Abstract
The central nervous system plays a key and important role in regulating dietary energy consumption. Studies in the literature have shown that high calorie intake is deleterious to the physiological function of neurons. On the other hand, low-calorie intake has demonstrated to be beneficial, protecting neurons against harmful effects that could lead to the development of neurodegeneration. This chapter aimed to review the main aspects of dietary energy restriction protocols, such as intermittent fasting and calorie restriction, in relation to neuronal plasticity, cognition, and neurodegeneration.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Dietary energy restriction
- Caloric restriction
- Intermittent fasting
- Cognition
- Cognitive function
- Memory
- Neurogenesis
- Neuroplasticity
- Brain
- Hormesis
- Hippocampus
- Neurodegeneration
- Alzheimer’s disease
- Parkinson’s disease
Introduction
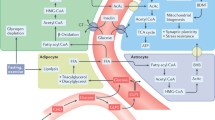
The central nervous system (CNS) plays a central role in regulating dietary energy consumption. Those individuals that are capable to overcome others in the acquisition of food (calorie) are evolutionary selected (Mattson 2002). Nevertheless, studies consistently show that persistent excessive calorie intake can impair cognitive performance. On the other hand, long-term restriction in calorie consumption, at lower levels than the habitual ad libitum intake, could improve cognitive function (Fig. 1) (Fontan-Lozano et al. 2007; Mattson 2010).
A schematic view of DER effects in neuronal plasticity, cognition , and neurodegeneration. High calorie intake (light blue scheme) is deleterious to physiological function of neurons. On the other hand, DER (dark blue scheme) has demonstrated to be beneficial, protecting neurons against harmful effects, which could lead to neurodegeneration (DER, dietary energy restriction) (Fontan-Lozano et al. 2007; Mattson 2010)
Dietary energy restriction (DER) stimulates neurogenesis and cognitive function. These beneficial effects of energy deprivation on the CNS are due to the induction of adaptive response pathways in a process called hormesis, which involves many mechanisms, including mitochondrial biogenesis, reduction of oxidative stress and inflammation, and increase of brain-derived neurotrophic factor (BDNF) levels, among others (Fig. 2) (Abrous et al. 2005; Mattson 2008a, b; Vasconcelos et al. 2014). The remarkable DER effects are often viewed as a response of adaptation to food deprivation (Mattson 2008a, b).
DER-related adaptive responses and cellular signaling pathways on the CNS that contribute to enhancement of cognitive function. In the left, DER changes brain signaling and neuronal network activity resulting in improved cognition. The hippocampus, a brain region responsible for cognitive processing, is particularly important for the adaptive responses to DER. In the right, the hippocampus neurons play central roles in learning and memory. These neurons have an increased activity in response to DER, resulting in augmented glutamate neurotransmitter release. NMDA receptors, leading to Ca2+ influx followed by CaM kinases activation which, in turn, can modulate activity of transcription factors such as CREB and NF-κB which induce an increase of BDNF levels and enhanced association with its receptor, TrkB (CaM, Ca2+/calmodulin-sensitive; CREB, cyclic AMP response element-binding protein; DER, dietary energy restriction; NMDA, N-methyl-D-aspartate; NF-κB, nuclear factor κB; TrkB, tropomyosin receptor kinase B) (Marosi and Mattson 2014; Longo and Mattson 2014)
There are two main protocols of DER that have been extensively studied due to promising effects on cognition and longevity: intermittent fasting (IF) and caloric restriction (CR) (Horne et al. 2015), the first associated with a restriction in frequency of feeding and the latter associated with restriction of calories ingested. While IF is a protocol that intercalates fasting periods with free access to food, CR refers to a chronic 20–40% decrease in calorie intake without malnutrition, both resulting in extended longevity, improved metabolic health and fitness, and improved physiological and molecular markers (Horne et al. 2015). Also, it has been demonstrated that DER could counteract many age-related diseases, such as reduction of the risk of metabolic deregulation, atherosclerosis, and neurological and cognitive diseases (Halagappa et al. 2007; Vasconcelos et al. 2014; Horne et al. 2015).
Although the term fasting is frequently interchanged by starvation, they do not have the same meaning. Starvation is a chronic nutritional insufficiency usually meaning extreme fasting, causing deterioration and death. On the other hand, fasting was shown to optimize general health and stress resistance and prevent many age-related diseases through ketogenesis and changes in metabolic signaling pathways (Muller et al. 2001; Hartman et al. 2013).
Many studies have indicated that DER protocols also enhance neuroplasticity, cognition, and behavioral outcomes (Zainuddin and Thuret 2012), as reviewed below.
