzed the DNA methylation status in soybean roots, stems, leaves, and cotyledons of developing seeds at single-base resolution. Profiling of DNA methylation in different organs revealed 2162 differentially methylated regions among organs. Recently, Takuno et al. (2016) studied the single-base resolution methylome that span the phylogenetic breadth of land plants using bisulfite conversion-sequencing. The results showed that a basal land plant, Marchantia polymorpha, lacks evident signal of gene-body methylation within exons, but conifers have high methylation levels in both CG and CHG sites in expressed genes, which indicated the evolutionary forces acting on DNA methylation vary substantially across species, genes, and methylation contexts.
The advantage of bisulfite-converted strategy is that it can provide single-base resolution for DNA and RNA methylation analysis. However, the sample preparation associated with bisulfite sequencing can be time-consuming, and the conversion process may result in DNA and RNA degradation and reduce sequence complexity. As bisulfite analysis depends on the complete conversion of unmethylated cytosines to uracil, incomplete conversion will cause error or inaccurate results. In addition, discrimination between dC, 5-mC, and 5-hmC cannot be accomplished by bisulfite sequencing. Therefore, these exiting issues need to be further addressed.
3.3 Single-Molecule Detection
DNA methylation analysis by single-molecule, real-time (SMRT) sequencing without bisulfite conversion was first established in 2010 (Flusberg et al. 2010). The SMRT sequencing is considered to be the third-generation sequencing technology and can realize the direct distribution study of 5-mC in DNA. In SMRT sequencing, DNA polymerases catalyze the incorporation of fluorescently labeled nucleotides into complementary DNA strands. The recording of nucleotide incorporations generates the sequence readout and information about the polymerase kinetics, which are used to discriminate different nucleotides.
In addition to SMRT method, nanopore sequencing technology also has been established to single-molecule detection of modified nucleosides (Branton et al. 2008). Nanopore analysis uses a voltage to drive molecules through a nanoscale pore and monitors how the ionic current through the nanopore changes as single molecules pass through it (Venkatesan and Bashir 2011). Different nucleotides passing through nanopores generate different electric currents, which can be measured and designated to the corresponding nucleotides or modified nucleotides. The methodology is successfully used to distinguish methylated from unmethylated cytosines without bisulfite conversion (Clarke et al. 2009; Mirsaidov et al. 2009).
Using the SMRT sequencing, Kim et al. (2014) obtained high-coverage SMRT sequence datasets from five organisms including Arabidopsis thaliana. Later, Berlin et al. (2015)introduced the MinHash Alignment Process (MHAP) and integrated MHAP with the Celera Assembler, enabling reference-grade de novo assemblies of Arabidopsis thaliana from SMRT sequencing. Although the SMRT sequencing and nanopore sequencing techniques haven’t been employed for DNA methylation analysis in plants so far, these powerful techniques are qualified for mapping of DNA methylation and we expect the technique will be used in plant samples in the near future.
4 Conclusions and Perspectives
DNA and RNA methylation are important epigenetic modifications in eukaryotes to maintain genome integrity and regulate gene expression. DNA methylation in plants is species-, tissue-, organelle- and age-specific. Although DNA cytosine methylome and RNA adenine methylome have been profiled in plants, however, these previous studies mainly used the mixture of different cell types for methylome analysis, which may shade somemethylation patterns in specific cell types. Therefore, the improvement on the isolation of cell type-specific nucleic acids and methylome sequencing in a single cell would facilitate to understand the dynamics of DNA and RNA methylation in plants.
The application of next-generation sequencing technology in DNA and RNA methylation studies has greatly contributed to our knowledge of DNA and RNA methylation. Future applications of some newly developed sequencing approaches such as single-molecule sequencing approaches are particularly well suited for the location study of DNA and RNA methylation. SMRT and nanopore sequencing offer the potential for direct sequencing of nucleic acid modifications without complicated pretreatment. The advancement of new technologies and methods may also lead to the discovery of novel epigenetic modifications in both DNA and RNA that will enhance our understanding of the fundamental issues in cellular developmental processing in plants.
