Abstract
In the last three decades, tremendous knowledge has been gained about human papillomavirus (HPV) infection and its associated diseases worldwide, and Latin American (LA) countries have made relevant contributions in the field. This chapter summarizes the major contributions made in LA on HPV epidemiology and prevention strategies. The region has one of the highest incidence and mortality rates from cervical cancer in the world. Overall, mortality rates are extremely high despite cytological screening in place in several countries. On the other hand, less is known about other HPV-associated tumors such as vulvar, vaginal, anal, penile, and oropharyngeal cancers. Overall, HPV DNA prevalence varies from 16% in normal cytology (substantially higher than worldwide prevalence estimates) to up to 90% in cervical cancer, HPV16 being the most frequently detected type in every cytological grade. Moreover, data on the natural history of HPV infections and risk of disease development are available from large cohort studies and served to propose new primary and secondary prevention modalities that include prophylactic HPV vaccination and HPV testing, respectively. The HPV vaccine was introduced in the national immunization programs of several LA countries, and multiple screening experiences using HPV testing were introduced in the region.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Papillomavirus
- Cancer prevention
- Cervical cancer
- Cytological screening
- Vaccines
- HPV testing
- Epidemiology
- Latin America
1 Introduction
Papillomaviruses are 8000-base-pair (8000-bp), double-stranded, circular DNA viruses that can cause warty and neoplastic changes in epithelia from many host species. Their genome consists of double-stranded DNA and encodes sequences for six early (E1, E2, E4, E5, E6, E7) and two late (L1, L2) proteins involved in capsid formation [30, 79]. Viral types are defined as a viral genome with an L1 late gene sequence that is at least 10% different from that of any other type. Interestingly, differing from most other viruses, papillomavirus infection is determined by DNA detection and not viral isolation.
Of the nearly 200 human papillomavirus (HPV) types identified, approximately 40 can infect human mucosa, particularly the anogenital and aerodigestive tracts [8], albeit cervical HPV infections are best understood.
The International Agency for Research on Cancer (IARC) has classified 12 HPV types as group 1 carcinogens; they are called “high-risk HPVs” (hr-HPVs) and include the following types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 [12]. Among the hr-HPVs, HPV16 is by far the most carcinogenic in terms of numbers of cervical cancer (CC) cases and its precursors [11, 52]. HPV16 also causes most HPV-related cancers in other anogenital epithelia and the oropharynx. HPV18 is classified second in terms of etiological importance but accounts for a high proportion of adenocarcinomas [52].
The varying carcinogenicity of HPV types partly relates to the expression of two early genes, the E6 and E7 oncogenes. Among other functions, E6 and E7 oncoproteins interfere with the functions of tumor suppressor proteins p53 and pRb, respectively. During the carcinogenic process, the HPV genome may integrate into the epithelial cell genome, and, during integration, parts of the HPV genome can be lost [76, 79]. However, the continued presence and expression of E6 and E7 gene regions are needed to sustain cancers and cancer cell lines.
Latin America (LA) has one of the highest incidence and mortality rates from CC in the world, with age-adjusted incidence rates ranging from 10 to 80 per 100,000 women/year [26, 32]. Overall, mortality rates are extremely high despite cytological screening in place in several countries. On the other hand, little is known about the rates of other HPV-associated tumors such as vulvar, vaginal, anal, penile, and oropharyngeal cancers. HPV DNA prevalence and type distribution are well known in many LA countries [15]. Moreover, data on the natural history of HPV infections and risk of disease development are available from large cohort studies and serve to propose new primary and secondary prevention modalities that include prophylactic HPV vaccination and HPV testing , respectively. The HPV vaccine was introduced in several LA national immunization programs, and multiple screening experiences using HPV testing were introduced in the region [45, 77]. Although promising, challenges to control HPV-related tumors are significant, mainly because as a comprehensive strategy it should include both components: vaccination and virological screening. Furthermore, information on HPV prevalence and type distribution in several LA countries is key both to measure the impact of HPV prophylactic vaccines and to establish appropriate post-vaccine epidemiological surveillance, with virological and disease endpoints.
2 HPV Natural History and Cervical Carcinogenicity
The cervix provides the best model of HPV and anogenital neoplasia natural history. The major stages in cervical carcinogenesis include infection of the cervical transformation zone metaplastic epithelium with one or more hr-HPV types, viral persistence, clonal progression of the persistently infected epithelium to cervical pre-cancer, and invasion.
Several epidemiological studies conducted in LA have contributed to establish these fundamental stages and to shed light on the factors that influence each of these transitions. Table 20.1 summarizes the main LA studies , pointing out their most relevant findings. The following findings should be highlighted: the cohort studies of the case-control studies conducted by IARC in Colombia, Paraguay, Brazil, and Peru; the cohort studies of Proyecto Guanacaste (Costa Rica) and Ludwig-McGill cohort (Brazil); and the HPV prevalence studies by IARC in Colombia, Mexico, Argentina, and Chile.