Effects of DER on Neurogenesis and Neuroplasticity
The hippocampus is an essential brain region for cognitive functions like learning and memory, in which neurogenesis occurs through life. During this process, proliferative cells form novel neurons in dentate gyrus (DG) region of the hippocampus, and these neurons integrate into the existing neuronal circuitry (Abrous et al. 2005). Another critical brain region involved in cognitive function is prefrontal cortex (Martinet et al. 2011), and it was shown a direct pathway communicating this brain area to the CA1 region of the hippocampus as a mechanism of memory and learning (Soares-Simi et al. 2013).
It has been shown a strong correlation between learning and memory abilities and the number of new neurons, pointing to a link between cognitive function and neurogenesis. For instance, Drapeau et al. (2003) evaluated in the same animals the amount of novel neurons and spatial navigation learning and found a positive correlation between these measurements.
Hormetic stimulus (mild stressors that induce hormesis), such as exercise, enriched environment, and DER were shown to increase new neuron formation (Lee et al. 2000; Abrous et al. 2005). Corroborating the association between cognition and neuroplasticity, Kaptan et al. (2015) showed that CR increased proliferation and the amount of neuronal cells in DG accompanied by an improvement in spatial learning and memory. Moreover, CR also mitigates the progressive neuronal loss, which is frequently associated with cognitive deficit (Graff et al. 2013).
BDNF is a neurotrophin that plays widespread roles in neuronal plasticity, survival, and differentiation, which is essential for learning and memory processes. It has been demonstrated that IF and CR can enhance BDNF expression in the hippocampus and other brain regions, which mediates adaptive responses. In turn, this neurotrophin seems to positively influence processes such as neurogenesis and cognition through stimulation of novel neuron survival and preventing cognitive dysfunction (Lee et al. 2000, 2002a; Marosi and Mattson 2014; Vasconcelos et al. 2014). Cyclic AMP response element-binding protein (CREB) transcription factor is the main regulator of BDNF expression (Lipsky and Marini 2007). Indeed, activation of CREB by phosphorylation was proven to be essential for memory formation. Furthermore, BDNF was shown to increase, in neurons, levels of N-methyl-D-aspartate receptor (NMDAR), which under physiological stimulation could contribute to induce plastic changes in synapses which are fundamental for memory and learning processes (Fig. 2) (Marosi and Mattson 2014).
Kaptan et al. (2015) showed that restriction of only 15% of calories during adolescence can increase cell proliferation in DG and BDNF levels in the prefrontal cortex and hippocampus, accompanied by an enhancement of learning and memory in female rats.
Effects of DER on Cognitive Function
Cognitive function can be defined as the ability to realize, memorize, and manage information from the environment (Shettleworth 2009). Cognitive abilities, such as learning and memory, play a central role in the success of a subject to continuously adapt to its changing surroundings (Morand-Ferron et al. 2016).
Severe DER results in a decrease of most organs size in mammals, except for the brain (Weindruch and Sohal 1997). This fact, from an evolutionary point of view, suggests that maintaining the cognitive function is of primordial importance under periods of limited food availability. Indeed, not only mammals are more active during periods of fasting, but also DER can improve cerebral functionality, as shown by a better execution of behavioral tasks of motor, sensory, learning, and memory abilities (Fontan-Lozano et al. 2007; Singh et al. 2012). These adaptive effects have been mostly associated with augmented generation of novel neurons from neuronal progenitor cells and improved synaptic plasticity (Lee et al. 2002b).
Although extremely important for adaptation, cognitive function and plasticity are very energetically costly (Kennedy et al. 1978; Leybaert et al. 2007). Brain consumes 20 to 25% of the glucose and oxygen, mostly for synapses and to restore resting membrane potential of axons (Attwell and Iadecola 2002), and it represents only 2% of the body weight (Clarke and Sokoloff 1999). Therefore, it is not surprising that changes in energy availability can impact cognition.
Numerous animal and human projects have attempted to study the effects of DER on cognition, as described below. Although the vast majority of DER research was performed with animals, there is also preliminary evidence in humans pointing to health enhancements (Horne et al. 2015).
Neurodegeneration
The neurodegenerative diseases consist of a group of neurological disorders that can affect different subsets of neurons in specific cerebral areas with no determined cause and slow progression. The neural degeneration can disrupt many levels of neuronal activity starting with molecular pathway impairment, disruption of synapses, and changes in local circuits and finally affecting higher-order neuronal networks (Fig. 3) (Palop et al. 2006). Regarding molecular level, some mechanisms seem to be more relevant to neural degeneration such as mitochondrial dysfunction, oxidative stress, and apoptosis (Przedborski et al. 2003). The most studied benefits of DER on neurodegenerative diseases include Alzheimer’s disease (AD) and Parkinson’s disease (PD), which will be the focus of this topic.
