Abstract
In recent years the interest in vitamin D is steadily growing. Numerous scientific publications and health guidebooks for the public have been published. Besides the well-known effects on calcium and bone metabolism, a positive impact of the sunshine vitamin on human wellbeing and health has been proposed. A high prevalence of vitamin D deficiency according to established optimal values has been described, although no increase of the classical diseases associated with vitamin D deficiency, e.g., rachitis and osteomalacia, has been observed. Besides infants and the elderly, populations at risk include women of childbearing age. The discovery that human tissues with a role in reproductive function, e.g., ovaries, endometrium, and placenta, express the vitamin D receptor and enzymes involved in vitamin D metabolism, has provoked studies on the role of vitamin D in reproductive health. While observational studies suggest an association between low vitamin D status and impaired reproductive outcomes, there are only few high quality randomized clinical trials available that could prove causality. This chapter summarizes the current knowledge on vitamin D metabolism, epidemiology, and treatment of vitamin D deficiency with focus on women’s reproductive health.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Vitamin D
- Metabolism
- Classification
- Reproduction
- Fertility
- Menstruation
- PCOS
- Endometriosis
- Assisted reproduction
- Vitamin D deficiency
Introduction
Since the early 2000s, a wealth of scientific articles about vitamin D and its role for human biology have been published. The “sunshine” vitamin is involved in various functions in the body. Increasingly, scientific investigations are revealing the importance of vitamin D and its role in health and disease prevention. For the time being, it is known that vitamin D is involved in immunomodulation processes, cell growth, differentiation, and hormonal balance. The secosteroid and prohormone is getting converted to its active metabolite, 1,25-alpha(OH)2D through multiple converting steps in the body. By binding to its high-affinity receptor, activation of transcription occurs, which causes certain target genes to be activated or inhibited. The relationship between vitamin D and the reproductive system was established after detecting 1-alpha-hydroxylase and the vitamin D receptor (VDR) to be expressed in many female organs like placenta, ovary, endometrium, and decidua. Since vitamin D deficiency has been detected increasingly over the past decades among all racial groups and globally, a large proportion of the female population in their reproductive age is affected. Therefore, this chapter is focusing on the novel findings on vitamin D in the area of human reproduction, fertility, and disease prevention.
Metabolism and Synthesis of Vitamin D
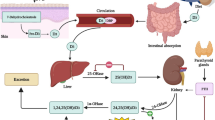
Vitamin D is a group of fat-soluble vitamins, with vitamin D2 and vitamin D3 being the most common forms. Vitamin D is absorbed from food (vitamin D3 [cholecalciferol], from animal sources, or from plant and fungi sources (vitamin D2 [ergocalciferol]), in the form of food supplements (either vitamin D2 or D3) or through the self-synthesis in the skin (vitamin D3) (Unholzer et al. 2017). Cholecalciferol, a prohormone and the physiologically most important representative, is formed from 7-dehydrocholesterol (7-DHC) by the action of UV-B rays in the keratinocytes of the skin (Unholzer et al. 2017) (Fig. 1). For its role in various processes in the body, cholecalciferol bound to its transport protein (vitamin D-binding protein (DBP)) is shuttled via the blood into the kidney, where it is metabolized into calcidiol (25(OH)D) (Unholzer et al. 2017) (a table of terms used in reference to vitamin D is given in Table 1 (modified after Unholzer et al. 2017)).The main steps of vitamin D metabolism include a multi-step hydroxylation process performed by cytochrome P450 mixed-function oxidases (CYPs). The enzymes responsible are located in the endoplasmic reticulum (e.g., CYP2R1) or in the mitochondria (e.g., CYP27A1, CYP27B1) and metabolize vitamin D2 or D3 into 25(OH)D2 or D3 (Bikle 2014).
Metabolism and synthesis of vitamin D. The formation of active 1,25(OH)2D is a multi-step hydroxylation process. First, cholecalciferol (vitamin D3) is formed in the skin after sufficient sunlight exposure from 7-DHC. After hydroxylation in the liver to calcidiol (25(OH)D), it gets metabolized in the kidney to the biological active form 1,25(OH)2D (calcitriol). 1,25(OH)2D is inactivated and degraded through further hydroxylation to calcitrioic acid
25(OH)D (calcidiol) is the major circulating and storage form of vitamin D in the body and serves as a marker of vitamin D status, while most of all circulating 25(OH)D is 25(OH)D3 (Unholzer et al. 2017). 25(OH)D is transported via its binding protein to the kidneys where it gets further hydroxylated by CYP27B1 (1-α hydroxylase), resulting in the biological active metabolite 1,25(OH)2D (Christakos et al. 2010) also known as calcitriol. Calcitriol is released into the blood and exerts its effects through a change in gene expression. While calcitriol is produced according to its specific needs in the body, its own degradation occurs to the inactive metabolite calcitroic acid via CYP24A1 (Christakos et al. 2016; Unholzer et al. 2017). The impact of the active vitamin D on cell functions is influenced by the bioavailability at the target sites and highly dependent upon DBP concentrations and its polymorphisms, which can vary among ethnicities.
Determination of Vitamin D Status
Vitamin D deficiency is still a large and global problem of the human population. Depending on the geographic location, populations in the northern latitudes, older people, children, as well as women in their reproductive period are potentially at high risk for vitamin D deficiency.
Reference intake values for an adequate vitamin D status are incongruent and despite years of research it is still not clearly defined how high an optimal vitamin D level should be to have an overall preventive effect. The determination of a serum concentration of 25(OH)D is the accepted approach to identify individual vitamin D status (Holick 2009). To date, according to different investigators, a 25(OH)D serum level in the range between 30 and 50 ng/ml can be regarded as adequately and physiologically sufficient. Values below 20 ng/ml are considered as deficient, 21–29 ng/ml as insufficient (Grant and Holick 2005; Holick 2009) (Table 2). Measurement of serum 25(OH)D concentrations is not subject to a uniform standard procedure. At present, enzyme-linked immunosorbent assays, high-performance liquid chromatography, liquid chromatography–mass spectrometry, and radioimmunoassays are generally used, which, due to different detection limits and variabilities, make a direct comparison of the values challenging (Thierfelder et al. 2008; Unholzer et al. 2017). Therefore, development of a uniform reference test for determination of serum 25(OH)D concentration is urgently necessary.