The great majority of sexually active women and men have been infected with HPV at least once in their lifetime [16]. HPV is the most common sexually transmitted infection; thus, HPV prevalence peaks around the sexual debut age, when exposure is high. Infections become undetectable within 2 years in more than 90% of individuals. Approximately 60% of these infections will prompt type-specific seroconversion , and if cervical samples are collected during productive viral infection, they may be associated with mild cervical abnormalities [i.e., low-grade squamous intraepithelial lesions (LSILs) or cervical intraepithelial neoplasia 1 (CIN1)] . Most of them are “transient” infections (cleared by the immune system) and do not result in clinical complications . Genital warts are other benign and common clinical sequelae in low-risk cases of HPV infection [66]. On the other hand, the hr-HPV infections that “persist” are more likely to progress to true cervical cancer (CC) precursor lesions, that is, high-grade squamous intraepithelial lesions (HSIL) or CIN3; progression to cervical cancer may take several years if left untreated.
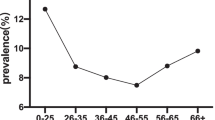
It is well known that cervical HPV infection is age dependent; an inverse relationship between age and HPV prevalence has been reported. HPV prevalence peaks below age 25 and declines with age. Using data from the Guanacaste cohort [39] and the TATI project (that only tested for 13 hr-HPVs) [2], the overall HPV prevalence was 26% in women younger than 25, dropping to 12% in those aged 35–44 and climbing again to 22% in those older than 54 (Table 20.1) [20]. This U-shaped age-specific curve of hr-HPV prevalence was previously shown by independent reports in Costa Rica [41], Mexico [49], Chile [34], Brazil (see r29, Table 20.1), and Colombia [57]. In Argentina, the curve peaked below age 25 and then dropped and plateaued around 30 to 35 years, reaching its minimum at 65 years of age or older [55]; this pattern resembles more those of Europe and North America.
CC risk is largely, and almost exclusively, defined by HPV natural history. Among HPV-infected women, the most important determinants of carcinogenic risk are persistence of infection and viral genotype, HPV16 being the most prominently carcinogenic [67].
Although hr-HPV DNA is detected in almost all CC cases, HPV infection alone is not sufficient to drive full carcinogenesis. A substantial part of the evidence of risk factors for HPV infection and progression to cervical cancer comes from studies conducted in LA. The lifetime number of male sexual partners and their sexual behavior are associated with an increased risk of HPV infection. High parity, long-term oral contraceptive use, and smoking are associated with an increased risk of HPV infection progression to CC ; the role of chronic inflammation, especially from coinfection with Chlamydia trachomatis, and certain dietary deficiencies have also been reported [1, 29].
Immunity is obviously an important risk factor ; an effective cell-mediated response to the early proteins is necessary for lesion regression. Host genetics and other influences on host immunity might affect the immune response to HPV infection; weak associations of HLA with risk of CIN3+ have been noted [31, 54]. Coinfection with HIV is important because HIV-induced immunosuppression impairs cell-mediated immune control of HPV infections [1].
3 HPV Prevalence and Type Distribution in Normal Cytology and Cervical Lesions in LA
The genotype distribution in normal cytology and LSIL reveals a wide spectrum of HPV types, both low- and high-risk types; as the severity of the cervical lesion increases, hr-HPVs become the most frequent types, being the only types in CC, with HPV16 and HPV18 accounting for about 70% of cases.
In one of the largest meta-analyses, including 48,171 women with normal cytology from studies in Trinidad and Tobago, Costa Rica, Honduras, Guatemala, Belize, Mexico, Argentina, Brazil, Chile, Colombia, Paraguay, and Peru, the prevalence of HPV (any type) was 16.1%. The vaccine-targeted HPV types (16 and/or 18) were identified in 4.3% of normal samples [15].
In LSIL, the most common viral types identified in samples from the LA region were HPV16 (26%), HPV33 (13%), HPV6 (11%), HPV58 (8%), and HPV31 (7%) [24].
In the regional meta-analyses including 2446 cases of HSIL and 5540 of CC, 46.5% of HSIL cases harbored HPV16 and 8.9% HPV18; in CC, 53.2% of cases harbored HPV16 and 13.2% HPV18, the next five most common types, in decreasing frequency, being HPV31, HPV58, HPV33, HPV45, and HPV52 [23].
The more recent worldwide meta-analysis of cross-sectional HPV-type distribution in HPV-positive women of all types of clinical status (from normal cytology to CC) included 35,895 samples from South and Central America studies, in which genotyping was performed by polymerase chain reaction (PCR)-based methods [36]. Overall HPV prevalence increased with growing severity of cervical disease from 24% in normal cytology (substantially higher than worldwide prevalence estimates) up to 90% in CC. HPV16 was the most frequently detected type in every grade. HPV16 positivity varied slightly across normal cytology (16.1%) and LSIL (25.1%), but increased substantially in HSIL (53.5%), to reach 59.5% in CC.
