Abstract
Arenaviruses are enveloped viruses that have a bi-segmented negative-stranded RNA genome. The genomic RNA segments, large (L) and small (S), use an ambisense coding strategy to encode two open reading frames in opposite orientation, separated by a noncoding intergenic region. Several arenaviruses are etiological agents of emerging diseases. At present, they are responsible for up to 500,000 zoonotic infections per year in endemic areas of Africa and South America and can lead to severe and lethal hemorrhagic fever as well as neurological symptoms. Arenaviridae represents the largest group of hemorrhagic fever (HF)-causing viruses: five of the South American arenaviruses (CHPV, GTOV, JUNV, MACV, and SABV) are associated with HF in humans.
The only locally licensed vaccine available is based on a live attenuated virus to prevent Argentine hemorrhagic fever (AHF). Immune therapy has been implemented to reduce AHF mortality rate significantly, and studies on small synthetic and natural chemicals have met variable success as antiviral agents. Rapid diagnosis and early treatment are essential to this end. In this chapter we review studies on virus discovery, molecular and cell biology of infection, pathogenesis, diagnosis, prevention, and treatment totally or partially conducted by Latin American scientists and medical personnel.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Arenaviridae
- Arenavirus taxonomy
- Hemorrhagic fevers
- Junín virus
- Arenavirus replication
- Pathogenesis
- Therapeutic targets
- Immune therapy
- Vaccines
1 Introduction and New Arenavirus Taxonomy
This chapter focuses on arenavirus studies carried out in past decades in Latin America. Some information comes also from reports of international collaborative research and study groups in which scientists from this region have participated.
The Arenaviridae family presently includes more than 30 viral species. The number of new arenaviruses isolated and characterized in the past few years has grown dramatically and led to the establishment of two genera: Mammarenavirus (known to infect mammals) and Reptarenavirus (identified in snakes) [71] (Table 10.1).
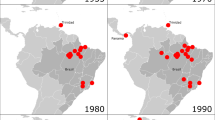
The mammarenaviruses (referred to as “arenaviruses” in the literature before 2016) are generally associated with infection in rodents and are divided into two major groups on the basis of serological cross-reactivity, phylogeny, and geographic site of isolation : the Old World (OW) complex (Africa, Europe, and Asia) and the larger New World (NW) complex (Americas), subdivided into clades. Lymphocytic choriomeningitis virus (LCMV), the type species, infects the common house mouse (Mus musculus), a fact that explains its global distribution. In contrast, all other arenaviruses show restricted geographic distribution coincident with the location of habitats of natural reservoir hosts. In rodents, arenaviruses usually establish an asymptomatic chronic infection. This fact has a correlation in vitro, where arenaviruses can produce persistent infections without associated cytopathic effects. However, occasionally some arenaviruses may be transmitted to humans through contact with urine or blood-contaminated materials and produce severe hemorrhagic fever. Included are Lassa (LASV) and Lujo (LUJV) viruses, in West Africa, and Junín (JUNV), Machupo (MACV), Guanarito (GTOV), Sabiá (SABV) , Chaparé (CHPV), and Whitewater Arroyo (WWAV) viruses, in the Americas (Fig. 10.1).
New World arenaviruses that cause hemorrhagic fevers . Endemic areas for American human pathogenic arenaviruses. The year of isolation/description of these viruses is indicated in parentheses. WWAV* includes several different North American arenaviruses, some of which are associated with cases of hemorrhagic fever with liver failure and belong to the species Whitewater Arroyo mammarenavirus
At present, arenaviruses cause as many as 500,000 zoonotic infections per year in endemic areas of Africa and South America that can lead to severe and lethal hemorrhagic fever symptoms. Human pathogenic arenaviruses are considered potential biological weapons.
Because of the number of cases of human disease in Latin America and the availability of locally and internationally published reports, this chapter discusses JUNV in more detail and refers to other arenaviruses when appropriate. By no means should this chapter be considered a thorough compilation of the research on arenaviruses conducted in Latin America; it is rather an overview of the diverse published studies in this field and a short perspective on future developments.
2 Virion Structure and Genome Organization
Members of the Mammarenavirus genus share the following features: virions are pleomorphic (mostly spherical), 50–300 nm in diameter (mean, ~120 nm); they contain several copies of circular nucleocapsids and include a variable number of ribosomes [74, 77]. They acquire a lipid envelope with club-shaped projections, 8–10 nm in length (spikes), during the budding process from the host cell membrane at the end of the infectious cycle.
Their genome consists of two single-stranded RNA segments : small (S) and large (L), about 3.5 kb and 7.5 kb in length, respectively. Each segment has two nonoverlapping open reading frames (ORF) of opposite sense, which was the origin of the term ambisense to describe this type of coding strategy [9]. The sizes of the gene products indicated in the following text are those of JUNV .
The ORFs of opposite polarity are separated in both RNAs by a noncoding intergenic region predicted to fold into a stable secondary structure [31]. The L segment codes for both the 94 amino acid (aa) zinc-binding Z-matrix protein that drives virus budding (~11 kDa), as well as for the RNA-dependent RNA polymerase L (2210 aa; ~250 kDa). The S RNA codes for both a nucleocapsid protein N (564 aa) as well as the glycoprotein precursor GPC (485 aa) [31, 77]. GPC is synthesized as a single polypeptide chain and is post-translationally cleaved to yield mature virion glycoproteins G1 (192 aa) and G2 (235 aa) and a stable signal peptide SSP (58 aa) [87]. G1/G2/SSP trimers form the spikes decorating the virus surface.
G1 is located at the top of the spike and mediates virus interaction with host cell-surface receptors, and G2 is similar to others class I viral fusion proteins [70]. SSP is generated by signal peptidase cleavage but, in contrast to conventional signal peptides, is stable, unusually long (58 aa vs. the usual 15–25 aa), and myristoylated; it contains two hydrophobic segments that span the lipid bilayer with both N- and C-termini residing in the cytosol; and contributes to G2 fusion activity through its C-terminal region [86, 87].
