Abstract
Despite the many positive impacts of petroleum hydrocarbons to human industrialization and activity, environmental contamination by petroleum hydrocarbons represents a major cause of marine and terrestrial pollution. Petroleum hydrocarbons contain various compounds such as alkanes, light aromatics (MAHs), cycloalkanes, heavy aromatics (PAHs) and asphaltenes, among others. A number of these compounds are potentially carcinogenic and mutagenic. Among the various remediation technologies, bioremediation or the use of microorganisms to degrade the hydrocarbons is considered a clean, cost-effective and environmentally friendly approach. Unlike other physical and chemical methods, it does not lead to secondary contamination, generally resulting in the complete mineralization of hydrocarbons. Several reports have now confirmed bioremediation as a promising technology to clean up the environments. This chapter presents an overview of current bioremediation approaches for the treatment of petroleum hydrocarbons.
Esmaeil Shahsavari and Gregory Poi contributed equally to this work.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
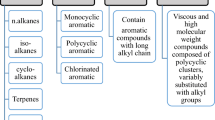
Petroleum hydrocarbon (PHC) pollution is commonly associated with operations at oil refineries, chemical plants and shipyards. Industrial activities and contingency situations such as tanker spills or leakage from storage tanks in aquatic and terrestrial environments pose significant hazards. The problem is compounded when petrol, diesel, gasoline and other petrochemical products contaminate groundwater (Andreoni and Gianfreda 2007). As a result, the release of petroleum hydrocarbon (e.g., crude oil) into the environment is a major cause of marine and terrestrial pollution (Kingston 2002; Macaulay and Rees 2014). The composition of crude oil varies, but on average there is a rough parity between paraffins, naphthenes and aromatic hydrocarbons. Paraffins are saturated linear and branched hydrocarbons, while naphthenes are cyclic saturated hydrocarbons (Fig. 1). Hydrocarbons are not all biodegraded at similar rates and not all hydrocarbons are readily degradable, but estimates for different crude oils range from 70 to 90% degradability, with the remaining hydrocarbons being primarily the asphaltenes and resins (Prince et al. 2003).
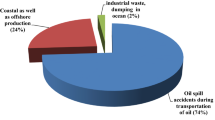
The susceptibility of crude oil components to microbial degradation has been described as follows: alkanes > light aromatics (MAHs) > cycloalkanes > heavy aromatics (PAHs) > asphaltenes (van Hamme et al. 2003). PAHs may contain one or more benzene rings, and they include naphthalene (two-ringed), phenanthrene (three-ringed) and anthracene (three-ringed) which are considered low molecular weight or light PAHs, while those with four or more rings such as pyrene (four-ringed), chrysenes (four-ringed), fluorenthene (five-ringed), benzo[a]pyrene (five-ringed) and coronenes (seven-ringed) are referred to as heavy PAHs. These common petroleum pollutants are considered to be potentially mutagenic and carcinogenic (Boonchan 2000; Mao et al. 2012). Consequently, the contamination of marine environments by hydrocarbons represents a global concern with potential consequences for both ecosystem and human health (Andersson et al. 2006). It is estimated that between 1.7 and 1.8 million metric tonnes of crude oil find their way into the world’s water every year, of which more than 90% is directly related to human activities (Nikolopoulou et al. 2007). Therefore, the bioremediation of contaminated environments is of great public concern. Petroleum hydrocarbons are only eliminated from the environment when converted to carbon dioxide and water by two processes, combustion and biodegradation . The remediation techniques used include are physical, chemical and biological (bioremediation) methods. Amongst these, bioremediation approaches or the use of microbes for the degradation of hydrocarbons are considered as clean and cost-effective technologies. In this book chapter, fundamental knowledge regarding the bioremediation of petroleum hydrocarbons in contaminated environments is presented.
Bioremediation Approaches
Bioremediation of contaminated environments relies on breaking down target pollutant compounds by microbial degradation. While biostimulation (BS) typically involves the addition of nutrients or substrates in the form of nitrogen and phosphate, bioaugmentation (BA) requires the addition of microbial cultures to the contaminated matrix, usually in combination with biostimulation (Boopathy 2000). This may be implemented as an in situ process that includes strategies such as soil amendment, bioventing or biosparging, bioslurping, phyto-/rhizoremediation and monitored natural attenuation. Ex situ processes require the soil materials to be excavated and loaded into a bioreactor pit or a treatment facility, and they include biopiling, composting, bioreactors and land farming (Macaulay and Rees 2014). An overview of bioremediation methods is presented in Fig. 2.
The treatment of waste solids, including soil containing hydrocarbon pollutants, can be relatively expensive making economic drivers the primary determinant behind the chosen options (Makadia et al. 2011). However, there are other considerations such as opportunity costs and the demands of the locality which pose practical restrictions on the application of certain technologies.
Bioremediation of Petroleum Hydrocarbon Pollution from Marine Oil Spills
In a review on the efficacy of bioremediation on marine oil spills (on the surface and on shorelines), bioremediation was determined to be effective, but no advantage was found with bioaugmentation with commercial microbial preparations over biostimulation of indigenous organisms. However, this was viewed from a perspective of a last resort technology following a marine oil spill that could not be collected or burnt and had to be dealt with in situ within a hostile environment either still floating on the surface at sea or on a shoreline (Prince et al. 2003).
The use of physical and chemical methods for petrogenic hydrocarbon remediation is inadequate, in that these methods do not completely remediate the hydrocarbons in the environment (Gavrilescu et al. 2014). Reports of large oil spills, marine or otherwise, often capture public attention followed by a demand for a prompt and environmentally sensitive response. In situations where the containment of the oil spill with booms or collection with skimmers is impractical, stimulating the natural biodegradation of oil offers the next best alternative. Such an approach would include strategies such as the spraying of dispersants to enhance the surface area for microbial colonization, as well as nutrient supplementation without the addition of cultures. In contrast to biostimulation, bioaugmentation involves the addition of exogenous cultures to initiate and accelerate the process of bioremediation but lacks effective, quantitative demonstration (Prince 2010).
