Abstract
Antiphospholipid syndrome (APS) is a clinical syndrome characterized by macro- and/or microvascular thrombosis and pregnancy morbidity, with laboratory criteria including persistently positive lupus anticoagulant and/or anticardiolipin antibody and/or anti-β2 glycoprotein-1 antibody. Patients with antiphospholipid antibodies (aPL) appear to be at increased risk of thrombosis. Primary prophylaxis with aspirin is probably indicated to reduce the risk of a first event in selected patients with positive aPL, though data are limited; aspirin may also be the treatment of choice in selected low-risk patients with prior APS-associated stroke. Generally, secondary prevention of thrombosis involves the administration of anticoagulation as well as, similar to primary prophylaxis, identification and management of modifiable risk factors. Patients with aPL and a history of prior venous thromboembolism without arterial thrombosis are adequately treated with warfarin administered to a target INR of 2.0–3.0. There is a lack of good-quality evidence to support any particular therapeutic strategy for patients with a history of arterial thrombosis. Standard-intensity warfarin (INR 2.0–3.0) is used in many centers, though some centers may choose to use higher-intensity warfarin (INR ≥3.0). All patients with aPL (with/without APS) should engage in regular cardiovascular disease (CVD) screening programs according to national guidelines. Any such patient with an inflammatory rheumatic disease should be managed to minimize inflammatory disease activity, so-called treat-to-target approaches. Hydroxychloroquine is an additional therapy that may facilitate achieving treat-to-targets but may have additional CVD protective properties. Aspirin is frequently added, particularly to patients with “traditional” atherosclerotic risk factors and/or demonstrated atherosclerotic lesions. While a lower threshold for instituting additional CVD risk interventions seems reasonable, currently there is no consensus to support a particular threshold or risk adjustment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Antiphospholipid syndrome (APS) is a clinical syndrome characterized by both clinical and laboratory manifestations. Clinical manifestations most commonly include macro- and/or microvascular thrombosis, which may occur in the arterial or venous circulation, pregnancy morbidity, and thrombocytopenia . Laboratory criteria require persistent antiphospholipid antibody (aPL) positivity over a 12-week period for the lupus anticoagulant (LA) , and/or anticardiolipin antibody (aCL), and/or anti-β2--glycoprotein-I antibody (aβ2GPI) at moderate-to-high titers. This chapter will cover the prevention and treatment of thrombotic manifestations of APS; therapies for the non-thrombotic manifestations of APS, pregnancy morbidity, and the special clinical circumstance of catastrophic APS are found in other chapters.
The prevention and treatment of thrombotic complications is focused on the use of anticoagulants and/or antiplatelet agents rather than other therapies such as immunomodulatory interventions. Antithrombotic therapy has been revolutionized in the last decade by the introduction of a variety of novel parenteral and oral anticoagulants. Despite ongoing studies, high-quality evidence for the efficacy and safety of many of these medications in patients with APS is still lacking, and in most cases older, but better studied, regimens remain the mainstay of anticoagulant therapy for patients with APS.
Importance of Cardiovascular Disease and Venous Thrombosis Prevention in Antiphospholipid Antibody-Positive Patients
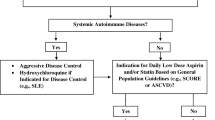
Risk modification strategies should always be considered for patients perceived to be at risk of atherosclerotic cardiovascular disease.
It has been noted in a number of studies that atherosclerotic cardiovascular events including stroke and myocardial infarction (MI) are increased in the presence of aPL. A case-control study nested within the Helsinki Heart Study noted higher titers of aCL in men who developed myocardial infarction (MI) or cardiac death, and men with MI/cardiac death had higher odds of having aCL titers in the upper quartile [1]. Similarly, Urbanus et al. [2] noted that young women with stroke or MI were more likely to have had LA/aβ2GPI. In both these studies, additional risk factors such as smoking (1,2), anti-oxidized LDL antibodies [1], or use of the oral contraceptive pill [2] further increased the odds ratio associated with MI or stroke.