4 HPV Genetic Variability
Comparative nucleotide sequence analysis of these viruses has elucidated some features of their phylogenetic relationship and pathogenesis implications.
HPV genomes have been classified into molecular variants when they present more than 98% similarity to the prototype in the L1 gene sequence [27]. Nevertheless, more recently , the comparison of the complete nucleotide sequence of HPV16 isolates from different phylogenetic branches showed that 4% of the full genome may vary in the eight genes and that 9.9% of amino acid positions are variable [22].
The most extensive worldwide studies concerning HPV intratypic nucleotide heterogeneity by far have been conducted for HPV16 because of its global predominance, followed by HPV18 and HPV45, HPV6 and HPV11, HPV5 and HPV8, and, more recently, HPV58, HPV31, HPV33, HPV35, and HPV52 [17]. Table 20.2 presents a selection of the main studies on HPV variability performed in LA.
Investigations of HPV type diversity have identified different phylogenetic branches (variants); particularly for HPV16, there are six branches : European (E), Asian (As), African-1 (Af-1), African-2 (Af-2), Asian-American (AA), and North American (NA) [17]. Different HPV16 variants exhibit differences in their biological and biochemical properties.
The prevalence of HPV variants and their association with cervical cancer has been reported in three case-control studies [9, 42, 69] and five cross-sectional studies in LA women with different grades of cervical lesions [18, 47, 56, 62, 78]. Follow-up studies have reported the role of HPV variants in the persistence of infection and disease progression [69, 74]. Overall, studies conducted in Argentina, Brazil, Costa Rica, Honduras, Mexico, and Paraguay have shown a large diversity of variants, with a higher frequency of E variants compared to other phylogenetic branches (Table 20.2). Interestingly, a high prevalence (>80%) of E variants was also observed in indigenous groups from Argentina [28, 62, 71]. These studies also suggested that the colonization of the American continent by Europeans and Africans is reflected in the composition of its variants.
Studies carried out in Mexico , Costa Rica, and Brazil have shown that non-E HPV16 variants, mostly AA variants, are associated with a higher risk of viral persistence and/or HSIL and CC [9, 18, 42, 68, 69, 74]. These studies with a large number of samples provide enough study power to detect associations between low-prevalence variants and persistent infections or disease risk [1].
In vitro functional assays show that several HPV variants differ in their ability to induce p53 degradation, Bax degradation, activation of mitogen-activated protein kinase (MAPK) signaling , E2-related transcription, and immortalization activity. Specific studies with non-E variants have shown enhanced transcription and replication efficiency in HPV16 and HPV18 AA variants compared to E variants [17]. This information may explain the increased oncogenic potential reported for these variants and their contribution for the high incidence of CC.
HPV vaccines are based on virus-like particles (VLPs) composed of L1 protein, the viral capsid main component. So far, serological studies of different HPV16 variants have shown that the humoral immune response to HPV16 does not seem to discriminate between different molecular variants [60]. The cross-protection between variants was confirmed by the near 100% prophylactic efficacy of vaccines in multicenter studies [1].
Although the coevolution of human populations and HPV16 and HPV18 variants is well supported, the geographic association for variants of other types remains unresolved. Global studies of HPV variant lineages from worldwide populations are needed to better understand the relationship between HPV and the recent and past evolution and dispersion of their human hosts, as well as the genetic basis of the pathogenesis of specific HPVs, viral–host interactions, and host evolution, among other applications and scientific inquiries. Multicenter studies and/or meta-analyses will be useful to validate the nucleotide level of pathogenesis and provide insights into the molecular basis of HPV-associated disease [17].
5 Epidemiology of HPV-Related Neoplasias
About 1.1 million new cancer cases and 600,000 cancer deaths per year are estimated in Latin America and the Caribbean [32]. Estimates indicate a 72% increase in the incidence of cancer and 78% increase in mortality between 2012 and 2030 [26]. Cancer rates vary considerably within LA: although breast cancer remains the leading cause of death for women worldwide, CC is the main cause of death from cancer in Bolivia, Honduras, and Nicaragua. Also, cervical cancer incidence rates vary considerably in the region, ranging from 11.4 cases per 100,000 in Costa Rica to 47.7 cases per 100,000 in Bolivia [59].
Decades of Papanicolaou-based screening to detect precancerous cervical lesions have not had a major impact in reducing CC incidence and mortality rates, which are still high in the region (Fig. 20.1). Despite efforts to reorganize screening programs in a few countries of the region, only a slight reduction in cervical cancer mortality has been noted [59]. Among the difficulties to control the burden of cancer in the region are the uneven allocation of resources, variable infrastructure and service availability, limited number of population-based cancer registries, and scarce distribution of public health posts, which is more evident in rural areas, distant from the large urban centers. These difficulties result in a scenario of disproportionate care provided to individuals affected by cancer.