Supplementation of Vitamin D and Prevention of Deficiency
Vitamin D deficiency may occur from inadequate intake or insufficient exposure to sun light. Synthesis of vitamin D mainly takes part in the skin after sufficient sunlight exposure (Böhles et al. 2011) and therefore is dependent on the season, daytime, and geographic factors. Other factors are the uncovered skin area, length of time staying outside, as well as the skin pigmentation which gets additionally influenced by decreasing age (Loomis 1967; Need et al. 1993; Holick 2002; Böhles et al. 2011; German Nutrition Society (DGE) 2012).
The exogenous intake of vitamin D, which accounts for about 10%, is influenced by the consumption of certain foods or dietary supplements (Böhles et al. 2011). Vitamin D is found only in few foods naturally, which includes oily fish, liver, and margarine (enriched) (D-A-CH 2008; Böhles et al. 2011) (Table 3) (USDA Food Composition Databases 1889).
In some countries, e.g., the US and Canada, certain foods such as milk, some breakfast cereals, and breads are enriched with vitamin D (Institute of Medicine 1999), while food supplementation is mostly prohibited in Europe (Böhles et al. 2011). The daily intake recommendations for vitamin D, available in many countries of Europe and the USA (e.g., World Health Organization/Food and Agriculture Organization, Institute of Medicine, German Nutrition Society) are not uniform (Lanham-New et al. 2011; Ross 2011). The Food and Nutrition Board at the IOM established a recommended dietary allowance (RDA) for vitamin D which is defined as “average daily level of intake sufficient to meet the nutrient requirements of nearly all (97–98%) healthy people” (Institute of Medicine 2010). The Recommendation of the Associations for Nutrition of German-speaking Countries for daily intake is much higher (Table 4) (Institute of Medicine 2010; German Nutrition Society (DGE) 2012). These recommendations are mainly based on dietary intake and because of the many variable factors, which influence the synthesis of vitamin D, a standard for recommended adequate intake for all ages, sexes, and races is hard to define (Institute of Medicine 1999). Therefore, intake reference values from different countries could be eventually too low to maintain healthiness.
Vitamin D and the Female Reproductive System
In northern countries, a peak in conception rates in the summer has been observed. Therefore, it seems likely that an association between vitamin D and fertility exists (Rojansky et al. 1992). The detection of the VDR in granulosa and cumulus oophorus cells of the human ovary (Perez-Fernandez et al. 1997) and enzymes involved in vitamin D metabolism in several female reproductive tissues (Thill et al. 2009; Parikh et al. 2010), e.g., the endometrium and fallopian epithelial cells (Agic et al. 2007), the decidua and placenta (Agic et al. 2007), and the pituitary gland, supports this assumption. Further, enzymes involved in vitamin D metabolism, e.g., 1-alpha hydroxylase, are expressed in female reproductive tissues indicating that locally the active 1,25(OH)2D can be synthesized (Vigano et al. 2006; Fischer et al. 2009; Tamblyn et al. 2017). It has been proposed that the ovary is a target organ for 1,25(OH)2D (Dokoh et al. 1983). In conjunction with estradiol, it labilizes lysosomes to weaken the tunica albuginea and to enhance ovum release during ovulation. Steroid hormone production (progesterone, estradiol, and estrone) of the ovary and placenta as well as the synthesis of chorionic gonadotropin and placental lactogen expression in human syncytiotrophoblasts is regulated by the vitamin (Barrera et al. 2008). In addition, 1,25(OH)2D alters the expression and activity of the estrogen biosynthesis catalyzing enzyme P450 (Sun et al. 1998) and affects anti-mullerian hormone (AMH) signaling and steroidogenesis in human cumulus granulosa cells, both pathways with importance for folliculogenesis (Merhi et al. 2014).
Vitamin D and Assisted Reproduction
Utilization of assisted reproductive technologies (ART), especially in vitro fertilization (IVF), is growing (Kamphuis et al. 2014). IVF is now being used not only by infertile couples but also by couples carrying single gene mutations who use IVF with pre-implantation embryo biopsy and transfer of unaffected embryos to the uterus (Berger and Baker 2014). ART allows the separate evaluation of the impact of vitamin D deficiency on various steps of reproduction from folliculogenesis to embryo implantation.
In several observational studies, serum 25(OH)D levels were highly deficient in women seeking medical help for couple’s infertility (Pagliardini et al. 2015). In a cohort of 1,072 women in Northern Italy, 11% of women reached a sufficient level in this prospective cross-sectional study (Pagliardini et al. 2015). At a higher latitude (50°N) in Germany, vitamin D insufficiency affected 81–93% of women presenting for infertility treatment (N = 312) (Dressler et al. 2016). As with the previous study BMI and limited exposure to sun were associated with an increased risk of vitamin D deficiency.
The relationship between vitamin D serum levels and IVF outcomes has been investigated with controversial results. One of the first studies that investigated IVF success and vitamin D status in 10 women found an association of raised estradiol levels during gonadotrophin-induced ovarian stimulation and a significant increase of serum 1,25(OH)2D (r = 0.787, p < 0.001) (Potashnik et al. 1992; Wehr et al. 2010). Several studies focused on 25(OH)D levels in follicular fluid as an independent predictor to success of an IVF-cycle. While Ozkan et al. showed higher pregnancy and implantation rates across tertiles of 25(OH)D in 84 infertile women undergoing IVF (Ozkan et al. 2010), two prospective studies with 101 and 82 women could not confirm these findings (Anifandis et al. 2010; Aleyasin et al. 2011). No significant differences in pregnancy rates and embryo quality were found between patients with low (<20 ng/ml) and moderate (20–30 ng/ml) 25(OH)D follicular fluid levels. Women with high vitamin D follicular fluid levels (>30 ng/ml) had even lower pregnancy rates and embryo quality (Anifandis et al. 2010; Aleyasin et al. 2011).