It has been reported that hydrocarbon-degrading bacteria are ubiquitous in the sea, and thus biostimulation would suffice. The problem lies in the slow-acting nature of the process. It may be too slow to prevent oil from reaching the shore and causing environmental damage as documented in the Exxon Valdez and Gulf of Mexico disasters. The introduction of uric acid has been recommended as a means to accelerate the process by providing the supply of nitrogen and phosphorus required, given that these constitute the rate-limiting factor for petroleum degradation at sea (Ron and Rosenberg 2014). Others suggest the addition of rhamnolipids as biosurfactants to enhance the rate of marine oil spill bioremediation (Chen et al. 2013). From a different perspective, it has been demonstrated that the process of bioaugmentation could be enhanced by using autochthonous bioaugmentation (ABA ) , defined as the exclusive use of adapted indigenous microorganisms for decontamination. The rate of hydrocarbon degradation was enhanced by the addition of lipophilic fertilizers (uric acid and lecithin) in combination with rhamnolipids acting as biosurfactants along with the addition of adapted indigenous microorganisms (Nikolopoulou et al. 2013).
The lack of quantitative demonstration of the efficacy on bioaugmentation in the field has been a considerable obstacle to the adoption of this technique as a tool for the biodegradation of petroleum hydrocarbon spills at sea (Prince 2010). This has been compounded by a gap between the availability of peer-reviewed documentation for laboratory-based work and that for field-based work to demonstrate effective translation and scale-up (Macaulay and Rees 2014). There has been an increase in attempts to bridge this gap as demonstrated by more recent work that follow up from bench-scale experimentation using shake flask to laboratory-based mesocosm experiments at pilot scale using large volumes such as 840 L (Bao et al. 2012) and 10,000 L (Hassanshahian et al. 2014). In the former, preliminary shake flask trials conducted on a mixed-species consortium containing four strains of marine bacterial isolates were found to be suitable candidates for the degradation of crude oil in a simulated marine environment and subsequently scaled up in a mesocosm experiment using a tank (1.5 m × 0.8 m × 0.7 m) with a volume of approximately 840 L. These four strains which included Ochrobactrum sp. (N1), Brevibacillus parabrevis (N2), B. parabrevis (N3) and B. parabrevis (N4) removed over 51.1% of crude oil from the simulated water body (Bao et al. 2012). In the latter, three different series of experiments were performed in a ‘mesocosm facility’ (10,000 L) where natural seawater was artificially polluted with crude oil (1000 ppm) and was amended with inorganic nutrients (Mesocosm 1, M1), inorganic nutrient plus an inoculum of Alcanivorax borkumensis SK2T (Mesocosm 2, M2) and inorganic nutrient plus an inoculum of A. borkumensis SK2T and Thalassolituus oleivorans MIL-1 T (Mesocosm 3, M3), respectively.
Experimental analyses performed in the mesocosms showed that the load of crude oil increased the total microbial abundance but inhibited the activity of some enzymes while stimulating some others. Bioaugmentation with only A. borkumensis SK2T produced the highest percentage of degradation (95%) in comparison with the biostimulation treatment (80%) and bioaugmentation using an Alcanivorax-Thalassolituus bacterial consortium (70%), which indicated an unfavourable interaction between the two bacterial genera used (Hassanshahian et al. 2014). This suggests that simply combining different species of hydrocarbonoclastic bacteria is not necessarily an advantage in the design of suitable consortia of biodegradation of hydrocarbons.
It was reported that Acinetobacter and Cloacibacterium were the dominant genera in freshwater microcosms, while the Oceanospirillales order and the Marinobacter, Pseudomonas and Cycloclasticus genera predominated in marine microcosms. It was also found that the Oceanospirillales order and the Marinobacter genus were selected in the different hydrocarbon-containing microcosms in hypersaline water. Pseudomonas appears to be the only genus of hydrocarbonoclastic bacteria present in freshwater, seawater and terrestrial systems (Afzal et al. 2007; Felföldi et al. 2010; Kadali et al. 2012; Mirdamadian et al. 2010; Zhang et al. 2011; Zhao et al. 2011). Nevertheless, the development of bioremediation as a technology for cleaning up oil spills is ongoing and has been driven by the relative low costs involved and the favourable impact it has on the environment as compared to alternative technologies (Macaulay and Rees 2014).
Bioremediation of Terrestrial Oil Spills
Growing industrialization and demands for energy have led to soil contamination by crude oil and refined products. If not mitigated, these petroleum hydrocarbon (PHC) pollutants pose a threat to both the environment and human health (Sanscartier et al. 2011). Diesel oil is a complex mixture of alkanes and aromatic compounds which is frequently reported in terrestrial hydrocarbon spills, often found leaking from storage tanks and pipelines or released in accidental spills and has been the subject of several pilot-scale to field-scale clean-up projects (Chemlal et al. 2013; Łebkowska et al. 2011).
Bioremediation Strategies to Treat PHC-Contaminated Soil
Landfarming is essentially a low-cost and low-technology method of ex situ biostimulation that has been successful in degrading PHC-contaminated soil, mainly in the superficial layer of soils since most oleophilic microbes are confined to the 15–30 cm region (Zouboulis and Moussas 2011). While reportedly effective for the degradation of low molecular weight PAHs (Picado et al. 2001), it has been shown to be unsuccessful in the degradation of heavy PAHs and requires a long residence time (Macaulay and Rees 2014). Composting is another simple ex situ aerobic biostimulation technology that uses organic amendments such as manure (Akinde and Obire 2008; Groudeva et al. 2001) and biowaste (Van Gestel et al. 2003) to provide both the microbial consortia and nutrients. The use of bioreactors for soil bioremediation overcomes some of the problems associated with the supply of oxygen and delivery of nutrients to the aerobic microorganisms, offering some degree of control over the environmental factors that influence biodegradation (Zouboulis and Moussas 2011).
Bioremediation Strategies to Treat PHC-Contaminated Soil and Groundwater
PHC contamination of soil and groundwater poses a major concern for human health and the environment (Andreoni and Gianfreda 2007; Paul et al. 2005; Dorn and Salanitro 2000). The release of fugitive PHC materials into the environment makes in situ bioremediation the only option where biostimulation or slow monitored natural attenuation is acceptable where no other options exist. In this context, the efficacy of bioremediation of groundwater (GW) in situ has been by most accounts attributed to natural managed attenuation and monitored natural attenuation (Aburto 2007; Aburto and Ball 2009; Aburto et al. 2009; Aburto and Peimbert 2011) as opposed to biodegradation and bioaugmentation (Chapelle 1999), with reports of effective bioremediation taking relatively long periods of 1–2 years when biostimulation was applied (Kao et al. 2008; Chen et al. 2010).