Cervical cancer mortality rates for selected countries in Latin America. (Adapted from Pan American Health Organization [59])
The global burden of HPV infections and related diseases is significant [35]. HPV was associated with 83,195 new cases of cervical cancer and 35,673 associated CC deaths in LA in 2012 [14]. Most of the cases are associated with HPV16 and HPV18, followed by five additional hr types (HPVs 31, 33, 45, 52, 58), which together account for about 90% of CC cases worldwide.
Information concerning HPV-related tumors outside the uterine cervix in LA countries is scarce [14, 32]. A recent systematic review of the presence of HPV in noncervical sites suggests a high HPV prevalence and higher clearance rates than in the uterine cervix [70]. Anal cancer incidence rates vary from as low as 0.2 × 100,000/year to 1.4 × 100,000/year in the northeast of Brazil and some areas of Argentina [26]. Estimates for other LA are limited or nonexistent. Similarly to high-income countries, anal cancer incidence is increasing with time in both women and men. This neoplasia is associated with hr-HPV types, particularly HPV16. In fact, most HPV-positive neoplasias outside the cervix are related to HPV16 [70].
Vulvar and vaginal cancers are relatively rare tumors with incidence rates less than 1 × 100,000/year [59]. Information is very limited in LA. Regional data show that HPV16 is the most prevalent type and is found in 75% to 100% of the basaloid/warty vulvar cancers that are more common in young women. About two thirds of vaginal cancers are linked to HPV, in particular HPV16 [14].
In some LA countries, the incidence of penile cancer is significantly higher than in more developed parts of the world: the central region of Brazil and some areas in Colombia and Paraguay account for about 2.0 × 100,000/year as compared to other countries with incidence rates around 0.4 × 100,000/year [26, 73]. Studies performed in LA show HPV DNA positivity in 30% and 50% of penile cancers [10, 70].
In the head and neck anatomical sites, some cancers are linked to HPV, although in variable frequencies, being more HPV associated in the base of the tongue and tonsils [19]. The most common HPV type found is HPV16 worldwide and in series of cases from LA [19, 46, 64, 70]. Notwithstanding, there have been reports of lower HPV positivity in oropharyngeal cancers from LA countries as compared to other countries in the Northern Hemisphere [37, 53, 65]. Further studies are warranted to better understand the basis for such differences and the impact on cancer patient management.
6 Control of HPV Infections and Related Diseases
6.1 Primary Prevention: HPV Vaccines
Since 2006, two vaccines composed of HPV L1 proteins self-assembled into virus-like particles (VLP) have been approved in LA: one containing VLPs of HPV types 6, 11, 16, and 18 (Merck & Co.) and one composed of HPV 16 and 18 VLPs (GlaxoSmithKline). Large phase II and III clinical trials to assess prophylactic efficacy have been conducted in which both HPV infection and cervical disease endpoints were evaluated, particularly HSIL (CIN2 or CIN3) as well as vulvar and vaginal intraepithelial neoplasias (VIN and VaIN, respectively) and genital warts for the quadrivalent vaccine [75]. Very high efficacy rates were noted in different populations that included young women between 16 and 26 years and older (up to 55 years). The quadrivalent HPV vaccine has also proven to be efficacious in men to prevent genital and anal infection and disease caused by the types included in the vaccine [13, 40]. Importantly, several clinical trials of HPV prophylactic vaccines conducted in LA clearly demonstrated the safety, immunogenicity, and efficacy of such recombinant vaccines among Latin Americans [61]. Furthermore, data collected in these clinical trials concerning the incidence and prevalence of genital HPV-associated infection and disease have provided important insights on the burden of genital HPV in the region [40, 61]. Moreover, seminal demonstration studies and surveys have shown that the HPV vaccine acceptability is very high in the region [3, 6, 50].
Most LA countries have a well-developed public immunization infrastructure including adolescent vaccination, which has facilitated the introduction of national immunization programs in the region (Fig. 20.2). Organizations such as the United Nations Children’s Emergency Fund (UNICEF) , Global Alliance for Vaccines and Immunization (GAVI) , and the PAHO revolving fund have enhanced HPV vaccine introduction in LA . After an initial phase, most countries are adopting the two-dose program supported by WHO [77] and are vaccinating girls aged 9 to 13 years at 0 and 6 months. Moreover, in several countries of the region, the HPV vaccine is offered to HIV-positive women up to 26 years of age.
Interestingly, a broad evaluation of the programs is driving a revision of the entire CC control strategies adopted by each country, which includes HPV vaccination for female adolescents and cytology/HPV testing for adult women [72]. Implementation of effective vaccine programs might seem straightforward and obvious in light of the vaccine efficacy and lack of serious adverse events to date; nonetheless, significant challenges remain. These problems include the cost of the program, covering two doses of vaccine and extending the vaccination to boys and other populations at risk including HIV-positive individuals. Equally important is to monitor the impact of this intervention that requires tools and strategies unavailable in many countries of the region. Introduction of cost-effective measures such as HPV vaccination only or vaccination supplemented with screening, with good coverage rates, will reduce HPV-related tumors in LA, as is happening in several countries of the world [13].