There are also increasing data that aPL may play a key role in the atherogenic process. Previous work from Hasunuma et al. demonstrated increased uptake of oxidized LDL by macrophage Fc-gamma receptors in the presence of aβ2GPI [3]. In addition, endothelial cell apoptosis and aβ2GPI may upregulate dendritic cells to drive inflammatory and oxidative stress responses, both of which may further increase endothelial dysfunction and damage [4]. Recent studies of monocyte gene expression profiling and miRNA analyses also have shown significant overlap between inflammation, oxidative stress, and atherogenic pathways in patients with primary APS, systemic lupus erythematosus (SLE) , and SLE-associated APS [5, 6]. In the context of SLE, aPL are associated with subclinical atherosclerosis [7, 8] and clinical cardiovascular disease (CVD) events [9]. It remains a matter of debate however to what extent these data from a high-risk condition, SLE, are generalizable to primary APS. Andrade et al. found no difference in aortic pulse wave velocity (PWV) or carotid intima-media thickness (IMT) in primary APS vs healthy controls. However a subset analysis did show higher aortic PWV in primary APS patients with index arterial vs venous events [10].
To date there have been no formal studies attempting to determine how best to translate these observations into formal guidelines for cardiovascular disease (CVD) risk prevention. In a number of other inflammatory rheumatologic diseases, adjustment of population risk assessments has been recommended. For example, in rheumatoid arthritis (RA) , European League Against Rheumatism (EULAR) recommends that all patients should be screened and their CVD risk calculated according to national CVD risk protocols [11]. The percentage 10-year risk calculated should then be adjusted by ×1.5 to adjust for the risk associated with RA if 2/3 of the following are present: RA for >10 years, anti-CCP antibodies, or rheumatoid factor. In SLE the excess risk is much higher and less consistent between studies; therefore, such a simple multiplication is inappropriate and likely to be highly inaccurate. The American Heart Association recommends SLE be considered as an additional risk factor in women, thus lowering the threshold and ideal targets for risk factors like lipids and blood pressure [12]. Wajed et al. proposed that SLE be considered a “coronary heart disease equivalent ” and therefore to adopt more stringent targets for other CVD risk factors [13]. In a recent study investigating the outcomes of SLE patients (with/without aPL) who participated in a 3-year free-of-charge CVD prevention counseling program, investigators demonstrated selected CVD risk factors that can be modified with continuous counseling [14].
Which, if any, of the above is most appropriate for patients with aPL is unclear. Many uncertainties remain, e.g., how to deal with single- vs triple-positive patients without previous thrombosis or obstetric complications, also whether any risk adjustment should be different for venous, obstetric, or arterial APS patients. It is likely that different targets, or risk score adjustments, will be needed for these different situations. However, in the absence of definitive studies, it is hard to draw firm conclusions.
After a review of the evidence to date , we recommend the following:
-
All patients with aPL (with/without APS) should engage in regular cardiovascular screening programs according to national guidelines. Blood pressure control, smoking cessation, cholesterol and triglyceride management, and optimal diabetic control will help reduce the risk of arterial thromboembolism.
-
Any such patient with an inflammatory rheumatic disease should be managed to minimize inflammatory disease activity, so-called treat-to-target (T-2-T) approaches. Hydroxychloroquine is an additional therapy that may facilitate achieving T-2-T targets and may have additional CVD protective properties (see below).
-
Aspirin (see below) is frequently added, particularly to patients with “traditional” atherosclerotic risk factors and/or demonstrated atherosclerotic lesions (further discussed below).
-
While a lower threshold for instituting additional CVD risk interventions seems reasonable, currently there is no consensus to support a particular threshold or risk adjustment.
Patients also should be counseled for other traditional thrombosis risk factors, such as oral contraceptive use, prolonged immobilization during a long flight, postmenopausal hormone therapy, pregnancy and postpartum period, and prophylaxis during surgical procedures. When aPL-positive patients undergo surgery, the most effective pharmacologic methods should be combined with physical anti-thrombosis methods such as intermittent venous compression [15].