General changes observed in neurodegeneration: from molecular to circuit disruption. (a) The neuronal degeneration can start with molecular pathway impairment that leads to an imbalance in neuronal environment and disruption of mitochondrial function triggering an increase in ROS and lipid peroxidation. All of these changes can result in neuronal apoptosis, synaptic disruption, and dysfunction of neuronal circuits. (b) Neurodegenerative diseases can affect different subsets of neurons in specific cerebral areas, such as dopaminergic neurons from mesolimbocortical pathways, including neurons from VTA to nucleus accumbens, hippocampus and frontal cortex or mesostriatal pathway, from substantia nigra to striatum in Parkinson’s disease and can affect cholinergic neurons and to a lesser extent non-cholinergic neurons in the hippocampus, in Alzheimer’s disease (ROS, reactive oxygen species; VTA, ventral tegmental area) (Przedborski et al. 2003)
The beneficial effects of DER on neurodegenerative process are mainly focused in its ability (1) to modulate some of the molecular pathways that are impaired in these illnesses and (2) to enhance neurogenesis and synaptic plasticity as previously presented in this chapter.
It is well established that along normal CNS aging numerous modifications occurred to nucleic acids, proteins, and lipids together with a cumulative burden of excitotoxic, metabolic, and oxidative stress. All these changes are intensified in the neurodegenerative process. However, animal studies have proposed that dietary changes such as in alimentary frequency and calorie intake could alter the incidence and severity of these disorders (Mattson et al. 2003). The mechanism proposed to the observed benefits of reduced dietary intake is based on energy availability changes that are perceived by neurons as mild cellular stress stimulus leading neurons to react increasing the production of stress resistance response proteins. The insulin-like/forkhead box O (FOXO) signaling pathway, sirtuins (SIRTs), and peroxisome proliferator-activated receptors (PPARs) are among the principal switches that stimulate the synthesis of neurotrophic factors such as BDNF, chaperones, and antioxidant enzymes (Gillette-Guyonnet and Vellas 2008).
Some studies performed in animal model of either excitotoxicity or metabolic insults as well as AD have proposed that short-term CR or IF could be neuroprotective and alleviate amyloid-β (Aβ) peptide aggregates and microglia activation, respectively (Patel et al. 2005; Halagappa et al. 2007). AD is histologically characterized by two well-known pathological hallmarks: senile plaques composed of Aβ peptide and the neurofibrillary tangles of abnormally phosphorylated tau. Both protocols (IF or CR) increase the expression of some key proteins such as sirtuin-SIR2 (first identified in yeasts subjected to CR with expanded lifespan) or SIRT1 (in mammals) (Fig. 4). This protein can regulate DNA repair, gene silencing, aging, and programmed cell death. In vitro studies suggest that increased levels of SIRT1 protect cells from apoptosis triggered by reactive oxygen species (ROS) generated by Aβ aggregates (Gillette-Guyonnet and Vellas 2008). Higher levels of SIRT1 lead to increased generation of catalytically active A disintegrin and metalloproteinase 10 (ADAM10) proteins that can maintain an anti-amyloidogenic activity either in rodent brains (Wang et al. 2005) or in monkey brains (Qin et al. 2006). ADAM10 is a constitutive α-secretase protein that cleaves the amyloid precursor protein (APP) generating a secreted and non-amyloidogenic product. Some studies have demonstrated that active ADAM10 not only reduces Aβ but also can reduce tau pathology, increase hippocampal neurogenesis, and help to maintain normal physiological synaptic functions (Yuan et al. 2017).
DER effects in Alzheimer’s disease animal models. In red, neurofibrillary tangles in cytosol and Aβ peptide aggregates that intensify microglia activation. In blue, DER can decrease microglia activation and due to an increase in SIRT1 can modulate ADAM10 activity avoiding Aβ peptide aggregation (Yuan et al. 2017). Furthermore, SIRT1 seems to inhibit apoptosis (Aβ, amyloid β; DER, dietary energy restriction; ADAM10, A disintegrin and metalloproteinase 10; SIRT1, sirtuin 1)
Notwithstanding the PD can be characterized by pathologic accumulation of presynaptic protein α-synuclein or microtubule-associated protein tau within vulnerable dopaminergic neurons and often glial cells, as well as in striatum (Fig. 5). In experimental models of PD, DER has also been associated with a reducing death vulnerability of substantia nigra (SN) dopaminergic neurons (Duan and Mattson 1999). A study performed in nonhuman primate rhesus monkey that received 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a neurotoxin that selectively degenerates dopaminergic neurons leading to PD-like motor, neurochemical, and histopathological alterations in humans and primates, showed that monkeys that were at a reduced caloric diet for up to 6 months before receiving neurotoxin injection had an increase in neurotrophin levels such as glial cell line-derived neurotrophic factor (GDNF) and BDNF in striatum that protected dopaminergic neurons from degeneration measured by less motor deficits and higher levels of dopamine (Maswood et al. 2004). The same protection was also observed in mouse model (Duan and Mattson 1999).