The nucleocapsid protein N is the most abundant virion protein, followed by G2, G1, Z, and L (~1500, 650, 650, 450, and 30 molecules per virion, respectively, as calculated per “old style” methods) [84]. The RNA–N interactions and zinc-binding capacity have been identified in computational and experimental studies [65, 80, 81].
3 Virus Entry and Cell Tropism
Specific virus interaction with receptor molecules on the cell membrane is a crucial step in the infectious process: it drives subsequent entry into the host cell, making cell receptors the major determinants of viral cell tropism, host range, and pathogenesis. Until 2005, little was known about the mechanism by which JUNV entered host cells. By using pseudo-typed retroviruses , several laboratories confirmed that JUNV, as well as other clade B NW arenaviruses, did not interact with α-dystroglycan, the known receptor for Old World (OW) arenaviruses, to enter the cells [72]. The next big breakthrough came 1 year later when using a proteomic pull-down approach , applying a recombinant receptor-binding G1 moiety of Machupo virus (MACV) as bait, transferrin receptor 1 (TfR1) was identified as the first known JUNV, MACV, GTOV, and SABV cell receptor [70]. A recent study has shown that a neutralizing monoclonal antibody directed toward G1 maps to the same site that makes contact with hTRf1, suggesting that this is the basis for immune therapy success [50].
Although the hTfR1 is definitely a major receptor that allows JUNV infection, there is information on additional or alternative cell-surface molecules that seem to promote virus entry. Dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) is a type II transmembrane lectin receptor. DC-SIGN is abundantly expressed on immature dendritic cells (iDCs) , one of the principal targets of JUNV. Previous reports showed that nonpermissive cells lacking TfR1 became significantly more susceptible for JUNV infection when transfected with a plasmid DNA construct expressing DC-SIGN receptor. In addition, pretreatment of these genetically modified cells with anti-DC-SIGN or mannan reduces the infection with JUNV. This work validates a direct cell-to-cell transmission of JUNV and supports the role of type C lectins in viral adsorption, internalization, and intracellular transport [25, 38].
After binding to its receptor, a virus can resort to different internalization mechanisms. Briefly, there are three general mechanisms for viral internalization: clathrin-mediated endocytosis , caveolar/raft pathway, and cholesterol-dependent endocytosis. It has been demonstrated in Vero cells that clathrin-mediated endocytosis is the main route used by JUNV and involves the cytoskeleton and a host of cellular proteins [55,56,57]. Finally, direct evidence of JUNV cell entry was obtained using transmission electron microscopy [55]. Pichinde virus (PICV) , another NW arenavirus , has also been shown to enter cells through a clathrin-dependent endocytic pathway, trafficked through the dynamin 2 endocytic pathway in which the virus travels through Rab5-mediated early endosomes and Rab7-mediated late endosomes [83]. Similar results have been obtained for JUNV [57]. JUNV internalization leads to PI3K/Akt signaling pathway activation [42], which requires both intact actin and a dynamic microtubule network [56]. An alternative virus internalization pathway has been recently described for TCRV [73].
Later, the fusion of the viral envelope with the endosomal membrane is essential for the progression of the infection cycle. Although G1 interacts with the TfR1, G2 is responsible for the fusion process. These studies revealed a crucial role of the SSP in pH-dependent membrane fusion [87]. It has been shown that sera from AHF patients inhibit fusion activity in an in vitro system [16].
4 RNA Transcription and Replication
The 3′- and 5′-terminal sequences of 19-nucleotide RNA segments are complementary and very well conserved in all arenaviruses. The base complementarity of these termini is probably the molecular basis for the circular conformation of the nucleocapsids that has been observed [29]. These termini are essential for replication and transcription and are believed to function as a binding site for viral polymerases (reviewed in [2]).
Genome replication and transcription take place in the cytoplasm of infected cells and require that viral proteins combine with viral RNA to form ribonucleoprotein (RNP) complexes. The L protein mediates viral transcription and replication using RNPs as templates. In TCRV , N and L proteins together with virus RNA are the minimal components of RNP complexes and are sufficient for genome replication and transcription [44]. Both N and L proteins are necessary and sufficient for these early steps in vivo in reconstructed JUNV transcription-replication [2]. During genome replication, full-length copies of genomic S and L RNAs are synthesized, generating the corresponding antigenomic S and L RNAs. In response to the ambisense coding strategy, both genomic and antigenomic RNAs serve as template for viral mRNA transcription (Fig. 10.2). Transcripts contain a cap but are not polyadenylated. The 3′-end sequences of the subgenomic mRNAs fall within the intergenic, suggesting that the stem-loop structure is involved in transcription termination regulated by interaction with N [36, 80]. However, elements regulating termination have not yet been well defined.
Schematic of the JUNV infection cycle . Virion adsorption to the cell surface is mediated by G1 (head of trimeric GP complex) interaction with the hTfR1 cell receptor. After receptor-mediated endocytosis, the drop in pH triggers conformational changes in G2 that drive virion and endosome membrane fusion. Uncoating releases the viral nucleoprotein, which serves as template for transcription and replication of the ambisense genomic RNAs. Translation of GPC mRNA and processing of the precursor via the secretory pathway yields the GP complexes (trimers of G1 + G2 + SSP) inserted in the cell membrane. The Z protein drives the budding process by curving the GP-containing membrane GP patches and interacting with the newly formed nucleocapsids. No nuclear phase is required for arenavirus replication
5 Glycoprotein Processing and Envelope Assembly
GPC is processed to yield SSP + G1 + G2 to form trimeric spikes protruding on the virus surface. SSP is required for transport of the G1–G2 precursor protein GPC from the endoplasmic reticulum (ER). After cleavage, JUNV SSP is retained and positioned in the GP complex through interaction with a zinc-binding domain in the cytoplasmic tail of G2. The G1–G2 precursor is cleaved by the cellular SKI-1/S1P protease in the Golgi compartment to form the mature G1 and G2 subunits. As with other class I viral fusion proteins, proteolytic cleavage of the GPC precursor is required to render the GP complex competent for membrane fusion [2, 87]. Other protein–protein interactions are necessary to package the genome and induce budding to generate virions. In addition, it has been shown that GP complexes become localized to cholesterol-rich lipid microdomains [18].