Primary Thrombosis Prevention
Patients with aPL appear to be at increased risk of thrombosis irrespective of the patient’s personal history of thromboembolism. In some studies, the risk of thrombosis in patients with positive aPL has been confounded by a high prevalence of SLE , which is in itself associated with thromboembolic complications [16]. However, a recent meta-analysis of thrombosis risk associated with aPL positivity in patients without SLE demonstrated that such patients also have significant increases in the risk of thrombosis compared to the general population [17]. Reynaud and colleagues examined 30 studies enrolling a total of 16,441 patients and found the odds ratio (OR) for venous and arterial thrombosis to be 6.14 and 3.58, respectively, in patients with a positive LA test. Anticardiolipin antibodies were associated with ORs of 1.46 and 2.65 for venous and arterial thrombosis, respectively, and aβ2GPI were associated with ORs of 3.12 for arterial thrombosis. The authors noted an overall low quality of evidence suggesting that the risk may have been inflated through reporting biases (i.e., the potential for event rates to have been inflated by reporting of patients with a history of the aPL and thrombosis, with a reduced likelihood of reporting of patients without these outcomes) [17]. The presence of more than one aPL probably increases the risk of thrombosis further. Pengo and colleagues examined 618 consecutive patients (of whom 55% had a prior history of thromboembolism) and compared patients with a history of thrombosis with those without [18]. They found that “triple positivity” (persistent positivity of LA, aCL, and aβ2GPI) was associated with an odds ratio of 33 for thrombosis (95% confidence interval 7.0–157.6). However, both male gender and venous thrombosis risk factors were additionally, and independently, associated with thrombosis supporting the multifactorial nature of thrombosis in aPL-positive patients . The recently developed Global APS Score (GAPSS) score may help estimate the risk of future thrombosis for individuals with positive aPL [19, 20] (discussed in Chap. 9).
The observation that patients with aPL are at increased risk of first thrombosis, especially in the setting of other risk factors, would suggest that primary prophylaxis (i.e., the administration of prophylactic treatment prior to a first episode of venous or arterial thromboembolism) may be of benefit. However any benefit of primary thromboprophylaxis in aPL-positive patients must be weighed against the risk of bleeding associated with anticoagulants and/or antiplatelet agents and be evaluated in light of the likelihood of reductions in the risk of thrombosis due to modification of other risk factors.
Aspirin is an optional preventative therapy in a variety of patients at risk of both arterial and venous thrombosis. Arnaud and colleagues examined ten observational studies and one randomized control trial including 1208 patients with positive aPL who experienced a total of 139 venous and arterial thromboembolic events. Aspirin-treated patients were protected against a first arterial event (OR 0.48 (0.28–0.82)) but not against a first venous event (0.58 (0.32–1.06)). Interpretation on this analysis is limited by heterogeneity, inclusion of both observational and interventional studies, and a lack of consistency in laboratory criteria for diagnosis . The finding that the beneficial effect of aspirin was confined to the non-prospective studies highlights the uncertainty about how (or whether) these observations should impact clinical practice [21]. Similar findings were reported in a more recent patient-level analysis [22].
The lack of convincing evidence of benefit of aspirin suggests that more intensive anticoagulant options should be undertaken with great care and only in highly selected patients. For most patients with aPL, it is likely that the bleeding risk associated with anticoagulant use would outweigh the small potential absolute benefit of primary thrombosis prevention. Cuadrado et al. examined the number of thrombotic events among patients with aPL and a history of SLE and/or prior obstetric morbidity randomized to receive low-dose aspirin or low-dose aspirin plus warfarin targeted to an international normalized ratio of 1.5. A total of 82 patients were allocated to low-dose aspirin and 84 to low-dose aspirin and warfarin . Over the total enrolment period of 5 years, eight patients had a thrombotic event (four per arm, p = NS). Eleven patients allocated to dual therapy reported abnormal bleeding compared with none allocated to low-dose aspirin. The authors concluded that low-dose aspirin plus warfarin cannot be justified as a primary prevention strategy given the lack of evidence of efficacy and reasonable evidence of toxicity [23].
Hydroxychloroquine has multiple beneficial effects in SLE patients , and it may reduce the levels and/or activity of aPL [24]. A prospective study of hydroxychloroquine in patients with aPL was terminated early due to a low recruitment rate exacerbated by a prolonged manufacturing shortage and price increase of hydroxychloroquine in the United States [25]. A recent systematic review found no evidence of a therapeutic benefit for hydroxychloroquine in patients with aPL concurrently treated with aspirin [22]. Toxicities include ocular abnormalities which increase toward 1% after 5–7 years of use or a cumulative dose of 1000 g, mandating regular ophthalmologic examinations [26].
Case reports and other very low-quality evidence have suggested that a variety of interventions, including intravenous immunoglobulin and immunosuppression, may be of benefit in selected patients with aPL; however, such evidence is highly prone to bias and is not relevant to the “average patient” with aPL and no prior history of thrombosis.