References
Alcazar Magana A, Wrobel K, Corrales Escobosa AR et al (2016) Application of liquid chromatography/electrospray ionization ion trap tandem mass spectrometry for the evaluation of global nucleic acids: methylation in garden cress under exposure to CuO nanoparticles. Rapid Commun Mass Spectrom 30:209–220
Berlin K, Koren S, Chin CS et al (2015) Assembling large genomes with single-molecule sequencing and locality-sensitive hashing. Nat Biotechnol 33:623–630
Branton D, Deamer DW, Marziali A et al (2008) The potential and challenges of nanopore sequencing. Nat Biotechnol 26:1146–1153
Brinkman AB, Simmer F, Ma K et al (2010) Whole-genome DNA methylation profiling using MethylCap-seq. Methods 52:232–236
Chan SW, Henderson IR, Jacobsen SE (2005) Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat Rev Genet 6:351–360
Chen ML, Shen F, Huang W et al (2013) Quantification of 5-methylcytosine and 5-hydroxymethylcytosine in genomic DNA from hepatocellular carcinoma tissues by capillary hydrophilic-interaction liquid chromatography/quadrupole TOF mass spectrometry. Clin Chem 59:824–832
Chen K, Zhao BS, He C (2016) Nucleic acid modifications in regulation of gene expression. Cell Chem Biol 23:74–85
Chow CS, Lamichhane TN, Mahto SK (2007) Expanding the nucleotide repertoire of the ribosome with post-transcriptional modifications. ACS Chem Biol 2:610–619
Clark SJ, Harrison J, Paul CL et al (1994) High sensitivity mapping of methylated cytosines. Nucleic Acids Res 22:2990–2997
Clarke J, Wu HC, Jayasinghe L et al (2009) Continuous base identification for single-molecule nanopore DNA sequencing. Nat Nanotechnol 4:265–270
Cokus SJ, Feng S, Zhang X et al (2008) Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 452:215–219
Down TA, Rakyan VK, Turner DJ et al (2008) A Bayesian deconvolution strategy for immunoprecipitation-based DNA methylome analysis. Nat Biotechnol 26:779–785
Edelheit S, Schwartz S, Mumbach MR et al (2013) Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genet 9:e1003602
Eichten SR, Briskine R, Song J et al (2013) Epigenetic and genetic influences on DNA methylation variation in maize populations. Plant Cell 25:2783–2797
Feng S, Cokus SJ, Zhang X et al (2010) Conservation and divergence of methylation patterning in plants and animals. Proc Natl Acad Sci U S A 107:8689–8694
Ferreira LJ, Azevedo V, Maroco J et al (2015) Salt tolerant and sensitive rice varieties display differential methylome flexibility under salt stress. PLoS One 10:e0124060
Flusberg BA, Webster DR, Lee JH et al (2010) Direct detection of DNA methylation during single-molecule, real-time sequencing. Nat Methods 7:461–465
Fraga MF, Rodriguez R, Canal MJ (2000) Rapid quantification of DNA methylation by high performance capillary electrophoresis. Electrophoresis 21:2990–2994
Fraga MF, Uriol E, Borja Diego L et al (2002) High-performance capillary electrophoretic method for the quantification of 5-methyl 2′-deoxycytidine in genomic DNA: application to plant, animal and human cancer tissues. Electrophoresis 23:1677–1681
Frommer M, McDonald LE, Millar DS et al (1992) A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A 89:1827–1831
He C (2010) Grand challenge commentary: RNA epigenetics? Nat Chem Biol 6:863–865
Helm M (2006) Post-transcriptional nucleotide modification and alternative folding of RNA. Nucleic Acids Res 34:721–733
Hu J, Chen X, Zhang H et al (2015) Genome-wide analysis of DNA methylation in photoperiod- and thermo-sensitive male sterile rice Peiai 64S. BMC Genomics 16:102
Huang W, Xiong J, Yang Y et al (2015) Determination of DNA adenine methylation in genomes of mammals and plants by liquid chromatography/mass spectrometry. RSC Adv 5:64046–64054
Huang W, Lan MD, Qi CB et al (2016a) Formation and determination of the oxidation products of 5-methylcytosine in RNA. Chem Sci 7:5495–5502
Huang W, Qi CB, Lv SW et al (2016b) Determination of DNA and RNA methylation in circulating tumor cells by mass spectrometry. Anal Chem 88:1378–1384
Ibarra CA, Feng X, Schoft VK et al (2012) Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science 337:1360–1364
Jiang HP, Qi CB, Chu JM et al (2015) Profiling of cis-diol-containing nucleosides and ribosylated metabolites by boronate-affinity organic-silica hybrid monolithic capillary liquid chromatography/mass spectrometry. Sci Rep 5:7785