6.2 Secondary Prevention: HPV Testing as Primary Screening
For more than 50 years, cervical cytology [the Papanicolaou (Pap) smear) has been the standard of care for CC screening. Cytology-based mass screening programs have been successful in reducing incidence and mortality in developed countries (such as the U.S. and European countries). Unfortunately, most LA countries tried unsuccessfully to replicate these results, evidencing, however, after decades of efforts, high incidence and mortality rates, with little impact on the disease burden [58].
The limitations inherent in cervical cytology prompted the development of new screening technologies: tests to detect the presence of hr-HPV DNA, which should be clinically validated for this purpose. HPV testing offers numerous potential advantages compared to cytology-based screening, such as greater sensitivity, high negative predictive value (which allows to extend the screening intervals in HPV-negative women), and automation [21]. However, even among women over 30 years of age, the cancer to transient infection ratio is low, and HPV assays must overcome the intrinsic problem of low positive predictive value. This lower specificity of HPV testing requires an additional test (“triage”) in women who are HPV DNA positive in the primary screening to identify those who are at risk of having a cervical cancer precursor and to reassure those who only have transient or low-risk infections. Triage includes visual inspection methods, cytology, and molecular biomarkers (high-risk HPV E6/E7 mRNA, high-risk HPV E6 proteins, p16, among others). Locally adapted algorithms employing primary screening with HPV testing are being developed in different settings [63]. The initial HPV tests were very expensive and unaffordable for several LA countries, but in recent years, more HPV tests became available and the prices have started to drop, making them more affordable.
During the past decade, there have been multiple experiences with HPV testing in LA, some as part of research studies and others to pilot the implementation of HPV tests in the public system and, more recently, the implementation of HPV testing as part of the public programs provided by the ministries of health [45]. Pilot studies that took place in Argentina [4, 5], Chile [33, 51], Colombia [58], El Salvador [25], Mexico [38, 48], and Nicaragua [7, 44] were highly efficacious to detect precancerous cervical lesions and good feasibility and acceptance of self-sampling. Similarly, in 2011 Argentina was the first country in the region to implement HPV DNA testing for primary screening within its public system for all women aged 30 or older. In recent years, Mexico has expanded the implementation of HPV DNA testing to 17 sites across the country, applying its extensive knowledge in this field. El Salvador, Guatemala, Honduras, and Nicaragua are beginning to institutionalize HPV testing at population level [45].
The need to develop a comprehensive quality assurance program associated with the specific HPV test to be implemented should also be considered to guarantee reliable test results in real-world settings . Despite the fact that most tests have their own internal quality control, quality control procedures should be put in place to ensure proper transportation and storage of reagents and samples, correct sample labeling and processing, suitable monitoring of positivity rates, and other test characteristics to rule out contamination [45, 63].
LA is slowly shifting toward HPV testing for cervical cancer screening, with the endorsement of several regional experiences that have resulted in increased coverage and better detection of pre-cancer lesions using HPV tests. In line with this, the ESTAMPA study, recently launched in LA countries by the International Agency for Cancer Research, will contribute valuable information about the performance of emerging CC screening and triage techniques and the feasibility of different approaches to implement organized HPV-based screening programs in the region [43].
Finally, it is important to emphasize that the screening test is important, but it is only one component of many other aspects of population-based programs that should be implemented to effectively impact CC cancer incidence and mortality.
7 Conclusions and Perspectives
The prevalence and incidence of HPV-related infection and disease in LA underscore the importance of supporting CC prevention strategies in the region. CC is one of the leading killers among women in LA, a region where many countries have not been successful in implementing population-level cytology-based screening programs. Hence, a more comprehensive CC control approach is required, wherein primary and secondary prevention strategies are implemented with both high coverage and sustainability.
Although regional data seem to indicate a favorable trend in prevention, significant challenges still remain. In primary prevention these include the cost of the program, covering two doses of vaccine, and extending the vaccination to boys and other populations at risk , including HIV-positive individuals. Equally important is to monitor the impact of this intervention that requires tools and strategies unavailable in many countries of the region.
In secondary prevention, it is crucial to change the paradigm by implementing HPV testing as primary screening in the most appropriate way. Among several challenges for its implementation, it should take into account the need to update screening guidelines, strengthen treatment capacity, and develop a comprehensive quality assurance plan for HPV testing.
Finally, gaps still exist in the knowledge and the future lines of research, policy, and advocacy for noncervical HPV cancer prevention , mainly anal and oropharyngeal cancers and precancers; further studies are warranted to better understand their pathogenesis and the impact on cancer patient management. Public health commitment and research to implement HPV-based preventive strategies, together with stronger and common advocacy to counter barriers affecting the adoption of these strategies, are likely to yield major benefits in reducing the burden of HPV-associated diseases in LA.