6 Z Protein at the Crossroads of the Infectious Cycle
López et al. (2001) initiated studies to establish a reverse genetic system for TCRV (a close nonpathogenic relative of JUNV ) focused initially at the replication and encapsidation of minigenomes [44]. In cells expressing N and L proteins, the coexpression of the small Z protein proved to be highly inhibitory to both transcription and replication via interaction with the L protein [39]. It has been shown that interaction between Z and N is required for assembly of both nucleocapsids and glycoproteins into infectious budding particles [15]. Z protein has been assigned a major role in virus particle budding. Later, the L-binding domain of Z protein and the structural requirements mediating Z homo-oligomerization were described for JUNV and TCRV [46]. N–N and N–L interactions are central during transcription and replication, and current evidence supports the notion that Z operates as a key modulator of viral RNA synthesis by directly interacting with L. When N and GP accumulate above a certain threshold, Z becomes engaged in virion assembly via Z–N-mediated recruitment of nucleocapsids and targeting of the plasma membrane, where Z–Z oligomerization and Z–G2 interactions lead to budding of complete virus particles [45]. Additionally, Z function may be related to cell response to viral infection .
7 Arenaviruses and Hemorrhagic Fevers in Latin America
Five NW arenaviruses are known to naturally cause severe febrile disease in humans in Latin America: the Guanarito (GTOV) , Junín (JUNV), Machupo (MACV), Sabiá (SABV), and Chaparé (CHPV) viruses [17, 20, 66, 79]. The diseases range from sporadic cases to small outbreaks to hyperendemic episodes. Humans usually become infected with arenaviruses by inhalation of virus in aerosolized droplets of rodent excreta.
In contrast, other arenaviruses are not associated with human disease, such as Mopeia virus (MOPV) in West Africa or Tacaribe (TCRV) , Pichinde (PICV) , and Oliveros (OLVV) , in the Americas.
Argentine hemorrhagic fever (AHF) is the best studied South American hemorrhagic fever (HF) and is similar to others in clinical presentation. It is a severe viral hemorrhagic syndrome endemic to the agricultural plains of central Argentina. Its incidence is mainly seasonal [24, 75].
The clinical symptoms of AHF include hematological, neurological, cardiovascular, renal, and immunological alterations. This emerging disease was first recognized in 1955, and its etiological agent was characterized and designated Junín virus (JUNV) for the geographic site where it was first isolated [66, 67]. JUNV is a rodent-borne virus and belongs to the clade B New World (NW) arenavirus within the Arenaviridae family [77].
The population of humans at risk is composed mainly of agricultural workers who become infected by inhaling aerosols of rodent excreta, although viral entry may occur by other routes, such as the conjunctival membranes, other mucous membranes, ingestion, and direct contact with damaged skin [60]. Transmission between humans has been reported even though AHF is usually not contagious from human to human. In patients with AHF, the viremia is present during the entire acute febrile period. Moreover, the virus was occasionally isolated from oral swabs, urine, and breast milk from infected subjects. Sexual transmission of JUNV was reported from convalescent men to women [13].
Since its emergence in the 1950s, annual epidemics of the disease have been recorded. The initially high case fatality rate of the disease was markedly reduced, first with adequate supportive measures and, more significantly, with the use of immune plasma [24].
Former endemic hotspots are currently cooling off; however, there is a steady and progressive geographic expansion of the endemic region into north-central Argentina, and currently almost 5 million people are considered to be at risk of contracting AHF [24].
A collaborative effort conducted by the U.S. and Argentine governments in the 1980s led to the production of a live attenuated Junín virus vaccine [51]. The availability of the live attenuated vaccine has contributed to a substantial reduction in the number of AHF cases in recent years [24].
JUNV may enter the body through the skin, respiratory tract, or gastrointestinal mucosa. After replication, generalized dissemination occurs, but gross pathology changes are nonspecific [34]. Capillary dilatation ensues with perivascular erythrocyte diapedesis and bleeding; minor edema of the vascular wall has also been observed. Erythroblastopenia with morphologically abnormal erythroid and leukopoietic cell lines and normal megakaryocytes has been described in bone marrow, as well as severe meningeal edema and hemorrhages in Virchow–Robin spaces in the central nervous system (CNS) (reviewed in [54]).
Decreased T- and B-lymphocyte counts and a diminished response to mitogens are expressions of immunosuppression during the acute phase of the disease. Low numbers of null, B, and T cells, as well as a lower T4/T8 ratio, have been observed during the acute phase of AHF. Null and T8 cell numbers improve after immune plasma infusion, and all cell subsets return to normal in early convalescence. It has been proposed that circulating monocytes (macrophages) are targets for JUNV replication, contributing to viral spread during the acute AHF [54]. However, at this stage as well as in early convalescence, patient peripheral-blood mononuclear cells may exert antibody-dependent cell cytotoxicity, suggesting JUNV replication in macrophages does not affect their killing capacity [54].
During the first week after symptom onset, AHF patients show very high serum interferon-alpha (IFN-α) titers. Even though these values slowly normalize during the second week of illness in survivors, they remain elevated in severe cases. Interferon levels at admission correlate with outcome and are significantly lower in patients who survive [41]. Other cytokines described as significantly elevated in the serum of acute AHF patients include tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, and IL-10 [53], although their individual function in disease pathogenesis has not been studied. For more details on human disease findings, readers should consult the review by Marta et al. [54].
Pathological lesions in fatal AHF include generalized vasocongestion with multiple hemorrhages in the gastrointestinal mucosa and different organs, such as the liver, kidney, and lungs, as well as in subcutaneous tissue. The highest virus titers are found in the spleen, lymph nodes, and lungs, and high levels of viral antigen are found in cells of the monocyte/macrophage lineage in peripheral blood, lymphatic tissue, lung, and liver [54].