Jiang HP, Chu JM, Lan MD et al (2016) Comprehensive profiling of ribonucleosides modification by affinity zirconium oxide-silica composite monolithic column online solid-phase microextraction—mass spectrometry analysis. J Chromatogr A 1462:90–99
Johnston JW, Harding K, Bremner DH et al (2005) HPLC analysis of plant DNA methylation: a study of critical methodological factors. Plant Physiol Biochem 43:844–853
Jones PA (2012) Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 13:484–492
Kim KE, Peluso P, Babayan P et al (2014) Long-read, whole-genome shotgun sequence data for five model organisms. Sci Data 1:140045
Law JA, Jacobsen SE (2010) Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet 11:204–220
Lechat MM, Brun G, Montiel G et al (2015) Seed response to strigolactone is controlled by abscisic acid-independent DNA methylation in the obligate root parasitic plant, Phelipanche ramosa L. Pomel. J Exp Bot 66:3129–3140
Li X, Zhu J, Hu F et al (2012) Single-base resolution maps of cultivated and wild rice methylomes and regulatory roles of DNA methylation in plant gene expression. BMC Genomics 13:300
Lister R, O’Malley RC, Tonti-Filippini J et al (2008) Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 133:523–536
Liu S, Dunwell TL, Pfeifer GP et al (2013) Detection of oxidation products of 5-methyl-2′-deoxycytidine in Arabidopsis DNA. PLoS One 8:e84620
Luo GZ, MacQueen A, Zheng G et al (2014) Unique features of the m6A methylome in Arabidopsis thaliana. Nat Commun 5:5630
Madlung A, Masuelli RW, Watson B et al (2002) Remodeling of DNA methylation and phenotypic and transcriptional changes in synthetic Arabidopsis allotetraploids. Plant Physiol 129:733–746
Mardis ER (2013) Next-generation sequencing platforms. Annu Rev Anal Chem (Palo Alto Calif) 6:287–303
Mattick JS, Amaral PP, Dinger ME et al (2009) RNA regulation of epigenetic processes. Bioessays 31:51–59
Matzke MA, Kanno T, Matzke AJ (2015) RNA-directed DNA methylation: the evolution of a complex epigenetic pathway in flowering plants. Annu Rev Plant Biol 66:243–267
Michalak M, Barciszewska MZ, Barciszewski J et al (2013) Global changes in DNA methylation in seeds and seedlings of Pyrus communis after seed desiccation and storage. PLoS One 8:e70693
Michalak M, Plitta-Michalak BP, Naskret-Barciszewska M et al (2015) Global 5-methylcytosine alterations in DNA during ageing of Quercus robur seeds. Ann Bot 116:369–376
Mirsaidov U, Timp W, Zou X et al (2009) Nanoelectromechanics of methylated DNA in a synthetic nanopore. Biophys J 96:L32–L34
Moricova P, Ondrej V, Navratilova B et al (2013) Changes of DNA methylation and hydroxymethylation in plant protoplast cultures. Acta Biochim Pol 60:33–36
Motorin Y, Helm M (2011) RNA nucleotide methylation. Wiley Interdiscip Rev RNA 2:611–631
Motorin Y, Lyko F, Helm M (2010) 5-methylcytosine in RNA: detection, enzymatic formation and biological functions. Nucleic Acids Res 38:1415–1430
Pandey N, Pandey-Rai S (2015) Deciphering UV-B-induced variation in DNA methylation pattern and its influence on regulation of DBR2 expression in Artemisia annua L. Planta 242:869–879
Plongthongkum N, Diep DH, Zhang K (2014) Advances in the profiling of DNA modifications: cytosine methylation and beyond. Nat Rev Genet 15:647–661
Rangwala SH, Richards EJ (2004) The value-added genome: building and maintaining genomic cytosine methylation landscapes. Curr Opin Genet Dev 14:686–691
Schaefer M (2015) RNA 5-methylcytosine analysis by bisulfite sequencing. Methods Enzymol 560:297–329
Serre D, Lee BH, Ting AH (2010) MBD-isolated genome sequencing provides a high-throughput and comprehensive survey of DNA methylation in the human genome. Nucleic Acids Res 38:391–399
Shen H, He H, Li J et al (2012) Genome-wide analysis of DNA methylation and gene expression changes in two Arabidopsis ecotypes and their reciprocal hybrids. Plant Cell 24:875–892
Shen L, Song CX, He C, Zhang Y (2014) Mechanism and function of oxidative reversal of DNA and RNA methylation. Annu Rev Biochem 83:585–614
Shen F, Huang W, Huang JT et al (2015) Decreased N(6)-methyladenosine in peripheral blood RNA From diabetic patients is associated with FTO expression rather than ALKBH5. J Clin Endocrinol Metab 100:E148–E154
Shen L, Liang Z, Gu X et al (2016) N(6)-methyladenosine RNA modification regulates shoot stem cell fate in Arabidopsis. Dev Cell 38:186–200