In summary, patients with aPL (particularly those with other systemic autoimmune diseases and multiple serologic abnormalities) are at an enhanced risk of both venous and arterial thrombosis compared with patients in the general population. Aspirin can be considered to reduce the risk of a first thrombotic event in patients with persistently positive aPL, especially in those with other cardiovascular risk factors; however, the net benefit of this intervention remains uncertain. More intensive prophylactic treatment , such as a combination of aspirin with low-intensity warfarin, appears ineffective and is associated with enhanced toxicity . Hydroxychloroquine should be used as part of a strategy to mitigate SLE complications , but the effectiveness is unknown in aPL-positive patients without other systemic autoimmune diseases. There is insufficient evidence to justify other prophylactic treatment strategies against thrombosis in patients with aPL, with or without underlying autoimmune diseases. Risk factor modification should be undertaken in all patients to reduce their risk of atherosclerotic vascular disease and venous thromboembolism.
Secondary Thrombosis Prevention
In general, secondary prevention of thromboembolism involves the administration of anticoagulation as well as the identification and management of modifiable risk factors as discussed above.
The risk of recurrent thrombosis in patients with aPL has not been clearly identified. In general, patients are divided into those with one or more venous thromboembolic events (without arterial events) or those with prior arterial events (irrespective of their history of venous events). Garcia et al. highlighted the poor quality of data describing the risk of recurrence in patients with aPL and prior venous thromboembolism. In their study of more than 500 patients with aPL (compared with more than 1900 patients without aPL), the unadjusted risk for recurrent venous thromboembolism after stopping anticoagulation was 1.53 (0.76–3.11) for patients with aCL and 2.83 (0.83–9.64) for patients with a positive LA test. Neither reached statistical significance, and the authors concluded that positive aPL tests increase the risk of recurrent venous thromboembolism; however, “the strength of this association is uncertain because the available evidence is of very low quality” [27].
Venous Thrombosis (Without a History of Arterial Thrombosis)
Patients with persistent positivity of aPL and a history of one or more venous thrombotic events are adequately treated with warfarin administered to achieve an international normalized ratio (INR) of 2.0–3.0. This conclusion is based upon two randomized controlled trials both of which were designed to demonstrate superiority of higher-intensity warfarin and both of which counterintuitively showed a nonsignificantly increased risk of recurrent thrombosis when high-intensity warfarin (target INR > 3.0) was compared to a more conventional approach (INR of 2.0–3.0) [28, 29]. No studies have examined the optimal duration of anticoagulation in patients with persistent positive aPL and one or more venous thromboembolic events; in the absence of such evidence, most “experts” support extended duration therapy for such patients [30]. There is no evidence to support the addition of aspirin to warfarin administered to achieve an INR of 2.0–3.0 in patients with a history of aPL and prior venous thromboembolism . Although the direct oral anticoagulants are appealing alternatives to warfarin in many clinical settings, there are only sporadic reports on their use in patients with aPL [31, 32]. The results of randomized controlled trials on their efficacy and safety in patients with APS are pending (discussed in Chap. 18).
Other agents, such as intravenous immunoglobulin or immunosuppression (e.g., with corticosteroids), are the subject of anecdotal reports of success in preventing recurrent venous thromboembolism; however, such reports should be regarded with skepticism, given small numbers, short follow-up periods , and exceptional case selection. Agents such as therapeutic dose low-molecular-weight heparin should be reserved for patients with recurrences despite usual therapeutic anticoagulation [33].
In summary, patients with aPL and a history of prior venous thrombosis and no previous arterial thrombosis can be adequately treated with warfarin with a target INR of 2.0–3.0. The direct oral anticoagulants are an appealing option for such patients; however, until reasonable evidence on their safety and efficacy is available, their use should be confined to selected patients who are fully informed with respect to the lack of good-quality evidence for their use.
Arterial Thrombosis (Irrespective of the History of Venous Thrombosis)
Patients with persistent aPL and a history of arterial thrombosis represent a more difficult clinical scenario. As previously discussed, risk factor modification is likely to be critical, particularly in older patients or those with additional risk factors.