Role of normal and mutated α-synuclein in synapse. (a) Presynaptic terminal of a dopaminergic neuron represents the well-known hallmark of Parkinson’s disease: α-synuclein is an important protein for presynaptic dopaminergic vesicle release. (b) The loss of normal function of this protein promotes the accumulation of dopamine in the cytoplasm that together with α-synuclein oligomers is toxic to neuron (DAT, dopamine active transporter; VMAT, vesicular monoamine transporter) (Duan and Mattson 1999)
Similar to exercise effect, DER, especially IF, upregulates the antioxidant enzyme expression such as superoxide dismutase 2 (SOD2), glutathione peroxidase and heme oxygenase 1 (HO1), chaperones such as heat-shock protein 70 (Hsp70) and glucose-regulated protein 78 (Grp78), proteins involved in stress resistance and mitochondrial bioenergetics, antiapoptotic proteins, and neurotrophins (Camandola and Mattson 2017) (Fig. 6). Increased levels of BDNF can, in turn, raise the glucose transporters expression in neurons (GLUT3) stimulating neuronal energy metabolism. Furthermore, BDNF can also induce mitochondrial biogenesis through peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) (Camandola and Mattson 2017). It is well established that in PD there are an increase in membrane lipid peroxidation, protein nitration and oxidation, and a remarkable decrease in mitochondrial activity (Duan and Mattson 1999).
DER effects in Parkinson’s disease animal models. In yellow, mutated α-synuclein promotes the accumulation of dopamine in the cytoplasm that together with α-synuclein oligomers is toxic and can decrease mitochondrial activity as well as increase ROS production. All this cellular changes can lead to neuronal apoptosis. In green, DER can increase antioxidant enzymes, antiapoptotic proteins, and neurotrophins protecting neurons from apoptosis (DER, dietary energy restriction; ROS, reactive oxygen species) (Duan and Mattson 1999)
Although no safe protocol of DER had already been established for humans, it is becoming clearer that this challenge induces a complex array of molecular mechanisms that seems to optimize brain function and protect against neurodegenerative diseases and injuries (Camandola and Mattson 2017).
Animal Studies
Animal studies are very critical to evaluate the potential effects of DER at the cellular level on CNS, which could guide us to a better understanding of its impact in clinical trials (Mattson 2010). Several animal studies have consistently shown that DER enhances cognitive function, as some of them described ahead.
Literature data have provided evidence that a lifelong CR in mice is able to increase memory and prevent age-related decline in long-term potentiation (LTP) (Eckles-Smith et al. 2000; Kuhla et al. 2013). Likewise, CR started at midlife promoted an extension of life and of cognitive function in aged mice (25-month-old) (Means et al. 1993).
Ferreira et al. (2006) showed that 7-week-old rats subjected to mild (30%) or strong (60%) CR presented improved learning compared to rats in ad libitum diet, although memory was ameliorated in rats fed a 30% CR diet only. Moreover, 10% to 30% CR improved learning ability of mice in the Morris water maze (MWM) test (Ma et al. 2014).
Xu et al. (2015) showed that 30% CR in mice, unlike 30% caloric enhancement, improved learning and memory abilities, also evaluated in the MWM test. Accordingly, mice in high calorie diet (30% higher calorie intake) for 10 months had decreased memory capacity and neurodegeneration, while 30% CR resulted in improved memory in the MWM test (Dong et al. 2015). It was also investigated the effects of 20 and 35% CR on cognitive function in mice, and improved learning was observed in male but not in female, which suggests a sex-dependent DER effect on cognitive function (Wu et al. 2003).
It is well known that inflammation can induce cognitive dysfunction. Vasconcelos et al. (2014) demonstrated that IF is able to mitigate memory impairment associated with neuroinflammation, induced by lipopolysaccharide (LPS, a component of gram-negative bacteria membrane) stimulus. These results seem to be due to the anti-inflammatory effects of this DER protocol, which involves reduction of circulating inflammatory cytokines and pro-inflammatory gene expression.
Obesity is an inflammatory disease associated with increased systemic inflammation and cognitive deficits. Jeon et al. (2016) showed that CR in obese mice promoted weight loss and reversed learning, metabolic, and brain glucose dysfunctions. Accordingly, dietary-induced obesity rats subjected to CR for 28 days had improved cognitive function and BNDF signaling (Kishi et al. 2015).
Human Studies
Numerous religious groups have to fast during specific periods of the day or of the year, including Christians, Buddhists, Jews, Hindus, and Muslims (Longo and Mattson 2014). In many clinics, patients are being advised to adopt a very low-calorie diet (less than 200 calories per day) or even water-only diet under medical supervision in order to prevent diseases or for weight control (Longo and Mattson 2014). In fact, many studies have confirmed the beneficial effects of DER in improving general health in humans, including reduction of body weight in obese subjects (Harvie et al. 2011, 2013), hypertension (Goldhamer et al. 2001; Al-Shafei 2014), rheumatoid arthritis (Kjeldsen-Kragh et al. 1991; Muller et al. 2001), and asthma (Johnson et al. 2007).