Song QX, Lu X, Li QT et al (2013) Genome-wide analysis of DNA methylation in soybean. Mol Plant 6:1961–1974
Squires JE, Preiss T (2010) Function and detection of 5-methylcytosine in eukaryotic RNA. Epigenomics 2:709–715
Stroud H, Greenberg MV, Feng S et al (2013) Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell 152:352–364
Takuno S, Ran JH, Gaut BS (2016) Evolutionary patterns of genic DNA methylation vary across land plants. Nat Plants 2:15222
Tang Y, Chu JM, Huang W et al (2013) Hydrophilic material for the selective enrichment of 5-hydroxymethylcytosine and its liquid chromatography-tandem mass spectrometry detection. Anal Chem 85:6129–6135
Tang Y, Xiong J, Jiang HP et al (2014) Determination of oxidation products of 5-methylcytosine in plants by chemical derivatization coupled with liquid chromatography/tandem mass spectrometry analysis. Anal Chem 86:7764–7772
Tang Y, Zheng SJ, Qi CB et al (2015) Sensitive and simultaneous determination of 5-methylcytosine and its oxidation products in genomic DNA by chemical derivatization coupled with liquid chromatography-tandem mass spectrometry analysis. Anal Chem 87:3445–3452
Thu KL, Pikor LA, Kennett JY et al (2010) Methylation analysis by DNA immunoprecipitation. J Cell Physiol 222:522–531
Torres AL, Barrientos EY, Wrobel K (2011) Selective derivatization of cytosine and methylcytosine moieties with 2-bromoacetophenone for submicrogram DNA methylation analysis by reversed phase HPLC with spectrofluorimetric detection. Anal Chem 83:7999–8005
Venkatesan BM, Bashir R (2011) Nanopore sensors for nucleic acid analysis. Nat Nanotechnol 6:615–624
Wang ST, Huang W, Lu W et al (2013) TiO2-based solid phase extraction strategy for highly effective elimination of normal ribonucleosides before detection of 2′-deoxynucleosides/low-abundance 2′-O-modified ribonucleosides. Anal Chem 85:10512–10518
Wu H, Zhang Y (2014) Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell 156:45–68
Xiong J, Jiang HP, Peng CY et al (2015) DNA hydroxymethylation age of human blood determined by capillary hydrophilic-interaction liquid chromatography/mass spectrometry. Clin Epigenetics 7:72
Yanez Barrientos E, Wrobel K, Lopez Torres A et al (2013) Application of reversed-phase high-performance liquid chromatography with fluorimetric detection for simultaneous assessment of global DNA and total RNA methylation in Lepidium sativum: effect of plant exposure to Cd(II) and Se(IV). Anal Bioanal Chem 405:2397–2404
Yao Q, Song CX, He C et al (2012) Heterologous expression and purification of Arabidopsis thaliana VIM1 protein: in vitro evidence for its inability to recognize hydroxymethylcytosine, a rare base in Arabidopsis DNA. Protein Expr Purif 83:104–111
Yu A, Lepere G, Jay F et al (2013) Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proc Natl Acad Sci U S A 110:2389–2394
Yuan BF (2014) 5-methylcytosine and its derivatives. Adv Clin Chem 67:151–187
Yuan BF, Feng YQ (2014) Recent advances in the analysis of 5-methylcytosine and its oxidation products. TrAC-Trend Anal Chem 54:24–35
Yuan BF, Zhang J, Wang H et al (2011) 6-Thioguanine reactivates epigenetically silenced genes in acute lymphoblastic leukemia cells by facilitating proteasome-mediated degradation of DNMT1. Cancer Res 71:1904–1911
Zemach A, Zilberman D (2010) Evolution of eukaryotic DNA methylation and the pursuit of safer sex. Curr Biol 20:R780–R785
Zhang HY, Xiong J, Qi BL et al (2016) The existence of 5-hydroxymethylcytosine and 5-formylcytosine in both DNA and RNA in mammals. Chem Commun (Camb) 52:737–740
Zhong S, Li H, Bodi Z et al (2008) MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 20:1278–1288
Zluvova J, Janousek B, Vyskot B (2001) Immunohistochemical study of DNA methylation dynamics during plant development. J Exp Bot 52:2265–2273
Acknowledgment
The author thanks the financial support from the National Natural Science Foundation of China (21522507, 21672166).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Yuan, BF. (2017). Analysis of Nucleic Acids Methylation in Plants. In: Rajewsky, N., Jurga, S., Barciszewski, J. (eds) Plant Epigenetics. RNA Technologies. Springer, Cham. https://doi.org/10.1007/978-3-319-55520-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-55520-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55519-5
Online ISBN: 978-3-319-55520-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)