The “aPL and Stroke Study” enrolled a selected group of patients who had a single positive aPL determination and a prior stroke, treated with aspirin or warfarin (administered to a nonstandard therapeutic intensity of 1.4–2.8), and followed for recurrent vascular events over a 2-year period. The rates of recurrent vascular events were similar in both groups. Criticisms of this study include the failure to enroll patients with persistent antibody positivity, the use of nonstandard warfarin intensity , and a demographic profile quite different from that generally seen in patients acknowledged to have APS [34]. A recently published follow-up analysis wherein selected patients underwent serial testing for aCL, aβ2GPI, and anti-phosphatidylserine antibodies from stored serum found that none of persistently present aCL and anti-phosphatidylserine antibodies or transiently positive aβ2GPI, anti-phosphatidylserine antibodies, or aCL were associated with an increased risk of recurrent thrombosis. Persistently positive aβ2GPI were associated with reduced time to event/death (hazard ratio 2.86 (1.21–6.76)). Unfortunately, LA, the antiphospholipid antibody test most strongly related to thrombosis, could not be determined [35].
The two randomized controlled trials which have established warfarin administered to a target INR 2.0–3.0 as the preferred treatment for patients with prior venous thromboembolism and persistent aPL positivity enrolled a relatively small number of patients with prior arterial thrombosis [28, 29]. As a result, there are limited data to support any particular treatment strategy in these patients. Furthermore, given the low rate of recurrent thrombosis observed to date in studies of anticoagulant strategies in such patients, it is unlikely that further methodologically rigorous evidence will become available – the required sample sizes for definitive studies are likely to make such studies unfeasible. Potential strategies include “usual-intensity warfarin” (target INR 2.0–3.0), with or without aspirin, higher-intensity warfarin (target INR greater than 3.0) with or without aspirin, alternate anticoagulant strategies (e.g., long-term therapeutic dose low-molecular-weight heparin), or the combination of one of these anticoagulant strategies with an alternate treatment designed to reduce the likelihood of recurrence [30].
“Usual-intensity warfarin” is the preferred treatment for the prevention of recurrent arterial thrombosis in many clinical centers and has been recommended by widely regarded, evidence-based guidelines [36]. The addition of aspirin is frequently considered, particularly in patients with additional atherosclerotic vascular risk factors who would be treated with antiplatelet therapy if they did not have persistent aPL positivity. As noted, the safety of this treatment remains controversial given the lack of good-quality, prospective data.
“Higher-intensity warfarin” is a preferred option in some clinical centers. However, maintaining an INR above 3.0 is technically difficult and may increase the risk of bleeding. Such therapy should be confined to specialized institutions familiar with the risks and benefits of more intense warfarin therapy. Addition of aspirin to “higher-intensity warfarin ” is likely to increase the risk of bleeding to an unreasonable degree in many patients; as a result, the combination of aspirin plus higher-intensity warfarin should be confined to those patients for whom other, less hazardous, antithrombotic strategies would be expected to fail.
Alternate therapeutic strategies, such as long-term therapeutic dose low-molecular-weight heparin, should be confined to patients with objectively confirmed failure of therapeutic anticoagulation with warfarin . The cost, complexity and potential for bleeding with such therapy make it an unreasonable “first choice” for such patients.
Certain patients with APS present with a fulminant “catastrophic” thrombotic course characterized by recurrent arterial and/or venous thrombosis despite adequate anticoagulation. Such patients may be treated with intensified low-molecular-weight heparin or more aggressive therapies such as immunomodulation with immunosuppressive drugs, intravenous immunoglobulin, plasma exchange or rituximab (discussed in a Chap. 17).
In summary, there is a lack of good-quality evidence to support any particular therapeutic strategy in patients with persistent aPL positivity and a history of arterial thrombosis (with or without prior venous thrombosis). Standard-intensity warfarin (administered to an INR of 2.0–3.0) is used in many centers based on limited data from two randomized controlled trials. Some centers may choose to use higher-intensity warfarin (administered to an INR of greater than 3.0). Aspirin is frequently added, particularly to patients with “traditional” atherosclerotic risk factors and/or with demonstrated atherosclerotic lesions with concomitant usual intensity warfarin; however, evidence for additional efficacy over and above that of warfarin is limited, and the addition of aspirin will increase the risk of bleeding. Aspirin alone may be the preferred agent in some patients presenting with stroke (e.g., perhaps those with low-risk serological characteristics). The direct oral anticoagulants are not indicated for the prevention of recurrent arterial thrombosis in most jurisdictions and as such should be avoided in these patients. Other agents, such as therapeutic dose low-molecular-weight heparin, may be considered in patients with objectively confirmed recurrent thrombosis despite adequate warfarin therapy. An additional role of hydroxychloroquine in the secondary prevention of arterial thrombosis is suggested; however, no studies have yet demonstrated such an effect, their use being thus restricted to empirical therapy for patients with recurrent thrombosis despite correct antithrombotic treatment or with bleeding complications or risk that preclude the use of anticoagulant drugs. Further research is required to better define “optimal therapy” for these patients, given the small number of patients currently enrolled in studies and the catastrophic negative outcomes of recurrent thrombosis.