Rudick et al. observed a relationship between serum 25(OH)D levels and implantation, clinical pregnancy, and live birth rates in 188 women (Rudick et al. 2012). These findings were significant in non-hispanic whites but did not apply to the Asian ethnicity (Rudick et al. 2012). The same group demonstrated a positive association between vitamin D status and clinical pregnancy rate among recipients of oocyte donation (Rudick et al. 2014). This observation is supportive of an effect of 25(OH)D levels on ART outcomes possibly due to a mediation of endometrial receptivity. Women with 25(OH)D > 20 ng/ml (n = 181) had a higher chance of obtaining top quality embryos, higher implantation (1.91 [95% confidence interval (CI): 1.20–3.05, P = 0.006]) and clinical pregnancy rates (adjusted odds ratio (aOR) 2.15 [95%]) compared to those with levels < 20 ng/ml (n = 154) (Paffoni et al. 2014). In a retrospective cohort of 368 women, vitamin D deficiency measured seven days’ prior blastocyst transfer, appeared as an independent predictor of lower clinical pregnancy rates (Polyzos et al. 2014). When the analysis was restricted to women undergoing elective single embryo transfer (274 patients), vitamin D deficiency was still independently associated with pregnancy rates [OR (95% CI) 0.56 (0.33–0.93), P = 0.024]. In a larger sample of 517 analyzed cycles, this finding was not confirmed (Franasiak et al. 2015). Vitamin D levels from serum samples obtained on the day of ovulation trigger in the fresh IVF cycle were analyzed. In this cohort with extended embryo culture, blastocyst biopsy for comprehensive chromosome screening and subsequent euploid embryo transfer vitamin D levels were unrelated to ongoing pregnancy rate (Franasiak et al. 2015). However, in a recent systematic review and meta-analysis of five studies, a deficient vitamin D level was related to lower live birth rate (relative risk (RR) 0.76, 95% CI 0.61–0.93) but not to a lower clinical pregnancy rate (RR 0.88, 95% CI 0.69–1.11) (Lv et al. 2016). Although a biologically plausible effect of vitamin D on reproductive tissues is very likely, it is still controversial whether vitamin D levels are reliable predictors of ART outcomes. Current evidence relies on heterogeneous results of small cohort studies while findings from RCTs are not yet available.
Vitamin D and Ovarian Reserve
AMH is an ovarian reserve marker and produced in the granulosa cells of the ovaries. While the AMH gene promoter contains a vitamin D response element, it is suggested that vitamin D might be involved in the regulation of the gonadal status. When granulosa cells were directly incubated with the active 1,25(OH)2D, changes in AMH receptor expression and downstream signaling were noted. In this experimental set-up direct effects on AMH levels were studied eliminating issues of vitamin DBP that affects the bioavailability of the active hormone in the circulation (Merhi et al. 2014). In a cross-sectional study including 388 premenopausal women with regular menstrual cycles, the authors observed a positive independent association of 25(OH)D levels with AMH in women aged 40 years and older (N = 141) (Merhi et al. 2012). In a prospective cross-sectional study of 283 infertile women starting their first cycle of infertility treatment serum AMH and vitamin D were determined and antral follicle count (AFC) measured on the second or third day. In contrast to the previous study, no significant association was observed between AMH levels or AFC and vitamin D concentrations, even after controlling for relevant co-variants (Drakopoulos et al. 2017). Thus, it is unclear from the available data if vitamin D deficiency is associated with lower ovarian reserve in reproductive aged women.
Vitamin D and Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is among the most common endocrine disorders affecting women of reproductive age and contributes to sub- and infertility. Clinical characteristics include hyperandrogenism, menstrual disturbances, and polycystic ovaries on ultrasound. The associated ovarian dysfunction is noticeable by oligo-/ anovulation. While many, but not all, women with PCOS are overweight or obese, insulin resistance (IR) and dyslipidemia are core pathophysiologic features of this syndrome. However, a substantial number of lean women affected by PCOS have IR as well, independent of obesity (Dunaif et al. 1989). There is increasing evidence that vitamin D affects insulin and glucose metabolism (Scragg et al. 2004; Liu et al. 2009). Vitamin D deficiency therefore has been proposed as the possible missing link between IR and PCOS. This assumption is supported by the finding that the active VDR regulates genes that are important for glucose and lipid metabolism as well as blood pressure regulation (Bouillon et al. 2008).
Several studies suggest associations between VDR polymorphisms and the development of PCOS and IR. Most of them had only modest sample sizes (Chiu et al. 2001; Mahmoudi 2009; Ranjzad et al. 2010; 2011; Wehr et al. 2011). Possible explanations of the role of VDR variants in the pathogenesis of PCOS include effects on luteinizing hormone, sex hormone binding globulin levels, and testosterone (Ranjzad et al. 2010; Wehr et al. 2011).
In a systematic review to gain insights into the association between vitamin D, BMI, and IR, 29 eligible trials with only one randomized controlled trial have been analyzed. Although univariate regression analyses revealed vitamin D to be a significant and independent predictor of IR in both PCOS and control women, the significance disappeared after adjustment for BMI in women with PCOS (Krul-Poel et al. 2013). Due to the heterogeneity of the available studies and the lack of randomized trials, it is currently hard to draw a definite conclusion about a causal relationship between vitamin D status and metabolic disturbances in PCOS.