8 Expansion of Agriculture and Emergence of AHF
This disease is endemic, with annual outbreaks from the end of summer until midwinter, coincident with the harvest of maize and with the increase in the population of the wild rodents Calomys musculinus, Calomys laucha, Akodon azarae, and Oryzomys flavescens [60]. It was assumed that appearance of AHF disease in the mid-1950s was caused by human changes made in natural habitats in relationship to agricultural practices. Those environmental modifications are thought to have favored the growth of the C. musculinus population and facilitated its contact with humans. The epidemiological features of AHF are determined by the natural cycle of JUNV and by the behavior of the rodent reservoirs [60]. AHF mainly affects rural workers from the agricultural region known as the humid pampa, in central-east Argentina [24]. There are, however, urban cases in which the origin of infection is not easy to establish.
9 Rodent Reservoirs
All arenaviruses pathogenic for humans are rodent viruses. Although each arenavirus can infect many species of rodents, in every geographic site there is one species that is the principal reservoir because of higher population density and the prevalence and characteristics of infection. Calomys musculinus (family Muridae, subfamily Sigmodontinae) has been identified as the principal reservoir of JUNV , although virus has also been isolated from the organs and body fluids of other rodents captured in the endemic area, including Calomys laucha and Akodon azarae, and occasionally from Mus musculus, Necromys benefactus, and Oligoryzomys flavescens [76].
Some of these animals develop an acute disease with antibody response and clearance of the virus, whereas others develop a persistent infection, with low titers or absence of antibodies, chronic viremia, and shedding of virus in urine, feces, and saliva [85]. The chronically infected rodents are usually asymptomatic and exhibit normal behavior. Field studies of natural populations demonstrated that infection with JUNV among C. musculinus was more frequent among males than females and was positively correlated with age and the presence of wounds and scars [60]. JUNV among rodents may be transmitted via aerosols and bites, as well as sexually .
10 Screening and Discovery of Arenaviruses in the Americas
Serological screening for arenavirus infection in wild rodents and patients, combined with reverse transcriptase (RT)-polymerase chain reaction (PCR), sequencing, and virus isolation, has been the basis for discovery and definition of new species or virus variants [33, 49, 59].
New monoclonal antibodies (mAbs) against JUNV N were obtained by Nakauchi et al. [63]. Three epitopes comprising residues 12–17 (WTQSLR), 72–79 (KEVDRLMS), and 551–558 (PPSLLFLP) are recognized by different mAbs with different degrees of specificity, that is, ranging from broadly reactive with South American arenaviruses to JUNV specific [63]. RT-PCR-based methods have been described using both Arenaviridae family-specific and species-specific methods that can be applied to detect arenaviruses in rodents captured in the field [37, 48].
Comparison of endpoint antibody titers to WWAV and AMAV in individual blood samples from nearly 5000 rodents indicated that the Tacaribe complex viruses that are enzootic in New Mexico, Texas, and Mexico are antigenically diverse [58]. In particular, the samples from Chiapas (Mexico) showed a strong reaction to AMAV antigen. Analyses of nucleotide and amino acid sequence data indicated that the deer mice were infected with a novel Tacaribe serocomplex virus (proposed name: Ocozocoautla de Espinosa virus, OCEV), which is phylogenetically closely related to Tacaribe serocomplex viruses that cause hemorrhagic fever in humans in South America (clade B) [14]. Hypothetically, OCEV or an arenavirus phylogenetically closely related to OCEV was the etiological agent in the hemorrhagic fever epidemic in Chiapas in 1967 and presently is the cause of a human disease that is clinically indistinct from dengue hemorrhagic fever and other severe febrile illnesses endemic to Chiapas. Moreover, it has been speculated that these findings support the notion that epidemics of highly lethal hemorrhagic fever(s) in the highlands of Mexico in the sixteenth century were caused by arenavirus(es) native to Mesoamerica [1, 52].
More recently, aiming at the identification of the natural rodent reservoir for SABV , a broadly cross-reactive enzyme-linked immunosorbent assay (ELISA) was used to screen for antibody-positive animals. RT-PCR amplification provided evidence of a new arenavirus (proposed name: Pinhal virus) of the lineage C and no evidence of involvement in human disease [11].
11 Clinical Presentation
The AHF incubation period ranges from 6 to 12 days, ending with the onset of fever, usually associated with a flu-like syndrome that may include myalgia, arthralgia, headache, relative bradycardia, conjunctivitis, nausea, vomiting, and diarrhea, with little central nervous system (CNS) or hematological involvement during the first week. The early symptoms of AHF differ from those of acute respiratory infections by an almost constant absence of sore throat, cough, or nasal congestion. At the end of the first week of evolution, oliguria and different degrees of dehydration are present; neurological symptoms are common, and, in female patients, mild to moderate metrorrhagia is always present, being in some cases the first symptom of this disease [34].
In the second week of the disease, about 75% of infected individuals begin to improve, whereas the remaining 25% manifest neurological disorders or severe bleeding. Overlapping shock and bacterial infections appear 6 to 12 days after the onset of symptoms. Fever persists, and petechiae in the oral mucosa and the axillary region as well as gingival bleeding can be observed. Less common and more severe hemorrhagic signs may be present including hematemesis, melena, hemoptysis, epistaxis, hematomas, metrorrhagia, and hematuria. CNS involvement can also be present during the second week in the form of hyporeflexia and mental confusion. When severe, this phase can progress to include areflexia, muscular hypotonia, ataxia, increased irritability, and tremors, followed by delirium, generalized seizures, and coma [34, 61].
Clinically apparent disease occurs in almost two thirds of infected subjects. The fatality rate is as high as 30% among untreated patients. Immune plasma therapy reduces mortality to less than 1%, although this specific therapy is effective only when started during the first week of illness.
In early convalescence, 10% of cases treated with immune plasma from convalescent patients develop a late neurological syndrome (LNS) . The LNS occurs after a period free of manifestations, differs from the neurological symptoms of the acute period of AHF, and is characterized by fever syndrome and manifestations from the cerebellar trunk [21]. Patients have a prolonged convalescence. Temporary loss of hair is common; many patients experience fatigue, irritability, and memory changes, but these symptoms are temporary and disappear gradually.
During the second week of illness, patients who are improving start to produce antibodies against JUNV as well as cellular immune response to clear up the virus. Moreover, robust titers of neutralizing antiviral antibodies can be detected in immune plasma from convalescent patients [reviewed in [24]].