Group Conclusion (Table 11.1)
Prevention of first and recurrent thrombosis in patients with aPL is a high clinical priority since such patients appear to be at increased risk of thrombosis. Primary prevention with aspirin is considered in patients with persistently positive aPL, especially in those with additional indications for aspirin, but should not be considered “standard” in patients who do not have such risk factors. Secondary prevention of patients with venous thrombosis should consist of warfarin administered to an INR of 2.0–3.0. Most patients with prior arterial thrombosis are treated with warfarin at a target INR of 2.0–3.0; however, evidence to support this intervention is weak. In some centers, there is a preference for higher-intensity warfarin or addition of low-dose aspirin to a standard intensity anticoagulation regime. The published experience with direct oral anticoagulants to treat thrombosis in patients with aPL is very limited. Good-quality studies are urgently needed for many clinical decisions relevant to patients with aPL who have, or at risk of, thrombosis as current evidence to guide practice is limited.
References
Vaarala O, Mänttäri M, Manninen V, et al. Anti-cardiolipin antibodies and risk of myocardial infarction in a prospective cohort of middle-aged men. Circulation. 1995;91:23–7.
Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol. 2009;8:998–1005.
Hasunuma Y, Matsuura E, Makita Z, Katahira T, Nishi S, Koike T. Involvement of beta 2-glycoprotein I and anticardiolipin antibodies in oxidatively modified low-density lipoprotein uptake by macrophages. Clin Exp Immunol. 1997;107:569–73.
Broder A, Chan JJ, Putterman C. Dendritic cells: an important link between antiphospholipid antibodies, endothelial dysfunction, and atherosclerosis in autoimmune and non-autoimmune diseases. Clin Immunol. 2013;146:197–206.
Perez-Sanchez C, Barbarroja N, Messineo S, et al. Gene profiling reveals specific molecular pathways in the pathogenesis of atherosclerosis and cardiovascular disease in antiphospholipid syndrome, systemic lupus erythematosus and antiphospholipid syndrome with lupus. Ann Rheum Dis. 2015;74:1441–9.
Pérez-Sánchez C, Aguirre MA, Ruiz-Limón P, et al. Atherothrombosis-associated microRNAs in antiphospholipid syndrome and systemic lupus erythematosus patients. Sci Rep. 2016;6:31375.
Ahmad Y, Shelmerdine J, Bodill H, et al. Subclinical atherosclerosis in systemic lupus erythematosus (SLE): the relative contribution of classic risk factors and the lupus phenotype. Rheumatology. 2007;46:983–8.
Kiani AN, Vogel-Claussen J, Arbab-Zadeh A, Magder LS, Lima J, Petri M. Semiquantified noncalcified coronary plaque in systemic lupus erythematosus. J Rheumatol. 2012;39:2286–93.
Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol. 2012;176:708–19.
Andrade D, Bortolotto L, Bonfá E, Borba E. Primary antiphospholipid syndrome: absence of premature atherosclerosis in patients without traditional coronary artery disease risk factors. Lupus. 2016;25:472–8.
Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–31.
Mosca L, Benjamin EJ, Berra K, et al. American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women – 2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57:1404–23.
Wajed J, Ahmad Y, Durrington PN, Bruce IN. Prevention of cardiovascular disease in systemic lupus erythematosus – proposed guidelines for risk factor management. Rheumatology. 2004;43:7–12.
Yelnik CM, Richey M, Haiduc V, Everett S, Zhang M, Erkan D. Cardiovascular disease prevention counseling program for systemic lupus erythematosus patients. Arthritis Care Res (Hoboken). 2016; doi:10.1002/acr.23128.