The most successful cases of bioaugmentation that have been documented are those using bioreactors to optimize the growth and activity of the microbial population to bioremediate the contaminated groundwater (El Fantroussi and Agathos 2005). This ex situ bioremediation strategy involved the pumping of the polluted groundwater for biotreatment followed by the injection of the treated groundwater back into the polluted site as part of a ‘pump and treat’ system. The integration of fixed-film microbial growth with such a system had been shown to be effective in the treatment of contaminated groundwater (Rodríguez-Martínez et al. 2006). Although effective, the costs associated with the building of the wells and the treatment process have been reported to be relatively high compared to other strategies (Macaulay and Rees 2014). However, these costs can be effectively mitigated using the same monitoring wells that would have already been in place for routine sample analyses for the ‘pump-out’ and recharge injection. The costs can be further reduced when coupled with a simplified modular bioreactor that has been designed for low operational costs that can be transported from site to site.
While the observation for bioremediation of groundwater has been attributed primarily to natural managed attenuation excluding the ex situ ‘pump and treat’ method (Chapelle 1999), this observation does not appear to be reflected in the literature in the case of land-based bioremediation of PHC-contaminated soil where the bioremediation is often carried both in situ and ex situ. An earlier study compared different approaches on the bioremediation of diesel-contaminated soil (Bento et al. 2005) using natural attenuation, biostimulation and bioaugmentation. The laboratory-scale study used a microbial consortia derived from hydrocarbonoclastic isolates sourced from Long Beach, California, USA, using 450 g soil taken from a beach in Hong Kong. The consortium had been shown to be effective for the degradation of TPH in diesel-contaminated Long Beach soil (California), where it was more effective than natural attenuation or biostimulation after 12 weeks. However, when the same consortium was applied to the diesel-contaminated soil from Hong Kong, Bento et al. (2005) reported that biostimulation (addition of nutrients) was less effective than natural attenuation or bioaugmentation, with natural attenuation being most effective at the degradation of diesel as measured by the reduction in light oil fraction for C12–C23. However, bioaugmentation for the degradation of the heavy oil fraction for C23–C40 was more effective than biostimulation, followed by natural attenuation in Hong Kong soil. It was in this context that Bento et al. (2005) reported that ‘the consortium degraded 73–75% of the light and heavy oil fraction of the TPH present in the Long Beach soil contaminated with diesel oil but had no effect on the Hong Kong soil”. Overall, optimum bioaugmentation performance occurs when the exogenous organisms are capable of competing with the indigenous microbes for nutrients resulting in increased abundance. This is consistent with the enrichment of indigenous microorganisms from a given microcosm to be used for inoculation for bioaugmentation in hydrocarbon-contaminated soil (Łebkowska et al. 2011), also referred to autochthonous bioaugmentation (Nikolopoulou et al. 2013). There have recently been several studies comparing the efficacy of different bioremediation approaches including natural attenuation, biostimulation and bioaugmentation of PHC-contaminated environments. The general finding has been that bioaugmentation in combination with biostimulation usually provides a faster rate of bioremediation than biostimulation on its own with a variety of hydrocarbon pollutants across a wide range of conditions (Calvo et al. 2009; Coulon et al. 2010; Kauppi et al. 2011; Łebkowska et al. 2011; Grace Liu et al. 2011; Sheppard et al. 2011; Zhao et al. 2011).
Translation and Scale-Up
Laboratory experiments have to be effectively extrapolated to the field scale (Diplock et al. 2009). The optimization of operational parameters is an important part of the process to evaluate the strategies in the implementation of a bioremediation process. While laboratory-scale experiments provide an opportunity to gain insights into the conditions for effective translation and scale-up for large-scale operations, it is with the caveat that the biotreatment can be accurately reproduced at laboratory scale (Lors et al. 2012). This is because it is not always possible to replicate field conditions in the lab, a key example being the absence of ecological considerations in most laboratory experiments, where the presence of predators and antagonistic microbes are capable of impacting on the efficacy of a given process (Macaulay and Rees 2014).
Hydrocarbonoclastic Bacteria in the Bioremediation of PHC-Contaminated Soil
TPH has been used to evaluate the efficacy of bioremediation as a means to bioremediate PHC-contaminated soil in bench-scale experiments (Aleer et al. 2010; Sheppard et al. 2011; Shahsavari et al. 2013; Adetutu et al. 2013) as well as field-scale experiments (Coulon et al. 2010; Gogoi et al. 2003; Mishra et al. 2001; Compeau et al. 1991). Field samples of soil contaminated with diesel oil collected from California, USA, and Hong Kong, China, showed that bioaugmentation showed the greatest degradation of the light (72.7%) and heavy (75.2%) fractions of TPH. The microbial consortium used for the bioaugmentation included Bacillus cereus, Bacillus sphaericus, Bacillus fusiformis, Bacillus pumilus, Acinetobacter junii and Pseudomonas sp. While the number of diesel-degrading microorganisms and heterotrophic population was not influenced by the bioremediation treatments, it was found that soil properties and the indigenous soil microbial population affected the degree of biodegradation (Bento et al. 2005). Contaminated soil sourced from a petroleum refinery in Portugal showed that factors such as exposure to the elements (air and sunlight) enhanced natural attenuation resulted in 30% TPH degradation as compared to bioaugmentation combined with nutrient and surfactant amendments which reached about 50% TPH degradation (Couto et al. 2010). The ability of bacterial groups such as Pseudomonas, Acinetobacter and Rhodococcus (Lin et al. 2010; Lee et al. 2012) as well as those from the Bacillus group has been identified as being important hydrocarbon degraders (Bento et al. 2005; Das and Mukherjee 2007; Łebkowska et al. 2011). These results have been translated and scaled up in the bioremediation of PHC- and oil-contaminated soil in the field with varying degrees of success (Menendez-Vega et al. 2007; Kauppi et al. 2011; Lee et al. 2012).