12 Diagnosis
Reporting of AHF disease is mandatory in Argentina. At present, the AHF diagnosis to establish specific therapy is based on clinical and laboratory data. During the early phase of the illness, the clinical manifestations of AHF are nonspecific and can be confused with several acute febrile conditions. Therefore, if platelet counts less than 100,000/mm3 in combination with white blood cell counts less than 2,500/mm3 are detected, when screening patients in endemic areas these criteria can be considered potentially useful to identify individuals at risk [reviewed in [24]].
Seroconversion occurs only late in the course of infection; serological tests are not useful markers in the early stages of the disease. Neutralizing anti-JUNV antibodies (Abs) consisting mainly of the IgG1 subtype are usually present from day 12 on (reviewed in [24]). Serological diagnosis can be done by complement fixation, indirect immunofluorescent antibody assays, neutralization tests, and ELISA. The sensitivity and specificity of ELISA make it the routine method of choice for the etiological diagnosis of reported cases retrospectively and for the surveillance of the zoonosis [62]. More recently, a more accurate ELISA was developed employing recombinant JUNV N protein [82]. Immunohistochemistry is used to examine organ specimens from autopsy and confirm etiology.
During the acute phase of infection, virus titers in blood are low. Therefore, JUNV antigen detection is not a method of choice for early diagnosis until more sensitive techniques become available. During this phase, virus isolation can be performed from whole blood or peripheral blood mononuclear cells (PBMCs), a useful (reliable, but lengthy and cumbersome) tool to retrospectively confirm the clinical diagnosis in addition to serological tests [7].
Because immune plasma therapy is able to reduce mortality when introduced during the first 8 days of infection, the availability of rapid and early diagnostic tests is fundamental. In this context, a RT-PCR-based assay has been established for rapid diagnosis [47] and has also been successfully applied to establish an etiological diagnostic in subjects who died before the appearance of the specific antibodies. At present, the RT-PCR analysis to detect JUNV genome seems to be the most sensitive, rapid, and early test for the specific diagnosis of the infection [40].
13 AHF Vaccine
A scientific collaboration between the U.S. and Argentine governments allowed the development of a live attenuated Junín virus vaccine, Candid#1 [51].
Nucleotide and amino acid sequence alignments, performed on Candid#1 and XJ ancestor strains, revealed several nucleotide substitutions throughout the GPC and L genes [5, 31, 32]. One of the changes proposed to affect infectivity was found in the G2 protein and later confirmed to be responsible for the attenuated phenotype by reverse genetics [3]. Candid#1 turned out to be safe, immunogenic, and effective in preventing AHF in preclinical studies in mice, guinea pigs, and rhesus monkeys. Guinea pigs and rhesus monkeys inoculated with increasing doses of Candid#1 developed neutralizing antibodies and became JUNV resistant if inoculated with highly virulent strains. Candid#1 also protected these animals against MACV, the etiological agent of Bolivian hemorrhagic fever [23]. These studies also showed the absence of neurovirulence, neurotropism, or hemorrhagic manifestations and the stability of the attenuated strain.
In phase III clinical trials conducted in the period 1988–1990, Candid#1 showed a protective efficacy ≥84% and no serious adverse effects. As expected, immune response to Candid#1 boosts preexisting immunity to JUNV but is not changed by previous exposure to Lymphocytic choriomeningitis virus (LCMV) [24].
The live attenuated JUNV vaccine, Candid#1, has proven effective during the past two decades in more than 100,000 persons [24]. The vaccine has been recently produced in Argentina and tested in a compatible clinical study with 946 healthy volunteers who participated to support the comparability of Candid#1 vaccine manufacturing in the U.S. and Argentina [6]. Results presented by Enria et al. (2010) showed that the vaccine produced in Argentina is equivalent to that manufactured in the U.S., both in ability to immunize against JUNV (immunogenicity ≥95.5%) and in the lack of promoting any serious adverse effects [22]. Candid#1 is the first effective vaccine against arenaviruses; it is effective to protect against AHF, promoting humoral- and cell-mediated responses, and, since January 2007 , in Argentina, is part of the National Immunization Program in the AHF risk area. The availability of such live attenuated vaccine has led to a substantial reduction in the incidence of AHF disease [22, 23].
14 Prognosis and Treatment
Without treatment, more than 80% of patients improve after the second week, although bacterial infection is a frequent complication.
Significant improvement in clinical AHF management has been achieved using immune plasma from convalescents, with mortality rates dropping from almost 30% to less than 1%. Additional administration of ribavirin may enhance these results even further (reviewed in [24]). Approximately 10% of cases treated with immune plasma develop late neurological syndrome (LNS). After a symptom-free period, LNS onset is characterized by fever, cerebellar signs, and cranial nerve palsies. LNS has never been registered among AHF patients recovering without specific treatment.
Current anti-arenaviral therapy is limited to an off-label use of ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide), which has had only mixed success in the treatment of severe infections and is associated with significant toxicity in humans [24].
15 Conclusions and Future Challenges
During the past years, impressive progress has been made in developing rapid diagnostic tools and preventive and therapeutic approaches supported by our increasing understanding of the basic molecular and cellular biology of JUNV.
Rapid and specific diagnostic tools have been developed that can be applied both for early detection of JUNV in AHF suspected patients and in epidemiological surveillance studies, including samples from field rodents.
A robust reverse genetic system for JUNV combined with new detailed knowledge on virus–host interactions has the potential to be utilized in a rational design of novel live attenuated virus vaccines with precisely engineered disruptions of pathogenic properties. The genomic sequence of Candid #1 can be used as starting information to precisely engineer a prototypic live attenuated vaccine incorporating these and other attenuating features, thereby improving the vaccine identity, efficacy, and safety [3]. Other approaches to safe and effective vaccines are being explored, including recombinant vectors expressing selected arenaviral proteins to generate immunogens based on live recombinant viruses, subunit vaccines, or virus-like particles (VLPs) [40].