Vitamin D and Menstrual Cycle
Animal data from knock-out mice models for 1-alpha hydroxylase, the enzyme converting vitamin D to its active form, show delayed puberty, anovulation, and irregular menstrual cycles (Panda et al. 2001; Dicken et al. 2012). Human data about vitamin D levels in the menstrual cycle and an association with irregularities are sparse and partially contradictory.
A study of 33 women included 202 serum samples collected at different time points in the follicular phase of the menstrual cycle found no differences in mean levels of 25(OH)D, free 25(OH)D, and bioavailable 25(OH)D (Franasiak et al. 2016). Several observational studies described a mid-cycle rise in the serum level of human 1,25(OH)2D with a near doubling of its concentration compared to early follicular levels (Pitkin et al. 1978; Gray et al. 1982; Buchanan et al. 1986). This finding, however, was not confirmed by other studies (Baran et al. 1980; Muse et al. 1986). The mid-cyclic peak was not associated with changes of serum calcium levels or other markers of bone health and not found in women on oral contraceptives (Gray et al. 1982; Tjellesen et al. 1983). Therefore, it was suggested that the mid-cycle endogenous estrogen increase induces the rise of 25(OH)D (Buchanan et al. 1986). As the vitamin D converting enzymes are found in human endometrium, the stage of menstrual cycle must be considered when serum concentrations of 1,25(OH)2D are analyzed. While 25(OH)D appears to be stable in the follicular phase, the measurement could be reliably performed during that time window of the menstrual cycle.
In a recent population-based study, lower levels of 25(OH)D were associated with irregular menstrual cycles of late reproductive-aged women (Jukic et al. 2015). However, the same group performed a community-based, cross-sectional study of 1,102 African American women. In this population a doubling of 25(OH)D serum levels was associated with half the odds of having long menstrual cycles (aOR 0.54, 95% CI 0.32–0.89) but not with the occurrence of short (aOR 1.03, 95% CI 0.82–1.29) or irregular (aOR 1.46, 95% CI 0.88–2.41) menstrual cycles (Jukic et al. 2016).
A role for vitamin D has been suggested in primary dysmenorrhea, as vitamin D inhibits synthesis of prostaglandins and VDR is located in the human uterus (Lerchbaum and Obermayer-Pietsch 2012). The first RCT investigating the effect of a single loading dose of vitamin D (300,000 IU) versus placebo on primary dysmenorrhea observed an inverse correlation of 25(OH)D levels with pain score as well as a significant reduction of pain in the vitamin D group with the greatest reduction in women with severe pain at baseline (Lasco et al. 2012). Although in another RCT the mean pain intensity in women with primary dysmenorrhea was lower in both the calcium-alone (1,000 mg calcium) and calcium-vitamin D (1,000 mg calcium + 5,000 IU vitamin D3) groups compared to placebo, the difference was statistically significant only in the calcium alone group (Zarei et al. 2016).
Vitamin D and Endometriosis
Endometriosis is a chronic and painful disease affecting up to 10% of women in their reproductive age (Eskenazi and Warner 1997). It leads to benign growths of endometrial cells outside the uterine cavity, such as in the pelvis, fallopian tubes, and ovaries. Endometriosis is strongly associated with female infertility (Eskenazi and Warner 1997).
Various studies suggest that there is a link between vitamin D and endometriosis. The endometrium represents a target tissue of vitamin D since it expresses the VDR as well as special vitamin D metabolizing enzymes. A dysregulation of vitamin D, vitamin D metabolites, and specific enzymes was observed in different studies of patients with endometriosis. The expression of 1α-hydoxylase and the VDR are increased in endometric tissue compared to normal endometrium (Agic et al. 2007). As part of a prospective cohort study with 1,385 endometric patients, a decrease of serum 25(OH)D in patients with the disease was observed (Harris et al. 2013). In contrast, serum levels of 25(OH)D have also been reported to be higher (Somigliana et al. 2007) or showed no difference (Agic et al. 2007) in patients suffering from endometriosis compared to healthy controls. Faserl et al. have found higher serum DBP levels in women with endometriosis in a cross-sectional study including 56 cases and 20 controls (Faserl et al. 2011). In the past years, in vivo and in vitro studies have been published suggesting a role of inflammation in endometriosis leading to the shift of anti-inflammatory treatments of the disease. Nevertheless, some results were found to be ambiguous (Ahn et al. 2015). Since these findings remain conflictive, further epidemiologic studies and clinical trials are needed to investigate this complex relationship and underlying mechanism of endometriosis and vitamin D.
Summary and Conclusions
In recent years, several studies suggest that vitamin D modulates female reproductive biology due to the expression of VDR and 1α-hydroxylase in reproductive tissues. This is supported by a regulation of steroidogenesis of sex hormones by the vitamin. A growing body of literature proposes that an individual’s vitamin D status may adversely impact reproductive functions (Fig. 2). Observational studies suggest a regulatory role of vitamin D in pathophysiological aspects of PCOS and endometriosis. Vitamin D might play a favorable role in IVF success and further beneficial effects include an improvement of primary dysmenorrhea following vitamin D supplementation and a possible association of high vitamin D levels with better ovarian reserve in women of late reproductive age. However, convincing evidence demonstrating a causal link between vitamin D and the pathophysiology of the diseases is still lacking. Most available studies are observational with an uneven distribution of populations, small numbers of participants, and different protocols for supplementation studies. Findings of observational studies must be confirmed by high quality randomized and interventional trials to obtain a better understanding of the underlying mechanisms and causality. Vitamin D supplementation is advised in the general population and in women planning on starting a family.
Policies and Protocols
-
Researchers and clinicians should agree on a uniform method to measure serum 25(OH)D concentrations. For this purpose, not only a uniform method (e.g., enzyme-linked immunosorbent assay or high performance liquid chromatography) but also a uniform procedure for the collection and processing of the samples should be carried out. The serum should be worked up within a specific time frame and stored under uniform conditions until testing. It is recommended to store the samples in a biobank which meets the uniform technical and practical requirements and which underlies quality controls. This will help with the interpretation and comparison of study results in the future.