References
Almonte M, Albero G, Molano M et al (2008) Risk factors for human papillomavirus exposure and co-factors for cervical cancer in Latin America and the Caribbean. Vaccine 26(suppl 11):L16–L36
Almonte M, Ferreccio C, Winkler JL et al (2007) Cervical screening by visual inspection, HPV testing, liquid-based and conventional cytology in Amazonian Peru. Int J Cancer 121:796–802
Arrossi S, Maceira V, Paolino M et al (2012) Acceptability and uptake of HPV vaccine in Argentina before its inclusion in the immunization program: a population-based survey. Vaccine 30:2467–2474
Arrossi S, Thouyaret L, Herrero R et al (2015) Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): a population-based cluster-randomised trial. Lancet Global Health 3(2):e85–e94
Arrossi S, Thouyaret L, Laudi R et al (2015) Implementation of HPV-testing for cervical cancer screening in programmatic contexts: the Jujuy demonstration project in Argentina. Int J Cancer 137:1709–1718
Baker ML, Figueiroa-Downing D, Chiang ED et al (2015) Paving pathways: Brazil’s implementation of a national human papillomavirus immunization campaign. Rev Panam Salud Publica 38:163–166
Bansil P, Wittet S, Lim JL et al (2014) Acceptability of self-collection sampling for HPV-DNA testing in low-resource settings: a mixed methods approach. BMC Public Health 14:596
Bernard HU, Burk RD, Chen Z et al (2010) Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401:70–79
Berumen J, Ordonez RM, Lazcano E et al (2001) Asian-American variants of human papillomavirus 16 and risk for cervical cancer: a case-control study. J Natl Cancer Inst 93:1325–1330
Bezerra AL, Lopes A, Landman G et al (2001) Clinicopathologic features and human papillomavirus DNA prevalence of warty and squamous cell carcinoma of the penis. Am J Surg Pathol 25:673–678
Bosch FX, Burchell AN, Schiffman M et al (2008) Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 26(suppl 10):K1–K16
Bouvard V, Baan R, Straif K et al (2009) A review of human carcinogens. Part B: Biological agents. Lancet Oncol 10:321–322
Brotherton JM, Ogilvie GS (2015) Current status of human papillomavirus vaccination. Curr Opin Oncol 27:399–404
Bruni L, Barrionuevo-Rosas L, Albero G et al. (2015) Human papillomavirus and related diseases in Americas. Summary report. In: ICO Information Centre on HPV and Cancer (HPV Information Centre). Available via DIALOG. http://www.who.int/hpvcentre of subordinate document. Accessed 5 June 2016
Bruni L, Diaz M, Castellsagué X et al (2010) Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 202:1789–1799
Burchell AN, Winer RL, de Sanjosé S et al (2006) Chapter 6: epidemiology and transmission dynamics of genital HPV infection. Vaccine 24(suppl 3):S3/52–S3/61
Burk RD, Harari A, Chen Z (2013) Human papillomavirus genome variants. Virology 445:232–243
Calleja-Macias IE, Kalantari M, Huh J et al (2004) Genomic diversity of human papillomavirus-16, 18, 31 and 35 isolates in a Mexican population and relationship to European, African, and Native American variants. Virology 319:315–323
Castellsagué X, Alemany L, Quer M, ICO International HPV in Head and Neck Cancer Study Group et al (2016) HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst 108(6):djv403
Castellsagué X, de Sanjosé S, Aguado T, Louie KS, Bruni L, Muñoz J, Diaz M, Irwin K, Gacic M, Beauvais O, Albero G, Ferrer E, Byrne S, Bosch FX (2007) HPV and cervical cancer in the world. 2007 Report. WHO/ICO information centre on HPV and cervical cancer (HPV Information Centre). Available at: www.who.int/hpvcentre
Castle PE, de Sanjosé S, Qiao YL et al (2012) Introduction of human papillomavirus DNA screening in the world: 15 years of experience. Vaccine 30(suppl 5):F117–F122
Chen Z, Terai M, Fu L et al (2005) Diversifying selection in human papillomavirus type 16 lineages based on complete genome analyses. J Virol 79:7014–7023
Ciapponi A, Bardach A, Glujovsky D et al (2011) Type-specific HPV prevalence in cervical cancer and high-grade lesions in Latin America and the Caribbean: systematic review and meta-analysis. PLoS One 6(10):e25493. doi: 10.1371/journal.pone.0025493
Clifford GM, Rana RK, Franceschi S et al (2005) Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 14:1157–1164
Cremer ML, Maza M, Alfaro KM et al (2016) Introducing a high-risk HPV DNA test into a public sector screening program in El Salvador. J Low Genit Tract Dis 20:145–150
Curado MP, Souza DLB (2014) Cancer burden in Latin America and the Caribbean. Ann Global Health 80:370–377
de Villiers EM, Fauquet C, Broker TR et al (2004) Classification of papillomaviruses. Virology 324:17–27
Deluca GD, Basiletti J, González JV et al (2012) Human papilloma virus risk factors for infection and genotype distribution in aboriginal women from Northern Argentina. Medicina (B Aires) 72:461–466