The VLPs can be generated from cells transfected with a JUNV Z expression vector in the absence of any other viral protein. In view of the capacity of Z protein to support fusions at the C-terminus without compromising its membrane budding properties, Borio et al. speculated on the possibility of using it as a vehicle of specific antigens to be included in eVLPs [12].
In particular, the development of a reverse genetic system for JUNV represented an important breakthrough and provided a powerful tool to precisely address questions regarding the biology and pathogenicity of JUNV (and other NW arenaviruses) [3, 4, 45].
Current studies are focused on the ability of arenaviruses to subvert the host cell innate antiviral defenses, the impact of arenavirus infection on the differentiation and function of cells targeted by hemorrhagic arenaviruses in vivo, including APCs such as macrophages and DCs, endothelial cells, and megakaryocytes involved in platelet formation [30, 64, 68, 69]. At the same time, novel animal models will provide important new information about the interaction of hemorrhagic arenaviruses with the host adaptive immune system, in particular, virus-induced immunosuppression, and understanding of the terminal hemorrhagic shock syndrome.
To study the direct effects of virus replication and gene expression that may be responsible for the perturbation of endothelial cell function, future research involving cell culture models for human endothelium that allow detailed analysis of virus-induced cell biological and biochemical alterations will have great importance.
Research on early molecular events of JUNV infection involving viral glycoprotein spikes and cell-surface receptors as well as virion and cell membrane fusion provided the basis for the development of novel therapeutic strategies [19, 35]. Other potential therapeutic targets being explored are specific steps for virus entry, processing, and replication [27, 78]. In particular, the multifunctional Z protein has been explored as target for antiviral compounds including siRNA [8, 26, 28]. Host cell factors have been also proposed as antiviral targets in arenavirus infection [43].
In addition to small antiviral molecules, immune therapy can be regarded as an alternative for AHF patients. Based on the success of immune therapy in controlling AHF mortality, it is possible to design a strategy replacing convalescent immune plasma, which is in short supply, with controlled humanized neutralizing monoclonal antibodies appropriately tested for efficacy [50, 88].
Further studies on new potential treatments are needed to block viral replication without causing toxicity and to prevent the increased vascular permeability that is responsible for hypotension and shock.
References
Acuna-Soto R, Romero LC et al (2000) Large epidemics of hemorrhagic fevers in Mexico 1545–1815. Am J Trop Med Hyg 62(6):733–739
Albarino CG, Bergeron E et al (2009) Efficient reverse genetics generation of infectious Junin viruses differing in glycoprotein processing. J Virol 83(11):5606–5614
Albarino CG, Bird BH et al (2011) The major determinant of attenuation in mice of the Candid1 vaccine for argentine hemorrhagic fever is located in the G2 glycoprotein transmembrane domain. J Virol 85(19):10404–10408
Albarino CG, Bird BH et al (2011) Reverse genetics generation of chimeric infectious Junin/Lassa virus is dependent on interaction of homologous glycoprotein stable signal peptide and G2 cytoplasmic domains. J Virol 85(1):112–122
Albarino CG, Ghiringhelli PD et al (1997) Molecular characterization of attenuated Junin virus strains. J Gen Virol 78(Pt 7):1605–1610
Ambrosio A, Saavedra M et al (2011) Argentine hemorrhagic fever vaccines. Hum Vaccin 7(6):694–700
Ambrosio AM, Enria DA et al (1986) Junin virus isolation from lympho-mononuclear cells of patients with Argentine hemorrhagic fever. Intervirology 25(2):97–102
Artuso MC, Ellenberg PC et al (2009) Inhibition of Junin virus replication by small interfering RNAs. Antivir Res 84(1):31–37
Auperin DD, Romanowski V et al (1984) Sequencing studies of pichinde arenavirus S RNA indicate a novel coding strategy, an ambisense viral S RNA. J Virol 52(3):897–904
Bao Y, Chetvernin V et al (2014) Improvements to pairwise sequence comparison (PASC): a genome-based web tool for virus classification. Arch Virol 159(12):3293–3304
Bisordi I, Levis S et al (2015) Pinhal virus, a new arenavirus isolated from Calomys tener in Brazil. Vector Borne Zoonotic Dis 15(11):694–700
Borio CS, Bilen MF et al (2012) Antigen vehiculization particles based on the Z protein of Junin virus. BMC Biotechnol 12(1):1–9
Briggiler AM, Enria DA et al (1987) Contagio interhumano e infección clfnica con virus Junin (V J) en matrimonios residentes en el area endémica de Fiebre Hemorráigica Argentina (FHA). Medicina (B Aires) 47:565
Cajimat MN, Milazzo ML et al (2012) Ocozocoautla de espinosa virus and hemorrhagic fever, Mexico. Emerg Infect Dis 18(3):401–405
Casabona JC, Levingston Macleod JM et al (2009) The RING domain and the L79 residue of Z protein are involved in both the rescue of nucleocapsids and the incorporation of glycoproteins into infectious chimeric arenavirus-like particles. J Virol 83(14):7029–7039
Castilla V, Contigiani M et al (2005) Inhibition of cell fusion in Junin virus-infected cells by sera from argentine hemorrhagic fever patients. J Clin Virol 32(4):286–288
Coimbra TLM, Nassar ES et al (1994) New arenavirus isolated in Brazil. Lancet 343(8894):391–392
Cordo SM, Valko A et al (2013) Membrane localization of Junin virus glycoproteins requires cholesterol and cholesterol rich membranes. Biochem Biophys Res Commun 430(3):912–917
Crispin M, Zeltina A et al (2016) Native functionality and therapeutic targeting of arenaviral glycoproteins. Curr Opin Virol 18:70–75