-
In addition to the uniform determination of the vitamin D status, a uniform procedure to control the effects of vitamin D therapy to increase vitamin D levels to a normal range should also be established. For this, patients should take vitamin D supplements long-term under observation because medication is very different and vitamin D deficiency has to be treated over a very long period of time.
-
The measurement of serum 25(OH)D has hitherto been the only method for determining vitamin D deficiency or sufficiency in the blood of patients. A further method or parameter for determination should be developed which can also be used as standard by the patient himself for the daily determination of vitamin D status, for example, via measurement in the urine or via skin.
-
Awareness should be raised in the society about the relevance of a sufficient vitamin D status by physicians and clinical staff. Especially in groups at high risk, like pregnant women or the elderly, regular supplement intake can prevent vitamin D deficiency.
-
Large scale randomized controlled trials (RCTs) need to be performed to prove a causal effect of vitamin D on reproductive health outcomes.
Dictionary of Terms
-
Active vitamin D – There is an active form of vitamin D, called calcitriol (1,25-dihydroxyvitamin D), in the body that is converted from the inactive form (25-hydroxyvitamin D (25-(OH)D)). The active form is needed to fulfill vitamin D function in human cells.
-
Inactive vitamin D – The inactive form of vitamin D is a precursor of the active form. It is used to determine a human’s vitamin D status as it is measured in the blood.
-
Bioavailability – If the active form of vitamin D can exert its actions in the body depends on the available free active vitamin D. A major proportion of the active vitamin D is bound to proteins in the blood (e.g., vitamin D-binding protein (DBP) and albumin) and therefore not readily available.
-
Vitamin D status – The individual vitamin D status is determined by the measurement of 25(OH)D in the blood serum by various methods.
-
Vitamin D deficiency – Serum levels of 25(OH)D below 20 ng/ml are considered as deficient.
-
Vitamin D insufficiency – Serum levels of 25(OH)D of 21–29 ng/ml are in the insufficient range.
-
Vitamin D sufficiency – A 25(OH)D serum level in the range of 30–50 ng/ml is considered physiologically sufficient.
Summary Points
-
A uniform standard method to measure 25(OH)D concentrations to determine a person’s vitamin D status does not exist, what makes a direct comparison of vitamin D levels between studies challenging.
-
Vitamin D insufficiency and deficiency have a high incidence in reproductive age women around the world.
-
Although the optimal level of vitamin D serum concentrations is still discussed among researchers, the currently widely accepted range for sufficient levels lies between 30 and 50 ng/ml.
-
Fortified foods or vitamin D supplements are a cost-efficient and safe way to increase serum vitamin D levels.
-
Vitamin D receptor and metabolizing enzymes are present in reproductive tissues and cells suggesting paracrine/autocrine functions of the vitamin.
-
An effect on steroid hormone synthesis (progesterone, estradiol, and estrone) and hormones related to pregnancy (placental lactogen, chorionic gonadotropin) has been reported in in vitro studies.
-
Vitamin D status is suggested to be associated with reproductive outcomes.
-
A positive effect of vitamin D on the metabolism of women suffering from PCOS, the outcome of in vitro fertilization, and dysmenorrhea has been reported in observational studies.
-
Available data are not consistent and limited due to methodological, ethnic, and racial differences and small sample sizes.
-
In order to determine causality and prove benefits of vitamin D in the prevention of diseases and therapeutic measures, further large-scale randomized controlled trials (RCTs) must be performed and uniform clinical guidelines are needed.
Abbreviations
- aOR:
-
Adjusted odds ratio
- AFC:
-
Antral follicle count
- AMH:
-
Anti-mullerian hormone
- CI:
-
Confidence interval
- DBP:
-
Vitamin D-binding protein
- 7-(DHC):
-
7-Dehydrocholesterol
- DGE:
-
German Nutrition Society
- FSH:
-
Follicle stimulating hormone
- 25(OH)D:
-
25-Hydroxyvitamin D
- 1,25(OH)2D:
-
1,25-Dihydoxyvitamin D
- IL-8:
-
Interleukin-8
- IR:
-
Insulin resistance
- IOM:
-
Institute of Medicine
- IVF:
-
In vitro-fertilization
- LH:
-
Luteinising hormone
- PCOS:
-
Polycystic ovary syndrome
- PTH:
-
Parathyroid hormone
- RCT:
-
Randomized controlled trial
- RDA:
-
Recommended dietary allowance
- RXR:
-
Retinoic acid receptor
- VDR:
-
Vitamin D receptor
References
Agic A, Xu H, Altgassen C, Noack F, Wolfler MM, Diedrich K, Friedrich M, Taylor RN, Hornung D (2007) Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci 14(5):486–497. https://doi.org/10.1177/1933719107304565
Ahn SH, Monsanto SP, Miller C, Singh SS, Thomas R, Tayade C (2015) Pathophysiology and immune dysfunction in endometriosis. Biomed Res Int 2015:795976. https://doi.org/10.1155/2015/795976
Aleyasin A, Hosseini MA, Mahdavi A, Safdarian L, Fallahi P, Mohajeri MR, Abbasi M, Esfahani F (2011.: Epub ahead of print) Predictive value of the level of vitamin D in follicular fluid on the outcome of assisted reproductive technology. Eur J Obstet Gynecol Reprod Biol. https://doi.org/10.1016/j.ejogrb.2011.07.006