Bioremediation of PHC-Contaminated Soil Using Biopiles and Windrowing
An earlier ex situ treatment of diesel-contaminated soil using 375 kg batches was performed to compare the efficacy of biopiles and windrows (1.5 m × 0.5 m × 0.5 m). Coarse wood chips and horse manure were used as a bulking agent for the contaminated soil, and they were compared with NPK fertilizer (7% each of N, P and K). Results provided evidence for the efficacy of bioaugmentation over biostimulation as a remediation strategy where rapid mineralization was achieved using static biopiles in contrast to windrow systems. The former was less labour intensive and did not require specialist soil-turning equipment and associated staff on-site to carry out translation to a full-scale remediation project (Cunningham and Philp 2000). It has been demonstrated that the process of bioaugmentation could be enhanced by using autochthonous bioaugmentation (Nikolopoulou et al. 2013). Łebkowska et al. (2011) reported that there was a lack of data in the literature concerning the efficiency of bioremediation of PHC-contaminated soil in relation to inoculation frequency , usually with reports of only a single application of (autochthonous) bioaugmentation and biostimulation. In one study, indigenous bacterial strains isolated from polluted soils were applied ex situ in high concentrations of 107–108 CFU g−1 dry weight to bioaugment soil contaminated by diesel oil, engine oil and aircraft fuel, with the inoculation performed every 3 days. Although the indigenous bacterial strains appeared to share some commonality for each mesocosm with Bacillus sp. and Pseudomonas sp. being dominant for all three mesocosms, there were significant differences in some of the key microorganisms in terms of distribution, with some microorganisms such as Pseudomonas alcaligenes, Sphingomonas paucimobilis, Alcaligenes xylosoxidans, and Comamonas testosteroni present only in the aircraft fuel-contaminated soil. The diesel-contaminated soil had an initial value of only 2509 mg kg−1 compared to 5568 mg kg−1 for the soil contaminated with aircraft fuel yet required more than double the residence time to achieve approximately 80% degradation. In contrast the aircraft fuel was degraded by 97.57% within only 22 days. This technology which had been previously patented was successfully scaled up to treat over 150 MT of soil (Łebkowska et al. 2011).
There have been several small-scale laboratory-based microcosm studies (less than 2.5 kg) conducted on PHC-contaminated soil to compare the efficacy of natural attenuation, biostimulation, bioaugmentation and biostimulation/bioaugmentation for bioremediation (Aburto-Medina et al. 2012; Aleer et al. 2010; Dandie et al. 2010; Sheppard et al. 2011; Makadia et al. 2011). While most of the studies have focused on comparing the different bioremediation approaches, Makadia et al. (2011) adopted the approach of recycling soil from an old biopile that was previously bioremediated to below 10,000 mg kg−1 to harness the hydrocarbon catabolic ability of the residual microbial population in lieu of BA using laboratory-cultured organisms to treat waste oil sludge sourced from crude oil tank bottom.
The advantage was twofold: firstly, the treated soil could be reused to reduce the landfill space required and, secondly, to exploit the hydrocarbon-degrading potential of the treated soil to reduce the cost of subsequent bioremediation projects. Four treatment strategies were employed: biostimulation (BS) , bioaugmentation (BA) , natural attenuation (NA) and a combination of BS and BA to assess the degradation of spiked waste oil sludge present in contaminated soil for a period of 12 weeks. Initial results in weeks 2 and 3 showed that both BS and the BA/BS samples had substantially higher rates of hydrocarbon reduction than BA or NA samples. However, this trend had changed by week 12; although there was substantial reduction in the TPH content of the soil microcosms, the percentage reduction for NA (86% reduction) was not significantly different (ANOVA, P > 0.05) to the reductions observed in the amended soil microcosms: BS (91%), BA (91%) and BS/BA (92%). Aleer et al. (2010) conducted work on 200 g lots of petroleum hydrocarbon-contaminated soils obtained from old hydrocarbon biopiles that were spiked with waste engine oil and monitored for 3 months. These were done to compare the efficacy of different types of treatment that included NA, BS, BS and combined treatment of BS/BA. TPH analyses showed that BS and BS/BA accelerated hydrocarbon degradation. Moreover, it was an effective treatment, with over 84% reduction to less than 10,000 mg kg−1 at week 8. However, a further 2 weeks of treatment was required for other microcosms to obtain the same level at week 10. The BS/BA microcosms yielded the highest degradation yield of 92% by week 10. It was determined that there were no significant differences in hydrocarbon levels in naturally attenuated and treated microcosms at week 12. The results for the 16S rRNA- and ITS-based denaturing gradient gel electrophoresis profiling showed diverse bacterial and fungal communities with some dominant members belonging to hydrocarbon-degrading Proteobacteria spp., Ascomycetes spp. and Basidiomycetes spp. The study showed that hydrocarbon-polluted soils possessed microbial hydrocarbon-degrading potential that could be recycled and harnessed for further application to the degradation of engine oil, with the combination of BS/BA microcosms giving the highest degradation yield, better than BS or BA. However, the results for NAT were better than BS or BA as a single treatment on its own (Aleer et al. 2010).
In another study by Sheppard et al. (2011), BS, using the addition of nutrients for fungi was compared with BA with the fungus Scedosporium apiospermum. The primary focus of this study was to use ecological toxicity as a means to complement chemical analyses to meet legislated guidelines for the disposal of bioremediated soil. This was performed in combination with biostimulation in the form of providing nutrients for fungi with soil maintained at approximately 50% water holding capacity, incubated at 30 °C. The results for NAT gave the highest degradation yield (43.42%) making the soil suitable for disposal as waste under current guidelines (as both pesticide and metal contents were within safe limits). This result was in contrast to the lower degradation values for BS (32.75%) and BA (31.98%). The BS/BA degradation value (37.20%) was lower than that for BS without BA (Sheppard et al. 2011). This would suggest that NA by the indigenous microorganisms plus BS performed better than BA with the fungi.
While the above studies relied on TPH analyses as the primary method to assess the end point of PHC-contaminated soil bioremediation, a separate study was done by Soleimani et al. (2013) to compare TPH concentrations and CHEMometric™ analysis of selected ion chromatograms (SIC) to assess the end point of biodegradation. The latter, termed the CHEMSIC method of petroleum biomarkers included terpanes and regular, diaromatic and triaromatic steranes used for determining the level and type of hydrocarbon contamination. Six methods for enhancing bioremediation were tested on oil-contaminated soils from three refinery areas in Iran (Isfahan, Arak and Tehran), including bacterial enrichment and planting and addition of nitrogen and phosphorus, molasses, hydrogen peroxide and a surfactant (Tween 80) at an incubation temperature of 28 ± 2 °C. Results demonstrated that bacterial enrichment (BA) and addition of nutrients (BS) were most efficient with 50–62% removal of TPH after 60 days. BA was performed using an inoculum based on a consortium containing five organisms: Bacillus, Listeria, Pseudomonas, Rothia and Corynebacterium spp. (Soleimani et al. 2013).