Delgado S, Erickson BR et al (2008) Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog 4(4):e1000047
Enria D, Briggiler AM et al (1998) An overview of the epidemiological, ecological and preventive hallmarks of Argentine hemorrhagic fever (Junin virus). Bull Inst Pasteur 96:12
Enria DA, Ambrosio AM et al (2010) Candid#1 vaccine against Argentine hemorrhagic fever produced in Argentina. Immunogenicity and safety. Medicina 70(3):215–222
Enria DA, Barrera Oro JG (2002) Junin virus vaccines. Curr Top Microbiol Immunol 263:239–261
Enria DA, Briggiler AM et al (2008) Treatment of argentine hemorrhagic fever. Antivir Res 78(1):132–139
Forlenza MB, Roldán JS et al (2011) Interacción del virus Junín con lectinas de tipo C y mecanismos endocíticos involucrados. In: X Congreso Argentino de Virología, pp 162–163
Garcia CC, Djavani M et al (2006) Arenavirus Z protein as an antiviral target: virus inactivation and protein oligomerization by zinc finger-reactive compounds. J Gen Virol 87(pt 5):1217–1228
García CC, Sepúlveda CS et al (2011) Novel therapeutic targets for arenavirus hemorrhagic fevers. Future Virol 6(1):27–44
Garcia CC, Topisirovic I et al (2010) An antiviral disulfide compound blocks interaction between arenavirus Z protein and cellular promyelocytic leukemia protein. Biochem Biophys Res Commun 393(4):625–630
Ghiringhelli PD, Rivera-Pomar RV et al (1991) Molecular organization of Junin virus S RNA: complete nucleotide sequence, relationship with other members of the Arenaviridae and unusual secondary structures. J Gen Virol 72(9):2129–2141
Gomez RM, Pozner RG et al (2003) Endothelial cell function alteration after Junin virus infection. Thromb Haemost 90(2):326–333
Goni SE, Iserte JA et al (2006) Genomic features of attenuated Junin virus vaccine strain candidate. Virus Genes 32(1):37–41
Goni SE, Iserte JA et al (2010) Molecular analysis of the virulence attenuation process in Junin virus vaccine genealogy. Virus Genes 40(3):320–328
Goni SE, Stephan BI et al (2011) Viral diversity of Junin virus field strains. Virus Res 160(1–2):150–158
Harrison LH, Halsey NA et al (1999) Clinical case definitions for argentine hemorrhagic fever. Clin Infect Dis 28(5):1091–1094
Helguera G, Jemielity S et al (2012) An antibody recognizing the apical domain of human transferrin receptor 1 efficiently inhibits the entry of all new world hemorrhagic fever arenaviruses. J Virol 86(7):4024–4028
Iapalucci S, Lopez N et al (1991) The 3′ end termini of the Tacaribe arenavirus subgenomic RNAs. Virology 182(1):269–278
Iserte JA, Stephan BI et al (2013) Family-specific degenerate primer design: a tool to design consensus degenerated oligonucleotides. Biotechnol Res Int 2013:383646
Iula LJ, Martínez MG et al (2011) La expresión del receptor celular DC-SIGN aumenta la capacidad infectiva del virus Junín. Interacción directa del receptor con la glicoproteína viral. In: X Congreso Argentino de Virología, p 42
Jacamo R, Lopez N et al (2003) Tacaribe virus Z protein interacts with the L polymerase protein to inhibit viral RNA synthesis. J Virol 77(19):10383–10393
Kerber R, Reindl S et al (2015) Research efforts to control highly pathogenic arenaviruses: a summary of the progress and gaps. J Clin Virol 64:120–127
Levis SC, Saavedra MC et al (1985) Correlation between endogenous interferon and the clinical evolution of patients with argentine hemorrhagic fever. J Interferon Res 5(3):383–389
Linero FN, Scolaro LA (2009) Participation of the phosphatidylinositol 3-kinase/Akt pathway in Junin virus replication in vitro. Virus Res 145(1):166–170
Linero FN, Sepulveda CS et al (2012) Host cell factors as antiviral targets in arenavirus infection. Viruses 4(9):1569–1591
Lopez N, Jacamo R et al (2001) Transcription and RNA replication of tacaribe virus genome and antigenome analogs require N and L proteins: Z protein is an inhibitor of these processes. J Virol 75(24):12241–12251
Loureiro ME, D’Antuono A et al (2012) Uncovering viral protein-protein interactions and their role in arenavirus life cycle. Viruses 4(9):1651–1667
Loureiro ME, Wilda M et al (2011) Molecular determinants of arenavirus Z protein homo-oligomerization and L polymerase binding. J Virol 85(23):12304–12314
Lozano ME, Enria D et al (1995) Rapid diagnosis of argentine hemorrhagic fever by reverse transcriptase PCR-based assay. J Clin Microbiol 33(5):1327–1332
Lozano ME, Posik DM et al (1997) Characterization of arenaviruses using a family-specific primer set for RT-PCR amplification and RFLP analysis. Its potential use for detection of uncharacterized arenaviruses. Virus Res 49(1):79–89
Machado AM, Figueiredo GG et al (2010) Standardization of an ELISA test using a recombinant nucleoprotein from the Junin virus as the antigen and serological screening for arenavirus among the population of Nova Xavantina, state of Mato Grosso. Rev Soc Bras Med Trop 43(3):229–233
Mahmutovic S, Clark L et al (2015) Molecular basis for antibody-mediated neutralization of New World hemorrhagic fever mammarenaviruses. Cell Host Microbe 18(6):705–713
Maiztegui JI, McKee KT Jr et al (1998) Protective efficacy of a live attenuated vaccine against argentine hemorrhagic fever. AHF study group. J Infect Dis 177(2):277–283
Marr JS, Kiracofe JB (2000) Was the huey cocoliztli a haemorrhagic fever? Med Hist 44(3):341–362
Marta RF, Montero VS et al (1999) Proinflammatory cytokines and elastase-alpha-1-antitrypsin in Argentine hemorrhagic fever. Am J Trop Med Hyg 60(1):85–89
Marta RF, Montero VS et al (1998) Systemic disorders in argentine haemorrhagic fever. Bull Inst Pasteur 96:115–124
Martinez MG, Cordo SM et al (2007) Characterization of Junín arenavirus cell entry. J Gen Virol 88(6):1776–1784