Anifandis GM, Dafopoulos K, Messini CI, Chalvatzas N, Liakos N, Pournaras S, Messinis IE (2010) Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Biol Endocrinol 8:91. https://doi.org/10.1186/1477-7827-8-91
Baran DT, Whyte MP, Haussler MR, Deftos LJ, Slatopolsky E, Avioli LV (1980) Effect of the menstrual cycle on calcium-regulating hormones in the normal young woman. J Clin Endocrinol Metab 50(2):377–379
Barrera D, Avila E, Hernandez G, Mendez I, Gonzalez L, Halhali A, Larrea F, Morales A, Diaz L (2008) Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reprod Biol Endocrinol 6:3. https://doi.org/10.1186/1477-7827-6-3
Berger VK, Baker VL (2014) Preimplantation diagnosis for single gene disorders. Semin Reprod Med 32(2):107–113. https://doi.org/10.1055/s-0033-1363552
Bikle DD (2014) Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 21(3):319–329. https://doi.org/10.1016/j.chembiol.2013.12.016
Böhles HJ, Fusch C, Genzel-Boroviczény O, Jochum F, Kauth T, Kersting M, Koletzko B, Lentze MJ, Moß AG, Mihatsch WA, Przyrembel H, Schnabel DG, Wabitsch M (2011) Vitamin-D-Versorgung im Säuglings-, Kindes- und Jugendalter. Monatsschr Kinderheilkd 159(11):1084–1084. https://doi.org/10.1007/s00112-011-2535-y
Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M (2008) Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 29(6):726–776. https://doi.org/10.1210/er.2008-0004. er.2008-0004 [pii]
Buchanan JR, Santen R, Cauffman S, Cavaliere A, Greer RB, Demers LM (1986) The effect of endogenous estrogen fluctuation on metabolism of 25-hydroxyvitamin D. Calcif Tissue Int 39(3):139–144
Chiu KC, Chuang LM, Yoon C (2001) The vitamin D receptor polymorphism in the translation initiation codon is a risk factor for insulin resistance in glucose tolerant Caucasians. BMC Med Genet 2:2
Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ (2010) Vitamin D: metabolism. Endocrinol Metab Clin North Am 39(2):243–253. https://doi.org/10.1016/j.ecl.2010.02.002
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G (2016) Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev 96(1):365–408. https://doi.org/10.1152/physrev.00014.2015
D-A-CH Referenzwerte für die Nährstoffzufuhr. 3.korrigierte Auflage, Nachdruck, Frankfurt am Main:Umschau/Braus: Deutsche Gesellschaft für Ernährung (DGE), Österreichische Gesellschaft für Ernährung (ÖGE), Schweizerische Gesellschaft für Ernährungsforschung (SGE), Schweizerische Vereinigung für Ernährung (SVE). DGE-Medienservice. 2008
Dicken CL, Israel DD, Davis JB, Sun Y, Shu J, Hardin J, Neal-Perry G (2012) Peripubertal vitamin D(3) deficiency delays puberty and disrupts the estrous cycle in adult female mice. Biol Reprod 87(2):51. https://doi.org/10.1095/biolreprod.111.096511
Dokoh S, Donaldson CA, Marion SL, Pike JW, Haussler MR (1983) The ovary: a target organ for 1,25-dihydroxyvitamin D3. Endocrinology 112(1):200–206
Drakopoulos P, van de Vijver A, Schutyser V, Milatovic S, Anckaert E, Schiettecatte J, Blockeel C, Camus M, Tournaye H, Polyzos NP (2017) The effect of serum vitamin D levels on ovarian reserve markers: a prospective cross-sectional study. Hum Reprod 32(1):208–214. https://doi.org/10.1093/humrep/dew304
Dressler N, Chandra A, Aguirre Davila L, Spineli LM, Schippert C, von Versen-Hoynck F (2016) BMI and season are associated with vitamin D deficiency in women with impaired fertility: a two-centre analysis. Arch Gynecol Obstet 293(4):907–914. https://doi.org/10.1007/s00404-015-3950-4
Dunaif A, Segal KR, Futterweit W, Dobrjansky A (1989) Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 38(9):1165–1174
Eskenazi B, Warner ML (1997) Epidemiology of endometriosis. Obstet Gynecol Clin North Am 24(2):235–258
Faserl K, Golderer G, Kremser L, Lindner H, Sarg B, Wildt L, Seeber B (2011) Polymorphism in vitamin D-binding protein as a genetic risk factor in the pathogenesis of endometriosis. J Clin Endocrinol Metab 96(1):E233–E241. https://doi.org/10.1210/jc.2010-1532
Fischer D, Thome M, Becker S, Cordes T, Diedrich K, Friedrich M, Thill M (2009) 25-Hydroxyvitamin D3 1alpha-hydroxylase splice variants in benign and malignant ovarian cell lines and tissue. Anticancer Res 29(9):3627–3633
Franasiak JM, Molinaro TA, Dubell EK, Scott KL, Ruiz AR, Forman EJ, Werner MD, Hong KH, Scott RT Jr (2015) Vitamin D levels do not affect IVF outcomes following the transfer of euploid blastocysts. Am J Obstet Gynecol 212(3):315.e311–315.e316. https://doi.org/10.1016/j.ajog.2014.09.029
Franasiak JM, Wang X, Molinaro TA, Green K, Sun W, Werner MD, Juneau CR, Scott RT (2016) Free vitamin D does not vary through the follicular phase of the menstrual cycle. Endocrine 53(1):322–326. https://doi.org/10.1007/s12020-016-0946-1
German Nutrition Society (DGE) (2012) New Reference Values for Vitamin D. Annals of Nutrition and Metabolism 60 (4):241-246
Grant WB, Holick MF (2005) Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev 10(2):94–111
Gray TK, McAdoo T, Hatley L, Lester GE, Thierry M (1982) Fluctuation of serum concentration of 1,25-dihydroxyvitamin D3 during the menstrual cycle. Am J Obstet Gynecol 144(8):880–884. https://doi.org/10.1016/0002-9378(82)90177-6