Although DER was shown to increase cognitive function in different species, studies in humans are limited and with conflicting results (Rashotte et al. 1998; Qasrawi et al. 2017). In one study, nonobese volunteers subjected to a controlled experimental underfeeding during 1 week showed, during fasting, enhanced concentration, energy during the day, and emotional balance (Michalsen et al. 2003). Witte et al. (2009) showed that 30% CR in elderly humans resulted in memory improvement. Conversely, Green et al. (1994) showed that women students under DER had impaired cognitive performances in vigilance and short-term memory parameters. Moreover, the same group showed 1 year later that meal skipping had no effect on cognition of women (Green et al. 1995).
One month a year, during the holy month of Ramadan, around 1.6 billion Muslims practice intermittent fasting , in which they are refrained to eat and drink from sunrise to sunset. This reoccurring fasting diet is an interesting model for studying IF. Several researches have aimed to check Ramadan effects on cognitive function (Qasrawi et al. 2017). Results are inconsistent and frequently controversial.
One of the aspects of cognitive function is alertness. Three studies reported a shift of the alertness to later hours of the day during Ramadan (Roky et al. 2000, 2003; Bahammam 2003). However, another study compared eight Muslim and eight non-Muslim volunteers before and on the first and second weeks of Ramadan and reported no change in sleepiness or reaction time during the day (Bahammam et al. 2013a). Accordingly, another study with controlled conditions (fixed caloric intake, sleep duration and schedule, and exposure to light and dark) also reported that there was no change in drowsiness or vigilance caused by Ramadan intermittent fasting, measured by the total blink duration and mean reaction time (Bahammam et al. 2013b).
It was also reported that there is no change in cognitive function in cyclists during Ramadan (Roky et al. 2000). However, Farooq et al. (2015) showed that 4 weeks of IF during Ramadan in teenager boys resulted in improved working memory and spatial planning ability. Another study demonstrated that in tasks which demand fast responses a better cognitive performance was observed in the morning, but no change in tasks non-dependent of speed (Tian et al. 2011). In the same study, it was also reported improved vigilance and psychomotor function in the morning (09:00 h) and poorer memory and visual and verbal learning in the afternoon (16:00 h) during fasting. However, the study did not assess sleep quality and duration (Tian et al. 2011).
Changes in sleep quality and deprived or disrupted sleep cannot be excluded as a possible reason for the findings cited above since most studies did not control sleep parameters (Devoto et al. 1999). Variations between results may also be attributed to different DER intensities, protocols, and duration before data collection. In hormesis theory, the stressful stimulus should not be so intense that the organism is not able to fully recover and should last enough to elicit an adaptive hormetic response (Mattson 2008a, b). Unfortunately, studies in humans are limited, and there is still no consensus on which DER protocol is the best one to achieve cognitive benefits.
Policies and Protocols
According to the World Health Organization (WHO), many chronic diseases are consequences of unhealthy diet. Opposite to malnutrition, high caloric intake that leads to obesity is the major risk factor to illnesses such as cardiovascular diseases, diabetes, and cancer and more recently has been associated with dementias. In 2014, around 39% of adults worldwide were overweight (Waqanivalu and Nederveen 2015).
In the face of such challenge, the University of Wisconsin–Madison and the National Institute on Aging have been studying the effects of DER on aging in rodents and nonhuman primates, and preliminary results suggest that a reduction in caloric intake can delay the onset of cardiovascular diseases and diabetes and control chronic inflammatory disorders and brain atrophy (Colman et al. 2009). Other groups have already established many protocols to deeply understand the changes promoted by caloric restriction and intermittent fasting on health.
Dictionary of Terms
-
Hormesis – is a process in which a moderate (usually intermittent) stressor elicits an adaptive beneficial response of the cell or organism.
-
Neuroplasticity, also known as neuronal or brain plasticity, is the ability of the brain to change, adapt, reorganize itself, and form new neural connections in response to various stimulus.
-
Neurogenesis – is the formation of new neurons from neural stem cells in the brain.
-
Hippocampus – is a brain region that plays a key role in memory consolidation and spatial navigation.
-
Cognitive function – is the ability to interpret, memorize, and use information from the environment and is primordial for the adaptation of an individual.
-
Morris water maze – is a classic and widely used test in behavioral neuroscience to access spatial learning and memory in rodents (hippocampus-related cognitive function).
-
Excitotoxicity – is a neurotoxicity process that can lead neurons to damage or death mediated by high levels of excitatory neurotransmitters being most commonly related to glutamate.
-
Apoptosis – is a type of programmed cell death.