The CHEMSIC results demonstrated that the bacterial enrichment was more efficient in the degradation of n-alkanes and low molecular weight PACs as well as alkylated PACs (e.g. naphthalenes, phenanthrenes and dibenzothiophenes), while nutrient addition led to a larger relative removal of isoprenoids (e.g. norpristane, pristane and phytane), with the conclusion that the CHEMSIC method could be used as a suitable tool for assessing bioremediation efficiency (Soleimani et al. 2013). However, while the study did not differentiate between the different BS and BA approaches used, the study did establish that bioremediation using the five-strain bacterial consortium was effective in the degradation of PHC contaminants as measured by TPH degradation . Table 1 summarizes laboratory-scale investigations on PHC-contaminated soil with volumes ranging from 1.0 to 149 kg for a variety of pollutants including diesel (Chemlal et al. 2012, 2013), PHC-contaminated soil from oil storage site (Grace Liu et al. 2011) and crude oil-spiked soil (Zhao et al. 2011). The small-scale study by Chemlal et al. (2012) on 2.0 kg of diesel-contaminated soil with an initial concentration of 5800 mg kg−1 showed 70.69% degradation within 40 days and was followed up with a scale-up to 149 kg at almost twice the concentration, 13,000 mg kg−1. This resulted in 85.38% degradation but with a longer residence time of 76 days, with the observation that alkanes were degraded before aromatics.
Liu et al. (2011) performed a series of experiments using 2.5 kg soil to compare various bioremediation combinations using BS, BA, BS/BA, and other additions including biosurfactants (BSF) and even kitchen waste (KW) as treatments for the bioremediation of PHC-contaminated soil from an oil storage site in Taiwan over 140 days. BA was performed using a microbial consortium which consisted of five strains of microorganisms including Gordonia alkanivorans (CC-JG39), Rhodococcus erythropolis (CC-BC11), Acinetobacter junii (CC- FH2), Exiguobacterium aurantiacum (CC-LSH4-1) and Serratia marcescens. The treatment using NA gave the lowest degradation yield at 15.6%, in sharp contrast to the highest degradation for KW at 81.9%. The next best yield was for BS using the lower concentrations of nitrogen and phosphate at 79.7%, while that using higher concentrations was lower, 58.9% suggesting that greater nutrient biostimulation did not correspond to improved yields (Zhao et al. 2011). A similar degradation of 61.90% was observed for crude oil-spiked soil with an initial concentration of 10,000 mg kg−1 using a consortium of five strains including Pseudomonas spp., Brucella spp., Bacillus spp., Rhodococcus spp., Microbacterium spp., Roseomonas spp. and Rhizobiales spp., at a shorter residence time of 60 days but with a smaller volume of only 1.0 kg of soil (Zhao et al. 2011). A summary of selected pilot-scale experiments that have been conducted on PHC-contaminated soil of up to 20 MT in mass per batch is shown in Table 2. The soils contained a variety of pollutants ranging from bunker fuel (Coulon et al. 2010), diesel (Lin et al. 2010) and PAHs (Sun et al. 2012). Coulon et al. (2010) performed a comparison of biopiled and windrowed soils in a full-scale trail where the end point of assessment targets was defined by human risk assessment and ecotoxicological hazard assessment approaches to compliment chemical analyses using TPH. The study reported that the amendment of nutrients significantly increased hydrocarbon degradation at the initial stages of the experiment, which was further enhanced by BA. Coulon et al. (2010) inferred that while the microbial population in the control soils was nutrient limited, there was already a capable microbial population present (Atlas 1981; Coulon et al. 2004; Bamforth and Singleton 2005; Delille and Coulon 2008) as the control soil with only an indigenous population was capable of degrading the hydrocarbon without any further treatment, but at a slower rate. The application of BS/BA to the bunker fuel-contaminated soil at field scale showed an increased rate of biodegradation, with windrow turning shown to be more effective than biopiling. Windrowing was effective for contaminated soil, which was more friable, in comparison with coarser soil, which may be more amenable to biopiling (Coulon et al. 2010).
A comparative pilot-scale study was conducted on the bioremediation of soil heavily contaminated by PAH soil in outdoor pot trials using three approaches: BA with bioemulsifier-producing microbial strain, BS and a combined BS/BA approach. The results for the BA approach showed that the concentration of total PAHs and 4–6 ring PAHs was reduced by 26.82% and 35.36%, respectively; BS at 33.9% and 11.0%, respectively; and BS/BA at 43.9% and 55.0%, respectively. The results showed that the combination of BS and BA had the highest percentage removal of PAHs in the contaminated soil.
The batch volumes of the PHC contamination for those references in Table 3 ranged from 50 MT to 990 MT in translation and scale-up experiments . The composition of the PHC contamination in the soil was varied and included mixtures of diesel, engine oil and aircraft fuel (Łebkowska et al. 2011), PAH (Lors et al. 2012), heavy residual fuel oil ‘mazut’ (Beskoski et al. 2011). An ex situ field-scale bioremediation was conducted on 600 MT of heavy residual fuel oil (mazut)-polluted soil from an energy power plant using BA, BS and a combination of BS/BA with multiple reinoculation of microbial consortia isolated from the mazut-contaminated soil compared with biostimulation using added nutritional elements (N, P and K). The biopile was comprised of mechanically mixed polluted soil with softwood sawdust and crude river sand with aeration aided by systematic mixing and protected from direct external influences by a polyethylene cover. Part (10 m3) of the material prepared for bioremediation was set aside uninoculated and maintained as an untreated control pile (CP) . Biostimulation and reinoculation with zymogenous microorganisms increased the number of hydrocarbon degraders after 50 days by more than 20 times in the treated soil. During the 5 months, the TPH content of the contaminated soil was reduced to 6% of the initial value, from 5.2 to 0.3 g kg−1 dry matter, while TPH reduced to only 90% of the initial value in the CP. After 150 days there were 96%, 97% and 83% reductions for the aliphatic, aromatic and nitrogen-sulphur-oxygen and asphaltene fractions, respectively. The isoprenoids, pristane and phytane fractions were more than 55% biodegraded, which indicated that they were not suitable biomarkers for following bioremediation. (Beskoski et al. 2011). An extended large-scale biopile comparing multiple inoculations to single inoculation of soil with indigenous microorganisms with suitable controls, to different lots of soil contaminated with diesel oil and aircraft fuel, respectively, was performed in Poland (Łebkowska et al. 2011). It was concluded that bioremediation was 50% more effective than the non-inoculated controls and 30% more effective than soil that had only a single inoculation. As part of the soil preparation procedure, stones and bigger solid particles were removed with the soil particle size reduced to about 5 cm, with the bioremediation conducted ex situ in biopiles at average temperatures of 15–30 °C (Łebkowska et al. 2011). Bioremediation depends not just on the intrinsic biodegradability of PHC fractions but also the availability of hydrocarbons, the weathered state of the hydrocarbons and the properties of the soil which support the biodegradation of the hydrocarbon contaminants (Gallego et al. 2011). Another large-scale study compared 3 MT biopiles with winnowing for the bioremediation of soil contaminated with bunker C fuel oil and found that soil which had a heavy texture was effectively remediated by windrowing and that coarser textures may be more amendable to biopiling. The amendment of treatments with nutrients was found to have significantly increased the rate of degradation at the initial stages, which was further increased, with the addition of inocula (Coulon et al. 2010).