Harris HR, Chavarro JE, Malspeis S, Willett WC, Missmer SA (2013) Dairy-food, calcium, magnesium, and vitamin D intake and endometriosis: a prospective cohort study. Am J Epidemiol 177(5):420–430. https://doi.org/10.1093/aje/kws247
Holick MF 2002 Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes Obes 9(1):87–98
Holick MF (2009) Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 19(2):73–78. https://doi.org/10.1016/j.annepidem.2007.12.001
Institute of Medicine (1999) Food and Nutrition Board: Dietary reference intakes: calcium, phosphorus. magnesium, vitamin D and fluoride. National Academy Press, Washington, DC
Institute of Medicine (2010) Food and nutrition board: dietary reference intakes for calcium and vitamin D. National Academy Press, Washington, DC
Jukic AM, Steiner AZ, Baird DD (2015) Lower plasma 25-hydroxyvitamin D is associated with irregular menstrual cycles in a cross-sectional study. Reprod Biol Endocrinol 13:20. https://doi.org/10.1186/s12958-015-0012-5
Jukic AM, Upson K, Harmon QE, Baird DD (2016) Increasing serum 25-hydroxyvitamin D is associated with reduced odds of long menstrual cycles in a cross-sectional study of African American women. Fertil Steril 106(1):172–179.e172. https://doi.org/10.1016/j.fertnstert.2016.03.004
Kamphuis EI, Bhattacharya S, van der Veen F, Mol BW, Templeton A, Evidence Based IVF Group (2014) Are we overusing IVF? BMJ 348:g252. https://doi.org/10.1136/bmj.g252
Krul-Poel YH, Snackey C, Louwers Y, Lips P, Lambalk CB, Laven JS, Simsek S (2013) The role of vitamin D in metabolic disturbances in polycystic ovary syndrome: a systematic review. Eur J Endocrinol 169(6):853–865. https://doi.org/10.1530/eje-13-0617
Lanham-New SA, Buttriss JL, Miles LM, Ashwell M, Berry JL, Boucher BJ, Cashman KD, Cooper C, Darling AL, Francis RM, Fraser WD, de Groot CP, Hypponen E, Kiely M, Lamberg-Allardt C, Macdonald HM, Martineau AR, Masud T, Mavroeidi A, Nowson C, Prentice A, Stone EM, Reddy S, Vieth R, Williams CM (2011) Proceedings of the rank forum on vitamin D. Br J Nutr 105(1):144–156. https://doi.org/10.1017/S0007114510002576
Lasco A, Catalano A, Benvenga S (2012) Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: results of a randomized, double-blind, placebo-controlled study. Arch Intern Med 172(4):366–367. https://doi.org/10.1001/archinternmed.2011.715
Lerchbaum E, Obermayer-Pietsch B (2012) Vitamin D and fertility: a systematic review. Eur J Endocrinol 166(5):765–778. https://doi.org/10.1530/EJE-11-0984
Liu E, Meigs JB, Pittas AG, McKeown NM, Economos CD, Booth SL, Jacques PF (2009) Plasma 25-hydroxyvitamin d is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr 139(2):329–334. https://doi.org/10.3945/jn.108.093831
Loomis WF (1967) Skin-pigment regulation of vitamin-D biosynthesis in man. Science 157(3788):501–506
Lv SS, Wang JY, Wang XQ, Wang Y, Xu Y (2016) Serum vitamin D status and in vitro fertilization outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet 293(6):1339–1345. https://doi.org/10.1007/s00404-016-4058-1
Mahmoudi T (2009) Genetic variation in the vitamin D receptor and polycystic ovary syndrome risk. Fertil Steril 92(4):1381–1383. https://doi.org/10.1016/j.fertnstert.2009.05.002. S0015-0282(09)01071-1 [pii]
Merhi ZO, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, Golub ET, Young M, Karim R, Greenblatt R, Minkoff H (2012) Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductive-aged women: women’s interagency HIV study. Fertil Steril 98(1):228–234. https://doi.org/10.1016/j.fertnstert.2012.03.029
Merhi Z, Doswell A, Krebs K, Cipolla M (2014) Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab 99(6):E1137–E1145. https://doi.org/10.1210/jc.2013-4161
Muse KN, Manolagas SC, Deftos LJ, Alexander N, Yen SS (1986) Calcium-regulating hormones across the menstrual cycle. J Clin Endocrinol Metab 62(6):1313–1316
Need AG, Morris HA, Horowitz M, Nordin C (1993) Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am J Clin Nutr 58(6):882–885
Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, Pal L (2010) Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril 94(4):1314–1319. https://doi.org/10.1016/j.fertnstert.2009.05.019. S0015-0282(09)01095-4 [pii]
Paffoni A, Ferrari S, Vigano P, Pagliardini L, Papaleo E, Candiani M, Tirelli A, Fedele L, Somigliana E (2014) Vitamin D deficiency and infertility: insights from in vitro fertilization cycles. J Clin Endocrinol Metab 99(11):E2372–E2376. https://doi.org/10.1210/jc.2014-1802
Pagliardini L, Vigano P, Molgora M, Persico P, Salonia A, Vailati SH, Paffoni A, Somigliana E, Papaleo E, Candiani M (2015) High prevalence of vitamin D deficiency in infertile women referring for assisted reproduction. Nutrients 7(12):9972–9984. https://doi.org/10.3390/nu7125516
Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D (2001) Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A 98(13):7498–7503. https://doi.org/10.1073/pnas.131029498
Parikh G, Varadinova M, Suwandhi P, Araki T, Rosenwaks Z, Poretsky L, Seto-Young D (2010) Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm Metab Res 42(10):754–757. https://doi.org/10.1055/s-0030-1262837
Perez-Fernandez R, Alonso M, Segura C, Munoz I, Garcia-Caballero T, Diguez C (1997) Vitamin D receptor gene expression in human pituitary gland. Life Sci 60(1):35–42