Summary Points
-
Dietary energy restriction is a hormetic stimulus that is associated with enhanced adult hippocampal neurogenesis and cognitive function.
-
The two main dietary energy restriction protocols are caloric restriction and intermittent fasting.
-
Caloric restriction involves the chronic restriction of 20 to 40% of the total calorie intake.
-
Intermittent fasting involves the restriction of the frequency of food consumption (for instance, every other day fasting or Ramadan intermittent fasting).
-
Many animal studies have proved the beneficial effects of dietary energy restriction on cognitive function and neural plasticity.
-
The brain region of the hippocampus plays a central role in learning and memory.
-
The neurodegenerative diseases consist of a group of different neurological disorders that affect different subsets of neurons in specific brain areas. They are usually characterized by slow progression with no determined cause.
-
In neurodegenerative diseases, some common molecular processes such as mitochondrial dysfunction, oxidative stress, and apoptosis are among the more relevant causes of neuronal degeneration.
-
Intermittent fasting and caloric restriction increase the production of stress resistance response proteins and neurotrophins.
-
Caloric restriction or intermittent fasting could alleviate amyloid-β peptide aggregates and microglia activation, respectively, in Alzheimer’s disease, and reduce neuronal death vulnerability in substantia nigra dopaminergic neurons in Parkinson’s disease.
Abbreviations
- Aβ:
-
Amyloid-β peptide
- AD:
-
Alzheimer’s disease
- ADAM10:
-
A disintegrin and metalloproteinase 10
- APP:
-
Amyloid precursor protein
- BDNF:
-
Brain-derived neurotrophic factor
- CaM:
-
Ca2+/calmodulin-sensitive
- CNS:
-
Central nervous system
- CR:
-
Caloric restriction
- CREB:
-
Cyclic AMP response element-binding protein
- DAT:
-
Dopamine active transporter
- DER:
-
Dietary energy restriction
- DG:
-
Dentate gyrus
- FOXO:
-
Forkhead box O
- GDNF:
-
Glial cell line-derived neurotrophic factor
- Grp78:
-
Glucose-regulated protein 78
- GLUT3:
-
Glucose transporter 3
- HO1:
-
Heme oxygenase 1
- Hsp70:
-
Heat-shock protein 70
- IF:
-
Intermittent fasting
- LPS:
-
Lipopolysaccharide
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MWM:
-
Morris water maze
- NF-κB:
-
Nuclear factor κB
- NMDAR:
-
N-Methyl-D-aspartate receptor
- PD:
-
Parkinson’s disease
- PGC-1α:
-
Peroxisome proliferator-activated receptor gamma coactivator 1-α
- PPARs:
-
Peroxisome proliferator-activated receptors
- ROS:
-
Reactive oxygen species
- SIRT:
-
Sirtuin
- SN:
-
Substantia nigra
- SOD2:
-
Superoxide dismutase 2
- TrkB:
-
Tropomyosin receptor kinase B
- VMAT:
-
Vesicular monoamine transporter
- VTA:
-
Ventral tegmental area
- WHO:
-
World Health Organization
References
Abrous DN, Koehl M, Le Moal M (2005) Adult neurogenesis: from precursors to network and physiology. Physiol Rev 85:523–569
Al-Shafei AI (2014) Ramadan fasting ameliorates arterial pulse pressure and lipid profile, and alleviates oxidative stress in hypertensive patients. Blood Press 23:160–167
Attwell D, Iadecola C (2002) The neural basis of functional brain imaging signals. Trends Neurosci 25:621–625
Bahammam A (2003) Sleep pattern, daytime sleepiness, and eating habits during the month of Ramadan. Sleep Hypnosis 5:165–174
Bahammam AS, Alaseem AM, Alzakri AA et al (2013a) The effects of Ramadan fasting on sleep patterns and daytime sleepiness: an objective assessment. J Res Med Sci 18:127–131
Bahammam AS, Nashwan S, Hammad O et al (2013b) Objective assessment of drowsiness and reaction time during intermittent Ramadan fasting in young men: a case-crossover study. Behav Brain Funct 9:32
Camandola S, Mattson MP (2017) Brain metabolism in health, aging, and neurodegeneration. EMBO J 36:1474–1492
Clarke DD, Sokoloff L (1999) Circulation and energy metabolism of the brain. In: Siegel GJ, Agranof BW, Albers RW, Fisher SK, Uhler MD (eds) Basic Neurochemistry. Lippincott-Raven, Philadelphia
Colman RJ, Anderson RM, Johnson SC et al (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325:201–204
Devoto A, Lucidi F, Violani C et al (1999) Effects of different sleep reductions on daytime sleepiness. Sleep 22:336–343
Dong W, Wang R, Ma LN et al (2015) Autophagy involving age-related cognitive behavior and hippocampus injury is modulated by different caloric intake in mice. Int J Clin Exp Med 8:11843–11853
Drapeau E, Mayo W, Aurousseau C et al (2003) Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A 100:14385–14390