Controlled field trials of the bioremediation of soils contaminated with petroleum hydrocarbons found bioremediation to be ecologically sound with a quantifiable reduction in the ecotoxicity observed as the measured TPH decreased. Ecotoxicological analysis of field work on biopiles in an oil refinery in Poland showed an 81% reduction in TPH versus 30% in the untreated biopile accompanied by a marked reduction in toxicity in the former, based on toxicity analysis including Microtox and phytotoxicity bioassays (Płaza et al. 2005). This approach was also used to assess the extent to which soil contaminated with bunker C fuel bioaugmentated in biopiles was remediated. In this case, a combination of chemical analysis and bioassays, including phytotoxicity assays as well as ecotoxicity evaluation with earthworms, was employed (Coulon et al. 2010). A key finding of the study was that although the bioremediated soil showed a significant ecological recovery, it was still relatively impaired with respect to human risk criteria with a need to perform further comparative studies to better assess the relationship and relative sensitivity of receptor-based end points (Coulon et al. 2010).
Conclusions
This chapter discussed recent literature in regard to the bioremediation of petroleum hydrocarbons. The bioremediation of PHC-contaminated soil has been extensively investigated at bench scale under controlled laboratory conditions. While the general finding has been that the degradation of PHC-contaminated soil has been more amenable to bioaugmentation than to either biostimulation or natural attenuation, there are a number of key exceptions, confirming the need to laboratory trials to be used to optimize treatment. However, the laboratory trials must try to emulate filed conditions and the results reviewed with caution. The translation and scale-up of bioremediation operations in the field have, on occasion, failed to measure up to expectations. There have also been more recent instances where the fieldwork has shown bioremediation to be effective, with bioaugmentation combined with some form of biostimulation showing the most biodegradation, particularly when performed ex situ. Recent developments in environmental microbiology, particularly next-generation sequencing, should play a key role in ensuring the commercial future of bioremediation technologies.
References
Aburto A (2007) Microbial diversity and factors affecting benzene degradation in a benzene-contaminated aquifer. Ph.D. thesis, University of Essex, Colchester
Aburto A, Ball AS (2009) Bacterial population dynamics and separation of active degraders by stable isotope probing during benzene degradation in a BTEX-impacted aquifer. Rev Int Contam Ambient 25(3):147–156
Aburto A, Peimbert M (2011) Degradation of a benzene–toluene mixture by hydrocarbon-adapted bacterial communities. Ann Microbiol 61(3):553–562
Aburto A, Fahy A, Coulon F, Lethbridge G, Timmis KN, Ball AS, McGenity TJ (2009) Mixed aerobic and anaerobic microbial communities in benzene-contaminated groundwater. J Appl Microbiol 106(1):317–328
Aburto-Medina A, Adetutu E, Aleer S, Weber J, Patil S, Sheppard P, Ball A, Juhasz A (2012) Comparison of indigenous and exogenous microbial populations during slurry phase biodegradation of long-term hydrocarbon-contaminated soil. Biodegradation 23(6):813–822
Adetutu E, Weber J, Aleer S, Dandie CE, Aburto-Medina A, Ball AS, Juhasz AL (2013) Assessing impediments to hydrocarbon biodegradation in weathered contaminated soils. J Hazard Mater 261:847–853
Afzal M, Iqbal S, Rauf S, Khalid ZM (2007) Characteristics of phenol biodegradation in saline solutions by monocultures of Pseudomonas aeruginosa and Pseudomonas pseudomallei. J Hazard Mater 149(1):60–66
Akinde SB, Obire O (2008) Aerobic heterotrophic bacteria and petroleum-utilizing bacteria from cow dung and poultry manure. World J Microbiol Biotechnol 24(9):1999–2002
Aleer S, Adetutu EM, Makadia TH, Patil S, Ball AS (2010) Harnessing the hydrocarbon-degrading potential of contaminated soils for the bioremediation of waste engine Oil. Water Air Soil Pollut 218(1–4):121–130
Andersson AJ, Mackenzie FT, Lerman A (2006) Coastal ocean CO2-carbonic acid-carbonate sediment system of the Anthropocene. Global Biogeochem Cycles. doi:10.1029/2005GB002506
Andreoni V, Gianfreda L (2007) Bioremediation and monitoring of aromatic-polluted habitats. Appl Microbiol Biotechnol 76(2):287–308
Atlas RM (1981) Microbial degradation of petroleum hydrocarbons: an environmental perspective. Microbiol Rev 45(1):180
Bamforth SM, Singleton I (2005) Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J Chem Technol Biotechnol 80(7):723–736
Bao MT, Wang LN, Sun PY, Cao LX, Zou J, Li YM (2012) Biodegradation of crude oil using an efficient microbial consortium in a simulated marine environment. Mar Pollut Bull 64(6):1177–1185
Bento FM, Camargo FA, Okeke BC, Frankenberger WT (2005) Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresour Technol 96(9):1049–1055
Beskoski VP, Gojgić-Cvijović G, Milić J, Ilić M, Miletić S, Šolević T, Vrvić MM (2011) Ex situ bioremediation of a soil contaminated by mazut (heavy residual fuel oil) – a field experiment. Chemosphere 83(1):34–40
Boonchan S, Britz ML, Stanley GA (2000) Degradation and mineralisation of high-molecular-weight polycyclic aromatic hydrocarbon by defined fungal-bacterial cocultures. Appl Environ Microbiol 66:1007–1019
Boopathy R (2000) Factors limiting bioremediation technologies. Bioresour Technol 74(1):63–67