Deluca GD, Basiletti J, Schelover E et al (2011) Chlamydia trachomatis as a probable cofactor in human papillomavirus infection in aboriginal women from northeastern Argentina. Braz J Infect Dis 15:567–572
Doorbar J (2007) Papillomavirus life cycle organization and biomarker selection. Dis Markers 23:297–313
Eiguchi K, Tatti S, Alonio LV et al (2008) Association of DRB1 and DQB1 HLA class II polymorphisms in high-grade and neoplastic cervical lesions of women from Argentina. J Low Genit Tract Dis 12:262–268
Ferlay J, Soerjomataram I, Ervik M et al. (2013) GLOBOCAN 2012 v1.2, Cancer incidence and mortality worldwide. In: IARC Cancer Base No. 11 [Internet]. Lyon: International Agency for Research on Cancer. Available from: http://globocan.iarc.fr . Accessed 15 June 2016
Ferreccio C, Barriga MI, Lagos M et al (2013) Screening trial of human papillomavirus for early detection of cervical cancer in Santiago, Chile. Int J Cancer 132:916–923. Erratum in: Int J Cancer 133:E1
Ferreccio C, Prado RB, Luzoro AV et al (2004) Population-based prevalence and age distribution of human papillomavirus among women in Santiago Chile. Cancer Epidemiol Biomarkers Prev 13:2271–2276
Forman D, de Martel C, Lacey CJ et al (2012) Global burden of human papillomavirus and related diseases. Vaccine 30(suppl 5):F12–F23
Guan P, Howell-Jones R, Li N et al (2012) Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 131:2349–2359
Heck JE, Berthiller J, Vaccarella S et al (2010) Sexual behaviours and the risk of head and neck cancers: a pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int J Epidemiol 39:166–181
Hernandez-Marquez CI, Salinas-Urbina AA, Cruz-Valdez A et al (2014) Conocimientos sobre virus del papiloma humano (VPH) y aceptación de auto-toma vaginal en mujeres mexicana. Rev Salud Pública 16:697–708
Herrero R, Castle PE, Schiffman M et al (2005) Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste. J Infect Dis 191:1796–1807
Herrero R, González P, Markowitz LE (2015) Present status of human papillomavirus vaccine development and implementation. Lancet 16:e206–e216
Herrero R, Hildesheim A, Bratti C et al (2000) Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 92:464–474
Hildesheim A, Schiffman M, Bromley C et al (2001) Human papillomavirus type 16 variants and risk of cervical cancer. J Natl Cancer Inst 93:315–318
International Agency for Research on Cancer. Latin American multicentre study of cervical cancer screening and triage with HPV testing (ESTAMPA). Available at: http://www.iarc.fr/en/research-groups/PRI/current-topics.php . Accessed 07 July 16
Jeronimo J, Bansil P, Lim J et al (2014) A multicountry evaluation of care HPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer. Int J Gynecol Cancer 24:576–585
Jeronimo J, Holme F, Slavkovsky R et al (2016) Implementation of HPV testing in Latin America. J Clin Virol 76(suppl 1):S69–S73
Kaminagakura E, Villa LL, Andreoli MA et al (2012) High-risk human papillomavirus in oral squamous cell carcinoma of young patients. Int J Cancer 130:1726–1732
Khouadri S, Villa LL, Gagnon S et al (2006) Human papillomavirus type 33 polymorphisms and high-grade squamous intraepithelial lesions of the uterine cervix. J Infect Dis 194:886–894
Lazcano-Ponce E, Lorincz AT, Torres L et al (2014) Specimen self-collection and HPV DNA screening in a pilot study of 100,242 women. Int J Cancer 135:109–116
Lazcano-Ponce E, Herrero R, Munoz N et al (2001) Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer 91:412–420
Lee FH, Paz-Soldan VA, Carcamo C et al (2010) Knowledge and attitudes of adult peruvian women vis-a-vis human papillomavirus (HPV), cervical cancer, and the HPV vaccine. J Low Genit Tract Dis 14:113–117
Léniz J, Barriga MI, Lagos M et al (2013) HPV vaginal self-sampling among women non-adherent to Papanicolaou screening in Chile. Salud Publica Mex 55:162–169
Li N, Franceschi S, Howell-Jones R et al (2011) Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer 128:927–935
López RV, Levi JE, Eluf-Neto J et al (2014) Human papillomavirus (HPV) 16 and the prognosis of head and neck cancer in a geographical region with a low prevalence of HPV infection. Cancer Causes Control 25:461–471
Maciag PC, Schlecht NF, Souza PS et al (2002) Polymorphisms of the human leukocyte antigen DRB1 and DQB1 genes and the natural history of human papillomavirus infection. J Infect Dis 186:164–172
Matos E, Loria D, Amestoy GM et al (2003) Prevalence of human papillomavirus infection among women in Concordia, Argentina: a population-based study. Sex Transm Dis 30:593–599