Duan W, Mattson MP (1999) Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res 57:195–206
Eckles-Smith K, Clayton D, Bickford P et al (2000) Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res Mol Brain Res 78:154–162
Farooq A, Herrera CP, Almudahka F et al (2015) A prospective study of the physiological and neurobehavioral effects of Ramadan fasting in preteen and teenage boys. J Acad Nutr Diet 115:889–897
Ferreira FR, VBMG S, Lopes EJ et al (2006) Effect of feed restriction on learning, memory and stress of rodents. Biosci J 22:91–97
Fontan-Lozano A, Saez-Cassanelli JL, Inda MC et al (2007) Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J Neurosci 27:10185–10195
Gillette-Guyonnet S, Vellas B (2008) Caloric restriction and brain function. Curr Opin Clin Nutr Metab Care 11:686–692
Goldhamer A, Lisle D, Parpia B et al (2001) Medically supervised water-only fasting in the treatment of hypertension. J Manip Physiol Ther 24:335–339
Graff J, Kahn M, Samiei A et al (2013) A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. J Neurosci 33:8951–8960
Green MW, Rogers PJ, Elliman NA et al (1994) Impairment of cognitive performance associated with dieting and high levels of dietary restraint. Physiol Behav 55:447–452
Green MW, Elliman NA, Rogers PJ (1995) Lack of effect of short-term fasting on cognitive function. J Psychiatr Res 29:245–253
Halagappa VK, Guo Z, Pearson M et al (2007) Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis 26:212–220
Hartman AL, Rubenstein JE, Kossoff EH (2013) Intermittent fasting: a “new” historical strategy for controlling seizures? Epilepsy Res 104:275–279
Harvie MN, Pegington M, Mattson MP et al (2011) The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes 35:714–727
Harvie M, Wright C, Pegington M et al (2013) The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr 110:1534–1547
Horne BD, Muhlestein JB, Anderson JL (2015) Health effects of intermittent fasting: hormesis or harm? A systematic review. Am J Clin Nutr 102:464–470
Jeon BT, Heo RW, Jeong EA et al (2016) Effects of caloric restriction on O-GlcNAcylation, Ca(2+) signaling, and learning impairment in the hippocampus of ob/ob mice. Neurobiol Aging 44:127–137
Johnson JB, Summer W, Cutler RG et al (2007) Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med 42:665–674
Kaptan Z, Akgun-Dar K, Kapucu A et al (2015) Long term consequences on spatial learning-memory of low-calorie diet during adolescence in female rats; hippocampal and prefrontal cortex BDNF level, expression of NeuN and cell proliferation in dentate gyrus. Brain Res 1618:194–204
Kennedy C, Sakurada O, Shinohara M et al (1978) Local cerebral glucose utilization in the normal conscious macaque monkey. Ann Neurol 4:293–301
Kishi T, Hirooka Y, Nagayama T et al (2015) Calorie restriction improves cognitive decline via up-regulation of brain-derived neurotrophic factor: tropomyosin-related kinase B in hippocampus of obesity-induced hypertensive rats. Int Heart J 56:110–115
Kjeldsen-Kragh J, Haugen M, Borchgrevink CF et al (1991) Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 338:899–902
Kuhla A, Lange S, Holzmann C et al (2013) Lifelong caloric restriction increases working memory in mice. PLoS One 8:e68778
Lee J, Duan W, Long JM et al (2000) Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci 15:99–108
Lee J, Duan W, Mattson MP (2002a) Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem 82:1367–1375
Lee J, Seroogy KB, Mattson MP (2002b) Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem 80:539–547
Leybaert L, De Bock M, Van Moorhem M et al (2007) Neurobarrier coupling in the brain: adjusting glucose entry with demand. J Neurosci Res 85:3213–3220
Lipsky RH, Marini AM (2007) Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci 1122:130–143
Longo VD, Mattson MP (2014) Fasting: molecular mechanisms and clinical applications. Cell Metab 19:181–192
Ma L, Zhao Z, Wang R et al (2014) Caloric restriction can improve learning ability in C57/BL mice via regulation of the insulin-PI3K/Akt signaling pathway. Neurol Sci 35:1381–1386
Marosi K, Mattson MP (2014) BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab 25:89–98
Martinet LE, Sheynikhovich D, Benchenane K et al (2011) Spatial learning and action planning in a prefrontal cortical network model. PLoS Comput Biol 7:e1002045
Maswood N, Young J, Tilmont E et al (2004) Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc Natl Acad Sci U S A 101:18171–18176
Mattson MP (2002) Brain evolution and lifespan regulation: conservation of signal transduction pathways that regulate energy metabolism. Mech Ageing Dev 123:947–953