Calvo C, Manzanera M, Silva-Castro G, Uad I, González-López J (2009) Application of bioemulsifiers in soil oil bioremediation processes. Future prospects. Sci Total Environ 407(12):3634–3640
Chapelle FH (1999) Bioremediation of petroleum hydrocarbon-contaminated ground water: the perspectives of history and hydrology. Ground Water 37(1):122–132
Chemlal R, Tassist A, Drouiche M, Lounici H, Drouiche N, Mameri N (2012) Microbiological aspects study of bioremediation of diesel-contaminated soils by biopile technique. Int Biodeter Biodegr 75:201–206
Chemlal R, Abdi N, Lounici H, Drouiche N, Pauss A, Mameri N (2013) Modeling and qualitative study of diesel biodegradation using biopile process in sandy soil. Int Biodeter Biodegr 78:43–48
Chen K-F, Kao C-M, Chen C-W, Surampalli RY, Lee M-S (2010) Control of petroleum-hydrocarbon contaminated groundwater by intrinsic and enhanced bioremediation. J Environ Sci 22(6):864–871
Chen Q, Bao M, Fan X, Liang S, Sun P (2013) Rhamnolipids enhance marine oil spill bioremediation in laboratory system. Mar Pollut Bull 71(1–2):269–275
Compeau GC, Mahaffey WD, Patras L (1991) Full-scale bioremediation of contaminated soil and water. In: Environmental biotechnology for waste treatment. Springer, Boston, pp 91–109
Coulon F, Pelletier E, Gourhant L, Louis RS, Delille D (2004) Degradation of petroleum hydrocarbons in two sub-antarctic soils: influence of an oleophilic fertilizer. Environ Toxicol Chem 23(8):1893–1901
Coulon F, Al Awadi M, Cowie W, Mardlin D, Pollard S, Cunningham C, Risdon G, Arthur P, Semple KT, Paton GI (2010) When is a soil remediated? Comparison of biopiled and windrowed soils contaminated with bunker-fuel in a full-scale trial. Environ Pollut 158(10):3032–3040
Couto MNP, Monteiro E, Vasconcelos MTS (2010) Mesocosm trials of bioremediation of contaminated soil of a petroleum refinery: comparison of natural attenuation, biostimulation and bioaugmentation. Environ Sci Pollut Res 17(7):1339–1346
Cunningham C, Philp J (2000) Comparison of bioaugmentation and biostimulation in ex situ treatment of diesel contaminated soil. Land Contam Reclam 8(4):261–269
Dandie CE, Weber J, Aleer S, Adetutu EM, Ball AS, Juhasz AL (2010) Assessment of five bioaccessibility assays for predicting the efficacy of petroleum hydrocarbon biodegradation in aged contaminated soils. Chemosphere 81(9):1061–1068
Das K, Mukherjee AK (2007) Crude petroleum-oil biodegradation efficiency of Bacillus subtilis and Pseudomonas aeruginosa strains isolated from a petroleum-oil contaminated soil from North-East India. Bioresour Technol 98(7):1339–1345
Delille D, Coulon F (2008) Comparative mesocosm study of biostimulation efficiency in two different oil-amended sub-Antarctic soils. Microb Ecol 56(2):243–252
Diplock E, Mardlin D, Killham K, Paton G (2009) Predicting bioremediation of hydrocarbons: laboratory to field scale. Environ Pollut 157(6):1831–1840
Dorn PB, Salanitro JP (2000) Temporal ecological assessment of oil contaminated soils before and after bioremediation. Chemosphere 40(4):419–426
El Fantroussi S, Agathos SN (2005) Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr Opin Microbiol 8(3):268–275
Felföldi T, Szekely AJ, Goral R, Barkacs K, Scheirich G, Andras J, Racz A, Marialigeti K (2010) Polyphasic bacterial community analysis of an aerobic activated sludge removing phenols and thiocyanate from coke plant effluent. Bioresour Technol 101(10):3406–3414
Gallego JLR, Sierra C, Permanyer A, Peláez AI, Menéndez-Vega D, Sánchez J (2011) Full-scale remediation of a jet fuel-contaminated soil: assessment of biodegradation, volatilization, and bioavailability. Water Air Soil Pollut 217(1):197–211
Gavrilescu M, Demnerova K, Aamand J, Agathos S, Fava F (2014) Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. Nat Biotechnol. doi:10.1016/j.nbt.2014.01.001
Gogoi BK, Dutta NN, Goswami P, Krishna Mohan TR (2003) A case study of bioremediation of petroleum-hydrocarbon contaminated soil at a crude oil spill site. Adv Environ Res 7(4):767–782
Grace Liu P-W, Chang TC, Whang L-M, Kao C-H, Pan P-T, Cheng S-S (2011) Bioremediation of petroleum hydrocarbon contaminated soil: effects of strategies and microbial community shift. Int Biodeter Biodegr 65(8):1119–1127
Groudeva VI, Groudev SN, Doycheva AS (2001) Bioremediation of waters contaminated with crude oil and toxic heavy metals. Int J Miner Process 62(1):293–299
Hassanshahian M, Emtiazi G, Caruso G, Cappello S (2014) Bioremediation (bioaugmentation/biostimulation) trials of oil polluted seawater: a mesocosm simulation study. Mar Environ Res 95:28–38
Kadali KK, Simons KL, Skuza PP, Moore RB, Ball AS (2012) A complementary approach to identifying and assessing the remediation potential of hydrocarbonoclastic bacteria. J Microbiol Methods 88(3):348–355
Kao CM, Chen CY, Chen SC, Chien HY, Chen YL (2008) Application of in situ biosparging to remediate a petroleum-hydrocarbon spill site: field and microbial evaluation. Chemosphere 70(8):1492–1499
Kauppi S, Sinkkonen A, Romantschuk M (2011) Enhancing bioremediation of diesel-fuel-contaminated soil in a boreal climate: comparison of biostimulation and bioaugmentation. Int Biodeter Biodegr 65(2):359–368
Kingston P (2002) Long-term environmental impact of oil spills. Spill Sci Technol Bull 7:53–61
Łebkowska M, Zborowska E, Karwowska E, Miaśkiewicz-Pęska E, Muszyński A, Tabernacka A, Naumczyk J, Jęczalik M (2011) Bioremediation of soil polluted with fuels by sequential multiple injection of native microorganisms: field-scale processes in Poland. Ecol Eng 37(11):1895–1900
Lee Y-C, Woo SG, Choi E-S, Ahn Y, Park J, Lee M, Yang J-W (2012) Bench-scale ex situ diesel removal process using a biobarrier and surfactant flushing. J Ind Eng Chem 18(3):882–887