Pitkin RM, Reynolds WA, Williams GA, Hargis GK (1978) Calcium-regulating hormones during the menstrual cycle. J Clin Endocrinol Metab 47(3):626–632
Polyzos NP, Anckaert E, Guzman L, Schiettecatte J, Van Landuyt L, Camus M, Smitz J, Tournaye H (2014) Vitamin D deficiency and pregnancy rates in women undergoing single embryo, blastocyst stage, transfer (SET) for IVF/ICSI. Hum Reprod 29(9):2032–2040. https://doi.org/10.1093/humrep/deu156
Potashnik G, Lunenfeld E, Levitas E, Itskovitz J, Albutiano S, Yankowitz N, Sonin Y, Levy J, Glezerman M, Shany S (1992) The relationship between endogenous oestradiol and vitamin D3 metabolites in serum and follicular fluid during ovarian stimulation for in-vitro fertilization and embryo transfer. Hum Reprod 7(10):1357–1360
Ranjzad F, Mahban A, Irani Shemirani A, Mahmoudi T, Vahedi M, Nikzamir A, Zali MR (2010) Influence of gene variants related to calcium homeostasis on biochemical parameters of women with polycystic ovary syndrome. J Assist Reprod Genet 28:225–232. https://doi.org/10.1007/s10815-010-9506-4 [doi]
Ranjzad F, Mahmoudi T, Irani Shemirani A, Mahban A, Nikzamir A, Vahedi M, Ashrafi M, Gourabi H (2011) A common variant in the adiponectin gene and polycystic ovary syndrome risk. Mol Biol Rep. https://doi.org/10.1007/s11033-011-0981-1. Epub ahead of print
Rojansky N, Brzezinski A, Schenker JG (1992) Seasonality in human reproduction: an update. Hum Reprod 7(6):735–745
Ross AC (2011) The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr 14(5):938–939. https://doi.org/10.1017/S1368980011000565
Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K (2012) Characterizing the influence of vitamin D levels on IVF outcomes. Hum Reprod 27(11):3321–3327. https://doi.org/10.1093/humrep/des280
Rudick BJ, Ingles SA, Chung K, Stanczyk FZ, Paulson RJ, Bendikson KA (2014) Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil Steril 101(2):447–452. https://doi.org/10.1016/j.fertnstert.2013.10.008
Scragg R, Sowers M, Bell C (2004) Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the third national health and nutrition examination survey. Diabetes Care 27(12):2813–2818
Somigliana E, Panina-Bordignon P, Murone S, Di Lucia P, Vercellini P, Vigano P (2007) Vitamin D reserve is higher in women with endometriosis. Hum Reprod 22(8):2273–2278. https://doi.org/10.1093/humrep/dem142
Sun T, Zhao Y, Mangelsdorf DJ, Simpson ER (1998) Characterization of a region upstream of exon I.1 of the human CYP19 (aromatase) gene that mediates regulation by retinoids in human choriocarcinoma cells. Endocrinology 139(4):1684–1691
Tamblyn JA, Susarla R, Jenkinson C, Jeffery LE, Ohizua O, Chun RF, Chan SY, Kilby MD, Hewison M (2017) Dysregulation of maternal and placental vitamin D metabolism in preeclampsia. Placenta 50:70–77. https://doi.org/10.1016/j.placenta.2016.12.019
Thierfelder W, Roth HJ, Laussmann D, Pientka L, Schumacher J, Schulz J (2008) Vitamin D and parathyoid hormone: a tool to determine assay-specific cutoff values for vitamin D. J Lab Med 32(6): 456-463. https://doi.org/10.1515/JLM.2008.060et
Thill M, Becker S, Fischer D, Cordes T, Hornemann A, Diedrich K, Salehin D, Friedrich M (2009) Expression of prostaglandin metabolising enzymes COX-2 and 15-PGDH and VDR in human granulosa cells. Anticancer Res 29(9):3611–3618
Tjellesen L, Christiansen C, Hummer L, Larsen NE (1983) Unchanged biochemical indices of bone turnover despite fluctuations in 1,25-dihydroxyvitamin D during the menstrual cycle. Acta Endocrinol 102(3):476–480
Unholzer S, Rothmund A, Haen E (2017) All-rounder vitamin D? Nervenarzt 88:489. https://doi.org/10.1007/s00115-016-0278-7
USDA Food Composition Databases (1889) United States Department of Agriculture, Beltsville. https://ndb.nal.usda.gov/ndb/. Accessed 1 Mar 2017
Vigano P, Lattuada D, Mangioni S, Ermellino L, Vignali M, Caporizzo E, Panina-Bordignon P, Besozzi M, Di Blasio AM (2006) Cycling and early pregnant endometrium as a site of regulated expression of the vitamin D system. J Mol Endocrinol 36(3):415–424. https://doi.org/10.1677/jme.1.01946
Wehr E, Pilz S, Boehm BO, Marz W, Obermayer-Pietsch B (2010) Association of vitamin D status with serum androgen levels in men. Clin Endocrinol (Oxf) 73(2):243–248. https://doi.org/10.1111/j.1365-2265.2009.03777.x
Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B (2011) Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol 164(5):741–749. https://doi.org/10.1530/EJE-11-0134
Zarei S, Mohammad-Alizadeh-Charandabi S, Mirghafourvand M, Javadzadeh Y, Effati-Daryani F (2016) Effects of calcium-vitamin D and calcium-alone on pain intensity and menstrual blood loss in women with primary dysmenorrhea: a randomized controlled trial. Pain Med. https://doi.org/10.1093/pm/pnw121
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Schröder-Heurich, B., von Versen-Höynck, F. (2019). Vitamin D Deficiency and Fertility: An Overview. In: Preedy, V., Patel, V. (eds) Handbook of Famine, Starvation, and Nutrient Deprivation. Springer, Cham. https://doi.org/10.1007/978-3-319-55387-0_44
Download citation
DOI: https://doi.org/10.1007/978-3-319-55387-0_44
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55386-3
Online ISBN: 978-3-319-55387-0
eBook Packages: MedicineReference Module Medicine