Erkan D, Leibowitz E, Berman J, et al. Perioperative medical management of antiphospholipid syndrome: hospital for special surgery experience, review of literature, and recommendations. J Rheumatol. 2002;29:843–9.
Hinojosa-Azaola A, Romero-Diaz J, Vargas-Ruiz AG, et al. Venous and arterial thrombotic events in systemic lupus erythematosus. J Rheumatol. 2016;43:576–86.
Reynaud Q, Lega JC, Mismetti P, et al. Risk of venous and arterial thrombosis according to type of antiphospholipid antibodies in adults without systemic lupus erythematosus: a systematic review and meta-analysis. Autoimmun Rev. 2014;13:595–608.
Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood. 2011;118:4714–8.
Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. The global anti-phospholipid syndrome score in primary APS. Rheumatology (Oxford). 2015;54:134–8.
Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. GAPSS: the global anti-phospholipid syndrome score. Rheumatology. 2013;52:1397–403.
Arnaud L, Mathian A, Ruffatti A, et al. Efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies: an international and collaborative meta-analysis. Autoimmun Rev. 2014;13:281–91.
Arnaud L, Mathian A, Devilliers H, et al. Patient-level analysis of five international cohorts further confirms the efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies. Autoimmun Rev. 2015;14:192–200.
Cuadrado MJ, Bertolaccini ML, Seed PT, et al. Low-dose aspirin vs low-dose aspirin plus low-intensity warfarin in thromboprophylaxis: a prospective, multicentre, randomized, open, controlled trial in patients positive for antiphospholipid antibodies (ALIWAPAS). Rheumatology (Oxford). 2014;53:275–84.
Broder A, Putterman C. Hydroxychloroquine use is associated with lower odds of persistently positive antiphospholipid antibodies and/or lupus anticoagulant in systemic lupus erythematosus. J Rheumatol. 2013;40:30–3.
Erkan D, Sciascia S, Unlu O, et al. A multicenter randomized controlled trial of hydroxychloroquine in primary thrombosis prophylaxis of persistently antiphospholipid antibody-positive patients without systemic autoimmune diseases. Lupus. 2016;25(Suppl 1S):88 (abstract).
Ding HJ, Denniston AK, Rao VK, Gordon C. Hydroxychloroquine-related retinal toxicity. Rheumatology (Oxford). 2016;55:957–67.
Garcia D, Akl EA, Carr R, Kearon C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood. 2013;122:817–24.
Finazzi G, Marchioli R, Brancaccio V, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost. 2005;3:848–53.
Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med. 2003;349:1133–8.
Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at the 13th international congress on antiphospholipid antibodies. Lupus. 2011;20:206–18.
Schaefer JK, McBane RD, Black DF, Williams LN, Moder KG, Wysokinski WE. Failure of dabigatran and rivaroxaban to prevent thromboembolism in antiphospholipid syndrome: a case series of three patients. Thromb Haemost. 2014;112:947–50.
Signorelli F, Nogueira F, Domingues V, Mariz HA, Levy RA. Thrombotic events in patients with antiphospholipid syndrome treated with rivaroxaban: a series of eight cases. Clin Rheumatol. 2016;35:801–5.
Dentali F, Manfredi E, Crowther M, Ageno W. Long-duration therapy with low molecular weight heparin in patients with antiphospholipid antibody syndrome resistant to warfarin therapy. J Thromb Haemost. 2005;3:2121–3.
Levine SR, Brey RL, Tilley BC, et al. Antiphospholipid antibodies and subsequent thrombo-occlusive events in patients with ischemic stroke. JAMA. 2004;291:576–84.
Amory CF, Levine SR, Brey RL, et al. Antiphospholipid antibodies and recurrent thrombotic events: persistence and portfolio. Cerebrovasc Dis. 2015;40:293–300.
Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e152S–84S.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Crowther, M. et al. (2017). Prevention and Treatment of Thrombotic Antiphospholipid Syndrome. In: Erkan, D., Lockshin, M. (eds) Antiphospholipid Syndrome. Springer, Cham. https://doi.org/10.1007/978-3-319-55442-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-55442-6_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55440-2
Online ISBN: 978-3-319-55442-6
eBook Packages: MedicineMedicine (R0)