Lin TC, Pan PT, Cheng SS (2010) Ex situ bioremediation of oil-contaminated soil. J Hazard Mater 176(1–3):27–34
Liu P-WG, Chang TC, Whang L-M, Kao C-H, Pan P-T, Cheng S-S (2011) Bioremediation of petroleum hydrocarbon contaminated soil: effects of strategies and microbial community shift. Int Biodeter Biodegr 65(8):1119–1127
Lors C, Damidot D, Ponge JF, Perie F (2012) Comparison of a bioremediation process of PAHs in a PAH-contaminated soil at field and laboratory scales. Environ Pollut 165:11–17
Macaulay BM, Rees D (2014) Bioremediation of oil spills: a review of challenges for research advancement. Ann Environ Sci 8(1):2
Makadia TH, Adetutu EM, Simons KL, Jardine D, Sheppard PJ, Ball AS (2011) Re-use of remediated soils for the bioremediation of waste oil sludge. J Environ Manage 92(3):866–871
Mao J, Luo Y, Teng Y, Li Z (2012) Bioremediation of polycyclic aromatic hydrocarbon-contaminated soil by a bacterial consortium and associated microbial community changes. Int Biodeter Biodegr 70:141–147
Menendez-Vega D, Gallego JLR, Pelaez AI, de Cordoba GF, Moreno J, Muñoz D, Sanchez J (2007) Engineered in situ bioremediation of soil and groundwater polluted with weathered hydrocarbons. Eur J Soil Biol 43(5–6):310–321
Mirdamadian SH, Emtiazi G, Golabi MH, Ghanavati H (2010) Biodegradation of petroleum and aromatic hydrocarbons by bacteria isolated from petroleum-contaminated soil. J Pet Environ Biotechnol 1:1–5
Mishra S, Jyot J, Kuhad RC, Lal B (2001) Evaluation of inoculum addition to stimulate in situ bioremediation of oily-sludge-contaminated soil. Appl Environ Microbiol 67(4):1675–1681
Nikolopoulou M, Pasadakis N, Kalogerakis K (2007) Enhanced bioremediation of crude oil utilizing lipophilic fertilizers. Desalination 211:286–295
Nikolopoulou M, Pasadakis N, Norf H, Kalogerakis N (2013) Enhanced ex situ bioremediation of crude oil contaminated beach sand by supplementation with nutrients and rhamnolipids. Mar Pollut Bull 77(1–2):37–44
Paul D, Pandey G, Pandey J, Jain RK (2005) Accessing microbial diversity for bioremediation and environmental restoration. Trends Biotechnol 23(3):135–142
Picado A, Nogueira A, Baeta-Hall L, Mendonça E, de Fátima RM, do Céu Sàágua M, Martins A, Anselmo AM (2001) Landfarming in a PAH-contaminated soil. J Environ Sci Health Part A 36(9):1579–1588
Płaza G, Nałęcz-Jawecki G, Ulfig K, Brigmon RL (2005) The application of bioassays as indicators of petroleum-contaminated soil remediation. Chemosphere 59(2):289–296
Prince RC (2010) Eukaryotic hydrocarbon degraders. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin/Heidelberg, pp 2065–2078
Prince RC, Garrett RM, Bare RE, Grossman MJ, Townsend T, Suflita JM, Lee K, Owens EH, Sergy GA, Braddock JF (2003) The roles of photooxidation and biodegradation in long-term weathering of crude and heavy fuel oils. Spill Sci Technol Bull 8(2):145–156
Rodríguez-Martínez EM, Pérez EX, Schadt CW, Zhou J, Massol-Deyá AA (2006) Microbial diversity and bioremediation of a hydrocarbon-contaminated aquifer (Vega Baja, Puerto Rico). Int J Environ Res Public Health 3(3):292–300
Ron EZ, Rosenberg E (2014) Enhanced bioremediation of oil spills in the sea. Curr Opin Biotechnol 27C:191–194
Sanscartier D, Reimer K, Zeeb B, Koch I (2011) The effect of temperature and aeration rate on bioremediation of diesel-contaminated soil in solid-phase bench-scale bioreactors. Soil Sediment Contam Int J 20(4):353–369
Shahsavari E, Adetutu EM, Anderson PA, Ball AS (2013) Plant residues – a low cost, effective bioremediation treatment for petrogenic hydrocarbon-contaminated soil. Sci Total Environ 443:766–774
Sheppard PJ, Adetutu EM, Makadia TH, Ball AS (2011) Microbial community and ecotoxicity analysis of bioremediated, weathered hydrocarbon-contaminated soil. Soil Res 49(3):261–269
Soleimani M, Farhoudi M, Christensen JH (2013) Chemometric assessment of enhanced bioremediation of oil contaminated soils. J Hazard Mater 254:372–381
Sun G-D, Xu Y, Jin J-H, Zhong Z-P, Liu Y, Luo M, Liu Z-P (2012) Pilot scale ex-situ bioremediation of heavily PAHs-contaminated soil by indigenous microorganisms and bioaugmentation by a PAHs-degrading and bioemulsifier-producing strain. J Hazard Mater 233:72–78
Van Gestel K, Mergaert J, Swings J, Coosemans J, Ryckeboer J (2003) Bioremediation of diesel oil-contaminated soil by composting with biowaste. Environ Pollut 125(3):361–368
van Hamme JD, Singh A, Ward OP (2003) Recent advance in petroleum microbiology. Microbiol Mol Biol Rev 67:503
Zhang Z, Hou Z, Yang C, Ma C, Tao F, Xu P (2011) Degradation of n-alkanes and polycyclic aromatic hydrocarbons in petroleum by a newly isolated Pseudomonas aeruginosa DQ8. Bioresour Technol 102(5):4111–4116
Zhao D, Liu C, Liu L, Zhang Y, Liu Q, Wu W-M (2011) Selection of functional consortium for crude oil-contaminated soil remediation. Int Biodeter Biodegr 65(8):1244–1248
Zouboulis AI, Moussas PA (2011) Groundwater and soil pollution: bioremediation. In: Nriagu J (ed) Encyclopedia of environmental health. Elsevier, Burlington, pp 1037–1044
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Shahsavari, E., Poi, G., Aburto-Medina, A., Haleyur, N., Ball, A.S. (2017). Bioremediation Approaches for Petroleum Hydrocarbon-Contaminated Environments. In: Anjum, N., Gill, S., Tuteja, N. (eds) Enhancing Cleanup of Environmental Pollutants. Springer, Cham. https://doi.org/10.1007/978-3-319-55426-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-55426-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55425-9
Online ISBN: 978-3-319-55426-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)