Mendoza L, Picconi MA, Mirazo S et al (2013) Distribution of HPV-16 variants among isolates from Paraguayan women with different grades of cervical lesion. Int J Gynaecol Obstet 122:44–47
Molano M, Posso H, Weiderpass E et al (2002) Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br J Cancer 87:324–333
Murillo R, Almonte M, Pereira A et al (2008) Cervical cancer screening programs in Latin America and the Caribbean. Vaccine Suppl 11:L37–L48
Pan American Health Organization (2013) Cancer in the Americas: country profiles 2013. Pan American Health Organization, Washington, DC
Pastrana DV, Vass WC, Lowy DR et al (2001) NHPV16 VLP vaccine induces human antibodies that neutralize divergent variants of HPV16. Virology 279:361–369
Perez G, Lazcano-Ponce E, Hernandez-Avila M et al (2008) Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like-particle vaccine in Latin American women. Int J Cancer 122:1311–1318
Picconi MA, Alonio LV, Sichero L et al (2003) Human papillomavirus type-16 variants in Quechua aboriginals from Argentina. J Med Virol 69:546–552
Picconi MA (2013) Human papillomavirus detection in cervical cancer prevention. Medicina (B Aires) 73:585–596
Quintero K, Giraldo GA, Uribe ML et al (2013) Human papillomavirus types in cases of squamous cell carcinoma of head and neck in Colombia. Braz J Otorhinolaryngol 79:375–381
Ribeiro KB, Levi JE, Pawlita M et al (2011) Low human papillomavirus prevalence in head and neck cancer: results from two large case-control studies in high-incidence regions. Int J Epidemiol 40:489–502
Rodríguez AC, Schiffman M, Herrero R et al (2008) Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst 100:513–517
Schiffman M, Castle PE, Jeronimo J et al (2007) Human papillomavirus and cervical cancer. Lancet 370:890–907
Schiffman M, Rodriguez AC, Chen Z et al (2010) A population-based prospective study of carcinogenic human papillomavirus variant lineages, viral persistence, and cervical neoplasia. Cancer Res 70:3159–3169
Sichero L, Ferreira S, Trottier H et al (2007) High grade cervical lesions are caused preferentially by non-European variants of HPVs 16 and 18. Int J Cancer 120:1763–1768
Taylor S, Bunge E, Bakker M et al (2016) The incidence, clearance and persistence of non-cervical human papillomavirus infections: a systematic review of the literature. BMC Infect Dis 16:293–314
Tonon SA, Basiletti J, Badano I et al (2007) Human papillomavirus type 16 molecular variants in Guarani Indian women from Misiones, Argentina. Int J Infect Dis 11:76–81
Tota JE, Ramana-Kumar AV, El-Khatib Z et al (2014) The road ahead for cervical cancer prevention and control. Curr Oncol 21:e255–e264
Velazquez EF, Cubilla AL (2007) Penile squamous cell carcinoma: anatomic, pathologic and viral studies in Paraguay (1993–2007). Anal Quant Cytol Histol 29:185–198
Villa LL, Sichero L, Rahal P et al (2000) Molecular variants of human papillomavirus types 16 and 18 preferentially associated with cervical neoplasia. J Gen Virol 81(Pt 12):2959–2968
Villa LL (2011) HPV prophylactic vaccination: the first years and what to expect from now. Cancer Lett 305:106–112
Wentzensen N, Vinokurova S, Von KnebelDoeberitz M (2004) Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res 64:3878–3884
WHO (2014) Human papillomavirus vaccines: WHO position paper. Wkly Epidemiol Rep 89:465–492
Yamada T, Manos MM, Peto J et al (1997) Human papillomavirus type 16 sequence variation in cervical cancers: a worldwide perspective. J Virol 71:2463–2472
zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2:342–350
Acknowledgments
We are grateful to the contribution of hundreds of investigators, physicians, and students who diligently dedicated their work to generate information about HPV infections and related diseases in the Latin American region. As the amount of quality information available is vast, apologies are extended to the numerous authors whose work is not mentioned.
We wish to dedicate this chapter to the memories of Dr. Angélica R. Teyssié (1922–2015), a prestigious Argentine virologist, a pioneer in the research of viral oncogenesis, particularly HPV, and in the training human resources, and to Dr. Xavier Castellsagué (1959–2016), one of the most productive and influential epidemiologists in the field of HPV-related neoplasias.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Picconi, M.A., Villa, L.L. (2017). Human Papillomavirus Research in Latin America. In: Ludert, J., Pujol, F., Arbiza, J. (eds) Human Virology in Latin America. Springer, Cham. https://doi.org/10.1007/978-3-319-54567-7_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-54567-7_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-54566-0
Online ISBN: 978-3-319-54567-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)