Mattson MP (2008a) Dietary factors, hormesis and health. Ageing Res Rev 7:43–48
Mattson MP (2008b) Hormesis defined. Ageing Res Rev 7:1–7
Mattson MP (2010) The impact of dietary energy intake on cognitive aging. Front Aging Neurosci 2:5
Mattson MP, Duan W, Guo Z (2003) Meal size and frequency affect neuronal plasticity and vulnerability to disease: cellular and molecular mechanisms. J Neurochem 84:417–431
Means LW, Higgins JL, Fernandez TJ (1993) Mid-life onset of dietary restriction extends life and prolongs cognitive functioning. Physiol Behav 54:503–508
Michalsen A, Schlegel F, Rodenbeck A et al (2003) Effects of short-term modified fasting on sleep patterns and daytime vigilance in non-obese subjects: results of a pilot study. Ann Nutr Metab 47:194–200
Morand-Ferron J, Cole EF, Quinn JL (2016) Studying the evolutionary ecology of cognition in the wild: a review of practical and conceptual challenges. Biol Rev Camb Philos Soc 91:367–389
Muller H, De Toledo FW, Resch KL (2001) Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Scand J Rheumatol 30:1–10
Palop JJ, Chin J, Mucke LA (2006) Network dysfunction perspective on neurodegenerative diseases. Nature 443:768–773
Patel NV, Gordon MN, Connor KE et al (2005) Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging 26:995–1000
Przedborski S, Vila M, Jackson-Lewis V (2003) Neurodegeneration: what is it and where are we? J Clin Invest 111:3–10
Qasrawi SO, Pandi-Perumal SR, Bahammam AS (2017) The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath 21:577
Qin W, Chachich M, Lane M et al (2006) Calorie restriction attenuates Alzheimer’s disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus). J Alzheimers Dis 10:417–422
Rashotte ME, Pastukhov IF, Poliakov EL et al (1998) Vigilance states and body temperature during the circadian cycle in fed and fasted pigeons (Columba livia). Am J Phys 275:R1690–R1702
Roky R, Iraki L, Hajkhlifa R et al (2000) Daytime alertness, mood, psychomotor performances, and oral temperature during Ramadan intermittent fasting. Ann Nutr Metab 44:101–107
Roky R, Chapotot F, Benchekroun MT et al (2003) Daytime sleepiness during Ramadan intermittent fasting: polysomnographic and quantitative waking EEG study. J Sleep Res 12:95–101
Shettleworth SJ (2009) Cognition, evolution, and behavior. Oxford University Press, Oxford
Singh R, Lakhanpal D, Kumar S et al (2012) Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (Dordr) 34:917–933
Soares-Simi SL, Pastrello DM, Ferreira ZS et al (2013) Changes in CREB activation in the prefrontal cortex and hippocampus blunt ethanol-induced behavioral sensitization in adolescent mice. Front Integr Neurosci 7:94
Tian HH, Aziz AR, Png W et al (2011) Effects of fasting during ramadan month on cognitive function in muslim athletes. Asian J Sports Med 2:145–153
Vasconcelos AR, Yshii LM, Viel TA et al (2014) Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflammation 11:85
Wang J, Ho L, Qin W et al (2005) Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J 19:659–661
Waqanivalu T, Nederveen L (2015) Fiscal policies for diet and prevention of noncommunicable diseases. World Health Organization. http://apps.who.int/iris/bitstream/10665/250131/1/9789241511247-eng.pdf?ua=1. Accessed 10 Nov 2017
Weindruch R, Sohal RS (1997) Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med 337:986–994
Witte AV, Fobker M, Gellner R et al (2009) Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A 106:1255–1260
Wu A, Sun X, Liu Y (2003) Effects of caloric restriction on cognition and behavior in developing mice. Neurosci Lett 339:166–168
Xu BL, Wang R, Ma LN et al (2015) Effects of caloric intake on learning and memory function in juvenile C57BL/6J mice. Biomed Res Int 2015:759803
Yuan XZ, Sun S, Tan CC et al (2017) The role of ADAM10 in Alzheimer’s disease. J Alzheimers Dis 58:303–322
Zainuddin MS, Thuret S (2012) Nutrition, adult hippocampal neurogenesis and mental health. Br Med Bull 103:89–114
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Vasconcelos, A.R., Orellana, A.M.M., Paixão, A.G., Scavone, C., Kawamoto, E.M. (2019). Intermittent Fasting and Caloric Restriction: Neuroplasticity and Neurodegeneration. In: Preedy, V., Patel, V. (eds) Handbook of Famine, Starvation, and Nutrient Deprivation. Springer, Cham. https://doi.org/10.1007/978-3-319-55387-0_99
Download citation
DOI: https://doi.org/10.1007/978-3-319-55387-0_99
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55386-3
Online ISBN: 978-3-319-55387-0
eBook Packages: MedicineReference Module Medicine