Martinez MG, Cordo SM et al (2008) Involvement of cytoskeleton in Junin virus entry. Virus Res 138(1–2):17–25
Martinez MG, Forlenza MB et al (2009) Involvement of cellular proteins in Junin arenavirus entry. Biotechnol J 4(6):866–870
Milazzo ML, Barragan-Gomez A et al (2010) Antibodies to Tacaribe serocomplex viruses (family Arenaviridae, genus Arenavirus) in cricetid rodents from New Mexico, Texas, and Mexico. Vector Borne Zoonotic Dis 10(6):629–637
Milazzo ML, Cajimat MN et al (2015) Epizootiology of Tacaribe serocomplex viruses (Arenaviridae) associated with neotomine rodents (Cricetidae, Neotominae) in southern California. Vector Borne Zoonotic Dis 15(2):156–166
Mills JN, Ellis BA et al (1994) Prevalence of infection with Junin virus in rodent populations in the epidemic area of Argentine hemorrhagic fever. Am J Trop Med Hyg 51(5):554–562
Molinas FC, de Bracco MM et al (1981) Coagulation studies in Argentine hemorrhagic fever. J Infect Dis 143(1):1–6
Morales MA, Calderon GE et al (2002) Evaluation of an enzyme-linked immunosorbent assay for detection of antibodies to Junin virus in rodents. J Virol Methods 103(1):57–66
Nakauchi M, Fukushi S et al (2009) Characterization of monoclonal antibodies to Junin virus nucleocapsid protein and application to the diagnosis of hemorrhagic fever caused by South American arenaviruses. Clin Vaccine Immunol CVI 16(8):1132–1138
Negrotto S, Mena HA et al (2015) Human plasmacytoid dendritic cells elicited different responses after infection with pathogenic and nonpathogenic Junin virus strains. J Virol 89(14):7409–7413
Parisi G, Echave J et al (1996) Computational characterisation of potential RNA-binding sites in arenavirus nucleocapsid proteins. Virus Genes 13(3):247–254
Parodi AS, Greenway DJ et al (1958) Sobre la etiología del brote epidémico de Junín. Dia Med 30:2300–2301
Parodi AS, Rugiero HR et al (1959) Isolation of the Junin virus (epidemic hemorrhagic fever) from the mites of the epidemic area (Echinolaelaps echidninus Barlese). Prensa Med Argent 46:2242–2244
Peña Cárcamo JR (2016) Viperina, un factor de restricción en la infección con el arenavirus Junín. PhD thesis, Universidad de Buenos Aires, Argentina
Pozner RG, Ure AE et al (2010) Junin virus infection of human hematopoietic progenitors impairs in vitro proplatelet formation and platelet release via a bystander effect involving type I IFN signaling. PLoS Pathog 6(4):e1000847
Radoshitzky SR, Abraham J et al (2007) Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature (Lond) 446(7131):92–96
Radoshitzky SR, Bao Y et al (2015) Past, present, and future of arenavirus taxonomy. Arch Virol 160(7):1851–1874
Reignier T, Oldenburg J et al (2006) Receptor use by pathogenic arenaviruses. Virology 353(1):111–120
Roldan JS, Martinez MG et al (2016) Human transferrin receptor triggers an alternative Tacaribe virus internalization pathway. Arch Virol 161(2):353–363
Romanowski V, Bishop DH (1983) The formation of arenaviruses that are genetically diploid. Virology 126(1):87–95
Romanowski V, Pidre ML et al (2013) Argentine hemorrhagic fever. In: Singh SK (ed) Viral hemorrhagic fevers. CRC Press, Boca Raton [ http://www.crcpress.com /]/Taylor & Francis Group, pp 317–337
Sabattini MS, Gonzalez de Rios LE et al (1977) Infección natural y experimental de roedores con virus Junin. Medicina (B Aires) 37:149–159
Salvato MS, Clegg JCS et al (2012) Family Arenaviridae. In: Virus taxonomy. Elsevier, San Diego, pp 715–723
Sepulveda CS, Garcia CC et al (2012) Inhibition of Junin virus RNA synthesis by an antiviral acridone derivative. Antiviral Res 93:16–22
Tesh RB, Jahrling PB et al (1994) Description of Guanarito virus (Arenaviridae: arenavirus), the etiologic agent of Venezuelan hemorrhagic fever. Am J Trop Med Hyg 50(4):452–459
Tortorici MA, Albarino CG et al (2001) Arenavirus nucleocapsid protein displays a transcriptional antitermination activity in vivo. Virus Res 73(1):41–55
Tortorici MA, Ghiringhelli PD et al (2001) Zinc-binding properties of Junin virus nucleocapsid protein. J Gen Virol 82(pt 1):121–128
Ure AE, Ghiringhelli PD et al (2008) Argentine hemorrhagic fever diagnostic test based on recombinant Junin virus N protein. J Med Virol 80(12):2127–2133
Vela EM, Colpitts TM et al (2008) Pichinde virus is trafficked through a dynamin 2 endocytic pathway that is dependent on cellular Rab5- and Rab7-mediated endosomes. Arch Virol 153(7):1391–1396
Vezza AC, Gard GP et al (1977) Structural components of the arenavirus Pichinde. J Virol 23(3):776–786
Vitullo AD, Hodara VL et al (1987) Effect of persistent infection with Junin virus on growth and reproduction of its natural reservoir, Calomys musculinus. Am J Trop Med Hyg 37(3):663–669
York J, Agnihothram SS et al (2005) Genetic analysis of heptad-repeat regions in the G2 fusion subunit of the Junin arenavirus envelope glycoprotein. Virology 343(2):267–274
York J, Romanowski V et al (2004) The signal peptide of the Junin arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature G1–G2 complex. J Virol 78(19):10783–10792
Zeitlin L, Geisbert JB et al (2016) Monoclonal antibody therapy for Junin virus infection. Proc Natl Acad Sci U S A 113(16):4458–4463
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Romanowski, V., Pidre, M.L., Lozano, M.E., Goñi, S.E. (2017). Arenaviruses and Hemorrhagic Fevers: From Virus Discovery to Molecular Biology, Therapeutics, and Prevention in Latin America. In: Ludert, J., Pujol, F., Arbiza, J. (eds) Human Virology in Latin America. Springer, Cham. https://doi.org/10.1007/978-3-319-54567-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-54567-7_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-54566-0
Online ISBN: 978-3-319-54567-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)